Abstract
Metabolic (dysfunction) associated fatty liver disease (MAFLD), previously known as non-alcoholic fatty liver disease, is the most common cause of chronic liver disease worldwide. Many risk factors contribute to the pathogenesis of MAFLD with metabolic dysregulation being the final arbiter of its development and progression. MAFLD poses a substantial economic burden to societies, which based on current trends is expected to increase over time. Numerous studies have addressed various aspects of MAFLD from its risk associations to its economic and social burden and clinical diagnosis and management, as well as the molecular mechanisms linking MAFLD to end-stage liver disease and hepatocellular carcinoma. This review summarizes current understanding of the pathogenesis of MAFLD and related diseases, particularly liver cancer. Potential therapeutic agents for MAFLD and diagnostic biomarkers are discussed.
Keywords: Metabolic (dysfunction) associated fatty liver disease, Liver disease, Liver cancer
Metabolic (dysfunction) Associated Fatty Liver Disease (MAFLD): Risk Factors and Impact on Human Health
MAFLD, previously known as non-alcoholic fatty liver disease, is the most common cause of chronic liver disease affecting up to half the world's population.[1–4] MAFLD is an umbrella term encompassing a spectrum of liver disease states ranging from steatosis to metabolic steatohepatitis (MeSH) (previously non-alcoholic steatohepatitis) to MAFLD-related cirrhosis and hepatocellular carcinoma (HCC).[5] MAFLD comes with a set of positive diagnostic criteria as recently espoused by Eslam et al,[6,7] and is consistent with our current understanding of its pathophysiology. MAFLD best describes the disease, which is the hepatic manifestation of systemic metabolic dysregulation.[2,4,7] The term also highlights the multifaceted and heterogenous nature of this disease and prevents over generalization as fatty liver disease in the absence of excess alcohol consumption.[2,4]
Many risk factors are associated with the presence of MAFLD [Figure 1A]. Systemic metabolic dysregulation is the principal proximate cause for its development and progression.[8] Indeed, MAFLD can be considered within the spectrum of metabolic syndrome and its constellation of associated abnormalities including elevated body mass index (BMI), insulin resistance, high fasting plasma glucose or diabetes mellitus, elevated systolic blood pressure, atherogenic dyslipidaemia, and chronic kidney disease.[1,9] Age and gender are also risk factors for MAFLD. The risk of MAFLD is lower in the pediatric population but increases with age and there is a considerable burden of MAFLD in children who are overweight or diagnosed with obesity.[8] MAFLD is more prevalent in males than in females in the younger age groups, although the opposite trend is seen in the oldest age groups (≥65 years).[10] It should be noted that up to a quarter of people with MAFLD have a BMI within the ethnic-specific health weight range.[11]
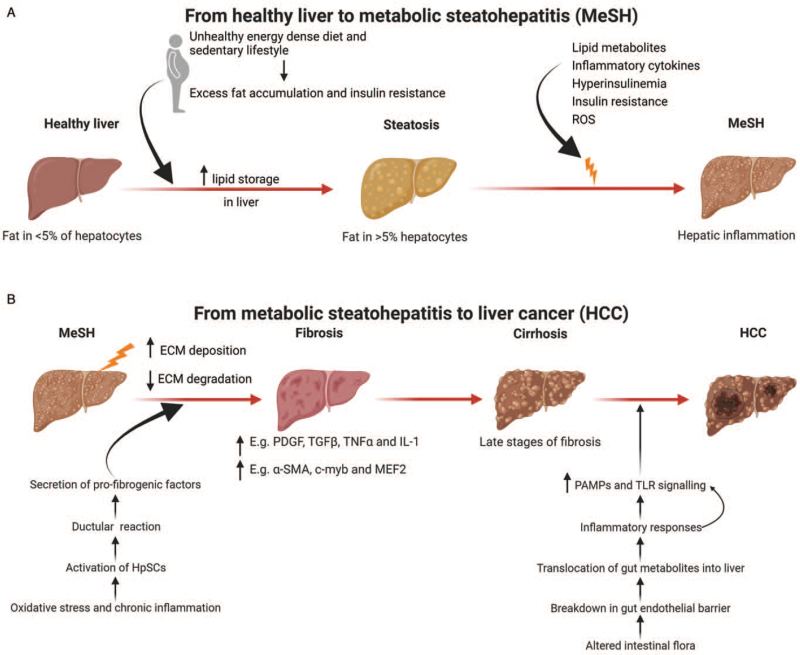
Figure 1.
Disease progression from a healthy liver to HCC. (A) A sedentary lifestyle, unhealthy, energy dense diets, and reduced physical activity are major contributors to obesity. This leads to insulin resistance and excess liver fat accumulation. Excess lipid storage in the liver in predisposed individuals triggers hepatic inflammation or MeSH. (B) Persistent MeSH leads to an increase in ECM deposition and a decrease in matrix degradation resulting in liver fibrosis. Hepatic fibrosis is driven by a variety of inflammatory molecules. Cirrhosis is late-stage liver fibrosis and is the substrate in most cases for the development of liver cancer, though in MAFLD, HCC can develop in the absence of cirrhosis. Adapted from “Non-Alcoholic Fatty Liver Disease (NAFLD) Spectrum”, by BioRender.com (2021). Diagram retrieved from https://app.biorender.com/biorender-templates. α-SMA: Alpha-smooth muscle actin; ECM: Extracellular matrix; HpSCs: Hepatic stem/progenitor cells; HCC: Hepatocellular carcinoma; IL-1: Interleukin-1; MAFLD: Metabolic (dysfunction) Associated Fatty Liver Disease; MEF2: Myocyte enhancer factor 2; MeSH: Metabolic steatohepatitis; NAFLD: Non-alcoholic fatty liver disease; PDGF: Platelet-derived growth factor; ROS: Reactive oxygen species; TLR: Toll-like receptor; TGFβ: Transforming growth factor beta; TNFα, tumor necrosis factor alpha.
