Abstract
PURPOSE
Addressing unwarranted clinical variation in oncology practices is expected to lead to improved cancer outcomes. Particularly, the application and impact of treatment guidelines on breast cancer outcomes are poorly studied in resource-limited settings. We measured adherence to a set of locally developed adjuvant treatment guidelines in a middle-income setting. Importantly, the impact of guidelines adherence on survival following breast cancer was determined.
METHODS
Data of 3,100 Malaysian women with nonmetastatic breast cancer diagnosed between 2010 and 2017 were analyzed. Adherence to the Malaysian Clinical Practice Guidelines for Management of Breast Cancer second Edition was measured. Outcomes comprised overall survival and event-free survival.
RESULTS
Guideline adherence for chemotherapy, radiotherapy, hormonal therapy, and targeted therapy were 61.7%, 79.2%, 85.1%, and 26.2%, respectively. Older age was generally associated with lower adherence to guidelines. Compared with patients who were treated according to treatment guidelines, overall survival and event-free survival were substantially lower in patients who were not treated accordingly; hazard ratios for all-cause mortality were 1.69 (95% CI, 1.29 to 2.22), 2.59 (95% CI, 1.76 to 3.81), 3.08 (95% CI, 1.94 to 4.88), and 4.48 (95% CI, 1.98 to 10.13) for chemotherapy, radiotherapy, hormone therapy, and targeted therapy, respectively. Study inferences remain unchanged following sensitivity analyses.
CONCLUSION
Our study findings appear to suggest that adherence to treatment guidelines that have been adapted for resource-limited settings may still provide effective guidance in improving breast cancer outcomes.
INTRODUCTION
Breast cancer is the most common cancer in females, worldwide.1 Wide survival disparities have been documented between the high-income countries (HICs) and the low- and middle-income countries (LMICs),2 attributed to lack of health care resources in the LMICs. Even within the LMICs, level of health care resources varies greatly from the upper middle-income countries to the low-income countries. This gap could potentially be reduced by improving delivery of high-quality breast cancer care in resource-limited settings. Particularly, addressing unwarranted clinical variation in oncology practices is expected to lead to improved cancer outcomes.3
CONTEXT
Key Objective
Does adherence to locally adapted breast cancer adjuvant treatment guidelines improve survival in a middle-income setting?
Knowledge Generated
Our findings suggest that adherence to treatment guidelines that have been adapted for resource-limited settings may still provide effective guidance in improving breast cancer outcomes. Rates of adherence were highest for hormonal therapy, followed by radiotherapy, chemotherapy, and finally targeted therapy.
Relevance
Study findings may facilitate development of treatment guidelines outside of high-income settings to improve standardization of cancer care, which in turn may improve treatment outcomes following cancer in these settings.
Unwarranted clinical variation is defined as patient care that differs in ways that are not a direct and proportionate response to available evidence; or to the health care needs and informed choices of patients.4 To this end, clinical practice guidelines (CPG) hold the potential to improve standardization of breast cancer care. Although CPG for breast cancer treatment may vary between nations, the recommendations are largely similar as guidelines are derived from the same base of evidence.5 Although CPGs are intended to guide administration of surgical procedures and cancer therapies, not all physicians may be able to adhere to them, especially in low-resource settings where access to adjuvant therapies may be a challenge because of the high costs of many cancer drugs, including those listed in the WHO Essential Medicine List.6,7 Apart from affordability, patient characteristics such as comorbidities may also influence treatment decision making.8
Regardless, nonadherence to treatment guidelines has been shown to worsen clinical outcomes following breast cancer in HICs.8-10 These findings may not be directly extrapolated to the LMICs where resource limitations may lead to systemic barriers in access to cancer treatment.11 Given that development of high-quality CPG requires great effort, several international guidelines such as from the Breast Health Global Initiative,12 and National Comprehensive Cancer Network,13 provide clear frameworks for adaptation across different geographical areas, resource levels, and practice settings. Nonetheless, what is presently lacking is the evidence on their uptake, and implementation in resource-limited settings. Evidence on impact of adherence to treatment guidelines on survival following breast cancer is also reprehensibly scarce in the LMICs.14 The latter may be especially useful in promoting application of treatment guidelines to standardize breast cancer care in other LMICs, and facilitate sharing of best practices.
Malaysia is an upper middle-income country with a dual public-private health care system comprising a government-led tax-funded public sector, and a predominantly for-profit private sector funded by out-of-pocket payments and private health insurance. Notably, subsidized cancer care is available via public tertiary hospitals to all Malaysian citizens regardless of their insurance status. Malaysia has its own CPG for breast cancer management, optimized on the basis of available health care resources, and is revised periodically.15,16 Key differences between the Malaysian CPG (second Edition) and other international guidelines for instance include the reservation of human epidermal growth factor receptor 2 (HER2)–targeted therapy use for only in the adjuvant settings, as well as the noninclusion of aromatase inhibitors and prognostic multigene assays.14 It is, however, unclear whether such adaptations may be detrimental to breast cancer outcomes. In the current study, we measured the association of guideline adherence with survival in Malaysian women with breast cancer.
