Abstract
Anthocyanins are components of the flavonoid group with different properties, such as antidiabetic properties. This study aimed to isolate anthocyanin from Berberis integerrima Bunge fruits and evaluate α-amylase and α-glucosidase inhibition by this mentioned anthocyanin. The anthocyanin of Berberis integerrima fruit was isolated using column chromatography, and the antidiabetic properties of the anthocyanin were determined by the levels of α-amylase and α-glucosidase inhibition. Km and Vmax were also evaluated using the GraphPad Prism 7. The results of this study showed that the anthocyanin content of the fruit extract was 14.36 ± 0.33 mg/g, and following purification, this amount increased to 34.51 ± 0.42 mg/g. The highest of α-glucosidase inhibition was observed in the purified anthocyanin with IC50 = 0.71 ± 0.085 mg/ml, compared to acarbose as the baseline with IC50 = 8.8 ± 0.14 mg/ml, p < 0.0001. Purified anthocyanin of the mentioned fruit with IC50 = 1.14 ± 0.003 mg/ml had the greatest α-amylase inhibition, which was similar to acarbose as the standard with IC50 = 1 ± 0.085 mg/ml, p < 0.05. The inhibition of α-glucosidase and α-amylase by purified anthocyanin showed uncompetitive inhibition, and the enzyme inhibition by unpurified anthocyanin showed mixed inhibition. The obtained findings showed that Berberis integerrima fruit can be mentioned as a source of anthocyanin with antidiabetic properties.
1. Introduction
The characteristics of the metabolic syndrome such as diabetes include high levels of glucose, obesity of the abdomen, and high level of cholesterol and high blood pressure, which increase the risk of cardiovascular disease. Insulin resistance is a significant feature of metabolic syndrome and type 2 diabetes. Nowadays, there are 246 million patients with type 2 diabetes in the world. The International Diabetes Federation (IDF) announced that 380 million people will be diabetic in 2025 [1]. Some plant extracts and plant compounds can reduce blood sugar and prevent diabetes.
In folk medicine, barberry fruits are consumed as an antidiabetic drug [2], which composed of great amounts of anthocyanin [3]. Anthocyanin pigments are globally well-known as medicinal agents.
Free radical scavengers are the most widely known properties of anthocyanins [3].
In addition, it was reported that the anthocyanin content of barberry fruits is similar to the anthocyanin content of blackberries fruits (Rubus glaucus) [4], nasturtium (Tropaeolum majus) [5], and a hybrid of strawberries (Fragaria ananassa) [6].
Some studies [7] showed that, in vitro, anthocyanins stimulate insulin secretion from the rodent pancreatic β-cells. Besides, consumption of fruits and vegetables rich in polyphenols reduces the incidence of type 2 diabetes [8, 9].
Anthocyanin compounds are abundant in food plants and appear in 27 plant families. The usage of anthocyanin has been estimated at 10000 tons of black grapes in one year. Anthocyanins are vital components of plant secondary metabolites and are also the most important coloring substances in wine [10].
The six anthocyanins usually presented in plants are classified according to the position and number of hydroxyl groups on the flavan nucleus and are nominated as cyanidin (cy), delphinidin (dp), malvidin (mv), peonidin (pn), pelargonidin (pg), and petunidin (pt). Anthocyanins are water-soluble glycosides of polyhydroxyl and polymethoxyl derivatives of 2-phenylbenzopyrylium or flavylium salts. The discrimination between anthocyanins is based on the position and number of hydroxyl groups; the level of methylation of these hydroxyl groups; the number, nature, and location of sugars attached to the molecule; and aromatic or aliphatic acids attached to the sugars in the molecule. The glycosylation increases water solubility and structural stability of the anthocyanidin [11].
Another study reported that, in vitro, the whole extract or pure form of anthocyanin has antioxidant properties, higher than the other natural antioxidants [12–14].
The purpose of this study was to evaluate the anthocyanin antidiabetic properties of Berberis integerrima Bunge fruits (BIBF) before loading on to the column chromatography and after being isolated from the column. For evaluation, the antidiabetic properties of BIBF, α-amylase, and α-glucosidase inhibition were assessed.
