INTRODUCTION
Sertoli-Leydig cell tumor (SLCT) is a rare mixed sex cord-stromal tumor comprising less than 0.5% of all ovarian cancers [1]. Somatic DICER1 hotspot variants in the RNase IIIb domain are identified in nearly all moderately and poorly differentiated SLCT [2-4]. The development of bilateral SLCT is extremely rare [1,3], typically seen as metachronous tumors arising 2 to 6 years apart in patients with DICER1 syndrome caused by germline pathogenic loss-of-function DICER1 variants [5]. In prior reports, bilateral SLCT tumors in four patients have been reported to be independent primary tumors [5,6]. However, determining whether a second SLCT in a DICER1 syndrome patient represents recurrence or an independent primary tumor can be challenging. Here we report a patient with three SLCT tumors arising within a span of 2.5 years. Germline and tumor profiling revealed a pathogenic DICER1 variant in the germline and three distinct somatic DICER1 hotspot variants in the three tumors, helping to clarify the clinical management plan for the patient.
CASE PRESENTATION
Clinical Presentation, Pathology and Family History
The patient is a 13-year-old female who presented with progressive abdominal pain and nausea for 2 weeks. A magnetic resonance imaging (MRI) scan revealed a 19 x 18.5 x 12.5 cm right ovarian solid/cystic mass (RT1). Following right salpingo-oophorectomy and surgical staging, pathologic examination revealed a moderately differentiated SLCT, FIGO stage 1a, with sheets of dark cells with small oval nuclei and focal poorly differentiated areas. Tubular structures and nests and focal heterologous elements including bland enteric type mucinous epithelium and foci of neuroendocrine differentiation were also seen (Figure 1). Computed tomography (CT) of the chest revealed a 2.2 cm cystic lesion in the lower lobe of the right lung concerning for type 1r pleuropulmonary blastoma (PPB). Post-operatively, the patient was monitored with surveillance MRI. After six months, a contralateral 5 cm left ovarian mass (LT1) was detected. Following ovarian sparing tumor resection of the left ovary, histologic examination again revealed a predominantly moderately differentiated SLCT similar to RT1; however, no heterologous elements were identified. Due to suspicion for tumor recurrence, adjuvant chemotherapy (four cycles of bleomycin/etoposide/cisplatin) was administered. No evidence of tumor was detected until one year after treatment, when surveillance MRI revealed a 4 cm left adnexal tumor (LT2). Following left salpingo-oophorectomy, histopathology revealed a predominantly poorly differentiated SLCT formed by sheets of atypical cells with angulated irregular nuclei admixed with some moderately differentiated areas and focal tubule formation. The SLCT and possible PPB raised concern for DICER1 syndrome, prompting genetic evaluation that revealed history of thyroid nodules in the maternal grandmother and great-grandmother per the patient’s mother. No pathology report was available. Paternal history was unremarkable with no known history consistent with DICER1 and no known family history of cancer. Pre-test counseling was provided for genetic testing to include analysis of the DICER1 gene.
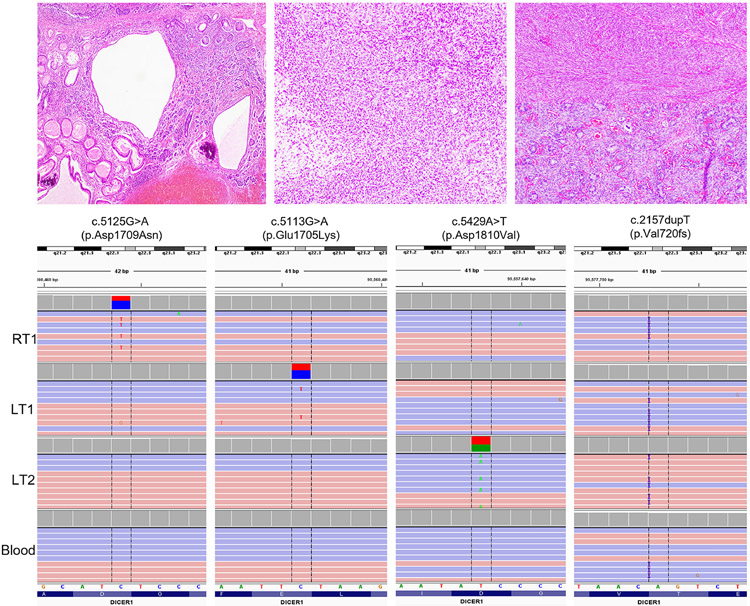
Figure 1. Representative hematoxylin and eosin (H&E)-stained images of the SLCT lesions with corresponding DICER1 alterations.