METHODS
Data for this study were retrieved from two registries: the Universiti Malaya Breast Cancer Registry17 and the Ramsay Sime Darby Breast Cancer Registry. The breast cancer registries used in this study have received ethics approval from their respective institutional review boards (Universiti Malaya IRB 733.23, Sime Darby IRB: 201208.1).
All women who were newly diagnosed with stage I-III breast cancer between January 2010 and December 2017, who underwent surgery as the primary treatment, were included. Patients with ductal carcinoma in situ, de novo metastatic breast cancer, previous cancers, nonepithelial breast cancer, bilateral breast cancer, or who received neoadjuvant treatment were excluded.
Independent variables included sociodemographic factors comprising age, ethnicity, menopausal status, parity, and type of hospital (public v private). Data on burden of comorbidity, which was only available for a subset of study population (n = 1,686), were presented using modified Charlson Comorbidity Index (CCI) score.18 Although the original CCI is a weighted score that is based on both the number and severity of 19 predefined comorbid conditions including cancer, in the current study, presence of cancer (weight = 1) was not scored. Tumor-related factors included surgical factors (type of surgery and surgical margin) and pathologic factors (histology, tumor size, lymph nodes involvement, grade, estrogen receptor [ER] status, progesterone receptor status, and HER2 status).
The key reference document that was used in this study is the Malaysian CPG on the Management of Breast Cancer (second edition), which was published in 2010, and was in effect up till the end of 2019.15 It was developed on the basis of the Canadian/US Preventive Services Task Force Grade A recommendations, while taking the health resource limitations into account. Details of the Malaysian CPGs can be found elsewhere.15,16 Briefly, the guidelines recommend chemotherapy when there is lymph node involvement, tumor size is more than 2 cm, tumor is poorly differentiated, absence of ER expression, or HER2 overexpression. Radiotherapy is required after breast-conserving surgery, or in the case of mastectomy, when surgical margins are positive or four or more axillary lymph nodes are involved. Hormone therapy is required for ER-positive tumors, whereas HER2-targeted therapy is recommended for HER2-overexpressing tumors. Generics for aromatase inhibitors or biosimilars for targeted therapy were not available during the study period.
In this study, four adjuvant treatments were investigated: chemotherapy, radiotherapy, hormonal therapy, and HER2-targeted therapy. Nonadherence to guideline was considered present if a patient was not started on an adjuvant treatment as recommended by the Malaysian CPG. When analyzing each treatment modality, patients who were treated without indications were excluded, as overtreatment may be driven by other clinical indications and also external factors, which may not have been adequately captured in the hospital-based registries, which in turn may lead to bias.
End point for overall survival (OS) was death from any cause. End point for event-free survival (EFS) was death, local recurrence, or occurrence of distant metastasis. Data on local recurrence or distant metastasis were obtained from medical records. Data on mortality were verified through linkage with the National Registration Department in Malaysia (updated until December 2019). To reduce immortal time bias as some patients who died early may not have received treatment, follow-up time was calculated one year from the date of diagnosis of breast cancer up until the date of death, or censored on December 1, 2019. Selection of potential confounders was based on prior literature.9,10
Missing data were < 10% for all variables except comorbidities (45.6%). After diagnosis, loss to follow-up before one year for OS and EFS were 46 and 102, respectively. In the main analyses, multiple imputation (MI) was used to address missing data for all variables except comorbidity, death, local recurrence, and distant metastasis.
Given that comorbidity is potentially an important confounder,8 we performed sensitivity analyses to compare the findings using complete case analysis (n = 1,686) versus findings from MI, where comorbidity was additionally imputed.
Data analysis was done using R. MI was done using the MICE package. Logistic regression was used to estimate odds ratios and corresponding 95% CI of variables associated with nonadherence to treatment guidelines. Kaplan-Meier curves were created for each of the adjuvant treatments (Data Supplement). Using the survival package, hazard ratios (HRs) and 95% CI for the effect of nonadherence (adherence to CPG as the reference group) were calculated for all-cause mortality and local/distant recurrences using Cox regression analyses. Proportional hazards assumption was checked with the cox.zph function, where all the imputed data sets were combined for analysis.
