Abstract
Background and study aims Rectal nonsteroidal anti-inflammatory drug (NSAID) prophylaxis reduces incidence of post-endoscopic retrograde cholangiopancreatography (ERCP) pancreatitis. Direct comparisons to the optimal timing of administration, before or after ERCP, are lacking. Therefore, we aimed to assess whether timing of rectal NSAID prophylaxis affects the incidence of post-ERCP pancreatitis.
Patients and methods We conducted an analysis of prospectively collected data from a randomized clinical trial. We included patients with a moderate to high risk of developing post-ERCP pancreatitis, all of whom received rectal diclofenac monotherapy 100-mg prophylaxis. Administration was within 30 minutes before or after the ERCP at the discretion of the endoscopist. The primary endpoint was post-ERCP pancreatitis. Secondary endpoints included severity of pancreatitis, length of hospitalization, and Intensive Care Unit (ICU) admittance.
Results We included 346 patients who received the rectal NSAID before ERCP and 63 patients who received it after ERCP. No differences in baseline characteristics were observed. Post-ERCP pancreatitis incidence was lower in the group that received pre-procedure rectal NSAIDs (8 %), compared to post-procedure (18 %) (relative risk: 2.32; 95% confidence interval: 1.21 to 4.46, P = 0.02). Hospital stays were significantly longer with post-procedure prophylaxis (1 day; interquartile range [IQR] 1–2 days vs. 1 day; IQR 1–4 days; P = 0.02). Patients from the post-procedure group were more likely to be admitted to the ICU (1 patient [0.3 %] vs. 4 patients [6 %]; P = 0.002).
Conclusions Pre-procedure administration of rectal diclofenac is associated with a significant reduction in post-ERCP pancreatitis incidence compared to post-procedure use.
Introduction
Pancreatitis is the most common complication of endoscopic retrograde cholangiopancreatography (ERCP) with an incidence rate of 3.2 % to 15 % 1 . Post-ERCP pancreatitis progresses to severe pancreatitis in 4.7 % of cases and carries a mortality rate of up to 0.7 % 1 2 .
A 2012 landmark trial positioned prophylactic rectal nonsteroidal anti-inflammatory drugs (NSAIDs) as the cornerstone in prevention of post-ERCP pancreatitis. Since then, rectal NSAIDs have been considered the standard of care in Europe, the United States, and Japan 3 4 5 .
Prevention of post-ERCP pancreatitis potentially can be improved by exploring and combining new prophylactic strategies, as well as optimizing current care 6 . Rectal NSAIDs are one of the most effective, cheap, and easy-to-use agents for preventing post-ERCP pancreatitis 7 . Although it is clear that prophylactic administration of rectal NSAIDs is beneficial, a direct head-to-head comparison about the most optimal time point in relation to the ERCP procedure (pharmacokinetic properties) has not been performed.
The European Society of Gastrointestinal Endoscopy (ESGE) advocates the use of rectal NSAIDs immediately before ERCP. In contrast, the American Society of Gastrointestinal Endoscopy and the Japanese Society of Gastrointestinal Endoscopy do not provide recommendations regarding the timing of administration 3 4 5 . To date, 100 mg has been considered the optimal rectal NSAID dose (indomethacin or diclofenac) 8 9 10 11 . The optimal timing of rectal NSAIDs in relation to ERCP has not been addressed in most studies and meta-analyses 12 13 14 15 16 17 18 .
We performed a randomized clinical trial (RCT) in which we compared aggressive periprocedural hydration in combination with rectal NSAID, compared with rectal NSAID monotherapy, for the prevention of post-ERCP pancreatitis in patients with a moderate to high risk 19 . For this trial, we prospectively identified whether the rectal NSAID was administered before or after the ERCP procedure. In this post-hoc analysis, we aimed to determine whether the timing of rectal NSAID administration affects the incidence of post-ERCP pancreatitis.
Patients and methods
Study design and setting
For this study, we selected patients from the FLUYT trial, a RCT conducted from June 2015 to June 2019 and coordinated by the Dutch Pancreatitis Study Group 19 . In this RCT, 826 patients were enrolled in 22 large teaching hospitals and university medical centers in the Netherlands. Patients were randomly assigned (1:1) to receive either the combination of aggressive periprocedural hydration and rectal NSAID 100 mg (hydration group) or rectal NSAID 100-mg monotherapy (control group). All patients received a rectal NSAID within 30 minutes before or after the ERCP procedure. The timing of administration was not dictated by the study design but was left to the discretion of the treating clinician, as guidelines did not define on preferred timing at that moment. Because concomitant use of a pancreatic duct stent and rectal NSAID is under discussion and merits further investigation, the decision to place a pancreatic duct stent was also left to the discretion of the treating clinician in the original trial. The study was performed in accordance with the Declaration of Helsinki and the ICH Guidelines for Good Clinical Practice. The Medical Research Ethics Committees United approved the protocol (NL52341.100.15). Patient demographics, patient- and procedure-related risk factors for post-ERCP pancreatitis, and follow-up data were collected prospectively using standardized digital case record forms. The study coordinator verified the data through a patient chart review of all hospital contacts between randomization and the end of follow-up (180 days post randomization). We adhered to the Strengthening the Reporting of Observational studies in Epidemiology (STROBE) guideline 20 .
