Abstract
Streptococcus gallolyticus is an uncommon cause of neonatal infections. We describe the first case of fulminant lethal neonatal sepsis due to S. gallolyticus reported in literature. Our patient was an extremely low birth weight premature infant born to a mother with prolonged rupture of amniotic membranes and chorioamnionitis. We also review the cases of neonatal S. gallolyticus infections reported in literature. Fifty-eight percent neonatal S. gallolyticus infections presented in the first week of life. Importantly, S. gallolyticus meningitis is more commonly reported with early-onset infections compared with group B streptococcal meningitis, which is more common with late-onset infections. Streptococcus gallolyticus should be included in differential for neonatal sepsis, particularly in the presence of meningitis in the first week of life. Most cases are sensitive to penicillin; however, cases of reduced sensitivity to penicillin have also been reported.
Keywords: neonatal sepsis, Streptococcus bovis, Streptococcus gallolyticus, Streptococcus pasteurianus, neonatal meningitis, chorioamnionitis, preterm
Streptococcus gallolyticus is a group of bacteria that belong to the nonenterococcal group D streptococci previously known as S. bovis/S. equinus complex (SBSEC). 1 Streptococcus gallolyticus has been reported as a cause of adult gastrointestinal tract infections and infective endocarditis for decades but is an uncommon cause of neonatal infections. 1 2 However, over the past decade, there have been increasing reports of neonatal sepsis, meningitis, and intrauterine infections due to S. gallolyticus. Here, we report an unusual case of a preterm, extremely low birth weight (ELBW) male neonate who developed fulminant early-onset sepsis due to S. gallolyticus . To the best of our knowledge, no prior cases of fulminant lethal sepsis due to S. gallolyticus have been reported to date. We also review the cases of neonatal S. gallolyticus infections reported in literature.
Case Presentation
A male infant was born at 26 weeks of gestation to a 25-year-old G5 now P5 mother with good prenatal care. Pregnancy was complicated by chronic hypertension and premature prolonged rupture of membranes 12 days before delivery for which the mother received 7 days of latency antibiotics (48 hours of intravenous ampicillin followed by 5 days of oral amoxicillin and a single dose of azithromycin) that was completed 5 days prior to delivery. She developed chorioamnionitis prior to delivery for which she received one dose of ampicillin.
Mother had a history of trichomonas infection during pregnancy which was treated. Her other prenatal infectious laboratories including group B streptococcal (GBS) culture were negative. The infant was born via spontaneous vaginal delivery with a birth weight of 950 g. In the delivery room, the infant required positive pressure ventilation and intubation. APGAR scores were 6, 3, and 4 at 1, 5, and 10 minutes, respectively. The infant was then admitted to the neonatal intensive care unit (NICU).
The infant's arterial cord blood gas pH was 6.96 with a pCO 2 of 76 mm Hg and a base excess of −15.9 mmol/L. His venous cord blood gas pH was 7.17 with a pCO 2 of 42 mm Hg and a base excess of −12.7 mmol/L.
The infant had mixed metabolic and respiratory acidosis on admission (pH 6.9) and required high ventilator support. The respiratory acidosis improved with ventilator adjustments and surfactant administration; however, he had persistent metabolic acidosis. He also had leukopenia (white blood cell count 1.5 × 10 3 /mm 3 ), thrombocytopenia (platelet count was 89 × 10 3 /mm 3 ), and an elevated C-reactive protein level of 21.1 mg/L. Blood culture was sent on admission and the infant was prescribed ampicillin and gentamicin.
Around 4.5 hours of life, the infant became hypotensive and rapidly deteriorated despite fluid resuscitation and receiving pressors (dopamine and dobutamine). A repeat blood culture and tracheal aspirate were obtained, but the infant died at 5.5 hours of life after he remained unresponsive to resuscitative efforts.
