Abstract
Type 2 inflammation is a complex immune response and primary mechanism for several common allergic diseases including allergic rhinitis, allergic asthma, atopic dermatitis, and chronic rhinosinusitis with nasal polyps. It is the predominant type of immune response against helminths to prevent their tissue infiltration and induce their expulsion. Recent studies suggest that epithelial barrier dysfunction contributes to the development of type 2 inflammation in asthma, which may partly explain the increasing prevalence of asthma in China and around the globe. The epithelial barrier hypothesis has recently been proposed and has received great interest from the scientific community. The development of leaky epithelial barriers leads to microbial dysbiosis and the translocation of bacteria to inter- and sub-epithelial areas and the development of epithelial tissue inflammation. Accordingly, preventing the impairment and promoting the restoration of a deteriorated airway epithelial barrier represents a promising strategy for the treatment of asthma. This review introduces the interaction between type 2 inflammation and the airway epithelial barrier in asthma, the structure and molecular composition of the airway epithelial barrier, and the assessment of epithelial barrier integrity. The role of airway epithelial barrier disruption in the pathogenesis of asthma will be discussed. In addition, the possible mechanisms underlying the airway epithelial barrier dysfunction induced by allergens and environmental pollutants, and current treatments to restore the airway epithelial barrier are reviewed.
Keywords: Airway epithelial barrier, Type 2 inflammation, Asthma, Allergen, Environmental pollutants
Type 2 Inflammation and Its Role in Asthma
Asthma is a common chronic inflammatory airway disease affecting all ages with an estimate of more than 300 million cases all around the world, varying widely between different countries.[1] In China, the prevalence of asthma in individuals older than 20 years was 4.2%, according to a recently published nationwide survey.[2] Noticeably, the ongoing increase in the prevalence of allergic asthma contributes to the growing number of asthma patients.[3]
Type 2 inflammation has been described as the underlying immune responses driving allergic asthma.[4] Type 1 immunity is mainly regulated by CD4+ T helper 1 cells (Th1), which secrete interleukin (IL)-2, interferon-γ and lymphotoxin-α. Th1 cells stimulate a type 1 immune response which is characterized by prominent phagocytotic activity. Type 2 inflammation originated as a response by the mucosal immunity against parasitic helminth infection that represents a very dedicated immune response to ameliorate the helminth burden in the tissues.[5] This type 2 cell-mediated immunity causes helminth expulsion or elimination, whilst simultaneously limits tissue injury, maintains tissue homeostasis, and contributes to regeneration and fibrosis.[6–8] Particularly, the expulsion response against helminth larvae represents all features of a full-blown type 2 immune response. An exciting series of molecular events to ensure the co-survival of the worm and the host are taking place. Löffler's pneumonia represents the basis for a type 2 immune response that was initially directed against Ascaris, hookworms, Toxocara and Schistosoma.[9,10] The life cycle of Ascaris infection is depicted in Figure 1. Similarly, an expulsion-like pathophysiology also occurs as an immune response to skin parasites, such as in scabies.[11]
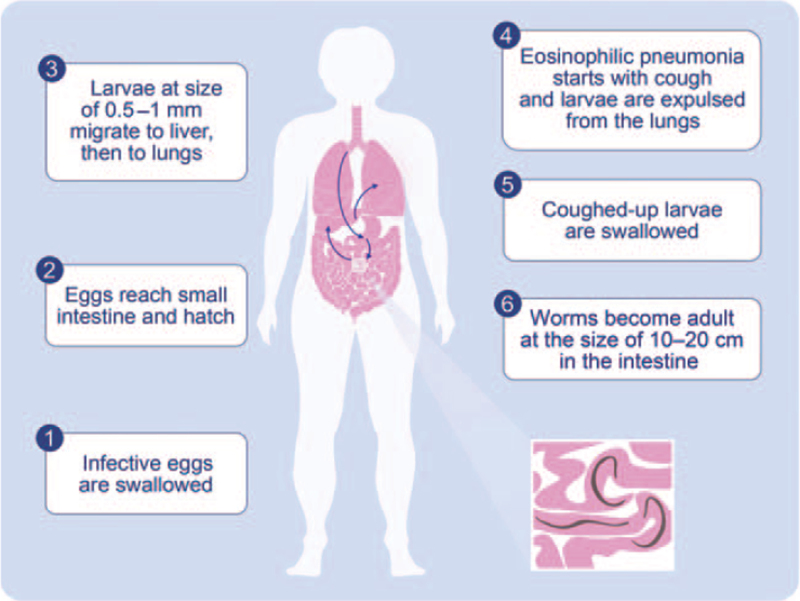
Figure 1.
Life circle of Ascaris in human body and Löffler's pneumonia. Ascaris infection occurs when their fertilized eggs are ingested. The eggs hatch in the intestine, and the larvae migrate to portal veins and then pass through the vena cava inferior, right heart, pulmonary artery, and enter the lungs. The size of the larvae ranges between 0.5 and 1 mm. The growing larvae of the worms cause an eosinophilic pneumonia with cough, as initially described by Löffler. As an essential mechanism of survival of the host, every single larva should be expulsed from the lungs, before they become adults. Because in the case of Ascaris, an adult is 15–20 cm long, and there is no space in the lungs for the adult worms to accommodate their substantially large size, which becomes a big threat to the survival of both the host and parasite. Accordingly, the larvae are fully expulsed from the lungs and swallowed, where they find sufficient space in the guts to become adults.
Type 2 immunity is associated with a wide range of allergic diseases such as allergic rhinitis (AR), allergic asthma, and atopic dermatitis (AD).[5] In asthma, airway type 2 inflammation is mediated by eosinophils, mast cells (MCs), basophils, CD4+ T helper 2 cells (Th2), group 2 innate lymphoid cells (ILC2) and immunoglobulin E (IgE)-expressing memory B cells.[4] Type 2 immunity is mainly regulated by Th2 cells secreting IL-4, IL-5, and IL-13 and stimulating antibody production and eosinophilia.[4] Type 2 cytokines promote hallmark features of asthma with a type 2-high signature, such as eosinophilia, mucus hypersecretion, bronchial hyperresponsiveness (BHR), IgE production, and susceptibility to exacerbations.[12] Clinically, biological agents that target type 2 inflammation showed remarkable clinical efficacy in moderate to severe asthma.[13] Currently, five monoclonal antibodies against IgE (omalizumab), IL-5 (mepolizumab and reslizumab), IL-5 receptor α (benralizumab), and IL-4 receptor α (dupilumab) have been approved for the treatment of severe or refractory asthma, and function by blocking the type 2 inflammatory pathways.[14] Some potentially effective biologicals targeting upstream proinflammatory mediators, such as thymic stromal lymphopoietin (TSLP) and IL-33, are also under clinical trials.[15,16]
Epithelial Barrier Dysfunction and Allergic Diseases
Epithelial barrier dysfunction has been demonstrated to participate in the development of allergic diseases.[17] Structural and functional disruption of the airway epithelial barrier was found in inflammatory and allergic respiratory diseases, i.e., asthma, AR, and chronic rhinosinusitis.[18] Studies showed that epithelial damage in allergic asthma was associated with tight junction (TJ) defects and decrease of adherence junctions.[19–21] The expression of TJ molecules, such as occludin and zonula occludens (ZO)-1, decreased in AR patients compared with healthy controls, which was associated with disease severity.[22] It is well-known that skin barrier dysfunction is a fundamental feature in AD. Filaggrin (FLG) loss-of-function gene mutations are the strongest known genetic risk factor for AD.[23] FLG deficiency is associated with impairment of keratinocyte differentiation, reduced inflammatory thresholds to irritants and haptens, and enhanced percutaneous microbial and allergen penetration.[24–26] In addition to FLG mutations, TJ barrier dysfunction has also been reported in AD.[27] Skin barrier dysfunction and AD is associated with an increased risk of food allergy and allergic asthma, and transcutaneous exposure of food or airborne allergens increases the risk of sensitization.[28–30] Moreover, skin barrier injury can induce intestinal MC expansion through skin-to-gut axis mediated by IL-33, IL-25, and ILCs.[31] Subsequently, degranulation of MCs causes increased intestinal permeability and leads to enhanced sensitization to food allergens in the intestinal tract.[31] Therefore, the dysfunction of the epithelial barrier in the airway, skin, and gut is closely associated with allergic diseases.
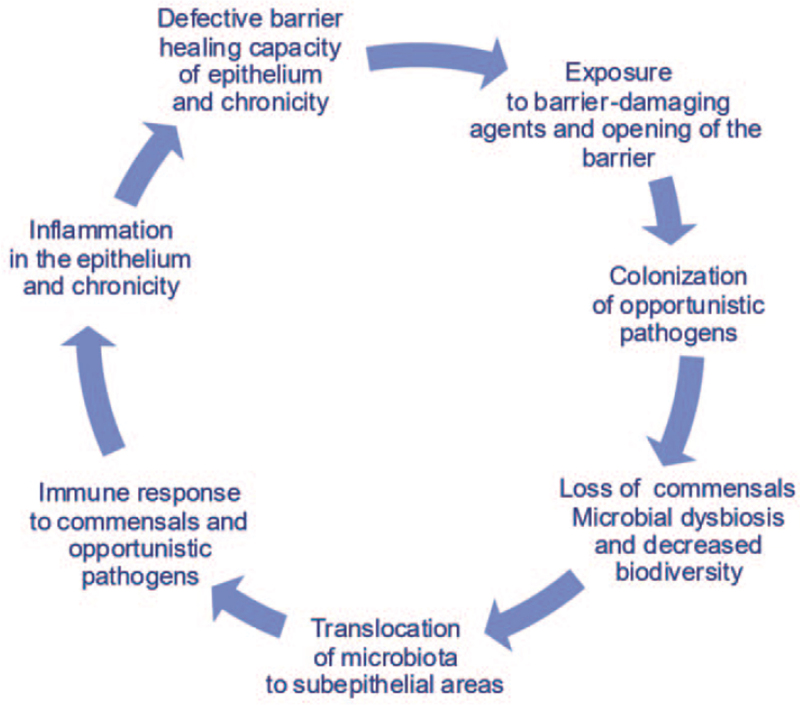
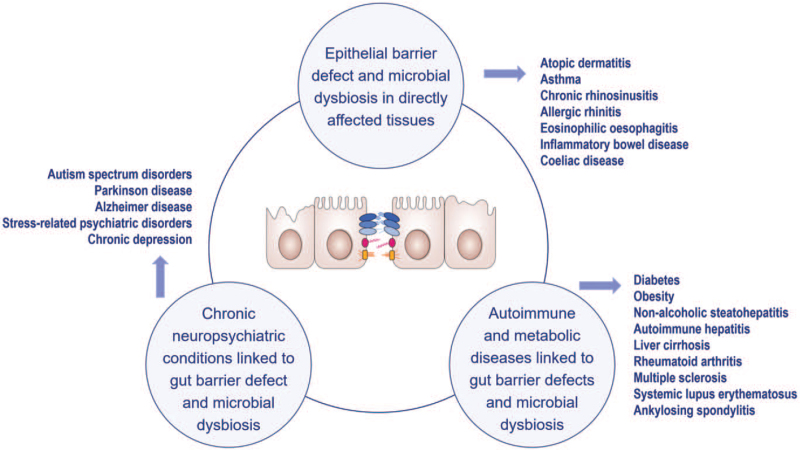
The “Epithelial Barrier Hypothesis” proposes that increased exposure to epithelial barrier damaging agents linked to industrialization, urbanization, and modern life underlies the rise in allergic, autoimmune, and other chronic conditions.[17,32] It discusses whether the immune responses to dysbiotic microbiota that cross the damaged barrier are involved in the development of these diseases.[33] Almost two billion patients are affected with diseases which can be initiated or exacerbated with the exposure to epithelial barrier damaging agents.[34] The development of leaky epithelial barriers then leads to microbial dysbiosis and the translocation of bacteria to interepithelial and subepithelial areas and the development of tissue microinflammation [Figure 2]. Studies on the epithelial barrier suggest that these processes underlie not only the development of allergy and autoimmune conditions in barrier-damaged tissues but also a wide range of diseases in which an immune response to commensal bacteria and opportunistic pathogens occurs[17] [Figure 3].
