Abstract
Oral infections caused by the yeast Candida albicans are some of the most frequent and earliest opportunistic infections in human immunodeficiency virus-infected patients. The widespread use of azole antifungal drugs has led to the development of drug resistance, creating a major problem in the treatment of yeast infections in AIDS patients and other immunocompromised individuals. Several molecular mechanisms that contribute to drug resistance have been identified. In C. albicans, the ability to morphologically switch from yeast cells (blastospores) to filamentous forms (hyphae) is an important virulence factor which contributes to the dissemination of Candida in host tissues and which promotes infection and invasion. A positive correlation between the level of antifungal drug resistance and the ability to form hyphae in the presence of azole drugs has been identified. Under hypha-inducing conditions in the presence of an azole drug, resistant clinical isolates form hyphae, while susceptible yeast isolates do not. This correlation is observed in a random sample from a population of susceptible and resistant isolates and is independent of the mechanisms of resistance. 35S-methionine incorporation suggests that growth inhibition is not sufficient to explain the inhibition of hyphal formation, but it may contribute to this inhibition.
Candidiasis, which is caused by the pathogenic yeast Candida albicans, is the most frequent fungal infection associated with AIDS and other immunocompromised states (6). C. albicans normally exists as a commensal organism that is part of the oral microbial flora and can cause oral infections in individuals with immune defects. Two classes of antifungal drugs are used to fight Candida infections. The fungicidal polyene drugs such as amphotericin B act by binding to sterols in the plasma membrane, disrupting membrane function. The fungistatic azoles, such as fluconazole and ketoconazole, act by inhibiting the target enzyme lanosterol demethylase (Erg11) in the ergosterol biosynthetic pathway. Amphotericin B is the most effective antifungal drug, but it is more toxic and is less tolerated by the body than the azoles. As a result, azoles have become the drug treatment of choice for many mucosal fungal infections (32).
In the last decade, the widespread use of azole drugs has led to the rapid development of azole drug resistance in patients with recurring oral candidiasis. The development of resistance depends on a number of factors such as drug-drug interaction, dosages and scheduling, host factors, and factors intrinsic to Candida (32). Only recently, molecular mechanisms and genetic alterations that render C. albicans strains resistant have been described (for a review, see reference 32). Many investigators have reported the increased overexpression of a class of genes encoding ATP binding cassette efflux pumps designated CDR (1, 13, 16, 24–26, 30) and increased expression of a gene encoding a major facilitator efflux pump called MDR1 (16, 25, 26, 30) in resistant clinical isolates of C. albicans. In addition, alterations in the target enzyme (lanosterol demethylase) for azole drugs, including point mutations and overexpression in the ERG11 gene, can contribute to the resistance of a clinical isolate (23, 31).
Unlike other Candida species such as C. glabrata and C. tropicalis, C. albicans is a dimorphic yeast (17). Its ability to switch from yeast cells (blastospores) to hyphae is considered important for the interactions of C. albicans with its host (3). Hyphae are long, slender, continuous tubules with septae that separate each of the nuclei but with no distinct indentation at the septae. Both yeast cells and hyphae are present in the host during commensal growth and during infection. Hyphae are thought to be an important virulence factor that promotes invasion of cells into the mucosa and that allows Candida cells to resist macrophage and neutrophil engulfment. The transition from yeast cell to hypha is influenced by cues such as temperature, pH, particular chemicals, carbon sources, and other components (17).
In 1985, the effects of azole antifungal agents on the yeast cell/hypha transition in susceptible isolates were studied in detail (19). At subinhibitory concentrations of azole drugs, hyphal branching was inhibited. At clinically relevant concentrations, hyphal development was arrested and the cells remained as yeast cells. This inhibition was independent of the inhibition of growth. At the time of this study, azole resistance had not been observed, and therefore, the effects of azole drugs on the yeast cell/hypha transition in resistant isolates was not investigated.
Many laboratories have studied the mechanisms of drug resistance, and many laboratories study the morphology of C. albicans as a virulence factor. However, the correlation between drug resistance and hyphal formation has not been examined in detail. Hyphal formation in susceptible strains is inhibited at clinically significant concentrations of azole drugs (2, 8, 12, 18, 19). A hyphal elongation assessment assay has been used to qualitatively separate susceptible and resistant strains (5, 9, 10, 15). Some quantitation of hyphal formation in resistant isolates in the presence of drugs has recently been reported (29). However, well-characterized strains with known mechanisms of resistance have not been characterized for their ability to form hyphae in the absence and presence of antifungal drugs. Furthermore, the effects of azole drugs on hyphal formation have not been correlated with the current National Committee for Clinical Laboratory Standards (NCCLS) standard for MIC testing (4). The aim of this study is to examine the ability of well-characterized susceptible and resistant strains of C. albicans to form hyphae in the absence or presence of azole drugs.
MATERIALS AND METHODS
Yeast strains.
