Abstract
In the on-going COVID-19 pandemic, pooled testing of samples by RT-PCR has been recommended at certain scenarios to increase labs’ testing capacity and reduce cost of testing. This paper describes the evaluation of bi-directional matrix pooling strategies with clinical samples in a 5 × 5 and 10 × 10 matrix. Nasopharyngeal swab samples in viral transport medium (VTM) previously tested (positive or negative) by real time RT-PCR for SARS-CoV-2 were used for these experiments. Ten sets of 5 × 5 (250 samples) and ten sets of 10 × 10 (1000 samples) pooling of samples in both directions was done with known positive samples introduced at random positions. Extracted nucleic acid was tested for SARS-CoV-2 E-gene by RT-PCR. Sensitivity or concordance and feasibility of matrix pooling were assessed in comparison to direct RT-PCR testing. In comparison to direct testing, the overall concordance was 86.6% for 5 × 5 pooling, 73.3% for 10 × 10 with 200 µL extraction volume and 86.6% for 10 × 10 with 400 µL extraction volume. Bi-directional matrix pooling can be adopted with advantage over conventional direct or pool testing for COVID-19 by RT-PCR under the following conditions: i) sample positivity rate of ≤ 5%, ii) matrix pool size of 8–10 samples, iii) use of min. 40 µL VTM from each sample and iv) utilization of automated liquid handling equipment, if available, for sample addition to avoid human errors.
Keywords: COVID-19, SARS-CoV-2, RT-PCR, Diagnosis, Screening, Pooling
1. Introduction
The current pandemic of coronavirus disease 2019 (COVID-19) is caused by the severe acute respiratory syndrome coronavirus (SARS-CoV-2) represents a significant challenge for treating physicians and other public health authorities. In order to combat the spread and ultimately contain the virus, it is important to quickly identify and isolate infected person(s) and their contacts (Koo et al., 2020). The World Health Organization (WHO) has recommended reliable diagnostic tests to distinguish SARS-CoV-2 from other respiratory infections and to help with appropriate care and treatment. The use of RT-PCR based assays to confirm the diagnosis of an infected person is crucial to control the spread of the virus, because despite the high viral load, the infection may be asymptomatic.
Across the world, efforts are being made to improve the capacity of testing, but at times the testing facilities are overwhelmed by sudden increase in load of samples, especially at the middle of pandemic waves. Furthermore, due to the high demand for testing worldwide, there was a shortage of labour and consumables, particularly RNA extraction kits, which added to the testing delay and inefficiency (Praharaj et al., 2020). Thus, it's become increasingly important to develop new methods for rational and effective use of available resources. At this point of time, many countries are undergoing a third wave of the pandemic and some countries, like India, are expecting the possibility of third wave. Apart from clinical infections, routine mass screening for COVID-19 may be required at airports, quarantine camps, healthcare settings, events, scholar acceptances, etc. and it is expected to continue in the future course of the COVID-19 pandemic.
In such cases, sample pooling can be a critical method for testing in large groups of people, where samples from a diverse population group are merged into a single tube for pooled polymerase chain reaction (PCR) analysis (Van et al., 2012). This technique has been demonstrated to be more cost-effective during bulk testing than individual testing, which is very important. Pooled testing of respiratory samples has the potential to significantly increase testing capacity due to the high sensitivity of real-time RT-PCR-based tests and the low prevalence of COVID-19 infection in areas or regions at a given point of time. Different pooling sizes for testing biological samples have been proposed by mathematical models and epidemiological forecasts as being practical and effective for meeting testing demands (Dorfman, 1943, Deckert et al., 2020, Deka and Kalita, 2020, Hanel and Thurner, 2020, Mutesa et al., 2021, Prakash et al., 2021).
An interesting mathematical model has been published by Fargion et al. (2020) which involves pooling and testing of samples in a bi-directional matrix (e.g. 9 ×9), claimed to have the advantage of time and cost compared to conventional pooling strategies. However, data on testing the matrix pooling strategy with clinical samples is not available. Hence, the present study was carried out to evaluate the performance, sensitivity and feasibility of bi- directional matrix pooling for COVID-19 testing by RT-PCR using known positive and negative clinical samples.