Race and genetics contribute to the development of MAFLD. A sequence variant (I148 allele) in the Patatin-like phospholipase domain-containing protein 3 (PNPLA3) is a well-known genetic risk factor for MAFLD[8] and is most prevalent in Hispanics[7] and lowest in African Americans.[8] African Americans, despite their high rates of obesity and diabetes, possess the protective variant (S453I allele).[8] Since this discovery, many other genetic variants, such as in TM6SF2, MBOAT7, APOC3, NCAN, GCKR, LYPLAL1, and PPP1R3B, have been identified as risk factors for MAFLD development and progression.[8]
MAFLD is characterized by liver lipid accumulation which in a proportion can progress to inflammation and subsequent hepatocyte injury.[5] Excess hepatic lipid deposition is manifested as steatosis but this in fact only has a minimal impact on liver-related mortality.[3,9,12] However, hepatic steatosis is a marker for increased extrahepatic disease-associated mortality from cardiovascular diseases and cancer. Chronic liver inflammation and hepatocyte injury induced by ectopic fat deposition in some individuals progresses to the development of steatohepatitis,[8] a major risk factor for cirrhosis and HCC.[3] Indeed, progression of MeSH to hepatic fibrosis is the main cause of end stage liver disease and related mortality and is the most important risk factor for MAFLD-related HCC.[8,13]
Epidemiology
The reported global incidence of MAFLD varies from 6% to 35%.[14] MAFLD is more prevalent in Western (20%–30%) than in Eastern countries (10%–20%),[15] probably a reflection of differential socioeconomic development. Currently, MAFLD is the most common cause of chronic liver diseases in Western nations.[3] In the US, MAFLD affects one-third of the population,[8] while in Europe, 24% of the population has the disease.[16] In Australia, the incidence of MAFLD is expected to increase from 22% in 2019 to 23.6% by 2030.[17] Similarly, in Canada the estimated incidence of MAFLD is 21.1% and is expected to increase by 20% by 2030.[18]
In the Asia-Pacific, the prevalence of MAFLD was previously considered lower than in Western countries. However, an increasing trend has been witnessed from 2009 to 2019.[7,8,19] In high-income regions of Asia such as Hong Kong (China), Taiwan (China), Singapore, and South Korea, the number of MAFLD cases and MAFLD-associated annual liver-related deaths is expected to substantially increase by 2030.[10] Chinese mainland has been the main contributor to the rapid rise in absolute numbers of MAFLD cases in Asia.[19] In 2016, the estimated prevalence of MAFLD in Chinese mainland across all age groups was 17.6%.[20] In Japan, MAFLD was reported to be present in 20% to 30% of the population and 10% of them developed steatohepatitis.[14]
In Middle East and North Africa (MENA) countries, the reported average rates for MAFLD incidence and related deaths were 8.9% and 8.6%, respectively.[19] In some MENA countries (eg, United Arab Emirates, Syria, and Sudan) the incidence of MAFLD has increased between 2009 and 2019 by >25%.[19]
The higher prevalence of MAFLD in Western countries can be attributed to multiple factors including the consumption of high-calorie, energy dense, nutritionally poor quality diets, reduced rates of physical activity, increased sedentariness, higher rates of obesity, and genetic factors.[14] Indeed, a close association between the incidence of MAFLD and obesity has been reported: 33% of obese patients and 59.1% of obese patients who underwent a liver biopsy were found to have MAFLD whereas only 3% to 5% of the general population have the disease.[14] However, obesity is not a pre-requisite for MAFLD as approximately 20% of MAFLD patients in the Asia-Pacific region are of healthy weight by BMI criteria (BMI < 23 kg/m2)[7] or overweight (BMI < 25 kg/m2) despite exhibiting the same characteristics of obese MAFLD.[7,10]
Impact of Sex on MAFLD
The prevalence and severity of the MAFLD may vary with sex with the largest difference seen in the 56 to 60 years age group.[21] Generally, during the reproductive ages, MAFLD is more common and tends to be more severe in men than in women, whereas the opposite trend is seen after menopause.[22–24] This can be attributed to differences in lifestyle, body composition, and distribution of body fat, and sex hormone metabolism.[25] Interestingly, as estrogen levels are higher during the reproductive years and reach a nadir after menopause, estrogen is thought to play a protective role in MAFLD development.[25] This has clearly been demonstrated in several Asian countries including South Korea, Hong Kong (China), Singapore, and Taiwan (China) where MAFLD cases in men outnumbered those in women through to late middle age (≤65 years) whereas female cases outnumbered males in the oldest age groups (>65 years).[10] In other studies, MALFD was shown to be more common in Asian or black women after the age of 50 years.[8]
MAFLD in Children
MAFLD is closely linked to abnormal lipid and glucose metabolism. With the increasing incidence of metabolic abnormalities and diabetes amongst children and adolescents, the incidence of MAFLD in this age bracket has also increased.[26] Interestingly, the prevalence of MAFLD in pediatric populations differs between regions with the highest prevalence in Asia.[27] In Asia, the prevalence of pediatric MAFLD has been increasing, particularly in obese male population over the age of 10.[28] In the US, as reported in the “Study of Child and Adolescent Liver Epidemiology (SCALE)”, MAFLD is present in 5% to 10% of children/adolescents aged 2 to 19 years.[26] A meta-analysis by Anderson et al[27] found that MAFLD prevalence in the general pediatric population ranged from 9% to 37%. Similar to the findings in adults, the prevalence of MAFLD in children and adolescents is higher in males than in females and incrementally increases with BMI.[27] Of note, 30% of the MAFLD population among children and adolescents are pre-diabetic or have already been diagnosed with type 2 diabetes.[26] This increased incidence of MAFLD was the leading cause of liver-related morbidity and mortality in children in developing countries.[27]
Financial and Social Impact of MAFLD
MAFLD results in a substantial economic burden to society. In the US, the direct cost for managing MAFLD-related complications reaches $103 billion/year.[16] In the UK, managing MAFLD and related complications incurs a cost of £5.24 billion/year. In France, Italy, and Germany combined, €27.7 billion/year is spent on managing MAFLD-related complications.[16] As the incidence of MAFLD increases, the 10-year economic burden of MAFLD and related conditions is predicted to increase to $908 billion in the US and to €302 billion in Europe.[16] If the increase in financial burden parallels the annual growth in obesity prevalence, the 10-year burden of MAFLD is predicted to reach approximately $1.005 trillion in the US and €334 billion in Europe.[16] Notably, the total economic burden of MAFLD is highest in adults aged 45 to 65 years, mirroring disease prevalence and duration in the same age group.[16] In 2019, the total lifetime cost of Thai MeSH patients was estimated to be $15.2 billion.[29]
Apart from a heavy financial burden, MAFLD poses a significant social burden. The estimated annual societal cost from MAFLD and its complications was $292.19 billion in the US and €227.84 billion in Europe.[16] In 2017, MAFLD-related disease-adjusted life years (DALYs) in Asia accounted for 66% of the global liver-related DALYs.[30] The MAFLD-related DALYs reached 3.3 million in Sub-Saharan Africa, 3 million in Eastern Europe, 2.7 million in Western Europe, and 2.5 million in high-income North America.[30] In most of Asia and MENA countries, a worsening trend of MAFLD-related DALYs was seen from 2009 to 2019[19] with highest DALYs reported in Cambodia, Egypt, Indonesia, Mongolia, Myanmar, Seychelles, Thailand, Turkmenistan, and Uzbekistan.[19]
A Brief Overview of MAFLD Pathogenesis and Natural History
MAFLD is the phenotypic manifestation of systemic metabolic dysregulation on a background of genetic predisposition and external environmental cues and is therefore heterogenous in its presentation.[4,12] In the now out dated “two-hit” model, insulin resistance coupled with a sedentary lifestyle and unhealthy dietary patterns is responsible for the excess hepatic accumulation of lipid.[31] Insulin resistance increases serum free fatty acid levels, de-novo lipogenesis from glucose and protein, and there is a relative reduction in hepatic lipid export.[12] The net effect is excess lipid storage in the liver.[12] The build-up of triglycerides promotes increased fatty acid oxidation and oxidative stress, otherwise known as the “second-hit” which results in steatohepatitis.[12] In simplistic terms, oxidative stress triggers lipid peroxidation, mitochondrial damage, and release of proinflammatory molecules which promotes progression of steatosis to steatohepatitis and fibrosis.[12,31] It is now appreciated however that MAFLD pathogenesis is best considered as arising from multiple, simultaneous hits, the outcome of systemic homeostatic dysregulation. The exact mechanisms for MAFLD progression have not been fully elucidated. It is believed that a combination of factors including genetic variations, oxidative stress, abnormal lipid metabolism, altered immune responses, mitochondrial and endoplasmic reticulum dysfunction, and an imbalance in gut microbiota (the so-called “multiple parallel hits” theory) is involved.[12] There is growing evidence that steatohepatitis especially that associated with fibrosis has a greater risk of adverse outcomes including cirrhosis and HCC.[32]
Clinical Aspects of MAFLD
Clinical features of MAFLD
MAFLD is usually asymptomatic. A clinical diagnosis is entertained when the newly proposed diagnostic definition is met.[33] In persons with imaging- or histologically-confirmed steatosis, a diagnosis of MAFLD is made if the following criteria are met: overweight/obesity, type 2 diabetes or in health weight individuals, and evidence of metabolic dysregulation with at least two of the following conditions: increased waist circumference, hypertriglyceridemia, hypertension, low serum high-density lipoprotein-cholesterol levels, prediabetes, insulin resistance, or chronic subclinical inflammation.[33]
In diagnosing MAFLD, serum biomarkers are useful but may not be pathognomic, since approximately 80% of patients have serum alanine aminotransferase (ALT) levels within laboratory reference ranges.[34,35] Of note, the serum level of ALT does not correlate with the severity of liver histology and therefore the ALT level cannot be used as a diagnostic or monitoring tool for MAFLD.[34,36] Other alternative serum biomarkers such as cytokeratin 18 fragments, adiponectin, thioredoxin, and manganese superoxide dismutase have been reported with varying diagnostic accuracies, but none are specific for MAFLD.[3] An increased ferritin level is observed in MAFLD patients and correlates with the degree of hepatic fibrosis.[14] It is important, once a diagnosis of MAFLD has been made, for concomitant liver diseases to be excluded and treated appropriately (eg, hepatitis B or C).
