Abstract
A 7-month-old female Holstein calf presented with bilateral microtia and absent external acoustic meatus. The real-time polymerase chain reaction test was negative for bovine viral diarrhea virus and bovine leukemia virus. The calf’s dam had a normal reproductive history. Computed tomography confirmed bilateral atresia of external auditory canals, aplasia of tympanic cavities and the ossicular chain, and temporomandibular joint abnormality. Necropsy revealed a severe malformation of the temporal bone. In the tympanic region, the external acoustic pore, tympanic bulla, and muscular process were absent bilaterally. The bilateral inner ear structure was normal. Based on these findings, we diagnosed the present case as congenital malformations of the external and middle ear accompanied by temporal bone anomaly.
Keywords: calf, conductive hearing loss, congenital ear malformation, temporal bone anomaly
Congenital malformations of the ear are seldom diagnosed in human or veterinary medicine [1, 2, 9,10,11, 13, 14]. The malformations can occur in the external, middle, and inner ear and cause conductive or sensorineural hearing loss. In many animals, including cattle, hereditary sensorineural deafness associated with loci for white pigmentation is reported, resulting from inner ear malformation [8, 12]. Although a few cases of atresia auris congenita, anotia, and microtia as congenital malformations of the external and/or middle ear have been reported in dogs [9,10,11], cats [2], cattle [3, 7], and sheep [5], few detailed pathological analyses of these malformations exist in the field of veterinary medicine. The present study is the first to precisely report the computed tomographic and pathological findings of congenital malformations of the external and middle ear accompanied by a temporal bone anomaly in a calf.
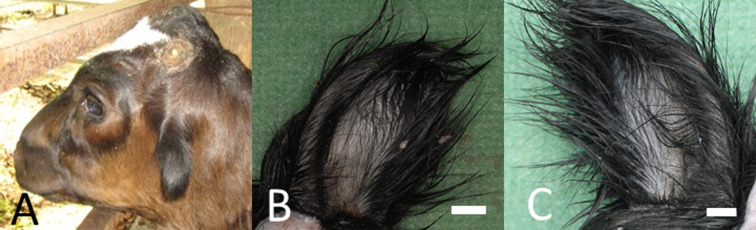
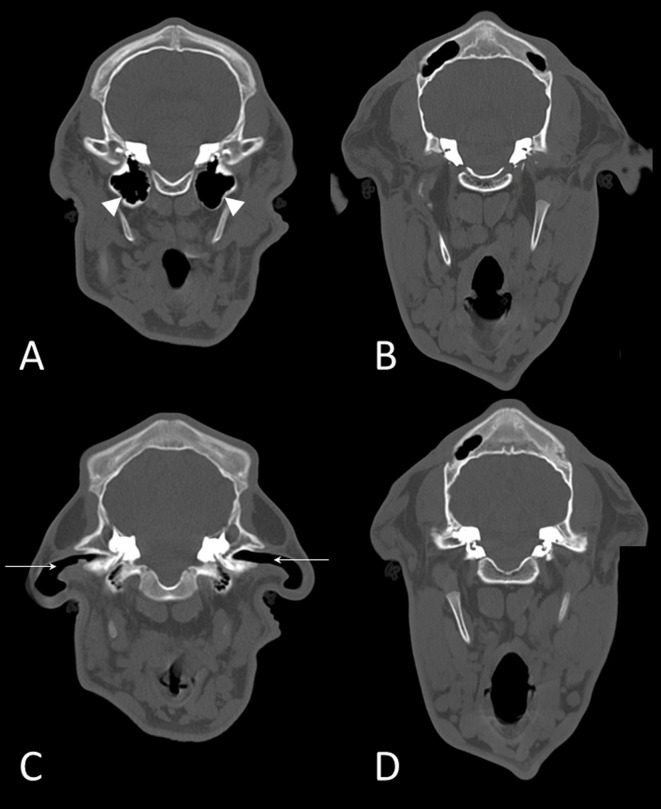
A 7-month-old female Holstein calf presented with bilateral microtia (Fig. 1A) and absence of the external acoustic meatus in both ears (Fig. 1B, 1C). The calf was negative for bovine viral diarrhea virus and bovine leukemia virus by real-time polymerase chain reaction test. The calf’s dam was nine years old and had 8 normal reproductive history. Bilateral microtia and absence of the external acoustic meatus in both ears were confirmed clinically at birth. Vomiting and salivation at rest, feeding disorder with regurgitation, and growth retardation had been confirmed from the age of 5 months. These clinical signs became severe over time, and the calf was emaciated; therefore, it was euthanized and necropsied at 7 months of age. The calf did not show any signs of motor dysfunction, and no hearing loss was noticed until necropsy. Radiographic examination confirmed deformation and shortening of the mandibles. Computed tomography confirmed bilateral atresia of external auditory canals, aplasia of tympanic cavities and the ossicular chain, and temporomandibular joint abnormality (Fig. 2A–D).
