ABSTRACT
Despite the existence of a highly efficient yellow fever vaccine, yellow fever reemergence throughout Africa and the Americas has put 900 million people in 47 countries at risk of contracting the disease. Although the vaccine has been key to controlling yellow fever epidemics, its live-attenuated nature comes with a range of contraindications that prompts advising against its administration to pregnant and lactating women, immunocompromised individuals, and those with hypersensitivity to chicken egg proteins. Additionally, large outbreaks have highlighted problems with insufficient vaccine supply, whereby manufacturers rely on slow traditional manufacturing processes that prevent them from ramping up production. These limitations have contributed to an inadequate control of yellow fever and have favored the pursuit of novel yellow fever vaccine candidates that aim to circumvent the licensed vaccine’s restrictions. Here, we review the live-attenuated vaccine’s limitations and explore the epitome of a yellow fever vaccine, whilst scrutinizing next-generation vaccine candidates.
KEYWORDS: Yellow fever, yellow fever vaccine, live-attenuated vaccine, vaccine shortage, vaccine contraindications, yellow fever next-generation vaccines, disease control, global health, emerging disease
Background
Yellow fever (YF) is considered one of the deadliest diseases in human history. The dramatic onset of symptoms and a case-fatality rate (CFR) of 30–60% during severe disease is substantially higher than the 1918s Spanish Influenza (>2.5%), making YF a feared infectious disease since the 18th century.1–5 Yellow fever decimated the human population in Africa and the Americas until the first half of the 20th century, when a safe and efficient vaccine was developed by Max Theiler and colleagues in 1936.2,3,6,7
Yellow fever has proven to be a difficult opponent to combat.8,9 YF vaccines have been a powerful tool to help controlling YF epidemics, yet the World Health Organization (WHO) still considers 47 countries in Africa and the Americas under high risk of YF epidemics.10,11 Nowadays, it is estimated that a total of 900 million people are at risk of YF across the globe, with 610 million people being at risk in Africa alone.10,12,13
The history of YF reveals that it has been a recurrent problem in those endemic regions of Africa and South America.3,7,14 Even in countries that were declared free of YF, reemergence has been observed.3,7,15,16 This is the case of Kenya, a country severely affected by YF throughout history and where a major YF outbreak struck the Rift Valley Province in 1992, 50 years after being considered free of the disease.7
In South America, Yellow fever virus (YFV) introduction to non-endemic regions has also caused large sylvatic and urban outbreaks. In 2016, two independent events of YFV introduction into Brazil’s Southeastern region of Minas Gerais resulted in the extensive dispersion of two YFV sub-lineages, which persisted until 2019 in some of Brazil’s most populated cities and other regions that had been considered free of YF for more than 80 years.17,18 Paraguay has also suffered from continuous re-emergence of YF in some of its regions.15,16 In 2008, YF resurfaced in Paraguay’s capital, Asuncion, becoming an urban epidemic after 34 years of YF absence.15,19
Throughout every outbreak, Theiler´s 17D vaccine has been crucial to control YF epidemics. Considered the most effective live-attenuated vaccine ever created, it confers life-long immunity upon a single dose, resulting in a seroconversion rate of 98% and induction of highly neutralizing antibodies in only 10 days after vaccination.11,20–22 Despite the unarguable vaccine efficacy, a range of contraindications and drawbacks have contributed to an inadequate control of yellow fever, restricting the possibilities to eliminate the yellow fever virus across the globe.
Limitations of the YF live-attenuated Vaccine
Little has changed in the yellow fever live-attenuated vaccine (YF-LAV) since the 1930s when it was derived from the original wild-type strain ‘Asibi’.21,23,24 Continuous virus serial passage in mouse and chicken tissues resulted in the multigenic attenuation of the YFV.6,24,25 A total of 176 serial passages rendered the virus less virulent due to the accumulation of at least 28 ‘naturally’ occurring substitutions across the whole YFV genome.6,24,25
The live-attenuated vaccine platform gives the YF vaccine their indisputable efficacy. Mimicking natural infection in a less pathogenic manner, it prompts mounting a complex immune response with a robust innate immune activation, strong antigen-specific T-cell responses, and production of persistent memory B cells.1,11 However, it is the live-attenuated nature of the vaccines that also result in most of its contraindications and inherent risks.9,16
Overall, and as advised by the Center of Disease Control of the USA ‘Yellow fever vaccination is contraindicated and should be avoided when the recipient has a condition that increases the risk for a serious adverse reaction’.26 Contraindications against the YF-LAV include pregnant and lactating women, infants under 6 months of age, senior citizens over 60 years of age; and subjects with severe immunodeficiency, conditions or treatments considered to induce a severe immunocompromised state, such as primary immunodeficiencies, HIV infection, transplantation, or recent chemotherapies and radiation therapies, among others.23,26,27 Furthermore, YF vaccines are also contraindicated in individuals suffering from hypersensitivity to egg proteins and their derivates.26
Except in the case of an outbreak emergency, contraindication of the YF-LAV in pregnant and lactating women is due to the theoretical risk of vertical transmission of the virus from mother to child.2,7,28–30 This recommendation is based on the suggestion that a higher risk is implied in young infants due to the vaccine neurotropism.9 Despite this, the World Health Organization (WHO) indicates that a risk-benefit assessment is to be made during outbreaks and when living in a YF endemic region, as the benefits of vaccination can compensate the risk of transmitting the attenuated virus to the fetus.7,9,28–30 According to the World Population Prospects of the Department of Economic and Social Affairs of the United Nations, from 2015 to 2020 there were an estimated 153.18 million births in YF endemic regions.31 This number, which still does not consider those women suffering miscarriages, provides insight into the number of pregnant women at a potential high risk of YF infection. This is especially relevant for countries struck by annual YF outbreaks, in which the administration of fractional doses of YF vaccines due to vaccine shortage, is suggested to result in protection of the recipients for approximately 12 months.32 In this scenario, women in reproductive age living in YF endemic regions are often put in the position of deciding whether or not to experience risk upon vaccination during pregnancy. Similarly, although vaccination of children under 6 months is contraindicated, vaccination of infants between 6 and 8 months may be considered when there is significant risk of YFV infection during epidemics or when travel to a YF endemic region is inevitable.26,33
Vaccination of immunocompromised subjects against YF poses an increased risk of developing vaccine-associated neurotropic and viscerotropic disease.2 Even if vaccination does not cause disease, the resulting immune response may not be adequate enough to result in protection.27,34 Thus, the benefits of administering the YF-LAV to immunocompromised patients may not outweigh the risks of developing disease.27 The number of immunocompromised or temporarily immunosuppressed individuals in YF risk areas can be counted in hundreds of millions. According to the International Agency for Research on Cancer of the WHO, in 2018, the estimated incidence of all cancers in both sexes and ages was of 487.76 million people living in YF risk areas.35 Likewise, data gathered by the Joint United Nations Program on HIV/AIDS on the prevalence of HIV among adults from 15 to 49 years of age in 2018, indicate that at least 64.6 million people living in YF endemic areas have been confirmed positive to HIV infection and are at risk of progressing to AIDS.36 UNAIDS also indicates that this number will increase yearly. In 2018, more than 300,000 new HIV infections were reported in YF high-risk areas.36 However, YF-LAV safety has been demonstrated when administered to HIV-infected adults under antiretroviral therapy with CD4+ cell counts above 350 cells/μl.37
Hypersensitivity to chicken eggs is the second most common food allergy in children.38 Although the prevalence of egg-hypersensitivity varies from region to region, it has been suggested it has a prevalence of 0.5–2.5% in young children.28,39 As a result of its manufacture using embryonated chicken eggs, individuals suffering of hypersensitivity to egg-derived proteins are not recommended to get full doses of the YF-LAV. Otherwise, chances of developing anaphylaxis after its administration are 1.8–3.2/100,000.2,7,38,40,41
While serious adverse events (SAE’s) associated with the YF-LAV are reported to be rare, their occurrence has been linked to the vaccine since its introduction.1,17,21,24 From 1945 to 2005, 23 cases of YF vaccine-associated neurotropic disease (YEL-AND) were reported.29 Whereas 65 cases of YF vaccine-associated viscerotropic disease (YEL-AVD) were reported from 2001 to 2011.1,19 The risk of developing YEL-AVD (0.4/100,000) or YEL-AND (0.8/100,000) can persuade many recipients against the benefits of vaccination;1 especially those of advanced age (>60), for whom the risk of acquiring SAE’s almost triples (1–2.3/100,000) due to immune senescence.9,16,28,34,42 According to the historical population estimates and projections based on the United Nations medium-fertility scenarios, at least 729.38 million people around the world over 65 years of age may be in this predicament in 2020.43
With previous arguments considered, the elimination of YF from endemic areas will surely remain a challenge until the large clusters of the population with current contraindications against the YF-LAV can access vaccination without further risks linked to the immunization.
