Abstract
Intravascular optical coherence tomography (IVOCT) provides high-resolution images of coronary calcifications and detailed measurements of acute stent deployment following stent implantation. Since pre- and post-stent IVOCT image “pull-back” acquisitions start from different locations, registration of corresponding pullbacks is needed for assessing treatment outcomes. In particular, we are interested in assessing finite element model (FEM) prediction of lumen gain following stenting, requiring registration. We used deep learning to segment calcifications in corresponding pre- and post-stent IVOCT pullbacks. We created 1D representations of calcium thickness as a function of the angle of the helical IVOCT scans. Registration of two scans was done by maximizing the cross correlation of these two 1D representations. Registration was accurate, as determined by visual comparisons of 2D image frames. We used our pre-stent calcification segmentations to create a lesion-specific FEM, which took into account balloon size, balloon pressure, and stent measurements. We then compared simulated lumen gain from FEM analysis to actual stent deployment results. Actual lumen gain across ~200 registered pre and post-stent images was 1.52 ± 0.51, while FEM prediction was 1.43 ± 0.41. Comparison between actual and FEM results showed no significant difference (p < 0.001), suggesting accurate prediction of FEM modeling. Registered image data showed good visual agreement regarding lumen gain and stent strut malapposition. Hence, we have developed a platform for evaluation of FEM prediction of lumen gain. This platform can be used to guide development of FEM prediction software, which could ultimately help physicians with stent treatment planning of calcified lesions.
Keywords: Vascular imaging, Stent deployment results, Image registration, Intravascular optical coherence tomography (IVOCT), Finite element model (FEM), Deep learning, coronary calcification
1. INTRODUCTION
Major calcifications are of great concern when performing percutaneous coronary intervention (PCI) because they can hinder stent deployment. Approximately 700,000 PCIs are performed each year to open up obstructed coronary arteries.1 Calcified plaques are found in 17% to 35% of patients undergoing PCI.2–4 Calcifications can lead to stent underexpansion and strut malapposition, which in turn can lead to increased risk of thrombosis and in-stent restenosis.5–10
Intravascular optical coherence tomography (IVOCT) has significant advantages for characterizing coronary calcification compared to other imaging modalities commonly used by interventional cardiologists. Although clinicians routinely use x-ray angiography to describe the vessel lumen for treatment planning, angiography does not provide specific information regarding vascular wall composition except in the case of severely calcified lesions.11 Intravascular ultrasound (IVUS) can identify the location of a coronary calcification but cannot assess the thickness because the radiofrequency signal is reflected from the calcium tissue interface giving an acoustic shadow.12 IVOCT, however, provides the location and often the thickness of a calcification.13 Our group has proposed multiple plaque characterization approaches on IVOCT images, including machine14,15 and deep learning16–20 methods as well as image registration.21
In this paper, we develop a co-registration method to register intravascular optical coherence tomography (IVOCT) images obtained before and after stenting. This is done to aid analysis of stent deployment, especially with regards to finite element modeling. Registration before and after stenting images will facilitate the computation of lumen gain, a metric of stent quality. We will use finite element model (FEM) to predict the lumen gain after applying post-stenting pressures. FEM results are compared to measured stent deployments in some elegant ex vivo experiments. The influence of the calcium on the stenting outcome was evaluated in terms of lumen gain. This work could provide insights for pre-clinical planning for complicated lesions.
2. METHODS
Image processing and learning techniques are applied to perform semantic segmentation of pixels in IVOCT images as calcified plaque, lumen, or other. The deep learning model trained on the in vivo data is used to classify the images from the ex vivo experiment before and after stenting. Calcification attributes are computed from the classified images. We create 1D representations of calcium thickness as a function of the angle of the IVOCT scan. The classified images are used to build the finite element model.