Figure 2.
Fact circle of epithelial barrier hypothesis.
Figure 3.
Conditions in which the pathogenesis is associated with epithelial barrier disruption.
Cellular and Molecular Components of the Airway Epithelial Barrier
The airway epithelium is a pseudostratified columnar structure composed of different types of cells. The predominant airway epithelial cells are ciliated epithelial cells, mucous-secreting goblet cells, airway basal cells, and club/clara cells;[35] and another three rare but specialized epithelial cells are neuroendocrine cells, solitary chemosensory cells, and ionocytes.[36,37] Airway basal cells are stem-cell-like progenitor cells that can differentiate to ciliated cells, mucus-secreting goblet cells, or other specialized epithelial cells.[38] Basal cells anchor the epithelium to the basal membrane via hemidesmosomes.[39] Ciliated epithelial cells originate from basal cells and/or club cells and contain abundant cilia that are necessary for the mucociliary clearance.[35] Mucus-secreting goblet cells are secretory cells that contain vesicles with tightly packed mucin granules and surfactant proteins.[40] Club cells, also called clara cells, are nonciliated secretory cells differentiated from basal cells in small airways, which can secrete a specific protein belonging to the secretoglobin family (secretoglobin family 1A member 1, SCGB1A1).[41] When the epithelium is injured, club cells are able to differentiate into ciliated and mucus-secreting goblet cells driven by the intercellular junctional protein E-cadherin.[42] Neuroendocrine cells are located at airway branch points with allergens and other harmful substances accumulating, contain dense granules of various neuropeptides, amines, and neurotransmitters regulated by the sympathetic and parasympathetic nervous system and serve as airway chemoreceptors.[43,44] Solitary chemosensory cells contain an apical microvilli tuft, and the function and signal pathways of these cells are similar to intestinal tuft cells, which can regulate type 2 immunity and produce epithelial IL-25.[45,46] The recently identified ionocytes account for only 1% of airway epithelial cells and lie in multiple levels of the respiratory tract. These cells originate from basal cells and highly express the cystic fibrosis transmembrane conductance regulator (CFTR).[36] The inhibition of CFTR has been found to reduce ZO-1 expression and epithelial differentiation, which implies that ionocytes play a role in regulating TJ assembly and epithelial barrier function.[47]
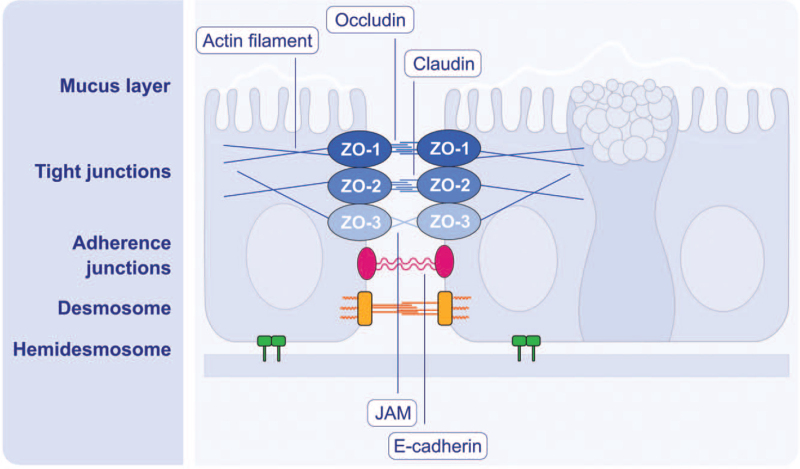
The chemical and physical barriers form the airway epithelial barrier function. Most exogenous substances are trapped in the mucus layer and cleared away by ciliary movements. The production and maintenance of the airway mucus is precisely regulated. It has been found that the balance between Muc5AC and Muc5B, major mucins secreted by goblet cells, can influence mucus viscosity, the ciliary beating and subsequently the likelihood of environmental molecules coming into contact with the airway epithelial cells.[48] On the other hand, the coordinated interaction between neighboring epithelial cells via cell-cell adhesion complexes is of great importance for the physical barrier function, including TJ, adherence junction, desmosome and hemidesmosome[49] [Figure 4]. These junctional structures not only build a physical barrier, but also play an important role in the regulation of epithelial permeability, cell proliferation and differentiation.[50]
Figure 4.
Schematic diagram of structure and molecular components of airway epithelial barrier. The mucus secreted by goblet cells forms the superficial mucus layer. The junctional structures between epithelial cells from the surface to base are tight junction (TJ), adherence junction (AJ), and desmosomes. TJs, located nearest to the epithelial surface, are key regulators of paracellular permeability depending on the size and ionize of molecules. TJs are constituted of transmembrane proteins including the claudin family (24 claudins), occludin, tricellulin, and junctional adhesion molecules, and the major TJ-associated cytoplasmic proteins are ZO-1, ZO-2, and ZO-3. AJs are located directly below TJs and composed of cadherin-catenin complexes. AJs provide intercellular adhesion to maintain epithelial integrity and perform multiple functions, such as initiation and stabilization of TJs, regulation of the actin cytoskeleton, intracellular signaling, and transcriptional regulation. Desmosomes are located around the midpoint of epithelial cells and contribute to the mechanical stability of airway epithelium due to their strong contact with the intermediate filaments. Hemidesmosomes make the epithelial layer attached to the basal membrane. JAM: Junction adhesion molecule; ZO: Zonula occludens.
Assessment of Epithelial Barrier Function
One of the direct implications of epithelial barrier damage is the increase in epithelial permeability leading to transepidermal water loss, which can be used as a measurable parameter for the assessment of epithelial barrier function. Although not available in routine clinical practice, some techniques can be used in research to evaluate epithelial permeability [Table 1]. For example, histological examination via airway mucosal tissue biopsy and/or cytological examination of epithelial cells can provide specific analysis of junctional structure and proteins, albeit it is an invasive method.[49] In addition, early studies have reported that compounds with traceable radioisotopes, e.g. iodine-125 and technetium-99, can be used to assess the permeability of the airway epithelium.[51,52] Mannitol, rarely metabolized and without radioactivity, was used in animal studies to evaluate the airway epithelial permeability.[53] However, a recent study showed no difference in serum mannitol levels between subjects with mild asthma and healthy controls after inhalation of mannitol.[54] Biomarkers for evaluating the epithelial barrier function are gaining research interest. One such potential biomarker of airway epithelial damage is club cell secretory protein-16 (CC16).[55] Studies have demonstrated that the levels of CC16 in serum and bronchoalveolar lavage fluid were elevated in subjects exposed to asbestos and ozone.[56,57] Recently, zonulin, identified as pre-haptoglobin-2 (pre-HP2), was shown to modulate intercellular TJs and reversibly regulate epithelial permeability in the intestine.[58,59] Studies in mice also indicated the involvement of zonulin in respiratory tract epithelial barrier damage. Rittirsch et al[60] reported that zonulin facilitated the development of acute lung injury (ALI) by enhancing albumin leak and complement activation. In addition, zonulin inhibitor was found to exhibit protective effects on influenza infection and mitigate pulmonary edema in ALI,[61] and might be a potential therapy for coronavirus disease 2019 according to a recent in silico analytic study.[62] It has to be noted here that by using electrical impedance spectroscopy, skin barrier integrity can be detected within 8 seconds in a robust and reliable manner.[63,64] There is a current need for similar devices for the assessment of mucosal epithelia.
Table 1.
Methods to assess airway permeability and reflect epithelial barrier function.
| Method | Sample-taking | Examination |
| Histology and/or cytology | Airway mucosal biopsyBronchial brushing | Specific analysis of junctional structure and proteins |
| Permeability assay in vivo | Serum | Tracking the metabolism of radioisotopes (e.g., iodine 125 and technetium 99) or mannitol to reflect the permeability of airway epithelium |
| Biomarkers | Serum | Testing in vitro the levels of CC16, zonulin |
| Electrical impedance spectroscopy | None | Direct assessment in vivo of epidermal barrier function in previous studies, implying a potential method to examine the airway epithelial barrier |
CC16: Club cell secretory protein-16.
Common Allergens and Environmental Factors that Induce Airway Epithelial Barrier Dysfunction
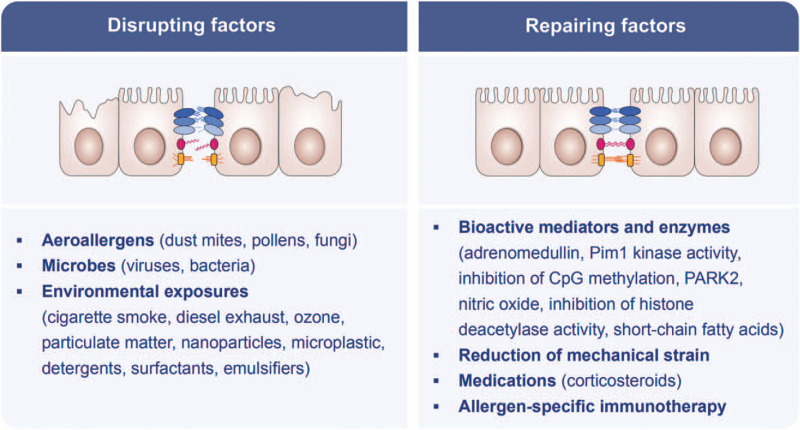
Many different exogeneous factors can open the skin and mucosal epithelial barriers. It must be emphasized that the substances mentioned in this review may cooperate in opening the barriers in a synergistic way together with epithelial inflammation. Airway epithelial barrier damage can be caused by a number of allergens, microbes, and environmental substances [Figure 5]. Common aeroallergens, such as dust mites, pollens, and fungi, can disrupt the airway epithelium barrier. The cysteine proteinase allergen Der p1 from house dust mite (HDM), Dermatophagoides pteronyssinus, can directly cleave the TJ adhesion protein occludin. The disruption of intercellular TJs subsequently increases the permeability of the epithelial barrier and induces an immune response.[65] Saito et al[66] recently found that the amount of peroxisome proliferator-activated receptor γ coactivator-1 alpha (PGC-1α) and E-cadherin decreased significantly in HDM-stimulated cells. The HDM allergen disrupted the airway epithelial barrier function through the protease-activated receptor 2 (PAR2)/Toll-like receptor 4/PGC-1α pathway. Similarly, pollens often contain proteases, for example, serine proteases and metalloproteinases, which act on transmembrane adhesion proteins E-cadherin, claudin-1, and occludin, as well as the cytosolic complex ZO-1, and then damage intercellular TJs, the anchorage of columnar epithelial cells and the integrity of epithelial barrier.[67,68] Proteases of Alternaria alternata can also induce the disruption of the airway epithelial barrier.[69]
Figure 5.