A. H&E image of RT1 (10x magnification) highlighting tumor with bland enteric-type heterologous epithelium adjacent to tubular and cystic structures.
B. H&E image of LT1 (10x magnification) demonstrating sheets of dark cells with angulated nuclei and moderate clear cytoplasm. No heterologous elements were present.
C. H&E image of LT2 (10x magnification) showing fascicles of spindle cells with oval nuclei and eosinophilic cytoplasm (top) consistent with poorly differentiated Sertoli-Leydig cell tumor. Focal well-differentiated area showing tubular structures was present (bottom).
D. Integrative Genomics Viewer (IGV) view of the 3 different somatic missense mutations and the germline variant in DICER1 as seen in each tumor and blood, respectively. Each somatic variant is unique to each tumor specimen, and the germline variant is present in all specimens.
RT1, right ovary tumor; LT1, left ovary tumor #1; LT2, left ovary tumor #2.
Genomic Analysis
Genomic analysis of tumor and blood specimens was performed as part of a Baylor College of Medicine institutional review board-approved childhood cancer study (Texas KidsCanSeq). Clinical next-generation sequencing of the first left ovarian tumor (LT1) using the Texas Children’s Hospital solid tumor DNA and RNA comprehensive panel (TCH Solid) revealed two DICER1 variants: a c.2157dupT (p.V720fs) frameshift variant in exon 14 of DICER1 (NM_177438.2) at a variant allele fraction (VAF) of 0.47 (47.6%), and a second c.5113G>A (p.E1705K) variant in exon 24 at a VAF of 0.41 (41.5%). The p.V720fs variant (submitted to ClinVar Variation ID 997605) is a novel variant that is predicted to lead to a premature truncation of the DICER1 protein and loss of function. The p.E1705K (COSMIC ID: COSM959251) variant affects a metal-binding site within the DICER1 RNase IIIb catalytic domain and is a recurrent hotspot variant in the gene. Clinical exome analysis of blood DNA revealed the p.V720fs variant to be a heterozygous germline variant in the patient that was absent in a maternal saliva sample (a paternal sample was not available). The p.E1705K variant was absent in the proband’s blood sample. Of note, the patient was also found to be a carrier for familial partial lipodystrophy-6 (OMIM# 615980) due to a pathogenic LIPE variant on the exome report. Tumor-only molecular profiling of the second left-sided tumor (LT2) and the original right-sided tumor (RT1) was then performed using the same TCH Solid panel. While both tumor specimens revealed the germline p.V720fs variant as expected, an additional distinct DICER1 hotspot variant was seen in each tumor: c.5429A>T (p.D1810V) in LT2 at VAF of 0.47 (47%) and c.5125G>A (p.D1709N) in RT1 at VAF of 0.376 (37.6%) (Figure 1). As for the p.E1705K variant seen in LT1, the p.D1810V (COSM4169903) and p.D1709N (COSM959249) are also well-known and recurrent hotspot variants that alter metal-binding sites in the RNAse IIIb domain, disrupting DICER1-mediated microRNA interaction and cleavage [2]. Since RNAse IIIb hotspot mutations have also been infrequently reported as somatic mosaic variants, manual review of the DICER1 sequence alignment was performed in all three tumors, demonstrating the three hotspot variants to be present in one tumor each and completely absent in the other tumors (variant allele count = 0; average coverage 350X) (Figure 1 and Table 1).
Table 1.
