Abstract
Background
Despite widespread availability of direct-acting antivirals including generic formulations, limited progress has been made in the global adoption of hepatitis C virus (HCV) treatment. Barriers to treatment scale-up include availability and access to diagnostic and monitoring tests, health-care infrastructure, and requirement for frequent visits during treatment.
Methods
ACTG A5360 was a phase 4, open-label, single-arm trial across 38 sites in Brazil, South Africa, Thailand, Uganda, and the USA. Key inclusion criteria were age of 18 years or older, evidence of active HCV infection (HCV RNA >1000 IU/mL) and HCV treatment-naive; patients with compensated cirrhosis and HIV/HCV co-infection were included but their enrolment was capped. All participants received a fixed dose combination of oral sofosbuvir (400 mg) and velpatasvir (100 mg) once daily for 12 weeks. The minimal monitoring (MINMON) approach consisted of four components: (1) there was no pre-treatment genotyping; (2) the entire treatment course (84 tablets) was dispensed at entry; (3) there were no scheduled visits or laboratory monitoring; and (4) there were two points of remote contact, at week 4 for adherence and week 22, to schedule outcome assessment at week 24 (−2 weeks to +4 weeks). Participants who missed the week 24 window could return for a visit to assess treatment response any time before week 72. Unplanned visits for any reason were permissible before the week 24 visit. The primary efficacy outcome was sustained virological response (SVR), defined as HCV RNA less than the lower limit of quantification measured at least 22 weeks post-treatment initiation; the primary safety outcome was serious adverse events. The primary efficacy analysis included all participants who initiated treatment, using a missing=failure approach. The primary safety analysis included all participants who initiated treatment and had at least one post-treatment assessment. This trial is registered at ClinicalTrials.gov, NCT03512210.
Findings
Between Oct 22, 2018, and July 19, 2019, 400 participants were enrolled across all 38 sites; 399 initiated treatment. At the SVR assessment visit, 355 (89%) of 397 participants reported taking 100% of the trial medication during the 12-week treatment period; two patients did not have any follow-up visits after the entry visit and were excluded from the safety analyses. Overall, 379 of the 399 who initiated treatment had an SVR (95·0%, 95% CI 92·4–96·7). 14 (4%) of 397 participants reported serious adverse events between treatment initiation and week 28; none were treatment related or led to treatment discontinuation or death. 15 (4%) of 399 participants had unplanned visits; none were related to treatment.
Interpretation
In this diverse global population of people with HCV, the MINMON approach with sofosbuvir–velpatasvir treatment was safe and achieved SVR comparable to standard monitoring observed in real-world data. Coupled with innovative case finding strategies, this strategy could be crucial to the global HCV elimination agenda.
Funding
US National Institutes of Health and Gilead Sciences.
Introduction
The introduction of direct-acting antivirals has revolutionised hepatitis C virus (HCV) treatment, providing a crucial tool for HCV elimination.1 WHO established ambitious targets to eliminate HCV by 2030, which requires that at least 90% of the 58 million individuals chronically infected with HCV are diagnosed and 80% treated.2 To date, based on modelling estimates only 11 of 45 high-income countries are on track to achieve HCV elimination; Egypt and Georgia are the only low-income and middle-income countries on track.3
Treatment cost and access are major challenges to widespread scale-up of HCV treatment. While costs have frequently been cited as a barrier, they have declined substantially in recent years,4 particularly with the production of generic formulations.4, 5 In low-income and middle-income countries, where over 80% of individuals with chronic HCV reside,6 costs associated with recommended diagnostics such as pre-treatment genotyping and on-treatment monitoring can be higher than the cost of medications or are often unavailable.5, 7 Additionally, overburdened health-care infrastructure is a key challenge to expanded access to HCV treatment, which is now further complicated by the COVID-19 pandemic threatening public health programmes globally. Progress toward HCV elimination has stalled during the pandemic;5 models suggest this will result in excess HCV-related mortality, underscoring the urgent need for simple, safe, and efficacious treatment algorithms.8 While US and European guidelines recommend simplified treatment algorithms in some patient populations,9, 10 there are limited data on simplified models of HCV treatment monitoring, particularly from low-income and middle-income settings.11, 12, 13
Research in context.
Evidence before this study
We searched PubMed on June 29, 2020, using the search terms “(hepatitis C[Title]) AND (treatment[Title] OR direct acting antivirals[Title])) AND (simplified[Title] OR minimal[Title])”, which returned 13 articles. No date or language restrictions were used. Since the introduction of all oral highly efficacious pangenotypic direct-acting antiviral (DAA) therapies for hepatitis C virus (HCV) infection, there have been calls for simplification of treatment delivery to facilitate achieving the WHO's ambitious HCV elimination targets. The American Association for the Study of Liver Disease (AASLD), European Association for the Study of Liver (EASL), and WHO have included in their guidelines language on the simplification of HCV treatment delivery in select subpopulations. However, most of this guidance is based on expert opinion. To, date there have been few well designed trials that have evaluated strategies to simplify HCV treatment delivery. Moreover, the data from these few trials have some limitations. First, all published trials required pre-treatment genotyping. Second, these studies used a variety of tools to classify cirrhosis including transient elastography, which may not be readily available in all settings globally. Third, individuals with compensated cirrhosis were excluded from most of these trials and there was limited representation of HIV/HCV co-infected individuals. Fourth, only one of these trials dispensed the entire treatment course at study entry. Fifth, and most importantly, the majority of these trials included only participants from high-income countries; more than 80% of people living with HCV reside in low-income and middle-income country settings. To our knowledge, no trial to date has evaluated a minimal in-person monitoring approach in a globally diverse population including individuals with compensated cirrhosis and a good representation of those living with HIV/HCV co-infection.
Added value of this study
The MINMON trial demonstrates that the delivery of HCV treatment can be simplified without compromising safety or efficacy in a globally diverse population of participants from high-income, middle-income, and low-income settings, including those with compensated cirrhosis and HIV/HCV co-infection. Further, the use of simple, easy to obtain laboratory-based tests such as FIB-4 to classify cirrhosis and elimination of pre-treatment genotyping improves the reproducibility of this study in primary-care settings globally. The dispensation of the entire study course of treatment without jeopardising efficacy also removes barriers associated with medication refills. Finally, while this study did not require pre-treatment genotyping, when samples were tested retrospectively, we identified participants with all genotypes 1–7.
Implications of all the available evidence
The data available cumulatively support the use of minimal monitoring approaches in the delivery of HCV therapy to individuals with non-decompensated cirrhosis living with HCV infection globally. Given the challenges with access to HCV genotyping and barriers associated with medication refill fulfilment, these data support the guidance to eliminate pre-treatment genotyping when using pangenotypic regimens, and suggest dispensation of the entire study treatment at initiation without compromising patient safety or treatment efficacy. Collectively these data support and provide added evidence for the use of simplified protocols for the delivery of HCV care. Such approaches will be crucial to the achievement of HCV elimination.
The AIDS Clinical Trials Group (ACTG) A5360 Minimal Monitoring (MINMON) trial examined the efficacy and safety of a minimal (in-person) monitoring strategy of HCV treatment delivery in a diverse global population living with HCV.