Parents declined an autopsy. Both blood cultures resulted positive for gram-positive cocci within 12 hours of incubation, which were further identified as S. gallolyticus using MALDI-TOF (matrix-assisted laser desorption/ionization time-of-flight) biotyper. Unfortunately, genotyping was not possible in our laboratory, and hence, we were unable to further classify this pathogen. The tracheal aspirate culture was negative. Placental pathology confirmed acute chorioamnionitis of the fetal membranes.
Discussion
This is the first case of S. gallolyticus in an ELBW infant leading to fulminant sepsis. Streptococcus gallolyticus has been often used interchangeably with S. bovis in literature. 2 Over the years, SBSEC has undergone numerous taxonomical changes. Based on their ability to ferment mannitol, S. bovis has been classified in the older literature into two biotypes, mannitol-fermenting biotype I and mannitol nonfermenting biotype II. Biotype II has been divided further into subtypes 1 and 2 based on starch and bile-esculin hydrolysis and trehalose acidification. 1 3 4 5 The most recent taxonomic classification uses genetic methodology to classify SBSEC into four species S. gallolyticus (further divided into subsp. gallolyticus , pasteurianus , and macedonicus ), S. alactolyticus , S. infantarius (divided into subsp. infantarius and coli ), and S. equinus . 1 3 4 5 Fig. 1 shows a simplified taxonomical classification of S. bovis .
Fig. 1.
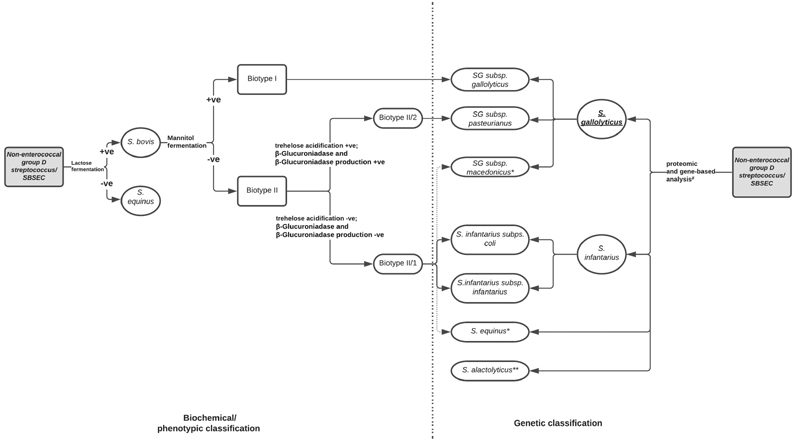
Taxonomical classification of S. bovis : This figure shows the correlation between the previously used biochemical/phenotypic classification and the new genetic classification of nonenterococcal group D Streptococcus . Reconstructed based on the description by Dekker and Lau, 1 Schlegel et al, 4 and Jans et al. 5 # Methods used for analysis include MALDI-TOF (proteomic-based), 16s rRNA, sodA, and groEL sequencing (single-gene-based) and/or whole genome sequencing. 1 5 * Streptococcus equinus and S. macedonicus likely belong to biotype II/1 based on phenotypic data. 5 **Phenotypic data for S. alactolyticus is variable, and hence, biotypic classification is not possible. 5 BE, bile-esculin; MALDI-TOF, matrix-assisted laser desorption/ionization time-of-flight; SBSEC, Streptococcus bovis/Streptococcus equinus complex; SG, Streptococcus gallolyticus ; subsp., subspecies.
Streptococcus gallolyticus is prevalent in the bowel flora in humans. In adults, S. gallolyticus subsp. gallolyticus is commonly associated with infective endocarditis, bacteremia, gastrointestinal infections including hepatobiliary infections, and colon cancer. 1 2 3 Streptococcus pasteurianus has been commonly associated with meningitis. 1 Although uncommon, S. gallolyticus subsp. gallolyticus and S. pasteurianus have emerged as important causes of neonatal sepsis in recent years. Streptococcus gallolyticus subsp. macedonicus has not been associated with infections in humans.