Disrupting and repairing factors for airway epithelial barrier. CpG: Repeated cytosine and guanine nucleotides linked with phosphate; PARK2: A Parkinson's disease-associated gene; Pim-1: Pim-1 proto-oncogene, serine/threonine kinase.
Importantly, increasing evidence indicates that exogenous noxious substances in the environment are risk factors for the airway epithelial barrier injury and leakiness, including cigarette smoke,[70,71] diesel exhaust,[72] ozone,[73] particulate matter,[74,75] nanoparticles,[76] microplastics,[77] detergents, surfactants, and proteolytic enzymes used in cleaning agents,[78–80] as well as emulsifiers in processed food.[81,82]
The skin epithelium is overwhelmingly exposed to toxic substances present in detergents and household cleaning products.[17] Increased use of detergents in general and the addition of surfactants to commercial detergents has significantly increased the daily exposure to tissue barrier-damaging substances.[83] An additional burden to the epithelial barrier was the introduction of proteolytic enzymes in washing powders in the mid-1960s to improve their cleaning efficiency.[84] Proteolytic enzymes derived from Bacillus subtilis have a direct disruptive effect on the airway epithelial barrier as observed in the development of asthma and rhinitis in employees of a detergent factory.[85,86] On the other hand, certain strains of Bacillus subtilis can serve as probiotics, regulating TJ proteins (ZO-1) and reducing death of intestinal epithelial cells.[87] A systematic review of epidemiological studies showed an association between exposure to cleaning products and asthma in four cross-sectional, longitudinal, and case-control studies.[88] Occupational allergies and asthma in the detergent industry have significantly decreased by adopting extensive measures and development of best practice guidelines focusing on exposure control in production facilities.[89] There has been extensive research on replacing nonbiodegradable products with more environmentally friendly and safer alternatives.[90] However, daily exposure to tissue barrier damaging doses of detergents and household cleaners continues today with the addition of household and professional dishwashers.
Viruses, such as rhinoviruses[91] and coronaviruses,[92,93] can disrupt TJs and epithelial barrier function, increasing epithelial permeability and viral invasion, and facilitating inflammatory reactions. A typical feature of chronic mucosal inflammation is the development of an immune response toward microbiome components or newly colonizing facultative pathogens, such as Staphylococcus aureus (S. aureus), moraxella, pneumococcus, hemophilus and Pseudomonas aeruginosa.[17,94]S. aureus is the most abundant bacteria that colonize barrier damaged tissues in the skin and respiratory mucosa. Increased colonization of S. aureus in the nose of asthma patients and increased serum levels of IgE against S. aureus enterotoxins have been repeatedly reported.[95–99] Prevalence of antibodies against S. aureus components has been linked to asthma severity and exacerbations.[97]S. aureus can enhance the TJ barrier integrity in nasal tissue in healthy individuals but not in nasal polyps.[100]S. aureus has also been shown to be dominant in skin microbiome of patients with AD, suggesting a role of this pathogen in skin barrier dysfunction.[101]
Role of Airway Epithelial Barrier Dysfunction in Type 2 Inflammation of Asthma
The development of asthma and respiratory allergies is a complex interaction between genes, immune system, and the environment whereby the airway epithelial barrier function plays a key role.[18,102] Airway epithelial damage leads to the loss of physical protection, facilitates the penetration of exogenous stimulants and allergens[103] and acts as an interface of innate and adaptive immunity.[104] Airway epithelial cells express pattern recognition receptors and detect environmental stimuli such as pathogens and allergens.[105] Epithelial barrier disruption has been the focus in understanding the pathogenesis of asthma with type 2 inflammation.[17]
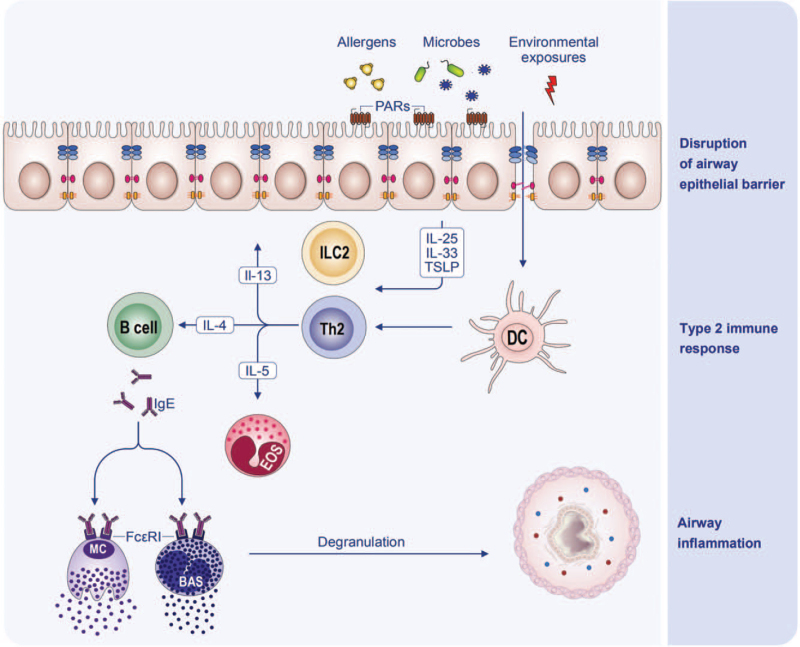
Aeroallergens, virus, bacteria, and environmental toxins can impair the epithelial barrier and promote airway epithelial cells to release alarmins IL-25, IL-33, and TSLP, as well as chemokines C-C motif chemokine ligand 2 (CCL2) and CCL20.[105] The alarmins can induce the differentiation of ILC2, which then releases type 2 cytokines IL-5 and IL-13. CCL2 and CCL20 can recruit immature dendritic cells (DCs) and monocytes, the precursors of DCs, to the lungs.[106,107] Epithelial cytokines IL-25, IL-33, and TSLP favor the development of a proallergic DC phenotype.[108] Activated DCs act as antigen presenting cells and migrate to the draining lymph node where they induce the differentiation of naïve T cells to Th2 cells. The interactions between the airway epithelial cells, DCs and the regional lymph node provide a cytokine milieu for Th2 cell differentiation.[108] IL-4 produced by basophils, together with IL-4 and IL-21 produced by follicular helper T cells, promotes immunoglobulin class switch to IgE in B cells. Effector cells including MC, basophils, and eosinophils are activated, degranulate, and release inflammatory mediators upon being re-exposed to allergens. IL-4 and IL-13 are cardinal type 2 cytokines and central to many aspects of airway changes in asthma, e.g., directly participating in type 2 inflammation, disrupting the epithelial barrier function, acting on basement membrane, and promoting airway remodeling. Therefore, a vicious cycle composed of IL-4, IL-13, epithelial barrier impairment, and type 2 inflammation has been suggested in asthma.[106] In addition, airway epithelial barrier damage will also enhance permeability to foreign substances including allergens,[109] which are uptaken, processed, and presented by DCs and initiate adaptive immune responses.[110] A recent study showed that allergen-induced degranulation of MCs was only observed in those with injured nasal epithelia, and epithelial barrier dysfunction promoted transepithelial allergen passage, sensitization, and MC degranulation even in the absence of an inflammatory condition.[111] In return, MC mediators could rapidly increase epithelial permeability, which facilitated allergen penetration again.[112] Moreover, airway epithelial barrier function can maintain the balance of immunomodulation. Restoring the epithelial barrier integrity reduced inflammation in models of Th2-mediated respiratory inflammation.[113] In a mouse model, the activation of MCs was elevated when the epithelial barrier was disrupted.[50] It is speculated that nasal epithelial barrier dysfunction is one of the crucial risk factors in the inflammatory progression from upper to lower airways.[114] Thus, airway epithelial barrier dysfunction may represent a cardinal pathophysiological mechanism of type 2 immunity. The physical barrier injury, allergic sensitization, and immunological dysregulation resulted from airway epithelial barrier disruption and dysfunction participate in the pathogenesis of asthma and respiratory inflammatory diseases [Figure 6].
Figure 6.
Schematic diagram of the interaction of type 2 inflammation and airway epithelial barrier in asthma. BAS: Basophil; DC: Dendritic cell; EOS: Eosinophils; FcεRI: High-affinity receptor for IgE; IgE: Immunoglobulin E; IL: Interleukin; ILC2: Group 2 innate lymphoid cell; MC: Mast cell; PARs: Protease-activated receptors; Th2: T helper 2 cell; TSLP: Thymic stromal lymphopoietin.
