Abstract
X-linked agammaglobulinemia (XLA) is a primary immunodeficiency disorder caused by mutations in the Bruton tyrosine kinase (BTK) gene leading to B lymphocyte deficiency and susceptibility to infection. A potential benefit of earlier diagnosis and treatment initiation on morbidity and mortality in XLA is incompletely understood. In the USIDNET Registry, we describe infection frequency and infection-related mortality in patients with XLA and their relationship to age of diagnosis and treatment initiation. Among the 231 XLA patients enrolled in the Registry, respiratory infections (N = 203, 88%) were the most commonly reported. Among those deceased (N = 20) where cause of death was known (N = 17), mortality was attributed to infection in most (N = 12, 71%). Chronic lung disease, often a consequence of repeated lower respiratory tract infection (LRTI), was also a frequent complication associated with mortality (N = 9, 53%). Age of diagnosis in years was lower for those without LRTI compared to those with (median 1.5 [IQR 0.5–3.3] vs. median 3.0 [IQR 1.0–5.0], p = 0.0026) and among living patients compared to deceased (median 1.8 [IQR 0.5–5.0] vs. median 2.7 [IQR 1.6–6.0], p = 0.04). Age at treatment initiation in years was lower among those without LRTIs compared to those with (median 1.0 [IQR 0.4–2.4] vs. median 2.8 [IQR 1.0–5.4], p = 0.0006). For every year increase in age at start of therapy, the odds of experiencing a LRTI was 1.216 (OR 1.216, 95% CI 1.048–1.411, p = 0.01). Given the expected finding of reduced LRTIs and mortality among those with earlier age at diagnosis, our study findings support inclusion of XLA in newborn screening programs.
Keywords: Immunoglobulin, Infection, Infectious organism, Primary immunodeficiency, USIDNET, X-linked agammaglobulinemia
Introduction
X-linked agammaglobulinemia (XLA) is a primary immunodeficiency disorder arising from a genetic defect in Bruton tyrosine kinase (BTK) and manifesting with B lymphocyte deficiency, agammaglobulinemia, and recurrent infection [1–4]. Infection and chronic lung disease, a consequence of repeated pulmonary infection, are the most frequently reported causes of death in XLA [5, 6]. Early initiation of immunoglobulin (Ig) replacement therapy and maintaining therapeutic IgG levels has been shown to effectively reduce infections [7, 8].
Despite advancements in diagnosis, XLA patients continue to experience infection-related morbidity and mortality as well as decreased quality of life [5, 9, 10]. Recent initiatives to add screening for B cell defects to standard newborn screening in the USA have made understanding the potential impact of earlier diagnosis on infection-related morbidity and mortality in XLA particularly relevant [11, 12]. Using the United States Immunodeficiency Network (USIDNET) Registry, we describe infections and infection-related mortality in XLA, and address how these may be modified by early diagnosis and treatment initiation.
Methods
The U.S. Immunodeficiency Network (USIDNET), a program of the Immune Deficiency Foundation (IDF), has been supported by a cooperative agreement, U24AI86837, from the National Institute of Allergy and Infectious Diseases (NIAID). The USIDNET Registry was established in 2003 and is a national patient-consented data base with clinical data from patients with primary immunodeficiency diseases. For this study, the USIDNET Registry was queried for infections reported in patients with XLA form of agammaglobulinemia through July 1, 2020. The data analyzed here includes all the information compiled in the USIDNET Registry at the time of our query. Patients were excluded if they did not have a known investigator designated BTK defect. Demographic, clinical, immune globulin treatment, and laboratory information was obtained. Infection and organism types and frequencies were recorded.