Supply and Demand must match
It has become obvious that having a very efficient vaccine available is not the only requirement to control effectively disease epidemics. As A.D.T. Barrett8 suggests, success against YF also comes down to vaccine supply and demand.8 For the last 20 years, vaccine supply has been insufficient.10,14,44 The scarcity of the YF-LAV has been accentuated especially during emergencies by the inability of the manufacturers to ramp up production. According to the WHO, the ‘vaccine needs’ to eliminate YF epidemics from 2017 to 2026, including doses for routine immunizations, mass campaigns, and emergencies, is of 1,384 million doses; corresponding to 138.4 million doses per year.10 However, the total vaccine production from all manufacturers running at full capacity only adds up to approximately 80 million doses annually.24,45 The unmet requirements of YF-LAV are of almost 60 million doses yearly (Figure 1).
Figure 1.
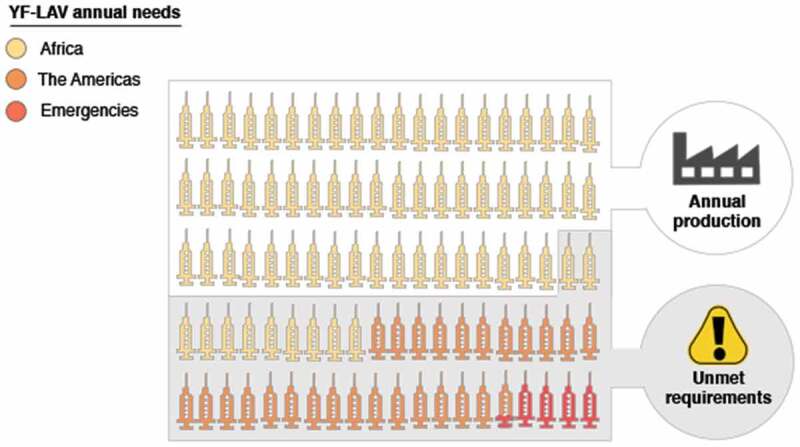
YF-LAV annual requirements to eliminate Yellow Fever. The World Health Organization suggests that a total of 138.4 million vaccine doses are required to eliminate Yellow Fever from Africa, Central, South America, and the Caribbean. However, the annual vaccine needs are significantly higher than the total production of all six manufacturers running at full capacity. Currently, an unmet requirement of 58.4 million doses remains per annum. In this graphic depiction of the annual vaccine needs each ‘syringe’ represents 1.3 million vaccine doses.
YF-LAVs are still produced using traditional manufacturing practices based on the propagation of attenuated YFV in chicken embryos.20 The extremely slow and time-consuming manufacturing process generates from 300 to 400 doses per egg.15,20,29,46 Besides the legacy technologies used for its manufacture, the YF-LAVs are dependent on the supply of one fundamental element: eggs.46,47 Following the biological standards defined by the WHO, these must be viable 12-day embryonated hen’s eggs from pathogen-free flocks.15,25,29 Since these are also required for the production of other live-attenuated vaccines such as the Influenza vaccine, their availability is limited. Nevertheless, unlike the YF-LAV, Influenza vaccines do not entirely rely on egg supply due to the existence of technologies available for their production using cell culture techniques.48
Currently, a total of five manufacturers in five countries (France, Senegal, the People’s Republic of China, the Russian Federation, and Brazil) produce YF vaccines.24,25,49,50 Nevertheless, only those qualified by the WHO (French, Russian, Senegalese and Brazilian) serve for international purposes and global supply, whilst vaccines manufactured in China are only deployed in domestic markets24,25,49,50 The United States was the sixth producer of YF vaccines until 2015, when their production was stopped as they transitioned to new manufacturing facilities.51 According to Sanofi U.S., the manufacturing of YF vaccines has resumed and are hoping to offer an update on their manufacture by the end of 2020.52
The inability to scale up vaccine production combined with the emergence of large outbreaks has resulted in vaccine shortage, which in turn has had a negative impact in vaccine coverage of YF endemic countries.10,45 From 2013 to 2015 at least seven countries at risk of YF reported the exhaustion of domestic vaccine stocks and received only half of their estimated vaccine requirements.10 In 2016, during an unprecedented outbreak in Angola, the spread of YF required the distribution of more than 12 million doses of vaccines, depleting WHO’s vaccine stockpiles twice.8,20,24,53–55 The Angola outbreak highlighted the inadequacy of the 6 million-dose vaccine stockpile during large outbreaks and the immunization with a fractional dose (one-fifth of the standard dose) was suggested as the best approach to fulfill the urgent vaccine demand.20,53,55,56
The use of fractional doses of YF-LAV has proven to be safe and effective in situations of vaccine shortage.20,57 It is suggested that administration of one-fifth of a dose results in comparable protection to the one induced with a standard dose, and is maintained 10 years after primary vaccination.58 A study that examined long-term immunity in adults after the administration of reduced doses (10,447 IU, 3,013 IU, and 587 IU) of YF-LAV, demonstrated that immunogenicity remained similar to immune responses mounted in subjects vaccinated with a standard dose (27,476 IU) after eight years, retaining an overall seroconversion rate of 87%.57
Dose fractioning practices have also been adopted to control large outbreaks in Brazil.9 Shortly after the 2016 large YFV outbreak in Southeastern Brazil concluded, the Brazilian government began a mass immunization campaign over fears of YF epizootic circulation in non-human primates.1,59 The emergence of a significant number of YF cases in non-endemic areas suggested the possibility of YFV establishment in highly populated regions with little vaccine coverage.1,18 Around 20 million people from 76 municipalities in the states of Sao Paolo, Rio de Janeiro, and Bahia were planned to be vaccinated from February to March 2018.59 With exhausted global vaccine stockpiles and undergoing substantial shortage, dose fractioning was a valuable strategy to expand vaccine coverage in order to reach all communities at risk, specially men aged 14 to 35, who due to occupational exposure are most vulnerable.1,18 Almost four weeks into the immunization campaign, one-quarter of the target population had been vaccinated, for which 92% of vaccine doses were fractionated.59 By April 2018, the use of fractioned doses allowed to increase vaccine coverage to 94.9% and 68.5% in Sao Paulo and Rio de Janeiro, respectively, reaching the necessary coverage levels (60–80%) described by the WHO to contain YF epidemics.9,10,60