Streptococcus infantarius has been associated with noncolonic cancers and is an uncommon cause of adult sepsis. 1 Only two cases of neonatal infections with S. infantarius subsp. coli (also known as S. lutetiensis ) have been reported in literature. 6
To review the cases of neonatal invasive S. gallolyticus infections reported in the English literature, we performed a MEDLINE search and found 66 cases of neonates (≤ 28 days old) reported to be infected by S. bovis . Twenty-nine cases were classified only as S. bovis and four cases as S. bovis biotype II. Infections with S. pasteurianus ( S. bovis biotype II/2) has been much more commonly reported compared with that with S. infantarius ( S. bovis biotype II/1) in both neonates and adults, thus making it likely that the cases without further identification beyond S. bovis / S. bovis biotype II belong to the S. gallolyticus species. However, due to lack of certainty, these cases ( n = 33) were not included in this review. Of the remaining 33 cases, two cases were reported as S. lutetiensis infection and one case as S. alactolyticus infection and hence were also not included. We analyzed the remaining 30 cases of neonatal S. gallolyticus infections reported in literature before November 30, 2021, and the present case ( n = 31) ( Table 1 ). 2 7 8 9 10 11 12 13 14 15 16 17 18 19 20 21 Of the 30 cases reported in literature, only 10 cases were reported from the United States. 10 13 14 21
Table 1. Summary of the neonatal cases of Streptococcus gallolyticus reported in literature .
| Reference | Number of pts reported | Birthweight (kg) | GA (wk) | Delivery type | Age of presentation | Organism | Clinical symptoms | Sites organism isolated from | Diagnosis | Final antibiotic therapy course | Final disposition |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Gavin et al (2003) 21 | 1 | 3.925 | Term | Vaginal | 3 d | SG subsp. pasteurianus | Fever, seizures | Blood + CSF | Bacteremia, meningitis | Penicillin G × 14 d | Survived |
| Onoyama et al (2009) 7 | 1 | 3.19 | Term | Vaginal | 4 d | SG subsp. pasteurianus | Fever, decreased activity | Blood + CSF | Bacteremia, meningitis | Cefotaxime × 14 d | Survived |
| Khan (2009) 8 | 1 | Not reported | Not reported | Not reported | 3 d | SG subsp. pasteurianus | Apnea, lethargy | Blood + CSF | Bacteremia, meningitis | Penicillin and gentamicin × 14 d | Survived |
| Floret et al (2010) 9 | 5 a | Not reported | Preterm | Not reported | 13–56 d | SG subsp. pasteurianus | Gastrointestinal symptoms in all pts including abdominal distension and diarrhea | Blood | Bacteremia | Cefotaxime × 10 d | Survived |
| Klatte et al. (2012) 10 | 4 | Not reported | Term | Vaginal | 2–13 d | SG subsp. pasteurianus | Pt 1: congestion, respiratory distress, increased sleepiness Pt 2: fever, seizures Pt 3: lethargy, seizures Pt 4: fever, congestion, lethargy |
CSF in all pts Blood in pts 2–4 |
Meningitis: all pts Bacteremia: pts 2–4 |
Pts 1 and4: Cefotaxime × 14–16 d Pts 2 and 3: ampicillin 14–16 d |
Survived |
