Abstract
Objectives/Hypothesis:
We hypothesize that treating hearing loss through cochlear implantation (CI) in older adults will improve cognitive function.
Study Type:
Prospective, interventional study.
Methods:
Thirty-seven participants age 65 and older who met criteria for CI were enrolled. Subjects underwent pre-operative cognitive testing with a novel arrangement of standard neuropsychologic tests including tests of general cognition and mood (Mini-Mental Status Exam [MMSE]), tests of verbally-based stimuli and responses (Digit span, Stroop, Hopkins Verbal Learning Test – Revised [HVLT-R], Hayling Sentence Completion) and comparable visually-based tests (Spatial span, d2 Test of Attention, Brief Visuospatial Memory Test [BVMT], Trails A and B). Testing was repeated twelve months post-operatively.
Results:
One year post-operatively, subjects showed a statistically significant improvement in hearing and on the following tests of cognitive function: Concentration performance of the d2 Test of Attention, Hayling Sentence Completion Test, HVLT-R (total and delayed recall), Spatial Span (backward), and Stroop Color Word Test. A subgroup analysis was performed comparing 13 participants with pre-operative cognitive impairment (MMSE ≤24) to 24 participants with normal cognition (MMSE≥25). In this subgroup analysis, a greater magnitude of improvement was seen in those with impaired cognition, with statistically significant improvement in Digit Span (scaled score), Stroop Word (T score); Stroop Color-Word (residual and T score), HVLT-R, and Hayling (overall). All verbally-based test scores improved, and 75% of the visually-based test scores improved.
Conclusions:
This study demonstrates the cognitive benefits of CI in older adults one year after surgery. For older adults with cognitive impairment prior to CI, the cognitive benefits were even greater than in subjects with normal cognition.
Level of Evidence:
3, non-randomized controlled cohort
Keywords: Cochlear implantation, hearing loss, presbycusis, cognition, dementia
Introduction
Age-related hearing loss is one of the most common chronic conditions among older adults.1,2 The prevalence of hearing loss increases from 25–40% in adults above 65 years old to greater than 80% in people older than 85 years.3 In addition to increased hearing loss, older adults also have a higher risk of dementia. In 2010, 4.7 million Americans aged 65 years or older were afflicted by the most common form of dementia, Alzheimer’s disease dementia. The prevalence of Alzheimer’s disease dementia is projected to increase to 13.8 million by 2050.4
Age-related hearing loss has been demonstrated to be a risk factor for cognitive decline and dementia in older adults.5–10 It has been estimated that the odds ratio for an older adult with hearing loss developing dementia compared to a normal hearing control is 1.24–1.8, and up to 4 for those with severe-profound sensorineural hearing loss.10 From a mechanistic perspective, multiple studies have shown how the auditory cortex atrophies in association with age-related hearing loss in the older adults.11–15 Hearing loss has also been shown to alter central pathways associated with emotional states, suggesting that hearing loss may induce brain changes that affect psychosocial function.16
Despite the epidemiologic data from observational studies and emerging imaging data, a gap in our current knowledge exists. We still do not know whether the association between hearing loss and Alzheimer’s disease dementia and related disorders of impaired cognition is due to neurobiological causation, psychosocial correlation, a “cognitive overload” phenomenon when individuals strain to hear, or an overlap of these factors.15,17 Moreover, due to a current paucity of data from large, controlled, interventional studies, it is unknown whether intervention to treat hearing loss in older adults with severe-profound hearing loss will impact cognitive function in a positive way.
Cochlear implantation is a well-established means of restoring hearing to individuals with severe-profound hearing loss.18 The most rapidly growing demographic of cochlear implant recipients is older adults.19 Many studies have shown the safety and hearing efficacy of cochlear implants in aging populations.20–28 Little is known, however, about the degree to which cochlear implants improves cognition in older adults. Mosnier et al. have shown that cochlear implantation improves cognitive function in older adults, especially in those patients who have mild cognitive impairment at the time of surgery.29 A recent systematic review of studies utilizing a variety of cognitive tests demonstrated the cognitive benefits of treating hearing loss with cochlear implantation.30 Other studies have generally shown that cochlear implantation can improve cognition, but the studies are often limited by small sample size, short-term (<1 year of follow up), or a limited cognitive battery.31–33
The goal of this study is to evaluate the impact of cochlear implants on cognition in older adults. Our hypothesis is that by restoring hearing through cochlear implantation, there will be a parallel benefit of improved cognitive function over a 6- and 12-month post-operative interval. We hypothesize that patients who have poorer cognitive function prior to implantation will show the most benefit. We have developed a novel arrangement of standard cognitive testing battery that includes both verbally- and visually-based cognitive tests which helps differentiate this study from prior studies. Verbally-based cognitive tests refer to tasks that rely to some relatively high degree on language processing and auditory input. Verbally-based tests may have some visual components, but they are primarily tests that require verbal or auditory input. Visually-based tasks are those in which the task is presented visually, though there still may be some verbal instructions. By utilizing this approach of comparable verbally- and visually-based tests, we hope to determine whether improved cognitive function is the result of simply improved hearing of test materials or if there is a genuine improvement in cognition. In other words, we aim to differentiate changes in test performance that may be due to improved hearing and understanding of the tests (verbally-based testing) vs. an improvement that may be attributed to more general improvement in cognition.
This study is different than prior work in that we are studying the effects of cochlear implants on cognition and multiple post-operative time points, we will analyze differences in patients who have impaired cognition (Mini-mental State Exam [MMSE] < 25) at baseline compared to those with normal cognition, and we will compare outcomes on verbally- vs. visually-based cognitive testing.
Methods
This was a prospective, interventional study. Institutional review board approval was obtained, and all participants provided informed consent before any research procedures commenced. Participants were recruited from a tertiary-care neurotology practice at an academic medical center and its affiliated Veterans Affairs Medical Center. The study was registered with clinicaltrials.gov. Patients who were age 65 years old or older were eligible to participate if they met all medical and audiological criteria for cochlear implantation. Participants needed to be medically stable to undergo surgery with low or acceptable risk. This would often include a pre-operative medical or cardiac evaluation. Audiological criteria for cochlear implantation included a four-frequency (0.5, 1, 2, and 3 kHz [or approximated 3kHz34]) pure tone average of greater than 70dB and speech testing - HINT (Hearing in Noise Test) or AzBio (Arizona Bioindustry Association) - scores worse than 40% in either quiet or with background noise (for Medicare criteria) or worse than 60% in quiet for the VA participants. Study participants were given a $50 gift card each time they participated in a cognitive assessment. In a shared-decision making model, patients were able to choose from among three cochlear implant manufacturers in consultation with their audiologist and cochlear implant surgeon. Cochlear implant surgery was performed after pre-operative cognitive testing. The cochlear implant surgery was performed under general anesthesia, using a post-auricular, transmastoid approach. The implant was secured in a tight, subperiosteal pocket, and the implant electrode was inserted via the round window of the inner ear. Surgeries were done on an outpatient basis or with overnight, 23-hour observation when needed. Visual impairment was noted per patient self-report.
Auditory testing
All pre- and post-operative testing was performed by licensed doctors of Audiology. Testing was conducted in a sound-proof booth. Pre-operative testing was done in the best aided condition. Post-operative testing was done in a sound field with bimodal testing (CI and hearing aid). Tests of word or speech comprehension (HINT and AzBio) were done with monitored live voice. Speech testing was performed in a sound field at distance of 1m at 0 degrees azimuth at an intensity level of 60 dBA.
Cognitive testing
All cognitive tests were administered by the same, certified neuropsychology technician under the supervision of a PhD neuropsychologist (KD). The tests were given in a quiet, clinical environment used for testing patients and research subjects. Instructions for the testing were given by live spoken voice, with adjustments being made to voice level based on how well the subject heard the instructions. Visual materials were presented to patients in a way they could clearly visualize the tests. Ten existing assessments of cognition and mood were included in our battery before and after implantation. The battery included measures of global cognition (Mini-Mental State Exam [MMSE]), mood (Geriatric Depression Scale [GDS]), attention (d2 Test of Attention, Trail Making Test Part A, Digit Span, Spatial Span), learning and memory (Hopkins Verbal Learning Test-Revised [HVLT-R], Brief Visuospatial Memory Test-Revised [BVMT-R]), and executive functioning (Hayling Sentence Completion Test, Trail Making Test Part B), and Stroop Color-Word Test). Executive function is a set of cognitive skills that include working memory, flexible thinking, and self-control. The entire battery was purposely designed to have tests that had both verbal/auditory and visual/non-auditory analogs, which are presented in Table I. Certain tests (d2 Test of Attention and Stroop test) were difficult to complete if patients endorsed a visual impairment or color blindness. If a patient endorsed difficulty with the test for this reason, they were allowed to skip that assessment and the data was not collected for analysis.
Table I.
This table lists the tests utilized in our cognitive testing protocol, the type of cognitive domain being tested, and whether the test was verbally- or visually-based in its stimulus and response.
| Cognitive domain | Verbal stimuli/responses | Visual stimuli/responses |
|---|---|---|
| Simple attention | Digit Span | Spatial Span |
| Sustained attention | Stroop Color Word Test | d2 Test of Attention |
| Learning and memory | HVLT-R | BVMT-R |
| Executive functioning | Hayling Sentence Completion Test | Trail Making Test Part B |
This cognitive battery represents a novel collection of standard cognitive tests that were chosen to differentiate performance that is verbally-based for both stimuli and responses, with comparable tests that are visually-based. This approach is meant to differentiate changes in test performance that may be due to improved hearing and understanding of the tests (verbally-based testing) vs. an improvement that may be attributed to more general improvement in cognition. When applicable, testing was done with vision correction. A brief description and summary of each test is included to provide some background.