RESULTS
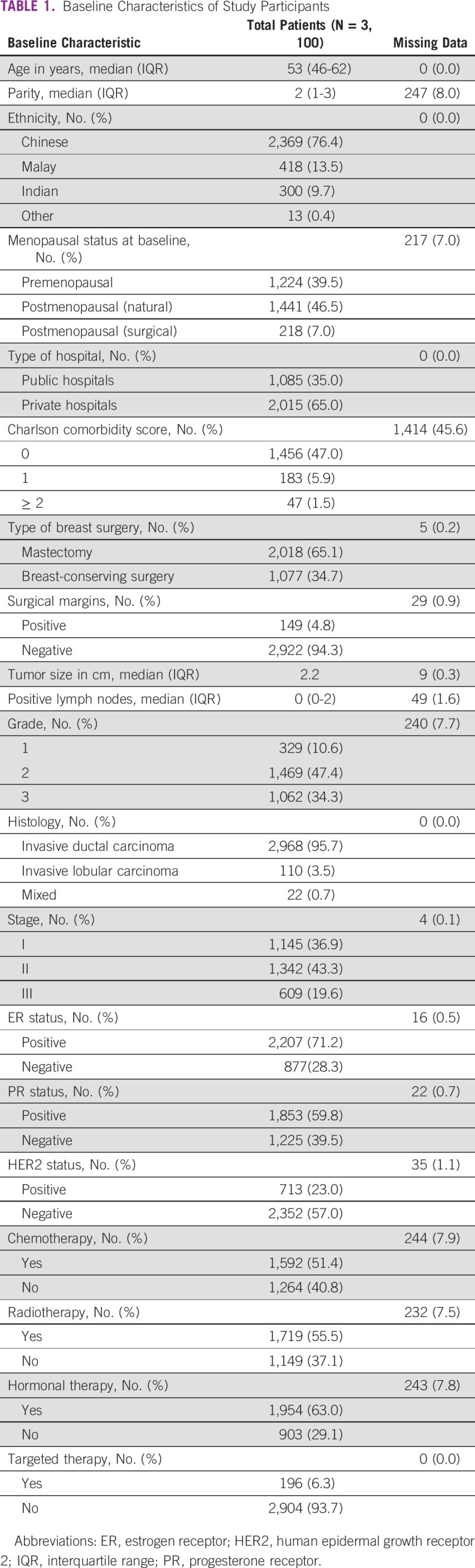
A total of 3,100 patients with stage I-III breast cancers were included in this study (Table 1). The cohort largely comprised middle-aged women (median age: 53 years, range: 22-89 years), with < 10% (269 patients) age older than 70 years. A majority (76.4%) were of Chinese ancestry, and two thirds (65.0%) were treated in private sector. Most patients presented with either stage I (36.9%) or stage II (43.3%) diseases at initial diagnosis. The majority of tumors were ER-positive (71.2%). Less than a quarter of tumors overexpressed HER2. Of 1,686 patients with data on comorbidities, 1,456 had a modified CCI of 0.
TABLE 1.
Baseline Characteristics of Study Participants
Overall, 278 deaths were observed over a median follow-up of 51 months (interquartile range: 29-75 months), whereas 338 deaths, local recurrences, and distant metastasis were reported over 49 months (interquartile range: 28-75 months) of follow-up. The proportional hazards assumption appeared to be largely satisfied for OS (Data Supplement) and EFS (Data Supplement).
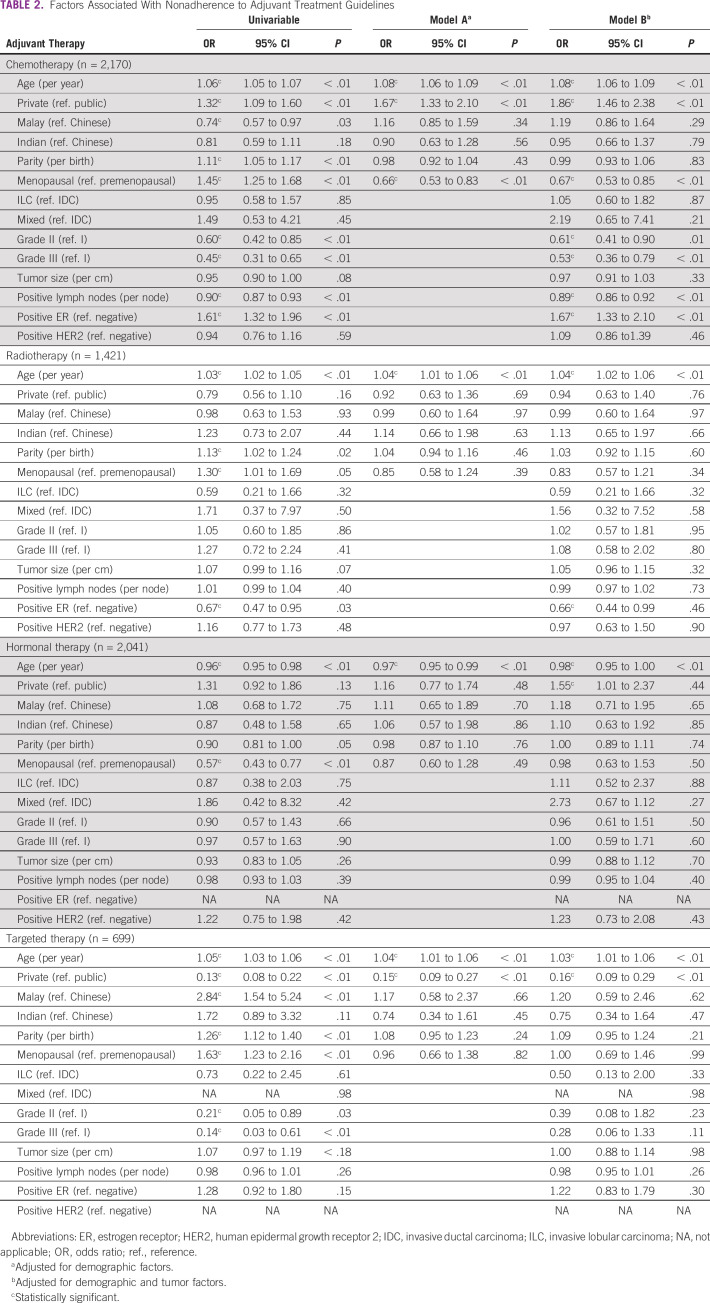
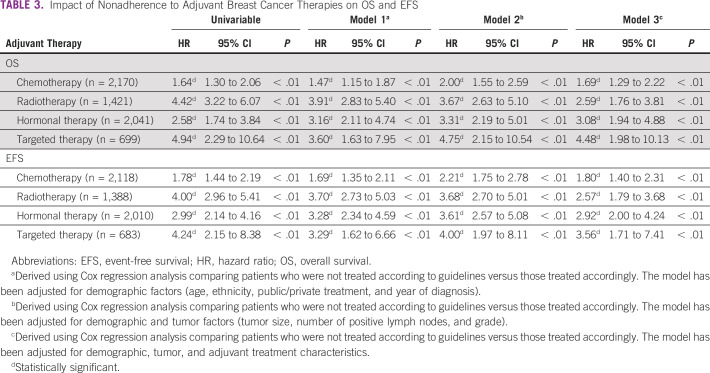
It was found that only 61.7% of the 2,396 patients with an indication for chemotherapy were treated according to the chemotherapy administration guideline (Data Supplement). After adjusting for demographic and tumor characteristics, it was found that older age, treatment in private hospital, lower grade tumor, fewer lymph node involvement, and ER-positive tumors were significantly associated with nonadherence to guidelines (Table 2). The 5-year OS was 89.6% (95% CI, 88.1 to 91.1) for those who were treated according to CPG, and 83.8% (95% CI, 81.0 to 86.5) for those who were not. The adjusted HR for OS was 1.69 (95% CI, 1.29 to 2.22; P value < 0.01), whereas the corresponding adjusted HR for EFS was 1.80 (95% CI, 1.40 to 2.31; P value < 0.01; Table 3).