Methods
Study design and participants
A5360 was a phase 4, international, open-label, single-arm trial designed to evaluate the efficacy and safety of a minimal monitoring approach of delivering interferon-free and ribavirin-free, pangenotypic direct-acting antivirals to HCV treatment-naive participants with evidence of active HCV infection. Participants were recruited at 38 ACTG-affiliated, National Institutes of Health Division of AIDS (DAIDS) certified clinical research sites (appendix p 4) across five countries (Brazil, South Africa, Thailand, Uganda, and the USA). All clinical research sites were nested within existing infectious diseases clinics and all provide clinical care to people living with HIV. Most sites were affiliated with universities. The clinicians at the sites varied depending on the study site and ranged from primary-care physicians to infectious disease specialists. Enrolment in the USA was limited to 132 participants across the 31 sites; four enrolment slots were reserved for each of the US sites. There were no caps or reserved slots for enrolment across the seven international sites.
Participants were either recruited from within the clinic population or from the community via referrals. All participants were aged 18 years or older with active HCV infection (RNA >1000 IU/mL within 35 days before study entry) and were HCV treatment-naive. Liver disease stage was determined by Fibrosis-4 (FIB-4) index,14 which is estimated using age, aspartate aminotransferase, alanine aminotransferase, and platelet count. Cirrhosis was defined as a FIB-4 score of 3·25 or more. Participants with compensated cirrhosis (FIB-4 ≥3·25 and Child-Turcotte-Pugh score ≤6) were eligible but limited to no more than 80 participants to reflect that 10–20% of people living with HCV worldwide have cirrhosis; individuals with decompensated liver disease were excluded. People with HIV were eligible if suppressed (HIV RNA <400 copies per mL) on non-efavirenz containing antiretrovirals, or HIV treatment-naive with CD4 of more than 350 cells per μL within 90 days of entry. Enrolment of people with HIV was limited to no more than 200 participants to ensure balance of HCV mono-infected and HIV/HCV co-infected participants. Pregnancy, breastfeeding, or evidence of chronic hepatitis B virus (HBV) infection (HBsAg positive) were exclusion criteria; participants with resolved HBV infection (anti-hepatitis B core [HBc] positive) with or without positive hepatitis B surface antibodies (anti-HBs) were eligible. Participants provided written informed consent, including permission for and preferred mode of remote contact. Other major investigations performed as part of screening for the trial included haematology, liver function tests, and blood chemistries. Detailed eligibility criteria and laboratory and clinical assessments performed as part of the trial are available in the study protocol.
The protocol was approved by institutional review or ethical review boards of all 38 participating sites. Interim reviews for conduct and safety were performed at least yearly by a network appointed independent study monitoring committee.
Procedures
All individuals consenting to be screened for the trial were evaluated for eligibility. Those eligible and willing to enroll in the trial were scheduled for an entry visit. Due to the requirement for documentation of active HCV infection (HCV RNA >1000 IU/mL) before enrolment, the median time from screening to enrolment was 19 days (range 0–35).
All participants received a single-tablet fixed dose combination containing 400 mg of sofosbuvir and 100 mg of velpatasvir taken by mouth once daily for 12 weeks with or without food. The MINMON intervention strategy constituted four elements: (1) no pre-treatment HCV genotype assessment; (2) dispensation of the entire treatment course at entry (three bottles containing 28 tablets each); (3) no scheduled clinic or laboratory monitoring visits before the efficacy outcome assessment; and (4) two points of remote contact—week 4 post-treatment initiation to assess adherence and update contact information, and week 22 to update contact information and schedule the outcome assessment visit at week 24 (−2 weeks to +4 weeks). Study staff could remotely contact participants using the participant's preferred method (eg, email, SMS text message, or social media platforms such as WhatsApp, Facebook Messenger, etc). All participants were provided with a telephone number that they could contact research staff if they had any questions or concerns related to the trial or trial medication. Unplanned clinic visits for any reason were allowed before the week 24 visit, including for repeat laboratory tests for laboratory abnormalities detected during study entry. To maintain compliance with our minimum monitoring intervention, no attempts were made to contact participants in person if remote contact failed. Participants were scheduled to be followed for 72 weeks following treatment initiation; results through the primary efficacy outcome assessment scheduled at week 24 are presented here.
HCV RNA for assessment of sustained virological response (SVR) was quantified using either the Roche COBAS HCV Quantitative nucleic acid assay (Roche Diagnostics, Indianapolis, IN, USA) with a limit of detection of 15 IU/mL, or the Abbott RealTime HCV assay (Abbott Laboratories, Abbott Park, IL, USA) with a limit of detection of 12 IU/mL. All testing was performed as per manufacturer's instructions at DAIDS-certified laboratories. HCV genotype was determined using stored baseline specimens after all participants completed their week 24/SVR primary outcome assessment visit. Sanger sequencing was used to obtain a short (approximately 325 bp) fragment sequence of the NS5B region and phylogenetic determination of genotype was performed using Basic Local Alignment Search Tool (BLAST).15
Site investigators were directed to report all serious and grade 3 or worse adverse events, and adverse events associated with study treatment changes. Adverse event severity was graded per the DAIDS grading table.16
Substance use, alcohol use, and smoking were assessed with the Alcohol, Smoking and Substance Involvement Screening Test (ASSIST),17, 18 which was interviewer-administered at study entry and week 24/SVR assessment visit. An aggregate substance use variable considered use of amphetamines, cocaine, hallucinogens, opioids, or sedatives. Active substance use was defined as self-reported use in the preceding 3 months.
Outcomes
The primary efficacy outcome was SVR, defined as plasma HCV RNA less than the assay's lower limit of quantitation (LLOQ) from the first sample obtained at least 22 weeks (and up to 72 weeks) following treatment initiation, corresponding to at least 10 weeks after the scheduled completion of treatment among all participants who initiated treatment, irrespective of subsequent treatment disposition. Participants who missed the week 24 window could return for a visit to assess treatment response any time before week 72. Participants with HCV RNA at or above the LLOQ, as well as those without any HCV RNA results in the time period specified above, were defined as non-responders. The primary safety outcome was any serious adverse event, defined as per the International Council for Harmonisation guidelines, occurring up to 28 weeks following treatment initiation, corresponding to the end of the scheduled week 24 visit window, among all participants who had at least one visit post-study entry.
Secondary outcomes of interest included occurrence of unplanned visits, adverse events, and premature discontinuation of study medication. Unplanned visits were defined as any visit a participant made to the study site before the week 24/SVR assessment. Adverse events were defined as any unfavourable and unintended sign (including an abnormal laboratory finding), symptom, or diagnosis that occurred in a study participant during the conduct of the study, regardless of the attribution (ie, relationship of event to study medication) between study entry and week 28 (the upper window for the week 24 visit where SVR was assessed). The week 24/SVR visit was also the first visit study participants were scheduled to be seen post-treatment completion where a history of adverse events could be elicited from the participants. These included any occurrences that were new in onset or aggravated in severity or frequency from the baseline condition. Premature discontinuation was assessed by self-report if the study participant reported stopping treatment before the completion of the 84 tablets.
Statistical analysis
Our sample size of 400 participants was based on the Wilson score 95% CI from an expected SVR from 92% to 99% where precision of the 95% CI ranged from 5·4% to 2·25% points, respectively. If eight or fewer SVR non-responders out of 400 were observed, the CI would be fully bounded above 96%. Conversely, if 29 or more SVR non-responders out of 400 were observed, the CI would be fully bounded below 95%. Under a true SVR of 98·5%, probability of the lower CI being more than 96% was 0·85; similarly, under a true SVR of 92%, probability of the upper CI being less than 95% was 0·74. The trial was sized on the single-group primary efficacy outcome of the entire sample, and not for any subgroups.