2. Materials and Methods
2.1. Chemicals
PNPG (4-nitrophenyl α-d-glucopyranoside), 3, 5-dinitro salicylic acid (DNSA), and α-glucosidase and α-amylase enzymes were obtained from Sigma Aldrich, USA. Other substances were purchased from Merck Chemicals (Darmstadt, Germany).
2.2. Plant Materials
Berberis integerrima Bunge was gathered from Kohmar (around Shiraz, capital of Fars province, Iran) in September 2018 and was identified and authenticated (Voucher No. Pm396) in the Medicinal Plants Museum, Department of Pharmacognosy, University of Medical Sciences, Shiraz, Iran [15].
2.3. Extraction and Purification
Three hundred and twenty-two grams of the frozen-dried fruit powder was percolated with ethanol, and the obtained extract was concentrated under vacuum in the rotary evaporator, followed by a speed vacuum to yield 140 g of the gummy substance. Thirty grams of crude extract was then suspended in 0.3% TFA. Afterward, it was filtered and mixed with ethyl acetate. Then, the liquid phase was gathered (this extraction repeated three times); this phase was packed on to the Amberlite column (2.5 × 45 cm). Following that, the column was washed out with distilled water containing 0.3% of TFA to discharge polysaccharides. The purified anthocyanin was eluted with 0.3% of TFA dissolved in methanol and then concentrated by the rotary evaporator [15]. The amount of obtained extracts and anthocyanin fraction were 20% and 2.57%, respectively [15].
2.4. Measurement of the Anthocyanin Content
A pH-differential was applied to determine the anthocyanin content in the BIBF fraction. 80.4 ml of the extract was combined with 3.6 ml of the buffer. The buffer was a mixture of 0.4 M sodium acetate solution and 0.025 M potassium chloride solution, and the buffer was fixed at pH 4.5. The absorbance of each sample was determined at 519 nm, and the distilled water was used as a blank. The anthocyanin content was calculated via the following equation:
| (1) |
where A is (A519 (pH 1.0)-A519 (pH 4.5)), MW is the molecular weight of anthocyanin (433.2 g/mol), DF is the dilution factor, ε is the extinction coefficient (31600 L cm−1mol−1), and L is the path length (1 cm) [15].
2.5. Determination of the Inhibition of α-Glucosidase
Inhibition of α-glucosidase was assessed according to the method reported by McCue et al. [16]. The enzyme solution included 125 μl of phosphate buffer (pH 6.9, 0.1 M) and 5 μl of α-glycosidase (25 unit/ml). In addition, 11 mM of P-nitrophenyl-α-D-glucopyranoside dissolved in the phosphate buffer (pH 6.9) was applied as a substrate solution. Then, 20 μl of the sample at various concentrations was mixed with the enzyme solution and incubated for 15 min at 37°C. The reaction was initiated by the addition of 20 μl of the substrate solution and incubation for an extra 15 min. The addition of sodium carbonate (0.2 M, 80 μl) retarded the reaction.
The absorbances of the samples were determined at 405 nm using a microplate reader, while the control did not contain the sample. The blank did not contain α-glucosidase, and acarbose was used as the standard sample. All the evaluations were performed three times. Finally, the enzyme inhibition rates of the samples were determined via the following equation:
| (2) |
2.6. Kinetics of α-Glucosidase Inhibition
The inhibition modes of α-glucosidase by the samples were assessed based on the method reported by Kim et al. [17]. In doing so, fixed amounts of α-glucosidase were blended with increasing concentrations of its substrates (PNPG) at 37°C for 15 min without and with the IC50 concentrations of the samples. After that, the reactions were stopped, and absorption was determined using the Michaelis–Menten curve. All the evaluations were repeated three times.
2.7. Determination Inhibition of α-Amylase
Inhibition of α-amylase (EC:3.2.1.1) by different concentrations of the samples according to the method reported by Maccu and Shetty was determined [16]. In this study, acarbose was used as a standard. First, the samples were mixed with distilled water and starch (as a substrate). The reaction started with the addition of α-amylase. Then, the mixture was incubated for 15 min at 37°C, and after that, dinitro salicylate alkaline potassium tartrate was added. Afterward, another incubation was performed for 15 minutes at 85°C, and finally, the activity of α-amylase was evaluated by determination of the absorbance at 540 nm (Biotek Corporation's synergy HTX multimode reader). The control contained DMSO (dimethyl sulfoxide) instead of the sample. Blank contained buffer instead of the enzyme. For evaluating the mode of α-amylase inhibition, the IC50 concentration of the sample and different concentrations of the starch were used according to the method mentioned previously. After obtaining the absorption rates for all of the substrate concentrations, the absorbance vs. substrate concentration was drawn until this curve reached saturation or plateau, indicating the maximum velocity.