Molecular Mechanisms Underlying the Disruption of Airway Epithelial Barriers
Currently, the precise underlying mechanisms leading to airway epithelial barrier disruption in asthma are under extensive research. Different mechanisms may be involved for the various damaging factors of the epithelial barrier, such as allergens, bacteria, virus, particulate matter, and other environmental pollutants.[34,115] Many allergens possess protease activity, which acts on protease-activated receptors (PARs) and induces airway epithelial barrier impairment. HDM allergens were reported to induce airway epithelial barrier dysfunction via proteolytic activity.[65] However, another report showed that HDM-induced airway inflammation and hypersensitivity was dependent on allergen sensitization but not to serine/cysteine protease activity, since HDM extract with the lowest serine protease activity still induced the most pronounced dysfunction of the epithelial barrier and CCL20 release in vitro.[116] Another study also demonstrated that inhalation of HDM allergens did not induce impairment of the airway epithelial barrier.[117] As non-allergic individuals mostly tolerate allergen exposure without or only with mild symptoms, mechanisms other than allergen-specific MC degranulation may have a relatively minor effect. It is suggested that HDM-induced PAR activation and epithelial barrier disruption depended on epidermal growth factor receptor (EGFR) signaling since EGFR inhibition reduced the HDM-triggered decrease in epithelial resistance and improved restoration of epithelial junctions.[118] Mitochondrial biogenesis and heat shock protein 90α have also been demonstrated to participate in HDM-induced airway epithelial barrier dysfunction with distinct signaling pathways.[66,119] Allergenic fungus A. alternata possesses serine protease activity and induces barrier disruption of airway epithelium in severe asthma patients.[69] German cockroach induced Ca2+ release from intracellular Ca2+ store by acting on PAR2 in the airway epithelium.[120] In addition, cockroach and HDM extracts also activated store-operated Ca2+ entry and thus sustained intracellular Ca2+ elevation in the airway epithelium,[121] which triggers proinflammatory cytokines release and airway epithelial barrier dysfunction.[122] Tumor necrosis factor (TNF)-α was shown to induce bronchial epithelial barrier dysfunction by activating Src-family kinase in severe asthma.[123]
The impact of type 2 cytokine IL-13 on epithelial barrier dysfunction has been well-established in air-liquid interface (ALI) cultures of bronchial epithelial cells and mouse models of lung inflammation.[124,125] IL-13, released both by ILC2 and Th2 cells, was shown to induce airway epithelial barrier disruption by targeting TJs in asthmatic patients.[124,125] By contrast, another study demonstrated that IL-13 plays an important role in restoration of airway epithelial barrier via IL-13 receptor α2.[126]
In addition to directly affecting TJ molecules in the epithelia, several programmed cell death processes have been suggested to contribute to airway epithelial barrier dysfunction. Both pyroptosis[127,128] and apoptosis[129] have been demonstrated to play a possible role in the airway epithelial barrier dysfunction and airway inflammation.[130,131] Similarly, ferroptosis and autophagy,[132] and their interactions[133] have also been suggested to contribute to airway epithelial barrier dysfunction in asthma. Particulate matter and respiratory syncytial virus-induced necroptosis of airway epithelial cells contribute to airway inflammation.[134,135] However, the role of necroptosis in airway epithelial barrier impairment needs to be clarified further.
Restoration of the Airway Epithelial Barrier
Epithelial barrier impairment is central to the pathogenesis of airway inflammation and may also be linked with severity and control of asthma, therefore, restoration of the barrier integrity may be a useful strategy in the treatment of asthma [Figure 5]. Deoxyribonucleic acid containing repeated cytosine and guanine nucleotides linked with phosphate (CpG DNA) treatment exhibited a barrier healing capacity in vitro.[136] Reduced adrenomedullin expression in airway epithelial cells was observed in asthma patients, and supplementation with adrenomedullin could promote airway epithelial wound repair.[137] It is reported that Pim1 kinase activity is essential to maintaining airway epithelial integrity and protects against HDM-induced proinflammatory cytokine secretion from airway epithelium.[138] Inhibition of CpG methylation was found to improve the integrity of the bronchial epithelial barrier in asthma.[139] Parkinson's disease-associated gene could also protect against HDM-induced airway epithelial barrier impairment by attenuating epithelial cell pyroptosis.[127] Nitric oxide promoted airway epithelial wound repair through increasing the activity of matrix metalloproteinases 9.[140] As also shown in vitro in bronchial epithelial cells,[125] inhibition of histone deacetylase activity could restore nasal epithelial integrity and prevent the development of allergic airway inflammation in patients with AR.[141] Therefore, further studies are warranted to provide evidence of the potential use of histone deacetylase activity inhibitors to restore the bronchial epithelial integrity in asthma patients. Mechanical strain inhibited airway epithelial repair as demonstrated in in vitro cultured epithelial cells,[142] thus maintaining well-control of asthma may reduce mechanical strain induced by hyperinflation secondary to airflow limitation.
As to the currently available treatments for asthma, corticosteroid dexamethasone was able to restore the expression of E-cadherin and beta- and gamma-catenin that was inhibited by TNF-α, as demonstrated in primary human bronchial epithelial cells.[143] A few studies demonstrated a protective effect of long-acting beta-agonists (LABA) on the airway epithelial barrier.[144,145] Montelukast could suppress cysteinyl leukotriene-induced disruption of TJs and adherence junctions (AJs) in human airway epithelial cells.[146] Allergen-specific immunotherapy (AIT) was also able to restore airway epithelial integrity that was damaged in mice exposed to HDM component Der f through inhibition of IL-25 expression and endoplasmic reticulum stress.[147] The effect of biologicals, such as anti-IgE, anti-IL-5/R, and anti-IL-4Rα monoclonal antibodies on airway epithelial barrier dysfunction in asthma patients, is not fully understood. Short-chain fatty acids propionate and butyrate were also capable of restoring HDM-induced bronchial epithelial barrier dysfunction and have been suggested for the potential treatment of asthma.[148] Even though there is limited evidence on the potential of probiotics in restoring the airway epithelial barrier integrity, a study showed a decrease in airway epithelial permeability in both animal models and in vitro cultured bronchial epithelial cells.[149]
Prospects
It should be noted that the airway epithelial barrier integrity is dynamically regulated by disrupting and repairing factors, both of which may coexist simultaneously. To date, most studies focus only on disruption or restoration of the barrier. Studies aiming to elucidate the imbalance between disruption and repair under different exposomes and its impacts on type 2 inflammation with state-of-the-art techniques will be of great importance to the development of new diagnostic and therapeutic strategies for asthma. A better understanding of the epithelial barrier hypothesis is needed for the prevention, early intervention, and development of novel therapeutic approaches.[17] Possible strategies to reduce diseases associated with a disrupted epithelial barrier include: avoidance and dose control of all of the above-mentioned noxious substances; development of safer, less-toxic products; discovery of biomarkers for the identification of barrier leaky subjects; development of novel therapeutic approaches for restoration of the expression of tissue-specific barrier molecules; strengthening other components of the mucosal barrier; blocking bacterial translocation; avoiding the colonization of opportunistic pathogens; interventions through diet and microbiome, and many more novel approaches. In addition, an international network has been initiated together with the development of the European Academy of Allergy and Clinical Immunology guidelines on environmental health, and a working group to target epithelial barrier related research, education, and communication to outreach regulatory authorities has been recently taken off[150] [Table 2].
Table 2.
The aims of EAACI working group targeting epithelial barrier related research, education and communication.
| Items | Contents |
| A | Coordination of research and education on the avoidance and dose control of all of the toxic substances |
| B | Coordination of research and education for the development of safer, less-toxic products |
| C | Coordination of research and education on the discovery of biomarkers for the identification of individuals with a leaky epithelial barrier |
| D | Coordination of research and education on the development of novel therapeutic approaches for strengthening the tissue-specific barriers |
| E | Coordination of research and education on understanding the changes in microbiome on epithelial barrier leaky areas, bacterial translocation, decreased biodiversity, colonization of opportunistic pathogens |
| F | Coordination of research and education on treatments and interventions through diet and the microbiome |
| G | Development of educational content on epithelial cell biology |
| H | Development of Schools and Focused Meetings on Epithelial Cells and Microbiome |
| I | Collaborative work with research groups from Immunology, Asthma, Pediatrics, Dermatology and ENT and Interest Groups of Aerobiology, Biologicals |
| J | Lobbying in throughout the whole world to have international projects and in the area |
EAACI: European Academy of Allergy and Clinical Immunology; ENT: Ear, nose and throat.
In summary, future studies are warranted to understand: (1) the imbalance between impairment and repair of the airway epithelial barrier; (2) the molecular components of different aeroallergens responsible for the induction of airway epithelial barrier damage; (3) exposomes including virus, bacteria, fungi, particulate matter, microplastics, and their interactions and contributions to airway epithelial barrier damage; (4) biomarkers of airway barrier dysfunction in asthma; (5) novel strategies to repair the airway epithelial barrier. Researches focusing on the interactions of airway epithelial barrier dysfunction and type 2 inflammation in the context of asthma will be helpful to find novel therapeutic targets for asthma. Adoption of state-of-art techniques such as single-cell sequencing, proteomics, airway organoids, Visium spatial imaging together with immunology and animal models will facilitate these studies.
Conclusions
The epithelial barriers of the skin, upper and lower airways, and gut mucosa have been severely impacted by the rapid change in the environment caused by industrialization, urbanization, and westernized lifestyle. The development of leaky epithelial barriers leads to the dysbiosis and translocation of microbiota to inter- and subepithelial areas, and the development of tissue microinflammation. Epithelial barrier dysfunction contributes to the development of type 2 inflammation in asthma, which then in turn aggravates barrier dysfunction. Allergens, bacteria, viruses, and environmental pollutants could cause epithelial barrier dysfunction by different mechanisms, such as proteases, Ca2+ signaling and programmed death of airway epithelial cells. Most currently available treatments for asthma, such as corticosteroids, LABA, montelukast, and AIT, are able to restore airway epithelial integrity. The interplay between the epithelial barrier and type 2 inflammation in asthma, as well as therapies aimed at regulating this balance is a promising field to be further explored.
Conflicts of interests
None.
