Dear editor
We read with interest the report in this journal by Zuo et al. regarding the effectiveness of bamlanivimab in patients with COVID-19.1 On April 16, 2021, the emergency use authorization for bamlanivimab monotherapy was rescinded by the FDA due to the evolution of SARS-CoV-2 variants. Biologic medications have captured attention as powerful therapeutic options that are engineered from human-synthesized proteins and target specific steps along immune system pathways.
Canakinumab is a human monoclonal antibody that was developed for use in auto-inflammatory syndromes and targets IL-1β, an inflammatory cytokine interleukin that is well-known to be elevated in patients with COVID-19 and plays a crucial role in the initiation of cytokine storm.2 , 3 The cytokine storms mediated by overproduction of proinflammatory cytokines have been observed in patients with COVID‐19, which is associated with the mortality and severity of COVID-19. IL-1β is thus a potential therapeutic target that can be inhibited by canakinumab to control cytokine storms. Through this mechanism, use of canakinumab may have prognostic benefits regarding patient outcomes with COVID-19 infection and serve as an additional treatment modality. Thus, we aim to perform a meta-analysis in the literature to evaluate the relationship between canakinumab administration and patient outcomes following COVID-19 infection.
An electronic search was performed using the electronic platforms (PubMed, Embase, and Cochrane Library databases) from December 1 2019 to February 21th, 2022. No language or publication restrictions were applied. The following subject heading search terms and key words were searched: (“SARS-CoV-2″ or “COVID-19″ or “2019-nCoV” or “novel coronavirus” or “coronavirus disease 2019″) AND (“canakinumab” or “interleukin 1β antibody” or “ACZ885”).
The inclusion criteria for this meta-analysis were as follows: (1) patients with confirmed COVID-19; (2) comparison was reported for clinical outcomes between canakinumab treatment (administered alone) and various control groups (placebo, standard care). Studies were excluded if they were (1) conference abstracts, case reports, editorials, non-clinical studies, and reviews; and (2) duplicated publications. We also extracted baseline information of first author's name, year of publication, study design, country of origin, number of participants, age, gender, dose of canakinumab used, outcomes (mortality, disease severity and change in anti-inflammatory factors).
Meta-analysis was conducted using Review Manager 5.2 (Cochrane Collaboration,
Oxford). We analyzed dichotomous data as a odds ratio (OR) with 95% confidence intervals (CIs) and continuous data as a standardized mean difference (SMD) with 95% CI. Heterogeneity was assessed using Cochran's Q test and the I2 statistic. We performed sensitivity analyses by sequentially omitting one study each time to assess the stability of the results. A p-value below 0.05 is considered to be statistically significant. “PROSPERO (International Prospective Register of Systematic Reviews) database” registration was done with study number as CRD42022314781.
After literature search, a total of 6 studies4, 5, 6, 7, 8, 9 comprising of 1121 adult patients with COVID-19, including 379 in the canakinumab (administered alone) and 742 in the control group arm, were included in this meta-analysis. The study characteristics of the included studies are listed in Table 1 . Four studies were from Italy. Two studies were RCTs, three studies were retrospective cohort and one studies was prospective case-control. All studies included mild to severe COVID-19 hospitalized patients. Canakinumab was intravenously or subcutaneously administered in the included studies. The eligible studies were published between 2020 and 2021 with different sample patient sizes that ranged from 20 to 520 patients with COVID-19.
Table 1.
Characteristics of included studies.
| Study | Country | Study design | Sample size | Canakinumab | Control | Usage of canakinumab | Patients included | ||
|---|---|---|---|---|---|---|---|---|---|
| Agea | Male (%) | Agea | Male (%) | ||||||
| Caricchio4 2021 | Europe and America | RCT | 454 | 59 (49–69) | 135 (59%) | 57 (50–68) | 132 (58%) | Canakinumab 450 mg for body weight of 40-<60 kg, 600 mg for 60–80 kg, and 750 mg for>80 kg, intravenous | Patients hospitalized with severe COVID-19 without invasive mechanical ventilation |
| Cremer5 2021 | America | RCT | 45 | NR | 20 (68.96%) | 68.2 (56.1, 83.3) | 13 (81.3%) | Canakinumab 300 mg (n = 14), Canakinumab 600 mg (n = 15), intravenous | Hospitalized patients |
| Generali6 2021 | Italy | Prospective case-control study | 48 | 70 (29–89) | 25 (76%) | 69 (44–85) | 13 (87%) | canakinumab (150 mg) was administered by subcutaneous injection on day 1 and on day 7 | Hospitalized patients |
| Katia7 2021 | Italy | Retrospective cohort | 34 | 53 (48, 62) | 15 (88.2%) | 59 (50, 72) | 13 (76.5%) | A subcutaneous single dose of canakinumab 300 mg | Hospitalized mild or |
| severe non ICU patients | |||||||||
| Mastroianni8 2021 | Italy | Retrospective cohort | 20 | 56 (46–82) | 4 (50%) | NR | NR | 150 mg BID for a body weight of 60–80 kg (or 2 mg/kg for participants weighing ≤40 kg), subcutaneous | Hospitalized patients |
| Potalivo9 2020 | Italy | Retrospective cohort | 520 | NR | NR | NR | NR | NR | Hospitalized patients |
Age data presented as median (IQR) or mean (SD); ICU: intensive care units; RCT: randomized controlled trial; NR: not reported.