Global cognition and mood tests
Mini-mental State Exam (MMSE)
The MMSE is a 30-point test of global cognition that is commonly used as part of the evaluation of patients with suspected dementia.35 A Cochrane review summarized the diagnostic accuracy of the MMSE use in the detection of dementia in people aged 65 and older.36 This Cochrane review highlighted ten studies in which an MMSE threshold of normal cognition at 25 showed a sensitivity of this point to be 0.87 and the specificity to be 0.82. Individuals with scores ≤24 are considered to have cognitive impairment. Subjects were given a written form of the MMSE with instructions presented verbally.
Geriatric Depression Scale (GDS)
One theory as to why hearing loss is associated with dementia is due to social isolation and depression.37–39 For this reason, we have included the GDS in this study. Originally described by Yesavage et al., the GDS is a 30-item questionnaire designed for rating depression for older adults.40 Scores considered “normal” range between 0–9. Scores 10–19 are considered “mild depression,” and 20–30 suggest “severe depression.” While the GDS is not a test of cognition, we felt like it would be important to include in a study on hearing loss and cognition. The GDS was administered with a written form and instructions presented verbally.
Verbally-based tests for stimuli and/or responses
Digit Span
Digit span is a term for several psychological tests which assess simple attention and working memory.41 The version which our study group used asked subjects to recall and repeat an increasing number of digits immediately after they are presented. The first of two parts requires subjects to recall a forward sequence of digits. Trials start with two digits and progress up to eight digits. Subjects are asked to recited them in a forward sequential order first and the number of digits increases as the subject successfully completes the task. There are two trials for each sequence of digits (two trials of two digits, two trials of three digits, etc.). A point is given for each successful trial. The test ends when the subject recalls a sequence of eight correctly or the subject cannot correctly recall the sequence after two attempts. In the forward sequence, a maximum of 16 points is awarded.
The second part is similar except that the subject responds by recalling and reciting the digits backwards. The same rules for progression and scoring apply. The test can go up to two trials of seven digits for a maximum score of 14. Although a combined score is often reported, the forward and backward scores are reported independently as well because the cognitive constructs that underlie performance is believed to be different in each part.42 The forward test condition is a measure of memory span or capacity whereas the backward test assesses mental manipulation or working memory. Further, the backward span represents an cognitive task dependent on working memory.
Stroop Color-Word Test (SCWT)
The Stroop Color-Word Test (SCWT) was designed to assess the ability of a subject to inhibit a habitual response for one that is less readily available.43 There are three components of the test: a word component, a color component, and the color-word component. The first two components inform the third. The word component consists of color words (“red,” “blue,” “green”) in black type while the color component consists of bars of color (red, blue, green). The color-word component consists of color words but each is printed in a color ink that differs from the printed word (e.g., “red” printed in blue ink).
The Stroop effect is the ability to inhibit cognitive interference which occurs when processing one specific stimulus feature impedes the simultaneous processing of another stimulus. In SCWT, the subject must inhibit the interference caused by processing the stimulus of the written word while processing and reporting the color of the word. For example, when given the word “red” in blue ink, the subject must report the color blue. The test is considered one measure of executive function, assessing selective attention, cognitive flexibility, and inhibition.44
In the SCWT, the raw score is the number of answers correct out of 100. The raw score is compared to a predicted score based on age and education level. The difference between the raw and predicted score is calculated and reported as a residual. This residual is subsequently converted to a T-score (normative mean of the raw score is converted to a T-score of 50, intervals of 10 represent a standard deviation from the mean).
Hopkins Verbal Learning Test- Revised (HVLT-R)
The HVLT-R was developed as a test of verbal memory and learning.45 The subject is presented 12 nouns, four each from three semantic categories (clothing, animals, gemstones), to be learned over the course of three learning trials. Learning is tested by presenting the words three times and asking for immediate recall. The total recall raw score is the sum of correctly recalled words after each of the three trials (36 possible correct). Memory is tested by having the test subject recall the words after a 20-minute delay (12 possible correct), as well as identifying the words from a list of words including the target and non-target words (reported as retention percentage).
Hayling Sentence Completion Test (Hayling)
The Hayling test initially designed to evaluate executive function in patients with frontal lobe lesions but has since been extended to other neurologic conditions.46 The test evaluates response initiation and response inhibition aspects of executive function as well as verbal reasoning. The task includes two sections, each containing 15 sentences in which the final word is omitted. In section A, the patient completes the sentence by providing a word which makes sense in the context of the sentence (initiation). In section B, the patient must complete the sentence with a completely unrelated word (inhibition). The time to respond and the appropriateness of the answer are factored in the evaluation. Scores based on the completion time and errors are totaled and scaled to produce an overall score ranging between 1 (indicating impaired function) and 10 (indicating very superior function).
Visually-based tests for both stimuli and responses
Spatial Span
The Spatial span test is a visuospatial analogue to the Digit span test which assesses attention, short-term and working memory.41 We utilized a Corsi block tapping method where blocks on a page are pointed out to the subject in a particular sequence and the subject repeats the sequence.47 The same rules for administration of the digit span are applied. The test begins with a sequence of two blocks and progresses up to a sequence of eight blocks with two trials per sequence (two trials of two blocks, two trials of three blocks, etc.). A point is awarded for each successful trial. The test concludes when a subject gets two consecutive trials incorrect or if they complete up to a sequence of 8 blocks (16 points total). Similar to the digit span, this is done in forward span and backward span sequences.
d2 Test of Attention (d2)
Introduced by Brickenkamp and Zillmer, the d2 is an assessment of attention and concentration processes.48 The objective of the test is to recognize and cancel out a target character (a “d” with a total of two dashes above and/or below the character) among visually similar nontarget characters (a “d” with more or a less dashes, a “p” with any number of dashes). This task requires sustained and selective attention as well as concentration. Sustained attention is employed to focus on a specific task for a certain extended amount of time. Selective attention is employed to focus on one task amid distraction. Among the several performance measures within the test, the “total correct” (total characters processed minus total errors) and “concentration performance” (total number of characters correctly cancelled minus total number incorrectly cancelled) were of highest interest. The “total correct” represents a measure of sustained attention and processing speed while “concentration performance” evaluates selective attention, processing speed as well as errors.
Brief Visuospatial Memory Test- Revised (BVMT)
While the HVLT-R tests verbal learning and memory, the BVMT is a visuospatial analogue.49 The test presents six simple geometric designs and locations on a page to be remembered over three trials. Learning is tested by having the subject draw the correct figures (1 point) in the appropriate location on the page (1 point) during each trial and is reported as the total recall raw score across the three learning trials (36 possible points). Memory is tested by having them repeat the task after a 25-minute delay, reported as the delayed raw score (12 possible points).
Trail Making Test Parts A and B (TMT)
The Trail Making Test (TMT) is a measure of attention, processing speed, and mental flexibility.50 It consists of Part A and Part B. Part A requires subjects to connect 25 encircled numbers randomly placed on a page in sequential order. Part A assesses linear processing and visual tracking. Part B further assesses multitasking and mental control by requiring the subject to connect encircled numbers and letters randomly arranged on a page in an alternating sequential order (1-A-2-B-3-C, etc.). The time to complete each task is recorded, with higher scores indicating poorer performance.
Audiometric testing
Pre- and post-op hearing was measured with standard audiometry, hearing in noise tests (HINT and AzBio), aided thresholds, and consonant-nucleus-consonant (CNC) words hearing testing pre-operatively, 6 and 12 months post-operatively, and as often as needed in the interim to determine function.51 Pure tone averages (PTA) were calculated using a four-frequency average of 0.5, 1, 2, and 4 kHz. A lower PTA is indicative of better hearing. A higher HINT, AzBio, and CNC score is indicative of better hearing. All speech testing was administered in English and in a sound-proof booth.
Statistical Data Analysis
Data analysis was performed using SPSS Statistics for Windows, Version 26.0 (IBM Corp., Armonk, NY). Pre- and post-operative audiometric data was reported as median and interquartile range and compared using a Wilcoxon signed-rank test as the data did not follow a normal distribution. Cognitive tests were analyzed using Wilcoxon signed-rank tests comparing pre-implant and one-year post-implant performance. A two-way mixed analysis of variance (ANOVA) was used to determine if there was an effect of cochlear implantation on cognitive test performance and if the effect differed based on pre-operative cognitive status (interaction effect). If an interaction was found, post-hoc analysis was performed to determine the direction of the interaction. When no interaction was found, a one-way ANOVA was used to analyze the main effects of cognitive status (MMSE) and cochlear implant independently. Thus, the interaction, MMSE and cochlear implant effects provide some insight into the results to determine if differences are due to cognitive grouping, the cochlear implant, both, or neither. In the case of data points assumed to be missing at random, these values were replaced using multiple imputation model via a Markov chain Monte Carlo method.
Results
Forty-eight patients were initially considered; however, eleven subjects did not complete the study. Nine participants did not complete the full battery of testing with cognitive assessment at 6 and/or 12 months after surgery, one ultimately chose not to pursue implantation, and one had surgery postponed past the enrollment and data collection window. Two of the patients were lost to follow up due to death from non-cochlear implant-related issues. Demographics of our study population (n=37) are described in Table II. Thirteen participants were classified with cognitive impairment (MMSE ≤24) at the beginning of the study and prior to cochlear implantation, and 24 were classified as cognitively intact and normal (i.e., MMSE>24).
Table II.
Patient characteristics
| − Patients initially enrolled | 48 | ||
| − Patients lost to follow-up prior to 12 months | 9 | ||
| − Patients who did not undergo surgery or did not have data available to analyze −Total number of patients |
2 37 |
||
|
| |||
| Age at implantation, mean (SD) | 79.4 (7.4) | ||
|
| |||
| Factor, n (%) | n | (%) | |
|
| |||
| Male | 32 | (86%) | |
| Veteran | 16 | (43%) | |
| Laterality, right | 16 | (43%) | |
|
| |||
| Pre-operative cognitive classification based on MMSE | Normal (≥25) | 24 | (65%) |
| Impaired cognition (≤24) | 13 | (35%) | |
|
| |||
| Visual impairment present | No | 26 | (70%) |
| Yes | 11 | (30%) | |
|
| |||
| Pre-operative depression classification based on GDS | Normal | 29 | (78%) |
| Mild | 8 | (22%) | |
|
| |||
| Manufacturer | Advanced Bionics | 12 | (32%) |
| Cochlear | 15 | (41%) | |
| Med-El | 10 | (27%) | |
This table shows the demographics of our study population. MMSE = mini-mental state exam, GDS = geriatric depression scale.