The use of fractional doses was suitable to help resolve the ongoing emergency, albeit it should only be considered as a temporary solution to vaccine shortage.9 Until sufficient data are gathered regarding the safety and efficacy of the use of fractional dosing in children, elderly, pregnant women, and immunocompromised individuals, the extensive use of fractional doses is not recommended.9,57 Furthermore, as of 2019, fractional dosing of the YF-LAV had not met the requirements of International Health Regulations, and therefore, the WHO does not recommend fractional-dose vaccination unless during extensive outbreaks.61
Recent YF outbreaks have further exhibited the challenging nature of managing infectious diseases in a modern super-connected world, emphasizing the risk of YF importation to non-endemic regions via travelers.9,19 YF widespread from Angola not only reached neighboring countries as the Democratic Republic of Congo and Kenya, but also distant countries like China, resulting in the first documented introduction of YF to Asia in human history.8,24,53,62 Alarmingly, a total of 11 confirmed cases were imported to China, putting at risk at least 1.8 billion naïve residents living in areas where vector-competent mosquitoes were present.8,62 The introduction of YF to China through travelers confirmed the potential of further widespread.8,11,24,44 According to the Yearbook of Tourism Statistics of the World Tourism Organization and the World Bank, at least 31.2 million arrivals of international travelers entered YF risk regions in 2017.63 Moreover, because of the extensive presence of the vector mosquitoes of the genres Aedes, almost half of the world’s population (more than 3 billion people) would be at risk upon YF importation (Figure 2).7,11,45,53,64–66 Given the vulnerability of Central, North America and Asia, if extensive concurrent outbreaks were to happen in one or more regions, the current YF-LAV vaccine supply would fall short again.13,45,62
Figure 2.
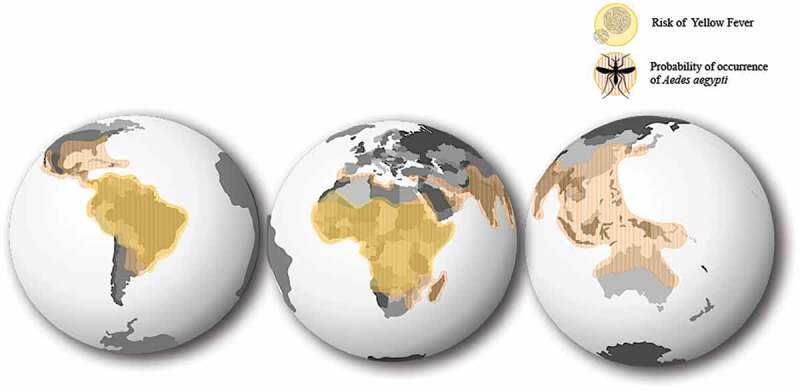
Overlapping distribution of Yellow Fever virus and Aedes aegypti’s probability of occurrence. According to the World Health Organization 47 countries in Africa, and Central and South America are considered Yellow Fever endemic countries (Yellow). YF endemic areas are regions where Yellow Fever virus circulation has been reported in either sylvatic or urban cycles, and therefore hold a potential risk of Yellow Fever virus transmission. Transmission of YF in non-endemic areas can be perpetuated upon importation of a YF infected subject to a region endemic to Yellow Fever’s main vector, the Aedes aegypti mosquito. Extensive widespread of Aedes mosquitoes (Orange) and the upsurge in international human migration puts half of the world’s population at risk upon YF importation.
A way out of Yellow fever: new generation Vaccines
YF most likely will never be eradicated.9,10 A combination of existing non-human primates animal reservoirs, an increase of abundance and dispersion of vector species; and the health authorities’ inability to control mosquito populations in sylvatic and urban infection cycles, has prevented the reduction of YFV transmission.9 Nevertheless, YF epidemics may be contained if we maintain high levels of immunity in the population (60–80%).9,10,60
The current limited vaccine production and shortage will hardly contribute to changing YF’s panorama. Hence, the production of novel YF vaccines using cutting-edge technologies is necessary to respond to further outbreak emergencies without risk of shortage.9 Ideally, the next generation YF vaccines would circumvent most of the YF-LAV manufacturing restrictions and contraindications, whilst maintaining a high safety profile and the ability to elicit a robust immune response upon a single vaccine dose.67 Most importantly, any new vaccine candidate must show equivalent immunogenicity and protective efficacy to the existing licensed vaccine.49 Currently, two surrogate markers for protection are used to assess protective efficacy: log10 neutralization index (LNI) ≥0.7 or a neutralization titer of ≥1:10.49,60
To date there are at least nine YF vaccine candidates under development, of which one-third have entered Phase I clinical trials. Further information of various vaccine candidates is described in Table 1. Several vaccine platforms are being tested, the use of inactivated virus being the most common approach, as it has been previously proved successful for the development of other arbovirus vaccines.67,68 XRX-001 is an inactivated vaccine candidate based on a β-propriolactone-inactivated virus generated in cell culture (Vero cells).40 This vaccine candidate conferred protection to hamsters against lethal challenge through passive immunization and resulted in the generation of neutralizing antibody titers in cynomolgus macaques.40,69 When XRX-001 progressed to a double-blind, placebo-controlled, dose-escalation Phase I safety trial with alum adjuvant, the induction of neutralizing antibodies and seroconversion (endpoint/baseline PRNT50 > 4) of subjects was reported.70 Nevertheless, two high doses (4.8 μg of antigen) were required for the development of neutralizing antibodies in 100% of the participants.70 Despite inactivated vaccines may require multiple doses to match the immune responses mounted by live virus vaccines, often they are preferred due to the improvement of the safety profile.25,71 The inactivated nature of XRX-001 prevents the inherent risks that live vaccines pose and therefore, it can be administered to those against the YF-LAV is contraindicated.9,67 If incorporated into mass preventive vaccination campaigns, inactivated vaccines like XRX-001 could be a useful tool to avoid severe adverse events in patients at risk.70,71 However, its requirement of multiple-dose administration would require further effort and expense, making them unsuitable for emergencies during large outbreaks.71
Table 1.