Imaging features for MAFLD
Conventional imaging techniques detecting liver fat accumulation are used when MAFLD is suspected.[37,38] Ultrasound is the first-line imaging tool and is widely used because of its availability, affordability, and lack of invasiveness.[37,38] Ultrasound of a suspected MAFLD liver will appear “bright” with increased echotexture, together with other radiological features.[36] Unenhanced computed tomography is generally more specific for MAFLD than ultrasound. As the degree of steatosis increases, the liver becomes hypo-attenuated on CT scan, appearing darker when compared to the adjacent fat free tissues such as the spleen.[37] Magnetic resonance imaging or magnetic resonance spectroscopy is the most sensitive and specific imaging technique for diagnosing the presence of liver fat as it identifies the difference in resonance of protons in fat and water.[37,38]
Histological features
Liver histology is the most accurate albeit invasive tool for MAFLD diagnosis.[8] Histological features of MeSH include the presence of hepatic steatosis, lobular inflammation, and the presence of ballooning.[8] Steatosis is generally most intense around the central veins, predominantly in zones 2 and 3.[39] Since many liver diseases manifest similar histology, at least 5% of the hepatocytes showing fatty change is needed to make a MAFLD diagnosis.[40,41] Steatosis is usually macrovesicular, composed of small or large vacuoles. Microvesicular steatosis may result in a foamy cytoplasmic appearance and is often seen in single hepatocytes or in patches.[40] Smaller lipid droplets set the nucleus in the center of the cell while larger lipid droplets (macrovesicular) can displace the nucleus to the periphery.[39]
MeSH if it persists is the harbinger of progressive liver disease and is usually associated with a degree of fibrosis.[39] Macrovesicular steatosis, ballooning degeneration of hepatocytes, scattered inflammation, apoptotic bodies, Mallory-Denk bodies (MDB), and zone 3 hepatocellular injury are typically seen in MeSH.[39,40] With the progression of MeSH, fibrosis worsens, leading to the development of bridging fibrosis and cirrhosis.
Interestingly, in children and adolescents, the typical histological changes of MeSH are seen in zone 1 rather than in zone 3.[39] The fat vacuoles are largest in hepatocytes around the portal regions. Mild lobular inflammation, portal inflammation, and MDBs are difficult to find, and fibrosis usually begins around the portal regions.[39] MAFLD-related cirrhosis is the end stage of the disease and histologically may lack perisinusoidal and pericellular fibrosis and other features of the disease.[39]
Management
Many guidelines have been published to provide guidance to clinicians for the management of patients with MAFLD [Table 1]. The guidelines are more for adult patients, whereas guidelines for pediatric patients are limited. Since most patients with early MAFLD have a good prognosis, management should focus on treating the underlying metabolic comorbidities,[42] which will reduce not only cardiovascular and cancer risk but also liver disease progression.[43]
Table 1.
Comparison of common guidelines for the management of MAFLD.
| Interventions | AASLD Practice Guideline[36] | EASL-EASD-EASO Clinical Practice Guideline[38] | The Asian Pacific Association for the Study of the Liver Clinical Practice Guideline[39] | AISF[40] | NICE[41] |
| Lifestyle intervention | |||||
| Exercise | 200 min/week, moderate intensity | Aerobic exercise and resistance training | Aerobic exercise and resistance training | Aerobic exercise and resistance training | Recommended |
| Diet | Low-calorie diet | Dietary restrictions | Energy restriction and exclusion of processed foods; avoid foods or drinks high in fructose. A Mediterranean-pattern composition is recommended. | Low-calorie, low-carb, low-fat and high fiber diet.Gold standard is the Mediterranean dietary pattern. | No specific outline |
| Target weight loss | 3% to 5% of body weight | 7% of body weight | 7% to 10% of body weight | Not recommended | Recommended |
| Pharmacological intervention | |||||
| Metformin | Not recommended | Insufficient evidence | May be beneficial for treating MAFLD-HCC patients with Type 2 diabetes | Not recommended | Not recommended |
| Pioglitazone | Only for biopsy proven MeSH | To treat diabetes in patient with concurrent MAFLD | Improves histological features of MAFLD | Could be beneficial but insufficient evidence | Only for adults with advanced fibrosis with or without diabetes |
| Vitamin E | Could be used for biopsy proven steatohepatitis | Could be beneficial but insufficient evidence | May improve liver histology; however, some concerns about safety | Not beneficial | Not mentioned |
| GLP-1 Agonists | Insufficient evidence | Insufficient evidence | Not mentioned | Insufficient evidence | Insufficient evidence |
| OCA | Insufficient evidence | Insufficient evidence | Awaiting study results | Awaiting study results | Not mentioned |
| Silymarin | Not mentioned | Not mentioned | Not mentioned | Not mentioned | Potential for use but insufficient evidence |
| Statins | Safe but not beneficial | Safe but not beneficial | Reduces cardiovascular morbidity and mortality | Safe but not beneficial | Safe but not beneficial |
| Surgical intervention | |||||
| Bariatric Surgery | Can consider foregut bariatric surgery for eligible obese patients with confirmed steatohepatitis | An option for patients that do not respond to lifestyle or pharmacological interventions | Decision should be individualized due to risk of post-operative complications | Not mentioned | Not mentioned |
AISF: Italian Association for the Study of the Liver; GLP-1: Glucagon-like peptide-1; HCC: Hepatocellular carcinoma; MAFLD: Metabolic (dysfunction) Associated Fatty Liver Disease; MeSH: Metabolic steatohepatitis; NICE: National Institute for Health and Care Excellence; OCA: Obeticholic acid.
The AASLD Practice Guidelines[40] for managing pediatric obesity was proposed as part of the MAFLD management plan, but no specific guidelines on diet and exercise related interventions are provided. Vitamin E (800 IU/day) is recommended for MAFLD patients with advanced fibrosis regardless of diabetes. However, the benefit of Vitamin E in pediatric patients with MeSH requires further evidence. Metformin is either not recommended or shows no evidence of benefit for the treatment of MeSH.[40]
Mechanisms of MAFLD Development and Progression
Critical role of inflammation
Hepatic inflammation occurs in stressed, de-differentiated adipose tissues in obese patients.[44] Inflammation in these tissue exacerbates hepatic steatosis and instigates innate inflammatory responses causing recruitment and activation of immune cells to the liver.[45] Activation of immune cells leads to the release of numerous pro-inflammatory chemokines (eg, macrophage chemokines, which are responsible for the recruitment of neutrophils and monocyte-derived macrophages) and cytokines (eg, monocyte chemoattractant protein-1 and tumor necrosis factor-α [TNF-α]).[44]
The liver is composed of multiple cell types including hepatocytes (60% of total cell mass), and other non-parenchymal cell types that comprise about 35% and consist of liver sinusoidal endothelial cells (LSECs, 44%), Kupffer cells (KCs) (33%), hepatic stellate cells (HSCs, 10%–25%), and NK cells (5%).[46–48] These cell types work synergistically during the transition to MeSH. During steatohepatitis, hepatocytes initially undergo “lipid mobilization and droplet remodeling”.[47] This is a protective mechanism. However, excess fat can turn the process into a toxic event resulting in decreased lipid droplet remodeling, disease progression, and death of hepatocytes via multiple mechanisms including apoptosis, necrosis, necroptosis, pyroptosis, and ferroptosis.[47] Injured or dead hepatocytes not only recruit macrophages but also release cellular components including damage-associated molecular pattern to trigger the innate immune response. Hepatocytes are capable of detecting pathogens and metabolic molecules via cytoplasmic pattern recognition receptor (PRR), which adds to the inflammatory response.