Audiometric results
The audiometric data of our study population are shown in Table III. There were statistically significant improvements in all measured audiometric outcomes post-implantation. There were two electric-acoustic stimulation (EAS) patients, meaning that there was enough residual post-operative acoustic hearing to wear a hybrid CI-hearing aid device.
Table III.
This table shows the pre- (within 3 months) and 1-year post-operative audiometric test results of the study population. A statistically significant improvement was seen for each test. Testing was done in the best aided condition.
| Pre-operative | Post-operative | |||||
|---|---|---|---|---|---|---|
| Non-implanted ear | Implanted ear | Bilateral | Implant only | Implant and aided | p-value | |
| Median (IQR) | Median (IQR) | Median (IQR) | Median (IQR) | Median (IQR) | ||
| 4f-PTA (dB HL) | 72.5 (62.8, 80.0) | 78.8 (70.6, 90.6) | 31.3 (26.9, 35) | <0.001 | ||
|
| ||||||
| CNC (%) | 35.2 (23.0, 44.2) | 54.4 (46.0, 64.0) | <0.001 | |||
|
| ||||||
| AzBio in Quiet (%) | 41.8 (37.1, 45.7) | 22.5 (15.3, 25.5) | 37.0 (21.5, 48.0) | 51.1 (30.0, 78.5) | 72.0 (65.1, 87.5) | <0.001 |
4f-PTA = four frequency pure tone average (0.5, 1, 2, and 3 kHz [or approximated 3kHz]); CNC = consonant-nucleus-consonant; IQR = interquartile range
Cognitive results
We obtained cognitive testing data pre-operatively and at 6 and 12 months post-operatively. Preliminary data analysis (unpublished) found no statistically significant difference between pre-operative testing and 6-month post-operative testing for any participant and test. For this reason, all post-operative results reported represent 12-month post-operative results. The results for the cognitive testing for all patients are shown in Table IV.
Table IV.
This table shows the cognitive test data for all subjects. Statistically significant results are highlighted in boldface.
| Pre-operative | One year post-operative | Wilcoxon Signed-Rank Test | ||||
|---|---|---|---|---|---|---|
| Cognitive Test | Outcome | Mean | (95% CI) | Mean | (95% CI) | p-value |
| MMSE | Score | 25.5 | (24.6, 26.5) | 25.8 | (24.8, 26.7) | 0.56 |
|
| ||||||
| GDS | Score | 5.7 | (4.3, 7.1) | 6.2 | (4.7, 7.7) | 0.67 |
|
| ||||||
| Verbally-based tests | ||||||
|
| ||||||
| Digit Span | Forward score | 8.8 | (8.0, 9.5) | 9.2 | (8.4, 9.9) | 0.18 |
| Backward score | 5.5 | (4.7, 6.3) | 6.0 | (5.5, 6.6) | 0.06 | |
| Total score | 14.2 | (12.8, 15.6) | 15.1 | (14.0, 16.2) | 0.03 | |
| Scaled score | 9.6 | (8.6, 10.7) | 13.9 | (10.2, 17.7) | <0.001 | |
|
| ||||||
| Stroop Color-Word | Raw score | 32.8 | (26.3, 39.3) | 32.5 | (30.4, 34.7) | 0.36 |
| Predicted score | 36.6 | (29.9, 43.4) | 30.3 | (29.8, 30.7) | 0.001 | |
| Residual (Raw minus predicted) | −2.3 | (−4.8, 0.3) | −1.2 | (−5.5, 3.1) | 0.41 | |
| T score | 49.6 | (45.2, 54.0) | 51.7 | (49.6, 53.9) | 0.26 | |
|
| ||||||
| HVLT-R | Total, raw score | 18.1 | (16.0, 20.2) | 21.5 | (19.5, 23.4) | 0.004 |
| Total, T score | 39.7 | (35.9, 43.5) | 46.1 | (43, 50) | 0.003 | |
| Delayed, raw score | 5.2 | (4.2, 6.3) | 6.6 | (5.6, 7.6) | 0.01 | |
| Delayed, T score | 39.6 | (36.1, 43.1) | 43.4 | (40, 47) | 0.018 | |
| Retention percentage | 0.62 | (0.51, 0.72) | 0.69 | (0.61, 0.78) | 0.14 | |
|
| ||||||
| Hayling | Total score | 11.3 | (10.3, 12.4) | 12.0 | (10.9, 13.2) | 0.23 |
| Overall score | 2.8 | (2.3, 3.4) | 3.3 | (2.8, 3.9) | 0.05 | |
|
| ||||||
| Visually-based tests | ||||||
|
| ||||||
| Spatial Span | Forward score | 7.5 | (6.9, 8.1) | 7.4 | (6.8, 7.9) | 0.69 |
| Backward score | 6.8 | (6.2, 7.4) | 7.4 | (6.8, 7.9) | 0.07 | |
| Total score | 14.3 | (13.2, 15.4) | 14.8 | (13.9, 15.7) | 0.35 | |
| Scaled score | 12.1 | (11.1, 13.1) | 12.4 | (11.6, 13.2) | 0.51 | |
|
| ||||||
| d2 | Raw score | 328.8 | (293.6, 364.0) | 339.3 | (305.5, 373.1) | 0.19 |
| Standardized score | 95.3 | (90.9, 99.6) | 96.1 | (91.9, 100.3) | 0.29 | |
| Errors | 41.7 | (25.5, 57.8) | 27.2 | (19.7, 34.6) | 0.39 | |
| Total Correct (Total minus errors) | 282.3 | (250.2, 314.4) | 308.5 | (275.9, 341.1) | 0.02 | |
| Concentration | 102.6 | (88.8, 116.4) | 117.6 | (104.5, 130.7) | 0.02 | |
|
| ||||||
| BVMT-R | Total, raw score | 19.1 | (16.4, 21.7) | 21.8 | (19.6, 24.0) | 0.01 |
| Total, T score | 47.6 | (43.2, 52.0) | 52.9 | (49.0, 56.9) | 0.003 | |
| Delayed, raw score | 7.8 | (6.8, 8.8) | 7.9 | (7.0, 8.8) | 0.91 | |
| Delayed, T score | 49.0 | (45.1, 52.9) | 50.3 | (46.6, 54.0) | 0.61 | |
|
| ||||||
| Trails A | Completion time (sec) | 41.5 | (35.9, 47.2) | 38.4 | (34.6, 42.3) | 0.37 |
| Scaled score | 10.6 | (9.6, 11.5) | 11.4 | (10.6, 12.1) | 0.15 | |
|
| ||||||
| Trails B | Completion time (sec) | 130.2 | (101.8, 158.6) | 123.9 | (96.1, 151.8) | 0.43 |
| Scaled score | 9.9 | (8.9, 10.9) | 10.7 | (9.8, 11.6) | 0.03 | |
MMSE = Mini-mental State Exam; GDS = Geriatric Depression Scale; HVLT-R = Hopkins Verbal Learning Test- Revised; BVMT = Brief Visuospatial Memory Test- Revised; CI = confidence interval; IQR = inter-quartile range
Our cohort was stratified into two groups based on pre-operative cognitive function – those with impaired cognition and those with normal cognition. A sub-analysis comparing subjects with cognitive impairment to those with normal cognition was conducted to determine if there was differential benefit of cochlear implantation based on pre-operative cognitive status. The results of the subgroup analysis are shown in Table V.
Table V.
This table shows the cognitive test data for subjects subdivided into groups of cognitive impairment (MMSE ≤24) and normal cognition (MMSE ≥25). Interaction effects to determine whether differences seen are due to cognitive function vs. the cochlear implant have been calculated. Statistically significant results are highlighted in boldface.