Participants
All patients were between 18 and 85 years, had an indication for ERCP, and provided written informed consent. Exclusion criteria were patients with a low risk of post-ERCP pancreatitis, for which they had to fulfill at least one of the following criteria: chronic pancreatitis (according to the MANNHEIM criteria) 21 , previous sphincterotomy, pancreatic head mass, or routine biliary stent exchange. Other exclusion criteria were: active pancreatitis prior to ERCP and contraindications to intensive hydration (e. g. cardiac/pulmonary/liver insufficiency, preexisting pitting edema, hyponatremia or hypernatremia) or rectal NSAIDs (e. g. renal insufficiency, allergy, active gastrointestinal bleeding, ulcer disease, and NSAID use for other indications [other than cardioprotective aspirin]). Because there is no international definition for classifying patients into low, moderate, or high risk of post-ERCP pancreatitis, risk stratification was estimated by adopting low-risk definitions used in the current literature 22 23 . By excluding low-risk patients, we only included moderate- to high-risk patients.
For the current study, we excluded patients who did not undergo an ERCP, because they were unable to develop the primary endpoint of post-ERCP pancreatitis. Also, we excluded patients for whom the timing of rectal NSAID administration was not available. We decided to use only patients in the control group (rectal NSAID monotherapy), because the randomization groups in the original RCT (aggressive periprocedural hydration plus rectal NSAID vs. rectal NSAID monotherapy) showed an interaction effect with the timing of rectal NSAID administration ( Table 1 ). In this way, we could avoid any potential influence of additional prophylaxis (aggressive periprocedural hydration) on the analyses.
Table 1. Interaction effect of randomization group on timing of rectal NSAIDs in participants of the FLUYT trial.
| Rectal NSAID before ERCP (n PEP/n total) | Rectal NSAID after ERCP (n PEP/n total) | Relative risk (95 % CI) | Interaction term | |
| Overall | 53/653 | 13/128 | ||
| Group | 0.017 | |||
|
27/307 | 2/65 | 0.35 (0.06–1.13) | |
|
26/346 | 11/63 | 2.32 (1.15–4.33) | |
ERCP − endoscopic retrograde cholangiopancreatography; PEP − post-ERCP pancreatitis; NSAIDs − nonsteroidal anti-inflammatory drugs; CI − confidence interval.
Outcomes and definitions
The primary outcome of this study was the proportion of patients that developed post-ERCP pancreatitis according to the Cotton criteria 24 . Briefly, these criteria included new onset of upper abdomen pain and elevation of pancreatic enzymes (amylase/lipase) of at least three times the upper limit of normal range at 24 hours after the procedure and hospitalization for at least two nights. Secondary outcomes included the severity of post-ERCP pancreatitis, defined according to Cotton and revised Atlanta criteria 24 25 , ERCP-related complications according to Cotton 24 , length of hospitalization, stay on the Intensive Care Unit, and mortality.
Statistical analysis
Because the current study is a non-randomized comparison, known prognostic factors (age, sex, body mass index [BMI], history of pancreatitis, trainee involvement, and pancreatic duct stent placement) for the primary outcome (post-ERCP pancreatitis) were tested for differences between the two groups. The variables that were deemed statistically ( P < 0.05) or with relevant differences were entered in a log-binominal regression model with post-ERCP pancreatitis as outcome and grouping variable as independent variable of main interest, thereby correcting the outcome for the potential confounders. Second, we performed predefined subgroup analyses for the same prognostic factors by entering interaction terms in the log-binominal regression analysis.
Continuous variables are presented as means with standard deviation (SDs) or medians with interquartile ranges (IQRs) and categorical variables as frequencies with percentages. Primary and secondary outcomes were assessed using the Mann-Whitney U test, Pearson X 2 test, or Fisher exact test as appropriate. The primary endpoint is presented as relative risk (RR) with corresponding 95 % confidence intervals. All analyses were performed by using R, version 3.6.2. A two-tailed P < 0.05 was regarded as statistically significance.
Results
Cohort identification and characteristics
The data used for our analyses originated from 826 patients. We excluded seven patients because they did not undergo an ERCP, six patients withdrew informed consent before the ERCP, 11 patients did not receive rectal NSAIDs, and in 21 patients, the exact timing of rectal NSAID administration was unknown ( Fig. 1 ). Of the remaining 781 patients, 372 were randomized to the aggressive hydration group, and therefore, excluded as well. Finally, 409 patients were included for the primary and secondary analyses.