LSECs represent approximately 50% of non-parenchymal liver cells[49] and function as gatekeepers of liver homeostasis involved in the maintenance of HSC quiescence.[47] During the early stages of MAFLD, LSEC fenestrae are lost, affecting the transfer of chylomicron remnants to hepatocytes for very low density lipoprotein synthesis. Insulin resistance and dyslipidaemia also impair the ability of LSECs to synthesize nitric oxide, which is considered a hepato-protective factor as it controls lipogenesis and enhances β-oxidation of fatty acids.[47] The inflammatory response and associated gut microbiota signals can activate the NF-κB pathway in LSECs causing the release of pro-inflammatory mediators like MCP-1, Interleukin-1 (IL-1), IL-6, and TNF-α with subsequent increases in the production of adhesion molecules (eg, intercellular adhesion molecule-1, vascular adhesion protein-1), and vascular cell adhesion.[47] Collectively, these events increase the activation of macrophage and neutrophils, amplifying inflammation and liver injury. Affected LSECs lose their ability to maintain HSC stability, thereby promoting fibrosis.
KCs are liver resident macrophages involved in the innate immune response and are sensitive to gut-derived endotoxins.[44,47] KCs are involved in the activation of the NF-κB pathway via CD14, Toll-like receptor (TLR) 2, TLR4, and adaptor proteins.[44] Lymphocyte accumulation is thought to contribute to inflammation in MeSH. The liver has many types of lymphocytes such as NK cells, NK T cells, and T cells. Cytokines released from KCs, mainly IL-1 and IL-18, regulate hepatic NK cell activity. In turn, NK cells produce IFN-γ which modulates T cell activity.[44]
Apart from KCs and lymphocytes, neutrophils are involved in the innate immune response and contribute to inflammation by secreting cytokines and bio-active molecules. Neutrophils are capable of releasing neutrophil extracellular traps (NETs) to capture pathogens and control infection.[45] In steatohepatitis, excess production of NETs coupled with their decreased clearance results in a chronic sterile inflammatory state.[45] However, the specific role of neutrophils in the pathogenesis of steatohepatitis is unclear.
Bone-marrow derived monocytes are recruited during the inflammatory process and are also critical for MAFLD progression.[45] KC-derived factors trigger the infiltration of these monocytes which later differentiate into liver macrophages, contributing to the inflammatory response. This suggests that the liver and bone-marrow communicate to maintain hepatic inflammation in MeSH.[45]
Fibrosis, a critical stage in the progression of MAFLD
Fibrosis is a wound healing response to persistent injury. It arises through increased deposition and decreased degradation of the extracellular matrix (ECM)[50] and is the precursor to cirrhosis.[51] The majority of MAFLD patients have simple steatosis with little or no fibrosis. In a minority (5%–10%), the disease will evolve to steatohepatitis with subsequent fibrosis, cirrhosis, and in some, HCC.[32,52] Mechanistically, the activation of HSCs plays a pivotal role in liver fibrosis.[53] In MAFLD, HSC activation releases pro-fibrogenic and inflammatory cytokines and mediators that drive fibrosis.[54] At the molecular level, hepatic fibrosis is driven by cytokines including platelet-derived growth factor (PDGF), transforming growth factor beta (TGF-β), TNF-α, and IL-1 secreted as part of the inflammatory response.[51] These cytokines injure HSCs and trigger their transformation from a quiescent to an activated state.[51] Activated HSC transdifferentiate into pro-fibrogenic myofibroblast-like cells promoting hepatic fibrosis.[50,51] These activated cells are characterized by increased expression of fibrogenic markers such as Alpha-smooth muscle actin, c-myb, and myocyte enhancer factor 2.[50] They also acquire features such as increased contractility and inflammatory properties and accumulate at sites of injury, producing excess ECM and collagen.[50]
PDGF produced by KCs is the dominant mitogen for activating HSCs and hence is an important driver of fibrosis.[51] PDGF upregulates the expression of matrix metallopeptidase 2 (MMP2), MMP9, and TIMP metallopeptidase inhibitor 1 (TIMP1) while it inhibits collagenase activity thereby increasing ECM deposition and decreasing degradation. In addition, PDGF activates various other signaling pathways such as extracellular signal-regulated kinase 1/2, mitogen-activated protein kinase (MAPK), c-Jun N-terminal kinase, and protein kinase B.[51] TGF-β is the strongest driver of fibrosis in the liver and is produced by activated HSCs, KCs, LSECs, and hepatocytes.[51] The level of TGF-β is elevated in fibrosis and is highest in cirrhosis. Similar to PDGF, TGF-β1 up-regulates the expression of matrix producing genes and inhibits matrix degradation.[51] TGF-β1 also induces hepatocyte apoptosis in fibrotic livers and is partly the explanation for tissue loss and a smaller liver size seen in patients with cirrhosis.[51] TNF-α is another pro-inflammatory mediator that is produced by HSCs, KCs, monocytes, and macrophages.[51] During hepatic fibrosis TNF-α contributes to the overproduction of ECM in the liver. Similarly, IL-1 activates HSCs, initiating ECM production.[51] All these contribute to the development of hepatic fibrosis, cirrhosis, and eventual loss of liver function.[51]
MAFLD-related HCC
An overview
MAFLD patients with advanced fibrosis and cirrhosis are at increased risk of HCC, though HCC can also arise in the absence of advanced fibrosis[8] [Figure 1B]. The increasing trend of MAFLD-induced HCC mirrors the increase in the incidence of obesity and metabolic syndrome.[1,13,14] HCC incidence is 12 times higher in patients with severe steatohepatitis than in the general MAFLD population and most cases occur in older males with the metabolic syndrome.[8] Cancer related mortality amongst MAFLD patients is among the top three causes of death and the third most common cause of HCC in the US.[55] Interestingly, MAFLD-related HCC can occur in the absence of cirrhosis.[14] The HCC incidence in MeSH patients with cirrhosis is lower than in hepatitis C (HCV) or hepatitis B (HBV) related cirrhosis.[13]
Most MAFLD-induced HCCs are at early/immediate stages. Approximately 43% of the cancers present with microinvasion.[5] Interestingly, MAFLD-HCC patients have less severe liver damage and dysfunction than HCV-related HCC patients as indicated by a higher serum albumin, lower serum bilirubin, and lower rates of ascites.[8] Pathologically, MAFLD-HCCs seem to be phenotypically different from HCCs of other etiologies and are generally well-differentiated with solitary lesions, with more inflammatory infiltration and less likelihood of extrahepatic metastases.[8] Interestingly, the size of MAFLD-HCC is generally larger than the HCCs originating from other chronic liver diseases.[8]
Role of oxidative stress and related injury
Oxidative stress and related injury play important roles in the pathogenesis of MAFLD-related HCC. Hyperinsulinemia, chronic inflammation, and insulin resistance are the major drivers for reactive oxygen species (ROS) production and subsequent oxidative stress.[56] Excess ROS injures mitochondria and DNA in hepatocytes[57] leading to hepatocyte apoptosis via activating caspase 3 and 9.[12] ROS also induces the secretion of inflammatory cytokines such as IL-6, TNF-α, leptin, and adiponectin,[58] all of which contribute to hepatic inflammation and cancer. Of particular note, IL-6 is a key signal for ROS-induced liver injury and HCC.[12,58–60] In the liver, the increased production of IL-6 stimulates cell proliferation and drives anti-apoptotic pathways via activating STAT3 signaling, a well-established oncogenic transcription factor[12,59,60]; TNF-α is involved in disease progression and hepatocarcinogenesis via activating the JAK2/STAT pathway.[12]
Uncontrolled cell proliferation is a feature of cancer development. In the pathogenesis of MAFLD-HCC, insulin resistance stimulates the expression of insulin and insulin-like growth factor-1 (IGF-1).[12] Binding of insulin and IGF-1 to their respective receptors provokes a signaling cascade via insulin receptor substrate 1 which in turn activates the phosphoinositide 3-kinase (PI3K) and MAPK pathways[12,56,61]; all of these are key pro-proliferative pathways implicated in tumorigenesis.[12] It has been shown that activation of PI3K pathway induces Mdm2/p53-dependent apoptosis and mTOR dependent cell proliferation,[12] while activation of MAPK pathway induces the transcription of proto-oncogenes such as c-fos and c-jun for cell proliferation and subsequent activation of the Wnt/β-catenin pathway.[12] These play a pivotal role in fibrosis and tumor development.[12]