The proportion of the trial sample experiencing SVR was calculated by the number who achieved SVR divided by all participants who initiated treatment using a missing=failure approach. The proportion experiencing the safety outcome was the number of participants with a serious adverse event divided by the number who initiated treatment and had at least one post-treatment assessment. Two-sided 95% CIs for each summary measure were calculated using Wilson score method for binomial proportions. SVR by select baseline characteristics was calculated; no hypothesis testing was performed. Subgroups defined a priori included country, cirrhosis classification, HIV infection status, sex at birth, and HCV genotype. All statistical analyses were done with SAS version 9.4.
There were two planned interim reviews for efficacy, with modification guidelines based on unacceptably low SVR. The first interim review of efficacy was planned at approximately 25% information (ie, SVR outcomes available for around 100 participants). Because recruitment from international locations occurred later in calendar time, the second review was planned at approximately 58% information (ie, SVR outcomes available on at least 230 participants), to allow at least 40% representation of the interim trial sample from participants from research locations outside the USA. The monitoring guideline of unacceptably low SVR was if the upper CI on a one-sided, 99·9% CI (by Wilson score method for binomial proportions) was less than 95%. With 100 participants, this guideline would have been met if 14 or more SVR non-responders were observed (ie, calculated SVR <86/100). Between reviews by the independent committee, the study team monitored study conduct and safety periodically as per the trial's monitoring plan. This trial is registered at ClinicalTrials.gov, NCT03512210.
Role of the funding source
The funders of the study had no role in study design, data collection, data analysis, data interpretation, or writing of the report, or in the decision to submit for publication.
Results
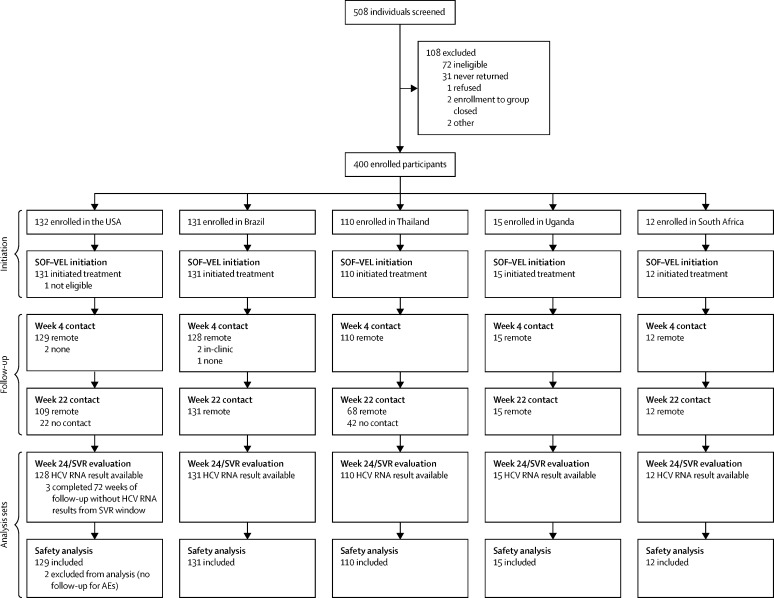
508 formal trial screenings were conducted to enroll 400 participants between Oct 22, 2018, and July 19, 2019; reasons for exclusion from enrolment are shown in figure 1 . Of 72 instances of screen failure, over half (39 [54%]) were due to undetectable HCV RNA. Of 400 participants enrolled in the trial, 399 initiated treatment; one participant reported use of a contraindicated medication at the entry visit and did not start treatment. Of 399 participants initiating treatment, 397 (99·5%) had at least one follow-up visit post-enrolment.
Figure 1.
Trial profile
AE=adverse event. SOF–VEL=sofosbuvir–velpatasvir.
Baseline characteristics are shown in table 1 . The median age of participants was 47 years and 139 (35%) of 399 individuals were assigned female sex at birth; 22 (6%) identified across the transgender spectrum. Most participants were either non-Hispanic Asian, non-Hispanic white, Hispanic/Latinx of any race, or non-Hispanic black. 56 (14%) of 397 participants self-reported current substance use; 161 (40%) of 397 participants reported current alcohol use. At entry, 34 (9%) of the 399 participants had compensated cirrhosis, and 121 (32%) of 374 with HBV panel (anti-HBs, anti-HBc, HBsAg) available had evidence of resolved HBV infection. Median HCV RNA was 6·1 log10 IU/mL (IQR 5·6–6·6). HCV genotype distribution is shown in table 1. 166 (42%) of 399 participants were living with HIV, and of these, 164 (99%) were on suppressive antiretroviral therapy.
Table 1.
Participant characteristics at baseline
| Overall (n=399) | USA (n=131) | Brazil (n=131) | Thailand (n=110) | Uganda (n=15) | South Africa (n=12) | ||
|---|---|---|---|---|---|---|---|
| Age, years | 47 (37–57) | 50 (37–58) | 51 (42–62) | 41 (33–47) | 35 (27–49) | 52 (44–58) | |
| Biological sex assigned at birth | |||||||
| Male | 260 (65%) | 86 (66%) | 59 (45%) | 91 (83%) | 15 (100%) | 9 (75%) | |
| Female | 139 (35%) | 45 (34%) | 72 (55%) | 19 (17%) | 0 | 3 (25%) | |
| Gender identity | |||||||
| Cisgender | 377 (94%) | 129 (98%) | 129 (98%) | 92 (84) | 15 (100) | 12 (100) | |
| Transgender spectrum* | 22 (6%) | 2 (2%) | 2 (2%) | 18 (16) | 0 | 0 | |
| Race/ethnicity | |||||||
| White, non-Hispanic | 99 (25%) | 72 (55%) | 23 (18%) | 0 | 0 | 4 (33%) | |
| Black, non-Hispanic | 57 (14%) | 29 (22%) | 6 (5%) | 0 | 15 (100%) | 7 (58%) | |
| Asian, non-Hispanic | 113 (28%) | 2 (2%) | 0 | 110 (100%) | 0 | 1 (8%) | |
| Hispanic/Latinx, any race | 95 (24%) | 23 (18%) | 72 (55%) | 0 | 0 | 0 | |
| Other | 35 (9%) | 5 (4%) | 30 (23%) | 0 | 0 | 0 | |
| History of substance use† | |||||||
| Currently | 56 (14%) | 27 (21%) | 17 (13%) | 10 (9%) | 0 | 2 (17%) | |
| Previously | 170 (43%) | 81 (62%) | 36 (28%) | 51 (46%) | 0 | 2 (17%) | |
| Never | 171 (43%) | 23 (18%) | 76 (58%) | 49 (45%) | 15 (100%) | 8 (67%) | |
| Alcohol use† | |||||||
| Currently | 161 (40%) | 60 (46%) | 43 (33%) | 46 (42%) | 7 (47%) | 5 (42%) | |
| Previously | 179 (45%) | 59 (45%) | 56 (43%) | 56 (51%) | 5 (33%) | 3 (25%) | |
| Never | 57 (14%) | 12 (9%) | 30 (23%) | 8 (7%) | 3 (20%) | 4 (33%) | |
| Tobacco use† | |||||||
| Currently | 140 (35%) | 83 (63%) | 29 (22%) | 25 (23%) | 0 | 3 (25%) | |
| Previously | 124 (31%) | 31 (24%) | 43 (33%) | 45 (41%) | 2 (13%) | 3 (25%) | |
| Never | 133 (33%) | 17 (13%) | 57 (44%) | 40 (36%) | 13 (87%) | 6 (50%) | |
| Compensated cirrhosis‡ | 34 (9%) | 9 (7%) | 13 (10%) | 10 (9%) | 0 | 2 (17%) | |
| HCV genotype§ | |||||||
| Genotype 1a | 177 (44%) | 72 (55%) | 45 (34%) | 59 (54%) | 0 | 1 (8%) | |