General features of novel vaccine candidates against Yellow fever
| Vaccine Candidate | Vaccine Platform | Strategy | Manufacturer | Country | Stage of development |
|---|---|---|---|---|---|
| XRX-001 | Inactivated | B-propiolactone virus inactivation. | Xcellerex, GE Healthcare, PnuVax. |
USA | Phase I Completed (NCT00995865) |
| pL/YFE | DNA | Expression of YF’s full-length envelope protein associated to the lysosomal membrane protein signal 1 (LAMP-1). | Oswaldo Cruz Foundation (Fiocruz) | Brazil | Preclinical |
| pYF17D-16 | Immunization DNA (i-DNA) |
DNA launched live-attenuated virus. | Medigen | Germany | Preclinical |
| MVA-BN-YF | Viral Vector | Attenuated, non-replicating MVA expressing YF’s envelope protein. |
Bavarian Nordic & National Institute of Allergy and Infectious Diseases (NIAID) | Denmark, USA | Phase I Completed (NCT02743455) |
The use of novel DNA vaccines encoding YFV’s proteins has also been investigated.72–75 Three doses of a DNA vaccine comprising the full-length envelope protein (prME) fused to the lysosomal-associated membrane protein signal 1 (LAMP-1), protected mice from a lethal challenge through the induction of robust T-cell responses and high neutralizing antibody titers. Nevertheless, the candidate’s neutralizing antibodies titers were 3.5-fold lower than the YF-LAV-induced titers.75 Another DNA vaccine based on the expression of the full-length genome of YF’s 17D live-attenuated strain has been evaluated. By combining the advantages of the DNA vaccine platform with the efficacy of live vaccines, this candidate uses ‘immunization DNA’ technology (i-DNA®) to launch live attenuated virus in recipients, eliciting an immune response in vivo after single vaccination.72,74 When tested in mice, seroconversion of all animals was observed 21 days after single vaccination, and neutralizing antibody titers in i-DNA® vaccinated mice were greater or equivalent to those vaccinated with the YF 17D vaccine.72 Although no DNA vaccine has been licensed to date, they represent a promising alternative with the potential to improve the safety of live vaccines by removing their infectious nature.73 Moreover, DNA vaccines benefit from simpler manufacture, have the potential to be scaled up during emergencies, and have lesser requirements for its manipulation, storage, and distribution.73,74
Surprisingly, no YF mRNA vaccine candidates have been proposed yet. Although initially mRNA vaccines were considered unworthy to be pursued as vaccine platform, today, one of the leading candidates to fight the emergence of SARS CoV-2, and the first to enter Phase I clinical trials (NCT04283461), is an mRNA vaccine.76–78 Improvements in stability and antigen-delivery efficiency have made of mRNA vaccines a promising technology with the potential to be rapidly designed, manufactured, and produced whilst retaining good safety profiles when tested in Phase I clinical trials.77–79
Recombinant viral vectors have also been considered for the development of safer YF vaccines with the potential of providing scalable production. The most advanced vaccine of this type is a non-replicating Modified Vaccinia Ankara developed by Bavarian Nordic A/S in collaboration with The National Institute of Allergy and Infectious Diseases (NIAID).23,80,81 After showing promising results in preclinical settings by inducing high levels of neutralizing antibodies (>1.0 log10 PRNT50) and conferring protection to challenged hamsters after a prime-boost regimen, the vaccine candidate was evaluated in a Phase I clinical trial.23,80,82 Despite its completion in June 2018, no further information on its progression into a Phase II clinical trial has been provided. Viral vector vaccines have proven to be very efficient in the delivery of antigens and generally do not present significant adverse effects after immunization.83–85 In particular, further attenuation of MVA into non-replicating live virus makes it suitable to be well tolerated by immunocompromised individuals and the elderly.83 No direct examination has been done regarding the use of recombinant poxvirus like MVA in pregnant or lactating women, except for their inclusion in the ongoing Ebola Vaccine Trial (NCT04521486) comprised of a heterologous, two-dose vaccination regimen consisting of Ad26.ZEBOV followed by MVA-BN-Filo.86,87 Vaccinated pregnant women will be followed to delivery to assess Ad26.ZEBOV and MVA-BN safety profiles. However, due to the inability of MVA to replicate in human cells, it is suggested that its administration to pregnant or lactating women would not represent a substantial risk.86
Despite proven safe and effective, Bavarian Nordic’s MVA vaccine is still not the ideal candidate to substitute the YF-LAV. MVA-BN-YF’s major disadvantage is the requirement of multiple doses to achieve comparable protection to a single dose of YF-LAV. Single immunization translates to fewer visits to healthcare centers. This is critical for the feasibility of large vaccination campaigns. In outbreak scenarios, the requirement of multiple doses may result in low compliance, and therefore, in recipients with only partial protection. Additionally, the requirement of boosters would result in an increase on packaging and transport costs, larger inventories, and the need of larger storage facilities. Therefore, further alternatives with equivalent safety and efficacy levels but that do not require the administration of a booster should be explored.
Other viral vectors, such as recombinant simian adenovirus, may represent a more appropriate platform to control YF epidemics. Simian Adenoviruses such as ChAdOx1 have been extensively studied as a vaccine platform.88 ChAdOx1 has been used as a vaccine vector with acceptable safety profiles for more than 10 different pathogens, including SARS-CoV-2 (NCT04324606).88–90 ChAdOx1 has also proved to be immunogenic when used as vector for vaccines against other Flavivirus.91 A ChAdOx1 expressing prME proteins of Zika virus provide protective immunity in mice from lethal challenge and is being evaluated in a Phase I clinical trials (NCT04015648).91,92 Based on the safety, efficiency, and the scalable manufacturing processes using validated cell lines, ChAdOx1 has been already tested in thousands of volunteers confirming acceptable safety profiles.88,93 Therefore, a ChAdOx1 encoding YFV proteins may have the potential to achieve durable and powerful humoral and cellular immune responses with a single non-adjuvanted dose, whilst being suitable for its administration to those with contraindications against live-attenuated vaccines due to their replication-defective nature. If ChAdOx1 nCoV-19 was to be extensively deployed, the generation of immunity against ChAdOx1 may result in the deterrence of the immunogenicity and protection efficacy upon the administration of a second vaccine based on the same vector. Nevertheless, alternatives to overcome preexisting immunity of Adenovirus (Ad) derived vectors have been thoroughly investigated since the failure of the Human serotype 5 Ad-based vaccines clinical trials in the late 2000s.94 One strategy to circumvent the preexisting immunity to Ad vectors is the use of different immunization routes to escape specific tissue-resident T cells that target the vector, or the use of further Ad serotypes with equivalent or greater potency than ChAdOx1.94 Other strategy to avoid preexisting immunity to Ad vectors is the use of homologous or heterologous prime boost-regimens that may induce sufficient immune responses to confer protection.94 However, as suggested previously, requiring multiple vaccine doses to achieve complete protection against YF may not be suitable in widespread outbreak scenarios.
To date, evidence of the use of viral vector vaccines in clinical settings indicates the requirement of boosters. MVA-BN-YF required the administration of two doses in preclinical studies to achieve 100% protection of golden hamsters after challenge.80 Similarly, evidence from the safety and efficacy trials of ChAdOx1 nCov-19 showed an efficacy of 70.4% only after the administration of two doses.95 However, the fact that a single dose of ChAdOx1 nCov-19 conferred protection (64.1%) against symptomatic disease is still encouraging.95 Whether a single immunization of a viral-vectored vaccine is able to match the YF-LAV efficacy, safety, and persistence of protection without the need of a booster is still to be determined. If this was not the case, it will be necessary to compromise on the expectations of the novel vaccines, and plan immunization schedules similar to those of licensed vaccines that require the administration of multiple doses, such as the oral polio vaccine, the pneumococcal conjugate vaccine, and the pentavalent vaccine (Diphtheria, Tetanus, Pertussis, Haemophilus influenzae and Hepatitis B).96 In many YF endemic countries, these vaccines are included in the recommended routine immunization of young infants at two, four, and six months after birth.96 Therefore, the logistics necessary for the inclusion of a YF viral-vectored vaccine in a prime-boost immunization regimen may not be unreasonable.