Role of gut microbiota
Gut microbiota is an integral part of the host immune system[12,62,63] and gut-derived bacterial metabolites and by-products contribute to MAFLD development and progression.[12,62,64] Altered intestinal flora in MAFLD including in pediatric MAFLD has been reported.[65] However, no single bacterium has been isolated but rather there is a change in the relative abundance of multiple types of microbiota including Escherichia, Prevotella, Streptococcus, Coprococcus, Faecalibacterium, and Ruminococcus.[66]
Gut microbiota influence insulin sensitivity and cholesterol metabolism.[67] Indeed, as the liver is constantly exposed to gut-derived toxins via the portal circulation, it is the frontline defense against gut-derived bacterial toxins.[12,63] Alterations of the gut microbiota profile are common in obese patients[12] and the resultant dysbiosis induces a breakdown in gut endothelial barrier function allowing bacteria and related metabolites (eg, lipopolysaccharide [LPS], bile acids, ethanol, and short chain fatty acids) to translocate to the liver, triggering inflammatory responses and immune cell infiltration.[62] Alterations in gut microbiota result in the release of pathogen-associated molecular patterns that are recognized by PRRs, and this further exacerbates the inflammation induced by the innate immune response.[12]
The effect of gut microbiota in MAFLD development is supported by animal studies. For example, germ-free mice colonized with microbiota extracted from MAFLD mice develop macrovesicular steatosis, higher levels of liver triglycerides, and increased expression of lipogenesis related genes.[68] In obese mice, there is a change in gut microbiota resulting in the accumulation of gut metabolites such as deoxycholic acid, which causes DNA damage.[69] The gut metabolites stimulate the secretion of pro-inflammatory and tumorigenic factors from affected HSCs.[69] Alterations of gut microbiota as a result of a high-fat diet have also been thought to facilitate hepatocarcinogenesis in chronically injured livers via TLR signaling.[70] Altered gut flora activate TLR signaling, resulting in the increased in expression of epiregulin which prevents apoptosis.[70] In the latter stages of HCC progression in mice, gut sterilization reduces tumor size and growth, suggesting that TLR signaling and microbiota play a pivotal role in HCC development.[70]
Direct evidence for the role of gut microbiota in the development of MAFLD-related HCC in humans is lacking. However, the above data[64] and the findings that high-fat diet fed mice develop a fatty liver phenotype with a greater abundance of lactobacillus gasseri and/or lactobacillus taiwanensis[71] clearly indicate a causal link between the microbiota and HCC in MAFLD. More studies are needed to clarify the impact of gut microbiota on the pathogenesis of human MAFLD-related HCC.
Role of liver stem cells
Hepatic stem/progenitor cells (HpSCs) are bipotent progenitor cells characterized by the expression of multiple markers including cytokeratin 7 and 19, biliary cytokeratins, SOX9, CD44, CD133, EpCAM, and neural cell adhesion molecule (NCAM).[72] Under physiological conditions, HpSCs are quiescent. Under pathological conditions HpSCs can be activated in response to liver injury.[72] Activation of HpSCs triggers the proliferation of reactive bile ducts, a condition termed “ductular reaction.”[72–74] The function and activation status of HpSCs, including their ability to produce humoral factors, is supported by a stem cell niche consisting of HSCs, KCs, portal myofibroblasts, Wnt/β-catenin, and Notch signaling genes. HPSCs regulate non-parenchymal cells by secreting hedgehog ligands, TGF-β1, and osteopontin.[72,73]
In patients with MeSH, chronic inflammation and associated persistent oxidative stress facilitates the activation and proliferation of HpSCs.[72,73] Activation of HpSCs and the associated ductular reaction contribute to HSC activation, ECM deposition, and MAFLD progression by secreting multiple factors such as TGF-β1, TNF-related weak inducer of apoptosis, PDGF and hedgehog ligands, thereby promoting fibrosis and angiogenesis.[72,75] These events increase fibrosis and inflammation in livers with steatohepatitis. Advanced fibrosis is a known risk factor for hepatocarcinogenesis. In a recent study, LPS infiltration as a result of gut-liver crosstalk was shown to activate TLR4 and NF-κB in HpSCs and macrophages leading to hepatocarcinogenesis.[72,76]
Potential Biomarkers and Therapeutics for MAFLD Treatment: Some Unmet Challenges
A number of unmet challenges exist for MAFLD. First, less invasive approaches for effective identification of MAFLD are needed. Fortunately, with technology advancements especially in high-throughput technologies such as “omics”, studies are underway to discover novel biomarkers specific for MAFLD detection.[77,78] Using a multiplexed proteomic assay, a few proteins including aminoacylase-1 receptor (MET), gelsolin (GSN), galectin-3 binding protein (LGALS3BP), NCAM L1-like protein (CHL1), and antithrombin III (SERPINC1) are associated with the development of steatosis.[78] In another study, 18 microRNAs have been linked to the development of MAFLD and nine of these 18 biomarkers could predict the severity of MAFLD better than aspartate aminotransferase (AST).[79] Recently, plasma proteome profiling of 48 MAFLD patients revealed significant changes in six proteins including fructose-1,6-bisphosphate aldolase, apolipoprotein M, LGALS3BP, polymeric immunoglobulin receptor, vitronectin, and afamin.[80] These representative plasma proteomic studies have the capacity to identify potential novel biomarkers for precise and early detection of MAFLD.[80]
Second, more effective therapies are needed for MAFLD. As of now, several classes of agents have shown promising effects. Obeticholic acid (OCA), a synthetic bile acid, is a Farnesoid X receptor (FXR) agonist shown to reduce MeSH.[81] The phase II (FLINT) study in non-cirrhotic, biopsy proven MeSH patients showed an improvement in MeSH and fibrosis in the OCA treated group.[81] The efficacy of OCA is currently being studied in a Phase III trial (REGENERATE) and another in those with compensated cirrhosis (REVERSE). Of note, adverse effects including pruritus and increased serum cholesterol levels in OCA treated patients have been reported.[81] Although the implications of the increased serum cholesterol level during OCA therapy for cardiovascular events is unclear, this adverse effect can be managed by statin therapy.[81] OCA is the most likely first candidate for approval as a therapeutic agent for MAFLD.[82] It is believed that FXR agonists, like OCA, will be beneficial for MAFLD patients; but the major challenge will be to find the optimal dose for efficacy with minimum side effects.[83] OCA monotherapy leads to histological steatohepatitis resolution in less than one-third of patients and other agents in combination will likely therefore be required.[82]
Another promising class of agents is the glucagon-like peptide-1 (GLP-1) receptor agonists such as Liraglutide, an incretin mimetic approved by the FDA for the treatment of type 2 diabetes mellitus.[84] Liraglutide improves liver histology in MeSH patients,[84] but semaglutide, another GLP-1 agnoist is being taken forward for the Phase III trial.[84] Semaglutide has proven benefits for patients with cardiac risk factors.[84] Resmetirom, a thyroid hormone receptor agonist resolved MeSH in a phase IIb trial.[84] A Phase III study is underway in non-cirrhotic MeSH and fibrosis stages 2 or 3.[84] Finally, arachidyl amido cholanoic acid (Aramchol), a partial inhibitor of hepatic stearoyl-CoA desaturase 1, significantly reduced liver fat content and improved metabolic parameters in a Phase IIa. A Phase III study is underway.[84]
Looking to the future, studies to identify better biomarkers and to contribute to the development of novel therapeutics, as well as studies to unveil the molecular mechanisms linking MAFLD to end stage liver disease and particularly HCC, are warranted.
Funding
JG is supported by the Robert W. Storr Bequest to the Sydney Medical Foundation, University of Sydney; and National Health and Medical Research Council of Australia (NHMRC) Program and Investigator Grants (AAP2008983, APP1053206, APP1196492). LQ is supported by NSW Cancer Council grants (APP1145008, APP1070076).
Conflicts of interest
None.