TABLE 2.
Factors Associated With Nonadherence to Adjuvant Treatment Guidelines
TABLE 3.
Impact of Nonadherence to Adjuvant Breast Cancer Therapies on OS and EFS
Of the 1,575 patients with indication for radiotherapy, 79.2% (1,267) of patients were treated according to guidelines (Data Supplement). Older age was found to be independently associated with nonadherence to guidelines (Table 2). The 5-year OS was 91.9% (95% CI, 90.5 to 93.4) for those who were treated according to CPG, and 67.8% (95% CI, 60.8 to 74.8) for those who were not. The adjusted HR for OS was 2.59 (95% CI, 1.76 to 3.81; P value < 0.01), whereas the corresponding adjusted HR for EFS was 2.57 (95% CI, 1.79 to 3.68; P value < 0.01; Table 3).
A majority of patients (85.1%) who had an indication for hormonal therapy were treated according to the treatment guideline (N = 2,204; Data Supplement). Here, older age was less likely to be associated with nonadherence to hormone therapy guidelines (Table 2). The 5-year OS was 94.0% (95% CI, 93.0 to 95.1) for those who were treated according to CPG, and 82.6% (95% CI, 76.8 to 88.5) for those who were not. The adjusted HR for OS was 3.08 (95% CI, 1.94 to 4.88; P value < 0.01), whereas the corresponding adjusted HR for EFS was 2.92 (95% CI, 2.00 to 4.24; P value < 0.01; Table 3).
In 713 patients who had an indication for HER2-targeted therapy, only 26.2% (187) were treated accordingly (Data Supplement). Multivariable analysis showed that older age and treatment in public hospitals were associated with nonadherence to targeted therapy (Table 2). The 5-year OS was 96.3% (95% CI, 93.5 to 99.0) for those who were treated according to CPG, and 81.9% (95% CI, 78.7 to 85.2) for those who were not. The adjusted HR for OS was 4.48 (95% CI, 1.98 to 10.13; P value < 0.01), whereas the corresponding adjusted HR for EFS was 3.56 (95% CI, 1.71 to 7.41; P value < 0.01; Table 3).