No further evidence of progression of any of the novel YF vaccine candidates described previously is available. The evaluation of the XRX-001 inactivated vaccine in a double-blind Phase 2 clinical study was suggested to be the way forward to its licensure in 2011.70 Unfortunately, similarly to MVA-BN-YF, no Phase II trial was scheduled. This decision may have been the result of the vaccine candidate’s possible inferiority to the live-attenuated vaccine. Despite XRX-001’s improved safety, its licensure may have been deterred by the production of lower antibody titers with fewer durability to those generated after immunization with the YF-LAV.67 Regarding possible reasons why MVA-BN-YF did not progress to a Phase II trial, we cannot be certain, as the outcome of the Phase I clinical trial is yet to be published.
The design of a Phase III clinical trial depends on the outcome of the Phase II study. Therefore, we cannot expect a Phase III study evaluating XRX-001 or MVA-BN-YF to happen soon.
Further progression in the development of novel YF vaccines may also have been obstructed by the limited availability of funding. It has been estimated that the cost of a Phase II clinical trial for vaccine development ranges from 16 to 28 million US dollars.97 Considering that Phase III trials usually include up to 10 times more participants than a Phase II, it would not be unreasonable to suggest that the cost of a Phase III clinical study may double the cost of a Phase II. Furthermore, as funding tends to be allocated based on current disease burden and pandemic potential, it comes and goes as new diseases emerge. With the rise of SARS-CoV-2, and the rearrangement of funds to research this pathogen, we cannot envision YF vaccine candidates moving forward any time soon. Securing continuity of funding through all stages of clinical development (Phase I to III–IV) might be the only way in which we could see more novel vaccines reaching licensure. The development of several vaccine candidates against SARS-CoV-2 in record time has highlighted how crucial the commitment of sponsors is to maintain momentum and advance swiftly through the development pipeline.
Any candidates that result in improved safety and are able to obtain equivalent levels of efficacy and protection to the live-attenuated vaccine should be considered worthy of support, regardless of booster requirements. Ultimately, if a novel YF vaccine was licensed, it would improve vaccine availability and position global health authorities one step closer to preventing YF outbreaks by reducing the burden of vaccine shortage. However, we must recognize that even when vaccine shortage is not a limiting factor, the deployment of YF vaccines to every corner of YF endemic countries will present itself with its own challenges, such as fighting vaccine hesitancy and ensuring access to vaccination.
This review is a descriptive study and the information presented only provides an overview of those vaccine candidates in the development pipeline which have published data. A patent search led to finding at least six more YF vaccine candidates going through preclinical testing. However, their description and evidence on their progress or outcome was scarce. Therefore, the scope of this study is limited by the availability of public data. In addition, restricted access to data hindered our ability to further investigate some of the novel technologies currently being examined for the development of the aforementioned new generation of YF vaccines.
Final Remarks
Yellow fever remains a global threat despite a very efficient live-attenuated vaccine having been available on the market for more than 80 years. Although eradicating YF may not be possible, the reemergence of its epidemics will only be controlled if mass vaccination is sustained and population immunity is maintained high. Because of the YF-LAV current contraindications, manufacturing, and supply limitations, the need for a new YF vaccine is undisputable.
Many vaccine platforms have been investigated to circumvent some YF-LAV restrictions, albeit none seem to fulfill all the requirements. Inactivated, DNA and poxvirus-based viral vector vaccines examined so far may avoid the risk inherent of live-attenuated vaccines but require the administration of multiple doses to achieve the same level of protective long-lasting immunity. Thus, further alternative strategies are still needed to improve YF vaccination and intervene in future outbreak emergencies.
Acknowledgments
The views expressed in this publication are those of the authors and not necessarily those of the Department of Health and Social Care. CR is a Jenner Institute investigator and is supported by the NIHR Biomedical Research Center Oxford vaccine theme.
Funding Statement
This report is independent research, funded by the UK Department of Health and Social Care through Innovate UK “New vaccines for global epidemics: development and manufacture” [Grant No. 972216], and also funded from an ODA budget [Global Health (ODA), 16/107/05 – Design, development and GMP manufacture of a Zika vaccine]. The views expressed in this publication are those of the authors and not necessarily those of the Department of Health and Social Care.
Abbreviations
| Ad | Adenovirus |
| CFR | Case-fatality rate |
| PRNT50 | Plaque reduction neutralization test by 50% |
| sAd | Simian adenovirus |
| SAE | Severe adverse events |
| YEL-AND | Yellow fever vaccine associated neurotropic disease |
| YEL-AVD | Yellow fever vaccine associated viscerotropic disease |
| YF | Yellow fever |
| YF-LAV | Yellow fever live-attenuated vaccine |
| YFV | Yellow fever virus |
Disclosure statement
No competing interests are reported by the authors.
References
- 1.Douam F, Ploss A.. Yellow fever virus: knowledge gaps impeding the fight against an old foe. Trends Microbiol. 2018;26:913–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Monath TP. Yellow fever: an update. Lancet Infect Dis. 2001;1:11–20. [DOI] [PubMed] [Google Scholar]
- 3.Staples JE, Monath TP. Yellow fever: 100 years of discovery. JAMA. 2008;300:960–62. [DOI] [PubMed] [Google Scholar]
- 4.Taubenberger JK, Morens DM. 1918 Influenza: the mother of all pandemics. Emerg Infect Dis. 2006;12:15–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yellow Fever , 2019 case definition. Atlanta (USA): Centers of Disease Control and Prevention (CDC), 2019 [accessed 2020 Oct 13]. https://wwwn.cdc.gov/nndss/conditions/yellow-fever/case-definition/2019/:NationalNotifiableDiseasesSurveillanceSystem(NNDSS).