The C. albicans isolates used in this study include two series of isolates obtained from single patients with disease (Table 1). Series 1 was obtained from an AIDS patient who experienced 14 episodes of oral candidiasis (20, 22, 33). The second series was obtained from a bone marrow transplant patient who experienced disseminated (bloodstream) Candida infection after transplantation (13, 14). Strain 3153A was obtained from the American Type Culture Collection (Rockville, Md.) and was used as a control strain. In addition, a random collection of susceptible, susceptible dose-dependent, and resistant isolates was assembled, including strains provided by Michael Pfaller (University of Iowa) and random isolates from our own laboratory collection. The isolates were grown at 30°C on YEPD agar plates (10 g of yeast extract, 20 g of peptone, 20 g of dextrose, 15 g of Bacto Agar per liter), stored at 4°C, and subcultured weekly or were stored at −70°C in YEPD containing 10% glycerol.
TABLE 1.
MICs, percent hyphal formation, and mechanisms of resistance of C. albicans isolates
| Strain designation
|
MIC (μg/ml)
|
% Hyphal formation in the presence of the following:
|
Mechanism(s) of resistance
|
|||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Series | This study | Previous publication | Fluconazole | Ketoconazole | Fluconazole (10 μg/ml) | Ketoconazole (2 μg/ml) | Increased MDR1 mRNA levels | Increased ERG11 mRNA levels | ERG11 R476K point mutation | Increased CDR mRNA levels |
| Series 1a | A1 | 1 | 0.25 | 0.010 | 0 | 6 | − | − | − | − |
| A2 | 4 | 8 | 0.023 | 29 | 17 | +++ | − | − | − | |
| A3 | 12 | 16 | 0.028 | 79 | 41 | +++ | + | − | − | |
| A4 | 13 | 32 | 0.056 | 88 | 86 | +++ | + | + | − | |
| A5 | 17 | >64 | 0.624 | 93 | 93 | +++ | + | + | ++ | |
| Series 2b | B1 | 1 | 2 | 0.03 | 5 | 21 | − | − | − | + |
| B2 | 3 | 8 | 0.25 | 77 | 86 | − | − | − | ++ | |
| B3 | 5 | 64 | 0.25 | 96 | 85 | − | − | − | +++ | |
| B4 | 8 | 16 | 0.25 | 92 | 85 | − | − | − | ++ | |
| B5 | 9 | 1 | 0.03 | 0 | 31 | − | − | − | + | |
Cell cultures were inoculated with a single colony grown on a YEPD agar plate. The culture medium components were obtained from Fisher Scientific Co., (Pittsburgh, Pa.). The MICs of fluconazole and ketoconazole were determined by the NCCLS broth macrodilution or microdilution methods (8) and were monitored or confirmed with E-test strips (AB Biodisk North America Inc., Piscataway, N.J.) following the manufacturer’s instructions.
Culture media.
Several different media were used for the testing of hyphal formation in this study.
(i) Growth media.
Cells were grown for 48 h prior to hyphal induction in YAD (1.7 g of yeast nitrogen base without amino acids and ammonium sulfate, 5.0 g of ammonium sulfate, and 5.4 g of dextrose per liter). For 35S-labeled methionine incorporation, the cells were grown in Lee’s medium without methionine (modified Lee’s medium at pH 4.5, without the addition of methionine) (28). Cultures were grown in these media for 48 h to the late stationary phase.
(ii) Induction media.
Cells were induced to form hyphae in medium 199 (M199) for 3 h at 37°C. M199 (10×) was obtained from Gibco (New York, N.Y.). For 35S-labeled methionine incorporation, cells were induced in RPMI medium 1640 without methionine (Gibco) for 3 h at 37°C to induce hyphae.
Antifungal agents.
Stock solutions of fluconazole (3.33 mg/ml in H2O; Pfizer, New York, N.Y.) and ketoconazole (1 mg/ml in ethanol; Sigma, St. Louis, Mo.) were prepared.
Yeast growth in the presence of an azole drug.
Cell cultures were inoculated from a single yeast colony on a YEPD agar plate and grown in 5 ml of growth medium for 48 h in the absence or presence of an azole drug at 30°C with shaking (180 rpm). Growth and hyphal induction were performed in 50-ml polypropylene tubes. Cell cultures were centrifuged at 5,644 × g for 5 min at 10°C and were washed twice with 5 ml of phosphate-buffered saline (PBS; 0.14 M NaCl, 2.7 mM KCl, 8.5 mM Na2HPO4, 1.8 mM KH2PO4 [pH 7.4]). The cells were resuspended in 1 ml of PBS.
Hyphal induction.
Induction media were prewarmed to 37°C. Cells from a 48-h stationary-phase culture were transferred to 5 ml of 1× M199 to a final concentration of 3 × 106 cells/ml. When indicated, the same concentration of azole drug used in the growth medium was added to the induction medium. The cells were incubated at 37°C with shaking (240 rpm). After 3 h (or other listed time intervals) over 250 cells were examined microscopically with a hemocytometer.
35S-labeled methionine incorporation.