| Pre-operative | One year post-operative | Interaction location, simple effect | Main effect | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cognitive test | Outcome | Cognitive status | Mean | (95% CI) | Mean | (95% CI) | Interaction | Pre-op score (Normal vs Cognitive Impairment) | Post-op score (Normal vs Cognitive Impairment) | Cognitive Impairment (Pre-op vs post-op) | Normal (Pre-op vs post-op) | MMSE effect | Cochlear Implant effect |
| MMSE | Score | Cognitive Impairment | 22.5 | (22 to 24) | 24.2 | (22 to 27) | 0.013 | <0.001 | 0.016 | 0.117 | 0.105 | <0.001 | 0.666 |
| Normal Cognition | 27.2 | (27 to 28) | 26.6 | (26 to 27) | |||||||||
| GDS | Score | Cognitive Impairment | 6.5 | (4 to 9) | 7.9 | (5 to 11) | 0.27 | -- | -- | -- | <0.001 | 0.434 | |
| Normal Cognition | 5.3 | (4 to 7) | 5.3 | (4 to 7) | |||||||||
| Verbally-based tests | |||||||||||||
| Digit Span | Forward Score | Cognitive Impairment | 7.8 | (7 to 9) | 8.5 | (7 to 10) | 0.673 | -- | -- | -- | -- | 0.069 | 0.211 |
| Normal Cognition | 9.3 | (8 to 10) | 9.6 | (9 to 11) | |||||||||
| Backward Score | Cognitive Impairment | 5.1 | (4 to 6) | 5.8 | (5 to 6) | 0.666 | -- | -- | -- | -- | 0.411 | 0.168 | |
| Normal Cognition | 5.8 | (5 to 7) | 6.1 | (5 to 7) | |||||||||
| Total score | Cognitive Impairment | 12.9 | (11 to 15) | 14.3 | (12 to 16) | 0.581 | -- | -- | -- | -- | 0.169 | 0.15 | |
| Normal Cognition | 14.9 | (13 to 17) | 15.6 | (14 to 17) | |||||||||
| Scaled score | Cognitive Impairment | 8.5 | (7 to 10) | 15.8 | (5 to 27) | 0.24 | -- | -- | -- | -- | 0.799 | 0.011 | |
| Normal Cognition | 10.2 | (9 to 12) | 13.0 | (12 to 14) | |||||||||
| Stroop Color-Word | Raw score | Cognitive Impairment | 23.3 | (18 to 29) | 31.2 | (27 to 36) | 0.066 | -- | -- | -- | -- | 0.07 | 0.49 |
| Normal Cognition | 36.7 | (28 to 45) | 33.0 | (30 to 36) | |||||||||
| Age/Ed. Predicted | Cognitive Impairment | 30.9 | (30 to 32) | 30.4 | (29 to 31) | 0.261 | -- | -- | -- | -- | 0.286 | 0.203 | |
| Normal Cognition | 39.0 | (30 to 49) | 30.3 | (30 to 31) | |||||||||
| Residual | Cognitive Impairment | −7.2 | (−13 to 2) | 0.3 | (−4 to 5) | 0.007 | 0.003 | 0.295 | 0.03 | 0.824 | 0.013 | 0.011 | |
| Normal Cognition | 3.1 | (−1 to 7) | 2.8 | (0 to 5) | |||||||||
| T-score | Cognitive Impairment | 40.4 | (31 to 50) | 50.2 | (46 to 55) | 0.007 | 0.005 | 0.367 | 0.068 | 0.504 | 0.013 | 0.026 | |
| Normal Cognition | 53.4 | (49 to 58) | 52.3 | (50 to 55) | |||||||||
| HVLT-R | Total raw score | Cognitive Impairment | 13.8 | (10 to 17) | 19.7 | (17 to 22) | 0.086 | -- | -- | -- | -- | 0.007 | 0.001 |
| Normal Cognition | 20.4 | (18 to 23) | 22.4 | (20 to 25) | |||||||||
| Total T-score | Cognitive Impairment | 32.2 | (26 to 39) | 43.2 | (38 to 48) | 0.088 | -- | -- | -- | -- | 0.009 | 0.001 | |
| Normal Cognition | 43.8 | (40 to 48) | 47.7 | (43 to 53) | |||||||||
| Delayed raw score | Cognitive Impairment | 3.5 | (2 to 5) | 5.8 | (4 to 8) | 0.209 | -- | -- | -- | -- | 0.031 | 0.009 | |
| Normal Cognition | 6.2 | (5 to 7) | 7.0 | (6 to 8) | |||||||||
| Delayed T-score | Cognitive Impairment | 33.2 | (27 to 39) | 41.5 | (36 to 47) | 0.076 | -- | -- | -- | -- | 0.024 | 0.016 | |
| Normal Cognition | 43.1 | (39 to 47) | 44.4 | (40 to 49) | |||||||||
| Retention percentage | Cognitive Impairment | 0.52 | (0.3 to 0.7) | 0.68 | (0.5 to 0.9) | 0.366 | -- | -- | -- | -- | 0.273 | 0.118 | |
| Normal Cognition | 0.67 | (0.5 to 0.8) | 0.71 | (0.6 to 0.8) | |||||||||
| Hayling | Total score | Cognitive Impairment | 10.1 | (8 to 12) | 11.0 | (9 to 13) | 0.751 | -- | -- | -- | -- | 0.079 | 0.236 |
| Normal Cognition | 12.0 | (11 to 13) | 12.6 | (11 to 14) | |||||||||
| Overall score | Cognitive Impairment | 2.1 | (1 to 3) | 2.7 | (2 to 4) | 0.696 | -- | -- | -- | -- | 0.042 | 0.048 | |
| Normal Cognition | 3.3 | (3 to 4) | 3.7 | (3 to 4) | |||||||||
| Visually-based tests | |||||||||||||
| Spatial Span | Forward Score | Cognitive Impairment | 7.1 | (6 to 8) | 7.1 | (6 to 8) | 0.837 | -- | -- | -- | -- | 0.221 | 0.793 |
| Normal Cognition | 7.8 | (7 to 9) | 7.6 | (7 to 8) | |||||||||
| Backward Score | Cognitive Impairment | 6.0 | (5 to 7) | 7.1 | (6 to 8) | 0.291 | -- | -- | -- | -- | 0.067 | 0.03 | |
| Normal Cognition | 7.2 | (6 to 8) | 7.6 | (7 to 8) | |||||||||
| Total score | Cognitive Impairment | 13.1 | (11 to 15) | 14.1 | (12 to 16) | 0.359 | -- | -- | -- | -- | 0.109 | 0.264 | |
| Normal Cognition | 15.0 | (14 to 16) | 15.1 | (14 to 16) | |||||||||
| Scaled score | Cognitive Impairment | 11.2 | (9 to 13) | 12.1 | (10 to 14) | 0.298 | -- | -- | -- | -- | 0.309 | 0.358 | |
| Normal Cognition | 12.6 | (11 to 14) | 12.5 | (12 to 14) | |||||||||
| d2 | Raw score | Cognitive Impairment | 350.1 | (279 to 422) | 303.8 | (260 to 347) | 0.025 | 0.348 | 0.097 | 0.282 | 0.019 | 0.692 | 0.604 |
| Normal Cognition | 316.1 | (275 to 357) | 360.6 | (313 to 408) | |||||||||
| Standardized score | Cognitive Impairment | 97.6 | (89 to 106) | 91.5 | (86 to 97) | 0.025 | 0.395 | 0.083 | 0.249 | 0.026 | 0.622 | 0.727 | |
| Normal Cognition | 93.8 | (89 to 99) | 98.8 | (93 to 105) | |||||||||
| Errors | Cognitive Impairment | 76.0 | (41 to 111) | 27.0 | (18 to 36) | <0.001 | <0.001 | 0.979 | 0.015 | 0.225 | 0.002 | 0.095 | |
| Normal Cognition | 21.1 | (13 to 30) | 27.2 | (16 to 39) | |||||||||
| Total Correct (Total minus errors) | Cognitive Impairment | 264.3 | (207 to 322) | 269.6 | (231 to 309) | 0.369 | -- | -- | -- | -- | 0.095 | 0.149 | |
| Normal Cognition | 293.0 | (252 to 334) | 331.8 | (286 to 378) | |||||||||
| Concentration (Correctly cancelled/incorrectly cancelled) | Cognitive Impairment | 76.1 | (54 to 98) | 103.0 | (89 to 118) | 0.145 | -- | -- | -- | -- | 0.004 | 0.023 | |
| Normal Cognition | 118.5 | (104 to 133) | 126.4 | (108 to 145) | |||||||||
| BVMT-R | Total raw score | Cognitive Impairment | 13.8 | (10 to 17) | 19.3 | (16 to 23) | 0.051 | -- | -- | -- | -- | 0.007 | 0.003 |
| Normal Cognition | 21.9 | (19 to 25) | 23.1 | (20 to 26) | |||||||||
| Total T-score | Cognitive Impairment | 38.7 | (33 to 45) | 48.5 | (42 to 55) | 0.055 | -- | -- | -- | -- | 0.007 | 0.001 | |
| Normal Cognition | 52.4 | (47 to 58) | 55.3 | (50 to 60) | |||||||||
| Delayed raw score | Cognitive Impairment | 5.0 | (3 to 7) | 6.8 | (5 to 9) | 0.005 | <0.001 | 0.07 | 0.062 | 0.067 | <0.001 | 0.279 | |
| Normal Cognition | 9.3 | (9 to 10) | 8.5 | (7 to 10) | |||||||||
| Delayed T-score | Cognitive Impairment | 38.8 | (32 to 45) | 45.8 | (39 to 53) | 0.026 | <0.001 | 0.068 | 0.053 | 0.401 | <0.001 | 0.186 | |
| Normal Cognition | 54.6 | (51 to 58) | 52.7 | (48 to 57) | |||||||||
| Trails A | Time | Cognitive Impairment | 42.0 | (31 to 53) | 41.9 | (33 to 51) | 0.452 | -- | -- | -- | -- | 0.463 | 0.428 |
| Normal Cognition | 41.3 | (34 to 48) | 36.6 | (33 to 41) | |||||||||
| Scaled score | Cognitive Impairment | 10.9 | (9 to 13) | 11.0 | (10 to 12) | 0.314 | -- | -- | -- | -- | 0.982 | 0.269 | |
| Normal Cognition | 10.4 | (9 to 12) | 11.5 | (11 to 12) | |||||||||
| Trails B | Time | Cognitive Impairment | 181.0 | (109 to 253) | 156.7 | (81 to 233) | 0.098 | -- | -- | -- | -- | 0.018 | 0.212 |
| Normal Cognition | 102.7 | (86 to 120) | 106.2 | (89 to 123) | |||||||||
| Scaled score | Cognitive Impairment | 8.1 | (6 to 10) | 9.5 | (8 to 11) | 0.297 | -- | -- | -- | -- | 0.008 | 0.03 | |
| Normal Cognition | 10.8 | (10 to 12) | 11.3 | (10 to 12) | |||||||||
MMSE = Mini-mental State Exam; GDS = Geriatric Depression Scale; HVLT-R = Hopkins Verbal Learning Test- Revised; BVMT = Brief Visuospatial Memory Test- Revised; CI = confidence interval; IQR = inter-quartile range; Ed. = education
A written description of the results of each test is provided as follows [note that the results of each cognitive test are presented in a uniform pattern of listing Interaction, MMSE effect, Cochlear implant effect, Entire cohort, Impaired cognition subgroup [see definitions in methods above]):
Global cognition and mood tests
Mini-mental State Exam (MMSE)
Interaction:
Yes
MMSE effect:
Yes
Cochlear implant effect:
No
Entire cohort:
For the entire cohort, the mean pre-operative MMSE score was 25.5 (95% CI 24.6–26.5) and mean post-operative MMSE score was 25.8 (95% CI 25–27) (p=0.56).