| Nagamatsu et al (2012) 11 | 1 | 3.092 | 40 | Vaginal | 8 d | SG subsp. pasteurianus | Seizures, fever, inconsolability, decreased oral intake | CSF | Meningitis | Ampicillin, panipenem/betamipron × 20 d | Survived |
| Thatrimontrichai et al (2012) 12 | 1 | 3.188 | 39 | Vaginal | 3 d | SG subsp. pasteurianus | Fever, lethargy, poor oral intake, bulging fontanelle | CSF | Meningitis | Cefotaxime × 14 d | Survived |
| Hede et al (2015) 13 | 2 (twins) | Pt 1: 2.12 Pt 2: 1.47 |
32 | C-section | 3 wk | SG subsp. pasteurianus | Pt 1: lethargy, irritability, respiratory distress, poor feeding, loose stools, seizures Pt 2: respiratory distress |
Pt 1: blood + CSF Pt 2: blood |
Meningitis in both pts Sepsis in pt 1 Bacteremia in pt 2 |
Ampicillin × 10–20 d | Both pts survived, but pt 1 was reported to have long-term neurologic deficits |
| Kennedy et al (2015) 14 | 1 | 3.05 | 37 | Vaginal | 4 d | SG subsp. gallolyticus | Fever, lethargy, irritability. | Blood + CSF | Bacteremia and meningitis | Ampicillin × 14 d | Survived |
| Park et al (2015) 15 | 1 | 3.6 | 38 | Vaginal | 28 d | SG subsp. pasteurianus | Fever, lethargy | CSF + blood + urine | Meningitis, UTI, bacteremia | Ampicillin + cefotaxime × 21 d | Survived, but rehospitalized 2 wk later due to fever and seizures, requiring 31 additional d of ampicillin |
| Saegeman et al (2016) 16 | 1 | Not reported | 30 | Not reported | 7 d | SG subsp. pasteurianus | Sepsis | Blood | Sepsis | Penicillin, duration unknown | Survived |
| Yamamura et al (2018) 17 | 1 | 3.68 | Term | Vaginal | 27 d | SG subsp. pasteurianus | Fever, lethargy, irritability, cold extremities | Blood + CSF | Sepsis, meningitis | Ampicillin × 22 d | Survived |
| Nguyen et al (2019) 18 | 2 | 3.25–4.19 | 39–40 | Vaginal | < 24 h | SG subsp. Pasteurianus | Pt 1: respiratory distress Pt 2: respiratory distress, sepsis |
Blood | Pt 1: sepsis, infective endocarditis, meningitis Pt 2: sepsis |
Pt 1: cefepime × 28 d, gentamicin × 14 d Pt 2: cefepime and clindamycin × 14 d |
Survived |
| Chen et al (2021) 19 | 3 (pt 2 and 3 were twins) | 1.86–2.58 | Pt 1: 35 Pts 2 and 3: 37 |
Pt 1: C-section Pts 2 and 3: not reported |
2–5 d | SG subsp. Pasteurianus | Pt 1: apnea, desaturation Pts 2 and 3: fever, tachypnea, desaturation |
Pt 1: blood Pt 2: blood + CSF Pt 3: blood |
Sepsis, meningitis | Ampicillin + cefotaxime × 14 d | Survived |
| Geetha et al (2021) 2 | 1 | 3.77 | 36 | Vaginal | < 24 h | SG subsp. pasteurianus | Respiratory distress, sepsis | Blood | Liver abscess, sepsis | Cefotaxime × 5 wk, coamoxiclav for 3 wk | Survived |
| Sim et al (2021) 20 | 4 | 1.81–3.37 | Pts 1–3: term; Pt 4: 34 |
Pts 1–3: vaginal Pt 4: C-section |
1–23 d | SG | Pts 1 and 2: respiratory distress Pts 2–4: fever Pts 3 and 4: lethargy/poor feeding |
Pts 1–4: blood Pts 1 and 2: CSF |
Sepsis: all pts Meningitis: pts 1 and 2 |
Ampicillin, clindamycin, or vancomycin × 7–14 d | Survived |
| This case | 1 | 0.95 | 26 | Vaginal | < 24 h | SG | Respiratory distress, sepsis | Blood | Sepsis | n/a | Died at 5 h |
Abbreviations: CSF, cerebrospinal fluid; GA, gestational age; GBS, group B Streptococcus ; pt, patient; S., Streptococcus ; SG, Streptococcus gallolyticus ; subsp., subspecies; UTI, urinary tract infection.