2.8. Statistical Analysis
The Tukey post hoc test was used to compare the IC50 means of the samples and the P value.
If the P value was <0.05, then the difference is considered as significant.
First, we converted the amount of sample absorption into enzyme activity using the product molar extinction coefficient. Then, we entered the values of the substrate concentrations and the activity level of the enzyme into the Graphpad Prism software, which calculated the values of Km and Vmax. After that, the mode of inhibition was evaluated.
3. Results and Discussion
In an in vivo study, it was significantly confirmed that dietary consumption of blueberry and black raspberry anthocyanin protects blood cells from oxidative stress and free radicals [18, 19]. Therefore, it shows the vital role of anthocyanin against reactive oxygen species [20].
The amount of anthocyanin can be affected by the alterations in the seasons [21]. The anthocyanin content may relate to some factors such as temperature, pH, and the presence of phenols and metals [22].
Furthermore, the usage of anthocyanins (ANCs) is supposed to be related to a reduced risk of degenerative diseases, such as cardiovascular diseases [23], cancer [24], atherosclerosis [25], and diabetes [26]. Also, in another research, it was reported that the compounds are responsible for the inhibition of α-amylase and α-glucosidase, included flavonoids, flavonol, phenolic acid, and anthocyanins [27].
On the basis of scientific documents, this may be the first time that the antidiabetic property of anthocyanin, which is isolated from Berberis integerrima Bunge fruits, was investigated.
We may accredit the hypoglycemic property of the anthocyanins to their hemiacetal transformation and/or chalcone compounds.
In addition, in another research, it was reported that blueberry fruits have plenty of anthocyanin compounds, and their anthocyanins have the potential to relieve symptoms of hyperglycemia in diabetic mice [26].
Other research reported that treatment by gavage (500 mg/kg body wt) with both the blueberry extract enriched with phenolic and the fraction enriched with anthocyanin decreased raised blood glucose levels by 33 and 51%, respectively, when combined with Labrasol. This hypoglycemic property was similar to that of metformin, known as an antidiabetic drug [26].
The results of other studies proposed that mulberry fruit anthocyanin extract showed significant antidiabetic activities by controlling blood glucose levels in rats as an animal model of type 2 diabetes [28, 29]. In addition, it was reported that in vitro, Calendula officinalis fruit anthocyanin increased the release of insulin from pancreatic β-cells [7].
In this study, after using an Amberlite column for anthocyanin purification and using the pH-differential method for determination anthocyanin [30], the amount of AFBI anthocyanin increased from 14.36 ± 0.33 mg/g to 34.51 ± 0.42 mg/g (P value <0.0001).
It was reported that anthocyanins in purple and red flesh purple potato tubers would have significant inhibitory effects on α-glucosidase and α-amylase [30]. One approach to the management of complications of diabetes is to inhibit α-amylase and α-glucosidase activities. In the present study, we evaluated the inhibition of α-amylase and α-glucosidase by BIBF extract and anthocyanin isolated from BiB fruits.
Based on the obtained data, purified anthocyanin has the most efficient inhibition feature on α-glucosidase activity (Figures 1 and 2). In other words, the lowest IC50 of α-glucosidase inhibition was observed in purified anthocyanin of BIBF (IC50 = 0.71 ± 0.085 mg/ml, Table 1). The IC50 of BIBF extract (1 ± 0.048 mg/ml, Table 1) was less than acarbose (IC50 = 8.8 ± 0.14 mg/ml, P < 0.001, Table 1). Also, there is a significant difference between IC50 of purified anthocyanin and BIBF extract (P < 0.05), and a significant difference is observed between IC50 of purified anthocyanin and acarbose (P < 0.001).
Figure 1.