Impaired cognition subgroup:
Upon subgroup analysis, mean MMSE score after cochlear implantation in patients with normal cognition remained stable (pre- and post-op means, 27.2 vs. 26.6, p=0.105). In contrast, mean MMSE score in subjects with pre-operative cognitive impairment increased after CI (pre- and post-op means, 22.5 vs. 24.2). However, this increase did not meet statistical significance (p=0.117). The interaction effect (p=0.013) suggests that cochlear implantation has a different effect on MMSE score in normal cognition patients compared to patients with pre-operative cognitive impairment.
Geriatric Depression Scale (GDS)
Interaction:
No
MMSE effect:
Yes
Cochlear implant effect:
No
Entire cohort:
For the entire cohort, the mean GDS was 5.7 (95% CI 4–7) pre-operatively and 6.2 one year post-operatively (95% CI 5–8). This difference was not statistically significant (p=0.67).
Impaired cognition subgroup:
Upon subgroup analysis, mean pre-operative GDS scores were higher in the cognitive impairment group (6.5, 95% CI 4–9) compared to patients with normal cognition (5.3, 95% CI 4.0–7.0) and this difference was statistically significant (p<0.001). There was no statistically significant interaction effect between cochlear implantation and pre-operative cognition (p=0.27). In both groups, most patients scored within the normal range before and after surgery. Only three patients in each group scored over 9 (i.e., the threshold for no depression) one year after cochlear implantation.
Verbally-based tests for both stimuli and responses
Digit Span
Interaction:
No
MMSE effect:
Yes
Cochlear implant effect:
Yes, for scaled score
Entire cohort:
For the entire cohort on Digit Span, mean pre-operative total raw scores were 14.2 (95% CI 13–16) and mean post-operative total scores improved to 15.1 (95% CI 14.0–16.2) (p=0.03). The mean pre-operative scaled score was 9.6 (95% CI 9–11) and the mean post-operative scaled scores improved to 13.9 (95% CI 10–18)(p<0.001).
Impaired cognition subgroup:
Upon subgroup analysis, although patients with normal pre-operative cognition performed slightly better than patients with cognitive impairment in the forward span test, the difference did not reach statistical significance (p=0.069). There was no statistically significant change in forward span score after cochlear implantation in either group (p=0.211). Backward span performance was similar in both groups (p=0.411) and did not change significantly after cochlear implantation (p=0.168). These findings resulted in no statically significant difference in total score both between and within groups. There was, however, a main effect for cochlear implants in improving the scaled scores for both subgroups (p=0.001).
Stroop Color-Word Test (SCWT)
Interaction:
Yes, for residual and T-scores
MMSE effect:
Yes, for residual and T-scores
Cochlear implant effect:
Yes, for residual and T-scores
Entire cohort:
For the entire cohort, the predicted pre-operative score was 36.6 (95% CI 29.9–43.4) which changed to a post-operative score of 30.3 (95% CI 29.8–30.7), which was statistically significant (p=0.001). None of the other SCWT subscores showed a statistically significant change.
Impaired cognition subgroup:
Upon subgroup analysis, there was no statistically significant interaction between cognitive status and cochlear implantation on the SCWT raw score (p=0.066) or age/education predicted score (p=0.261). There was also no significant main effect on MMSE or cochlear implantation on raw score or age/education predicted score.
When looking at the residual and T-scores, there was a significant interaction between pre-operative cognitive status and cochlear implantation (p=0.007 for both tests). Patients with pre-operative cognitive impairment performed nearly a standard deviation below the mean pre-operatively which was significantly different from our patients with normal cognition (T-score, means, 40.4 [95% CI 31–50] for cognitive impairment group vs. 53.4 [95% CI 49–58] for the normal cognition group, p=0.005). One year after cochlear implantation, there was no statistically significance in T-score (means, 50.2 [95% CI 46–55] for the cognitive impairment group vs. 52.3 [95% CI 50–55] for the normal cognition group). A similar effect was seen for residual SCWT subscore. In other words, the cognitive impairment group improved in their post-operative performance such that there was no difference post-operatively from the cognitive impairment and normal cognition groups. This represents a normalization of a previously abnormal cognitive test following cochlear implantation for those with cognitive impairment.
Hopkins Verbal Learning Test- Revised (HVLT-R)
Interaction:
Yes, for total T-score and retention percentage
MMSE effect:
Yes, for total raw and T-scores and delayed raw and T-scores
Cochlear implant effect:
Yes, for total raw and T-scores and delayed raw and T-scores
Entire cohort:
For the entire cohort, there were multiple domains of the HVLT-R that showed a statistically significant improvement after surgery. Mean pre-operative scores on the total raw were 18.1 (95% CI 16.0– 20.2) which improved post-operatively to 21.5 (95% CI 19.5–23.4)(p=0.004). Mean pre-operative total T scores were 39.7 (95% CI 35.9–43.5) which improved post-operatively to 46.1 (95% CI 43–50)(p=0.003). Mean pre-operative delayed raw scores were 5.2 (95% CI 4.2–6.3) which improved post-operatively to 6.6 (95% CI 5.6–7.6)(p=0.01). Mean pre-operative delayed T scores were 39.6 (95% CI 36.1–43.1) which improved post-operatively to 43.4 (95% CI 40–47)(p=0.018).
Impaired cognition subgroup:
Upon subgroup analysis, there was also a statistically significant difference in performance on total recall based on pre-operative cognition (cognitive impairment mean, 13.8 [95% CI 10–17] vs. normal cognition mean, 20.4 [95% CI 18–23], p=0.007). Both groups experienced a similar statistically significant improvement in performance after implantation cognition (cognitive impairment means, 19.7 [95% CI 17–22] vs. normal cognition mean 22.4 [95% CI 20–25], p=0.007). There was a statistically significant difference in performance on delayed recall based on pre-operative cognition (cognitive impairment mean, 3.5 [95% CI 2–5] vs. normal cognition mean of 6.2 [95% CI 5–7], p=0.007). Both groups experienced a similar statistically significant improvement in performance after implantation cognition (cognitive impairment mean, 5.8 [95% CI 4–8] vs. normal cognition mean, 7.0 [95% CI 6–8], p=0.009). Although there was a difference in mean gain between the groups in both total and delayed recall with the group with mild cognitive impairment improving more, the difference did not reach statistical significance (no interaction effect, p>0.05). There was no statistically significant difference in retention percentage between groups or within groups (pre- vs. post-implantation).
Hayling Sentence Completion Test (Hayling)
Interaction:
No
MMSE effect:
Yes, for overall score
Cochlear implant effect:
Yes, for overall score
Entire cohort:
For the entire cohort, the pre-operative mean overall score was 2.8 (95% CI 2.3–34) which improved to a post-operative mean overall score of 3.3 (95% CI 3–4) (p=0.05). There was not a statistically significant improvement in the total score.
Impaired cognition subgroup:
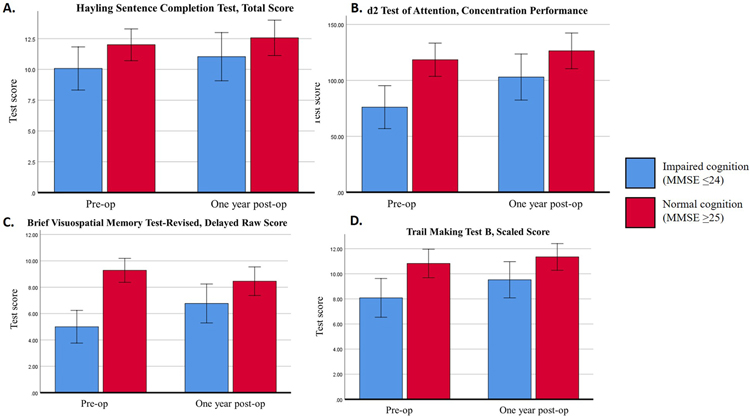
Upon subgroup analysis, there was no interaction effect between cochlear implantation and pre-operative cognition on total scaled score (p=0.751) or overall score (p=0.696) on the Hayling test. In the overall score, there was a statistically significant difference in performance between patients with pre-operative cognitive impairment (mean, 2.1 [95% CI 1–3]) and normal cognition (mean, 3.3 [95% CI 3–4]) (p=0.042). There was a statistically significant increase in performance in both groups after CI (post-operative cognitive impairment mean, 2.7 [95% CI 2–4] vs. normal cognition mean 3.7 [95% CI 3–4], p=0.048). (Figure 1A.)
Figure 1.
This figure shows the pre-and post-operative differences cognitive test scores for two subgroups of subjects, those with cognitive impairment (MMSE≤24) and normal cognition (MMSE≥25). Each of the test that are highlighted showed a statistically significant improvement. The tests included are A. Hayling Sentence Completion Test, Total Score, B. d2 Test of Attention, C. Brief Visuospatial Memory Test-Revised, Delayed Raw Score and D. Trail Making Test Part B, Scaled Score.
Visually-based tests for both stimuli and responses
Spatial Span
Interaction:
No
MMSE effect:
No
Cochlear implant effect:
Yes, for backward score
Entire cohort:
For the entire cohort, there were no statistically significant changes in pre- to post-operative Spatial Span scores.