Cell cultures were inoculated from a single yeast colony on a YEPD agar plate and were grown in 5 ml of Lee’s medium without methionine (pH 4.5) for 48 h to the late stationary phase. The cells were inoculated in 5 ml of RPMI medium 1640 without methionine to a density of 3 × 106 cells/ml. 35S-labeled methionine (final concentration, 1.5 nM; 1,175 Ci/mmol; 1.8 μCi/ml) and nonlabeled methionine (final concentration, 300 nM) were added to the RPMI 1640 medium. The cells were incubated for up to 4 h. At each time interval, cells were counted to determine the percent hyphal formation, and 0.3 ml of each cell culture was spotted onto GF/C filters in triplicate. The filters were washed twice with 3 ml of ice-cold 5% trichloroacetic acid–1% sodium pyrophosphate and then three times with 3 ml of ice-cold 100% ethanol and were dried. The filters were placed in a vial containing 4 ml of Ecoscint scintillation fluid (National Diagnostics, Atlanta, Ga.), and the counts were determined in a scintillation counter.
RESULTS
To determine the effects of azole antifungal agents on hyphal formation, two series of well-characterized clinical isolates (Table 1) and a random collection of clinical isolates were analyzed for hyphal formation in the absence and presence of antifungal drugs. A standard protocol for large-scale hyphal formation was used. The cells are grown to stationary phase (48 h) at 30°C followed by washing in PBS and resuspension to a final concentration of less than 3 × 106 cells/ml in an induction medium (usually M199) at 37°C for 3 h (see Materials and Methods). Under these conditions, we usually observe greater than 90% hyphal formation of all cells in the absence of drug (7). Hyphae are defined as tubular projections from a mother cell that extend greater than one cell diameter from the mother cell. Usually, the length of the hyphae is over five cell diameters after 3 h. It is noteworthy that in the determination of percent hyphal formation, the number of germinated cells is determined. During a 3-h induction, the number of total cells in both yeast and hyphal cultures will increase, but the number of hyphae will not increase. Instead, the number of cells per hypha will increase. Hyphae germinate from a single cell and continue to extend, consisting of several septated hyphal cells. Therefore, the number of cells in a hyphal state is larger than the percent hyphal formation that was measured in these experiments.
Fluconazole inhibits hyphal formation when present during both 48 h of growth and 3 h of induction.
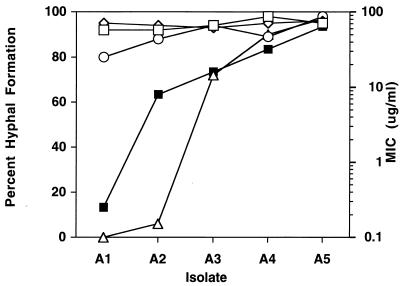
To investigate the effects of azole drugs on hyphal formation, the five isolates in series 1 were analyzed (Fig. 1, isolates A1 to A5). The isolates differ in level of resistance as measured by their fluconazole MICs (Fig. 1). In the absence of drug, all five isolates formed hyphae at comparable percentages (Fig. 1). Fluconazole was added at a concentration of 10 μg/ml, an intermediate value between the MIC for the susceptible isolate (0.25 μg/ml) and the MIC for the resistant isolates (>64 μg/ml). The presence of fluconazole during growth alone or during induction alone (Fig. 1) had no effect on the percentage of hyphal formation for the five isolates. However, when fluconazole was present during both growth and induction (Fig. 1), hyphal formation was severely inhibited in susceptible isolates while hyphal formation was unaffected in the resistant isolates. Thus, by this protocol, azole drugs are necessary during both growth and induction to show an effect on hyphal formation in susceptible isolates.
FIG. 1.
Hyphal formation in the presence of fluconazole during growth and/or induction. Five isolates (isolates A1 to A5) (Table 1) were tested for hyphal formation. The isolates differed in their level of resistance (solid squares) as determined from the MICs. Isolates were tested for hyphal formation in the absence of fluconazole (open squares), with fluconazole added only during growth (open circles), with fluconazole added only during hyphal induction (diamonds), and with fluconazole added during both growth and induction (triangles). Cells were grown in YAD and were induced in M199. The fluconazole concentration used in this experiment was 10 μg/ml.
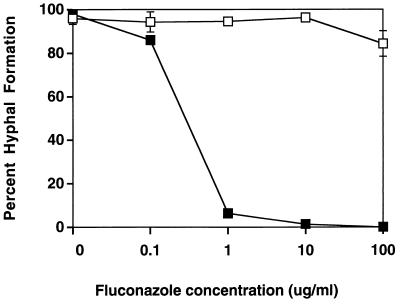
Hyphal formation in susceptible and resistant strains in the presence of increasing concentrations of fluconazole.
Hyphal formation in susceptible and resistant isolates was tested with increasing concentrations of fluconazole during growth and induction (Fig. 2). The addition of only 0.1 μg of fluconazole per ml and subsequent higher concentrations was effective in reducing hyphal formation in a susceptible isolate (isolate A1; MIC, 0.25 μg/ml). However, hyphal formation in a resistant isolate (isolate A5; MIC, >64 μg/ml) was unaffected by the presence of fluconazole, with only a small reduction in hyphal formation in the presence of fluconazole at 100 μg/ml.
FIG. 2.
Hyphal formation in a susceptible isolate and a resistant isolate in the presence of increasing concentrations of fluconazole. Different concentrations of fluconazole were added during growth and hyphal induction of susceptible isolate A1 (black squares) and resistant isolate A5 (open squares). Hyphal formation was observed at 3 h. Mean and standard deviations are shown for all points. For most points, the standard deviation is smaller than the symbol. Cells were grown in YAD and were induced in M199.