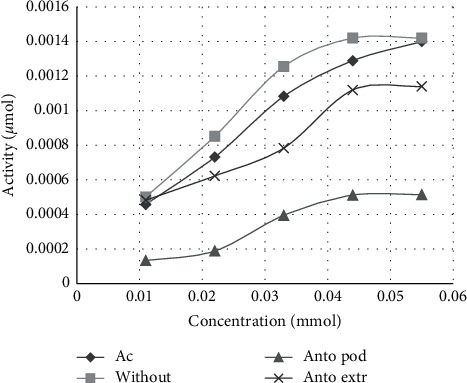
Michaelis–Menten curves of α-glucosidase inhibition by anthocyanin extract and purified anthocyanin in comparison to reaction without the inhibitor and acarbose as a standard. Antoextr, anthocyanin extract; Ant pod, purified anthocyanin; without, reaction without the inhibitor; Ac, acarbose.
Figure 2.
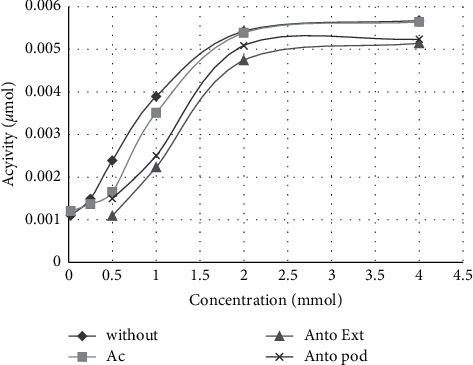
Michaelis–Menten curves of α-amylase inhibition by anthocyanin extract and purified anthocyanin in comparison to reaction without the inhibitor and acarbose as a standard. Antoextr, anthocyanin extract; Ant pod, purified anthocyanin; without, reaction without the inhibitor; Ac, acarbose.
Table 1.
IC50 ± SD of the enzymes inhibition of the purified anthocyanin and anthocyanin extract in comparison to acarbose.
| Samples | IC50 of α-glucosidase inhibition (mg/ml) | IC50 of α-amylase inhibition (mg/ml) |
|---|---|---|
| Anthocyanin content after using column | 0.71 ± 0.085 | 1.14 ± 0.003 |
| Anthocyanin extract | 1 ± 0.048 | 1.4 ± 0.01 |
| Acarbose (standard) | 8.82 ± 0.14 | 1 ± 0.085 |
In addition, the results of this study showed that purified anthocyanin of BIBF (IC50 = 1.14 ± 0.003 mg/ml, Table 1) inhibits α-amylase more than BIBF extract (IC50 = 1.4 ± 0.01 mg/ml, P < 0.001, Table 2), and the IC50 of acarbose (1 ± 0.085 mg/ml, P < 0.05, Table 1) was higher than purified anthocyanin of BIBF. The IC50 of BIBF extract (1.4 ± 0.01 mg/ml, Table 1) was less than acarbose (1 ± 0.085 mg/ml, P < 0.001, Table 1).
Table 2.
Km ± SD and Vmax ± SD and the type of α-glucosidase inhibition of the studied samples in comparison to acarbose.
| Samples | Km ± SD (mmol) | Vmax ± SD (µmol/min) | Kind of inhibition |
|---|---|---|---|
| Anthocyanin content after using column | 3.03 ± 0.6↓ | 0.0009 ± 0.0001↓ | Uncompetitive |
| Anthocyanin extract | 4.54 ± 0.9↑ | 0.002 ± 0.0002↓ | Mixed |
| Without inhibition | 4.47 ± 0.01 | 0.0028 ± 0.0003 | — |
| Acarbose (standard) | 5.22 ± 1.04↑ | 0.0028 ± 0.0003 (constant) | Competitive |
In another research, it was reported that extracts of Rumex maderensis exhibited a moderate α-amylase inhibitory potential. Flowers of Rumex maderensis presented the strongest inhibition, while leaves showed lowest inhibition. All mentioned samples showed higher IC50 values than acarbose as a commercial drug (P < 0.05). Also, flower extract of Rumex maderensis showed the most inhibition of α-glucosidase [31].