2. Materials and methods
2.1. Sample processing and nucleic acid extraction
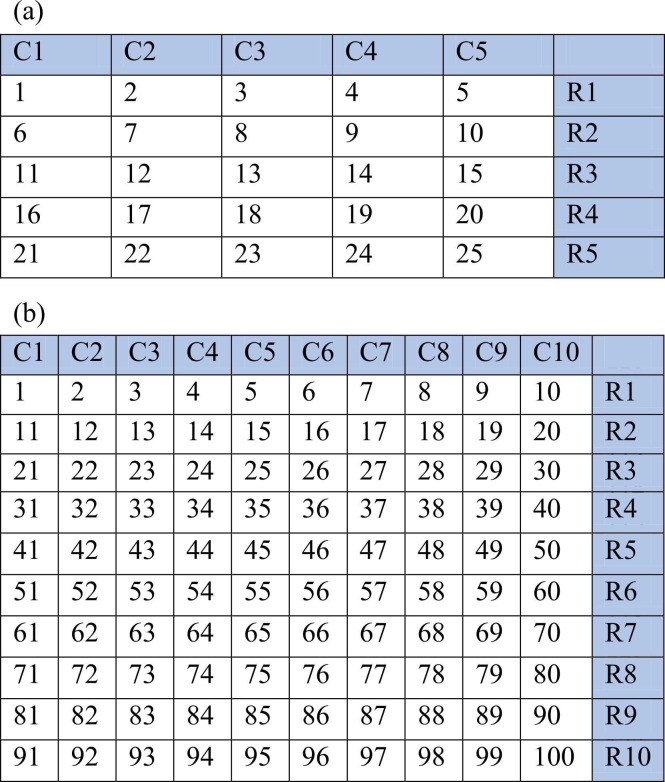
Nasopharyngeal swab samples in viral transport medium (VTM) previously tested (positive and negative) by real time RT-PCR for SARS-CoV-2, and archived at − 80 °C in our COVID-19 testing laboratory within the last one month of study initiation, were used for these experiments. The person pooling the samples was blinded for the patient details and previous test outcome, i.e., positive or negative. Ten sets of 5 × 5 (250 samples) and 10 × 10 (1000 samples) pooling of samples in both directions was done ( Fig. 1). For 5 × 5 pooling, 40 µL of sample from each VTM (5 ×40 µL = 200 µL) was added directly to the extraction lysis buffer. For 10 × 10 pooling, 20 µL of sample from each VTM (10 ×20 µL = 200 µL) was added directly to the extraction lysis buffer; similarly, 40 µL sample volume per VTM (10 ×40 µL = 400 µL) also used for the 10-sample matrix pooling experiment to assess any gain in sensitivity when sample volume is doubled. In this matrix testing, each sample is included in two different pools. Each 5 × 5 and 10 × 10 pool had one to three positives introduced at random positions, and remaining were negative samples. The Ct values of introduced positive samples ranged between 20 and 35. Total nucleic acid extraction was done using the MGIEasy Nucleic Acid Extraction Kit (Shenzhen, China) as per manufacturer instructions. Nucleic acid elution volume was kept constant at 60 µL for all 5 × 5, 10 × 10 (20 µL sample) and 10 × 10 (40 µL sample) pools. The introduced-positive samples were also extracted individually using the same procedure.
Fig. 1.
(a) 5×5 sample pool: A total of 25 samples were included in 5 × 5 matrix pool. Forty µL from each sample was added in two pools i.e row and column. e.g. 40 µL VTM from sample no. 1 was added to pool no. C1 and R1; Sample no.6 was added in pool no. C1 and R2, and so on. One to three positives were introduced at random positions in an operator- blinded manner; (b) 10 × 10 sample pool: A total of 100 samples were included in 10 × 10 pool. Twenty or Forty µL from each VTM was added in two pools. e.g. 40 µL VTM from Sample no. 1 was added in pool no. C1 and R1; Sample no.11 was added in pool no. C1 and R2, and so on. 1–3 positives were introduced at random positions in an operator-blinded manner [C- column, R-Row, numerical- sample nos.].