Impaired cognition subgroup:
Upon subgroup analysis, there was no statistically significant interaction effect or main effect on forward span score based on pre-operative cognitive status or cochlear implantation. Regarding backward span score, there was no significant interaction effect (p=0.291). Although patients with normal cognition had a higher mean backward span score both pre-operatively (7.2 vs. 6.0) and post-operatively (7.6 vs. 7.1), the main effect of cognitive status was not statistically significant (p=0.067). In subgroup analysis of cognitive impairment vs. normal cognition participants, there was a statistically significant main effect of cochlear implantation on backward span (p=0.03), suggesting similar improvement in the assessment regardless of pre-operative cognitive status.
d2 Test of Attention (d2)
Interaction:
Yes, for raw score, standardized score, and errors
MMSE effect:
Yes, for errors and concentration
Cochlear implant effect:
Yes, for concentration
Entire cohort:
In the d2 test, there are scores for “total correct” and for “concentration performance.” For the entire cohort, for the “total correct” score, there was an improvement in performance after cochlear implantation from a mean score of 282.3 (95% CI 250.2–314.4) to a mean post-operative score of 308.5 (95% CI 275.9–341.1) (p=0.02). For tests of concentration, pre-operative mean for the entire group was 102.6 (95% CI 88.8–116.4) which improved to a post-operative mean of 117.6 (95% CI 104.5, 130.7) (p=0.02).
Impaired cognition subgroup:
Upon subgroup analysis, both the cognitive impairment and normal cognition groups improved, however, the improvement did not reach statistical significance. Pre- and post-op means for the cognitive impairment group were 264 to 297 compared to the pre- and post-op means for the normal cognition group which were 293 to 332 (p=0.369). In the “concentration performance” category of the d2 test, pre-operative scores differed between groups of different cognitive level (means, 76.1 [95% CI 54–98] for cognitive impairment vs. 118.5 [95% CI 104–133] for normal cognition, p=0.004]. There was a similar and statistically significant improvement in score in both groups one year after cochlear implantation (pre- and post-operative means, 76.1 [95% CI 54–98] to 103.0 [95% CI 89–118] for cognitive impairment vs. 118.5 [95% CI 104–133] to 126.4 [95% CI 108–145] for the normal cognition group, p=0.023]. (Figure 1B.) The other d2 test scores of raw score, standardized score, and errors did not show a statistically significant difference for the whole group or on subgroup analysis.
Brief Visuospatial Memory Test- Revised (BVMT)
Interaction:
Yes, in delayed raw and delayed T-scores
MMSE effect:
Yes, in total raw and total T-scores, and delayed raw and delayed T-scores
Cochlear implant effect:
Yes, in total raw and total T-scores
Entire cohort:
For the entire cohort, there was a statistically significant improvement in the Total raw and T scores on the BVMT after surgery. The total raw pre-operative score was 19.1 (95% CI 16.4–21.7) which improved post-operatively to 21.8 995% CI 19.6–24.0) (p=0.01). The mean pre-operative total T score was 47.6 (95% CI 43.2–52.0) which improved post-operatively to 52.9 (95% CI 49.0–56.9)(p=0.003). There was no statistically significant change in the delayed raw and T scores.
Impaired cognition subgroup:
Upon subgroup analysis, there was no interaction effect on total recall raw score between pre-operative cognitive group and cochlear implantation (p=0.051) so the main effects are reported. There was a statistically significant difference in total raw recall scores based on pre-operative cognition (pre-operative means were 13.8 [95% CI 10–17] for cognitive impairment vs. 21.9 [95% CI 19–25] for normal cognition, p=0.007]. There was a statistically significant improvement in raw score in both groups after cochlear implantation [post-operative score, means, 19.3 [95% CI 16–23] for cognitive impairment vs. 23.9 [95% CI 20–26] for normal cognition, p=0.003].
There was a statistically significant interaction effect on the raw score of delayed recall from pre-operative cognition and cochlear implantation (p=0.005). Delayed recall (raw) improved significantly in the group with pre-operative cognitive impairment (pre-operative mean, 5.0 [95% CI 3–7] vs. post-operative mean 6.8 [95% CI 5–9], p<0.001). There was no statistically significant change in performance in the group with normal cognition pre-operatively (means, 9.3 [95% CI 9–10] vs. 8.5 [95% CI 7–10], p=0.07). Nearly every patient was able to correctly identify the figures accurately in the delayed forced choice recognition trial (6 of 6) at each time point so comparative analysis was not performed. (Figure 1C.)
Trails Making Test Parts A and B (TMT)
Interaction:
No
MMSE effect:
Yes, in Trails B time and scaled score
Cochlear implant effect:
Yes, in scaled score
Entire cohort:
For the entire cohort, there was not a statistically significant change for TMT-A scores, however, for TMT-B, the mean pre-operative scaled score was 9.9 (95% CI 9–11) and the mean post-operative scaled score was 10.7 (95% CI 10–12) (p=0.03).
Impaired cognition subgroup:
Upon subgroup analysis, there was no statistically significant interaction effect or main effect of pre-operative cognition and cochlear implantation on TMT-A time. Mean times ranged between 36.6 seconds (normal cognition group, post-operative) to 42.0 seconds (cognitive impairment group, pre-operative) which were within less than a standard deviation from published normative means. Comparing the normal cognition to the impaired cognition groups, there was a statistically significant difference in TMT-B time to completion with pre-operative means of 181.0 sec (95% CI 109–253) for the cognitive impairment group vs. 102.7 sec (95% CI 86–120 sec) for the normal cognition group (p=0.018). The completion time did not change significantly after cochlear implantation (p=0.212), however, the scaled score of the TMT-B test did show a main effect of cochlear implantation and improvement in test score due to the cochlear implant (p=0.03). (Figure 1D.)
Discussion
In multiple studies, hearing loss has been identified as a risk factor for cognitive decline and dementia in older adults.5–10 The odds of developing dementia in older adults with hearing loss has been reported as 1.24–4 fold compared to normal hearing older adults.10 Hearing loss has been identified as the largest potentially modifiable risk factor for developing Alzheimer’s disease dementia, accounting for 8% of the attributable risk of all individuals who develop Alzheimer’s.52 Moreover, Alzheimer’s disease has been reported to differentially affect the auditory association cortex as an area of unique vulnerability to decreased gray matter density.53
Cochlear implantation is a well-established intervention for the treatment of individuals with severe-profound sensorineural hearing loss. Cochlear implants are indicated for patients of any age, and have been demonstrated to be effective in restoring hearing in older adults, even octo- and nonagenarians.19,21 Regardless of chronologic age, cochlear implants are also safe and effective in patients who have increased frailty.54 Despite the growing body of literature that has identified hearing loss as a risk factor for dementia, there is a paucity of data that demonstrates the impact of treating hearing loss on mitigating the risk of developing dementia. It has been shown that pre-operative cognitive function can affect cochlear implant hearing outcomes, but how do cochlear implants affect cognition?55,56
In recent systematic reviews evaluating whether treating hearing loss improves cognition, studies have suggested the cognitive benefits of cochlear implantation.30,57 Mosnier et al. have shown in both short- and long-term follow-up, cochlear implantation results in improved cognition and a low rate of progression from mild cognitive impairment (MCI) to dementia.29,58 Knopke et al. have shown improvement in working memory and processing speed in older patients following cochlear implantation.59 Issing et al. have shown that following cochlear implantation, older adults improve on dementia screening tests and TMT 6 months after surgery.60 Moberly et al. have done extensive work evaluating how cognition also affects cochlear implant performance.55,56,61 In the context of these studies and growing body of literature, the strengths of the current study are the novel and expansive use of standard cognitive tests to evaluate older cochlear implant candidates, the long duration of follow up, and the subgroup analysis of patients who have impaired cognition when they undergo surgery compared to a group of older adults with normal cognition at the time of surgery.
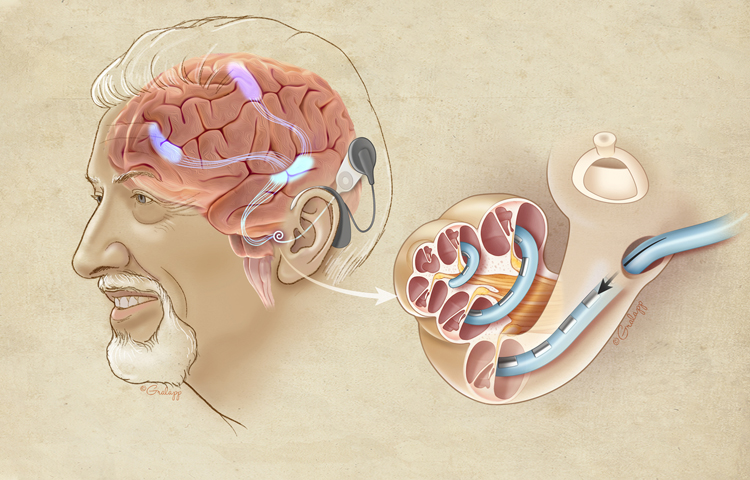
In this report, we have demonstrated the benefit of cochlear implantation on cognitive function in older adults. A visual abstract of this finding can be seen in Figure 2. One year after implantation, participants showed improvement on several cognitive tests of simple attention (spatial span [backward]), sustained attention (Stroop Color Word Test, d2 Test of Attention), executive function (Hayling sentence completion test), and learning and memory (Hopkins Verbal Learning Test).
Figure 2.
This figure depicts how a cochlear implant can activate various brain regions in older adults with severe-profound hearing loss. The cut out cochlea with an implant electrode is highlighted and enlarged to show intracochlear details. Projections from the auditory association cortex activating neural networs to the frontal and pre-motor cortex are shown. From prior work,62 it has been demonstrated that auditory stimuli will activate the pre-motor (attention) cortex in patients with Alzheimer’s disease. (Reproduced with permission from Chris Gralapp, copyright retained by artist).