Infection types were defined by the following terms, which were selected and entered into the database by clinicians: respiratory infections which included lower respiratory tract infections (abbreviated LRTIs, including entry terms “pneumonia,” “empyema,” “lung abscess”); sinusitis, ear infections, and complications (including entry terms “otitis media,” “mastoiditis”); upper respiratory tract infections (abbreviated URTIs, including entry terms “URI,” “cough/rhinitis,” “pharyngitis,” “strep throat,” “croup,” “bronchitis”); gastrointestinal infections (including entry terms “acute gastroenteritis,” “infectious diarrhea disease,” “infectious colitis,” “peritonitis,” “hepatitis”); skin infections (including entry terms “abscess,” “cellulitis,” “impetigo,” “varicella,” “dermal mycosis,” “pyoderma gangrenosum”); eye infections (including entry terms “conjunctivitis,” “keratoconjunctivitis”); central nervous system infections (including entry terms “meningitis,” “encephalitis,” “meningoencephalitis,” “brain abscess,” “acute poliomyelitis”); blood infections (including entry terms “sepsis,” “bacteremia,” “viremia”); musculoskeletal infections (including entry terms “septic arthritis,” “osteomyelitis”); genitourinary infections (including entry terms “urinary tract infection,” “pyelonephritis,” “urethritis”); oral infections (including entry terms “candidiasis”); and lymph node infections (including entry terms “lymphadenitis,” “lymphangitis”).
Statistical Analysis
Associations between Ig replacement therapy and the most frequently reported infection types were analyzed using a chi-squared test; if indicative of statistical significance, a simple logistic regression was performed. Age was compared between patients receiving intravenous Ig (IVIg) and patients receiving subcutaneous (SQIg) using Wilcoxon rank-sum scores. Age at diagnosis and age at treatment initiation were compared between living and deceased patients, patients with and without pulmonary infections, and patients with and without a family history of agammaglobulinemia using Wilcoxon rank-sum scores. Where applicable, age was compared utilizing odds ratios. Age for each patient in the cohort was calculated using year of birth and most recent visit date. Birth year was compared among living and deceased patients and patients with and without LRTIs and URTIs using Wilcoxon rank-sum scores. The data analysis for this paper was generated using SAS software, version 9.4 of the SAS System for Windows. Copyright © 2016 SAS Institute Inc. SAS and all other SAS Institute Inc. product or service names are registered trademarks or trademarks of SAS Institute Inc., Cary, NC, USA.
Results
Demographic Data and Infection and Organism Types and Frequencies
An investigator identified BTK alteration was noted in 231 patients with a diagnosis of X-linked agammaglobulinemia. Fifty-one physicians contributed to patient data. Demographic information is presented in Table 1. All 231 patients in the cohort were male. The most commonly identified race/ethnicity was White/Caucasian (N = 143, 61.9%). The median age in years at time of last entry into the Registry, calculated based on year of birth and most recent visit date, was 11.7 (IQR 4.6–21.9). The median age in years at diagnosis was 2.0 (IQR 0.8–5.0) and at symptom onset was 0.8 (IQR 0.3–2.0). Of 170 patients where family history of primary immunodeficiency was reported, as positive or negative, 106 patients (62.4%) were identified as having a positive family history and 64 patients (37.6%) were identified as having a negative family history. In the 231-patient cohort, 61 patients (26.4%) had an unknown or not reported family history. Based on reported birth year, for the 231 patients in the cohort, the number of patients born in each decade is shown in Fig. 1.
Table 1.
