To the editor:
Studies analyzing humoral immunity against the most recent severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) variant of concern omicron (B.1.1.529) in the general population demonstrate a clear benefit of an additional boost with respect to neutralization capacity,1 and several national health authorities recommend the fourth vaccination dose. For immunocompromised patients, including kidney transplant and hemodialysis patients, the fourth vaccination is of special relevance.
The aim of the present study was to evaluate the immunogenicity of a fourth mRNA-based vaccination (BNT162b2; Pfizer–BioNTech) in comparison to 3 vaccination doses in hemodialysis patients. We analyzed the adaptive immunity against SARS-CoV-2 wild-type (WT) and omicron variants of concern in a cohort of hemodialysis patients (HDP; n = 40) following the third vaccination dose compared with the fourth dose. Titers of binding antibodies as well as neutralizing antibodies against WT and omicron were estimated by enzyme-linked immunosorbent assay and SARS-CoV-2 spike-protein (S-protein) pseudovirus assays, respectively. T-cell immunity reactive against WT- and omicron-derived S-protein was analyzed by multiparameter flow cytometry. The analyses were performed 4 to 6 weeks following the third doses in all patients (HDP3x; n = 40). Patients who received an additional dose after 6 to 8 weeks (HDP4x; n = 19) were analyzed 4 to 6 weeks thereafter, and patients who received only 3 doses (n = 21) were analyzed after 12 weeks (Supplementary Figure S1).
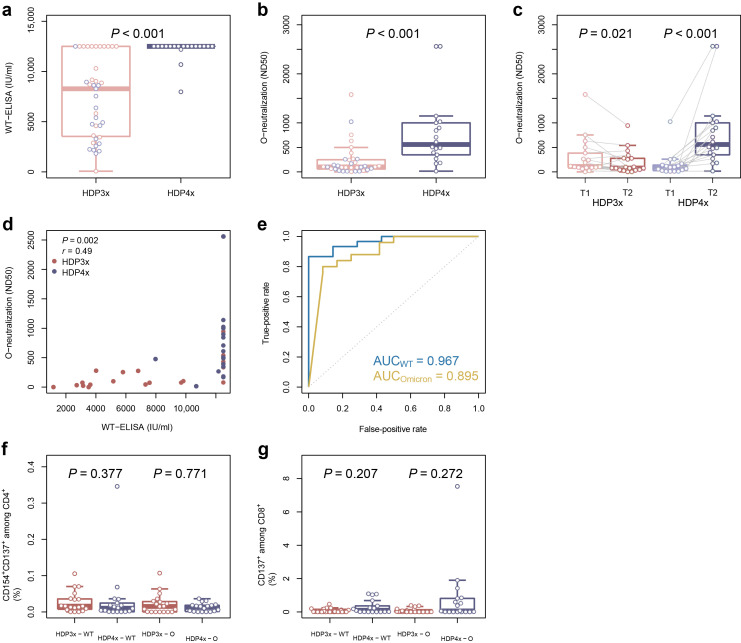
All patients demonstrated seroconversion with significantly higher titers of binding and omicron-specific neutralizing antibodies in the HDP4x group compared with HDP3x (Figure 1 a and b). As expected, the fourth vaccination led to a significant increase in titers of omicron-specific neutralizing antibodies (HDP4x, T2), whereas a significant decline in neutralizing antibody titers was observed between 6 and 12 weeks following the third vaccination (HDP3x, T2), if the fourth dose was not applied (Figure 1c and Supplementary Figure S1). Of interest, we demonstrated a significant correlation between titers of binding and omicron-specific neutralizing antibodies (Figure 1d), with receiver operating characteristic curve demonstrating a higher area under the curve for WT compared with omicron (Figure 1e). Notably, we did not detect significant differences in humoral or cellular immunity in patients who received coronavirus disease 2019 (COVID-19) vector vaccine from Johnson & Johnson or mRNA vaccine BNT162b2 as a third vaccine dose (Figure 1c, f, and g), as was previously reported.2
Figure 1.