Fig. 1.
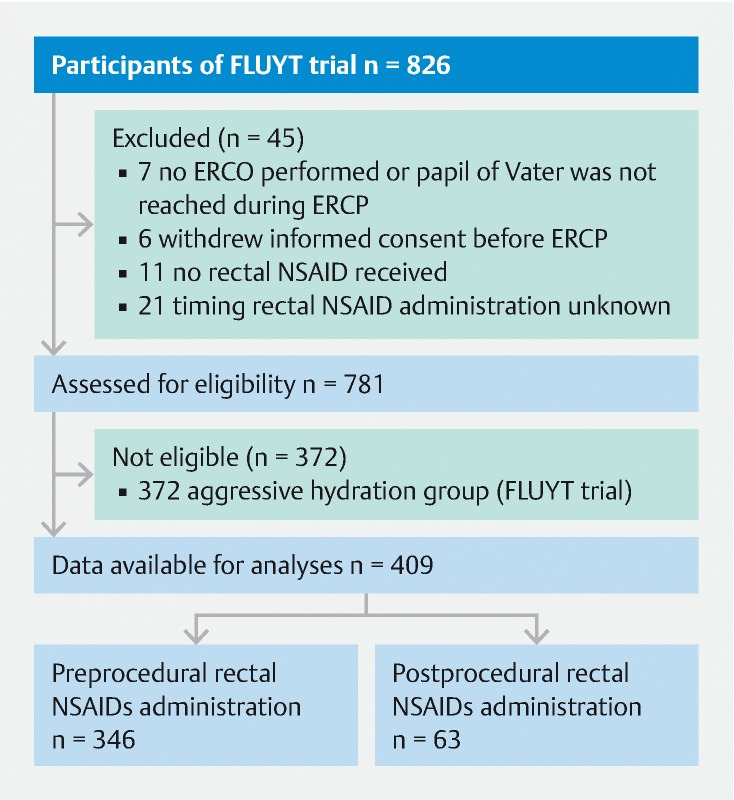
Patient recruitment flow diagram. ERCP − endoscopic retrograde cholangiopancreatography; NSAIDs − nonsteroidal anti-inflammatory drugs.
In 346 patients, the rectal NSAID was administered within 30 minutes before the start of the ERCP procedure (preprocedural group) and in 63 patients within 30 minutes after the end of the ERCP procedure (postprocedural group). Timing of rectal NSAID administration was equally distributed between hospitals and clinicians, and was often influenced by logistics around the ERCP procedure and independent of the ERCP indication. All rectal NSAIDs administered were diclofenac 100 mg.
Baseline and ERCP characteristics
Baseline and ERCP characteristics are summarized in Table 2 and Supplementary Table S1 . The median age was 59 years (IQR 49–71) and 237 patients (58 %) were women. The overall mean BMI was 27.45 kg/m 2 (±4.95). Choledocholithiasis was the most frequent indication for ERCP (80 %). No statistically significant differences at baseline were observed between the two groups, although BMI showed a potential clinically relevant difference ( P = 0.07).
Table 2. Baseline and ERCP characteristics.
| Total (N = 409) | Pre-ERCP (N = 346) | Post- ERCP (N = 63) | P value | |
| Age (yr) – median (IQR) | 59 (49–71) | 59.5 (49–71) | 56.0 (47.5–70) | 0.72 |
| Female sex | 237 (58 %) | 203 (59 %) | 34 (54 %) | 0.58 |
| Body mass index (kg/m 2 ) – mean (SD) 1 | 27.5 (4.95) | 27.3 (4.98) | 28.5 (4.66) | 0.07 |
| Previous cholecystectomy | 112 (27 %) | 96 (28 %) | 16 (25 %) | 0.82 |
| ASA class on admission | 0.52 | |||
|
101 (25 %) | 89 (26 %) | 12 (19 %) | |
|
245 (60 %) | 204 (59 %) | 41 (65 %) | |
|
63 (15 %) | 53 (15 %) | 10 (16 %) | |
| Smoker 2 | 0.22 | |||
|
187 (46 %) | 162 (47 %) | 25 (40 %) | |
|
92 (22 %) | 76 (22 %) | 16 (25 %) | |
|
84 (21 %) | 77 (22 %) | 7 (11 %) | |
| Alcohol abuse 3 , 4 | 64 (16 %) | 52 (15 %) | 12 (19 %) | 0.19 |
| ERCP indication | ||||
| (Suspicion of) common bile duct stones | 329 (80 %) | 279 (81 %) | 50 (79 %) | 0.95 |
|
46 (11 %) | 36 (10 %) | 10 (16 %) | 0.30 |
|
8 (2 %) | 6 (2 %) | 2 (3 %) | 0.79 |
|
5 (1 %) | 4 (1 %) | 1 (2 %) | 1.00 |
|
7 (1 %) | 7 (2 %) | 0 | 0.54 |
|
5 (1 %) | 4 (1 %) | 1 (2 %) | 1.00 |
|
13 (3 %) | 12 (3 %) | 1 (2 %) | |
| Complexity of ERCP 35 | 0.50 | |||
|
29 | 23 | 6 | |
|
341 | 287 | 54 | |
|
37 | 34 | 3 | |
|
2 | 2 | 0 | |
| Common bile duct cannulation achieved | 380 (93 %) | 322 (93 %) | 58 (92 %) | 0.79 |
| Difficult cannulation 5 , 6 | 117 (29 %) | 98 (29 %) | 19 (31 %) | 0.83 |
| (unintentional) pancreatic duct cannulation | 153 (38 %) | 127 (37 %) | 26 (41 %) | 0.58 |
| Pancreatic duct stent placement | 24 (6 %) | 20 (6 %) | 4 (6 %) | 0.77 |
Data are expressed as n (%). IQR interquartile range. ASA American Society of Anesthesiologists. ERCP Endoscopic retrograde cholangiopancreatography.