2.1. Calcification segmentation and measurements
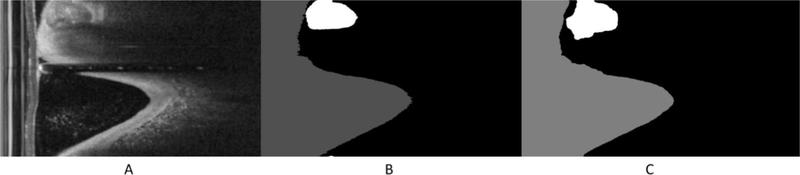
We applied a deep learning segmentation model trained on pre-stent data set to segment both pre- and post-stent images in the (r, θ) representation. Figure 1 shows the (r, θ) representation in (A), the corresponding manually annotated label in (B), and the segmentation results in (C). We concatenated labels across the whole pull back (540 labels) to form one large 2-D array as in figure 2. Then we computed the calcification thickness for each A-line, indicated by the orange arrow, in both pre- and post-stent.
Figure 1.
Automated segmentation results. Images are: (A) is the IVOCT image, (B) is the ground truth annotation, and (C) is the corresponding automatic segmentation result. The white region is the calcifications. This example shows good agreement between manual and automated assessments. Lumen is shown in gray and calcification in white.
Figure 2.
Calcification thickness quantification. (A) The original spiral data set is rather arbitrarily split into (r, θ) label frames. The orange arrow represents a single A-line. The thin orange line crossing the calcification (white) represents one A-line. (B) We concatenated labels to form one large 2-D array.
2.2. Co-registration framework
We created 1D representations of calcium thickness as a function of the angle of the helical IVOCT scan. Figure 3 shows calcification thicknesses as a function of A-line position (angle) for pre-stent (A) and post-stent (B). The horizontal axis is the A-line position (angle) across the concatenated data along the entire pull back. The vertical axis represents the calcification thickness associated with its A-line. We normalize these graphs so that their maximum value is one. The pre-stent image is the fixed image. We compute the cross-correlation between the pairs of 1D graphs. The location of the maximum values of the cross-correlation indicates the time lead or lag. We align the graphs and the corresponding IVOCT scans.
Figure 3.
Calcification thicknesses as a function of A-line position (angle) for (A) pre-stent. (B) post stent pullback. When a calcification is extremely thick and its back border was not clear due to attenuation, the maximum thickness was limited to 1 mm.
2.3. Finite element model construction
We constructed lesion-specific finite element models from IVOCT images. To create a finite element mesh, there were several steps. They are as follows: (1) Process images using the semantic segmentation deep learning as above. (2) Manually correct labels if necessary. (3) Reconstruct the surface from segmentation results by computing a triangular approximation of the interfaces between different materials. (4) Smooth the generated surfaces to eliminate any staircase-like surfaces. (5) Generate the FEM mesh where the volume enclosed by the generated surface is filled with tetrahedron, using Amira software 6.5 (Thermo Fisher Scientific, Waltham, MA, USA).
Other details of finite element modeling follow. The stent was made of 316 L stainless steel, which was described as a perfect linear elastoplastic material with a Young’s modulus of 190 GPa, Poisson’s ratio of 0.3, and yield strength of 207 MPa. The mechanical behaviors of lesions were described using a hyperelastic constitutive model. The strain energy density function U was defined by a reduced third-order polynomial
where I1 and I2 are the first and second invariants of the Cauchy–Green deformation tensor, λ1, λ2, and λ3 are the principal stretches. The coefficients Cij are adopted from the literature.21, 22 In addition, perfect plastic behavior was prescribed for fibrotic plaque with a yield stress of 0.07 MPa and a corresponding yield strain of 34%.24
Symmetric constraints were enforced at both ends of the artery. The stent was first crimped from its nominal diameter of 3.00 mm to mimic its catheter delivery state and plastic deformation.23 At the stenotic location, the stent was radially expanded to a diameter of 3mm to push the stenotic lesion outwards.25 After unloading the displacement of the balloon, the stent recoiled to its final deployment shape. A frictionless contact was enforced between the stent and the lesion.26 The model was solved using commercial software ABAQUS (Dassault Systèmes Simulia Corp., Providence, RI). The mechanics of stenting was governed by the dynamic equilibrium equation
where ∇ is the Laplace operator, σ is the Cauchy stress tensor, ρ is the density, b is the body force, and a is the acceleration.27