Comparison of humoral and cellular immunity of hemodialysis patients vaccinated with 3 (HDP3x) or 4 (HDP4x) severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) vaccine doses. Isolated serum samples from hemodialysis patients, vaccinated with 3 doses (n = 40) or 4 doses (n = 19), were analyzed for (a) titers (IU/ml) of binding antibodies against SARS-CoV-2 wild-type (WT) glycoprotein S and (b) omicron-specific neutralizing antibodies (ND50). (c) Comparison of omicron-specific neutralizing antibodies (ND50) after the third doses (T1, respectively), after 12 weeks (HDP3x, T2), or after the fourth doses (HDP4x, T2). Correlation between titers of SARS-CoV-2 WT binding and omicron-specific neutralizing antibodies (d) and receiver operating characteristic curve (e) for the predictive capacity of binding antibody titers against WT glycoprotein S for strong (>100 ND50) WT-specific (blue) and omicron-specific (yellow) neutralizing response, including the value for the area under the curve (AUC). (f,g) Isolated peripheral blood mononuclear cells from hemodialysis patients, vaccinated with 3 doses (HDP3x; n = 19) or 4 doses (HDP4x; n = 18), were stimulated for 16 hours with 1 μg/ml SARS-CoV-2 overlapping peptide pool from WT (left box plots) or the mutated regions of SARS-CoV-2 omicron lineage (O; right box plots). SARS-CoV-2–reactive T helper cells were identified as life/dead-marker–CD3+CD4+CD137+CD154+ (f), and SARS-CoV-2–reactive cytotoxic T cells were identified as life/dead-marker–CD3+CD8+CD137+ (g). In all box plots, red corresponds to the patients who received only 3 doses and blue to those who received all 4 doses; light colors refer to the time point after the third dose, whereas dark colors denote the time point after the fourth dose or 12 weeks after the third. Groups were compared using 2-sided, unpaired Mann-Whitney U-test, except for (c), where Wilcoxon signed-rank paired test was employed; the correlation (d) was evaluated employing the Pearson correlation coefficient. P ≤ 0.050 was defined as significant. ELISA, enzyme-linked immunosorbent assay.
In contrast to the humoral immunity, the cellular immunity remained stable in follow-up, without magnitude increase of WT or omicron S-protein–specific T cells in the HDP4x group (Figure 1f and g).
In line with the improved seroconversion rate in transplant patients,3, 4, 5 our data demonstrate an improved omicron-specific humoral immunity following the fourth mRNA vaccination dose compared with the 3-dose vaccination regimen in hemodialysis patients. Even though adequate neutralization titers have been observed following the third dose, the significant titer decline observed in follow-up justifies the fourth dose in this vulnerable population subgroup.
Disclosure
All the authors declared no competing interests
Data Statement
The data will be available on request.
Acknowledgments
We feel deep gratitude to the patients who donated their blood samples and clinical data for this project. We would like to acknowledge the excellent assistance of dialysis staff (Jacqueline Wellenkötter and Tina Giglio) of Dialysezentrum Schwerte as well as the expertise and technical assistance of immune diagnostic laboratory (Sviatlana Kaliszczyk, Patrizia Wehler, Sarah Skrzypczyk, Eva Kohut, Julia Kurek, and Jan Zapka) of Center for Translational Medicine at Marien Hospital Herne. This work was supported by grants of Mercator Foundation, COVIDDataNet.NRW, and AIF/ZIM project EpiCov.
Footnotes
Supplementary Methods.
Table S1. Study population.
Figure S1. Schematic illustration of the course of vaccination.
Figure S2. Gating strategy to identify spike protein (S-protein) reactive T cells among CD4+ T and CD8+ T cells.
Supplementary Material
References
- 1.Garcia-Beltran W.F., St Denis K.J., Hoelzemer A., et al. mRNA-based COVID-19 vaccine boosters induce neutralizing immunity against SARS-CoV-2 Omicron variant. Cell. 2022;185:457–466.e4. doi: 10.1016/j.cell.2021.12.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Munro A.P.S., Janani L., Cornelius V., et al. Safety and immunogenicity of seven COVID-19 vaccines as a third dose (booster) following two doses of ChAdOx1 nCov-19 or BNT162b2 in the UK (COV-BOOST): a blinded, multicentre, randomised, controlled, phase 2 trial. Lancet. 2021;398:2258–2276. doi: 10.1016/S0140-6736(21)02717-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Caillard S., Thaunat O., Benotmane I. Antibody response to a fourth messenger RNA COVID-19 vaccine dose in kidney transplant recipients: a case series. Ann Intern Med. 2022;175:455–456. doi: 10.7326/L21-0598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kamar N., Abravanel F., Marion O., et al. Assessment of 4 doses of SARS-CoV-2 messenger RNA-based vaccine in recipients of a solid organ transplant. JAMA Network Open. 2021;4 doi: 10.1001/jamanetworkopen.2021.36030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Roch T., Rohn B., Blazquez-Navarro A., et al. A vector-based vaccine dose after 3 doses of mRNA-based COVID-19 vaccination does not substantially improve humoral SARS-CoV-2 immunity in renal transplant recipient. Kidney Int Rep. 2022;7:932–934. doi: 10.1016/j.ekir.2022.01.1068. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.