2.2. Real-time PCR for SARS-CoV-2 E-gene
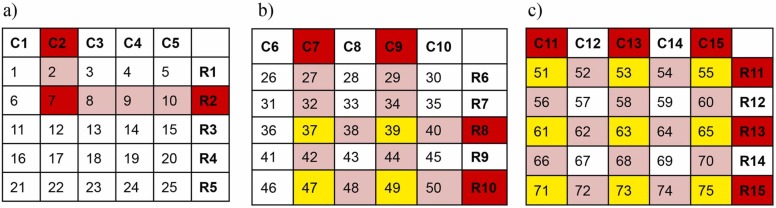
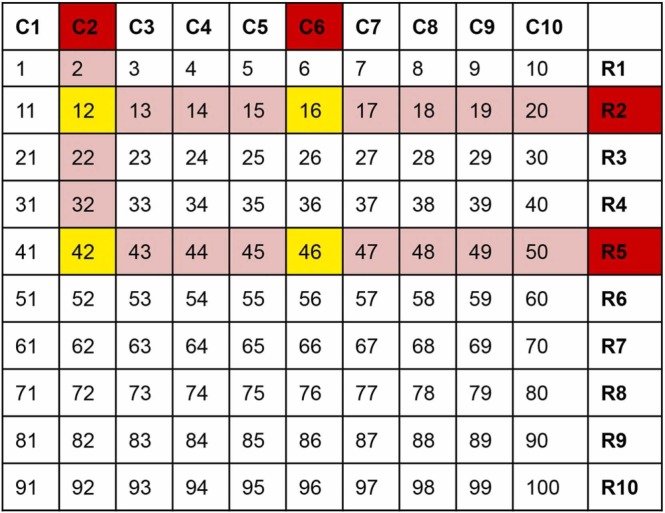
Single step real-time PCR for SARS-CoV-2 targeting the E-gene and RdRp gene (nCoV Real-Time-Detection kit, SD biosensor, Republic of Korea) was performed on the extracted nucleic acid from pooled and individual samples. Ct value of E-gene was considered for identifying positive samples, and for analysis and comparison of results. Any row or column pool with noticeable amplification with Ct values up to 40 including ambiguous amplification curves were considered positive (highlighted in red in Fig. 2). Identification of specific positive samples in a pool was done as described previously (Fargion et al., 2020). Briefly, the intersects of positive row and column pools are identified as shown in Fig. 2. Both true positive samples and some negative samples are expected to be chosen as intersect (s). Single clear intersect directly identifies the positive sample; more than one intersect between a positive column and a row requires additional direct sample testing of all intersects to identify the true positive sample, as shown below. De-pooling or additional direct testing sample numbers can be determined by multiplying the number of positive column(s) and row(s) in a matrix i.e., a matrix with 2 column pool and 2 row pool flagging will require 2×2 = 4 sample individual testing ( Fig. 2, Fig. 3).
Fig. 2.
Identification of intersects of positive pools in 5-sample matrix. (a) 1 × 1 = 1 intersect (red) directly identified as positive; (b) 2 × 2 = 4 intersects (yellow) to be tested to identify true positives; (c) 3 × 3 = 9 intersects (yellow) to be tested to identify true positives.
Fig. 3.
Identification of intersects of positive pools in 10-sample matrix. 2 × 2 = 4 intersects (yellow) to be tested to identify true positives.
3. Results and discussions
The RT-PCR results of sample pools were analyzed to identify whether both row and/or column pools flagged positive corresponding to the positive samples introduced at random positions and also to identify any false positive pools which did not have known positive samples. The results for 5 × 5 and 10 × 10 pools are summarized in Table 1. In 5 × 5 matrix testing, 86.6% of the positive sample introduced-pools have flagged in bi-directional manner; in 10 × 10 matrix with 200 µL and 400 µL pooled sample testing, 73.3% and 86.6% of the positive sample introduced-pools have flagged in bi-directional manner, respectively.
Table 1.