Fig. 1.
Gross findings. Microtia (A) and absence of the external acoustic meatus in both ears (B, C). Bar=1 cm.
Fig. 2.
Computed tomographic images (bone window) of a normal calf (A, C) and the present case (B, D). Tympanic cavities (arrowheads in A) are absent in this case (B). External auditory canals (arrows in C) are absent in this case (D).
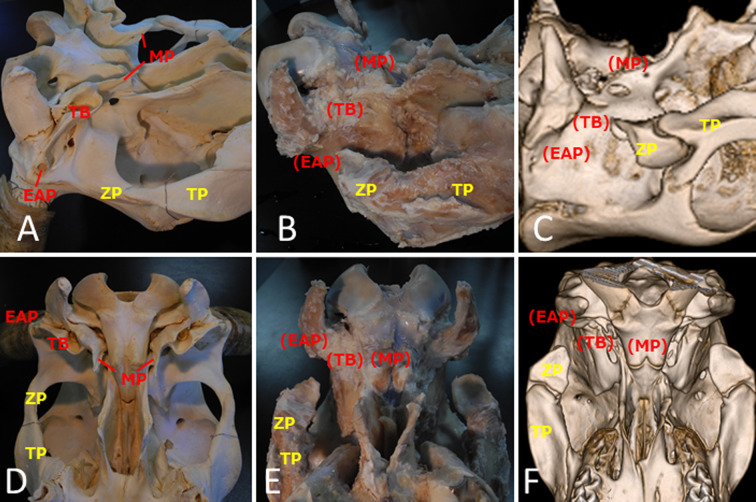
The external auditory canal were absent at necropsy, and the pinnae were small and deformed (Fig. 1B, 1C). On the right-hand side, a stenosed duct, 1.5 cm in length, was found on the lateral side of the external auditory canal. There was no duct on the left-hand side. There were thin restiform collagen fibers in place of the external auditory canal, and adipose tissue filled the space. There were no tympanic cavities or eustachian tubes. Severe malformation was observed at the temporal bone (Fig. 3A–F). At the petrous part, the mastoid process and styloid process were absent bilaterally. The facial canal and internal acoustic pore were intact, and facial nerves and vestibulocochlear nerves entered, respectively. At the tympanic part, the external acoustic pore, tympanic bulla, and muscular process were absent bilaterally. The zygomatic processes were partially absent at the squamous part. They connected to the zygomatic bone’s temporal processes rostrally but did not connect to the squamous parts caudally. Most of the mandibular fossa was absent, and it articulated with the mandible by the lateral one-third of the articular surface. The lateral opening of the temporal meatus was not confirmed bilaterally. The zygomatic bone, pterygoid bone, and mandible were deformed, consistent with the temporal bone malformation. The deformed and shortened mandibles showed a left–right asymmetry with a severely deformed condylar process. The necropsy revealed no other skeletal or organ malformations.
Fig. 3.