One of the aims of this study was to differentiate improvement on cognitive tests that are verbally-based that rely primarily on auditory stimuli and verbal responses compared to tests that are visually-based. Four tests were chosen in each category with a matching analog in the other category. Of those, three of the verbally-based tests showed improvement (Hayling, HVLT-R, and Stroop), and two of the visually-based tests showed improvement (spatial span, and d2 test of attention). Although greater changes were shown for verbally-based cognitive tasks, there were several visually-based tasks that also showed improved scores. In other words, the auditory stimulation from the cochlear implant may be improving cognitive function by activating non-auditory brain regions. This phenomenon has been previously reported in which auditory stimuli have been shown to activate and engage many non-auditory brain networks.62,63
In clinical trials involving older adults, such as this study, there will inevitably be some degree of variability in baseline cognitive performance. Our pre-operative cognitive screening revealed patients with cognitive impairment and individuals with normal cognition. Based on this finding, we evaluated whether those who started the study with lower function would have greater benefit to cognition with cochlear implants than those who started with normal cognitive function. We found that the cognitive impairment groups did have greater improvement than the normal cognition group on many of the cognitive tests. On some tests, such as the Stroop Color Word Test, the cognitive impairment group started at a much lower, and statistically significantly different baseline. In fact, the cognitive impairment group’s average pre-operative test result was nearly one standard deviation below the mean. However, this difference was overcome by cochlear implantation. One year after implantation, the cognitive impairment group had improved so much that there was no statistically significant difference between the groups. The HVLT-R delayed recall score of the cognitive impairment group was 1.7 standard deviations below normal value. Following cochlear implantation, this score improved nearly one standard deviation based on raw scores to nearly the baseline of the normal cognition group. This is a compelling finding in a population at risk for progression of cognitive impairment to Alzheimer’s disease.
There are some limitations to this study. This study incorporated a large dataset through evaluating the audiologic and cognitive performance in 37 patients across multiple timepoints. We acknowledge the possibility of false positive findings as a limitation to our study since no specific procedure such as a Bonferroni correction was performed.
Another limitation is that we did not have a true control group in the study. Ideally, a well-matched control group would consist of matched individuals who also have severe-profound sensorineural hearing loss and meet criteria for cochlear implantation, but do not have the surgery. While scientifically rigorous, it would be unethical to withhold cochlear implant surgery to a group of candidates because of the known benefits of cochlear implantation. To overcome this limitation, each study subject served as his or her own control with post-operative cognitive status being compared to the pre-operative baseline cognitive test results. Another limitation of the study is that cognitive decline typically occurs over many years. In this study, we have only followed patients for one year. One could argue that this is not enough time to evaluate whether or not cochlear implants truly mitigate the risk of cognitive decline in older adults. The improvement that we have demonstrated is compelling, however, and likely represents a true improvement in certain cognitive domains. A longitudinal study following this cohort of patients for a longer period of time is currently underway. Finally, we used a psychometric definition of cognitive impairment (i.e., falling below a specific cutoff on the MMSE) as opposed to a clinical definition. It is possible that some of these individuals with cognitive impairment by cutoffs of cognitive screening tests would revert back to normal on follow-up without intervention. Future studies would more formally diagnose mild cognitive impairment (MCI), especially of the amnestic subtype.
Another limitation of the study is that we did not control for potential confounders like CI usage via data logging or age. All CI devices from the three United States, FDA approved manufacturers were used, so there was some variability in technology available for data logging. Not all manufacturers offered data logging features on their external processors when this study began.
Repeated, sequential cognitive testing introduces the potential of “practice effects.” This is when improvement occurs simply because the test is taken repeatedly and the test taker becomes more familiar with the questions and/or answers. Practice effects have been shown to diminish when extended periods of time (6 months or one year) between testing sessions, which allows more confidence in predicting cognitive performance on repeatedly tested measures.64,65 In a study by Hammers et al. of patients with mild cognitive impairment, even repeat testing at 1 week intervals showed incrementally small-than-expected benefit from practice.66 The many months between testing in our study likely diminished the influence of practice effects, but we acknowledge this as a potential limitation. Ongoing and future work using alternate forms of the tests, when available, will hopefully mitigate the impact of practice effects.
This study provides the rationale for additional study on the cognitive impact of cochlear implantation in older adults. We noted particular improvement among patients who began the study with lower cognitive function, even cognitive impairment. Diminished cognition should not be a considered a contraindication to cochlear implantation in older adults. In fact, this group with diminished cognition may stand to gain the most from improved hearing. Future studies will focus on the long-term (>5 year) effect of cochlear implantation on cognition, and evaluate for the conversion rates of mild cognitive impairment to dementia and Alzheimer’s disease. A greater mechanistic understanding of how hearing loss is associated with dementia is also needed.
Conclusion
This study has demonstrated improvement in multiple cognitive domains, based on both verbally- and visually-based cognitive tests, following cochlear implantation in older adults. In addition to the well-established benefits to auditory function, there is a growing body of evidence, including the findings of this study, that demonstrate the cognitive benefits of cochlear implantation. While more research is needed, the restoration of hearing via cochlear implantation does seem to improve cognition within a one-year time interval. Future prospective studies are needed to further evaluate whether cochlear implants mitigate the risks of developing dementia in older adults.
Acknowledgments:
We acknowledge Diane Tyler, RN, our study coordinator who assisted in the patient recruitment and data management, and Morgan Richards, our neuropsychiatric technician who did all of the cognitive testing.
Funding and Conflicts of Interest:
This study was funded by the American Otological Society Clinician Scientist Grant and funding from the National Institute of Aging (1 R03 AG056458–01). Dr. Gurgel is on the surgical advisory board for Med-El, and received institutional research funding from Cochlear and Advanced Bionics.
References
- 1.Cruickshanks KJ, Wiley TL, Tweed TS et al. Prevalence of hearing loss in older adults in Beaver Dam, Wisconsin. The Epidemiology of Hearing Loss Study. Am J Epidemiol 1998; 148:879–886. [DOI] [PubMed] [Google Scholar]
- 2.Gurgel RK, Briggs SE, Dhepyasuwan N, Rosenfeld RM. Quality Improvement in Otolaryngology-Head and Neck Surgery: Age-Related Hearing Loss Measures. Otolaryngol Head Neck Surg 2021:1945998211000442. [DOI] [PubMed] [Google Scholar]
- 3.Yueh B, Shapiro N, MacLean CH, Shekelle PG. Screening and management of adult hearing loss in primary care: scientific review. JAMA 2003; 289:1976–1985. [DOI] [PubMed] [Google Scholar]
- 4.Hebert LE, Weuve J, Scherr PA, Evans DA. Alzheimer disease in the United States (2010–2050) estimated using the 2010 census. Neurology 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gurgel RK, Ward PD, Schwartz S, Norton MC, Foster NL, Tschanz JT. Relationship of Hearing Loss and Dementia: A Prospective, Population-Based Study. Otol Neurotol 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lin FR. Hearing loss and cognition among older adults in the United States. J Gerontol A Biol Sci Med Sci 2011; 66:1131–1136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lin FR, Ferrucci L, Metter EJ, An Y, Zonderman AB, Resnick SM. Hearing loss and cognition in the Baltimore Longitudinal Study of Aging. Neuropsychology 2011; 25:763–770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lin FR, Yaffe K, Xia J et al. Hearing Loss and Cognitive Decline in Older Adults. JAMA internal medicine 2013:1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gallacher J, Ilubaera V, Ben-Shlomo Y et al. Auditory threshold, phonologic demand, and incident dementia. Neurology 2012; 79:1583–1590. [DOI] [PubMed] [Google Scholar]
- 10.Thomson RS, Auduong P, Miller AT, Gurgel RK. Hearing loss as a risk factor for dementia: A systematic review. Laryngoscope investigative otolaryngology 2017; 2:69–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lin FR, Ferrucci L, An Y et al. Association of hearing impairment with brain volume changes in older adults. NeuroImage 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Eckert MA, Cute SL, Vaden KI Jr., Kuchinsky SE, Dubno JR. Auditory cortex signs of age-related hearing loss. J Assoc Res Otolaryngol 2012; 13:703–713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Husain FT, Medina RE, Davis CW et al. Neuroanatomical changes due to hearing loss and chronic tinnitus: a combined VBM and DTI study. Brain research 2011; 1369:74–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Peelle JE, Troiani V, Grossman M, Wingfield A. Hearing loss in older adults affects neural systems supporting speech comprehension. J Neurosci 2011; 31:12638–12643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wong PC, Ettlinger M, Sheppard JP, Gunasekera GM, Dhar S. Neuroanatomical characteristics and speech perception in noise in older adults. Ear and hearing 2010; 31:471–479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Husain FT, Carpenter-Thompson JR, Schmidt SA. The effect of mild-to-moderate hearing loss on auditory and emotion processing networks. Frontiers in systems neuroscience 2014; 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Parham K, Lin FR, Coelho DH, Sataloff RT, Gates GA. Comprehensive Management of Presbycusis: Central and Peripheral. Otolaryngol Head Neck Surg 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Buchman CA, Gifford RH, Haynes DS et al. Unilateral Cochlear Implants for Severe, Profound, or Moderate Sloping to Profound Bilateral Sensorineural Hearing Loss: A Systematic Review and Consensus Statements. JAMA otolaryngology-- head & neck surgery 2020. [DOI] [PubMed] [Google Scholar]