Footnotes
How to cite this article: Bae SDW, George J, Qiao L. From MAFLD to hepatocellular carcinoma and everything in between. Chin Med J 2022;135:547–556. doi: 10.1097/CM9.0000000000002089
References
- 1.Huang DQ, El-Serag HB, Loomba R. Global epidemiology of NAFLD-related HCC: trends, predictions, risk factors, and prevention. Nat Rev Gastroenterol Hepatol 2021; 18:223–238. doi: 10.1038/s41575-020-00381-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zheng KI, Fan JG, Shi JP, Wong VW, Eslam M, George J, Zheng MH. From NAFLD to MAFLD: a “redefining” moment for fatty liver disease. Chin Med J 2020; 133:2271–2273. doi: 10.1097/CM9.0000000000000981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Marengo A, Rosso C, Bugianesi E. Liver cancer: connections with obesity, fatty liver, and cirrhosis. Annu Rev Med 2016; 67:103–117. doi: 10.1146/annurev-med-090514-013832. [DOI] [PubMed] [Google Scholar]
- 4.Younossi ZM, Rinella ME, Sanyal AJ, Harrison SA, Brunt EM, Goodman Z, et al. From NAFLD to MAFLD: implications of a premature change in terminology. Hepatology 2021; 73:1194–1198. doi: 10.1002/hep.31420. [DOI] [PubMed] [Google Scholar]
- 5.Pinyol R, Torrecilla S, Wang H, Montironi C, Pique-Gili M, Torres-Martin M, et al. Molecular characterisation of hepatocellular carcinoma in patients with non-alcoholic steatohepatitis. J Hepatol 2021; 75:865–878. doi: 10.1016/j.jhep.2021.04.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Eslam M, Sanyal AJ, George J. Toward more accurate nomenclature for fatty liver diseases. Gastroenterology 2019; 157:590–593. doi: 10.1053/j.gastro.2019.05.064. [DOI] [PubMed] [Google Scholar]
- 7.Eslam M, Sanyal AJ, George J. International Consensus Panel. MAFLD: A consensus-driven proposed nomenclature for metabolic associated fatty liver disease. Gastroenterology 2020; 158:1999–2014. e1. doi: 10.1053/j.gastro.2019.11.312. [DOI] [PubMed] [Google Scholar]
- 8.Yu J. Obesity, Fatty Liver and Liver Cancer. 2018; Singapore: Springer, 157. [Google Scholar]
- 9.Michelotti GA, Machado MV, Diehl AM. NAFLD, NASH and liver cancer. Nat Rev Gastroenterol Hepatol 2013; 10:656–665. doi: 10.1038/nrgastro.2013.183. [DOI] [PubMed] [Google Scholar]
- 10.Estes C, Chan HLY, Chien RN, Chuang WL, Fung J, Goh GBB, et al. Modelling NAFLD disease burden in four Asian regions-2019–2030. Aliment Pharmacol Ther 2020; 51:801–811. doi: 10.1111/apt.15673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kuchay MS, Martinez-Montoro JI, Choudhary NS, Fernandez-Garcia JC, Ramos-Molina B. Non-alcoholic fatty liver disease in lean and non-obese individuals: current and future challenges. Biomedicines 2021; 9:1346.doi: 10.3390/biomedicines9101346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kutlu O, Kaleli HN, Ozer E. Molecular pathogenesis of nonalcoholic steatohepatitis- (NASH-) related hepatocellular carcinoma. Can J Gastroenterol Hepatol 2018; 2018:8543763.doi: 10.1155/2018/8543763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sagnelli E, Macera M, Russo A, Coppola N, Sagnelli C. Epidemiological and etiological variations in hepatocellular carcinoma. Infection 2020; 48:7–17. doi: 10.1007/s15010-019-01345-y. [DOI] [PubMed] [Google Scholar]
- 14.Oda K, Uto H, Mawatari S, Ido A. Clinical features of hepatocellular carcinoma associated with nonalcoholic fatty liver disease: a review of human studies. Clin J Gastroenterol 2015; 8:1–9. doi: 10.1007/s12328-014-0548-5. [DOI] [PubMed] [Google Scholar]
- 15.Wang J, He W, Tsai PJ, Chen PH, Ye M, Guo J, et al. Mutual interaction between endoplasmic reticulum and mitochondria in nonalcoholic fatty liver disease. Lipids Health Dis 2020; 19:72.doi: 10.1186/s12944-020-01210-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Younossi ZM, Koenig AB, Abdelatif D, Fazel Y, Henry L, Wymer M. Global epidemiology of nonalcoholic fatty liver disease-Meta-analytic assessment of prevalence, incidence, and outcomes. Hepatology 2016; 64:73–84. doi: 10.1002/hep.28431. [DOI] [PubMed] [Google Scholar]
- 17.Adams LA, Roberts SK, Strasser SI, Mahady SE, Powell E, Estes C, et al. Nonalcoholic fatty liver disease burden: Australia, 2019–2030. J Gastroenterol Hepatol 2020; 35:1628–1635. doi: 10.1111/jgh.15009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Swain MG, Ramji A, Patel K, Sebastiani G, Shaheen AA, Tam E, et al. Burden of nonalcoholic fatty liver disease in Canada, 2019–2030: a modelling study. CMAJ Open 2020; 8:E429–E436. doi: 10.9778/cmajo.20190212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Golabi P, Paik JM, AlQahtani S, Younossi Y, Tuncer G, Younossi ZM. Burden of non-alcoholic fatty liver disease in Asia, the Middle East and North Africa: data from Global Burden of Disease 2009–2019. J Hepatol 2021; 75:795–809. doi: 10.1016/j.jhep.2021.05.022. [DOI] [PubMed] [Google Scholar]
- 20.Estes C, Anstee QM, Arias-Loste MT, Bantel H, Bellentani S, Caballeria J, et al. Modeling NAFLD disease burden in China, France, Germany, Italy, Japan, Spain, United Kingdom, and United States for the period. J Hepatol 2018; 69:896–904. doi: 10.1016/j.jhep.2018.05.036. [DOI] [PubMed] [Google Scholar]
- 21.Zhang X, Wu M, Liu Z, Yuan H, Wu X, Shi T, et al. Increasing prevalence of NAFLD/NASH among children, adolescents and young adults from 1990 to 2017: a population-based observational study. BMJ Open 2021; 11:e042843.doi: 10.1136/bmjopen-2020-042843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fan JG, Wei L, Zhuang H. National Workshop on Fatty Liver and Alcoholic Liver Disease, Chinese Society of Hepatology, Chinese Medical Association; Fatty Liver Disease Expert Committee, Chinese Medical Doctor Association. Guidelines of prevention and treatment of nonalcoholic fatty liver disease (2018, China). J Dig Dis 2019; 20:163–173. doi: 10.1111/1751-2980.12685. [DOI] [PubMed] [Google Scholar]
- 23.Lonardo A, Nascimbeni F, Ballestri S, Fairweather D, Win S, Than TA, et al. Sex differences in nonalcoholic fatty liver disease: state of the art and identification of research gaps. Hepatology 2019; 70:1457–1469. doi: 10.1002/hep.30626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Villanueva-Ortega E, Garces-Hernandez MJ, Herrera-Rosas A, Lopez-Alvarenga JC, Laresgoiti-Servitje E, Escobedo G, et al. Gender-specific differences in clinical and metabolic variables associated with NAFLD in a Mexican pediatric population. Ann Hepatol 2019; 18:693–700. doi: 10.1016/j.aohep.2019.04.012. [DOI] [PubMed] [Google Scholar]
- 25.Hörist-Kollmann S, Strametz-Juranek J. Female dietary patterns and the pathogenesis of NAFLD. Gend Genome 2018; 2:49–55. doi: 10.1177/2470289718787091. [Google Scholar]
- 26.Suri A, Song E, van Nispen J, Voigt M, Armstrong A, Murali V, et al. Advances in the epidemiology, diagnosis, and management of pediatric fatty liver disease. Clin Ther 2021; 43:438–454. doi: 10.1016/j.clinthera.2021.01.006. [DOI] [PubMed] [Google Scholar]