3. EXPERIMENTAL METHODS
3.1. Ex-vivo experimental data
All ex-vivo cadaveric hearts were first CT scanned to choose a good candidate that has large deposits of calcium. PCI was then performed using an 8-Fr guiding catheter. We deployed a 3.0 mm diameter stent (Xience Sierra [3.0 mm diameter, 18 mm long], Abbott Vascular, Santa Clara, CA) using a non-compliant balloon dilated to its nominal pressure. This was followed by post-dilations at 3.5, 4.0 and 4.5 mm, each at the following balloon pressures: 10, 20, and 30 atm, for a total of 11 pullbacks (1 pre-stenting pullback; 1 post stenting pullback; and 9 post-stenting pullbacks). Maximal balloon pressure and maximal balloon size were recorded. IVOCT was performed after stent implantation. A 2.7-Fr IVOCT catheter (Dragonfly or Dragonfly JP; Abbott Vascular, Santa Clara, CA) was advanced proximal to the lesion, and automated pullback was performed with contrast injection through the guiding catheter. IVOCT images were recorded and analyzed using the IVOCT console. All IVOCT images were manually labeled by two skilled cardiologists from the Cardiovascular Imaging Core Laboratory, Harrington Heart and Vascular Institute, University Hospitals Cleveland Medical Center, Cleveland, OH, USA.
3.2. Finite element model experiment
A calcified coronary plaque model was reconstructed based on 280 OCT images with a pixel size of 9.76 μm and spacing of 200 μm, as illustrated in Fig. 1(a). OCT images were acquired from a stent implantation experiment in a cadaveric coronary artery (left anterior descending artery, 63-year-old male). The cadaveric heart was acquired from Restore Life U.S. (Elizabethton, TN). The institutional review board of University Hospital determined that this activity was not human subject research and did not require approval. Calcified and fibrotic plaque in each image was first labeled using the automated method. Then the results were minimally edited manually using the commercial software AMIRA (Thermo Fisher Scientific, Waltham, MA, USA).
4. RESULTS
The qualitative results show the ability of the deep learning model to classify calcifications in IVOCT images while producing a smooth segmentation of the overall image. All predicted images for all training sets were compared with their corresponding manual segmentation. In Figure 1, segmentation of the lumen and the calcification are shown. Both lumen and calcification regions show good agreement with the ground truth labels. Figure 5 shows that calcification thickness graphs are much better aligned following registration (B) as compared to before registration (A). Blue is the pre-stent pullback while the orange is the post stent. The dark blue region is where the two pullbacks align.
Figure 5.
Calcification thicknesses as a function of frame number. (A) representing the calcification thickness for the pre-stent. (B) is for the post stent and (C) after registration. Blue is the present pullback while the orange is the post stent.
Figure 6 shows the anatomical representation of IVOCT images before (A and C) and after (B and D) stenting. A and C are the same pre-stent image frame. The unregistered post-stent image frame B has the same frame number as A. We can see that the image frame in D matched the one in C after the registration.
Figure 6.
Automatic registration of IVOCT images. A and C are the same pre stent image. C is the unregistered post-stent image frame with the same frame number as A (images A and B has frame number of 243 in the pre- and post-stent respectively). D is the registered image frame corresponding to C.
Additionally, in a sequence of registered images, we compared the frames before and after the corresponding image (Figure 7). We found that each frame matched its corresponding image better than the frame before and the frame after. This suggests that the accuracy of our registration is within 1 frame interval (±200 μm).
Figure 7.
Qualitative analysis of registered pre- and post-stent IVOCT image datasets suggests z-registration accuracy is within 1 IVOCT frame interval (±200 μm). We visually compared registered pre- and post-stent IVOCT image pairs, as well as images located immediately before and after within the registered datasets.
The performance of the finite element model was measured by comparing the lumen area from the IVOCT experiment and the predicted lumen area from the FEM. Table 1 shows FEM predictions of the lumen gain as compared to actual lumen gain from IVOCT measurements. The FEM results agree well with the measurement from the IVOCT images.