Eight missing: six in the preprocedural group and two in the postprocedural group.
Forty-six missing: 31 in the preprocedural group and 15 in the postprocedural group.
According to National Institute on Alcohol Abuse and Alcoholism (Women: more than three drinks on any single day and more than seven drinks per week. Men: more than four drinks on any single day and more than 14 drinks per week).
Fifty-five missing: 38 in the preprocedural group and 17 in the postprocedural group.
Difficult cannulation was defined as > 5 attempts.
Eight missing. 6 missing in the preprocedural group and 2 missing in the postprocedural group.
Effectiveness and safety
Post-ERCP pancreatitis occurred in 37 of 409 patients (9 %, 95 % CI: 6.6–12.2) ( Table 3 ). Twenty-six of the 346 patients in the pre-procedure group had pancreatitis, compared with 11 of 63 patients in the post-procedure group (RR: 2.32; 95 % CI: 1.21 to 4.46, P = 0.020). When adjusted for BMI, a relative risk of 2.36; 95 % CI: 1.12 to 4.55 ( P = 0.015) was found. We found fewer patients with mild pancreatitis in the pre-procedure group according to Cotton ( P = 0.007) or Atlanta ( P = 0.011) criteria. No differences between the groups were observed in development of moderate or severe pancreatitis according to Cotton ( P = 0.16) or Atlanta criteria ( P = 0.49), and other ERCP-related complications ( P = 1.00). However, the median length of hospital stay of all patients in the post-procedure group was longer: 1.0 (IQR 1–4) vs. 1.0 (IQR 1–2), respectively ( P = 0.022). Also, patients in the post-procedure group were more frequently admitted to the ICU (1 vs. 4; P = 0.002). A significant interaction was absent for all predefined subgroups ( Fig. 2 ). All subgroups appeared to benefit from administering rectal NSAIDs pre-procedure, although a statistically significant result was lacking. This may be explained by a type II error because we did find a statistically significant benefit for preprocedural NSAIDs in the total cohort.
Table 3. Primary and secondary outcomes for timing of rectal NSAID administration.
| Pre-ERCP (N = 346) | Post ERCP (N = 63) | P value | |
| Primary outcome | |||
| Post-ERCP pancreatitis | 26 (7.5 %) | 11 (17.5 %) | 0.020 |
| Adjusted for BMI | 0.015 | ||
| Secondary outcomes | |||
| Post-ERCP pancreatitis severity Cotton | |||
|
3 (< 1 %) | 4 (6 %) | 0.007 |
|
23 (7 %) | 7 (11 %) | 0.16 |
| Post-ERCP pancreatitis severity Atlanta | |||
|
19 (5 %) | 9 (14 %) | 0.011 |
|
7 (2 %) | 2 (3 %) | 0.49 |
| ERCP-related complications | 16 (5 %) | 3 (5 %) | 1 |
|
5 (1 %) | 0 | 0.53 |
|
9 (3 %) | 2 (3 %) | 1 |
|
3 (< 1) | 1 (2 %) | 0.53 |
| Length of hospital stay (days) – median (IQR) | 1 (1–2) | 1 (1–4) | 0.022 |
| ICU admission | 1 (< 1 %) | 4 (6 %) | 0.002 |
| Length of ICU stay (days) – median (IQR) | 33 | 2 (1.75–2.5) | 0.59 |
| 30 day mortality | 3 (< 1 %) | 2 (3 %) | 0.17 |
| Mortality during 180 days of follow-up | 6 (1 %) | 2 (3 %) | 0.36 |
Data are n (%) or median (IQR). BMI − body mass index; IQR − interquartile range; ERCP − endoscopic retrograde cholangiopancreatography.
Fig. 2.