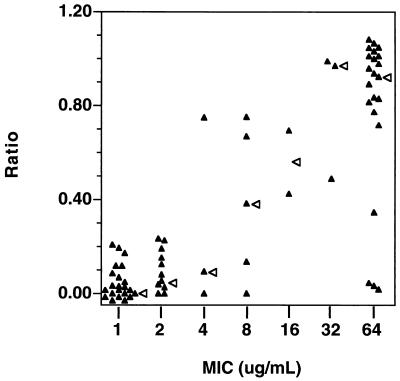
Hyphal formation in the presence of fluconazole correlates with MIC for a random sample of clinical isolates.
A sample population of clinical isolates including a large percentage of resistant isolates was tested for hyphal formation in the absence and presence of fluconazole (10 μg/ml) (Fig. 3). The relative sensitivities of the isolates were determined as a ratio (percent hyphal formation in the presence of drug divided by the percent hyphal formation in the absence of drug) (Fig. 3). Isolates against which fluconazole had no effect would have a ratio of approximately 1. In the absence of fluconazole, percent hyphal formation was 87.2 ± 9.7 (range, 55 to 100). There was a positive correlation between MIC and the ability to form hyphae in the presence of drug (R = 0.757; P < 0.0001). For all 22 isolates for which the MIC was ≤1 μg/ml, fluconazole inhibited hyphal formation. There was some variation for the 22 isolates for which the MIC was ≥64 μg/ml. However, all of these resistant isolates showed significant hyphal formation in the presence of drug. There is also considerable variation in hyphal formation in isolates of intermediate sensitivity. This may be due in part to the concentration of fluconazole used in these determinations (10 μg/ml) and the small number of available isolates for which MICs were intermediate. Isolates for which the fluconazole MIC was near 10 μg/ml may have variable responses to this concentration of fluconazole.
FIG. 3.
Hyphal formation in a random sample from a population of clinical isolates in the absence or presence of fluconazole. A collection of randomly selected isolates was tested for hyphal formation in the absence and presence of fluconazole at 10 μg/ml. The ratio (percent hyphal formation in the presence of fluconazole/percent hyphal formation in the absence of fluconazole) was graphed versus the MICs for the isolates. Each filled triangle represents the data for a separate clinical isolate. The absolute values on both the x and y axes have been slightly modified for the graph so that all the datum points are distinct. An open arrowhead to the right of the value marks the median value. A positive correlation was observed between the ratio of hyphal formation (y axis) and the MIC (x axis)(R = 0.757; P < 0.0001). Percent hyphal formation was observed at 3 h. Cells were grown in YAD and were induced in M199.
Hyphal formation in the presence of fluconazole and ketoconazole correlates with MICs of azoles for two series of isolates.
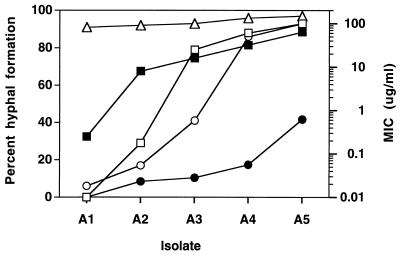
Two series of isolates (Table 1), each obtained from a single individual, were tested for hyphal formation in the presence of both fluconazole and ketoconazole. In both series, the level of resistance increases throughout the series, with the exception that the final isolate in the second series is susceptible to azole drugs (13, 14). Each set of isolates represents a single strain of C. albicans, as determined by restriction fragment length polymorphism analysis with the Ca3 probe (14, 33).
In series 1, hyphal development in the presence of fluconazole or ketoconazole increased with the increase in the MICs of these drugs for the isolates (Fig. 4 and Table 1). Each of the isolates in the series differs from the previous isolate by an additional molecular mechanism of resistance. Those mechanisms include increased expression of two types of efflux pump, increased expression of the target enzyme ERG11, and a point mutation within ERG11 (Table 1) (32). Each isolate appears to increase its hyphal formation and its resistant phenotype. No one isolate appears to be solely responsible for the increased hyphal formation. The large rise in hyphal formation in the presence of fluconazole between isolates A2 and A3 and the large rise in hyphal formation between isolates A3 and A4 in the presence of ketoconazole is a function of the drug concentration used in the assays (7).
FIG. 4.
Hyphal formation in series 1 isolates in the presence of fluconazole and ketoconazole correlates with MICs of azoles. Five isolates (isolates A1 to A5) from a series of 17 isolates (Table 1) were tested for hyphal formation. Each isolate differed in its level of resistance (MIC) to fluconazole (solid square) and ketoconazole (solid circle). The isolates were tested for hyphal formation in the absence of an azole drug (open triangle), with fluconazole at a concentration of 10 μg/ml (open square), and with ketoconazole at a concentration of 2 μg/ml (open circle). Cells were grown in YAD and were induced in M199.
In series 2, hyphal development in the presence of fluconazole or ketoconazole again parallels the MICs of the drugs for the isolates (Table 1). In this series, the only identified mechanism of resistance is CDR overexpression, which parallels the MICs of both drugs for the isolates. This series confirms that hyphal formation correlates with increased resistance, independent of the mechanism.