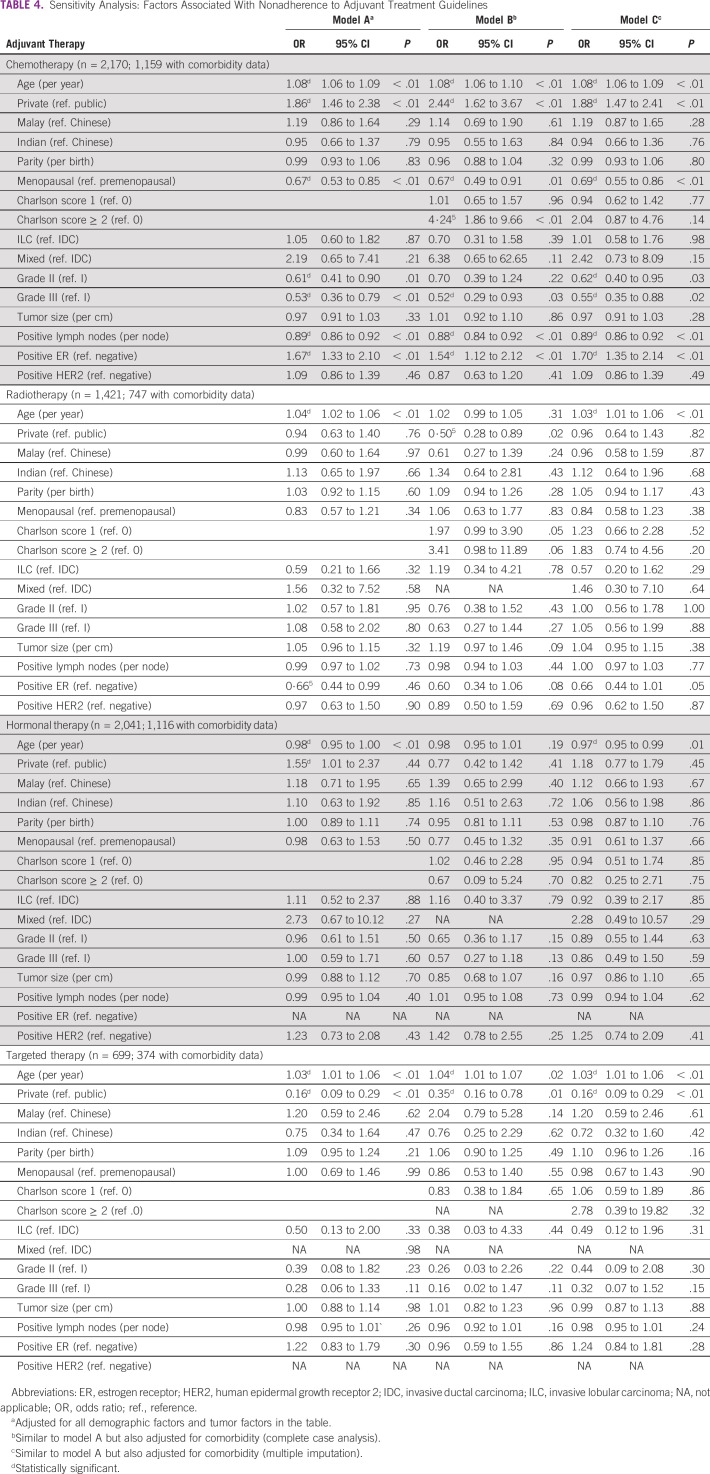
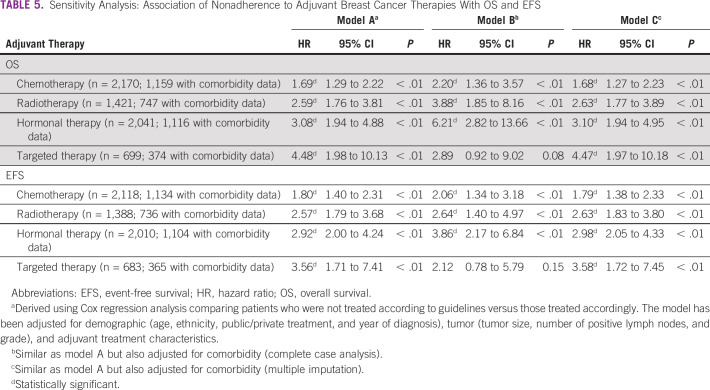
Sensitivity analyses showed that higher burden of comorbidities was substantially associated with nonadherence to chemotherapy guidelines, and marginally associated with nonadherence to radiotherapy guidelines in the complete case analyses, but not in the MI models (Table 4). Adjustment for comorbidity in the Cox regression models measuring the association between guidelines adherence and OS, and EFS using complete case analyses, and MI nonetheless revealed that the main study inferences remain unchanged (Table 5).
TABLE 4.
Sensitivity Analysis: Factors Associated With Nonadherence to Adjuvant Treatment Guidelines
TABLE 5.
Sensitivity Analysis: Association of Nonadherence to Adjuvant Breast Cancer Therapies With OS and EFS
DISCUSSION
We observed that adherence to adjuvant treatment guidelines tended to vary, from low adherence for targeted therapy to moderate adherence for chemotherapy, and high adherence for radiotherapy and hormonal therapy guidelines. Our study findings importantly demonstrated that nonadherence to CPG was associated with worse OS and EFS following breast cancer in Malaysia.