| Genotype 1b | 72 (18%) | 18 (14%) | 49 (37%) | 4 (4%) | 0 | 1 (8%) | |
| Genotype 2 | 26 (7%) | 20 (15%) | 4 (3%) | 0 | 0 | 2 (17%) | |
| Genotype 3 | 80 (20%) | 17 (13%) | 28 (21%) | 33 (30%) | 0 | 2 (17%) | |
| Genotype 4 | 26 (7%) | 3 (2%) | 5 (4%) | 1 (1%) | 14 (93%) | 3 (25%) | |
| Genotype 5 | 3 (1%) | 0 | 0 | 0 | 0 | 3 (25%) | |
| Genotype 6 | 11 (3%) | 0 | 0 | 11 (10%) | 0 | 0 | |
| Genotype 7 | 1 (<1%) | 0 | 0 | 0 | 1 (7%) | 0 | |
| Unknown | 3 (1%) | 1 (<1%) | 0 | 2 (2%) | 0 | 0 | |
| Log10HCV RNA, IU/mL | 6·1 (5·6–6·6) | 6·2 (5·6–6·6) | 5·8 (5·4–6·2) | 6·6 (6·0–6·9) | 5·8 (5·4–6·2) | 6·1 (6·0–6·6) | |
| BMI, kg/m2 | 25·0 (22·2–28·7) | 26·6 (23·7–31·9) | 26·0 (22·3–29·8) | 23·1 (20·5–25·2) | 22·9 (21·9–25·6) | 24·3 (22·3–27·1) | |
| Individuals with HIV | |||||||
| Number with HIV | 166 (42%) | 43 (33%) | 28 (21%) | 89 (81%) | 1 (7%) | 5 (42%) | |
| Currently on antiretroviral therapy | 164/166 (99%) | 42/43 (98%) | 28/28 (100%) | 88/89 (99%) | 1/1 (100%) | 5/5 (100%) | |
| HIV RNA <400 copies per mL | 164/166 (99%) | 42/43 (98%) | 28/28 (100%) | 88/89 (99%) | 1/1 (100%) | 5/5 (100%) | |
Data are median (IQR), n (%), or n/N individuals with HIV (%).
Identities across the transgender spectrum included genderqueer, female or transgender female, gender non-conforming, and transgender male.
Missing data for two participants from Brazil.
Based on FIB-4 and Child-Turcotte-Pugh scoring.
Missing data for two participants from Thailand and one from the USA.
Week 4 remote contact was successful in 394 (99%) of 399 participants; week 22 remote contact was successful in 335 (84%). Three participants reported losing study medications. One reported the loss 14 days after the interruption occurred and, per protocol, study medications were not replaced. The two other participants reported losing their third bottle after interruptions of 4 and 7 days, and were provided with replacements. Most (362 [91%] of 397) self-reported completing all study medications between 77 and 91 days (expected 84 days), 24 (6%) reported taking between 92 and 105 days, and nine (2%) reported taking 106 days or longer to complete their study medications. Overall, 355 (89%) of 397 individuals self-reported taking 100% of medications within the treatment period at the week 24/SVR visit, and 30 (8%) of 397 reported taking between 90 and 99%; the remainder reported taking less than 90% of study medication.
15 (4%) of 399 participants had 21 unplanned in-person visits between entry and week 22 (table 2 ). The most common reasons were abnormal laboratory results at entry (n=8) or non-adverse event related clinical events (n=6). Three unplanned visits were associated with an adverse event, none of which were related to study medication. None of the unplanned visits were associated with treatment discontinuation. Details of all unplanned study visits are provided in the appendix (p 6).
Table 2.
Unplanned visits and adverse events occurring from enrolment through week 28 (n=397)
| Overall (n=397) | United States (n=129) | Brazil (n=131) | Thailand (n=110) | Uganda (n=15) | South Africa (n=12) | ||
|---|---|---|---|---|---|---|---|
| Unplanned visits* | |||||||
| Participants reporting at least one unplanned visits* | 15 (4%) | 3 (2%) | 3 (2%) | 4 (4%) | 3 (20%) | 2 (17%) | |
| Total number of unplanned visits | 21 | 3 | 5 | 4 | 3 | 6 | |
| Reason for unplanned visits | |||||||
| Lab evaluations | 8 | 1 | 0 | 3 | 0 | 4 | |
| Adverse event | 3 | 1 | 1 | 0 | 0 | 1 | |
| Clinical event (non-AE) | 6 | 0 | 3 | 0 | 3 | 0 | |
| Other† | 4 | 1 | 1 | 1 | 0 | 1 | |
| Serious adverse events (primary safety outcome) | |||||||
| Total number of participants reporting at least one SAE | 14 (4%) | 7 (5%) | 0 | 5 (5%) | 0 | 2 (17%) | |
| Toxicity grade | |||||||
| Grade 3 | 12 | 7 | 0 | 4 | 0 | 1 | |
| Grade 4 | 2 | 0 | 0 | 1 | 0 | 1 | |
| SAE occurring while on study medication | 5 | 1 | 0 | 3 | 0 | 1 | |
| SAE leading to discontinuation of study drug | 0 | 0 | 0 | 0 | 0 | 0 | |
| SAE related to study medication | 0 | 0 | 0 | 0 | 0 | 0 | |
| Death | 0 | 0 | 0 | 0 | 0 | 0 | |
| Adverse events (excluding serious adverse events) (secondary safety outcome) | |||||||
| Participants reporting at least one AE (excluding SAEs) | 23 (6%) | 7 (5%) | 8 (6%) | 7 (6%) | 0 | 1 (8%) | |
| Total number of adverse events | 28 | 12 | 8 | 7 | 0 | 1 | |
| AE occurred while on study medication | 8 | 7 | 1 | 0 | 0 | 0 | |
| AE related to study medication | 5 | 4 | 1 | 0 | 0 | 0 | |
| AE leading to discontinuation of study drug‡ | 1 | 1 | 0 | 0 | 0 | 0 | |
Data are n or n (%). AE=adverse event. SAE=serious adverse event.
All participants who initiated treatment were included in the denominator for unplanned visits (n=399).
Other included unscheduled study visit outside of window (n=1), visited site to clarify query on study treatment (n=1), visited site for counseling and collect vitamin B12 for management of pernicious anaemia (n=1), participant newly diagnosed with HIV and wanted to initiate antiretroviral therapy (n=1).
One case of abdominal distension attributed to study product resulted in discontinuation.
Two participants were lost to follow-up before primary outcome assessment with no information available after the entry visit; these two participants were considered non-responders in the primary efficacy analyses. One additional participant returned for the SVR assessment before the SVR visit window and did not return thereafter; this participant was also considered as missing the SVR visit. Additionally, two participants discontinued treatment early, one due to medication loss and the other due to a grade 1 adverse event.