RT-PCR results of matrix pool testing.
| Matrix | 5 × 5 (200 µL) | 10 × 10 (200 µL) | 10 × 10 (400 µL) |
|---|---|---|---|
| Bi-directional positive pools detected / expected positive pools | 26/30 | 22/30 | 26/30 |
| Concordance/sensitivity | 86.6% | 73.3% | 86.6% |
| False-positive pools | 2 | 0 | 3 |
The pool testing concordance with respect to the Ct value ranges of known positive samples is detailed in Table 2. The concordance was 100% for Ct value range of ≤ 25 cycles across all three models i.e. 5 × 5, 10 × 10 (200 µL), 10 × 10 (400 µL). For Ct range 26–30, concordance was 93.3%, 86.6% and 100% for 5 × 5, 10 × 10 (200 µL) and 1 × 1 (400 µL), respectively. For Ct range 30–35, concordance was 75.0%, 50.0% and 66.6% for 5 × 5, 10 × 10 (200 µL) and 10 × 10 (400 µL), respectively.
Table 2.
Concordance in pool testing with respect to individual sample Ct values.
| Ct value range for Individual positive sample | Number of introduced positive samples | Concordance between matrix pool testing and individual testing (%) |
||
|---|---|---|---|---|
| 5-sample matrix |
10-sample matrix |
|||
| 200 µL | 200 µL | 400 µL | ||
| ≤ 25 cycles | 3 | 3/3 (100%) | 3/3 (100%) | 3/3 (100%) |
| 26–30 cycles | 15 | 14/15 (93.3%) | 13/15 (86.6%) | 15/15 (100%) |
| 31–35 cycles | 12 | 9/12 (75.0%) | 6/12 (50.0%) | 8/12 (66.6%) |
| Total | 30 | 26/30 (86.6%) | 22/30 (73.3%) | 26/30 (86.6%) |
The average Ct values were compared for direct and matrix testing and detailed in Table 3. On an average the Ct values obtained for 5 × 5 matrix pooling exceeded individual sample testing by more than 2.6 ± 0.67 cycles; Whereas for 10 × 10 matrix pooling the Ct values exceeded more than 4.63 ± 1.8 for 200 µL and 4.3 ± 1.47 for 400 µL extraction.
Table 3.
Ct value comparison for direct and matrix pool testing.
| Ct value range for individual positive sample | Ct (Mean±SD) |
|||
|---|---|---|---|---|
| Direct |
5 × 5 |
10 × 10 |
||
| 200 µL | 200 µL | 400 µL | ||
| ≤ 25 | 23.3 ± 0.9 | 28.8 ± 1.8 | 30.4 ± 0.9 | 30.2 ± 1.3 |
| 26–30 | 27.8 ± 1.7 | 29.4 ± 3.0 | 32.6 ± 2.3 | 32.2 ± 2.6 |
| 31–35 | 31.2 ± 0.8 | 31.9 ± 3.4 | 33.2 ± 1.6 | 32.8 ± 1.9 |
| Overall | 27.43 ± 3.4 | 30.03 ± 2.73 | 32.06 ± 1.6 | 31.73 + 1.93 |
Pooled sample testing has been regarded as a straightforward and practical method for increasing testing output while reducing the amount of resources used in order to perform RT-PCR in real-time (Lohse et al., 2020). The gold standard test for the detection of viral genome with good sensitivity and specificity from nasopharyngeal swabs is considered to be RT-PCR (Shen et al., 2020). The speed of diagnosis during the epidemic can be greatly enhanced by the strategy of pooling (Abel et al., 1999). Pooling can be performed using different strategies which have been described in various studies (Lohse S et al., 2020; Yelin et al., 2020). It can be done by pooling of nasopharyngeal swabs in a universal container or pooling of VTM or pooling of extracted RNA. In our study, before RNA extraction, samples in VTM were pooled, which helped in saving reagents both for extraction and PCR. For reagent use and time required, the RNA extraction step remains one of the most rate limiting stages for SARS-CoV-2 RT-PCR (Grant et al., 2020). At this point in time, number of studies have been published on conventional pooling strategies for COVID-19 RT-PCR testing (Praharaj et al., 2020, Barak et al., 2021, Prakash et al., 2021, Sawicki et al., 2021, Shukla et al., 2021 Jul).