Footnotes
How to cite this article: Dong X, Ding M, Zhang J, Ogülür I, Pat Y, Akdis M, Gao Y, Akdis CA. Involvement and therapeutic implications of airway epithelial barrier dysfunction in type 2 inflammation of asthma. Chin Med J 2022;135:519–531. doi: 10.1097/CM9.0000000000001983
References
- 1.Asher MI, García-Marcos L, Pearce NE, Strachan DP. Trends in worldwide asthma prevalence. Eur Respir J 2020; 56:2002094.doi: 10.1183/13993003.02094-2020. [DOI] [PubMed] [Google Scholar]
- 2.Huang K, Yang T, Xu J, Yang L, Zhao J, Zhang X, et al. Prevalence, risk factors, and management of asthma in China: a national cross-sectional study. Lancet 2019; 394:407–418. doi: 10.1016/S0140-6736(19)31147-X. [DOI] [PubMed] [Google Scholar]
- 3.Backman H, Räisänen P, Hedman L, Stridsman C, Andersson M, Lindberg A, et al. Increased prevalence of allergic asthma from 1996 to 2006 and further to 2016-results from three population surveys. Clin Exp Allergy 2017; 47:1426–1435. doi: 10.1111/cea.12963. [DOI] [PubMed] [Google Scholar]
- 4.Fahy JV. Type 2 inflammation in asthma–present in most, absent in many. Nat Rev Immunol 2015; 15:57–65. doi: 10.1038/nri3786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hammad H, Lambrecht BN. Barrier epithelial cells and the control of type 2 immunity. Immunity 2015; 43:29–40. doi: 10.1016/j.immuni.2015.07.007. [DOI] [PubMed] [Google Scholar]
- 6.Mukai K, Tsai M, Starkl P, Marichal T, Galli SJ. IgE and mast cells in host defense against parasites and venoms. Semin Immunopathol 2016; 38:581–603. doi: 10.1007/s00281-016-0565-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Akdis M, Aab A, Altunbulakli C, Azkur K, Costa RA, Crameri R, et al. Interleukins (from IL-1 to IL-38), interferons, transforming growth factor β, and TNF-α: receptors, functions, and roles in diseases. J Allergy Clin Immunol 2016; 138:984–1010. doi: 10.1016/j.jaci.2016.06.033. [DOI] [PubMed] [Google Scholar]
- 8.Lambrecht BN, Hammad H, Fahy JV. The cytokines of asthma. Immunity 2019; 50:975–991. doi: 10.1016/j.immuni.2019.03.018. [DOI] [PubMed] [Google Scholar]
- 9.Löffler W. Zur differential-diagnose der lungeninfiltrierungen. Beiträge zur Klinik der Tuberkulose und spezifischen Tuberkulose-Forschung 1932; 79:338–367. doi: 10.1007/BF02079220. [Google Scholar]
- 10.Cottin V. Eosinophilic lung diseases. Clin Chest Med 2016; 37:535–556. doi: 10.1016/j.ccm.2016.04.015. [DOI] [PubMed] [Google Scholar]
- 11.Hashimoto T, Satoh T, Yokozeki H. Pruritus in ordinary scabies: IL-31 from macrophages induced by overexpression of thymic stromal lymphopoietin and periostin. Allergy 2019; 74:1727–1737. doi: 10.1111/all.13870. [DOI] [PubMed] [Google Scholar]
- 12.Hammad H, Lambrecht BN. The basic immunology of asthma. Cell 2021; 184:1469–1485. doi: 10.1016/j.cell.2021.02.016. [DOI] [PubMed] [Google Scholar]
- 13.Yang X, Feng H, Chen Z, Ying S. Biologic targeting: new and effective therapeutic approaches against severe asthma. Chin Med J 2018; 131:1009–1012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cevhertas L, Ogulur I, Maurer DJ, Burla D, Ding M, Jansen K, et al. Advances and recent developments in asthma in 2020. Allergy 2020; 75:3124–3146. doi: 10.1111/all.14607. [DOI] [PubMed] [Google Scholar]
- 15.Menzies-Gow A, Corren J, Bourdin A, Chupp G, Israel E, Wechsler ME, et al. Tezepelumab in adults and adolescents with severe, uncontrolled asthma. N Engl J Med 2021; 384:1800–1809. doi: 10.1056/NEJMoa2034975. [DOI] [PubMed] [Google Scholar]
- 16.Porsbjerg CM, Sverrild A, Lloyd CM, Menzies-Gow AN, Bel EH. Anti-alarmins in asthma: targeting the airway epithelium with next-generation biologics. Eur Respir J 2020; 56:2000260.doi: 10.1183/13993003.00260-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Akdis CA. Does the epithelial barrier hypothesis explain the increase in allergy, autoimmunity and other chronic conditions? Nat Rev Immunol 2021; 21:739–751. doi: 10.1038/s41577-021-00538-7. [DOI] [PubMed] [Google Scholar]
- 18.Heijink IH, Kuchibhotla V, Roffel MP, Maes T, Knight DA, Sayers I, et al. Epithelial cell dysfunction, a major driver of asthma development. Allergy 2020; 75:1902–1917. doi: 10.1111/all.14421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Xiao C, Puddicombe SM, Field S, Haywood J, Broughton-Head V, Puxeddu I, et al. Defective epithelial barrier function in asthma. J Allergy Clin Immunol 2011; 128:549–556.e1-12.doi: 10.1016/j.jaci.2011.05.038. [DOI] [PubMed] [Google Scholar]
- 20.Shahana S, Björnsson E, Lúdvíksdóttir D, Janson C, Nettelbladt O, Venge P, et al. Ultrastructure of bronchial biopsies from patients with allergic and non-allergic asthma. Respir Med 2005; 99:429–443. doi: 10.1016/j.rmed.2004.08.013. [DOI] [PubMed] [Google Scholar]
- 21.de Boer WI, Sharma HS, Baelemans SM, Hoogsteden HC, Lambrecht BN, Braunstahl GJ. Altered expression of epithelial junctional proteins in atopic asthma: possible role in inflammation. Can J Physiol Pharmacol 2008; 86:105–112. doi: 10.1139/y08-004. [DOI] [PubMed] [Google Scholar]
- 22.Steelant B, Farré R, Wawrzyniak P, Belmans J, Dekimpe E, Vanheel H, et al. Impaired barrier function in patients with house dust mite-induced allergic rhinitis is accompanied by decreased occludin and zonula occludens-1 expression. J Allergy Clin Immunol 2016; 137:1043–1053. e5. doi: 10.1016/j.jaci.2015.10.050. [DOI] [PubMed] [Google Scholar]
- 23.Irvine AD, McLean WH, Leung DY. Filaggrin mutations associated with skin and allergic diseases. N Engl J Med 2011; 365:1315–1327. doi: 10.1056/NEJMra1011040. [DOI] [PubMed] [Google Scholar]
- 24.Pendaries V, Malaisse J, Pellerin L, Le Lamer M, Nachat R, Kezic S, et al. Knockdown of filaggrin in a three-dimensional reconstructed human epidermis impairs keratinocyte differentiation. J Invest Dermatol 2014; 134:2938–2946. doi: 10.1038/jid.2014.259. [DOI] [PubMed] [Google Scholar]
- 25.Scharschmidt TC, Man MQ, Hatano Y, Crumrine D, Gunathilake R, Sundberg JP, et al. Filaggrin deficiency confers a paracellular barrier abnormality that reduces inflammatory thresholds to irritants and haptens. J Allergy Clin Immunol 2009; 124:496–506. doi: 10.1016/j.jaci.2009.06.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fallon PG, Sasaki T, Sandilands A, Campbell LE, Saunders SP, Mangan NE, et al. A homozygous frameshift mutation in the mouse Flg gene facilitates enhanced percutaneous allergen priming. Nat Genet 2009; 41:602–608. doi: 10.1038/ng.358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Duan S, Wanke K, Wawrzyniak P, Meng Y, Kast JI, Rückert B, et al. Platelet-activating factor decreases skin keratinocyte tight junction barrier integrity. J Allergy Clin Immunol 2016; 138:1725–1728. e3. doi: 10.1016/j.jaci.2016.05.037. [DOI] [PubMed] [Google Scholar]
- 28.Werfel T, Allam JP, Biedermann T, Eyerich K, Gilles S, Guttman-Yassky E, et al. Cellular and molecular immunologic mechanisms in patients with atopic dermatitis. J Allergy Clin Immunol 2016; 138:336–349. doi: 10.1016/j.jaci.2016.06.010. [DOI] [PubMed] [Google Scholar]
- 29.Berin MC. Pathogenesis of IgE-mediated food allergy. Clin Exp Allergy 2015; 45:1483–1496. doi: 10.1111/cea.12598. [DOI] [PubMed] [Google Scholar]
- 30.van Ginkel CD, Flokstra-de Blok BM, Kollen BJ, Kukler J, Koppelman GH, Dubois AE. Loss-of-function variants of the filaggrin gene are associated with clinical reactivity to foods. Allergy 2015; 70:461–464. doi: 10.1111/all.12569. [DOI] [PubMed] [Google Scholar]
- 31.van Splunter M, Liu L, van Neerven R, Wichers HJ, Hettinga KA, de Jong NW. Mechanisms underlying the skin-gut cross talk in the development of IgE-mediated food allergy. Nutrients 2020; 12:3830.doi: 10.3390/nu12123830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pat Y, Ogulur I. The epithelial barrier hypothesis: a 20-year journey. Allergy 2021; 76:3560–3562. doi: 10.1111/all.14899. [DOI] [PubMed] [Google Scholar]
- 33.Sugita K, Soyka MB, Wawrzyniak P, Rinaldi AO, Mitamura Y, Akdis M, et al. Outside-in hypothesis revisited: the role of microbial, epithelial, and immune interactions. Ann Allergy Asthma Immunol 2020; 125:517–527. doi: 10.1016/j.anai.2020.05.016. [DOI] [PubMed] [Google Scholar]
- 34.Celebi Sözener Z, Cevhertas L, Nadeau K, Akdis M, Akdis CA. Environmental factors in epithelial barrier dysfunction. J Allergy Clin Immunol 2020; 145:1517–1528. doi: 10.1016/j.jaci.2020.04.024. [DOI] [PubMed] [Google Scholar]
- 35.Knight DA, Holgate ST. The airway epithelium: structural and functional properties in health and disease. Respirology 2003; 8:432–446. doi: 10.1046/j.1440-1843.2003.00493.x. [DOI] [PubMed] [Google Scholar]
- 36.Montoro DT, Haber AL, Biton M, Vinarsky V, Lin B, Birket SE, et al. A revised airway epithelial hierarchy includes CFTR-expressing ionocytes. Nature 2018; 560:319–324. doi: 10.1038/s41586-018-0393-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Seumois G, Vijayanand P. Single-cell analysis to understand the diversity of immune cell types that drive disease pathogenesis. J Allergy Clin Immunol 2019; 144:1150–1153. doi: 10.1016/j.jaci.2019.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rock JR, Randell SH, Hogan BL. Airway basal stem cells: a perspective on their roles in epithelial homeostasis and remodeling. Dis Model Mech 2010; 3:545–556. doi: 10.1242/dmm.006031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Evans MJ, Cox RA, Shami SG, Wilson B, Plopper CG. The role of basal cells in attachment of columnar cells to the basal lamina of the trachea. Am J Respir Cell Mol Biol 1989; 1:463–469. doi: 10.1165/ajrcmb/1.6.463. [DOI] [PubMed] [Google Scholar]
- 40.Rogers DF. The airway goblet cell. Int J Biochem Cell Biol 2003; 35:1–6. doi: 10.1016/s1357-2725(02)00083-3. [DOI] [PubMed] [Google Scholar]