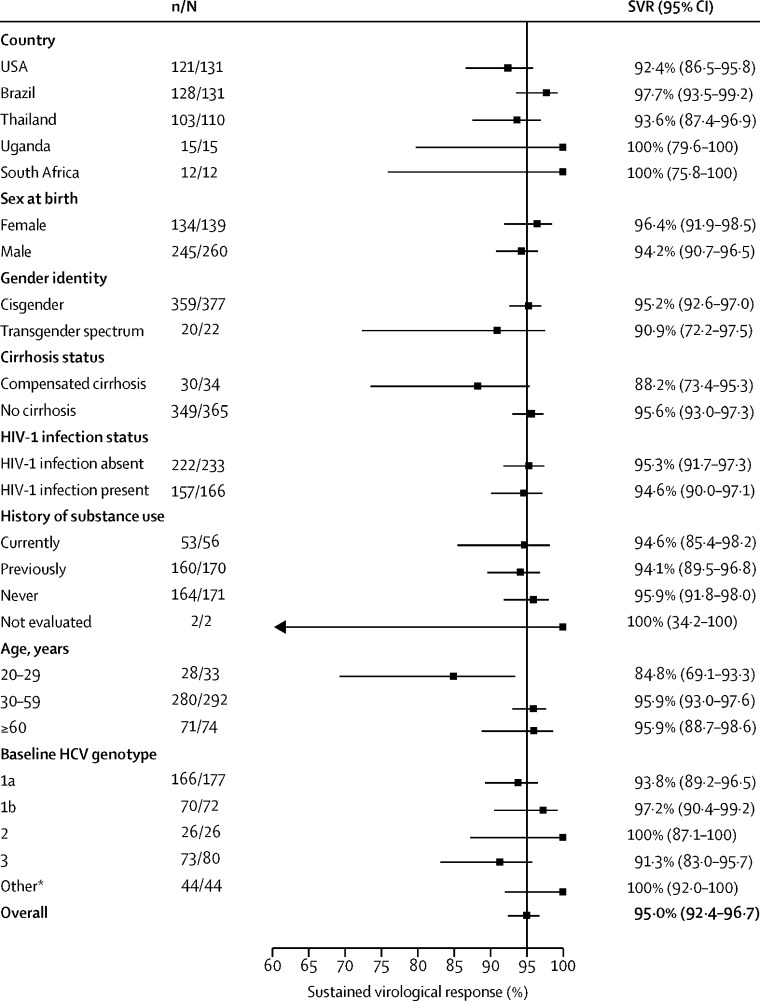
Of 399 participants who initiated treatment, 379 (95·0%, 95% CI 92·4–96·7) had an SVR. By country, SVR ranged from 92·4% (86·5–95·8) in the USA to 100% (75·8–100) in South Africa and 100% (79·6–100) in Uganda (figure 2 ). 30 (88·2%, 95% CI 73·4–95·3) of 34 participants with cirrhosis and 349 (95·6%, 95% CI 93·0–97·3) of of 365 without cirrhosis achieved SVR (figure 2). Among the 166 participants living with HIV, 94·6% (90·0–97·1) achieved SVR (figure 2). Across genotypes, SVR ranged from 91·3% (83·0–95·7) in HCV genotype 3 to 100% (87·1–100) for genotype 2. SVR was also 100% (87·1–100) for genotype 4 (26/26), 100% (43·9–100) for genotype 5 (3/3), 100% (74·1–100) for genotype 6 (11/11), and 100% (20·7–100) for genotype 7 (1/1). SVR among participants with current, former, and no history of substance use are shown in figure 2.
Figure 2.
SVR overall and by subgroups defined by various participant baseline characteristics
*Other included genotypes 4–7, and three participants with unknown genotype.
14 (4%) of 397 participants experienced at least one serious adverse event (table 2; appendix p 7); five occurred while participants were on treatment and none were associated with study medication or resulted in treatment discontinuation or death. Two grade 4 adverse events were reported: one myocardial infarction and one acute exacerbation of anaemia in a participant with known pernicious anaemia. 23 (6%) of 397 participants reported 28 adverse events. Eight occurred while on study medication and five (diarrhoea [n=2], headache [n=1], fatigue [n=1], and abdominal bloating [n=1; resulting in discontinuation reported above]) were attributed to the study medication.
20 participants did not achieve protocol-defined SVR; two were lost to follow-up after entry, and one participant who discontinued treatment returned for SVR evaluation early. 17 participants had detectable HCV RNA greater than the lower limit of quantification at the week 24/SVR visit, of whom one discontinued treatment after 6 days due to loss of study medication. The remaining 16 participants completed treatment and had a valid week 24/SVR sample; ten had genotype 1 and six had genotype 3 infections. Four participants had compensated cirrhosis. 12 (75%) of the 16 non-responders who completed treatment and returned for their week 24/SVR assessment reported taking 100% of their study medication, two (13%) reported taking 90–99%, and two (13%) reported taking less than 90%. Three (15%) of the 20 non-responders reported current substance use. Detailed information about the 20 non-responders is provided in table 3 .
Table 3.
Select characteristics of participants who did not achieve sustained virological response
| Age, years | Sex at birth (M/F) | Current substance use at entry (Y/N) | Compensated Cirrhosis based on FIB-4/CTP (Y/N) | HIV co-infection (Y/N) | Self-reported time to completion of SOF–VEL, days | Self-reported adherence at week 24/SVR visit | HCV genotype at entry | HCV RNA at entry, log10 IU/mL | HCV RNA at week 24/SVR assessment, log10 IU/mL | Adverse event (Y/N) | Unplanned visit (Y/N) | Lost medication/premature discontinuation (Y/N) | Lost to follow-up (Y/N) | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Brazil | 38 | M | N | N | Y | 84 | 100% | 1a | 5·6 | 5·5 | N | N | N | N |
| Brazil | 66 | M | Y | N | N | 84 | 100% | 3a | 6·0 | 5·6 | N | N | N | N |
| Brazil | 59 | F | N | Y | N | 85 | 100% | 3a | 5·5 | 6·1 | N | N | N | N |
| Thailand | 27 | M | N | N | Y | 83 | 100% | 1a | 6·4 | 6·7 | N | N | N | N |
| Thailand | 47 | M | N | N | Y | 84 | 100% | 3b | 6·3 | 6·5 | N | N | N | N |
| Thailand | 25 | M | N | N | Y | 84 | 100% | 1a | 5·9 | 5·4 | N | N | N | N |
| Thailand | 51 | M | N | Y | Y | 84 | 100% | 3a | 6·5 | 5·8 | N | N | N | N |
| Thailand | 59 | M | N | Y | N | 85 | 100% | 3b | 6·5 | 4·1 | Y | N | N | N |
| Thailand | 33 | M | N | N | Y | 88 | 100% | 1a | 6·8 | 7·1 | Y | N | N | N |
| Thailand | 20 | F | N | N | N | 157 | 50–74% | 3b | 5·8 | 3·3 | N | N | N | N |
| USA | 24 | F | N | N | N | 6 | <50% | 3a | 4·1 | 5·5 | N | N | Y | N |
| USA | 46 | M | N | N | N | 85 | 100% | 1a | 6·5 | 6·7 | N | N | N | N |
| USA | 62 | M | N | Y | N | 85 | 100% | 1a | 6·1 | 6·5 | N | N | N | N |
| USA | 60 | M | N | N | N | 85 | 100% | 1a | 5·5 | 4·6 | N | N | N | N |
| USA | 58 | M | Y | N | N | 87 | 90–99% | 1a | 6·2 | 4·7 | N | N | N | N |
| USA | 51 | M | N | N | Y | 95 | 90–99% | 1a | 6·2 | 6·3 | N | N | N | N |
| USA | 34 | M | N | N | Y | 110 | 75–89% | 1b | 5·8 | 5·4 | N | N | N | N |
| USA | 25 | F | N | N | Y | .. | .. | 1a | 5·3 | NA | N | N | .. | Y |
| USA | 55 | M | Y | N | N | .. | .. | 1a | 5·5 | NA | N | N | .. | Y |
| USA | 59 | F | N | N | N | 51 | <50% | 1b | 6·4 | NA | Y | Y | Y | Y |
Two participants were lost to follow-up post-entry with no follow-up information beyond baseline. CTP=Child-Turcotte-Pugh. M=male. F=female. SOF–VEL=sofosbuvir–velpatasvir. Y=yes. N=no. NA=sample not available within SVR window.