Malformation of the temporal bone. Skeletal specimens of normal cattle (A, D). Skeletal specimens (B, E) and 3D computed tomography images (C, F) of the present case. At the tympanic part, external acoustic pore (EAP), tympanic bulla (TB), and muscular process (MP) are completely absent bilaterally. Zygomatic processes (ZP) are partially absent at the squamous part, which connect to zygomatic bone temporal processes (TP) rostrally, but not to the squamous part.
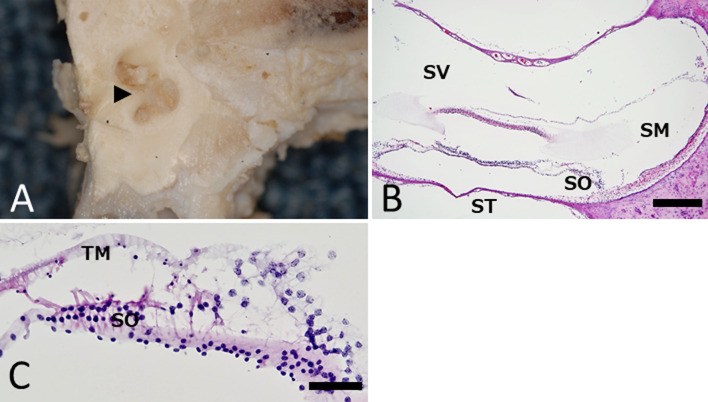
After formalin fixation, the temporal bone was cut at the tympanic part level by the frontal plane, and normal bilateral inner ear structure was confirmed (Fig. 4A). After decalcification, the tissues were embedded in paraffin, sectioned at 4 µm thickness, and stained them with hematoxylin and eosin stain. The normal structure of the inner ear (scala media, scala vestibuli, scala tympani, and spiral organ) was histologically confirmed (Fig. 4B, 4C).
Fig. 4.
Frontal plane of tympanic part. (A) Gross findings. Normal inner ear structure (arrow head) is confirmed. (B) Histological findings. Normal structure of the inner ear is confirmed: scala media (SM), scala vestibuli (SV), scala tympani (ST), and spiral organ (SO). Hematoxylin and eosin stain. Bar=500 μm. (C) High-magnification image of SO. SO and tectorial membrane (TM) is confirmed. Hematoxylin and eosin stain. Bar=50 μm
Based on these findings, we diagnosed the present case as congenital malformations of the external and middle ear accompanied by temporal bone anomaly. Many parts of temporal bone developed incompletely in the present case, so deformation of the zygomatic bone, pterygoid bone, and mandible was considered secondary to temporal bone malformation. The feeding disorder and vomiting were caused by malocclusion secondary to the bone deformation.
Embryologically, external auditory canal, middle ear, and ossicular chain develop concomitantly from the first branchial groove, first and second branchial arches, and first pharyngeal pouch. While the inner ear development occurs independently of external and middle ear structures, it develops from the auditory placode at an earlier stage than external and middle ear development [1, 14]. Therefore, concomitant anomalies of the outer/middle ear and the inner ear are unusual [1, 14]. This calf also had malformations of the external and middle ear but a normal inner ear structure. Therefore, the developmental defect must have occurred at the time of outer/middle ear development in the present case.
The ear malformations can cause hearing loss categorized as conductive, sensorineural, or both. Conductive hearing loss occurs when sound conduction is impeded through the external ear, the middle ear, or both. Sensorineural hearing loss occurs when there is a problem within the inner ear: the cochlea or the neural pathway to the auditory cortex [4]. Because the inner ear is involved in sound and balance, developmental defects in the inner ear can also cause balance disorders [6]. The present case, which did not show any motor dysfunction, had a functionally and structurally normal inner ear. Although no hearing loss was noticed until necropsy in this calf, the lack of the external and middle ear indicated the presence of conductive hearing loss.