Our finding that older age was persistently associated with worse adherence to adjuvant treatment guidelines, even following adjustment for comorbidity, is corroborated by prior findings.10 Furthermore, certain treatments such as trastuzumab may be associated with increased risk of cardiovascular events. As such, it is conceivable that the oncologists may have been cautious in prescribing HER2-targeted therapy in older patients, and those with comorbidities, as shown in our prior research.19 Although concerns on severity of therapy-related adverse events in older patients are valid, treating oncologists must be aware of the fact that existing treatment guidelines have been developed on the basis of sound scientific evidence derived from studies that included a wide range of patients including older women. Therefore, it is paramount that oncology teams aim to adhere to these guidelines when making adjuvant treatment recommendations for older women, and facilitate informed decision making. Nonetheless, it is acknowledged that cancer treatment decision making in older individuals is fraught with complexities, given that older women may have different priorities in life compared with their younger counterparts, where quality of life may take center stage in comparison to prolongation of survival.20
Although we did not examine psychologic factors, previous studies have suggested that poor adherence to chemotherapy may be attributed to prevailing fear and overestimation of adverse effects.21,22 In comparison, radiotherapy and hormonal therapy are less likely to produce such severe adverse effects, explaining the relatively higher rates of guideline adherence in the current study.23 It is also conceivable that in multicultural settings, preference for complementary and alternative medicine may also explain refusal of cancer treatment.24
The apparent lack of adherence to adjuvant chemotherapy guidelines in private hospitals to some extent may be explained by the fact that some patients may have undergone genomic-based, individualized risk assessment using prognostic multigene assays.25 As prognostic multigene assays were not included in the second edition of the Malaysian CPG, some private patients who were candidates for chemotherapy may not have received chemotherapy following the test.
Apart from clinical complications, patients may also refuse chemotherapy as they fear that its adverse effects may delay return to work, leading to income loss.26 Although many of the employees working in private sectors in Malaysia may have some form of employer-provided health insurance that enables them to seek cancer care in private hospitals, they have limited medical leave and therefore, are forced to go on unpaid leave.27
This study seems to suggest that in resource-limited settings, adherence to targeted therapy may be substantially low because of the high cost of trastuzumab. Out of the 252 patients in our study with indications for HER2-targeted therapy in the public sector, only 18 had received it. In Malaysia, access to trastuzumab in the public health care system is currently rationed to a limited number of clinically qualified patients on the basis of available annual national budgets. Hence, for a majority of Malaysian women with HER2-overexpressed breast cancer; to obtain access to the drug would mean paying out of pocket or medical insurance coverage.23 Nevertheless, the proportion of patients who received trastuzumab in our study remains higher than previously reported (26% v 19%),28 which is potentially explained by the higher socioeconomic standing of the subgroup of patients. This notion is supported by our finding that private treatment was associated with greater adherence to targeted therapy. When taken together, these observations serve to underscore that lack of affordability of cancer drugs is a key driver of nonadherence to cancer treatment guidelines in the LMICs. By contrast, affordability of hormonal therapy such as tamoxifen may also largely explain the high adherence to hormonal therapy in this study.29
The finding that nonadherence to CPG is associated with worse breast cancer outcomes is corroborated by studies in HICs.8-10 In particular, our study and a Singaporean study both observed that patients who were not treated according to CPG were associated with poorer survival. Although it may be argued that resource-stratified treatment guidelines developed for LMICs detract from the standard care in HICs, and even criticized as condoning substandard treatment, their superseding aim is to provide patients with the highest level of care reasonably available.30 Hence, our study demonstrates that adherence to a CPG that has been adapted for LMICs may still be associated with improved clinical outcomes.
As patterns of adherence to cancer therapies also reflect the inability of the national health care systems in the LMICs to provide expensive cancer drugs, there is an urgent need for governments to work toward a common goal of improving access to life-saving therapies. Pragmatic solutions for health policymakers in Asia among others include having joint negotiations at regional level with pharmaceutical industries to lower the prices of cancer drugs for LMICs, establishing clear policies in developing, reviewing, and approving cancer generics and biosimilars, as well as addressing outdated insurance reimbursement policies.3
To the best of our knowledge, this is the first study of its kind in a middle-income setting. A strength of our study is its relatively large sample size. As data on mortality were obtained from a central registry, we are confident with the validity of our findings pertaining to OS. Although no central registry exists for local recurrences or metastasis, a majority of patients in the current study returned to the same hospital where they were diagnosed and treated, making EFS fairly valid.
However, it is acknowledged that our data did not allow us to distinguish the reasons for deviation from the local CPG (physician-related, patient-related, or health system–related). Furthermore, limitations in our data did not permit investigation on treatment abandonment, which represents a potential area for future research. As patients receiving neoadjuvant (chemo)therapy were excluded in this study, our findings may not be generalizable to subgroups where it is indicated such as women with locally advanced breast cancer. Although the current study was conducted in a relatively affluent region of the country, where private health care utilization tends to be higher and this may somewhat appear to limit its generalizability to lower-income settings, our findings should in fact be considered as conservative, where nonadherence to treatment guidelines is expected to be higher in the less affluent regions.
It is hence strongly felt that our present findings may facilitate development of policies to address the systemic disparities in access to oncology services within the national health care system. In this instance, improving access to radiotherapy services may be one of the best buys. Large modeling initiatives for instance have provided compelling evidence that scaling up of radiotherapy capacities in resource-limited settings will not only improve treatment access, but also results in substantial economic gains.31 In the local context, the average distance and travel time for Malaysians living in the less affluent regions to the closest radiotherapy center currently stands at 124.2 km/108.8 minutes in East Coast and 228.1 km/236.1 minutes in East Malaysia,32 highlighting the urgent need to address these disparities.