Discussion
The MINMON trial recruited a diverse global sample of people living with HCV, including people with compensated cirrhosis and HIV infection, and HCV cure (ie, SVR) was achieved in 95·0% of participants despite minimal laboratory monitoring and in-person visits. This finding has important implications for HCV elimination, particularly in the face of public health emergencies such as the COVID-19 pandemic. The MINMON approach can specifically inform HCV elimination programmes by providing a solution that overcomes barriers of in-person contact and limited resources.
In other large trials of HCV treatment with sofosbuvir–velpatasvir, observed SVR was 95–99%.19, 20 Unlike MINMON, however, in these trials, participants were seen at weeks 1, 2, 4, 6, 8, 10, and 12 post-entry. Such intensive follow-up requires extensive resources that are not feasible outside of clinical trials, especially in resource-constrained settings where most people living with HCV reside. A recent meta-analysis of more than 5500 individuals enrolled in 12 clinical cohorts across Canada, Europe, and the USA observed an SVR of 92·3% when accounting for losses to follow-up, non-adherence, treatment discontinuation, and death.21 Similarly, the SVR from a Canadian cohort treated with sofosbuvir–velpatasvir was 94·6%.22 The 95·0% overall SVR observed in MINMON is comparable to the registrational trials in which sofosbuvir–velpatasvir was delivered with intensive clinical and laboratory monitoring and more frequent in-person visits for medication refills, and observational cohorts in the setting of a standard of care with similar levels of clinical visits and monitoring.
Few studies have evaluated simplified HCV treatment delivery.11, 12, 13 Most reduced the frequency of on-treatment laboratory monitoring but still required pre-treatment HCV genotyping and medication dispensation at multiple timepoints.11, 13 Most comparable to MINMON is the SMART-C trial, a randomised trial comparing standard to simplified monitoring using a fixed-dose combination of glecaprevir and pibrentasvir, which required three tablets to be taken orally daily for 8 weeks.12 However, this trial excluded people with cirrhosis, included only 27 (7%) individuals living with HIV and HCV, and required pre-treatment HCV genotyping. While similar to MINMON in that all medications were dispensed at entry, the on-treatment remote contact in the simplified arm of SMART-C was at weeks 4 and 8. SMART-C used transient elastography or aspartate aminotransferase to platelet ratio index to stage fibrosis. By contrast, in MINMON, cirrhosis classification was based on the readily accessible and low-cost FIB-4 score, which is based on age, routine laboratory assessment of liver enzyme levels, and platelet count, and among those with FIB-4 score of more than 3·25, the Child-Turcotte-Pugh score, which is based on presence of signs or symptoms and routine laboratory assessments of total bilirubin, albumin, and international normalised ratio. The overall SVR observed in the simplified arm of the SMART-C trial was 92%, compared with 95% that was observed in the standard monitoring arm.
There are several strengths of the MINMON trial. This is the first trial of a simplified monitoring approach to eliminate pre-treatment HCV genotyping and to include people with evidence of compensated cirrhosis. The elimination of pre-treatment genotyping is crucial to the scale-up of HCV therapy, particularly in low-income and middle-income countries where this testing is often unavailable, and when available, is usually associated with high cost. Second, this is the first simplified monitoring study to include a racially and globally diverse population from high-income and low-income settings. Third, dispensation of the full treatment course at baseline and elimination of all on-treatment clinical and laboratory monitoring reduces patient, provider, and health system costs. This is also particularly relevant to populations who are mobile, such as long-distance truckers who have a high HCV prevalence and may not be able to accommodate appointments every 4 weeks ot more frequently for refills given the nature of their occupation.23 Although this trial was designed before the COVID-19 pandemic, the elimination of in-person contact is particularly relevant now in the context of broad-based public health interventions during state and nationwide lockdowns which limit mobility. Further, telemedicine and remote contact have become the norm in the delivery of health care in several countries, especially during lockdowns. Fourth, tests used here to assess cirrhosis (FIB-4) and HBsAg positivity are routinely available globally. Despite 32% of the participants having resolved HBV infection and 9% with compensated cirrhosis, we did not observe any serious adverse events related to liver dysfunction.24, 25 Fifth, participants from each genotype 1–7 achieved SVR of more than 91%, with CIs including 95%. Sixth, this trial included people living with HIV (42% of the study sample) and current substance use (14%), with observed SVR over 94% within each of these subgroups. SVR in participants with HIV was similar to the 95% SVR observed in the ASTRAL-5 HIV/HCV trial, which required more intensive follow-up and monitoring.26
There are, however, some limitations that need to be considered in the interpretation of these findings. A weakness of this trial was the absence of a concurrent comparator group—ie, this was a single-arm trial with no control group receiving standard monitoring. However, extensive experience with sofosbuvir–velpatasvir and other HCV regimens in observational cohorts provided for an expected SVR of approximately 95%.21, 22, 27
The small number of participants in certain subgroups limits the ability to make strong conclusions regarding these populations. Of particular importance are individuals with HCV genotype 3 infection or compensated cirrhosis in whom the observed SVR was lower than 95% in this trial; however, of importance, the 95% CI for SVR in these groups included 95%. HCV genotype 3 infection is common in some regions where this trial was conducted and 80 study participants had genotype 3 infection. Of these, nine had genotype 3b infection, which may be more likely to harbour NS5A resistance-associated substitutions;28 of these nine participants, three did not achieve SVR. The significance of this observation in a small subgroup of participants is uncertain but is unlikely to be the consequence of the minimal monitoring strategy. From the perspective of global HCV elimination, treatment implementation in the absence of HCV genotype testing is a crucial element of the simplification strategy, reducing cost, and increasing uptake, especially in settings where genotyping may not be available.
While this trial was not designed or powered to examine subgroup differences, we are able to examine the SVR in MINMON in the context of SVR reported from other trials and large cohorts of people taking sofosbuvir–velpatasvir. For example, among participants with genotype 3 infection, the SVR of 91·3% observered here was numerically lower than the 95% observed in ASTRAL-3, but the 95% CI for the two estimates overlapped. In an analysis of individuals with genotype 2 and 3 infection outside of a trial setting, the SVR among those with genotype 3 and taking sofosbuvir–velpatasvir was 92%.29 Similarly, in MINMON, SVR of 88·2% among those with compensated cirrhosis can be compared with a range of 90–99% (depending on genotype) in the ASTRAL 2–4 trials. Additionally, in a large observational cohort of individuals taking sofosbuvir–velpatasvir, 126 (10·9%) of 1147 with cirrhosis failed to achieve SVR, which is comparable to the 88·2% SVR observed in this trial. Therefore, although the observed point estimate of SVR in certain subgroups was lower than 95%, the observed SVRs were comparable to other observational and clinical trial data using sofosbuvir–velpatasvir with standard monitoring.