- 41.Zhu L, An L, Ran D, Lizarraga R, Bondy C, Zhou X, et al. The club cell marker SCGB1A1 downstream of FOXA2 is reduced in asthma. Am J Respir Cell Mol Biol 2019; 60:695–704. doi: 10.1165/rcmb.2018-0199OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ceteci F, Ceteci S, Zanucco E, Thakur C, Becker M, El-Nikhely N, et al. E-cadherin controls bronchiolar progenitor cells and onset of preneoplastic lesions in mice. Neoplasia 2012; 14:1164–1177. doi: 10.1593/neo.121088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Van Lommel A. Pulmonary neuroendocrine cells (PNEC) and neuroepithelial bodies (NEB): chemoreceptors and regulators of lung development. Paediatr Respir Rev 2001; 2:171–176. doi: 10.1053/prrv.2000.0126. [DOI] [PubMed] [Google Scholar]
- 44.Klein Wolterink R, Pirzgalska RM, Veiga-Fernandes H. Neuroendocrine cells take your breath away. Immunity 2018; 49:9–11. doi: 10.1016/j.immuni.2018.06.010. [DOI] [PubMed] [Google Scholar]
- 45.Gerbe F, Jay P. Intestinal tuft cells: epithelial sentinels linking luminal cues to the immune system. Mucosal Immunol 2016; 9:1353–1359. doi: 10.1038/mi.2016.68. [DOI] [PubMed] [Google Scholar]
- 46.Kohanski MA, Workman AD, Patel NN, Hung LY, Shtraks JP, Chen B, et al. Solitary chemosensory cells are a primary epithelial source of IL-25 in patients with chronic rhinosinusitis with nasal polyps. J Allergy Clin Immunol 2018; 142:460–469.e7.doi: 10.1016/j.jaci.2018.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ruan YC, Wang Y, Da Silva N, Kim B, Diao RY, Hill E, et al. CFTR interacts with ZO-1 to regulate tight junction assembly and epithelial differentiation through the ZONAB pathway. J Cell Sci 2014; 127:4396–4408. doi: 10.1242/jcs.148098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bonser LR, Erle DJ. Airway mucus and asthma: the role of MUC5AC and MUC5B. J Clin Med 2017; 6:112.doi: 10.3390/jcm6120112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Steelant B, Seys SF, Boeckxstaens G, Akdis CA, Ceuppens JL, Hellings PW. Restoring airway epithelial barrier dysfunction: a new therapeutic challenge in allergic airway disease. Rhinology 2016; 54:195–205. doi: 10.4193/Rhin15.376. [DOI] [PubMed] [Google Scholar]
- 50.Hellings PW, Steelant B. Epithelial barriers in allergy and asthma. J Allergy Clin Immunol 2020; 145:1499–1509. doi: 10.1016/j.jaci.2020.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Buckle FG, Cohen AB. Nasal mucosal hyperpermeability to macromolecules in atopic rhinitis and extrinsic asthma. J Allergy Clin Immunol 1975; 55:213–221. doi: 10.1016/0091-6749(75)90139-6. [DOI] [PubMed] [Google Scholar]
- 52.Ilowite JS, Bennett WD, Sheetz MS, Groth ML, Nierman DM. Permeability of the bronchial mucosa to 99mTc-DTPA in asthma. Am Rev Respir Dis 1989; 139:1139–1143. doi: 10.1164/ajrccm/139.5.1139. [DOI] [PubMed] [Google Scholar]
- 53.Taylor SM, Downes H, Hirshman CA, Peters JE, Leon D. Pulmonary uptake of mannitol as an index of changes in lung epithelial permeability. J Appl Physiol Respir Environ Exerc Physiol 1983; 55:614–618. doi: 10.1152/jappl.1983.55.2.614. [DOI] [PubMed] [Google Scholar]
- 54.Georas S, Ransom N, Hillman S, Eliseeva S, Veazey J, Smyth T, et al. The leaky lung test: a pilot study using inhaled mannitol to measure airway barrier function in asthma. J Asthma 2019; 56:1257–1265. doi: 10.1080/02770903.2018.1536145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Broeckaert F, Clippe A, Knoops B, Hermans C, Bernard A. Clara cell secretory protein (CC16): features as a peripheral lung biomarker. Ann N Y Acad Sci 2000; 923:68–77. doi: 10.1111/j.1749-6632.2000.tb05520.x. [DOI] [PubMed] [Google Scholar]
- 56.Petrek M, Hermans C, Kolek V, Fialová J, Bernard A. Clara cell protein (CC16) in serum and bronchoalveolar lavage fluid of subjects exposed to asbestos. Biomarkers 2002; 7:58–67. doi: 10.1080/13547500110086892. [DOI] [PubMed] [Google Scholar]
- 57.Blomberg A, Mudway I, Svensson M, Hagenbjörk-Gustafsson A, Thomasson L, Helleday R, et al. Clara cell protein as a biomarker for ozone-induced lung injury in humans. Eur Respir J 2003; 22:883–888. doi: 10.1183/09031936.03.00048203. [DOI] [PubMed] [Google Scholar]
- 58.Tripathi A, Lammers KM, Goldblum S, Shea-Donohue T, Netzel-Arnett S, Buzza MS, et al. Identification of human zonulin, a physiological modulator of tight junctions, as prehaptoglobin-2. Proc Natl Acad Sci U S A 2009; 106:16799–16804. doi: 10.1073/pnas.0906773106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Sturgeon C, Fasano A. Zonulin, a regulator of epithelial and endothelial barrier functions, and its involvement in chronic inflammatory diseases. Tissue Barriers 2016; 4:e1251384.doi: 10.1080/21688370.2016.1251384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Rittirsch D, Flierl MA, Nadeau BA, Day DE, Huber-Lang MS, Grailer JJ, et al. Zonulin as prehaptoglobin2 regulates lung permeability and activates the complement system. Am J Physiol Lung Cell Mol Physiol 2013; 304:L863–872. doi: 10.1152/ajplung.00196.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Shirey KA, Lai W, Patel MC, Pletneva LM, Pang C, Kurt-Jones E, et al. Novel strategies for targeting innate immune responses to influenza. Mucosal Immunol 2016; 9:1173–1182. doi: 10.1038/mi.2015.141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Di Micco S, Musella S, Scala MC, Sala M, Campiglia P, Bifulco G, et al. In silico analysis revealed potential anti-SARS-CoV-2 main protease activity by the zonulin inhibitor larazotide acetate. Front Chem 2020; 8:628609.doi: 10.3389/fchem.2020.628609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Rinaldi AO, Morita H, Wawrzyniak P, Dreher A, Grant S, Svedenhag P, et al. Direct assessment of skin epithelial barrier by electrical impedance spectroscopy. Allergy 2019; 74:1934–1944. doi: 10.1111/all.13824. [DOI] [PubMed] [Google Scholar]
- 64.Rinaldi AO, Korsfeldt A, Ward S, Burla D, Dreher A, Gautschi M, et al. Electrical impedance spectroscopy for the characterization of skin barrier in atopic dermatitis. Allergy 2021; 76:3066–3079. doi: 10.1111/all.14842. [DOI] [PubMed] [Google Scholar]
- 65.Wan H, Winton HL, Soeller C, Tovey ER, Gruenert DC, Thompson PJ, et al. Der p 1 facilitates transepithelial allergen delivery by disruption of tight junctions. J Clin Invest 1999; 104:123–133. doi: 10.1172/JCI5844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Saito T, Ichikawa T, Numakura T, Yamada M, Koarai A, Fujino N, et al. PGC-1α regulates airway epithelial barrier dysfunction induced by house dust mite. Respir Res 2021; 22:63.doi: 10.1186/s12931-021-01663-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Gaspar R, de Matos MR, Cortes L, Nunes-Correia I, Todo-Bom A, Pires E, et al. Pollen proteases play multiple roles in allergic disorders. Int J Mol Sci 2020; 21:3578.doi: 10.3390/ijms21103578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Van Cleemput J, Poelaert K, Laval K, Impens F, Van den Broeck W, Gevaert K, et al. Pollens destroy respiratory epithelial cell anchors and drive alphaherpesvirus infection. Sci Rep 2019; 9:4787.doi: 10.1038/s41598-019-41305-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Leino MS, Loxham M, Blume C, Swindle EJ, Jayasekera NP, Dennison PW, et al. Barrier disrupting effects of alternaria alternata extract on bronchial epithelium from asthmatic donors. PLoS One 2013; 8:e71278.doi: 10.1371/journal.pone.0071278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Petecchia L, Sabatini F, Varesio L, Camoirano A, Usai C, Pezzolo A, et al. Bronchial airway epithelial cell damage following exposure to cigarette smoke includes disassembly of tight junction components mediated by the extracellular signal-regulated kinase 1/2 pathway. Chest 2009; 135:1502–1512. doi: 10.1378/chest.08-1780. [DOI] [PubMed] [Google Scholar]
- 71.Aghapour M, Raee P, Moghaddam SJ, Hiemstra PS, Heijink IH. Airway epithelial barrier dysfunction in chronic obstructive pulmonary disease: role of cigarette smoke exposure. Am J Respir Cell Mol Biol 2018; 58:157–169. doi: 10.1165/rcmb.2017-0200TR. [DOI] [PubMed] [Google Scholar]
- 72.Lehmann AD, Blank F, Baum O, Gehr P, Rothen-Rutishauser BM. Diesel exhaust particles modulate the tight junction protein occludin in lung cells in vitro. Part Fibre Toxicol 2009; 6:26.doi: 10.1186/1743-8977-6-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Michaudel C, Mackowiak C, Maillet I, Fauconnier L, Akdis CA, Sokolowska M, et al. Ozone exposure induces respiratory barrier biphasic injury and inflammation controlled by IL-33. J Allergy Clin Immunol 2018; 142:942–958. doi: 10.1016/j.jaci.2017.11.044. [DOI] [PubMed] [Google Scholar]
- 74.Caraballo JC, Yshii C, Westphal W, Moninger T, Comellas AP. Ambient particulate matter affects occludin distribution and increases alveolar transepithelial electrical conductance. Respirology 2011; 16:340–349. doi: 10.1111/j.1440-1843.2010.01910.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Xian M, Ma S, Wang K, Lou H, Wang Y, Zhang L, et al. Particulate matter 2.5 causes deficiency in barrier integrity in human nasal epithelial cells. Allergy Asthma Immunol Res 2020; 12:56–71. doi: 10.4168/aair.2020.12.1.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Vita AA, Royse EA, Pullen NA. Nanoparticles and danger signals: oral delivery vehicles as potential disruptors of intestinal barrier homeostasis. J Leukoc Biol 2019; 106:95–103. doi: 10.1002/JLB.3MIR1118-414RR. [DOI] [PubMed] [Google Scholar]
- 77.Jin Y, Lu L, Tu W, Luo T, Fu Z. Impacts of polystyrene microplastic on the gut barrier, microbiota and metabolism of mice. Sci Total Environ 2019; 649:308–317. doi: 10.1016/j.scitotenv.2018.08.353. [DOI] [PubMed] [Google Scholar]