Exact number of neonates (≤ 28 days) unknown.
Fifty-eight percent of the patients presented during the first week of life. There was a slightly higher incidence of early-onset (≤ 6 days) ( n = 17; 55%) and late-onset infections (> 6 days) ( n = 14; 45%) due S. gallolyticus . Gestational age was unknown for one infant and 12 of 30 (40%) infants were premature (gestational age 26–36 weeks). Presenting symptoms included respiratory distress, apnea, metabolic acidosis, fever, lethargy, abdominal distension, loose stools, congestion, poor feeding, and seizures. In five cases, further identification beyond S. gallolyticus was not performed. When classification was available, S. pasteurianus was more common than S. gallolyticus subsp. gallolyticus (25 vs. 1).
Meningitis was reported in 19 patients (18 due to S. pasteurianus and 1 due to S. gallolyticus subsp. gallolyticus ). Hede et al 13 hypothesized that S. gallolyticus infection in neonates follows the pattern of early- and late-onset GBS diseases. However, unlike GBS infection, S. gallolyticus infection was associated with a higher rate of meningitis (63%), and early-onset S. gallolyticus infection was more likely to be associated with meningitis compared with late-onset infection (76 vs. 43%). 22 Meningitis was more commonly reported in term neonates (16 of 18; 89%) compared with preterm neonates (2 of 12; 17%).
In one patient, bacteremia was associated with infective endocarditis, and in another patient with liver abscess. 2 18 Park et al 15 reported a case of urinary tract infection due to S. pasteurianus in the absence of pyuria.
Most patients were treated with a penicillin and/or a third-generation cephalosporin and the duration of antimicrobial therapy ranged from 7 days to 8 weeks (median: 14 days; average: 15 days). Although all patients had a severe clinical course, most patients had a favorable outcome. All patients, except this case, survived the acute infection. One patient had to be rehospitalized 2 weeks postdischarge due to partially treated meningitis but had no long-term neurological sequalae. 15 Only one patient with meningitis was reported to have long-term neurological deficits. 13
This is the first reported case of fulminant lethal sepsis due to S. gallolyticus . Our patient was an ELBW preterm infant with history of prolonged rupture of amniotic membranes (12 days) and acute chorioamnionitis which may have resulted in the particularly severe course in this case.
The exact route of S. gallolyticus infection in neonates remains uncertain. It is presumed that like GBS, S. gallolyticus infection occurs either vertically via transvaginal transmission or postnatal horizontal transmission. 13 Fikar and Levy 23 reported positive rectal and vaginal cultures from the patient's mother 2 weeks following the onset of symptoms in a neonate with S. bovis meningitis. In another report, mother of the infant with S. pasteurianus meningitis grew Escherichia coli and Group D Streptococcus in the urine culture collected on fourth postpartum day. 12 A case of intrapartum infection and postpartum bacteremia without neonatal infection has also been reported. 24 Floret et al 9 and Saegeman et al 16 reported clusters of neonatal infections due to S. pasteurianus in their respective NICUs, likely due to horizontal transmission from health care workers. In one case series, one of the four patients had history of maternal contact with chicken who died 1 week prior to patient's birth; however, postmortem testing of chickens was not performed. 10
Similar to GBS, S. gallolyticus is often sensitive to penicillin; however, cases with reduced susceptibility to penicillin have been reported including two cases of neonatal meningitis due to S. pasteurianus . 3 8 10 This organism is also susceptible to aminoglycosides, cephalosporin, and vancomycin, and high rates of resistance to quinolones, macrolides, and tetracyclines have been reported. 3
Conclusion
Streptococcus gallolyticus must be considered an important differential for neonatal sepsis particularly, in the presence of meningitis in the first week of life when maternal GBS is negative. Appropriate identification and classification of the organism are important to further understand the epidemiology of neonatal infections due to S. gallolyticus . Culture sensitivity should be performed to determine appropriate antibiotic for treatment due to the increasing rates of reduced susceptibility to penicillin. Although no mortality was reported in previous cases of neonatal S. gallolyticus infections, this case shows that S. gallolyticus in ELBW infants may be lethal.