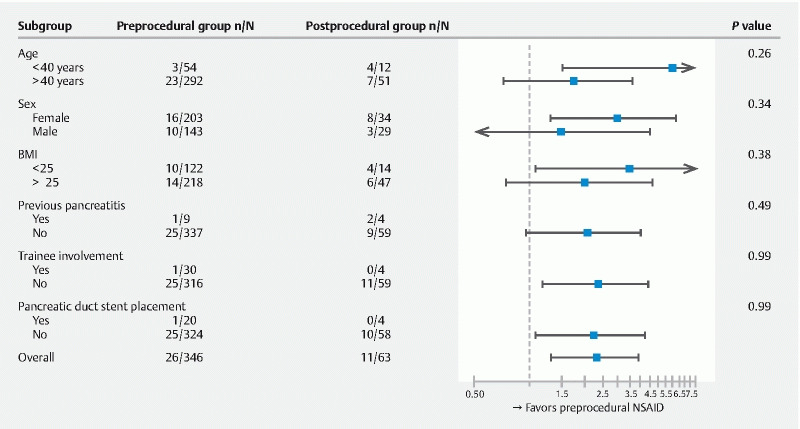
Forest plot of subgroup data. The position of the square indicates the relative risk of developing post-ERCP pancreatitis in each subgroup; the horizontal lines indicate 95 % confidence intervals. In three patients pancreatic duct stent placements technically failed: two in the preprocedural group and one in the postprocedural group. BMI − body mass index.
Discussion
In this multicenter, prospective study, we observed that pre-procedure rectal diclofenac administration is associated with a lower risk of post-ERCP pancreatitis, a shorter hospital stay, and a lower risk of being admitted to the ICU in patients with presumed moderate to high risk compared to post-procedure use. The severity of the pancreatitis was not associated with administration timing.
A direct head-to-head comparison to assess optimal timing of rectal NSAID administration has not been performed. Several meta-analyses have evaluated the optimal timing of rectal NSAID administration by indirect comparisons. Four studies suggest that administering rectal NSAIDs before ERCP might achieve a greater reduction in post-ERCP pancreatitis incidence when comparing pre-ERCP and post-ERCP administration separately to placebo 16 17 18 26 . Others, however, did not confirm this preference for pre-procedure administration 12 13 14 15 . The result of our study is in line with the only indirect, risk-stratified, RCT (n = 2600) on the timing of administering rectal NSAIDs to date 27 . This trial demonstrated that universal pre-procedure administration of rectal NSAIDs, rather than risk-stratified post-procedure administration, provides better protection against post-ERCP pancreatitis (relative risk [RR] 0.47; 95 % CI: 0.36–0.66, P < 0.0001). In high-risk patients, there was a RR of 0.47 (95 % CI: 0.27–0.82, P = 0.006) that favored preprocedural administration. This study, which provides the most robust evidence with respect to timing of rectal NSAIDs thus far, prompted the ESGE to recommend the preprocedural administration of rectal NSAID 3 . The revised ESGE guideline was published at the end of 2019, and therefore, not influence endoscopists in the original trial in their decision to administer the rectal NSAID pre-procedure or post-procedure. Our results are in line with this prospective study and confirm the revised ESGE guidelines.
The peak plasma concentration of NSAIDs occurs within 30 minutes from rectal administration 28 29 . Furthermore, pancreatic injury starts early after induction of pancreatitis 30 . For this reason, it is logical to expect a more optimal peak serum concentration attained in the early phase of pancreatic injury to prevent pancreatic inflammation. In this regard, it is important to consider that the therapeutic window for prevention of post-ERCP pancreatitis may be narrow once the inflammatory cascade becomes activated.
A strength of this study is that the endpoints of the analysis (e. g. post-ERCP pancreatitis, severity, ERCP-related complications) were those used in the original RCT. As such, data on them were all collected prospectively on case record forms and there is no retrospective interpretation and judgment involved, which limits bias. In addition, a blinded adjudication committee evaluated all primary and secondary outcomes. Second, baseline characteristics did not differ between the two groups, and therefore, we assume only a minor risk of confounding by indication regarding the timing of administration of the rectal NSAID. Nevertheless, BMI showed a potentially clinically relevant difference, and for that reason, we decided to correct for BMI. Moreover, as we had specific data on patient- and procedure-related risk factors for post-ERCP pancreatitis, we were able to consider confounding factors and identify subgroups. This gave us an advantage over the meta-analyses that addressed the subject of rectal NSAID timing. Last, we included patients with moderate to high risk for post-ERCP pancreatitis in a multicenter setting, which increased the generalizability of our findings.
Several limitations must be acknowledged. First, we performed a non-randomized comparison. The ideal design to attain the highest level of evidence would be a randomized controlled trial. However, subjecting patients to such a trial may be deemed unethical, considering the current evidence favoring pre-procedure NSAIDs in our study, concomitant with the trial of Luo et al 27 . Second, we included a relatively small group of patients who received a rectal NSAID after the ERCP, which may potentially contribute to a type two error. Nevertheless, we deemed it justifiable to perform the analysis solely in the control group to rule out (possible) interactions of the aggressive hydration in the prevention of post-ERCP pancreatitis and rectal NSAID pharmacokinetics. Because international guidelines recommend using prophylactic rectal NSAID monotherapy, this seems even more appropriate.