35S-labeled methionine incorporation.
The inhibition of hyphal formation by azole drugs may be due either to an overall inhibition of growth or to an interference with the mechanism of hyphal formation. To distinguish between these two possibilities, 35S-labeled methionine incorporation was used to measure the growth of the cells during induction (Fig. 5). Resistant isolate A5 and susceptible isolate A1 were grown for 48 h in the absence of methionine and in the absence and presence of fluconazole. The lack of methionine did not influence the growth of the cells nor the subsequent hyphal formation (7). 35S-labeled and nonlabeled methionine were added during hyphal induction, and incorporation into cells was measured over 4 h. As a control, cells were treated for 10 min at 65°C, which causes cell death, as determined by the numbers of CFU on agar plates. These control cells did not incorporate 35S-labeled methionine above the background level (7). Therefore, incorporation of 35S-labeled methionine is dependent on living cells.
FIG. 5.
Incorporation of 35S-labeled methionine in the absence or presence of fluconazole. 35S-labeled methionine incorporation (open symbols and solid lines) and percent hyphal production (filled symbols and dotted lines) were measured at 37°C over 4 h (x axis). Susceptible isolate A1 was monitored in the absence (squares) and presence (circles) of fluconazole (10 μg/ml). Resistant isolate A5 was monitored in the absence (diamonds) and presence (triangles) of fluconazole (10 μg/ml). For methionine incorporation, the background incorporation (time zero) was subtracted from the incorporation at subsequent points. Values are the averages for three samples. The standard deviations are not shown because they were smaller than the sizes of the symbols for most datum points. Hyphal formation of the susceptible isolate in the presence of fluconazole was 0% for the entire 4 h (closed circles). Cells were grown in Lee’s medium at pH 4.5 without methionine and were induced in RPMI 1640 medium without methionine.
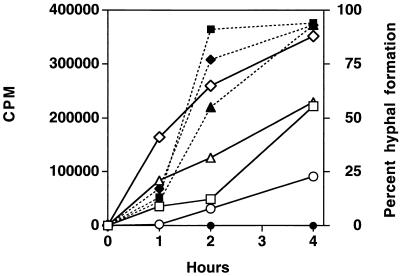
35S-methionine incorporation and hyphal induction were monitored for both susceptible and resistant isolates in the presence and absence of fluconazole at 37°C (Fig. 5). As expected, fluconazole had no effect on hyphal formation in the resistant isolate, while it inhibited hyphal formation in the susceptible isolate. In monitoring 35S-methionine incorporation, the resistant isolate incorporated 147% more label than the susceptible isolate in the absence of fluconazole and 170% more label in the presence of fluconazole. At 4 h, fluconazole inhibited methionine incorporation in the sensitive isolate by 52% and in the resistant isolate by 60%. Therefore, fluconazole had a similar effect on both sensitive and resistant isolates compared to its effect on the controls not treated with the drug. Despite comparable reductions (52 versus 60%) in methionine incorporation due to fluconazole in both sensitive and resistant isolates, hyphal formation was completely blocked in the sensitive isolates (<1%) but was unaffected in the resistant isolate (100%). This indicates that inhibition of hyphal formation is not solely due to growth inhibition.
Incorporation of 35S-methionine was also monitored at 30°C for both susceptible and resistant isolates in the absence and presence of fluconazole (7). At 30°C, all cells grew as yeast cells with some production of pseudohyphae. The pattern of incorporation of label at 30°C in both isolates was not significantly different from the pattern of incorporation at 37°C. This lends additional support to the observation that inhibition of hyphal production is not simply the result of growth inhibition.
DISCUSSION
In this series of experiments, we have demonstrated a strong positive correlation between the level of resistance, as determined by the MIC, and the ability to form hyphae in the presence of azole antifungal drugs. This correlation is valid for a collection of susceptible, susceptible dose-dependent, and resistant clinical isolates. The inhibition of hyphal formation is independent of the molecular mechanisms of resistance that have been identified; that is, the effect is seen with cells that exhibit each of the mechanisms. The azole drug inhibition of hyphal formation is not simply due to an inhibition of growth, as monitored by measurement of the level of 35S-labeled methionine incorporation. This suggests that the azoles have a direct inhibitory effect on hyphal formation.
The effect of azole drugs on hyphal formation is most likely the result of the increased surface area of hyphal cells compared to that of yeast cells. Hyphal structures would require more plasma membrane per cell. The bulk sterol of the plasma membrane is ergosterol, the end product of the ergosterol biosynthetic pathway. The target of the azoles, the product of ERG11, is an essential component of this pathway. Thus, the effect of azoles on hyphal formation may be due to limiting amounts of ergosterol. Previous work has shown that a strain defective in ERG11 activity (strain D10) is severely impaired in hyphal formation (11), presumably because of the lack of ergosterol. In the current experiments, defined media lacking ergosterol or other sterols were used for both growth and induction. The inhibition of hyphal formation was not altered by the addition of exogenous ergosterol (7), although it is not clear if the cells can use the exogenous ergosterol. We have observed similar effects in Lee’s medium, another defined medium that induces hyphae, although we have had limited success with YEPD, which is a rich, undefined medium that likely contains bulk sterols (7). We have observed similar effects on azole-resistant isolates with other azoles (voriconazole) (7).