Footnotes
Conflict of Interest None declared.
References
- 1.Dekker J P, Lau A F. An update on the Streptococcus bovis group: classification, identification, and disease associations . J Clin Microbiol. 2016;54(07):1694–1699. doi: 10.1128/JCM.02977-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Geetha O, Cherie C, Natalie T WH, Merchant K, Chien C M, Chandran S. Streptococcus gallolyticus subspecies pasteurianus causing early onset neonatal sepsis complicated by solitary liver abscess in a preterm infant . Access Microbiol. 2021;3(03):200. doi: 10.1099/acmi.0.000200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pompilio A, Di Bonaventura G, Gherardi G. An overview on Streptococcus bovis/Streptococcus equinus complex isolates: identification to the species/subspecies level and antibiotic resistance . Int J Mol Sci. 2019;20(03):480. doi: 10.3390/ijms20030480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Schlegel L, Grimont F, Ageron E, Grimont P AD, Bouvet A. Reappraisal of the taxonomy of the Streptococcus bovis / Streptococcus equinus complex and related species: description of Streptococcus gallolyticus subsp. gallolyticus subsp. nov., S. gallolyticus subsp. macedonicus subsp. nov. and S. gallolyticus subsp. pasteurianus subsp. nov Int J Syst Evol Microbiol 200353(Pt 3):631–645. [DOI] [PubMed] [Google Scholar]
- 5.Jans C, Meile L, Lacroix C, Stevens M J. Genomics, evolution, and molecular epidemiology of the Streptococcus bovis / Streptococcus equinus complex (SBSEC) . Infect Genet Evol. 2015;33:419–436. doi: 10.1016/j.meegid.2014.09.017. [DOI] [PubMed] [Google Scholar]
- 6.Yu A T, Shapiro K, Beneri C A, Wilks-Gallo L S. Streptococcus lutetiensis neonatal meningitis with empyema . Access Microbiol. 2021;3(09):264. doi: 10.1099/acmi.0.000264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Onoyama S, Ogata R, Wada A, Saito M, Okada K, Harada T. Neonatal bacterial meningitis caused by Streptococcus gallolyticus subsp. pasteurianus J Med Microbiol 200958(Pt 9):1252–1254. [DOI] [PubMed] [Google Scholar]
- 8.Khan A. Relative penicillin resistance in Streptococcus bovis . A case of neonatal meningitis J Paediatr Child Health 200945(7-8):474–475. [DOI] [PubMed] [Google Scholar]
- 9.Floret N, Bailly P, Thouverez M. A cluster of bloodstream infections caused by Streptococcus gallolyticus subspecies pasteurianus that involved 5 preterm neonates in a university hospital during a 2-month period . Infect Control Hosp Epidemiol. 2010;31(02):194–196. doi: 10.1086/650380. [DOI] [PubMed] [Google Scholar]
- 10.Klatte J M, Clarridge J E, III, Bratcher D, Selvarangan R. A longitudinal case series description of meningitis due to Streptococcus gallolyticus subsp. pasteurianus in infants . J Clin Microbiol. 2012;50(01):57–60. doi: 10.1128/JCM.05635-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nagamatsu M, Takagi T, Ohyanagi T. Neonatal meningitis caused by Streptococcus gallolyticus subsp. pasteurianus. J Infect Chemother. 2012;18(02):265–268. doi: 10.1007/s10156-011-0320-4. [DOI] [PubMed] [Google Scholar]
- 12.Thatrimontrichai A, Chanvitan P, Janjindamai W, Dissaneevate S, Maneenil G. Early onset neonatal bacterial meningitis caused by Streptococcus gallolyticus subsp. pasteurianus. Southeast Asian J Trop Med Public Health. 2012;43(01):145–151. [PubMed] [Google Scholar]