DICER1 (NM_177438.2) germline and somatic variants in the SLCT tumors
| SLCT | Germline p.Val720fs |
Somatic p.Asp1709Asn |
Somatic p.Glu1705Lys |
Somatic p.Asp1810Val |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Detected | VAF | Detected | Total Read Depth |
VAF | Detected | Total Read Depth |
VAF | Detected | Total Read Depth |
VAF | |
| RT1 | Yes | 0.515 | Yes | 628 | 0.376 | No | 619 | 0 | No | 561 | 0 |
| LT1 | Yes | 0.476 | No | 1750 | 0 | Yes | 1772 | 0.41 | No | 757 | 0 |
| LT2 | Yes | 0.474 | No | 983 | 0 | No | 959 | 0 | Yes | 681 | 0.47 |
RT1, right ovary tumor; LT1, left ovary tumor #1; LT2, left ovary tumor #2; VAF, variant allele fraction
Discussion
This is the first report of three SLCT primaries harboring distinct somatic hotspot DICER1 missense mutations in one patient with DICER1 syndrome. Each SLCT demonstrated the classic DICER1-associated tumor pattern of a germline loss-of-function variant combined with somatic missense variants in the RNAse IIIb domain. Similar to the p.V720fs frameshift variant in this case, reported germline DICER1 variants are most often loss-of-function frameshift variants dispersed throughout the gene [7]; however, there is no known clear genotype-phenotype relationship with regard to the location of the truncating germline variant within the DICER1 gene and the corresponding clinical features (age at disease onset, number of organs involved, specific tissue sites involved or survival) [7].
While DICER1 tumor predisposition is inherited in an autosomal dominant manner with reduced penetrance, approximately 20% of cases are believed to arise de novo in the proband [8]. In this report, the mother was negative for the DICER1 germline mutation seen in the proband, paternal history was unremarkable and paternal germline status was unavailable. While history of thyroid nodules in maternal grandmother and great grandmother is a recognized clinical manifestation of DICER1 syndrome, this can be a non-specific finding frequently reported in the general population. The negative germline testing of the proband’s mother clearly documents that the variant is not maternally inherited. However, without paternal testing, we cannot conclusively determine if the variant was de novo.
The development of multiple SLCT in an individual is uncommon, with only 3 of 49 patients with SLCT reported to have metachronous tumors in a series published by Schultz et al from the International Ovarian and Testicular Stromal Tumor Registry, even though ~60% of cases had a confirmed germline DICER1 mutation [3]. Nevertheless, all three patients in the Registry with metachronous SLCT were found to have germline DICER1 mutation [3]. More recently, McCluggage et al. reported germline and somatic DICER1 mutation status in three bilateral SLCT along with a review of the literature on bilateral SLCT, in which all bilateral SLCT cases (n=8) where DICER1 germline status had been ascertained, were found to have a pathogenic germline variant [5]. Including the case reported here, therefore, bilateral SLCT when assessed for germline DICER1 status have been reported to harbor a pathogenic germline variant in 12/12 cases to date. Age at onset of SLCT was available for 9 of these 12 cases, of which 8/9 patients have developed bilateral tumors on or before 18 years. Similar to other cancer predisposition syndromes, e.g., hereditary retinoblastoma, diagnosis of bilateral SLCT, especially in children, is highly likely to represent DICER1 Syndrome and germline analysis is recommended.
In a patient with multiple SLCT tumors, independent primary tumors may have a different prognosis than recurrent tumors. Standard treatment of primary SLCT consists of surgical resection, with adjuvant chemotherapy reserved for the minority of cases demonstrating high-risk histologic features (e.g., poorly differentiated morphology, retiform pattern, heterologous elements) or advanced staged disease. Prognosis for recurrent SLCT is poor, with mortality reported as 50-70% in small series [3,9]. The unique nature of the somatic variants in this case allowed rapid confirmation that these tumors were, in fact, three primary lesions. Along with this case, tumor sequencing of bilateral SLCT tumors in 4 other cases have been reported, and in all 5 cases, multiple SLCT have been found to be independent primary lesions with distinct DICER1 somatic mutations [5,6]. Routine tumor sequencing of DICER1-related tumors can be specifically useful to differentiate recurrent or metastatic disease from distinct primary lesions. If there are distinct somatic missense variants and a shared loss of function variant then germline analysis should be performed to confirm the diagnosis of DICER1 syndrome with implications for genetic counselling, potential identification of at-risk family members and appropriate cancer surveillance.