Under the conditions used in the present study, the azole must be present during both growth and induction of hyphae (Fig. 1). It is likely that the drug must be present during growth to exhaust the population of endogenous ergosterol that would allow the cells to continue to grow and divide. Removal of the drug pressure during induction would allow the cells to start making ergosterol at sufficient levels to produce hyphae.
We have not quantitated the length or the branching of the hyphal projections or the number of cells within each projection. There was no noticeable difference in the hyphal projections between susceptible cells in the absence of drug and resistant cells in the absence or presence of drug. The concentrations that we have used to study hyphal formation are significantly higher than the concentrations at which effects on length and branching of hyphae were seen (19).
On the basis of drug resistance theories for other organisms, it is predicted that resistant isolates of Candida would be less virulent than susceptible isolates. One possible virulence factor that might be affected in resistant isolates is the ability to form hyphae. In the absence of drugs there is no difference in hyphal formation between susceptible and resistant clinical isolates (Fig. 1 and 2). This has not been previously reported and indicates that resistant isolates are not altered in one virulence trait: their ability to form hyphae.
The concentration dependence of the azole inhibition of hyphal formation and the range of the concentration effect (Fig. 2 and 3) are consistent with the level of resistance as determined by the MICs for the isolates. This suggests that the inhibition of hyphal formation is directly related to the MIC for the isolate and not to a side reaction or a secondary effect. The large variability of isolates for which MICs are intermediate (Fig. 3) is most likely due to the similarity of the MIC to the concentration used to monitor hyphal formation. The large variability of isolates for which MICs are high is due to resistant isolates in which hyphal formation is still inhibited by azole drugs (7). The resistant isolates, which do not form hyphae in the presence of drug, may be altered such that their hyphal formation remains susceptible to azole drugs, while their growth is resistant to these drugs. The resistance mechanisms of these isolates will be of interest but have not yet been determined.
Previous work with hyphal formation and resistance did not use isolates for which the molecular mechanisms of resistance had been identified. In this work, we used two series of isolates in which the resistance mechanisms have been well characterized (Table 1). Hyphal formation in the presence of azole drugs shows that each of the molecular mechanisms contributes to the inhibition of hyphal formation, but no mechanism is dominant in this inhibition. The two series represent two types of clinical isolates: genetically stable isolates such as those in series 1 (33) and transiently resistant isolates such as those in series 2 (13). Both genetically stable isolates and transiently resistant isolates display the same correlation between level of resistance and inhibition of hyphal formation.
Incorporation of 35S-labeled methionine during hyphal formation (Fig. 5) suggests that inhibition of growth is not sufficient to explain the lack of hyphal formation in susceptible cells in the presence of azole drugs. This has been observed previously (19) in a study in which ATP levels were used to measure the growth of the cells.
The interpretation of the level of 35S-labeled methionine incorporation has several complications. First, the absolute levels of incorporation differ between the sensitive and the resistant isolates in the absence or presence of drug (147 to 170% more incorporation in the resistant isolate). This appears to be a genetic characteristic of the isolates, which are the same strain (33) but which differ in azole resistance (30, 31). The resistance mechanisms in these series might alter the lipid content of the plasma membrane, which might have effects on amino acid transport (21). The addition of azoles may further affect the sterol components of the plasma membrane, resulting in alterations in amino acid transport that might affect methionine import (21). There is no report of alterations in methionine transport in the yeast cell/hypha transition. It has been shown previously that methionine transport is reduced in cells with higher ergosterol contents and is increased in cells with lower ergosterol contents. Nystatin-resistant isolates with lower ergosterol contents have increased levels of import of methionine (27). It is likely that azole-treated cells have lower ergosterol contents, which would predict an increased level of import of methionine. A complete analysis of methionine import in susceptible and resistant isolates is beyond the scope of this series of experiments.
Despite the absolute differences in methionine incorporation between susceptible and resistant isolates, the addition of fluconazole reduced the level of methionine incorporation by approximately half in both susceptible and resistant isolates. This equivalent reduction cannot easily explain the complete inhibition of hyphal formation in the susceptible isolate, while hyphal formation in the resistant isolate is unaffected. This suggests that fluconazole inhibits hyphal formation, in addition to its inhibition of cell growth. An alternate hypothesis is that there is a threshold value of growth above which hyphal formation can occur. The susceptibilities isolate may not reach that growth threshold in the presence of fluconazole and may be unable to form hyphae.
There are several important clinical implications of this positive correlation between MIC and inhibition of hyphal formation. A simplified and shortened measurement of the percent inhibition of hyphal formation might be developed on the basis of these observations. It could use microscopic examination or antigen detection of hypha-specific proteins. Such a method might be an alternative to the current NCCLS method. In addition, the correlation described above gives us further insight into the effects of antifungal drugs on the pathogenic yeast C. albicans.
ACKNOWLEDGMENTS
We thank Spencer Redding (University of Texas Health Science Center at San Antonio), Kieren Marr (Fred Hutchinson Cancer Research Center), and Michael Pfaller (University of Iowa) for the use of their isolates. We thank Paula Sundstrom (Ohio State University) for her protocol for hyphal formation and the use of her Hwp1 antibody. We thank Jo Beth Harry (Seattle Biomedical Research Institute) for assistance with Western blotting and the other members of our laboratory for their support and comments on the manuscript.