Demographics and family history among 231 patients with X-linked agammaglobulinemia from the USIDNET Registry
| Patient characteristics | N (%) or median (IQR) | |
|---|---|---|
| Gender, N (%) | Male | 231 (100) |
| Female | 0 (0) | |
| Living (at time of last entry), N (%) | Yes | 148 (64.1) |
| No | 20 (8.7) | |
| Unspecified | 63 (27.3) | |
|
Age, years Median (IQR) |
Onset | 0.8 (0.3–2.0) |
| Diagnosis | 2.0 (0.8–5.0) | |
| Last entry | 11.7 (4.6–21.9) | |
| Race and ethnicity, N (%) | White/Caucasian | 143 (61.9) |
| Black/African American | 20 (8.7) | |
| Hispanic or Latino | 20 (8.7) | |
| Asian or Pacific Islander | 6 (2.6) | |
| American Indian/Alaska | 0 (0.0) | |
| Other or more than one race | 6 (2.6) | |
| Unknown or not reported | 56 (24.2) | |
| Family history of primary immunodeficiency, N (%) | Positive | 106 (45.9) |
| Negative | 64 (27.7) | |
| Unknown or not reported | 61 (26.4) |
IQR interquartile ratio, USIDNET United States Immunodeficiency Network
Fig. 1.
Birth year among 231 patients with X-linked agammaglobulinemia from the USIDNET Registry
Table 2 summarizes infection types and frequency among the 231 XLA patients in the Registry. The most frequent infections were respiratory infections, identified in 203 patients (87.9%) which included lower respiratory tract infections (N = 129, 55.8%), sinusitis (N = 128, 55.4%), ear infections and complications (N = 124, 53.7%), and upper respiratory tract infections (N = 96, 41.6%). Skin infections were identified in 83 patients (35.9%), gastrointestinal infections in 61 patients (26.4%), conjunctivitis in 59 patients (25.5%), and central nervous system infections in 34 patients (14.7%). Less frequent infections included sepsis (N = 16, 6.9%), septic arthritis (N = 15, 6.5%), genitourinary tract infections (N = 12, 5.2%), osteomyelitis (N = 9, 3.9%), oral candidiasis (N = 9, 3.9%), and lymphadenitis/lymphangitis (N = 7, 3.0%).
Table 2.
Infection frequency and types among 231 patients with X-linked agammaglobulinemia in the USIDNET Registry
| Infection | Number of patients (%)* | Infection cont | Number of patients (%)* |
|---|---|---|---|
| Respiratory | 203 (87.9) | Eye | 59 (25.5) |
| LRTI | 129 (55.8) | Conjunctivitis | 52 (22.5) |
| Pneumonia | 129 (55.8) | Keratoconjunctivitis | 1 (0.4) |
| Empyema | 5 (2.2) | Other | 8 (3.5) |
| Lung abscess | 1 (0.4) | Central nervous system | 34 (14.7) |
| Recurrent pneumonias | 1 (0.4) | Meningitis | 17 (7.4) |
| Sinus infections | 128 (55.4) | Encephalitis | 10 (4.3) |
| Sinusitis | 128 (55.4) | Meningoencephalitis | 4 (1.7) |
| Recurrent/chronic sinusitis | 5 (2.2) | Acute poliomyelitis | 4 (1.7) |
| Ear infections and complications | 124 (53.7) | Brain abscess | 1 (0.4) |
| Acute otitis media | 124 (53.7) | Blood | 23 (10.0) |
| Recurrent otitis media | 5 (2.2) | Sepsis | 16 (6.9) |
| Mastoiditis | 5 (2.2) | Bacteremia | 5 (2.2) |
| URTI/pharyngitis | 96 (41.6) | Viremia | 3 (1.3) |
| URI | 73 (31.6) | Musculoskeletal | 20 (8.7) |
| Bronchitis | 29 (12.6) | Septic arthritis | 15 (6.5) |
| Croup | 11 (4.8) | Osteomyelitis | 9 (3.9) |
| Pharyngitis | 6 (2.6) | Genitourinary | 12 (5.2) |
| Cough/rhinitis | 4 (1.7) | Urinary tract infection | 7 (3.0) |
| Strep throat | 2 (0.9) | Pyelonephritis | 3 (1.3) |
| Recurrent URI | 2 (0.9) | Urethritis | 2 (0.9) |