Table 1.
Average lumen gain across the registered stenting area.
| Lumen gain (mean ± SD) | |
|---|---|
| Actual measurement | 1.52 ± 0.51 |
| FEM prediction | 1.43 ± 0.41 |
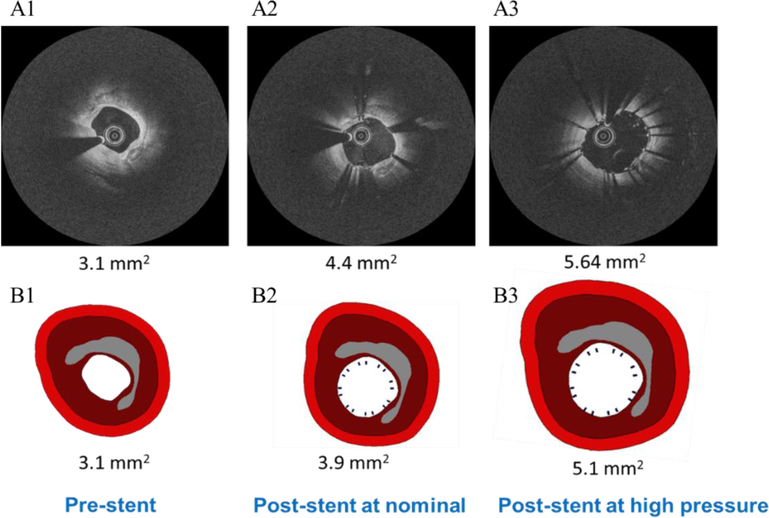
The upper panel in Figure 8 shows the registered pre-stent IVOCT image with post-stent images at different pressure steps. The lower panel shows the corresponding FEM predictions at the same pressure steps as the upper panel.
Figure 8.
The registered IVOCT images and the corresponding prediction from the FEM. The first IVOCT image in the upper panel is the pre-stent image. A2 and A3 are the post stent images at nominal and 20 atm respectively. B1 is the FE model based on the pre-stent labeled images. B2 and B3 are the simulation results that matched the procedure conditions as in A2 and A3.
We can also analyze the predictions over an entire stenting area. In Figure 9, we show two lines comparing actual measurements (dark blue) with the FEM prediction (orange) for registered pre and post-stent. In the bottom line (yellow), we show the pre-stent lumen area across all frames. Here we see our FEM model almost exactly matches the experimental data.
Figure 9.
Comparison between prediction results from the FEM after stenting with the one from the actual data.
FEM simulation of stent deployment is in Figure 10. A and B columns show stent deployment and IVOCT images at a pressure of 11-atm. Predicted lumen shape is consistent, with non-circular irregularities present at the same locations in FEM and IVOCT. Malapposed struts (red arrows) not flush with the artery wall are visible in this frame. Calcifications hinder deployment as (proximal, min, distal) IVOCT areas are (4.2, 3.11, 3.8) mm2, less than the manufacturer’s predicted 7.0 mm2 at 11-atm.
Figure 10.
IVOCT images and corresponding cuts of the 3D FEM show excellent correspondence of stent struts malapposition (red arrow). A and B are the registered pre and post stent image frames. C and D are the corresponding FEM prediction of stent deployment at 11 atm.
5. DISCUSSION
Deep learning calcification segmentation was an important step. We were able to quantify calcification attributes based on the automated segmentations (Figure 1). For the lumen area, the automated measurements were excellent (good precision and bias) compared to manual assessments. Segmentation errors are mostly related to calcification deposits that have small arc angles (<40°), which have less impact on clinical decision-making. Our algorithm tends to agree with manual determination of the calcification front border but has less agreement with the back border. This is due to the IVOCT signal having limited depth penetration, making determination of the calcification back border difficult even for manual assessments. This is especially the case after stenting, when calcifications will be hidden behind the stent strut shadow.