In conclusion, our findings support the notion that adherence to a CPG that has been adapted for resource-limited settings may still be associated with improved survival following breast cancer. At the national level, our findings help validate the clinical utility of the Malaysian CPG for breast cancer. The present findings may also be useful for cancer control experts and key opinion leaders in oncology in other LMICs to advocate for development or adaptation of evidence-based treatment guidelines to facilitate standardization of breast cancer care, with the aim of improving breast cancer outcomes in these settings.
Nur Aishah Mohd Taib
Consulting or Advisory Role: Roche, AstraZeneca, MSD
Marniza Saad
Honoraria: Astellas Pharma, Novartis, Johnson & Johnson, Ipsen, Cipla, AstraZeneca, Eisai, Pfizer, Amgen
Consulting or Advisory Role: Johnson & Johnson, MSD, AstraZeneca, Novartis, Merck, BMS, Viatris
Research Funding: Johnson & Johnson, MSD
Nirmala Bhoo-Pathy
Honoraria: Roche, Novartis
Speakers' Bureau: Roche, Novartis
Research Funding: Pfizer, Novartis
No other potential conflicts of interest were reported.
DISCLAIMER
The views expressed in this article are the authors' own and not an official position of their institutions.
AUTHOR CONTRIBUTIONS
Conception and design: Chin Vern Song, Cheng-Har Yip, Nur Aishah Mohd Taib, Nirmala Bhoo-Pathy
Provision of study materials or patients: Cheng-Har Yip, Nur Aishah Mohd Taib, Mee Hoong See, Li Ying Teoh, Marniza Saad
Collection and assembly of data: Chin Vern Song, Cheng-Har Yip, Mee Hoong See, Li Ying Teoh
Data analysis and interpretation: Chin Vern Song, Cheng-Har Yip, Nur Aishah Mohd Taib, Evelyn M. Monninkhof, Marniza Saad, Cuno S.P.M. Uiterwaal, Nirmala Bhoo-Pathy
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/go/authors/author-center.
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (Open Payments).
Nur Aishah Mohd Taib
Consulting or Advisory Role: Roche, AstraZeneca, MSD
Marniza Saad
Honoraria: Astellas Pharma, Novartis, Johnson & Johnson, Ipsen, Cipla, AstraZeneca, Eisai, Pfizer, Amgen
Consulting or Advisory Role: Johnson & Johnson, MSD, AstraZeneca, Novartis, Merck, BMS, Viatris
Research Funding: Johnson & Johnson, MSD
Nirmala Bhoo-Pathy
Honoraria: Roche, Novartis
Speakers' Bureau: Roche, Novartis
Research Funding: Pfizer, Novartis
No other potential conflicts of interest were reported.
REFERENCES
- 1.Bray F, Ferlay J, Soerjomataram I, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries CA Cancer J Clin 68394–4242018 [DOI] [PubMed] [Google Scholar]
- 2.Allemani C, Matsuda T, Di Carlo V, et al. Global surveillance of trends in cancer survival 2000-14 (CONCORD-3): Analysis of individual records for 37 513 025 patients diagnosed with one of 18 cancers from 322 population-based registries in 71 countries Lancet 3911023–10752018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cardoso F, MacNeill F, Penault-Llorca F, et al. Why is appropriate healthcare inaccessible for many European breast cancer patients?—The EBCC 12 manifesto Breast 55128–1352021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sutherland K, Levesque JF.Unwarranted clinical variation in health care: Definitions and proposal of an analytic framework J Eval Clin Pract 26687–6962020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wolters R, Regierer AC, Schwentner L, et al. A comparison of international breast cancer guidelines—Do the national guidelines differ in treatment recommendations? Eur J Cancer 481–112012 [DOI] [PubMed] [Google Scholar]
- 6.de Vries E. EML Cancer Medicines Working Group. Geneva, WHO, 2021; https://cdn.who.int/media/docs/default-source/essential-medicines/2021-eml-expert-committee/open-session/os_devries.pdf?sfvrsn=c19e4517_4 [Google Scholar]
- 7.Yip CH, Buccimazza I, Hartman M, et al. Improving outcomes in breast cancer for low and middle income countries World J Surg 39686–6922015 [DOI] [PubMed] [Google Scholar]
- 8.Wollschläger D, Meng X, Wöckel A, et al. Comorbidity-dependent adherence to guidelines and survival in breast cancer-Is there a role for guideline adherence in comorbid breast cancer patients? A retrospective cohort study with 2137 patients Breast J 24120–1272018 [DOI] [PubMed] [Google Scholar]
- 9.Hill DA, Friend S, Lomo L, et al. Breast cancer survival, survival disparities, and guideline-based treatment Breast Cancer Res Treat 170405–4142018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Ho PJ, Ow SGW, Sim Y, et al. Impact of deviation from guideline recommended treatment on breast cancer survival in Asia. Sci Rep. 2020;10:1330. doi: 10.1038/s41598-020-58007-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Brand NR, Qu LG, Chao A, et al. Delays and barriers to cancer care in low- and middle-income countries: A systematic review Oncologist 24e1371–e13802019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Anderson BO, Yip CH, Smith RA, et al. Guideline implementation for breast healthcare in low-income and middle-income countries: Overview of the Breast Health Global Initiative Global Summit 2007 Cancer 1132221–224320088 suppl [DOI] [PubMed] [Google Scholar]