In the current study on analyzing 5 and 10 sample pooling in bi-directional matrix method, 5 sample pool matrix had a similar concordance rate (86.6%) with 10 sample pooling when 400 µL of pooled sample was used instead of 200 µL which had concordance of only 73.3%. In a multicentric study (n = 10) which also included our laboratory, direct 5-sample and 10-sample pooling strategies for COVID-19 RT-PCR testing were evaluated (Praharaj et al., 2020). When compared to direct testing, concordance for 5-sample pooling ranged between 70% and 100% among different laboratories (overall 88%) and for 10-sample pooling concordance ranged between 50% and 90% (overall 66%). The results of the present study also have similar concordance range with matrix testing. Doubling the sample volume i.e., 40 µL instead of 20 µL from each sample in 10-sample matrix pooling has increased the overall detection by 13.3%, especially due to detection of samples with low viral loads. Dilution effect in 5 or 10 sample direct pooling and matrix pooling strategy is expected to be similar, but the advantage of testing fewer samples during de-pooling and direct identification of some positive samples prevails in the later.
On an average (row and column), Ct values obtained with the 5-sample pooled testing exceeded individual sample testing by 2.6 ± 0.67 cycles, while Ct values obtained with the 10- sample pooling exceeded individual sample testing by 4.63 ± 1.8 (200 µL extraction) and 4.3 ± 1.47 (400 µL extraction) cycles in this study. Praharaj et al. (2020) recorded Ct value differences of 2.18 ± 1.86 and 3.81 ± 2.26 cycles for 5-sample pool and 10-sample pool, respectively. In a real-time PCR assay with 100 per cent efficiency, Ct difference of approximately 3.3 cycles is expected between neat sample and 10-fold dilution. However different assay variables including PCR efficiency, and more importantly the total RNA content and/or inhibitors from co-extracted samples can greatly affect the overall Ct of a pool with a positive sample. In the present study, for a given positive sample the Ct value in row and column pool had considerable variation suggesting the effect of co-extracted samples. Similar observations in varying Ct differences have been reported from different laboratories when pooling is attempted from VTM samples (Praharaj et al., 2020, Prakash et al., 2021, Shukla et al., 2021 Jul, Volpato et al., 2021 Apr).
Comparison of conventional pool testing and bi-directional pool testing with respect to number of samples that would require individual testing in second round after initial pool testing at different positivity rate is detailed in Table 4.
Table 4.
Maximum no. of samples that would require de-pooling per 100 sample in different pooling strategies.
| Sample positivity rate | Maximum no. of samples that would require de-pooling |
|||
|---|---|---|---|---|
| 5 sample pool | 5 × 5 Matrix | 10 sample pool | 10 × 10 Matrix | |
| 1% | 5 | 1 | 10 | 1 |
| 2% | 10 | 4 | 20 | 4 |
| 3% | 15 | 9 | 30 | 9 |
| 4% | 20 | 16 | 40 | 16 |
| 5% | 25 | 25 | 50 | 25 |
It is evident that for 5-sample pooling, the advantage of matrix testing over direct pooling exists theoretically till sample positivity rate of 4%. However, the number of samples that will need to be de-pooled in both conventional and matrix pool testing will vary depending on the distribution of positive samples across the samples to be tested. The 10 × 10 matrix testing has a clear advantage over conventional 10-sample pool testing, and based on the current study findings, the sensitivity of the 10 × 10 matrix can be increased by doubling the sample (extraction volume). The matrix testing can be done with 5–10 samples in a bi- directional manner as per the maximum pooling number decided by the testing laboratory. However, the maximum sample size in a pool without compromising sensitivity should be worked out in each laboratory and adopted for matrix testing in order to be effective. The disadvantages of matrix testing are the initial additional time consumed for sample addition, i.e., each sample has to be added to two different wells or tubes (row and column), human error during sample addition and additional workload on technicians during sample addition and de-pooling. In the current study, five pools which did not have any introduced positive sample had flagged in single direction (either row or column) but did not yield any positive sample on de-pooling. The occurrence of false-positive pools might be due to the co-extracted sample’s carrier RNA effect on any low viral load sample which was negative on direct testing or due to the human error occurred during sample addition. Barak et al. (2021) have used and highlighted that automation of both sample handling, processing, and result reporting by use of automated liquid handlers and software is crucial for delivering test results quickly and minimizing laboratory errors during pool testing.