- 27.Anderson EL, Howe LD, Jones HE, Higgins JPT, Lawlor DA, Fraser A. The prevalence of non-alcoholic fatty liver disease in children and adolescents: a systematic review and meta-analysis. PLoS One 2015; 10:e0140908.doi: 10.1371/journal.pone.0140908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zou ZY, Zeng J, Ren TY, Huang LJ, Wang MY, Shi YW, et al. The burden and sexual dimorphism with nonalcoholic fatty liver disease in Asian children: a systematic review and meta-analysis. Liver Int 2021; doi: 10.1111/liv.15080. Online ahead of print. [DOI] [PubMed] [Google Scholar]
- 29.Phisalprapa P, Prasitwarachot R, Kositamongkol C, Hengswat P, Srivanichakorn W, Washirasaksiri C, et al. Economic burden of non-alcoholic steatohepatitis with significant fibrosis in Thailand. BMC Gastroenterol 2021; 21:135.doi: 10.1186/s12876-021-01720-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Paik JM, Golabi P, Younossi Y, Srishord M, Mishra A, Younossi ZM. The growing burden of disability related to nonalcoholic fatty liver disease: data from the Global Burden of Disease 2007–2017. Hepatol Commun 2020; 4:1769–1780. doi: 10.1002/hep4.1599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Benedict M, Zhang X. Non-alcoholic fatty liver disease: an expanded review. World J Hepatol 2017; 9:715–732. doi: 10.4254/wjh.v9.i16.715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Farrell GC, Larter CZ. Nonalcoholic fatty liver disease: from steatosis to cirrhosis. Hepatology 2006; 43:S99–S112. doi: 10.1002/hep.20973. [DOI] [PubMed] [Google Scholar]
- 33.Eslam M, Newsome PN, Sarin SK, Anstee QM, Targher G, Romero-Gomez M, et al. A new definition for metabolic dysfunction-associated fatty liver disease: an international expert consensus statement. J Hepatol 2020; 73:202–209. doi: 10.1016/j.jhep.2020.03.039. [DOI] [PubMed] [Google Scholar]
- 34.Dyson JK, Anstee QM, McPherson S. Non-alcoholic fatty liver disease: a practical approach to diagnosis and staging. Frontline Gastroenterol 2014; 5:211–218. doi: 10.1136/flgastro-2013-100403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mantovani A. MAFLD vs NAFLD: where are we? Dig Liver Dis 2021; 53:1368–1372. doi: 10.1016/j.dld.2021.05.014. [DOI] [PubMed] [Google Scholar]
- 36.Mahady SE, Adams LA. Burden of non-alcoholic fatty liver disease in Australia. J Gastroenterol Hepatol 2018; 33:1–11. doi: 10.1111/jgh.14270. [DOI] [PubMed] [Google Scholar]
- 37.Hamer OW, Aguirre DA, Casola G, Lavine JE, Woenckhaus M, Sirlin CB. Fatty liver: imaging patterns and pitfalls. Radiographics 2006; 26:1637–1653. doi: 10.1148/rg.266065004. [DOI] [PubMed] [Google Scholar]
- 38.Zhang YN, Fowler KJ, Hamilton G, Cui JY, Sy EZ, Balanay M, et al. Liver fat imaging-a clinical overview of ultrasound, CT, and MR imaging. Br J Radiol 2018; 91:20170959.doi: 10.1259/bjr.20170959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Brown GT, Kleiner DE. Histopathology of nonalcoholic fatty liver disease and nonalcoholic steatohepatitis. Metabolism 2016; 65:1080–1086. doi: 10.1016/j.metabol.2015.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chalasani N, Younossi Z, Lavine JE, Diehl AM, Brunt EM, Cusi K, et al. The diagnosis and management of non-alcoholic fatty liver disease: Practice Guideline by the American Association for the Study of Liver Diseases, American College of Gastroenterology, and the American Gastroenterological Association. Hepatology 2012; 55:2005–2023. doi: 10.1002/hep.25762. [DOI] [PubMed] [Google Scholar]
- 41.Rios RS, Zheng KI, Zheng MH. Non-alcoholic steatohepatitis and risk of hepatocellular carcinoma. Chin Med J 2021; 134:2911–2921. doi: 10.1097/CM9.0000000000001888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Eslam M, Sarin SK, Wong VWS, Fan JG, Kawaguchi T, Ahn SH, et al. The Asian Pacific Association for the Study of the Liver clinical practice guidelines for the diagnosis and management of metabolic associated fatty liver disease. Hepatol Int 2020; 14:889–919. doi: 10.1007/s12072-020-10094-2. [DOI] [PubMed] [Google Scholar]
- 43.Chitturi S, Wong VWS, Chan WK, Wong GLH, Wong SKH, Sollano J, et al. The Asia-Pacific working party on non-alcoholic fatty liver disease guidelines-Part 2: management and special groups. J Gastroenterol Hepatol 2018; 33:86–98. doi: 10.1111/jgh.13856. [DOI] [PubMed] [Google Scholar]
- 44.Parthasarathy G, Revelo X, Malhi H. Pathogenesis of nonalcoholic steatohepatitis: an overview. Hepatol Commun 2020; 4:478–492. doi: 10.1002/hep4.1479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Luci C, Bourinet M, Leclere PS, Anty R, Gual P. Chronic inflammation in non-alcoholic steatohepatitis: molecular mechanisms and therapeutic strategies. Front Endocrinol (Lausanne) 2020; 11:597648.doi: 10.3389/fendo.2020.597648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Farrell GC, van Rooyen D, Gan L, Chitturi S. NASH is an inflammatory disorder: pathogenic, prognostic and therapeutic implications. Gut Liver 2012; 6:149–171. doi: 10.5009/gnl.2012.6.2.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Schuster S, Cabrera D, Arrese M, Feldstein AE. Triggering and resolution of inflammation in NASH. Nat Rev Gastroenterol Hepatol 2018; 15:349–364. doi: 10.1038/s41575-018-0009-6. [DOI] [PubMed] [Google Scholar]
- 48.Vekemans K, Braet F. Structural and functional aspects of the liver and liver sinusoidal cells in relation to colon carcinoma metastasis. World J Gastroenterol 2005; 11:5095–5102. doi: 10.3748/wjg.v11.i33.5095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ding C, Li Y, Guo F, Jiang Y, Ying W, Li D, et al. A cell-type-resolved liver proteome. Mol Cell Proteomics 2016; 15:3190–3202. doi: 10.1074/mcp.M116.060145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Seshachalam VP, Sekar K, Hui KM. Insights into the etiology-associated gene regulatory networks in hepatocellular carcinoma from The Cancer Genome Atlas. J Gastroenterol Hepatol 2018; 33:2037–2047. doi: 10.1111/jgh.14262. [DOI] [PubMed] [Google Scholar]
- 51.Anstee QM, Targher G, Day CP. Progression of NAFLD to diabetes mellitus, cardiovascular disease or cirrhosis. Nat Rev Gastroenterol Hepatol 2013; 10:330–344. doi: 10.1038/nrgastro.2013.41. [DOI] [PubMed] [Google Scholar]
- 52.Zhou WC, Zhang QB, Qiao L. Pathogenesis of liver cirrhosis. World J Gastroenterol 2014; 20:7312–7324. doi: 10.3748/wjg.v20.i23.7312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Bataller R, Brenner DA. Liver fibrosis. J Clin Invest 2005; 115:209–218. doi: 10.1172/JCI24282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ramos-Lopez O, Martinez-Lopez E, Roman S, Fierro NA, Panduro A. Genetic, metabolic and environmental factors involved in the development of liver cirrhosis in Mexico. World J Gastroenterol 2015; 21:11552–11566. doi: 10.3748/wjg.v21.i41.11552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Farrell GC, Wong VWS, Chitturi S. NAFLD in Asia - as common and important as in the West. Nat Rev Gastroenterol Hepatol 2013; 10:307–318. doi: 10.1038/nrgastro.2013.34. [DOI] [PubMed] [Google Scholar]