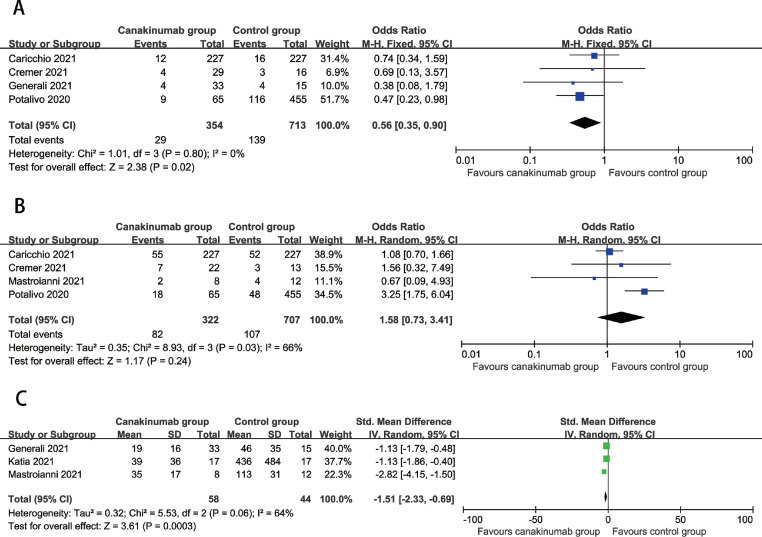
The meta-analysis showed the overall mortality was lower in the canakinumab group compared to control group (OR=0.56, 95%CI: 0.35, 0.90, P = 0.02; I2=0%) (Fig 1 A). Moreover, canakinumab treatment were not associated with developing severe COVID-19 disease (OR=1.58, 95%CI: 0.73 to 3.41, P = 0.24; I2=66%) (Fig.1B). Compared with control group, CRP levels were significantly decreased in the canakinumab group (SMD=−1.51, 95%CI: −2.33 to −0.96, P = 0.0003; I2=64%) (Fig.1C). In addition, sensitivity analyses by excluding each study at a time did not materially change the overall results, indicating that our results were statistically stable.
Fig. 1.
. A Association between canakinumab treatment and mortality, Fig. 1B. Association between canakinumab treatment and developing severe COVID-19, Fig. 1C Association between canakinumab treatment and CRP levels.
In this study, we find that treatment with canakinumab is associated with improvements in overall mortality as well as decreased serum CRP levels, suggesting lower levels of acute inflammation.
The association between treatment with canakinumab and decreased mortality and serum CRP concentration is likely mediated through the mechanism of action of the monoclonal antibody. By inhibiting IL-1β, a key inflammatory response mediator in the cytokine storm triggered by infection by COVID-19, there is a decreased likelihood of systemic hyperinflammation, a well-known predictor of all-cause mortality.10 , 11 C-reactive protein (CRP) is an inflammatory biomarker that serves many functions during episodes of acute inflammation, including promoting the secretion of pro-inflammatory cytokines, enhancing leukocyte function and activating the complement cascade.12 Higher serum concentrations of acute phase reactants indicate more severe inflammatory episodes, allowing for CRP to be used as a marker of inflammation in COVID-19 infection and extrapolated to determine potency and response to canakinumab. Furthermore, by limiting the level of acute inflammation and propensity for the activation of a cytokine storm, canakinumab is thus potentially able to mitigate and even prevent immune-mediated tissue damage and organ dysfunction, both factors which improve overall mortality.13 , 14 These restrictions of inflammatory activity are supported by the negative association of canakinumab and serum CRP levels in COVID-19 patients, an outcome that is well documented for other indications of canakinumab as well.15 , 16 Altogether, canakinumab serves as a powerful anti-inflammatory therapeutic option that is able to specifically target and limit inflammatory mechanisms.
There are several limitations that should be noted with our study. There was a relatively small sample size for use in the meta-analysis with 6 included articles. There were other inflammatory factors investigated in the included studies, however, the sample size was too small for a meta-analysis to be conducted. However, despite these limitations, our study is the first meta-analysis to explore the association between treatment with canakinumab and patient outcomes following COVID-19 infection.