- 6.Theiler MSH. The effect of prolonged cultivation in vitro upon the pathogenicity of Yellow fever virus. J Exp Med. 1937;65:767‐86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tomori O. Yellow fever: the recurring plague. Crit Rev Clin Lab Sci. 2004;41:391–427. [DOI] [PubMed] [Google Scholar]
- 8.Barrett AD. Yellow fever in angola and beyond–the problem of vaccine supply and demand. N Engl J Med. 2016;375:301–03. [DOI] [PubMed] [Google Scholar]
- 9.Tomashek KM, Challberg M, Nayak SU, Schiltz HF. Disease resurgence, production capability issues and safety concerns in the context of an aging population: is there a need for a new yellow fever vaccine? Vaccines (Basel). 2019;7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.A global strategy to eliminate Yellow fever epidemics 2017–2026. Geneva (Switzerland): World Health Organization, 2018. [Google Scholar]
- 11.Collins ND, Barrett AD. Live attenuated yellow fever 17D vaccine: a legacy vaccine still controlling outbreaks in modern day. Curr Infect Dis Rep. 2017;19:14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.The L. Yellow fever: a global reckoning. Lancet. 2016;387:1348. [DOI] [PubMed] [Google Scholar]
- 13.Yellow fever: a current threat . Emergencies preparedness, response. World Health Organization. [accessed 2020 Apr 3]. https://www.who.int/csr/disease/yellowfev/impact1/en/. [Google Scholar]
- 14.Nathan N, Barry M, Van Herp M, Zeller H. Shortage of vaccines during a yellow fever outbreak in Guinea. Lancet. 2001;358:2129–30. [DOI] [PubMed] [Google Scholar]
- 15.Gardner CL, Ryman KD. Yellow fever: a reemerging threat. Clin Lab Med. 2010;30:237–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Seligman SJ, Casanova JL. Yellow fever vaccine: worthy friend or stealthy foe? Expert Rev Vaccines. 2016;15:681–91. [DOI] [PubMed] [Google Scholar]
- 17.Delatorre E, De Abreu FVS, Ribeiro IP, Gomez MM, Aac DS, Ferreira-de-brito A, Neves M, Bonelly I, de Miranda RM, Furtado ND, et al. Distinct YFV lineages co-circulated in the Central-Western And Southeastern Brazilian regions from 2015 to 2018. Front Microbiol. 2019;10:1079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Silva NIO, Sacchetto L, De Rezende IM, Trindade GS, LaBeaud AD, De Thoisy B, Drumond BP. Recent sylvatic yellow fever virus transmission in Brazil: the news from an old disease. Virol J. 2020;17:9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Monath TP, Vasconcelos PF. Yellow fever. J Clin Virol. 2015;64:160–73. [DOI] [PubMed] [Google Scholar]
- 20.Visser LG, Roukens AH. Modelling a way out of yellow fever. Lancet. 2016;388:2847–48. [DOI] [PubMed] [Google Scholar]
- 21.Barrett AD, Teuwen DE. Yellow fever vaccine - how does it work and why do rare cases of serious adverse events take place? Curr Opin Immunol. 2009;21:308–13. [DOI] [PubMed] [Google Scholar]
- 22.Van Epps HL. Broadening the horizons for yellow fever: new uses for an old vaccine. J Exp Med. 2005;201:165–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Silva JVJ Jr., Lopes TRR, Oliveira-Filho EF, Oliveira RAS, Duraes-Carvalho R, Gil L. Current status, challenges and perspectives in the development of vaccines against yellow fever, dengue, Zika and chikungunya viruses. Acta Trop. 2018;182:257–63. [DOI] [PubMed] [Google Scholar]
- 24.Barrett ADT. Yellow fever live attenuated vaccine: a very successful live attenuated vaccine but still we have problems controlling the disease. Vaccine. 2017;35:5951–55. [DOI] [PubMed] [Google Scholar]
- 25.Beck AS, Barrett AD. Current status and future prospects of yellow fever vaccines. Expert Rev Vaccines. 2015;14:1479–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Contraindications administering yellow fever vaccine. Atlanta (USA): Center of Disease Control. [accessed 2020 May 19]. https://www.cdc.gov/travel-training/local/HistoryEpidemiologyandVaccination/contraindications-administering-yellow-fever-vaccine.pdf. [Google Scholar]
- 27.De Jong W, De Man RA, Dalm V, Reusken C, Goeijenbier M, Van Gorp ECM. Yellow fever vaccination for immunocompromised travellers: unjustified vaccination hesitancy? J Travel Med. 2019;26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Porudominsky R, Gotuzzo EH. Yellow fever vaccine and risk of developing serious adverse events: a systematic review. Rev Panam Salud Publica. 2018;42:e75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Monath TP. Yellow fever vaccine. Expert Rev Vaccines. 2005;4. [DOI] [PubMed] [Google Scholar]
- 30.Krubiner CB, Schwartz DA. Viral hemorrhagic fevers in pregnant women and the vaccine landscape: comparisons between yellow fever, Ebola, and lassa fever. Curr Trop Med Rep. 2019;6:186–96. [Google Scholar]
- 31.United Nations . Fertility Data. World population prospects 2019. Depeartment of Economic and Social Affairs. Population Dynamics, 2020[accessed 2020 Apr 3]. https://population.un.org/wpp/Download/Standard/Fertility/. [Google Scholar]
- 32.Q&A: fractional doses of the yellow fever vaccine. World Health Organization, 2017. [accessed 2020 Apr 3]. https://www.who.int/emergencies/yellow-fever/mediacentre/qa-fractional-dosing/en/. [DOI] [PubMed] [Google Scholar]
- 33.Yellow fever . International travel and health. World Health Organization. [accessed 2021 Jan 4] https://www.who.int/ith/vaccines/yf/en/#:~:text=The%20YF%20vaccine%20is%20contraindicated,transmission%20may%20be%20very%20high. [Google Scholar]
- 34.Visser LG. The immunosuppressed traveler. Infect Dis Clin North Am. 2012;26:609–24. [DOI] [PubMed] [Google Scholar]
- 35.World Health Organization . Cancer today. International Agency for Research on Cancer, 2020. [accessed 2020 Apr 3]. https://gco.iarc.fr/today/online-analysis-map?v=2018&mode=population&mode_population=continents&population=900&populations=900&key=asr&sex=0&cancer=39&type=0&statistic=5&prevalence=0&population_group=0&ages_group%5B%5D=0&ages_group%5B%5D=17items=10&group_cancer=1&include_nmsc=1&include_nmsc_other=1&projection=globe&color_palette=default&map_scale=quantile&map_nb_colors=5&continent=0&rotate=%255B10%252C0%255D. [Google Scholar]
- 36.United Nations . AIDSinfo. UNAIDS, 2020. [accessed 2020 Apr 3]. http://aidsinfo.unaids.org/. [Google Scholar]
- 37.Colin De Verdiere N, Durier C, Samri A, Meiffredy V, Launay O, Matheron S, Mercier-Delarue S, Even S, Aboulker JP, Molina JM, et al. Immunogenicity and safety of yellow fever vaccine in HIV-1-infected patients. AIDS. 2018;32:2291–99. [DOI] [PubMed] [Google Scholar]
- 38.Dhanapala P, De Silva C, Doran T, Suphioglu C. Cracking the egg: an insight into egg hypersensitivity. Mol Immunol. 2015;66:375–83. [DOI] [PubMed] [Google Scholar]
- 39.Rona RJ, Keil T, Summers C, Gislason D, Zuidmeer L, Sodergren E, Sigurdardottir ST, Lindner T, Goldhahn K, Dahlstrom J, et al. The prevalence of food allergy: a meta-analysis. J Allergy Clin Immunol. 2007;120:638–46. [DOI] [PubMed] [Google Scholar]