The eligibility requirement of HIV RNA suppression among participants with HIV selected for a subset of individuals with documented medication adherence and may not be generalisable to all people living with HIV and HCV. Since all trial participants received sofosbuvir–velpatasvir, these results may not apply to other HCV regimens. Further, although we included people with a history of drug use, we did not have an adequate number of participants who reported a history of active injection; future studies should evaluate this approach among people who inject drugs who may face additional adherence challenges but are a key population to be reached for HCV elimination. The small number of participants aged 18–29 years limited the ability to make generalisations about the use of MINMON in young adult populations who may need additional interventions to improve adherence. Finally, another subgroup that will also need to be considered in future research are treatment-experienced individuals.
Last, this trial was implemented at NIH-certified research sites, which may not reflect the care available in other treatment settings. However, the approach and laboratory assays used in the trial are readily available in many primary health-care settings; the introduction of novel point-of-care and high throughput diagnostics for confirmation of active infection by RNA or HCV core antigen could accelerate the implementation of such a simplified treatment approach globally.30, 31 Further, in several national HCV programmes across the world, task-shifting, transition to point-of-care testing, and elimination of certain monitoring components are already being implemented with promising results.32, 33 Nevertheless, all programmes require some level of on-treatment, in-person contact at least for medication refills. It is crucial to evaluate the utility of the MINMON approach in such programmes to further simplify HCV treatment by eliminating all on-treatment visits to help accelerate the global HCV elimination agenda.
Limitations notwithstanding, the MINMON approach with sofosbuvir–velpatasvir used in this study is a simple, safe, and efficacious way to deliver treatment globally to HCV-treatment-naive or HCV/HIV co-infected individuals without evidence of decompensated cirrhosis. Coupled with innovative case finding strategies and point-of-care diagnostics, this streamlined approach could have a crucial role in achieving global HCV elimination.
Data sharing
The authors confirm that all data underlying the findings are fully available without restriction. Due to ethical restrictions, study data are available upon request from sdac.data@sdac.harvard.edu with the written agreement of the AIDS Clinical Trials Group.
Declaration of interests
SSS declares grants and study products to the institution from Gilead Sciences related to the submitted work as well as grants and study product to the institution from Gilead Sciences and Abbott Laboratories not related to the submitted work, and honoraria from Gilead Sciences. GKR declares grants to his institution from Gilead Sciences, Citius Pharmaceuticals, Pfizer, Emergent Biosolutions, and Leonard Meron Bioscience, outside of the submitted work, and consulting fees from Teradyne Inc, Massachusetts Executive Office of Energy and Environmental Affairs, and Massachusetts Interscholastic Athletic Association. AS and NC are employees of Gilead Sciences and hold stock in the company. EPN declares honoraria from Gilead and AbbVie. DK declares honoraria and support to attend conferences from Gilead Sciences. JS declares speaker and advisory board honoraria from ViiV, Merck, Gilead, and AbbVie. PT declares consulting fees from ViiV and Merck. CB declares grants from Gilead Sciences paid to her institution, outside of the submitted work, and served as the chair of a data safety and monitoring board (DSMB) for GlaxoSmithKline. SN declares grants from Gilead Sciences and AbbVie to her institution, outside of the submitted work, personal consulting fees from BioMarin and Theratechnologies, support to attend meetings from Gilead Sciences, and has participated on DSMB for Bristol Myers Squibb, FHI 360, and Personal Health Insights Inc, and holds stock options in Vir Bio. DW declares grants to his institution from Gilead Sciences outside of the submitted work and discloses royalties/licenses from UpToDate. MS declares grants to his institution from Gilead Sciences, AbbVie, Janssen, and Assembly Biosciences outside of the submitted work, personal consulting fees from AbbVie, Gilead Sciences, Assembly Biosciences, Arbutus, Virion, Antios, and GlaxoSmithKline, has received honoraria from Practice Point Communication, DKB, and Clinical Care Options, and served on DSMBs and holds stock in Gilead Sciences and AbbVie. All other authors declare no competing interests.
Acknowledgments
Acknowledgments
We thank people living with HCV worldwide, especially the ACTG A5360 participants, who graciously participate in research studies globally. We would also like to acknowledge the ACTG leadership, the hepatitis transformative science group of the ACTG, and the statistical and data management centre of the ACTG for their continued support and guidance. We also acknowledge the contributions of all members of the ACTG A5360 team who oversaw the implementation of this trial. Research reported in this publication was supported by the National Institute of Allergy and Infectious Diseases of the National Institutes of Health under Award Number UM1 AI068634, UM1 AI068636, and UM1 AI106701. Additional funding support and study product was provided by Gilead Sciences. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Contributors
SSS, SWC, LS, LAS, CW, GR, CS, AA, BL, NC, SN, DW, and MS contributed to the study design. SSS served as the protocol chair; SWC and MS served as protocol co-vice chairs. SSS, SWC, LS, LAS, CW, GR, IB, CS, AS, AA, BL, DA, EPN, DAK, KS, CK, PT, JAB, JSB, CAB, MVS, NC, SN, DW, and MS were involved in the implementation of the trial and data collection. LS and IB had full access to all the data, verified the underlying data, and led all the statistical analysis. All authors contributed to the data interpretation. SSS wrote the first draft of the manuscript. All authors reviewed and approved the final manuscript. SSS had final responsibility for the decision to submit for publication.