| Skin | 83 (35.9) | Other | 1 (0.4) |
| Abscess | 33 (14.3) | Oral | 10 (4.3) |
| Cellulitis | 18 (7.8) | Candidiasis | 9 (3.9) |
| Impetigo | 14 (6.1) | Other | 5 (2.2) |
| Dermal mycosis | 8 (3.5) | Lymph node | 7 (3.0) |
| Pyoderma gangrenosum | 7 (3.0) | Lymphadenitis | 5 (2.2) |
| Varicella | 6 (2.6) | Lymphangitis | 2 (0.9) |
| Other | 48 (20.8) | ||
| Gastrointestinal | 61 (26.4) | ||
| Acute gastroenteritis | 54 (23.4) | ||
| Infectious diarrheal illness | 31 (13.4) | ||
| Infectious colitis | 8 (3.5) | ||
| Hepatitis | 8 (3.5) | ||
| Peritonitis | 2 (0.9) | ||
| Other | 2 (0.9) |
LRTI lower respiratory tract infection, URTI upper respiratory tract infection
*Frequency of infections as number and percentage of patients acquiring. The percentage adds up to more than 100 because some patients had multiple infections and organisms
In Table 3, we describe organism type and frequency among our cohort. Of 231 patients with XLA, 105 patients (45.5%) had an identified organism. Bacterial, viral, parasitic, and fungal organisms were identified in 85 (36.8%), 26 (11.3%), 10 (4.3%), and 7 (3.0%) patients, respectively. The most frequently identified organisms were Haemophilus influenzae (N = 24, 10.4%), Staphylococcus aureus (N = 24, 10.4%), Streptococcus pneumoniae (N = 18, 7.8%), Pseudomonas aeruginosa (N = 14, 6.1%), and Giardia (N = 10, 4.3%). Less common organisms (< 2% of patients) included Clostridioides difficile, Pneumocystis jirovecii, Campylobacter jejuni, Escherichia coli, Shigella, Salmonella, and Echovirus.
Table 3.
Organism frequency and types among 231 patients with X-linked agammaglobulinemia in the USIDNET Registry
| Organism | Number of patients (%)* |
|---|---|
| Any organism | 105 (45.5) |
| Bacteria | 85 (36.8) |
| H. influenzae | 24 (10.4) |
| S. aureus (unspecified MSSA or MRSA) | 24 (10.4) |
| S. pneumoniae | 18 (7.8) |
| P. aeruginosa | 14 (6.1) |
| S. epidermidis | 4 (1.7) |
| C. difficile | 3 (1.3) |
| Shigella | 3 (1.3) |
| Salmonella | 3 (1.3) |
| Campylobacter | 3 (1.3) |
| E. coli | 3 (1.3) |
| S. aureus (methicillin-resistant) | 2 (0.9) |
| Other | 13 (5.6) |
| Virus | 26 (11.3) |
| Echovirus | 4 (1.7) |
| Respiratory syncytial virus | 2 (0.9) |
| Cytomegalovirus | 2 (0.9) |
| Influenza A | 2 (0.9) |
| Influenza B | 1 (0.4) |
| Other | 16 (6.9) |
| Parasite | 10 (4.3) |
| Giardia | 10 (4.3) |
| Fungus/yeast | 7 (3.0) |
| Pneumocystis jirovecii | 3 (1.3) |
| Aspergillus | 1 (0.4) |
| Candida | 1 (0.4) |
| Other/unspecified | 2 (0.9) |
MRSA methicillin-resistant Staphylococcus aureus, MSSA methicillin-susceptible Staphylococcus aureus
*Frequency of organisms as number and percentage of patients acquiring. The percentage adds up to more than 100 because some patients had multiple infections and organisms
Ig Replacement Therapy, Stem Cell Transplant, and Comparison Among Infection Types
We describe Ig replacement therapy and allogeneic hematopoietic stem cell transplant (HSCT) in our XLA cohort in Table 4. Four patients (1.7%) received a HSCT. While the reason for HSCT was not documented in our cohort, prior studies have indicated that patients with XLA undergo HCST to avoid significant infection, chronic lung disease, neurogenerative disease, or malignancy that develop despite appropriate therapy [13].