For pre and post stent registration, we created a specialized image registration method. Our solution consisted of creating 1D representations of calcium thickness as a function of the angle of the helical IVOCT scan. Qualitative analysis of registered pre- and post-stent IVOCT image datasets suggests z-registration accuracy is within 1 IVOCT frame interval (±200 μm). We visually compared registered pre- and post-stent IVOCT image pairs, as well as images located immediately before and after within the registered datasets. Qualitative features suggested that registration accuracy was within 1 frame interval. In Figure 7, this is especially apparent when considering the side branch located within the registered pair. In the bottom pre-stent image, located one frame away from the registered pair, we see that the side branch has not yet appeared, as it has in the registered pair. Moreover, in the top pre-stent, located one frame away from the registered pair, we see that the side branch is more pronounced than in the registered pair. Clearly, the best match occurs within the registered pair, suggesting that registration accuracy is within the IVOCT frame interval of one frame apart.
We quantify the accuracy of our model by computing the lumen gain and comparing it with the simulation results from the finite element model. Lumen gain, a metric of stent quality, is the ratio between the lumen area before and after stenting. In our data, comparison between actual and FEM results showed no significant difference (p < 0.001), suggesting accurate prediction of FEM modeling. In the present finite element model, we have adopted a straight artery since the OCT datasets could not provide information related to artery curvature. The actual curvature could be obtained by co-registration with the angiogram.28
Our method is easy to implement and able to perform registration while requiring far less computation time to register volume pairs. The proposed framework is generic and can be used to register images from other modalities (i.e. IVUS). oving forward, we believe this platform can be used to guide development of FEM prediction software, which could ultimately help physicians with stent treatment planning for lesions containing calcifications.
Figure 4.
The finite element model with stent at the diseased lesion. The model was generated from the labeled pre-stent IVOCT volume. The artery wall (green) was generated by offsetting the out surface of the plaque with a uniform thickness of 0.75mm.
ACKNOWLEDGEMENTS
This project was supported by the National Heart, Lung, and Blood Institute through U.S. National Institutes of Health (NIH) Grants R21HL108263, R01HL114406, and R01HL143484, by NIH construction Grant (C06 RR12463), and by the Choose Ohio First Scholarship. These grants were attained via collaboration between Case Western Reserve University and University Hospitals of Cleveland. The content of this report is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. The grants were obtained via collaboration between Case Western Reserve University and University Hospitals of Cleveland. This work made use of the High-Performance Computing Resource in the Core Facility for Advanced Research Computing at Case Western Reserve University. The veracity guarantor, Juhwan Lee, affirms to the best of his knowledge that all aspects of this paper are accurate.
REFERENCES
- [1].Kim LK, Feldman DN, Swaminathan RV, Minutello RM, Chanin J, Yang DC, Lee MK, Charitakis K, Shah A, Kaple RK, Bergman G, Singh H and Wong SC, “Rate of percutaneous coronary intervention for the management of acute coronary syndromes and stable coronary artery disease in the United States (2007 to 2011),” Am. J. Cardiol. 114(7), 1003–1010 (2014). [DOI] [PubMed] [Google Scholar]
- [2].Mosseri M, Satler LF, Pichard AD and Waksman R, “Impact of vessel calcification on outcomes after coronary stenting,” Cardiovascular Revascularization Medicine 6(4), 147–153 (2005). [DOI] [PubMed] [Google Scholar]