Despite the use of rectal NSAIDs, the risk of post-ERCP pancreatitis remains relevant. Furthermore, the effect of rectal NSAIDs is mostly limited to prevention of mild pancreatitis 15 19 31 32 33 . Based on the results of the current study, we recommend administering a rectal NSAID before the start of ERCP to reach an optimal prophylactic effect. For future post-ERCP pancreatitis studies, we need to consider other strategies for optimizing current preventive care. A previous study established that there is an association between body weight and the effect of diclofenac 34 . Two other studies showed that a dose escalation to rectal indomethacin 200 mg administered after ERCP did not confer any advantage compared with the standard regime of 100 mg 8 10 . It will be interesting to investigate whether the incidence of post-ERCP pancreatitis decreases when people with high body weight receive a higher dose of rectal NSAID administered before ERCP, as compared to the standard dose (100 mg). Perhaps it must also be taken into account that the pharmacodynamics of rectal NSAIDs may differ between individuals.
Conclusions
In conclusion, this study demonstrates that pre-procedure administration of prophylactic rectal NSAIDs in moderate- to high-risk patients is associated with a lower risk of post-ERCP pancreatitis, shorter hospital stay, and lower chance of ICU admittance compared to post-procedure administration. In all probability, it is a highly cost-effective intervention. These findings confirm the ESGE 2019 guideline's recommendation and guide clinicians in optimizing prophylactic care for ERCP procedures.
Acknowledgments
The authors wish to thank all the principal investigators of the study sites involved in the FLUYT trial. The FLUYT study was funded by the Netherlands Organisation for Health Research and Development (ZonMw; grant number 837001506) and the Radboud university medical centre. ZonMw had no role in study design, data collection, data analysis, data interpretation, or preparation of the report. The corresponding author has full access to all the data in the study and bears final responsibility for the decision to submit for publication.
Footnotes
Conflicts of interest Dr. van Hooft has received research funding from Cook Medical and served as a consultant for Medtronic, Cook Medical and Boston Scientific, outside the submitted work. Dr. Besselink has received research funding form Intuitive, Ethicon Endo-Surgery, and Medtronic, outside the submitted work. Dr. Bruno has received research funding from Boston Scientific, Cook Medical, Pentax Medical, InterScope, ChiRho- Clin, and 3M and served as a consultant for Boston Scientific, Cook Medical, and Pentax Medical, outside the submitted work. Dr. Fockens has received consultancy fees from Cook Medical and Olympus, outside the submitted work. Dr. Drenth has received research funding from Gilead to support Hepatitis C elimination in the Netherlands, outside the submitted work. Dr. van Geenen has received research funding from Mylan, Boston Scientific, and Olympus and served as a consultant for MTW-Endoskopie, outside the submitted work. Christina J. Sperna Weiland, Xavier J.N.M. Smeets, Robert C. Verdonk, Alexander C. Poen, Abha Bhalla, Niels G. Venneman Wietske Kievit, Hester C. Timmerhuis, Devica S. Umans, and Hjalmar C. van Santvoort do not have potential conflicts of interest or disclosures to report.