Based on Tables 2 and 3, the enzyme inhibition modes of purified anthocyanin (with Km = 3.03 ± 0.6 mmol and Vmax = 0.0009 ± 0.0001 μmol/min for α-glucosidase and with Km = 0.8 ± 0.4 mmol and Vmax = 0.007 ± 0.0008 μmol/min for α-amylase) were uncompetitive. The enzyme inhibition modes of BIBF extract (with Km = 4.54 ± 0.9 mmol and Vmax = 0.002 ± 0.0002 μmol/min for α-glucosidase and with Km = 2.05 ± 0.57 mmol and Vmax = 0.0074 ± 0.0001 μmol/min for α-amylase) were mixed.
Table 3.
Km ± SD and Vmax ± SD and the type of α-amylase inhibition of the studied samples and acarbose.
| Samples | Km ± SD (mmol) | Vmax ± SD (µmol/min) | Kind of inhibition |
|---|---|---|---|
| Anthocyanin content after using column | 0.8 ± 0.4↓ | 0.007 ± 0.0008↓ | Uncompetitive |
| Anthocyanin extract | 2.05 ± 0.57↑ | 0.0074 ± 0.001↓ | Mixed |
| Reaction without inhibitor | 1.01 ± 0.213 | 0.0076 ± 0.0006 | – |
| Acarbose (standard) | 1.264 ± 0.301↑ | 0.0076 ± 0.0007 (constant) | Competitive |
It was reported that the anthocyanin extract of purple fleshed potato including petunidin, cyanidin, and malvidin compounds showed a noncompetitive inhibition in inhibition of α-glucosidase. In addition, Narita et al. reported that chlorogenic acid showed a mixed inhibition in inhibition of pancreatic α-amylase [32].
In comparison to acarbose as a competitive inhibitor, in mixed and uncompetitive inhibitions, the sample binds to broad regions of the enzyme other than the active site [33]. Another advantage of mixed inhibition is that it may not be affected by higher substrate concentrations and would still be effective at lower concentrations of the substrate [33].
4. Conclusion
Anthocyanin of Berberis integerrima fruit could inhibit α-glucosidase and α-amylase.
There are different responses to the plant extracts rich in anthocyanin, determined by certain remarkable biomarkers. The present study and similar studies provide a basis for further clinical trials. Berberis ssp. fruits possess different components with potential health benefits [34, 35].
Acknowledgments
The authors acknowledge the Shiraz University of Medical Sciences (17493) for performing this project.
Data Availability
The data used to support the findings of this study are available from the corresponding author upon request.
Conflicts of Interest
The authors declare that they have no conflicts of interest.
References
- 1.Saeedi P., Petersohn I., Salpea P., Malanda B., Karuranga S., Unwin N. Global and regional diabetes prevalence estimates for 2019 and projections for 2030 and 2045. Results from the International Diabetes Federation Diabetes Atlas . 2019;157:p. 107843. doi: 10.1016/j.diabres.2019.107843. [DOI] [PubMed] [Google Scholar]
- 2.Ashraf H., Heidari R., Nejati V. Anti hyperglycemic and anti hyperlipidemic effects of fruit aqueous extract of Berberis integerrima Bge. In Streptozotocin- induced diabetic rats. Iranian Journal of Pharmaceutical Research . 2014;13:1313–1318. [PMC free article] [PubMed] [Google Scholar]
- 3.Lila M. A. Anthocyanins and human health: an in vitro investigative approach. Journal of Biomedicine and Biotechnology . 2004;2004:306–313. doi: 10.1155/s111072430440401x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Moein M., Moein S., Mousavi F. Study the relationship between antioxidant potential and phenolic contents of Juniperus excelsa fruit. International Journal of Pharmacy and Pharmaceutical . 2014;6(7):192–194. [Google Scholar]
- 5.Rezaeian S., Pourianfar H. R., Janpoor J. Antioxidant properties of several medicinal plants growing wild in northeastern Iran. Asian Journal of Plant Science & Research . 2015;5(2):63–68. [Google Scholar]
- 6.Ngo T., Wrolstad R. E., Zhao Y. Color quality of Oregon strawberries—impact of genotype, composition, and processing. Journal of Food Science . 2007;72:C25–C32. doi: 10.1111/j.1750-3841.2006.00200.x. [DOI] [PubMed] [Google Scholar]