- [3].Farag M, Costopoulos C, Gorog DA, Prasad A and Srinivasan M, “Treatment of calcified coronary artery lesions,” Expert Rev Cardiovasc Ther, Expert review of cardiovascular therapy, Expert review of cardiovascular therapy. 14(6), 683–690 (2016). [DOI] [PubMed] [Google Scholar]
- [4].Kawaguchi R, Tsurugaya H, Hoshizaki H, Toyama T, Oshima S and Taniguchi K, “Impact of lesion calcification on clinical and angiographic outcome after sirolimus-eluting stent implantation in real-world patients,” Cardiovasc Revasc Med 9(1), 2–8 (2008). [DOI] [PubMed] [Google Scholar]
- [5].Dangas GD, Claessen BE, Caixeta A, Sanidas EA, Mintz GS and Mehran R, “In-stent restenosis in the drug-eluting stent era,” J. Am. Coll. Cardiol. 56(23), 1897–1907 (2010). [DOI] [PubMed] [Google Scholar]
- [6].Fujii K, Carlier SG, Mintz GS, Yang Y, Moussa I, Weisz G, Dangas G, Mehran R, Lansky AJ, Kreps EM, Collins M, Stone GW, Moses JW and Leon MB, “Stent underexpansion and residual reference segment stenosis are related to stent thrombosis after sirolimus-eluting stent implantation: an intravascular ultrasound study,” J. Am. Coll. Cardiol. 45(7), 995–998 (2005). [DOI] [PubMed] [Google Scholar]
- [7].Attizzani GF, Capodanno D, Ohno Y and Tamburino C, “Mechanisms, pathophysiology, and clinical aspects of incomplete stent apposition,” J. Am. Coll. Cardiol. 63(14), 1355–1367 (2014). [DOI] [PubMed] [Google Scholar]
- [8].Doi H, Maehara A, Mintz GS, Yu A, Wang H, Mandinov L, Popma JJ, Ellis SG, Grube E, Dawkins KD, Weissman NJ, Turco MA, Ormiston JA and Stone GW, “Impact of post-intervention minimal stent area on 9-month follow-up patency of paclitaxel-eluting stents: an integrated intravascular ultrasound analysis from the TAXUS IV, V, and VI and TAXUS ATLAS Workhorse, Long Lesion, and Direct Stent Trials,” JACC Cardiovasc Interv 2(12), 1269–1275 (2009). [DOI] [PubMed] [Google Scholar]
- [9].Hong M-K, Mintz GS, Lee CW, Park D-W, Choi B-R, Park K-H, Kim Y-H, Cheong S-S, Song J-K, Kim J-J, Park S-W and Park S-J, “Intravascular ultrasound predictors of angiographic restenosis after sirolimus-eluting stent implantation,” Eur. Heart J. 27(11), 1305–1310 (2006). [DOI] [PubMed] [Google Scholar]
- [10].Uren NG, Schwarzacher SP, Metz JA, Lee DP, Honda Y, Yeung AC, Fitzgerald PJ, Yock PG and POST Registry Investigators., “Predictors and outcomes of stent thrombosis: an intravascular ultrasound registry,” Eur. Heart J. 23(2), 124–132 (2002). [DOI] [PubMed] [Google Scholar]
- [11].Mintz GS, Popma JJ, Pichard AD, Kent KM, Satler LF, Chuang YC, Ditrano CJ and Leon MB, “Unstable Angina/Myocardial Infarction/Atherosclerosis: Patterns of Calcification in Coronary Artery Disease A Statistical Analysis of Intravascular Ultrasound and Coronary Angiography in 1155 Lesions,” Circulation 91(7), 1959–1965 (1995). [DOI] [PubMed] [Google Scholar]
- [12].Ma T, Yu M, Li J, Munding CE, Chen Z, Fei C, Shung KK and Zhou Q, “Multi-frequency intravascular ultrasound (IVUS) imaging,” IEEE Trans Ultrason Ferroelectr Freq Control 62(1), 97–107 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Akiko F, S MG, Mitsuaki M, Tetsumin L, Song-Yi K, Masahiro, Eisuke U, Taishi Y, S HE, A SR, Tsunekazu K and Akiko M, “A new optical coherence tomography-based calcium scoring system to predict stent underexpansion,” Eurointervention Journal 13(18) (2018). [DOI] [PubMed] [Google Scholar]