4. Conclusions
The matrix pooling strategy would considerably increase the laboratories' test capacity and reduce the testing cost per sample. When the prevalence or sample positivity rate of SARS- CoV-2 is low, using matrix pooling strategy to test clinical samples will be far more effective, as many of the positive samples in a pool can be identified directly, or testing fewer samples in de-pooling. It should also be noted that pooling sizes will vary depending on the populations and groups of people being tested, as well as the positivity rates. Based on our laboratory experience in conventional pool testing and the current study findings, the authors are of the opinion that matrix pooling can be adopted with advantage over conventional direct or pool testing for COVID-19 RT-PCR testing under the following conditions: i) sample positivity rate of ≤ 5%, ii) matrix pool size of 8–10 samples, iii) use of min. 40 µL VTM from each sample for RNA extraction and iv) utilization of automated liquid handling equipment, if available, for sample addition to avoid human errors.
Funding
We thank Department of Health Research (DHR), Government of India for funding this project (Grant ID: No. R. 15012/04/2019-HR-VRDL) through “Establishing network of laboratories for management of epidemics and natural calamities” & Viral Research and Diagnostic Laboratory (VRDL) network program.
CRediT authorship contribution statement
RD, RB conceived the study and planned the detailed experimental strategy. FMS, RVR, GD and GA have identified the samples and conducted the study. RB coordinated all the experiments analyzed with help from FMS, RVR, GD and GA. RB wrote the first draft of the manuscript with help from GD and GA. RD has finalized the manuscript and figures. All authors participated in the review and approval of final manuscript.
Declaration of Competing Interest
The authors declare that we have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
We would like to thank our laboratory research assistant Ms. K. Indumathy and laboratory technicians Mr. Bhuvaneshwar, Ms. Sukanya, Ms. Preethi and Ms. Ramani for their help and dedication towards identification of samples and conducting the laboratory experiments.
References
- Abel U., Schosser R., Süss J. Estimating the prevalence of infectious agents using pooled samples: biometrical considerations. Zentralblatt Bakteriol. 1999;289(5–7):550–563. doi: 10.1016/s0934-8840(99)80009-7. [DOI] [PubMed] [Google Scholar]
- Barak N., Ben-Ami R., Sido T., Perri A., Shtoyer A., Rivkin M., Licht T., Peretz A., Magenheim J., Fogel I., Livneh A. Lessons from applied large-scale pooling of 133,816 SARS-CoV-2 RT-PCR tests. Sci. Transl. Med. 2021;13(589):eabf2823. doi: 10.1126/scitranslmed.abf2823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deckert A., Bärnighausen T., Kyei N. Pooled-sample analysis strategies for COVID-19 mass testing: a simulation study. Bull. World Health Organ. 2020;98(9):590–598. doi: 10.2471/BLT.20.257188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deka S., Kalita D. Effectiveness of sample pooling strategies for SARS-CoV-2 mass screening by RT-PCR: a scoping review. J. Lab Physicians. 2020;12(03):212–218. doi: 10.1055/s-0040-1721159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorfman R. The detection of defective members of large populations. Ann. Math. Stat. 1943;14:436–440. [Google Scholar]
- Fargion B.I., Fargion D., Lucentini P.G., Habib E. Purim: a rapid method with reduced cost for massive detection of CoVid-19. arXiv. 2020 [Google Scholar]
- Grant P.R., Turner M.A., Shin G.Y., Nastouli E., Levett L.J. Extraction-free COVID-19 (SARS-CoV-2) diagnosis by RTPCR to increase capacity for national testing programmes during a pandemic. bioRxiv. 2020 doi: 10.1101/2020.04.06.028316. [DOI] [Google Scholar]