This research was supported by grant RO1 DE-11367, from the National Institute of Dental Research. K.C.H. acknowledges the support of the Mary Gates Endowment for Undergraduate Research (1996–1997 and 1997–1998) and the John and Dorothy Franco Award for Excellence in Biological Sciences Research (1998). T.C.W. is supported by a New Investigator Award from the M. J. Murdock Charitable Trust and is the recipient of a New Investigator Award in Molecular Pathogenic Mycology from the Burroughs Wellcome Fund.
REFERENCES
- 1.Albertson G D, Niimi M, Cannon R D, Jenkinson H F. Multiple efflux mechanisms are involved in Candida albicans fluconazole resistance. Antimicrob Agents Chemother. 1996;40:2835–2841. doi: 10.1128/aac.40.12.2835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Borgers M, Van de Ven M A. Mode of action of intraconazole: morphological aspects. Mycoses. 1989;1:53–59. doi: 10.1111/j.1439-0507.1989.tb02294.x. [DOI] [PubMed] [Google Scholar]
- 3.Cutler J E. Putative virulence factors of Candida albicans. Annu Rev Microbiol. 1991;45:187–218. doi: 10.1146/annurev.mi.45.100191.001155. [DOI] [PubMed] [Google Scholar]
- 4.Galgiani J, Bartlett M, Espinel-Ingroff M, Fromtling R, Pfaller M, Rinaldi M. Reference method for broth dilution antifungal susceptibility testing of yeasts: proposed standard. Vol. 12. Wayne, Pa: National Committee for Clinical Laboratory Standards; 1992. [Google Scholar]
- 5.Gallagher P J, Bennett D E, Henman M C, Russell R J, Flint S R, Shanley D B, Coleman D C. Reduced azole susceptibility of oral isolates of Candida albicans from HIV-positive patients and a derivative exhibiting colony morphology variation. J Gen Microbiol. 1992;138:1901–1911. doi: 10.1099/00221287-138-9-1901. [DOI] [PubMed] [Google Scholar]
- 6.Greenspan D, Greenspan J, Schiodt M, Pindborg J. AIDS and the mouth. Copenhagen, Denmark: Munksgaard; 1990. pp. 91–102. [Google Scholar]
- 7.Ha, K. C., and T. C. White. Unpublished data.
- 8.Haller I. Mode of action of clotrimazole: implications for therapy. Am J Obstet Gynecol. 1985;152:939–944. doi: 10.1016/s0002-9378(85)80005-3. [DOI] [PubMed] [Google Scholar]
- 9.Johnson E M, Richardson M D, Warnock D W. Effect of imidazole antifungals on the development of germ tubes by strains of Candida albicans. J Antimicrob Chemother. 1983;12:303–316. doi: 10.1093/jac/12.4.303. [DOI] [PubMed] [Google Scholar]
- 10.Johnson E M, Richardson M D, Warnock D W. In-vitro resistance to imidazole antifungals in Candida albicans. J Antimicrob Chemother. 1984;13:547–558. doi: 10.1093/jac/13.6.547. [DOI] [PubMed] [Google Scholar]
- 11.Lees N D, Broughton M C, Sanglard D, Bard M. Azole susceptibility and hyphal formation in a cytochrome P-450-deficient mutant of Candida albicans. Antimicrob Agents Chemother. 1990;34:831–836. doi: 10.1128/aac.34.5.831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Marichal P, Gorrens J, Van C J, Vanden B H. Culture media for the study of the effects of azole derivatives on germ tube formation and hyphal growth of C. albicans. Mykosen. 1986;29:76–81. doi: 10.1111/j.1439-0507.1986.tb03753.x. [DOI] [PubMed] [Google Scholar]
- 13.Marr K A, Lyons C N, Rustad T, Bowden R A, White T C. Rapid, transient fluconazole resistance in Candida albicans is associated with increased mRNA levels of CDR. Antimicrob Agents Chemother. 1998;42:2584–2589. doi: 10.1128/aac.42.10.2584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Marr K A, White T C, vanBurik J A H, Bowden R A. Development of fluconazole resistance in Candida albicans causing disseminated infection in a patient undergoing marrow transplantation. Clin Infect Dis. 1997;25:908–910. doi: 10.1086/515553. [DOI] [PubMed] [Google Scholar]
- 15.Moran G P, Sullivan D J, Henman M C, McCreary C E, Harrington B J, Shanley D B, Coleman D C. Antifungal drug susceptibilities of oral Candida dubliniensis isolates from human immunodeficiency virus (HIV)-infected and non-HIV-infected subjects and generation of stable fluconazole-resistant derivatives in vitro. Antimicrob Agents Chemother. 1997;41:617–623. doi: 10.1128/aac.41.3.617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Niimi, M., F. J. Fischer, J. Piper, H. F. Jenkinson, M. Arisawa, and R. D. Cannon. 1997. Presented at the 13th Congress of the International Society for Human and Animal Mycology.