- 56.Leung C, Yeoh SW, Patrick D, Ket S, Marion K, Gow P, et al. Characteristics of hepatocellular carcinoma in cirrhotic and non-cirrhotic non-alcoholic fatty liver disease. World J Gastroenterol 2015; 21:1189–1196. doi: 10.3748/wjg.v21.i4.1189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Frades I, Andreasson E, Mato JM, Alexandersson E, Matthiesen R, Martinez-Chantar ML. Integrative genomic signatures of hepatocellular carcinoma derived from nonalcoholic fatty liver disease. PLoS One 2015; 10:e0124544.doi: 10.1371/journal.pone.0124544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Park EJ, Lee JH, Yu GY, He G, Ali SR, Holzer RG, et al. Dietary and genetic obesity promote liver inflammation and tumorigenesis by enhancing IL-6 and TNF expression. Cell 2010; 140:197–208. doi: 10.1016/j.cell.2009.12.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.He G, Karin M. NF-κB and STAT3 - key players in liver inflammation and cancer. Cell Res 2011; 21:159–168. doi: 10.1038/cr.2010.183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Min HK, Mirshahi F, Verdianelli A, Pacana T, Patel V, Park CG, et al. Activation of the GP130-STAT3 axis and its potential implications in nonalcoholic fatty liver disease. Am J Physiol Gastrointest Liver Physiol 2015; 308:G794–G803. doi: 10.1152/ajpgi.00390.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Donohoe F, Wilkinson M, Baxter E, Brennan DJ. Mitogen-Activated Protein Kinase (MAPK) and obesity-related cancer. Int J Mol Sci 2020; 21:1241.doi: 10.3390/ijms21041241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Bashiardes S, Shapiro H, Rozin S, Shibolet O, Elinav E. Non-alcoholic fatty liver and the gut microbiota. Mol Metab 2016; 5:782–794. doi: 10.1016/j.molmet.2016.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Noverr MC, Huffnagle GB. Does the microbiota regulate immune responses outside the gut? Trends Microbiol 2004; 12:562–568. doi: 10.1016/j.tim.2004.10.008. [DOI] [PubMed] [Google Scholar]
- 64.Margini C, Dufour JF. The story of HCC in NAFLD: from epidemiology, across pathogenesis, to prevention and treatment. Liver Int 2016; 36:317–324. doi: 10.1111/liv.13031. [DOI] [PubMed] [Google Scholar]
- 65.Michail S, Lin M, Frey MR, Fanter R, Paliy O, Hilbush B, et al. Altered gut microbial energy and metabolism in children with non-alcoholic fatty liver disease. FEMS Microbiol Ecol 2015; 91:1–9. doi: 10.1093/femsec/fiu002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Li F, Ye J, Shao C, Zhong B. Compositional alterations of gut microbiota in nonalcoholic fatty liver disease patients: a systematic review and Meta-analysis. Lipids Health Dis 2021; 20:22.doi: 10.1186/s12944-021-01440-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Rabot S, Membrez M, Bruneau A, Gerard P, Harach T, Moser M, et al. Germ-free C57BL/6J mice are resistant to high-fat-diet-induced insulin resistance and have altered cholesterol metabolism. FASEB J 2010; 24:4948–4959. doi: 10.1096/fj.10-164921. [DOI] [PubMed] [Google Scholar]
- 68.Le Roy T, Llopis M, Lepage P, Bruneau A, Rabot S, Bevilacqua C, et al. Intestinal microbiota determines development of non-alcoholic fatty liver disease in mice. Gut 2013; 62:1787–1794. doi: 10.1136/gutjnl-2012-303816. [DOI] [PubMed] [Google Scholar]
- 69.Yoshimoto S, Loo TM, Atarashi K, Kanda H, Sato S, Oyadomari S, et al. Obesity-induced gut microbial metabolite promotes liver cancer through senescence secretome. Nature 2013; 499:97–101. doi: 10.1038/nature12347. [DOI] [PubMed] [Google Scholar]
- 70.Dapito DH, Mencin A, Gwak GY, Pradere JP, Jang MK, Mederacke I, et al. Promotion of hepatocellular carcinoma by the intestinal microbiota and TLR4. Cancer Cell 2012; 21:504–516. doi: 10.1016/j.ccr.2012.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Zeng H, Liu J, Jackson MI, Zhao FQ, Yan L, Combs GF, Jr. Fatty liver accompanies an increase in lactobacillus species in the hind gut of C57BL/6 mice fed a high-fat diet. J Nutr 2013; 143:627–631. doi: 10.3945/jn.112.172460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Overi D, Carpino G, Franchitto A, Onori P, Gaudio E. Hepatocyte injury and hepatic stem cell niche in the progression of non-alcoholic steatohepatitis. Cells 2020; 9:590.doi: 10.3390/cells9030590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Carpino G, Renzi A, Onori P, Gaudio E. Role of hepatic progenitor cells in nonalcoholic fatty liver disease development: cellular cross-talks and molecular networks. Int J Mol Sci 2013; 14:20112–20130. doi: 10.3390/ijms141020112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Sato K, Marzioni M, Meng F, Francis H, Glaser S, Alpini G. Ductular reaction in liver diseases: pathological mechanisms and translational significances. Hepatology 2019; 69:420–430. doi: 10.1002/hep.30150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Williams MJ, Clouston AD, Forbes SJ. Links between hepatic fibrosis, ductular reaction, and progenitor cell expansion. Gastroenterology 2014; 146:349–356. doi: 10.1053/j.gastro.2013.11.034. [DOI] [PubMed] [Google Scholar]
- 76.Carpino G, Del Ben M, Pastori D, Carnevale R, Baratta F, Overi D, et al. Increased liver localization of lipopolysaccharides in human and experimental NAFLD. Hepatology 2020; 72:470–485. doi: 10.1002/hep.31056. [DOI] [PubMed] [Google Scholar]
- 77.Giraudi PJ, Stephenson AM, Tiribelli C, Rosso N. Novel high-throughput applications for NAFLD diagnostics and biomarker discovery. Hepatoma Res 2021; 7:2.doi: 10.20517/2394-5079.2020.92. [Google Scholar]
- 78.Wood GC, Chu X, Argyropoulos G, Benotti P, Rolston D, Mirshahi T, et al. A multi-component classifier for nonalcoholic fatty liver disease (NAFLD) based on genomic, proteomic, and phenomic data domains. Sci Rep 2017; 7:43238.doi: 10.1038/srep43238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Lopez-Riera M, Conde I, Quintas G, Pedrola L, Zaragoza A, Perez-Rojas J, et al. Non-invasive prediction of NAFLD severity: a comprehensive, independent validation of previously postulated serum microRNA biomarkers. Sci Rep 2018; 8:10606.doi: 10.1038/s41598-018-28854-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Niu L, Geyer PE, Albrechtsen NJW, Gluud LL, Santos A, Doll S, et al. Plasma proteome profiling discovers novel proteins associated with non-alcoholic fatty liver disease. Mol Syst Biol 2019; 15:e8793.doi: 10.15252/msb.20188793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Reimer KC, Wree A, Roderburg C, Tacke F. New drugs for NAFLD: lessons from basic models to the clinic. Hepatol Int 2020; 14:8–23. doi: 10.1007/s12072-019-10001-4. [DOI] [PubMed] [Google Scholar]
- 82.Rau M, Geier A. An update on drug development for the treatment of nonalcoholic fatty liver disease - from ongoing clinical trials to future therapy. Expert Rev Clin Pharmacol 2021; 14:333–340. doi: 10.1080/17512433.2021.1884068. [DOI] [PubMed] [Google Scholar]
- 83.Kremoser C. FXR agonists for NASH: how are they different and what difference do they make? J Hepatol 2021; 75:12–15. doi: 10.1016/j.jhep.2021.03.020. [DOI] [PubMed] [Google Scholar]
- 84.Vuppalanchi R, Noureddin M, Alkhouri N, Sanyal AJ. Therapeutic pipeline in nonalcoholic steatohepatitis. Nat Rev Gastroenterol Hepatol 2021; 18:373–392. doi: 10.1038/s41575-020-00408-y. [DOI] [PubMed] [Google Scholar]