- 78.Wang M, Tan G, Eljaszewicz A, Meng Y, Wawrzyniak P, Acharya S, et al. Laundry detergents and detergent residue after rinsing directly disrupt tight junction barrier integrity in human bronchial epithelial cells. J Allergy Clin Immunol 2019; 143:1892–1903. doi: 10.1016/j.jaci.2018.11.016. [DOI] [PubMed] [Google Scholar]
- 79.Xian M, Wawrzyniak P, Rückert B, Duan S, Meng Y, Sokolowska M, et al. Anionic surfactants and commercial detergents decrease tight junction barrier integrity in human keratinocytes. J Allergy Clin Immunol 2016; 138:890–893.e9. doi: 10.1016/j.jaci.2016.07.003. [DOI] [PubMed] [Google Scholar]
- 80.Brant A, Hole A, Cannon J, Helm J, Swales C, Welch J, et al. Occupational asthma caused by cellulase and lipase in the detergent industry. Occup Environ Med 2004; 61:793–795. doi: 10.1136/oem.2003.011288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Aguayo-Patrón SV, Calderón de la Barca AM. Old Fashioned vs. ultra-processed-based current diets: possible implication in the increased susceptibility to type 1 diabetes and celiac disease in childhood. Foods 2017; 6:100.doi: 10.3390/foods6110100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Alemao CA, Budden KF, Gomez HM, Rehman SF, Marshall JE, Shukla SD, et al. Impact of diet and the bacterial microbiome on the mucous barrier and immune disorders. Allergy 2021; 76:714–734. doi: 10.1111/all.14548. [DOI] [PubMed] [Google Scholar]
- 83.Speel HC. Surface active agents; chemical types and applications. J Invest Dermatol 1945; 6:293–304. doi: 10.1038/jid.1945.27. [DOI] [PubMed] [Google Scholar]
- 84.Bajpai D, Tyagi VK. Laundry detergents: an overview. J Oleo Sci 2007; 56:327–340. doi: 10.5650/jos.56.327. [DOI] [PubMed] [Google Scholar]
- 85.Flindt ML. Pulmonary disease due to inhalation of derivatives of bacillus subtilis containing proteolytic enzyme. Lancet 1969; 1:1177–1181. doi: 10.1016/s0140-6736(69)92165-5. [DOI] [PubMed] [Google Scholar]
- 86.Adisesh A, Murphy E, Barber CM, Ayres JG. Occupational asthma and rhinitis due to detergent enzymes in healthcare. Occup Med (Lond) 2011; 61:364–369. doi: 10.1093/occmed/kqr107. [DOI] [PubMed] [Google Scholar]
- 87.Peng M, Liu J, Liang Z. Probiotic bacillus subtilis CW14 reduces disruption of the epithelial barrier and toxicity of ochratoxin A to caco-2 cells. Food Chem Toxicol 2019; 126:25–33. doi: 10.1016/j.fct.2019.02.009. [DOI] [PubMed] [Google Scholar]
- 88.Folletti I, Zock JP, Moscato G, Siracusa A. Asthma and rhinitis in cleaning workers: a systematic review of epidemiological studies. J Asthma 2014; 51:18–28. doi: 10.3109/02770903.2013.833217. [DOI] [PubMed] [Google Scholar]
- 89.Sarlo K. Control of occupational asthma and allergy in the detergent industry. Ann Allergy Asthma Immunol 2003; 90:32–34. doi: 10.1016/s1081-1206(10)61646-8. [DOI] [PubMed] [Google Scholar]
- 90.Pinto IS, Neto IF, Soares HM. Biodegradable chelating agents for industrial, domestic, and agricultural applications–a review. Environ Sci Pollut Res Int 2014; 21:11893–11906. doi: 10.1007/s11356-014-2592-6. [DOI] [PubMed] [Google Scholar]
- 91.Sajjan U, Wang Q, Zhao Y, Gruenert DC, Hershenson MB. Rhinovirus disrupts the barrier function of polarized airway epithelial cells. Am J Respir Crit Care Med 2008; 178:1271–1281. doi: 10.1164/rccm.200801-136OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Teoh KT, Siu YL, Chan WL, Schlüter MA, Liu CJ, Peiris JS, et al. The SARS coronavirus E protein interacts with PALS1 and alters tight junction formation and epithelial morphogenesis. Mol Biol Cell 2010; 21:3838–3852. doi: 10.1091/mbc.E10-04-0338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Shepley-McTaggart A, Sagum CA, Oliva I, Rybakovsky E, DiGuilio K, Liang J, et al. SARS-CoV-2 envelope (E) protein interacts with PDZ-domain-2 of host tight junction protein ZO1. PLoS One 2021; 16:e0251955.doi: 10.1371/journal.pone.0251955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Tuli JF, Ramezanpour M, Cooksley C, Psaltis AJ, Wormald PJ, Vreugde S. Association between mucosal barrier disruption by Pseudomonas aeruginosa exoproteins and asthma in patients with chronic rhinosinusitis. Allergy 2021; 76:3459–3469. doi: 10.1111/all.14959. [DOI] [PubMed] [Google Scholar]
- 95.Kim YC, Won HK, Lee JW, Sohn KH, Kim MH, Kim TB, et al. Staphylococcus aureus nasal colonization and asthma in adults: systematic review and meta-analysis. J Allergy Clin Immunol Pract 2019; 7:606–615.e9. doi: 10.1016/j.jaip.2018.08.020. [DOI] [PubMed] [Google Scholar]
- 96.Bachert C, Gevaert P, Holtappels G, Johansson SG, van Cauwenberge P. Total and specific IgE in nasal polyps is related to local eosinophilic inflammation. J Allergy Clin Immunol 2001; 107:607–614. doi: 10.1067/mai.2001.112374. [DOI] [PubMed] [Google Scholar]
- 97.Sintobin I, Siroux V, Holtappels G, Pison C, Nadif R, Bousquet J, et al. Sensitisation to staphylococcal enterotoxins and asthma severity: a longitudinal study in the EGEA cohort. Eur Respir J 2019; 54:1900198.doi: 10.1183/13993003.00198-2019. [DOI] [PubMed] [Google Scholar]
- 98.Sørensen M, Klingenberg C, Wickman M, Sollid J, Furberg AS, Bachert C, et al. Staphylococcus aureus enterotoxin sensitization is associated with allergic poly-sensitization and allergic multimorbidity in adolescents. Allergy 2017; 72:1548–1555. doi: 10.1111/all.13175. [DOI] [PubMed] [Google Scholar]
- 99.Friedman SJ, Schroeter AL, Homburger HA. IgE antibodies to Staphylococcus aureus. Prevalence in patients with atopic dermatitis. Arch Dermatol 1985; 121:869–872. [PubMed] [Google Scholar]
- 100.Altunbulakli C, Costa R, Lan F, Zhang N, Akdis M, Bachert C, et al. Staphylococcus aureus enhances the tight junction barrier integrity in healthy nasal tissue, but not in nasal polyps. J Allergy Clin Immunol 2018; 142:665–668.e8. doi: 10.1016/j.jaci.2018.01.046. [DOI] [PubMed] [Google Scholar]
- 101.Altunbulakli C, Reiger M, Neumann AU, Garzorz-Stark N, Fleming M, Huelpuesch C, et al. Relations between epidermal barrier dysregulation and staphylococcus species-dominated microbiome dysbiosis in patients with atopic dermatitis. J Allergy Clin Immunol 2018; 142:1643–1647.e12. doi: 10.1016/j.jaci.2018.07.005. [DOI] [PubMed] [Google Scholar]
- 102.Davies DE. Epithelial barrier function and immunity in asthma. Ann Am Thorac Soc 2014; 11: (Suppl 5): S244–251. doi: 10.1513/AnnalsATS.201407-304AW. [DOI] [PubMed] [Google Scholar]
- 103.Steelant B. Epithelial dysfunction in chronic respiratory diseases, a shared endotype. Curr Opin Pulm Med 2020; 26:20–26. doi: 10.1097/MCP.0000000000000638. [DOI] [PubMed] [Google Scholar]
- 104.Schleimer RP, Kato A, Kern R, Kuperman D, Avila PC. Epithelium: at the interface of innate and adaptive immune responses. J Allergy Clin Immunol 2007; 120:1279–1284. doi: 10.1016/j.jaci.2007.08.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Lambrecht BN, Hammad H. The airway epithelium in asthma. Nat Med 2012; 18:684–692. doi: 10.1038/nm.2737. [DOI] [PubMed] [Google Scholar]
- 106.Akdis CA, Arkwright PD, Brüggen MC, Busse W, Gadina M, Guttman-Yassky E, et al. Type 2 immunity in the skin and lungs. Allergy 2020; 75:1582–1605. doi: 10.1111/all.14318. [DOI] [PubMed] [Google Scholar]
- 107.Yao X, Liu X, Wang X. Potential role of interleukin-25/interleukin-33/thymic stromal lymphopoietin-fibrocyte axis in the pathogenesis of allergic airway diseases. Chin Med J (Engl) 2018; 131:1983–1989. doi: 10.4103/0366-6999.238150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Shamji MH, Durham SR. Mechanisms of allergen immunotherapy for inhaled allergens and predictive biomarkers. J Allergy Clin Immunol 2017; 140:1485–1498. doi: 10.1016/j.jaci.2017.10.010. [DOI] [PubMed] [Google Scholar]
- 109.Gon Y, Hashimoto S. Role of airway epithelial barrier dysfunction in pathogenesis of asthma. Allergol Int 2018; 67:12–17. doi: 10.1016/j.alit.2017.08.011. [DOI] [PubMed] [Google Scholar]
- 110.Hammad H, Lambrecht BN. Dendritic cells and airway epithelial cells at the interface between innate and adaptive immune responses. Allergy 2011; 66:579–587. doi: 10.1111/j.1398-9995.2010.02528.x. [DOI] [PubMed] [Google Scholar]
- 111.Kortekaas Krohn I, Seys SF, Lund G, Jonckheere AC, Dierckx de Casterlé I, Ceuppens JL, et al. Nasal epithelial barrier dysfunction increases sensitization and mast cell degranulation in the absence of allergic inflammation. Allergy 2020; 75:1155–1164. doi: 10.1111/all.14132. [DOI] [PubMed] [Google Scholar]
- 112.Steelant B, Seys SF, Van Gerven L, Van Woensel M, Farré R, Wawrzyniak P, et al. Histamine and T helper cytokine-driven epithelial barrier dysfunction in allergic rhinitis. J Allergy Clin Immunol 2018; 141:951–963.e8. doi: 10.1016/j.jaci.2017.08.039. [DOI] [PubMed] [Google Scholar]
- 113.Kortekaas Krohn I, Callebaut I, Alpizar YA, Steelant B, Van Gerven L, Skov PS, et al. MP29-02 reduces nasal hyperreactivity and nasal mediators in patients with house dust mite-allergic rhinitis. Allergy 2018; 73:1084–1093. doi: 10.1111/all.13349. [DOI] [PubMed] [Google Scholar]
- 114.Hellings PW, Borrelli D, Pietikainen S, Agache I, Akdis C, Bachert C, et al. European summit on the prevention and self-management of chronic respiratory diseases: report of the European union parliament summit (29 March 2017). Clin Transl Allergy 2017; 7:49.doi: 10.1186/s13601-017-0186-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Breiteneder H, Peng YQ, Agache I, Diamant Z, Eiwegger T, Fokkens WJ, et al. Biomarkers for diagnosis and prediction of therapy responses in allergic diseases and asthma. Allergy 2020; 75:3039–3068. doi: 10.1111/all.14582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Post S, Nawijn MC, Jonker MR, Kliphuis N, van den Berge M, van Oosterhout AJ, et al. House dust mite-induced calcium signaling instigates epithelial barrier dysfunction and CCL20 production. Allergy 2013; 68:1117–1125. doi: 10.1111/all.12202. [DOI] [PubMed] [Google Scholar]