- 40.Monath TP, Lee CK, Julander JG, Brown A, Beasley DW, Watts DM, Hayman E, Guertin P, Makowiecki J, Crowell J, et al. Inactivated yellow fever 17D vaccine: development and nonclinical safety, immunogenicity and protective activity. Vaccine. 2010;28:3827–40. [DOI] [PubMed] [Google Scholar]
- 41.Kelso JM, Mootrey GT, Tsai TF. Anaphylaxis from yellow fever vaccine. J Allergy Clin Immunol. 1999;103:698–701. [DOI] [PubMed] [Google Scholar]
- 42.Lindsey NP, Schroeder BA, Miller ER, Braun MM, Hinckley AF, Marano N, Slade BA, Barnett ED, Brunette GW, Horan K, et al. Adverse event reports following yellow fever vaccination. Vaccine. 2008;26:6077–82. [DOI] [PubMed] [Google Scholar]
- 43.Our Wolrd in Data . Population by age bracket with UN projections, World, 1950 to 2020. Oxford Martin School, University of Oxford, 2020. [accessed 2020 Apr 3]. https://ourworldindata.org/population-aged-65-outnumber-children. [Google Scholar]
- 44.Wu JT, Peak CM, Leung GM, Lipsitch M. Fractional dosing of yellow fever vaccine to extend supply: a modelling study. Lancet. 2016;388:2904–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Monath TP, Woodall JP, Gubler DJ, Yuill TM, Mackenzie JS, Martins RM,Reiter P, Heymann DL. Yellow fever vaccine supply: a possible solution. Lancet. 2016;387:1599–600. [DOI] [PubMed] [Google Scholar]
- 46.Kraus B, Von Fircks S, Feigl S, Koch SM, Fleischanderl D, Terler K,Dersch-Pourmojib M, Konetschny C, Grillberger L, Reiter M. Avian cell line - Technology for large scale vaccine production. BMC Proc. 2011;5(Suppl 8):52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Nikolay A, Leon A, Schwamborn K, Genzel Y, Reichl U. Process intensification of EB66(R) cell cultivations leads to high-yield yellow fever and Zika virus production. Appl Microbiol Biotechnol. 2018;102:8725–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Organization WH Types of seasonal influenza vaccine. World Health Organization. [accessed 2021 Jan 6]. https://www.euro.who.int/en/health-topics/communicable-diseases/influenza/vaccination/types-of-seasonal-influenza-vaccine. [Google Scholar]
- 49.Ishikawa T, Yamanaka A, Konishi E. A review of successful flavivirus vaccines and the problems with those flaviviruses for which vaccines are not yet available. Vaccine. 2014;32:1326–37. [DOI] [PubMed] [Google Scholar]
- 50.Collins MH, Metz SW. Progress and works in progress: update on flavivirus vaccine development. Clin Ther. 2017;39:1519–36. [DOI] [PubMed] [Google Scholar]
- 51.Temporary total depletion of US licensed yellow fever vaccine addressed by availability of stamaril vaccine at selected clinics. Centers for Disease Control and Prevention, 2020. [accessed 2020 Nov 6]. https://wwwnc.cdc.gov/travel/news-announcements/yellow-fever-vaccine-access. [Google Scholar]
- 52.Yellow Fever Vaccine Information . Sanofi U.S., 2020[accessed 2020 Nov 6]. https://www.sanofi.us/en/products-and-resources/vaccines/yellow-fever-vaccine-information#:~:text=Sanofi%20Pasteur’s%20new%20U.S.%20YF,YF%2DVAX%20in%20December%202020. [Google Scholar]
- 53.Wilder-Smith A. Yellow fever vaccination: estimating coverage. Lancet Infect Dis. 2017;17:1109–11. [DOI] [PubMed] [Google Scholar]
- 54.Yellow fever global vaccine stockpile in emergencies. World Health Organization, 2016. [accessed 2020 Apr 3]. https://www.who.int/news-room/feature-stories/detail/yellow-fever-global-vaccine-stockpile-in-emergencies:. [Google Scholar]
- 55.Vannice K, Wilder-Smith A, Fractional-Dose Yellow HJ. Fever vaccination — advancing the evidence base. N Engl J Med. 2018;379. [DOI] [PubMed] [Google Scholar]
- 56.The L. Yellow fever: a major threat to public health. Lancet. 2018;391:402. [DOI] [PubMed] [Google Scholar]
- 57.Da Costa-rocha IA, Campi-Azevedo AC, Peruhype-Magalhaes V, Coelho-Dos-Reis JG, Fradico JRB, Souza-Lopes T, Reis LR, Freire LC, Costa-Pereira C, Mambrini JVM, et al. Duration of humoral and cellular immunity 8 years after administration of reduced doses of the 17DD-Yellow fever vaccine. Front Immunol. 2019;10:1211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Roukens AHE, Van Halem K, De Visser AW, Visser LG. Long-term protection after fractional-dose yellow fever vaccination: follow-up study of a randomized, controlled, noninferiority trial. Ann Intern Med. 2018;169:761–65. [DOI] [PubMed] [Google Scholar]
- 59.Callender DM. Management and control of yellow fever virus: Brazilian outbreak January-April, 2018. Glob Public Health. 2019;14:445–55. [DOI] [PubMed] [Google Scholar]
- 60.Gotuzzo E, Yactayo S, Cordova E. Efficacy and duration of immunity after yellow fever vaccination: systematic review on the need for a booster every 10 years. Am J Trop Med Hyg. 2013;89:434–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Roukens AHE, Visser LG. Fractional-dose yellow fever vaccination: an expert review. J Travel Med. 2019;26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Wilder-Smith A, Leong WY. Importation of yellow fever into China: assessing travel patterns. J Travel Med. 2017;24. [DOI] [PubMed] [Google Scholar]
- 63.World Tourism Organization, World Bank . Yearbook of tourism statistics. International arrivals. World Tourism Organization. [accessed 2020 Apr 3]. https://data.worldbank.org/indicator/st.int.arvl. [Google Scholar]
- 64.Kraemer MU, Sinka ME, Duda KA, Mylne AQ, Shearer FM, Barker CM, Moore CG, Carvalho RG, Coelho GE, Van Bortel W, et al. The global distribution of the arbovirus vectors Aedes aegypti and Ae. Albopictus. Elife. 2015;4:e08347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.WHO report on global surveillance of epidemic-prone infectious diseases - Yellow fever. Emergencies preparedness, response. World health Organization. https://www.who.int/csr/resources/publications/yellowfev/CSR_ISR_2000_1/en/index1.html. [Google Scholar]
- 66.Liu-Helmersson J, Brannstrom A, Sewe MO, Semenza JC, Estimating Past RJ. Present, and future trends in the global distribution and abundance of the arbovirus vector aedes aegypti under climate change scenarios. Front Public Health. 2019;7:148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Pereira RC, Silva AN, Souza MC, Silva MV, Neves PP, Silva AA, Matos DD, Herrera MA, Yamamura AM, Freire MS, et al. An inactivated yellow fever 17DD vaccine cultivated in Vero cell cultures. Vaccine. 2015;33:4261–68. [DOI] [PubMed] [Google Scholar]
- 68.Pugachev KV, Guirakhoo F, Monath TP. New developments in flavivirus vaccines with special attention to yellow fever. Curr Opin Infect Dis. 2005;18:387–94. [DOI] [PubMed] [Google Scholar]