- 7.Jayaprakasam B., Vareed S. K., Olson L. K., Nair M. G. Insulin secretion by bioactive anthocyanins and anthocyanidins present in fruits. Journal of Agricultural and Food Chemistry . 2005;53:28–31. doi: 10.1021/jf049018+. [DOI] [PubMed] [Google Scholar]
- 8.Anderson R. A., Polansky M. M. Tea enhances insulin activity. Journal of Agricultural and Food Chemistry . 2002;50:7182–7186. doi: 10.1021/jf020514c. [DOI] [PubMed] [Google Scholar]
- 9.Landrault N., Poucheret P., Azay J., Krosniak M., Krosniak F. M., Gasc F. Effect of a polyphenols-enriched chardonnay white wine in diabetic rats. Journal of Agricultural and Food Chemistry . 2003;51:311–318. doi: 10.1021/jf020219s. [DOI] [PubMed] [Google Scholar]
- 10.Tian B., Yuan L., Zheng M.-Y., Xi Z.-M. Differences in anthocyanin accumulation profiles between teinturier and non-teinturier cultivars during ripening Meng. Foods . 2021;10(5):p. 1073. doi: 10.3390/foods10051073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dangles O., Saito N. Brouillard anthocyanin intramolecular copigment effect. Phytochemistry . 1993;34:119–124. doi: 10.1016/s0031-9422(00)90792-1. [DOI] [Google Scholar]
- 12.Dilip G., Tetsuya K. Anthocyanins and anthocyanin-rich extracts: role in diabetes and eye function. Asia Pacific Journal of Clinical Nutrition . 2007;16(2):200–208. [PubMed] [Google Scholar]
- 13.Yu M., Gouvinhas I., Rocha J., Barros A. I. R. N. A. Phytochemical and antioxidant analysis of medicinal and food plants towards bioactive food and pharmaceutical resources. Scientific Reports . 2021;11:p. 10041. doi: 10.1038/s41598-021-89437-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mattioli R., Francioso A., Mosca L., Silva P. Anthocyanins: a comprehensive review of their chemical properties and health effects on cardiovascular and neurodegenerative diseases. Molecules . 2020;25(17):p. 3809. doi: 10.3390/molecules25173809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang E., Yin Y., Xu C., Liu J. Isolation of high-purity anthocyanin mixtures and monomers fromblueberries using combined chromatographic techniques. Journal of Chromatography A . 2014;1327:39–48. doi: 10.1016/j.chroma.2013.12.070. [DOI] [PubMed] [Google Scholar]
- 16.McCue P., Kwon Y. I., Shetty K. Anti-amylase, anti-glucosidase and anti-angiotensin converting enzyme potential of selected foods. Journal of Food Biochemistry . 2005;29:278–294. doi: 10.1111/j.1745-4514.2005.00020.x. [DOI] [Google Scholar]
- 17.Kim Y. M., Jeong Y. K., Wang M. H., Lee W. Y., Rhee H. I. Inhibitory effect of pine extract on alpha-glucosidase activity and postprandial hyper hyperglycemia. Nutrition . 2005;21:756–761. doi: 10.1016/j.nut.2004.10.014. [DOI] [PubMed] [Google Scholar]
- 18.Wu X., Pittman H. E., Prior R. L. Fate of anthocyanins and antioxidant capacity in contents of the gastrointestinal tract of weanling pigs following black Raspberry consumption. Journal of Agricultural and Food Chemistry . 2006;54(2):583–589. doi: 10.1021/jf052108+. [DOI] [PubMed] [Google Scholar]
- 19.Pap N., Fidelis M., Azevedo L., Araújo M., Carmo V., Wang D. Berry polyphenols and human health: evidence of antioxidant, anti-inflammatory, microbiota modulation, and cell- protecting effects. Current Opinion in Food Science . 2021;42:167–186. doi: 10.1016/j.cofs.2021.06.003. [DOI] [Google Scholar]
- 20.Kong J. M., Chia L. S., Goh N. K., Chia T. F., Brouillard R. A., Brouillard R. Analysis and biological activities of anthocyanins. Phytochemistry . 2003;64(5):923–933. doi: 10.1016/s0031-9422(03)00438-2. [DOI] [PubMed] [Google Scholar]
- 21.Jagetia G. C., Rao S. K., Baliga M. S., Babu S. K. The evaluation of nitric oxide scavenging activity of certain herbal formulations in vitro: a preliminary study. Phytotherapy Research . 2004;18(7):561–565. doi: 10.1002/ptr.1494. [DOI] [PubMed] [Google Scholar]