- Hanel R., Thurner S. Boosting test-efficiency by pooled testing for SARS-CoV-2— formula for optimal pool size. PLoS One. 2020;15(11) doi: 10.1371/journal.pone.0240652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koo J.R., Cook A.R., Park M., Sun Y., Sun H., Lim J.T., Tam C., Dickens B.L. Interventions to mitigate early spread of SARS-CoV-2 in Singapore: a modelling study. Lancet Infect. Dis. 2020;20(6):678–688. doi: 10.1016/S1473-3099(20)30162-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lohse S., Pfuhl T., Berkó-Göttel B., Rissland J., Geißler T., Gärtner B., Becker S.L., Schneitler S., Smola S. Pooling of samples for testing for SARS-CoV-2 in asymptomatic people. Lancet Infect. Dis. 2020;20(11):1231–1232. doi: 10.1016/S1473-3099(20)30362-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mutesa L., Ndishimye P., Butera Y., Souopgui J., Uwineza A., Rutayisire R., Ndoricimpaye E.L., Musoni E., Rujeni N., Nyatanyi T., Ntagwabira E. A pooled testing strategy for identifying SARS-CoV-2 at low prevalence. Nature. 2021;589(7841):276–280. doi: 10.1038/s41586-020-2885-5. [DOI] [PubMed] [Google Scholar]
- Praharaj I., Jain A., Singh M., Balakrishnan A., Dhodapkar R., Borkakoty B., Ashok M., Das P., Biswas D., Kalawat U., Turuk J. Pooled testing for COVID-19 diagnosis by real-time RT-PCR: A multi-site comparative evaluation of 5-& 10-sample pooling. Indian J. Med. Res. 2020;152(1–2):88–94. doi: 10.4103/ijmr.IJMR_2304_20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prakash S., Prakash O., Mishra H., Khan D.N., Shukla S., Pandey A., Rade K., Gupta N., Bhatt M.L., Jain A. Feasibility, efficiency & effectiveness of pooled sample testing strategy (pooled NAAT) for molecular testing of COVID-19. Indian J. Med. Res. 2021;153(1–2):227. doi: 10.4103/ijmr.IJMR_2333_20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawicki R., Korona-Glowniak I., Boguszewska A., Stec A., Polz-Dacewicz M. Sample pooling as a strategy for community monitoring for SARS-CoV-2. Sci. Rep. 2021;11(1):1–8. doi: 10.1038/s41598-021-82765-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen M., Zhou Y., Ye J., Al-maskri A., A.A., Kang Y., Zeng S., Cai S. Recent advances and perspectives of nucleic acid detection for coronavirus. J. Pharm. Anal. 2020;10:97–101. doi: 10.1016/j.jpha.2020.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shukla S., Upadhyay V., Maurya V.K. Evaluating the efficiency of specimen (sample) pooling for real-time PCR based diagnosis of COVID-19. Indian J. Med. Microbiol. 2021;39(3):339–342. doi: 10.1016/j.ijmmb.2021.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van T.T., Miller J., Warshauer D.M., Reisdorf E., Jernigan D., Humes R., Shult P.A. Pooling nasopharyngeal/throat swab specimens to increase testing capacity for influenza viruses by PCR. J. Clin. Microbiol. 2012;50:891–896. doi: 10.1128/JCM.05631-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volpato F., Lima-Morales D., Wink P.L., Willig J., de-Paris F., Ashton-Prolla P., Barth A.L. Pooling of samples to optimize SARS-CoV-2 diagnosis by RT-qPCR: comparative analysis of two protocols. Eur. J. Clin. Microbiol. Infect. Dis. 2021;40(4):889–892. doi: 10.1007/s10096-020-04071-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yelin I., Aharony N., Tamar E.S., Argoetti A., Messer E., Berenbaum D., Shafran E., Kuzli A., Gandali N., Shkedi O., Hashimshony T., Mandel-Gutfreund Y., Halberthal M., Geffen Y., Szwarcwort-Cohen M., Kishony R. Evaluation of COVID-19 RT-qPCR test in multi sample pools. Clin. Infect. Dis. 2020;71(16):2073–2078. doi: 10.1093/cid/ciaa531. [DOI] [PMC free article] [PubMed] [Google Scholar]