- [14].Kolluru C, Prabhu D, Gharaibeh Y, Wu H and Wilson DL, “Voxel-based plaque classification in coronary intravascular optical coherence tomography images using decision trees,” Proc SPIE Int Soc Opt Eng 10575 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Prabhu DS, Bezerra HG, Kolluru C, Gharaibeh Y, Mehanna E, Wu H and Wilson DL, “Automated A-line coronary plaque classification of intravascular optical coherence tomography images using handcrafted features and large datasets,” JBO 24(10), 106002 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Kolluru C, Prabhu D, Gharaibeh Y, Bezerra H, Guagliumi G and Wilson D, “Deep neural networks for A-line-based plaque classification in coronary intravascular optical coherence tomography images,” JMI 5(4), 044504 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Gharaibeh Y, Dong P, Prabhu D, Kolluru C, Lee J, Zimin V, Mozafari H, Bizzera H, Gu L and Wilson D, “Deep learning segmentation of coronary calcified plaque from intravascular optical coherence tomography (IVOCT) images with application to finite element modeling of stent deployment,” Medical Imaging 2019: Image-Guided Procedures, Robotic Interventions, and Modeling 10951, 109511C, International Society for Optics and Photonics (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Lee J, Prabhu D, Kolluru C, Gharaibeh Y, Zimin VN, Bezerra HG, Wilson DL and Wilson DL, “Automated plaque characterization using deep learning on coronary intravascular optical coherence tomographic images,” Biomed. Opt. Express, BOE 10(12), 6497–6515 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Gharaibeh Y, Prabhu DS, Kolluru C, Lee J, Zimin V, Bezerra HG and Wilson DL, “Coronary calcification segmentation in intravascular OCT images using deep learning: application to calcification scoring,” JMI 6(4), 045002 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Lee J, Prabhu D, Kolluru C, Gharaibeh Y, Zimin VN, Dallan LAP, Bezerra HG and Wilson DL, “Fully automated plaque characterization in intravascular OCT images using hybrid convolutional and lumen morphology features,” Scientific Reports 10(1), 1–13 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Prabhu D, Mehanna E, Gargesha M, Wen D, Brandt E, van Ditzhuijzen NS, Chamie D, Yamamoto H, Fujino Y, Farmazilian A, Patel J, Costa M, Bezerra HG and Wilson DL, “3D registration of intravascular optical coherence tomography and cryo-image volumes for microscopic-resolution validation,” Proc SPIE Int Soc Opt Eng 9788 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Zhao S, Gu L and Froemming SR, “Finite element analysis of the implantation of a self-expanding stent: impact of lesion calcification,” Journal of Medical Devices 6(2), 021001 (2012). [Google Scholar]
- [23].Zhao S, Gu L and Froemming SR, “On the importance of modeling stent procedure for predicting arterial mechanics,” Journal of biomechanical engineering 134(12), 121005 (2012). [DOI] [PubMed] [Google Scholar]
- [24].Gastaldi D, Morlacchi S, Nichetti R, Capelli C, Dubini G, Petrini L and Migliavacca F, “Modelling of the provisional side-branch stenting approach for the treatment of atherosclerotic coronary bifurcations: effects of stent positioning,” Biomech Model Mechanobiol 9(5), 551–561 (2010). [DOI] [PubMed] [Google Scholar]
- [25].Zhao S, Gu L and Froemming SR, “Experimental investigation of the stent–artery interaction,” Journal of Medical Engineering & Technology 37(7), 463–469 (2013). [DOI] [PubMed] [Google Scholar]
- [26].Pericevic I, Lally C, Toner D and Kelly DJ, “The influence of plaque composition on underlying arterial wall stress during stent expansion: The case for lesion-specific stents,” Medical Engineering & Physics 31(4), 428–433 (2009). [DOI] [PubMed] [Google Scholar]
- [27].Dong P, Mozafari H, Prabhu D, Bezerra HG, Wilson DL and Gu L, “Optical Coherence Tomography-Based Modeling of Stent Deployment in Heavily Calcified Coronary Lesion,” J Biomech Eng 142(5) (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Mortier P, Wentzel J, Santis GD, Chiastra C, Migliavacca F, Beule MD, Louvard Y and Dubini G, “Patient-specific computer modelling of coronary bifurcation stenting: the John Doe programme,” EuroIntervention, <https://eurointervention.pcronline.com/article/patient-specific-computer-modelling-of-coronary-bifurcation-stenting-the-john-doe-programme> (12 February 2020. ). [DOI] [PubMed]