- 22.Mokhber-Dezfuli N., Saeidnia S., Gohari A. R., Kurepaz-Mahmoodabadi M. Phytochemistry and pharmacology of Berberis species. Pharmacognosy Reviews . 2014;8(15):8–15. doi: 10.4103/0973-7847.125517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wallace T. C. Anthocyanins in cardiovascular disease. Advances in Nutrition . 2011;2(1–7) doi: 10.3945/an.110.000042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wang L. S., Stoner G. D. Anthocyanins and their role in cancer prevention. Cancer Letters . 2008;269:281–290. doi: 10.1016/j.canlet.2008.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Xia X., Ling W., Ma J., et al. An anthocyanin-rich extract from black rice enhances atherosclerotic plaque stabilization in apolipoprotein E-deficient mice. Journal of Nutition . 2006;136:2220–2225. doi: 10.1093/jn/136.8.2220. [DOI] [PubMed] [Google Scholar]
- 26.Grace M. H., Ribnicky D. M., Kuhn P., Poulev A., Logendra S., Yousef G. G. Hypoglycemic activity of a novel anthocyanin-rich formulation from lowbush blueberry, Vaccinium angustifolium Aiton. Phytomedicine . 2009;16:406–415. doi: 10.1016/j.phymed.2009.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ghosh S., More P., Derle A., Patil A. B., Markad P. Novel hit for the treatment of type II diabetes Mellitus with inhibitory activity against α-amylase and α-glucosidase. PLoS One . 2014;9:p. e106039. doi: 10.1371/journal.pone.0106039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Du Q., Zheng J., Xu Y. Composition of anthocyanins in mulberry and their antioxidant activity. Journal of Food Composition and Analysis . 2008;21:90–395. doi: 10.1016/j.jfca.2008.02.007. [DOI] [Google Scholar]
- 29.Peng C. H., Liu L. K., Chuang C. M., Chyau C. C., Huang C. N., Wang C. J. Mulberry water extracts possess an anti-obesity effect and ability to inhibit hepatic lipogenesis and promote lipolysis. Journal of Agricultural and Food Chemistry . 2011;59:2663–2671. doi: 10.1021/jf1043508. [DOI] [PubMed] [Google Scholar]
- 30.Kalita D., Holm D. G., LaBarbera D. V., Petrash J. M., Jayanty S. S. Inhibition of α-glucosidase, α-amylase, and aldose reductase by potato polyphenolic compounds. PLoS One . 2018;13(1):p. e0191025. doi: 10.1371/journal.pone.0191025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Spínola V., Lorent-Martínez E. J., Castilho P. C. Inhibition of α-amylase, α-glucosidase by phenolic compounds of Rumex maderensis (Madeira sorrel). Influence of simulated gastrointestinal digestion on hyperglycaemia-related damage linked with aldose reductase activity and protein glycation. LWT- Food Science and Technology . 2020;118:p. 108727. [Google Scholar]
- 32.Narita Y., Inouye K. Kinetic analysis and mechanism on the inhibition of chlorogenic acid and its components against porcine pancreas α-amylase isozymes I and II. Journal of Agricultural and Food Chemistry . 2009;57:9218–9225. doi: 10.1021/jf9017383. [DOI] [PubMed] [Google Scholar]
- 33.Yin Z., Zhang W., Feng F., Zhang Y. α-Glucosidase inhibitors isolated from medicinal plants”. Food Sci Hum Well . 2014;3:136–174. doi: 10.1016/j.fshw.2014.11.003. [DOI] [Google Scholar]
- 34.Ghosh D., Konishi T. Anthocyanins and anthocyanin-rich extracts: role in diabetes and eye function. Asia Pacific Journal of Clinical Nutrition . 2007;16(2):200–208. [PubMed] [Google Scholar]
- 35.Ali A., Qazalbash M. A., Ali S., Hussain A. A comparative study of barberry fruits in terms of its nutritive and medicinal contents from CKNP region, Gilgit-Baltistan, Pakistan. Journal of Biodiversity and Environmental Sciences . 2014;5(2):9–17. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data used to support the findings of this study are available from the corresponding author upon request.