Additional research is needed to further probe this association and provide a more diverse and sufficiently large sample size to provide a better understanding of what circumstances provide optimal clinical utility.
In conclusion, treatment with canakinumab in patients with COVID-19 infection is associated with a mortality benefit and lower levels of acute inflammation. Additional studies are required to confirm these findings.
Declaration of Competing Interest
The authors declare that they have no competing interest
Acknowledgments
Funding Information
None declared.
Acknowledgments
None
References
- 1.Zuo L., Ao G., Wang Y., Gao M., Qi X. Bamlanivimab improves hospitalization and mortality rates in patients with COVID-19: a systematic review and meta-analysis. J Infect. 2022;84(2):248–288. doi: 10.1016/j.jinf.2021.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dhimolea E. Canakinumab. mAbs. 2010;2(1):3–13. doi: 10.4161/mabs.2.1.10328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lucas C., Wong P., Klein J., et al. Longitudinal analyses reveal immunological misfiring in severe COVID-19. Nature. 2020;584(7821):463–469. doi: 10.1038/s41586-020-2588-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Caricchio R., Abbate A., Gordeev I., et al. Effect of canakinumab vs placebo on survival without invasive mechanical ventilation in patients hospitalized with severe COVID-19: a randomized clinical trial. JAMA. 2021;326(3):230–239. doi: 10.1001/jama.2021.9508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cremer P.C., Sheng C.C., Sahoo D., et al. Double-blind randomized proof-of-concept trial of canakinumab in patients with COVID-19 associated cardiac injury and heightened inflammation. Eur Heart J Open. 2021;1(1) doi: 10.1093/ehjopen/oeab002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Generali D., Bosio G., Malberti F., et al. Canakinumab as treatment for COVID-19-related pneumonia: a prospective case-control study. Int J Infect Dis. 2021;104:433–440. doi: 10.1016/j.ijid.2020.12.073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Katia F., Myriam D.P., Ucciferri C., et al. Efficacy of canakinumab in mild or severe COVID-19 pneumonia. Immun Inflamm Dis. 2021;9(2):399–405. doi: 10.1002/iid3.400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mastroianni A., Greco S., Chidichimo L., et al. Early use of canakinumab to prevent mechanical ventilation in select COVID-19 patients: a retrospective, observational analysis. Int J Immunopathol Pharmacol. 2021;35 doi: 10.1177/20587384211059675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Potalivo A., Montomoli J., Facondini F., et al. Sixty-day mortality among 520 Italian hospitalized COVID-19 patients according to the adopted ventilatory strategy in the context of an integrated multidisciplinary clinical organization: a population-based cohort study. Clin Epidemiol. 2020;12:1421–1431. doi: 10.2147/CLEP.S278709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Proctor M.J., McMillan D.C., Horgan P.G., Fletcher C.D., Talwar D., Morrison D.S. Systemic inflammation predicts all-cause mortality: a glasgow inflammation outcome study. PLoS ONE. 2015;10(3) doi: 10.1371/journal.pone.0116206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Davidson M., Menon S., Chaimani A., et al. Interleukin-1 blocking agents for treating COVID-19. Cochrane Database Syst Rev. 2022;1(1) doi: 10.1002/14651858.CD015308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Del Giudice M., Gangestad S.W. Rethinking IL-6 and CRP: why they are more than inflammatory biomarkers, and why it matters. Brain Behav Immun. 2018;70:61–75. doi: 10.1016/j.bbi.2018.02.013. [DOI] [PubMed] [Google Scholar]
- 13.Koritala T., Pattan V., Tirupathi R., et al. Infection risk with the use of interleukin inhibitors in hospitalized patients with COVID-19: a narrative review. Infez Med. 2021;29(4):495–503. doi: 10.53854/liim-2904-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sheng C.C., Sahoo D., Dugar S., et al. Canakinumab to reduce deterioration of cardiac and respiratory function in SARS-CoV-2 associated myocardial injury with heightened inflammation (canakinumab in Covid-19 cardiac injury: the three C study) Clin Cardiol. 2020;43(10):1055–1063. doi: 10.1002/clc.23451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Church L.D., McDermott M.F. Canakinumab, a fully-human mAb against IL-1beta for the potential treatment of inflammatory disorders. Curr Opin Mol Ther. 2009;11(1):81–89. [PubMed] [Google Scholar]
- 16.Capodanno D., Angiolillo D.J. Canakinumab for secondary prevention of atherosclerotic disease. Expert Opin Biol Ther. 2018;18(2):215–220. doi: 10.1080/14712598.2018.1420776. [DOI] [PubMed] [Google Scholar]