Supplementary Material
References
- 1.Thomas DL. Global elimination of chronic hepatitis. N Engl J Med. 2019;380:2041–2050. doi: 10.1056/NEJMra1810477. [DOI] [PubMed] [Google Scholar]
- 2.WHO Interim guidance for country validation of viral hepatitis elimination. https://www.who.int/publications/i/item/9789240028395 [DOI] [PubMed]
- 3.Gamkrelidze I, Pawlotsky JM, Lazarus JV, et al. Progress towards hepatitis C virus elimination in high-income countries: an updated analysis. Liver Int. 2021;41:456–463. doi: 10.1111/liv.14779. [DOI] [PubMed] [Google Scholar]
- 4.Linas BP, Nolen S. A guide to the economics of hepatitis C virus cure in 2017. Infect Dis Clin North Am. 2018;32:447–449. doi: 10.1016/j.idc.2018.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.WHO Accelerating access to hepatitis C diagnostics and treatment. Overcoming barriers in low and middle-income countries. Global progress report 2020. https://apps.who.int/iris/rest/bitstreams/1328465/retrieve
- 6.Polaris Observatory HCV Collaborators Global prevalence and genotype distribution of hepatitis C virus infection in 2015: a modelling study. Lancet Gastroenterol Hepatol. 2017;2:161–176. doi: 10.1016/S2468-1253(16)30181-9. [DOI] [PubMed] [Google Scholar]
- 7.Chaillon A, Mehta SR, Hoenigl M, et al. Cost-effectiveness and budgetary impact of HCV treatment with direct-acting antivirals in India including the risk of reinfection. PLoS One. 2019;14:e0217964. doi: 10.1371/journal.pone.0217964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Blach S, Kondili LA, Aghemo A, et al. Impact of COVID-19 on global HCV elimination efforts. J Hepatol. 2021;74:31–36. doi: 10.1016/j.jhep.2020.07.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.AASLD-IDSA Hepatitis C Guidance Panel Hepatitis C guidance 2019 update: American Association for the Study of Liver Diseases–Infectious Diseases Society of America recommendations for testing, managing, and treating hepatitis C virus infection. Hepatology. 2020;71:686–721. doi: 10.1002/hep.31060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.European Association for the Study of the Liver EASL recommendations on treatment of hepatitis C: final update of the series. J Hepatol. 2020;73:1170–1218. doi: 10.1016/j.jhep.2022.10.006. [DOI] [PubMed] [Google Scholar]
- 11.Davis JS, Young M, Marshall C, et al. Minimal compared with standard monitoring during sofosbuvir-based hepatitis C treatment: a randomized controlled trial. Open Forum Infect Dis. 2020;7:ofaa022. doi: 10.1093/ofid/ofaa022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dore GJ, Feld JJ, Thompson A, et al. Simplified monitoring for hepatitis C virus treatment with glecaprevir plus pibrentasvir, a randomised non-inferiority trial. J Hepatol. 2020;72:431–440. doi: 10.1016/j.jhep.2019.10.010. [DOI] [PubMed] [Google Scholar]
- 13.Sood A, Duseja A, Kabrawala M, et al. Sofosbuvir-velpatasvir single-tablet regimen administered for 12 weeks in a phase 3 study with minimal monitoring in India. Hepatol Int. 2019;13:173–179. doi: 10.1007/s12072-019-09927-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sterling RK, Lissen E, Nathan Clumeck N, et al. Development of a simple noninvasive index to predict significant fibrosis in patients with HIV/HCV coinfection. Hepatology. 2006;43:1317–1325. doi: 10.1002/hep.21178. [DOI] [PubMed] [Google Scholar]
- 15.NIH BLAST® Basic Local Alignment Search Tool. https://blast.ncbi.nlm.nih.gov/Blast.cgi
- 16.DAIDS Division of AIDS (DAIDS) table for grading the severity of adult and pediatric adverse events. corrected version 2.1, July 2017. https://rsc.niaid.nih.gov/sites/default/files/daidsgradingcorrectedv21.pdf
- 17.Humeniuk R, Ali R, Babor TF, et al. Validation of the Alcohol, Smoking and Substance Involvement Screening Test (ASSIST) Addiction. 2008;103:1039–1047. doi: 10.1111/j.1360-0443.2007.02114.x. [DOI] [PubMed] [Google Scholar]
- 18.WHO The Alcohol, Smoking and Substance Involvement Screening Test (ASSIST). Manual for use in primary care. https://www.who.int/publications/i/item/978924159938-2
- 19.Foster GR, Afdhal N, Roberts SK, et al. Sofosbuvir and velpatasvir for HCV genotype 2 and 3 infection. N Engl J Med. 2015;373:2608–2617. doi: 10.1056/NEJMoa1512612. [DOI] [PubMed] [Google Scholar]
- 20.Feld JJ, Jacobson IM, Hezode C, et al. Sofosbuvir and velpatasvir for HCV genotype 1, 2, 4, 5, and 6 infection. N Engl J Med. 2015;373:2599–2607. doi: 10.1056/NEJMoa1512610. [DOI] [PubMed] [Google Scholar]
- 21.Mangia A, Milligan S, Khalili M, et al. Global real-world evidence of sofosbuvir/velpatasvir as simple, effective HCV treatment: analysis of 5552 patients from 12 cohorts. Liver Int. 2020;40:1841–1852. doi: 10.1111/liv.14537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wilton J, Wong S, Yu A, et al. Real-world effectiveness of sofosbuvir/velpatasvir for treatment of chronic hepatitis C in British Columbia, Canada: a population-based cohort study. Open Forum Infect Dis. 2020;7:ofaa055. doi: 10.1093/ofid/ofaa055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Valway S, Jenison S, Keller N, Vega-Hernandez J, Hubbard McCree D. Risk assessment and screening for sexually transmitted infections, HIV, and hepatitis virus among long-distance truck drivers in New Mexico, 2004-2006. Am J Public Health. 2009;99:2063–2068. doi: 10.2105/AJPH.2008.145383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pockros PJ. Black box warning for possible HBV reactivation during DAA therapy for chronic HCV infection. Gastroenterol Hepatol. 2017;13:536–540. [PMC free article] [PubMed] [Google Scholar]
- 25.Bersoff-Matcha SJ, Cao K, Jason M, et al. Hepatitis B virus reactivation associated with direct-acting antiviral therapy for chronic hepatitis C virus: a review of cases reported to the U.S. Food and Drug Administration adverse event reporting system. Ann Intern Med. 2017;166:792–798. doi: 10.7326/M17-0377. [DOI] [PubMed] [Google Scholar]
- 26.Wyles D, Brau N, Kottilil S, et al. Sofosbuvir and velpatasvir for the treatment of hepatitis C virus in patients coinfected with human immunodeficiency virus type 1: an open-label, phase 3 study. Clin Infect Dis. 2017;65:6–12. doi: 10.1093/cid/cix260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Falade-Nwulia O, Suarez-Cuervo C, Nelson DR, Fried MW, Segal JB, Sulkowski MS. Oral direct-acting agent therapy for hepatitis C virus infection: a systematic review. Ann Intern Med. 2017;166:637–648. doi: 10.7326/M16-2575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Smith D, Magri A, Bonsall D, et al. Resistance analysis of genotype 3 hepatitis C virus indicates subtypes inherently resistant to nonstructural protein 5A inhibitors. Hepatology. 2019;69:1861–1872. doi: 10.1002/hep.29837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Belperio PS, Shahoumian TA, Loomis TP, Mole LA, Backus LI. Real-world effectiveness of daclatasvir plus sofosbuvir and velpatasvir/sofosbuvir in hepatitis C genotype 2 and 3. J Hepatol. 2019;70:15–23. doi: 10.1016/j.jhep.2018.09.018. [DOI] [PubMed] [Google Scholar]
- 30.Grebely J, Lamoury FMJ, Hajarizadeh B, et al. Evaluation of the Xpert HCV Viral Load point-of-care assay from venipuncture-collected and finger-stick capillary whole-blood samples: a cohort study. Lancet Gastroenterol Hepatol. 2017;2:514–520. doi: 10.1016/S2468-1253(17)30075-4. [DOI] [PubMed] [Google Scholar]
- 31.Freiman JM, Tran TM, Schumacher SG, et al. Hepatitis C core antigen testing for diagnosis of hepatitis C virus infection: a systematic review and meta-analysis. Ann Intern Med. 2016;165:345–355. doi: 10.7326/M16-0065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhang M, O'Keefe D, Craig J, et al. Decentralised hepatitis C testing and treatment in rural Cambodia: evaluation of a simplified service model integrated in an existing public health system. Lancet Gastroenterol Hepatol. 2021;6:371–380. doi: 10.1016/S2468-1253(21)00012-1. [DOI] [PubMed] [Google Scholar]
- 33.Shiha G, Soliman R, Serwah A, Mikhail NNH, Asselah T, Easterbrook P. A same day ‘test and treat’ model for chronic HCV and HBV infection: results from two community-based pilot studies in Egypt. J Viral Hepat. 2020;27:593–601. doi: 10.1111/jvh.13268. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The authors confirm that all data underlying the findings are fully available without restriction. Due to ethical restrictions, study data are available upon request from sdac.data@sdac.harvard.edu with the written agreement of the AIDS Clinical Trials Group.