Ear malformations or hearing loss may have a genetic or an acquired background [1, 12]. In many animals, including cattle, hereditary sensorineural deafness associated with loci for white pigmentation is reported, resulting from inner ear malformation [8]. Acquired ear malformations originate from exogenic injury during pregnancy, such as infections, chemical agents, malnutrition, irradiation, Rh incompatibility, hypoxia, atmospheric pressure changes, and noise exposure in humans and animals [1, 12]. In the present case, investigations of the dam and pathogens were limited, and the actual cause was unknown.
CONFLICT OF INTEREST
There are no conflicts of interest on this article.
Acknowledgments
This work was supported by JSPS KAKENHI Grant Number 19K16006.
REFERENCES
- 1.Bartel-Friedrich S., Wulke C.2007. Classification and diagnosis of ear malformations. GMS Curr. Top. Otorhinolaryngol. Head Neck Surg. 6: Doc05. [PMC free article] [PubMed] [Google Scholar]
- 2.Coomer A. R., Bacon N.2009. Primary anastomosis of segmental external auditory canal atresia in a cat. J. Feline Med. Surg. 11: 864–868. doi: 10.1016/j.jfms.2009.02.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hill F. I.2003. A triad of bovine inherited diseases (abstract). N. Z. Vet. J. 51: 46. doi: 10.1080/00480169.2003.36338 [DOI] [PubMed] [Google Scholar]
- 4.Isaacson J. E., Vora N. M.2003. Differential diagnosis and treatment of hearing loss. Am. Fam. Physician 68: 1125–1132. [PubMed] [Google Scholar]
- 5.Jawasreh K., Boettcher P. J., Stella A.2016. Genome-wide association scan suggests basis for microtia in Awassi sheep. Anim. Genet. 47: 504–506. doi: 10.1111/age.12431 [DOI] [PubMed] [Google Scholar]
- 6.Nakajima Y.2015. Signaling regulating inner ear development: cell fate determination, patterning, morphogenesis, and defects. Congenit. Anom. (Kyoto) 55: 17–25. doi: 10.1111/cga.12072 [DOI] [PubMed] [Google Scholar]
- 7.Newman S. J., Bailey T. L., Jones J. C., DiGrassie W. A., Whittier W. D.1999. Multiple congenital anomalies in a calf. J. Vet. Diagn. Invest. 11: 368–371. doi: 10.1177/104063879901100414 [DOI] [PubMed] [Google Scholar]
- 8.Philipp U., Lupp B., Mömke S., Stein V., Tipold A., Eule J. C., Rehage J., Distl O.2011. A MITF mutation associated with a dominant white phenotype and bilateral deafness in German Fleckvieh cattle. PLoS One 6: e28857. doi: 10.1371/journal.pone.0028857 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rezaei M., Mahmoudi T., Ebrahimi M., Vosugh D.2015. First report of microtia in dog. Comp. Clin. Pathol. 24: 699–702. doi: 10.1007/s00580-014-2036-1 [DOI] [Google Scholar]
- 10.Schmidt K., Piaia T., Bertolini G., De Lorenzi D.2007. External auditory canal atresia of probable congenital origin in a dog. J. Small Anim. Pract. 48: 233–236. doi: 10.1111/j.1748-5827.2006.00241.x [DOI] [PubMed] [Google Scholar]
- 11.Simpson D.1997. Atresia of the external acoustic meatus in a dog. Aust. Vet. J. 75: 18–20. doi: 10.1111/j.1751-0813.1997.tb13820.x [DOI] [PubMed] [Google Scholar]
- 12.Strain G. M.2015. The genetics of deafness in domestic animals. Front. Vet. Sci. 2: 29. doi: 10.3389/fvets.2015.00029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Strain G. M.2012. Canine deafness. Vet. Clin. North Am. Small Anim. Pract. 42: 1209–1224. doi: 10.1016/j.cvsm.2012.08.010 [DOI] [PubMed] [Google Scholar]
- 14.Swartz J. D., Faerber E. N.1985. Congenital malformations of the external and middle ear: high-resolution CT findings of surgical import. AJR Am. J. Roentgenol. 144: 501–506. doi: 10.2214/ajr.144.3.501 [DOI] [PubMed] [Google Scholar]