- 13.Koh WJ, Anderson BO, Carlson RW.NCCN resource-stratified and harmonized guidelines: A paradigm for optimizing global cancer care Cancer 12612416–24232020suppl 10 [DOI] [PubMed] [Google Scholar]
- 14.Coughlin SS, Ekwueme DU.Breast cancer as a global health concern Cancer Epidemiol 33315–3182009 [DOI] [PubMed] [Google Scholar]
- 15.Ministry of Health Malaysia Management of Breast Cancer ed 2Putrajaya, Ministry of Health Malaysia, 2010 http://www.moh.gov.my/moh/attachments/6915.pdf [Google Scholar]
- 16.Ministry of Health Malaysia Management of Breast Cancer ed 3Putrajaya, Ministry of Health Malaysia, 2019 https://www.moh.gov.my/moh/resources/Penerbitan/CPG/Kanser/Breast%20Cancer/CPG_Management_of_Breast_Cancer_(Third_Edition)_130720.pdf [Google Scholar]
- 17.Pathy NB, Yip CH, Taib NA, et al. Breast cancer in a multi-ethnic Asian setting: Results from the Singapore-Malaysia hospital-based breast cancer registry Breast 20S75–S802011suppl 2 [DOI] [PubMed] [Google Scholar]
- 18.Charlson ME, Pompei P, Ales KL, et al. A new method of classifying prognostic comorbidity in longitudinal studies: Development and validation J Chronic Dis 40373–3831987 [DOI] [PubMed] [Google Scholar]
- 19. Subramaniam S, Kong YC, Zaharah H, et al. Baseline cardiovascular comorbidities, and the influence on cancer treatment decision-making in women with breast cancer. Ecancer. 2021;15:1293. doi: 10.3332/ecancer.2021.1293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Angarita FA, Elmi M, Zhang Y, et al. Patient-reported factors influencing the treatment decision-making process of older women with non-metastatic breast cancer: A systematic review of qualitative evidence Breast Cancer Res Treat 171545–5642018 [DOI] [PubMed] [Google Scholar]
- 21.Rajah HDA, Chan CMH, Kong YC, et al. Insights on emotional distress following cancer, sources of support and the unmet needs in a setting with limited supportive care services for people living with cancer Support Care Cancer 295811–58192021 [DOI] [PubMed] [Google Scholar]
- 22.Nies YH, Ali AM, Abdullah N, et al. A qualitative study among breast cancer patients on chemotherapy: Experiences and side-effects Patient Prefer Adherence 121955–19642018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Eniu A, Cherny NI, Bertram M, et al. Cancer medicines in Asia and Asia-Pacific: What is available, and is it effective enough? ESMO Open. 2019;4:e000483. doi: 10.1136/esmoopen-2018-000483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Mohd Mujar NM, Dahlui M, Emran NA, et al. Complementary and alternative medicine (CAM) use and delays in presentation and diagnosis of breast cancer patients in public hospitals in Malaysia. PLoS One. 2017;12:e0176394. doi: 10.1371/journal.pone.0176394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.McVeigh TP, Kerin MJ.Clinical use of the Oncotype DX genomic test to guide treatment decisions for patients with invasive breast cancer Breast Cancer (Dove Med Press) 9393–4002017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Neuner JM, Kong A, Blaes A, et al. The association of socioeconomic status with receipt of neoadjuvant chemotherapy Breast Cancer Res Treat 173179–1882019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kong YC, Rauf N, Subramaniam S, et al. Working after cancer: In-depth perspectives from a setting with limited employment protection policies J Cancer Surviv 15706–7122021 [DOI] [PubMed] [Google Scholar]
- 28. Lim GC, Aina EN, Cheah SK, et al. Closing the global cancer divide—Performance of breast cancer care services in a middle income developing country. BMC Cancer. 2014;14:212. doi: 10.1186/1471-2407-14-212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lundgren C, Lindman H, Rolander B, et al. Good adherence to adjuvant endocrine therapy in early breast cancer—A population-based study based on the Swedish Prescribed Drug Register Acta Oncol 57935–9402018 [DOI] [PubMed] [Google Scholar]
- 30.Kerr S, Jazieh AR, Kerr D.How useful are international treatment guidelines in low- and middle-income countries? JCO Glob Oncol 3441–4432017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Atun R, Jaffray DA, Barton MB, et al. Expanding global access to radiotherapy Lancet Oncol 161153–11862015 [DOI] [PubMed] [Google Scholar]
- 32. Yahya N, Sukiman NK, Suhaimi NA, et al. How many roads must a Malaysian walk down? Mapping the accessibility of radiotherapy facilities in Malaysia. PLoS One. 2019;14:e0213583. doi: 10.1371/journal.pone.0213583. [DOI] [PMC free article] [PubMed] [Google Scholar]