Supplementary material :
References
- 1.Kochar B, Akshintala V S, Afghani E et al. Incidence, severity, and mortality of post-ERCP pancreatitis: A systematic review by using randomized, controlled trials. Gastrointest Endosc. 2015;81:143–1.49E11. doi: 10.1016/j.gie.2014.06.045. [DOI] [PubMed] [Google Scholar]
- 2.Andriulli A, Loperfido S, Napolitano G et al. Incidence rates of post-ERCP complications: A systematic survey of prospective studies. Am J Gastroenterol. 2007;102:1781–1788. doi: 10.1111/j.1572-0241.2007.01279.x. [DOI] [PubMed] [Google Scholar]
- 3.Dumonceau J-M, Kapral C, Aabakken L et al. ERCP-related adverse events: European Society of Gastrointestinal Endoscopy (ESGE) Guideline. Endoscopy. 2019;52:127–149. doi: 10.1055/a-1075-4080. [DOI] [PubMed] [Google Scholar]
- 4.Chandrasekhara V, Khashab M A, Muthusamy V R et al. Adverse events associated with ERCP. Gastrointest Endosc. 2017;85:32–47. doi: 10.1016/j.gie.2016.06.051. [DOI] [PubMed] [Google Scholar]
- 5.Mine T, Morizane T, Kawaguchi Y et al. Clinical practice guideline for post-ERCP pancreatitis. J Gastroenterol. 2017;52:1013–1022. doi: 10.1007/s00535-017-1359-5. [DOI] [PubMed] [Google Scholar]
- 6.Coté G A, Elmunzer B J. Nonsteroidal anti-inflammatory drugs for prevention of post-ERCP pancreatitis: sooner rather than later during ERCP? Gastroenterology. 2016;151:1027–1028. doi: 10.1053/j.gastro.2016.09.026. [DOI] [PubMed] [Google Scholar]
- 7.Akshintala V S, Hutfless S M, Colantuoni E et al. Systematic review with network meta-analysis: pharmacological prophylaxis against post-ERCP pancreatitis. Aliment Pharmacol Ther. 2013;38:1325–1337. doi: 10.1111/apt.12534. [DOI] [PubMed] [Google Scholar]
- 8.Fogel E L, Lehman G A, Tarnasky P et al. Rectal indometacin dose escalation for prevention of pancreatitis after endoscopic retrograde cholangiopancreatography in high-risk patients: a double-blind, randomized controlled trial. Lancet Gastroenterol Hepatol. 2020;5:132–141. doi: 10.1016/S2468-1253(19)30337-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yoshihara T, Horimoto M, Kitamura T et al. 25 mg versus 50 mg dose of rectal diclofenac for prevention of post-ERCP pancreatitis in Japanese patients: A retrospective study. BMJ Open. 2015;5:1–6. doi: 10.1136/bmjopen-2014-006950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lai J H, Hung C Y, Chu C H et al. A randomized trial comparing the efficacy of single-dose and double-dose administration of rectal indomethacin in preventing post-endoscopic retrograde cholangiopancreatography pancreatitis. Medicine (Baltimore) 2019;98:e15742. doi: 10.1097/MD.0000000000015742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Katoh T, Kawashima K, Fukuba N et al. Low-dose rectal diclofenac does not prevent post-ERCP pancreatitis in low- or high-risk patients. J Gastroenterol Hepatol. 2020;35:1247–1253. doi: 10.1111/jgh.14948. [DOI] [PubMed] [Google Scholar]
- 12.Liu L, Li C, Huang Y et al. Nonsteroidal Anti-inflammatory drugs for endoscopic retrograde cholangiopancreatography postoperative pancreatitis prevention: a systematic review and meta-analysis. J Gastrointest Surg. 2019;23:1991–2001. doi: 10.1007/s11605-018-3967-7. [DOI] [PubMed] [Google Scholar]
- 13.Yang C, Zhao Y, Li W et al. Rectal nonsteroidal anti-inflammatory drugs administration is effective for the prevention of post-ERCP pancreatitis: An updated meta-analysis of randomized controlled trials. Pancreatology. 2017;17:681–688. doi: 10.1016/j.pan.2017.07.008. [DOI] [PubMed] [Google Scholar]
- 14.Patai Á, Solymosi N, Mohácsi L et al. Indomethacin and diclofenac in the prevention of post-ERCP pancreatitis: a systematic review and meta-analysis of prospective controlled trials. Gastrointest Endosc. 2017;85:1144–11560. doi: 10.1016/j.gie.2017.01.033. [DOI] [PubMed] [Google Scholar]
- 15.Ding X, Chen M, Huang S et al. Nonsteroidal anti-inflammatory drugs for prevention of post-ERCP pancreatitis: A meta-analysis. Gastrointest Endosc. 2012;76:1252–1259. doi: 10.1016/j.gie.2012.08.021. [DOI] [PubMed] [Google Scholar]
- 16.Wan J, Ren Y, Zhu Z et al. How to select patients and timing for rectal indomethacin to prevent post-ERCP pancreatitis: A systematic review and meta-analysis. BMC Gastroenterol. 2017;17:1–9. doi: 10.1186/s12876-017-0599-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rustagi T, Njei B. Factors affecting the efficacy of nonsteroidal anti-inflammatory drugs in preventing post-endoscopic retrograde cholangiopancreatography pancreatitis: A systematic review and meta-analysis. Pancreas. 2015;44:859–867. doi: 10.1097/MPA.0000000000000326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sun H L, Han B, Zhai H P et al. Rectal NSAIDs for the prevention of post-ERCP pancreatitis: A meta-analysis of randomized controlled trials. Surgeon. 2014;12:141–147. doi: 10.1016/j.surge.2013.10.010. [DOI] [PubMed] [Google Scholar]