- 69.Julander JG, Trent DW, Monath TP. Immune correlates of protection against yellow fever determined by passive immunization and challenge in the hamster model. Vaccine. 2011;29:6008–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Monath TP, Fowler E, Johnson CT, Balser J, Morin MJ, Sisti M, Trent DW. An inactivated cell-culture vaccine against yellow fever. N Engl J Med. 2011;364:1326–33. [DOI] [PubMed] [Google Scholar]
- 71.Hayes EB. Is it time for a new yellow fever vaccine? Vaccine. 2010;28:8073–76. [DOI] [PubMed] [Google Scholar]
- 72.Tretyakova I, Nickols B, Hidajat R, Jokinen J, Lukashevich IS, Plasmid PP. DNA initiates replication of yellow fever vaccine in vitro and elicits virus-specific immune response in mice. Virology. 2014;468-470:28–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Jiang X, Dalebout TJ, Lukashevich IS, Bredenbeek PJ, Franco D. Molecular and immunological characterization of a DNA-launched yellow fever virus 17D infectious clone. J Gen Virol. 2015;96:804–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Pushko P, Lukashevich IS, Weaver SC, Tretyakova I. DNA-launched live-attenuated vaccines for biodefense applications. Expert Rev Vaccines. 2016;15:1223–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Maciel M Jr., Cruz Fda S, Cordeiro MT, Da Motta MA, Cassemiro KM, Maia Rde C, de Figueiredo RC, Galler R, Freire Mda S, August JT, et al. A DNA vaccine against yellow fever virus: development and evaluation. PLoS Negl Trop Dis. 2015;9:e0003693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Safety and immunogenicity study of 2019-nCoV vaccine (mRNA-1273) for prophylaxis of SARS-CoV-2 infection (COVID-19). ClinicalTrials.gov, NIH U.S. National Library fo Medicine, 2020. [accessed 2020 Oct 13]. https://clinicaltrials.gov/ct2/show/NCT04283461. [Google Scholar]
- 77.Erasmus JH, Fuller DH. Preparing for pandemics: RNA Vaccines at the forefront. Mol Ther. 2020;28:1559–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Fuller DH, Berglund P. Amplifying RNA Vaccine Development. N Engl J Med. 2020;382:2469–71. [DOI] [PubMed] [Google Scholar]
- 79.Self-Amplifying LK. RNA Viruses as RNA Vaccines. Int J Mol Sci. 2020;21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Julander JG, Testori M, Cheminay C, Volkmann A. Immunogenicity and protection after vaccination with a modified vaccinia virus ankara-vectored yellow fever vaccine in the Hamster model. Front Immunol. 2018;9:1756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Priyamvada L, Hudson W, Ahmed R, Wrammert J. Humoral cross-reactivity between Zika and dengue viruses: implications for protection and pathology. Emerg Microbes Infect. 2017;6:e33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.A phase I trial to evaluate the safety, reactogenicity, and immunogenicity of MVA-BN yellow fever vaccine with and without montanide ISA-720 adjuvant in 18-45 year old healthy volunteers (NCT02743455). ClinicalTrials.gov, NIH U.S. National Library fo Medicine, 2016. [accessed 2020 Apr 3]. https://clinicaltrials.gov/ct2/show/NCT02743455. [Google Scholar]
- 83.Schafer B, Holzer GW, Joachimsthaler A, Coulibaly S, Schwendinger M, Crowe BA, Kreil TR, Barrett PN, Falkner FG. Pre-clinical efficacy and safety of experimental vaccines based on non-replicating vaccinia vectors against yellow fever. PLoS One. 2011;6:e24505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Rollier CS, Reyes-Sandoval A, Cottingham MG, Ewer K, Hill AV. Viral vectors as vaccine platforms: deployment in sight. Curr Opin Immunol. 2011;23:377–82. [DOI] [PubMed] [Google Scholar]
- 85.Imler JL. Adenovirus vectors as recombinant viral vaccines. Vaccine. 1995;13:1143–51. [DOI] [PubMed] [Google Scholar]
- 86.Schwartz DA. Being pregnant during the Kivu Ebola virus outbreak in DR Congo: the rVSV-ZEBOV vaccine and its accessibility by mothers and infants during humanitarian crises and in conflict areas. Vaccines (Basel). 2020;8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Effectiveness and safety of a heterologous, two-dose Ebola vaccine in the DRC. ClinicalTrials.gov, NIH U.S. National Library fo Medicine, 2019. [accessed 2020 Oct 14]. https://clinicaltrials.gov/ct2/show/NCT04152486?term=Ad26.ZEBOV%2FMVA-BN-Filo&cond=Ebola&cntry=CD&draw=2&rank=2. [Google Scholar]
- 88.Kim YC, Dema B, Reyes-Sandoval ACOVID-19. vaccines: breaking record times to first-in-human trials. NPJ Vaccines. 2020;5:34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.A study of a candidate COVID-19 vaccine (COV001). ClinicalTrials.gov, NIH U.S. National Library fo Medicine, 2020 [accessed 2020 May 18]. https://clinicaltrials.gov/ct2/show/NCT04324606. [Google Scholar]
- 90.Folegatti PM, Ewer KJ, Aley PK, Angus B, Becker S, Belij-Rammerstorfer S, Bellamy D, Bibi S, Bittaye M, Clutterbuck EA, et al. Safety and immunogenicity of the ChAdOx1 nCoV-19 vaccine against SARS-CoV-2: a preliminary report of a phase 1/2, single-blind, randomised controlled trial. Lancet. 2020;396:467–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Lopez-Camacho C, Abbink P, Larocca RA, Dejnirattisai W, Boyd M, Badamchi-Zadeh A, Wallace ZR, Doig J, Velazquez RS, Neto RDL, et al. Rational Zika vaccine design via the modulation of antigen membrane anchors in chimpanzee adenoviral vectors. Nat Commun. 2018;9:2441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Safety and immunogenicity of a candidate ZIKV vaccine (ZIKA001). ClinicalTrials.gov, NIH U.S. National Library fo Medicine, 2019. [accessed 2020 Apr 3]. https://clinicaltrials.gov/ct2/show/NCT04015648. [Google Scholar]
- 93.Dicks MD, Spencer AJ, Edwards NJ, Wadell G, Bojang K, Gilbert SC,Hill AV, Cottingham MG. A novel chimpanzee adenovirus vector with low human seroprevalence: improved systems for vector derivation and comparative immunogenicity. PLoS One. 2012;7:e40385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Fausther-Bovendo H, Kobinger GP. Pre-existing immunity against Ad vectors: humoral, cellular, and innate response, what’s important? Hum Vaccin Immunother. 2014;10:2875–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Voysey M, Clemens SAC, Madhi SA, Weckx LY, Folegatti PM, Aley PK, Angus B, Baillie VL, Barnabas SL, Bhorat QE, et al. Safety and efficacy of the ChAdOx1 nCoV-19 vaccine (AZD1222) against SARS-CoV-2: an interim analysis of four randomised controlled trials in Brazil, South Africa, and the UK. Lancet. 2021;397:99–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Immunization Schedules - Africa . University of Cape Town. [accessed 2021 Jan 17]. http://www.vacfa.uct.ac.za/immunization-schedules-africa. [Google Scholar]
- 97.Gouglas D, Thanh Le T, Henderson K, Kaloudis A, Danielsen T, Hammersland NC,Robinson JM, Heaton PM, Rottingen JA. Estimating the cost of vaccine development against epidemic infectious diseases: a cost minimisation study. Lancet Glob Health. 2018;6:e1386–e96. [DOI] [PMC free article] [PubMed] [Google Scholar]