ACKNOWLEDGEMENT
The Texas KidsCanSeq study is a Clinical Sequencing Evidence-Generating Research (CSER) consortium project supported by the NHGRI/NCI grant U01HG006485 (DWP and SEP). DWP is the recipient of a St Baldrick's Innovation Award with additional support from the Chance for Hope Foundation. AG, SEP, DWP and AR were involved in study concept and design. All authors were involved in acquisition, analysis, or interpretation of data. All authors were involved in critical revision of the manuscript for important intellectual content.
Footnotes
DATA DEPOSITION
The germline variant was submitted to ClinVar by the Texas KidsCanSeq study – ClinVar allele ID 985319.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- [1].Karnezis AN, Buza N, Shen D, Kommoss F. Sertoli-Leydig cell tumour. In: WHO Classification of Tumours Editorial Board. Female genital tumours [Internet]. Lyon (France): International Agency for Research on Cancer; 2020. [cited 2021 Oct 25], (WHO classification of tumours series, 5th ed.; vol. 4). Available from: https://tumourclassification.iarc.who.int/chapters/34. [Google Scholar]
- [2].Heravi-Moussavi A, Anglesio MS, Cheng S-WG, Senz J, Yang W, Prentice L, et al. Recurrent somatic DICER1 mutations in nonepithelial ovarian cancers. N Engl J Med 2012;366:234–42. 10.1056/NEJMoa1102903. [DOI] [PubMed] [Google Scholar]
- [3].Schultz KAP, Harris AK, Finch M, Dehner LP, Brown JB, Gershenson DM, et al. DICER1-related Sertoli-Leydig cell tumor and gynandroblastoma: Clinical and genetic findings from the International Ovarian and Testicular Stromal Tumor Registry. Gynecol Oncol 2017;147:521–7. 10.1016/j.ygyno.2017.09.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].de Kock L, Terzic T, McCluggage WG, Stewart CJR, Shaw P, Foulkes WD, et al. DICER1 Mutations Are Consistently Present in Moderately and Poorly Differentiated Sertoli-Leydig Cell Tumors. Am J Surg Pathol 2017;41:1178–87. 10.1097/PAS.0000000000000895. [DOI] [PubMed] [Google Scholar]
- [5].McCluggage WG, Chong A-L, de Kock L, Foulkes WD. Somatic tumour testing establishes that bilateral DICER1-associated ovarian Sertoli-Leydig cell tumours represent independent primary neoplasms. Histopathology 2020;77:223–30. 10.1111/his.14123. [DOI] [PubMed] [Google Scholar]
- [6].Chen KS, Stuart SH, Stroup EK, Shukla AS, Wang J, Rajaram V, et al. Distinct DICER1 Hotspot Mutations Identify Bilateral Tumors as Separate Events. JCO Precis Oncol 2018;2. 10.1200/PO.17.00113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].De Paolis E, Paragliola RM, Concolino P. Spectrum of DICER1 Germline Pathogenic Variants in Ovarian Sertoli-Leydig Cell Tumor. J Clin Med. 2021. Apr 23;10(9):1845. doi: 10.3390/jcm10091845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Hill DA, Wang JD, Schoettler P, Dehner L, Ivanovich J, Bracamontes D, Williams G, Messinger Y, Goodfellow PJ, Priest J. Germline DICER1 mutations are common in both hereditary and presumed sporadic pleuropulmonary blastoma. Lab Invest. 2010;90:311. [Google Scholar]
- [9].Gouy S, Arfi A, Maulard A, Pautier P, Bentivegna E, Leary A, et al. Results from a Monocentric Long-Term Analysis of 23 Patients with Ovarian Sertoli-Leydig Cell Tumors. Oncologist 2019;24:702–9. 10.1634/theoncologist.2017-0632. [DOI] [PMC free article] [PubMed] [Google Scholar]