- 19.Sperna Weiland C J, Smeets X JNM, Kievit W. Aggressive fluid hydration plus non-steroidal anti-inflammatory drugs versus non-steroidal anti-inflammatory drugs alone for post-endoscopic retrograde cholangiopancreatography pancreatitis (FLUYT): a multicentre, open-label, randomised, controlled trial. Lancet Gastroenterol Hepatol. 2021;5:350–358. doi: 10.1016/S2468-1253(21)00057-1. [DOI] [PubMed] [Google Scholar]
- 20.Vandenbroucke J P, Von Elm E, Altman D G et al. Strengthening the Reporting of Observational Studies in Epidemiology (STROBE): Explanation and elaboration. Epidemiology. 2007;18:805–835. doi: 10.1097/EDE.0b013e3181577511. [DOI] [PubMed] [Google Scholar]
- 21.Schneider A, Löhr J M, Singer M V. The M-ANNHEIM classification of chronic pancreatitis: Introduction of a unifying classification system based on a review of previous classifications of the disease. J Gastroenterol. 2007;42:101–119. doi: 10.1007/s00535-006-1945-4. [DOI] [PubMed] [Google Scholar]
- 22.Buxbaum J, Yan A, Yeh K et al. Aggressive hydration with lactated ringer’s solution reduces pancreatitis after endoscopic retrograde cholangiopancreatography. Clin Gastroenterol Hepatol. 2014;12:303–3070. doi: 10.1016/j.cgh.2013.07.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Elmunzer B J, Scheiman J M, Lehman G A et al. A Randomized Trial of Rectal Indomethacin to Prevent Post-ERCP Pancreatitis. N Engl J Med. 2012;366:1414–1422. doi: 10.1056/NEJMoa1111103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cotton P B, Lehman G, Vennes J et al. Endoscopic sphincterotomy complications and their management: an attempt at consensus. Gastrointest Endosc. 1991;37:383–393. doi: 10.1016/s0016-5107(91)70740-2. [DOI] [PubMed] [Google Scholar]
- 25.Banks P A, Bollen T L, Dervenis C et al. Classification of acute pancreatitis – 2012: Revision of the Atlanta classification and definitions by international consensus. Gut. 2013;62:102–111. doi: 10.1136/gutjnl-2012-302779. [DOI] [PubMed] [Google Scholar]
- 26.Yang J, Wang W, Liu C et al. Rectal nonsteroidal anti-inflammatory drugs for endoscopic retrograde cholangiopancreatography postoperative pancreatitis prevention: a network meta-analysis. J Clin Gastroenterol. 2020;54:305–313. doi: 10.1097/MCG.0000000000001322. [DOI] [PubMed] [Google Scholar]
- 27.Luo H, Zhao L, Leung J et al. Routine pre-procedural rectal indometacin versus selective post-procedural rectal indometacin to prevent pancreatitis in patients undergoing endoscopic retrograde cholangiopancreatography: a multicentre, single-blinded, randomised controlled trial. Lancet. 2016;387:2293. doi: 10.1016/S0140-6736(16)30310-5. [DOI] [PubMed] [Google Scholar]
- 28.Tammaro S, Caruso R, Pallone F et al. Post-endoscopic retrograde cholangio-pancreatography pancreatitis: Is time for a new preventive approach? World J Gastroenterol. 2012;18:4635–4638. doi: 10.3748/wjg.v18.i34.4635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Van Der Marel C D, Anderson B J, Rømsing J et al. Diclofenac and metabolite pharmacokinetics in children. Paediatr Anaesth. 2004;14:443–451. doi: 10.1111/j.1460-9592.2004.01232.x. [DOI] [PubMed] [Google Scholar]
- 30.Cuthbertson C M, Christophi C. Disturbances of the microcirculation in acute pancreatitis. Br J Surg. 2006;93:518–530. doi: 10.1002/bjs.5316. [DOI] [PubMed] [Google Scholar]
- 31.Elmunzer B J, Scheiman M J, Lehman A G et al. A randomized trial of rectal indomethacin to prevent post-ERCP pancreatitis. N Engl J Med. 2012;366:1414–1422. doi: 10.1056/NEJMoa1111103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sethi S, Sethi N, Wadhwa V et al. A meta-analysis on the role of rectal diclofenac and indomethacin in the prevention of post-endoscopic retrograde cholangiopancreatography pancreatitis. Pancreas. 2014;43:190–197. doi: 10.1097/MPA.0000000000000090. [DOI] [PubMed] [Google Scholar]
- 33.Puig I, Calvet X, Baylina M et al. How and when should NSAIDs be used for preventing post-ERCP pancreatitis? A systematic review and meta-analysis. PLoS One. 2014;9:1–8. doi: 10.1371/journal.pone.0092922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Leerhøy B, Nordholm-Carstensen A, Novovic S et al. Effect of body weight on fixed dose of diclofenac for the prevention of post-endoscopic retrograde cholangiopancreatography pancreatitis. Scand J Gastroenterol. 2016;51:1007–1012. doi: 10.3109/00365521.2016.1172338. [DOI] [PubMed] [Google Scholar]
- 35.Cotton P B, Calvet X, Eisen G, Romagnuolo J et al. Grading the complexity of endoscopic procedures: results of an ASGE working party. Gastrointest Endosc. 2011;73:868–874. doi: 10.1016/j.gie.2010.12.036. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