- 17.Odds F C. Candida and Candidosis: a review and bibliography. London, United Kingdom: Bailliere Tindall; 1988. [Google Scholar]
- 18.Odds F C, Cheesman S L, Abbott A B. Antifungal effects of fluconazole (UK 49858), a new triazole antifungal, in vitro. J Antimicrob Chemother. 1986;18:473–478. doi: 10.1093/jac/18.4.473. [DOI] [PubMed] [Google Scholar]
- 19.Odds F C, Cockayne A, Hayward J, Abbott A B. Effects of imidazole- and triazole-derivative antifungal compounds on the growth and morphological development of Candida albicans hyphae. J Gen Microbiol. 1985;131:2581–2589. doi: 10.1099/00221287-131-10-2581. [DOI] [PubMed] [Google Scholar]
- 20.Pfaller M A, Rhine C J, Redding S W, Smith J, Farinacci G, Fothergill A W, Rinaldi M G. Variations in fluconazole susceptibility and electrophoretic karyotype among oral isolates of Candida albicans from patients with AIDS and oral candidiasis. J Clin Microbiol. 1994;32:59–64. doi: 10.1128/jcm.32.1.59-64.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Prasad R. Nutrient transport in Candida albicans, a pathogenic yeast. Yeast. 1987;3:209–221. doi: 10.1002/yea.320030402. [DOI] [PubMed] [Google Scholar]
- 22.Redding S, Smith J, Farinacci G, Rinaldi M, Fothergill A, Rhine C J, Pfaller M. Resistance of Candida albicans to fluconazole during treatment of oropharyngeal candidiasis in a patient with AIDS: documentation by in vitro susceptibility testing and DNA subtype analysis. Clin Infect Dis. 1994;18:240–242. doi: 10.1093/clinids/18.2.240. [DOI] [PubMed] [Google Scholar]
- 23.Sanglard D, Ischer F, Koymans L, Bille J. Amino acid substitutions in the cytochrome P-450 lanosterol 14 alpha-demethylase (CYP51A1) from azole-resistant Candida albicans clinical isolates contribute to resistance to azole antifungal agents. Antimicrob Agents Chemother. 1998;42:241–253. doi: 10.1128/aac.42.2.241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sanglard D, Ischer F, Monod M, Bille J. Cloning of Candida albicans genes conferring resistance to azole antifungal agents: characterization of CDR2, a new multidrug ABC transporter gene. Microbiology. 1997;143:405–416. doi: 10.1099/00221287-143-2-405. [DOI] [PubMed] [Google Scholar]
- 25.Sanglard D, Ischer F, Monod M, Bille J. Susceptibilities of Candida albicans multidrug transporter mutants to various antifungal agents and other metabolic inhibitors. Antimicrob Agents Chemother. 1996;40:2300–2305. doi: 10.1128/aac.40.10.2300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sanglard D, Kuchler K, Ischer F, Pagani J L, Monod M, Bille J. Mechanisms of resistance to azole antifungal agents in Candida albicans isolates from AIDS patients involve specific multidrug transporters. Antimicrob Agents Chemother. 1995;39:2378–2386. doi: 10.1128/aac.39.11.2378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Singh M, Jayakumar A, Prasad R. The effect of altered ergosterol content on the transport of various amino acids in Candida albicans. Biochim Biophys Acta. 1979;555:42–55. doi: 10.1016/0005-2736(79)90070-1. [DOI] [PubMed] [Google Scholar]
- 28.Staab J F, Ferrer C A, Sundstrom P. Developmental expression of a tandemly repeated, proline-and glutamine-rich amino acid motif on hyphal surfaces on Candida albicans. J Biol Chem. 1996;271:6298–6305. doi: 10.1074/jbc.271.11.6298. [DOI] [PubMed] [Google Scholar]
- 29.Wakabayashi H, Abe S, Teraguchi S, Hayasawa H, Yamaguchi H. Inhibition of hyphal growth of azole-resistant strains of Candida albicans by triazole antifungal agents in the presence of lactoferrin-related compounds. Antimicrob Agents Chemother. 1998;42:1587–1591. doi: 10.1128/aac.42.7.1587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.White T C. Increased mRNA levels of ERG16, CDR, and MDR1 correlate with increases in azole resistance in Candida albicans isolates from a patient infected with human immunodeficiency virus. Antimicrob Agents Chemother. 1997;41:1482–1487. doi: 10.1128/aac.41.7.1482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.White T C. The presence of an R467K amino acid substitution and loss of allelic variation correlate with an azole-resistant lanosterol 14α demethylase in Candida albicans. Antimicrob Agents Chemother. 1997;41:1488–1494. doi: 10.1128/aac.41.7.1488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.White T C, Marr K A, Bowden R A. Clinical, cellular, and molecular factors that contribute to antifungal drug resistance. Clin Microbiol Rev. 1998;11:382–402. doi: 10.1128/cmr.11.2.382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.White T C, Pfaller M A, Rinaldi R G, Smith J, Redding S W. Stable azole drug resistance associated with a substrain of Candida albicans from an HIV-infected patient. Oral Dis. 1997;3:S102–S109. doi: 10.1111/j.1601-0825.1997.tb00336.x. [DOI] [PubMed] [Google Scholar]