- 19.Miller G, Miller C, Marrone N, Howe C, Fain M, Jacob A. The impact of cochlear implantation on cognition in older adults: a systematic review of clinical evidence. BMC geriatrics 2015; 15:16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Alice B, Silvia M, Laura G, Patrizia T, Roberto B. Cochlear implantation in the elderly: surgical and hearing outcomes. BMC surgery 2013; 13 Suppl 2:S1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Carlson ML, Breen JT, Gifford RH et al. Cochlear implantation in the octogenarian and nonagenarian. Otol Neurotol 2010; 31:1343–1349. [DOI] [PubMed] [Google Scholar]
- 22.Choi JS, Contrera KJ, Betz JF, Blake CR, Niparko JK, Lin FR. Long-term use of cochlear implants in older adults: results from a large consecutive case series. Otol Neurotol 2014; 35:815–820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cloutier F, Bussieres R, Ferron P, Cote M. OCTO “Outcomes of cochlear implant for the octogenarians: audiologic and quality-of-life”. Otol Neurotol 2014; 35:22–28. [DOI] [PubMed] [Google Scholar]
- 24.Lenarz M, Sonmez H, Joseph G, Buchner A, Lenarz T. Cochlear implant performance in geriatric patients. The Laryngoscope 2012; 122:1361–1365. [DOI] [PubMed] [Google Scholar]
- 25.Lin FR, Chien WW, Li L, Clarrett DM, Niparko JK, Francis HW. Cochlear implantation in older adults. Medicine 2012; 91:229–241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Migirov L, Taitelbaum-Swead R, Drendel M, Hildesheimer M, Kronenberg J. Cochlear implantation in elderly patients: surgical and audiological outcome. Gerontology 2010; 56:123–128. [DOI] [PubMed] [Google Scholar]
- 27.Ramos A, Guerra-Jimenez G, Rodriguez C, Borkoski S, Falcon JC, Perez D. Cochlear implants in adults over 60: a study of communicative benefits and the impact on quality of life. Cochlear implants international 2013; 14:241–245. [DOI] [PubMed] [Google Scholar]
- 28.Vermeire K, Brokx JP, Wuyts FL, Cochet E, Hofkens A, Van de Heyning PH. Quality-of-life benefit from cochlear implantation in the elderly. Otol Neurotol 2005; 26:188–195. [DOI] [PubMed] [Google Scholar]
- 29.Mosnier I, Vanier A, Bonnard D et al. Long-Term Cognitive Prognosis of Profoundly Deaf Older Adults After Hearing Rehabilitation Using Cochlear Implants. Journal of the American Geriatrics Society 2018; 66:1553–1561. [DOI] [PubMed] [Google Scholar]
- 30.Amieva H, Ouvrard C. Does Treating Hearing Loss in Older Adults Improve Cognitive Outcomes? A Review. J Clin Med 2020; 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Issing C, Baumann U, Pantel J, Stover T. Impact of Hearing Rehabilitation Using Cochlear Implants on Cognitive Function in Older Patients. Otol Neurotol 2021. [DOI] [PubMed] [Google Scholar]
- 32.Sarant J, Harris D, Busby P et al. The Effect of Cochlear Implants on Cognitive Function in Older Adults: Initial Baseline and 18-Month Follow Up Results for a Prospective International Longitudinal Study. Front Neurosci 2019; 13:789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cosetti MK, Pinkston JB, Flores JM et al. Neurocognitive testing and cochlear implantation: insights into performance in older adults. Clin Interv Aging 2016; 11:603–613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gurgel RK, Popelka GR, Oghalai JS, Blevins NH, Chang KW, Jackler RK. Is it valid to calculate the 3-kilohertz threshold by averaging 2 and 4 kilohertz? Otolaryngol Head Neck Surg 2012; 147:102–104. [DOI] [PubMed] [Google Scholar]
- 35.Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”: a practical method for grading the cognitive state of patients for the clinician. Journal of psychiatric research 1975; 12:189–198. [DOI] [PubMed] [Google Scholar]
- 36.Creavin ST, Wisniewski S, Noel-Storr AH et al. Mini-Mental State Examination (MMSE) for the detection of dementia in clinically unevaluated people aged 65 and over in community and primary care populations. The Cochrane database of systematic reviews 2016:CD011145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Shukla A, Harper M, Pedersen E et al. Hearing Loss, Loneliness, and Social Isolation: A Systematic Review. Otolaryngol Head Neck Surg 2020:194599820910377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mick P, Kawachi I, Lin FR. The association between hearing loss and social isolation in older adults. Otolaryngol Head Neck Surg 2014; 150:378–384. [DOI] [PubMed] [Google Scholar]
- 39.Sharma RK, Chern A, Golub JS. Age-Related Hearing Loss and the Development of Cognitive Impairment and Late-Life Depression: A Scoping Overview. Semin Hear 2021; 42:10–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yesavage JA, Brink TL, Rose TL et al. Development and validation of a geriatric depression screening scale: a preliminary report. J Psychiatr Res 1982; 17:37–49. [DOI] [PubMed] [Google Scholar]
- 41.Wechsler D WMS-III: Wechsler memory scale administration and scoring manual. Psychological Corporation, 1997. [Google Scholar]
- 42.Kaplan E, Fein D, Morris R, Delis D. The WAIS-R as a Neuropsychological Instrument San Antonio. TX, Psychological Corporation; 1991. [Google Scholar]
- 43.Golden CJ, Freshwater SM. Stroop color and word test 1978.
- 44.Homack S, Riccio CA. A meta-analysis of the sensitivity and specificity of the Stroop Color and Word Test with children. Archives of clinical neuropsychology : the official journal of the National Academy of Neuropsychologists 2004; 19:725–743. [DOI] [PubMed] [Google Scholar]
- 45.Brandt J, Benedict RH. Hopkins verbal learning test--revised: professional manual. Psychological Assessment Resources, 2001. [Google Scholar]
- 46.Burgess PW, Shallice T. Response suppression, initiation and strategy use following frontal lobe lesions. Neuropsychologia 1996; 34:263–272. [DOI] [PubMed] [Google Scholar]
- 47.Kessels RP, van Zandvoort MJ, Postma A, Kappelle LJ, de Haan EH. The Corsi Block-Tapping Task: standardization and normative data. Appl Neuropsychol 2000; 7:252–258. [DOI] [PubMed] [Google Scholar]
- 48.Brickenkamp R, Zillmer E. The d2 test of attention, 1st US Edn. eds Hogrefe and Huber. WA, Seattle: 1998. [Google Scholar]
- 49.Benedict R Brief Visuospatial Memory Test-Revised Psychological Assessment Resources. Inc, Odessa, FL: 1997. [Google Scholar]
- 50.Reitan R The relationship of the Trail Making Test to organic brain damage. Journal of Consulting Psychology 1955; 19:393–394. [DOI] [PubMed] [Google Scholar]
- 51.Adunka OF, Gantz BJ, Dunn C, Gurgel RK, Buchman CA. Minimum Reporting Standards for Adult Cochlear Implantation. Otolaryngol Head Neck Surg 2018:194599818764329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Livingston G, Huntley J, Sommerlad A et al. Dementia prevention, intervention, and care: 2020 report of the Lancet Commission. Lancet 2020; 396:413–446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Aylward A, Auduong P, Anderson JS et al. Changes in the Auditory Association Cortex in Dementing Illnesses. Otol Neurotol 2020. [DOI] [PubMed] [Google Scholar]
- 54.Aylward A, Murphy-Meyers M, Allen CM, Patel NS, Gurgel RK. Frailty and Quality of Life After Cochlear Implantation in Older Adults. Otolaryngol Head Neck Surg 2021:1945998211004589. [DOI] [PubMed] [Google Scholar]
- 55.Skidmore JA, Vasil KJ, He S, Moberly AC. Explaining Speech Recognition and Quality of Life Outcomes in Adult Cochlear Implant Users: Complementary Contributions of Demographic, Sensory, and Cognitive Factors. Otol Neurotol 2020; 41:e795–e803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zhan KY, Lewis JH, Vasil KJ et al. Cognitive Functions in Adults Receiving Cochlear Implants: Predictors of Speech Recognition and Changes After Implantation. Otol Neurotol 2020; 41:e322–e329. [DOI] [PubMed] [Google Scholar]
- 57.Dawes P Hearing interventions to prevent dementia. Hno 2019; 67:165–171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Mosnier I, Bebear JP, Marx M et al. Improvement of Cognitive Function After Cochlear Implantation in Elderly Patients. JAMA otolaryngology-- head & neck surgery 2015. [DOI] [PubMed] [Google Scholar]
- 59.Knopke S, Schubert A, Haussler SM, Grabel S, Szczepek AJ, Olze H. Improvement of Working Memory and Processing Speed in Patients over 70 with Bilateral Hearing Impairment Following Unilateral Cochlear Implantation. J Clin Med 2021; 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Issing C, Baumann U, Pantel J, Stover T. Impact of Hearing Rehabilitation Using Cochlear Implants on Cognitive Function in Older Patients. Otol Neurotol 2021; 42:1136–1141. [DOI] [PubMed] [Google Scholar]
- 61.Kramer S, Vasil KJ, Adunka OF, Pisoni DB, Moberly AC. Cognitive Functions in Adult Cochlear Implant Users, Cochlear Implant Candidates, and Normal-Hearing Listeners. Laryngoscope investigative otolaryngology 2018; 3:304–310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.King JB, Jones KG, Goldberg E et al. Increased Functional Connectivity After Listening to Favored Music in Adults With Alzheimer Dementia. J Prev Alzheimers Dis 2019; 6:56–62. [DOI] [PubMed] [Google Scholar]
- 63.Wang D, Belden A, Hanser SB, Geddes MR, Loui P. Resting-State Connectivity of Auditory and Reward Systems in Alzheimer’s Disease and Mild Cognitive Impairment. Front Hum Neurosci 2020; 14:280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Hammers DB, Porter S, Dixon A, Suhrie KR, Duff K. Validating 1-Year Reliable Change Methods. Archives of clinical neuropsychology : the official journal of the National Academy of Neuropsychologists 2021; 36:87–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Duff K, Atkinson TJ, Suhrie KR, Dalley BC, Schaefer SY, Hammers DB. Short-term practice effects in mild cognitive impairment: Evaluating different methods of change. J Clin Exp Neuropsychol 2017; 39:396–407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Hammers DB, Suhrie KR, Dixon A, Porter S, Duff K. Reliable change in cognition over 1 week in community-dwelling older adults: a validation and extension study. Archives of clinical neuropsychology : the official journal of the National Academy of Neuropsychologists 2021; 36:347–358. [DOI] [PMC free article] [PubMed] [Google Scholar]