- 117.Turi GJ, Ellis R, Wattie JN, Labiris NR, Inman MD. The effects of inhaled house dust mite on airway barrier function and sensitivity to inhaled methacholine in mice. Am J Physiol Lung Cell Mol Physiol 2011; 300:L185–190. doi: 10.1152/ajplung.00271.2010. [DOI] [PubMed] [Google Scholar]
- 118.Heijink IH, van Oosterhout A, Kapus A. Epidermal growth factor receptor signalling contributes to house dust mite-induced epithelial barrier dysfunction. Eur Respir J 2010; 36:1016–1026. doi: 10.1183/09031936.00125809. [DOI] [PubMed] [Google Scholar]
- 119.Dong HM, Le YQ, Wang YH, Zhao HJ, Huang CW, Hu YH, et al. Extracellular heat shock protein 90α mediates HDM-induced bronchial epithelial barrier dysfunction by activating RhoA/MLC signaling. Respir Res 2017; 18:111.doi: 10.1186/s12931-017-0593-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Hong JH, Lee SI, Kim KE, Yong TS, Seo JT, Sohn MH, et al. German cockroach extract activates protease-activated receptor 2 in human airway epithelial cells. J Allergy Clin Immunol 2004; 113:315–319. doi: 10.1016/j.jaci.2003.11.026. [DOI] [PubMed] [Google Scholar]
- 121.Jairaman A, Maguire CH, Schleimer RP, Prakriya M. Allergens stimulate store-operated calcium entry and cytokine production in airway epithelial cells. Sci Rep 2016; 6:32311.doi: 10.1038/srep32311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Samanta K, Parekh AB. Store-operated Ca2+ channels in airway epithelial cell function and implications for asthma. Philos Trans R Soc Lond B Biol Sci 2016; 371:20150424.doi: 10.1098/rstb.2015.0424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Hardyman MA, Wilkinson E, Martin E, Jayasekera NP, Blume C, Swindle EJ, et al. TNF-(-mediated bronchial barrier disruption and regulation by src-family kinase activation. J Allergy Clin Immunol 2013; 132:665–675.e8. doi: 10.1016/j.jaci.2013.03.005. [DOI] [PubMed] [Google Scholar]
- 124.Sugita K, Steer CA, Martinez-Gonzalez I, Altunbulakli C, Morita H, Castro-Giner F, et al. Type 2 innate lymphoid cells disrupt bronchial epithelial barrier integrity by targeting tight junctions through IL-13 in asthmatic patients. J Allergy Clin Immunol 2018; 141:300–310.e11. doi: 10.1016/j.jaci.2017.02.038. [DOI] [PubMed] [Google Scholar]
- 125.Wawrzyniak P, Wawrzyniak M, Wanke K, Sokolowska M, Bendelja K, Rückert B, et al. Regulation of bronchial epithelial barrier integrity by type 2 cytokines and histone deacetylases in asthmatic patients. J Allergy Clin Immunol 2017; 139:93–103. doi: 10.1016/j.jaci.2016.03.050. [DOI] [PubMed] [Google Scholar]
- 126.Yang SJ, Allahverdian S, Saunders A, Liu E, Dorscheid DR. IL-13 signaling through IL-13 receptor α2 mediates airway epithelial wound repair. FASEB J 2019; 33:3746–3757. doi: 10.1096/fj.201801285R. [DOI] [PubMed] [Google Scholar]
- 127.Ge X, Cai F, Shang Y, Chi F, Xiao H, Xu J, et al. PARK2 attenuates house dust mite-induced inflammatory reaction, pyroptosis and barrier dysfunction in BEAS-2B cells by ubiquitinating NLRP3. Am J Transl Res 2021; 13:326–335. [PMC free article] [PubMed] [Google Scholar]
- 128.Tsai YM, Chiang KH, Hung JY, Chang WA, Lin HP, Shieh JM, et al. Der f1 induces pyroptosis in human bronchial epithelia via the NLRP3 inflammasome. Int J Mol Med 2018; 41:757–764. doi: 10.3892/ijmm.2017.3310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Sebag SC, Koval OM, Paschke JD, Winters CJ, Comellas AP, Grumbach IM. Inhibition of the mitochondrial calcium uniporter prevents IL-13 and allergen-mediated airway epithelial apoptosis and loss of barrier function. Exp Cell Res 2018; 362:400–411. doi: 10.1016/j.yexcr.2017.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Zhuang J, Cui H, Zhuang L, Zhai Z, Yang F, Luo G, et al. Bronchial epithelial pyroptosis promotes airway inflammation in a murine model of toluene diisocyanate-induced asthma. Biomed Pharmacother 2020; 125:109925.doi: 10.1016/j.biopha.2020.109925. [DOI] [PubMed] [Google Scholar]
- 131.Das S, Miller M, Beppu AK, Mueller J, McGeough MD, Vuong C, et al. GSDMB induces an asthma phenotype characterized by increased airway responsiveness and remodeling without lung inflammation. Proc Natl Acad Sci U S A 2016; 113:13132–13137. doi: 10.1073/pnas.1610433113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Li K, Li M, Li W, Yu H, Sun X, Zhang Q, et al. Airway epithelial regeneration requires autophagy and glucose metabolism. Cell Death Dis 2019; 10:875.doi: 10.1038/s41419-019-2111-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Zhao J, Dar HH, Deng Y, St Croix CM, Li Z, Minami Y, et al. PEBP1 acts as a rheostat between prosurvival autophagy and ferroptotic death in asthmatic epithelial cells. Proc Natl Acad Sci U S A 2020; 117:14376–14385. doi: 10.1073/pnas.1921618117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Simpson J, Loh Z, Ullah MA, Lynch JP, Werder RB, Collinson N, et al. Respiratory syncytial virus infection promotes necroptosis and hmgb1 release by airway epithelial cells. Am J Respir Crit Care Med 2020; 201:1358–1371. doi: 10.1164/rccm.201906-1149OC. [DOI] [PubMed] [Google Scholar]
- 135.Xu F, Luo M, He L, Cao Y, Li W, Ying S, et al. Necroptosis contributes to urban particulate matter-induced airway epithelial injury. Cell Physiol Biochem 2018; 46:699–712. doi: 10.1159/000488726. [DOI] [PubMed] [Google Scholar]
- 136.Kubo T, Wawrzyniak P, Morita H, Sugita K, Wanke K, Kast JI, et al. CpG-DNA enhances the tight junction integrity of the bronchial epithelial cell barrier. J Allergy Clin Immunol 2015; 136:1413–1416.e1-8. doi: 10.1016/j.jaci.2015.05.006. [DOI] [PubMed] [Google Scholar]
- 137.Hagner S, Welz H, Kicic A, Alrifai M, Marsh LM, Sutanto EN, et al. Suppression of adrenomedullin contributes to vascular leakage and altered epithelial repair during asthma. Allergy 2012; 67:998–1006. doi: 10.1111/j.1398-9995.2012.02851.x. [DOI] [PubMed] [Google Scholar]
- 138.de Vries M, Hesse L, Jonker MR, van den Berge M, van Oosterhout AJ, Heijink IH, et al. Pim1 kinase activity preserves airway epithelial integrity upon house dust mite exposure. Am J Physiol Lung Cell Mol Physiol 2015; 309:L1344–1353. doi: 10.1152/ajplung.00043.2015. [DOI] [PubMed] [Google Scholar]
- 139.Wawrzyniak P, Krawczyk K, Acharya S, Tan G, Wawrzyniak M, Karouzakis E, et al. Inhibition of CpG methylation improves the barrier integrity of bronchial epithelial cells in asthma. Allergy 2021; 76:1864–1868. doi: 10.1111/all.14667. [DOI] [PubMed] [Google Scholar]
- 140.Bove PF, Wesley UV, Greul AK, Hristova M, Dostmann WR, van der Vliet A. Nitric oxide promotes airway epithelial wound repair through enhanced activation of MMP-9. Am J Respir Cell Mol Biol 2007; 36:138–146. doi: 10.1165/rcmb.2006-0253SM. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Steelant B, Wawrzyniak P, Martens K, Jonckheere AC, Pugin B, Schrijvers R, et al. Blocking histone deacetylase activity as a novel target for epithelial barrier defects in patients with allergic rhinitis. J Allergy Clin Immunol 2019; 144:1242–1253.e7. doi: 10.1016/j.jaci.2019.04.027. [DOI] [PubMed] [Google Scholar]
- 142.Savla U, Waters CM. Mechanical strain inhibits repair of airway epithelium in vitro. Am J Physiol 1998; 274:L883–892. doi: 10.1152/ajplung.1998.274.6.L883. [DOI] [PubMed] [Google Scholar]
- 143.Carayol N, Campbell A, Vachier I, Mainprice B, Bousquet J, Godard P, et al. Modulation of cadherin and catenins expression by tumor necrosis factor-alpha and dexamethasone in human bronchial epithelial cells. Am J Respir Cell Mol Biol 2002; 26:341–347. doi: 10.1165/ajrcmb.26.3.4684. [DOI] [PubMed] [Google Scholar]
- 144.Winter MC, Shasby SS, Ries DR, Shasby DM. PAR2 activation interrupts E-cadherin adhesion and compromises the airway epithelial barrier: protective effect of beta-agonists. Am J Physiol Lung Cell Mol Physiol 2006; 291:L628–635. doi: 10.1152/ajplung.00046.2006. [DOI] [PubMed] [Google Scholar]
- 145.Coraux C, Kileztky C, Polette M, Hinnrasky J, Zahm JM, Devillier P, et al. Airway epithelial integrity is protected by a long-acting beta2-adrenergic receptor agonist. Am J Respir Cell Mol Biol 2004; 30:605–612. doi: 10.1165/rcmb.2003-0056OC. [DOI] [PubMed] [Google Scholar]
- 146.Trinh H, Pham DL, Choi Y, Kim HM, Kim SH, Park HS. Epithelial folliculin enhances airway inflammation in aspirin-exacerbated respiratory disease. Clin Exp Allergy 2018; 48:1464–1473. doi: 10.1111/cea.13253. [DOI] [PubMed] [Google Scholar]
- 147.Yuan X, Wang J, Li Y, He X, Niu B, Wu D, et al. Allergy immunotherapy restores airway epithelial barrier dysfunction through suppressing IL-25 -induced endoplasmic reticulum stress in asthma. Sci Rep 2018; 8:7950.doi: 10.1038/s41598-018-26221-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Richards LB, Li M, Folkerts G, Henricks P, Garssen J, van Esch B. Butyrate and propionate restore the cytokine and house dust mite compromised barrier function of human bronchial airway epithelial cells. Int J Mol Sci 2020; 22:65.doi: 10.3390/ijms22010065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Martens K, Pugin B, De Boeck I, Spacova I, Steelant B, Seys SF, et al. Probiotics for the airways: potential to improve epithelial and immune homeostasis. Allergy 2018; 73:1954–1963. doi: 10.1111/all.13495. [DOI] [PubMed] [Google Scholar]
- 150.Akdis CA. Agache I, Akdis CA. The defective bronchial epithelial barrier and epithelial barrier hypothesis in asthma. Global Atlas of Asthma 2nd Edition.Zurich: European Academy of Allergy and Clinical Immunology; 2021. 105–107. [Google Scholar]