- 13.Hede S V, Olarte L, Chandramohan L, Kaplan S L, Hulten K G. Streptococcus gallolyticus subsp. pasteurianus infection in twin infants . J Clin Microbiol. 2015;53(04):1419–1422. doi: 10.1128/JCM.02725-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kennedy G J, Kavanagh K L, Kashimawo L A, Cripe P J, Steele R W. An unlikely cause of neonatal sepsis. Clin Pediatr (Phila) 2015;54(10):1017–1020. doi: 10.1177/0009922815591894. [DOI] [PubMed] [Google Scholar]
- 15.Park J W, Eun S H, Kim E C, Seong M W, Kim Y K. Neonatal invasive Streptococcus gallolyticus subsp. pasteurianus infection with delayed central nervous system complications . Korean J Pediatr. 2015;58(01):33–36. doi: 10.3345/kjp.2015.58.1.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Saegeman V, Cossey V, Loens K, Schuermans A, Glaser P. Streptococcus Gallolyticus subsp. pasteurianus infection in a neonatal intensive care unit . Pediatr Infect Dis J. 2016;35(11):1272–1275. doi: 10.1097/INF.0000000000001290. [DOI] [PubMed] [Google Scholar]
- 17.Yamamura Y, Mihara Y, Nakatani K, Nishiguchi T, Ikebe T. Unexpected ventriculitis complication of neonatal meningitis caused by Streptococcus gallolyticus subsp. pasteurianus : a case report . Jpn J Infect Dis. 2018;71(01):68–71. doi: 10.7883/yoken.JJID.2017.053. [DOI] [PubMed] [Google Scholar]
- 18.Nguyen M T, Idriss S, Guzman E, De Oliveira E R. Neonatal meningitis, endocarditis, and pneumonitis due to Streptococcus gallolyticus subsp. pasteurianus : a case report . BMC Pediatr. 2019;19(01):265. doi: 10.1186/s12887-019-1645-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chen W C, Lee P I, Lin H C. Clustering of Streptococcus gallolyticus subspecies pasteurianus bacteremia and meningitis in neonates . J Microbiol Immunol Infect. 2021;54(06):1078–1085. doi: 10.1016/j.jmii.2020.07.004. [DOI] [PubMed] [Google Scholar]
- 20.Sim J Y, Wang L W, Chow J C. Streptococcus gallolyticus - a potentially neglected pathogen causing neonatal sepsis not covered by routine group B Streptococcus screening . J Microbiol Immunol Infect. 2021;54(06):1190–1192. doi: 10.1016/j.jmii.2021.05.003. [DOI] [PubMed] [Google Scholar]
- 21.Gavin P J, Thomson R B, Jr, Horng S J, Yogev R. Neonatal sepsis caused by Streptococcus bovis variant (biotype II/2): report of a case and review . J Clin Microbiol. 2003;41(07):3433–3435. doi: 10.1128/JCM.41.7.3433-3435.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.COMMITTEE ON FETUS AND NEWBORN; COMMITTEE ON INFECTIOUS DISEASES Puopolo K M, Lynfield R, Cummings J J.Management of Infants at Risk for Group B Streptococcal Disease [published correction appears in Pediatrics 2019 Oct;144(4): e20192350 ] Pediatrics 201914402e20191881. [DOI] [PubMed] [Google Scholar]
- 23.Fikar C R, Levy J. Streptococcus bovis meningitis in a neonate . Am J Dis Child. 1979;133(11):1149–1150. doi: 10.1001/archpedi.1979.02130110057009. [DOI] [PubMed] [Google Scholar]
- 24.Binghuai L, Wenjun S, Xinxin L. Intrauterine infection and post-partum bacteraemia due to Streptococcus gallolyticus subsp. pasteurianus J Med Microbiol 201362(Pt 10):1617–1619. [DOI] [PubMed] [Google Scholar]