Table 4.
Immunoglobulin (Ig) replacement therapy and hematopoietic stem cell transplant history among 231 patients with X-linked agammaglobulinemia from the USIDNET Registry
| Feature of therapy | N (%) or median (IQR) | |
|---|---|---|
| Ig therapy | Received | 187 (81.0%) |
| Did not receive | 1 (0.4%) | |
| Unknown or not reported | 43 (18.6%) | |
| Ig route (specified in 187 of 225 patients) | Intravenous | 83 (44.4%) |
| Subcutaneous | 45 (24.1%) | |
| Intramuscular | 1 (0.5%) | |
| Other | 2 (1.1%) | |
| Unknown/not reported | 56 (29.9%) | |
| Median age, years | Ig started | 1.8 (0.7–4.0) |
| Median age, years | Patients receiving intravenous Ig | 16.2 (8.2–25.0) |
| Patients receiving subcutaneous Ig | 15.6 (9.2–25.0) | |
| Median frequency, days | Intravenous Ig | 28 (21–30) |
| Subcutaneous Ig | 7 (7–14) | |
| Median dose, mg/kg | Intravenous Ig | 490 (351–699) |
| Subcutaneous Ig | 157 (121–192) | |
| Hematopoietic stem cell transplant, N (%) | Received | 4 (1.7%) |
| Did not receive | 121 (52.4%) | |
| Unknown or not reported | 106 (45.9%) |
Ig immunoglobulin, IQR interquartile ratio, USIDNET United States Immunodeficiency Network
Of 231 included XLA patients, 187 (81.0%) were reported to be on Ig therapy and 43 patients (18.6%) had unknown or not reported Ig therapy. Of the 187 on Ig replacement therapy, 83 patients (44.4%) received IVIg, 45 patients (24.1%) received SQIg, 56 patients (29.9%) had unknown or unreported administration routes, 1 patient (0.5%) received IM Ig, and 2 patients (1.1%) had an administration route reported as other. The frequency of IVIg therapy was a median interval of 28 days (IQR 21–30) and the frequency of SQIg therapy was a median interval of 7 days (IQR 7–14). The median monthly IVIg dose was 490 mg/kg (IQR 351–699) and the median weekly SQIg dose was 157 mg/kg (IQR 121–192). Median age in years at time of most recent visit among patients receiving IVIg was 16.2 (IQR 8.2–25.0) and among patients receiving SQIg was 15.6 (IQR 9.2–25.0) (p = 0.97).
We analyzed our cohort to determine if there was an association between Ig replacement therapy type (defined as IVIg vs. SQIg) and the most frequently reported infection types (respiratory, gastrointestinal, and skin). There were no significant associations found between LRTIs (p = 0.40), ear infections (p = 0.39), sinus infections (p = 0.53), gastrointestinal (p = 0.37), skin infections (p = 0.67), and Ig replacement therapy type.
Associations Between Infection, Mortality, Family History, and Age at Diagnosis and Treatment Initiation
We analyzed our 231-patient cohort to understand the association between infection and mortality in the Registry. Of 168 patients where living or deceased status was specified, 148 (88%) were reported as living and 20 (12%) were reported as deceased. The median age of death was 21 years (IQR 15–28 years). By comparison, the median age of those living was 16 years (IQR 9–25). Among the 17 for whom a cause of death was specified, 12 (71%) were attributed partially or fully to infection and 9 (53%) were partially or fully attributed to pulmonary conditions (Table 5); often, patients had multiple listed causes of death (N = 14, 82%). Of note, 63 patients in our 231-patient cohort (27%) were missing data on living or deceased status.
Table 5.
Cause of death among 20 patients with X-linked agammaglobulinemia in the USIDNET Registry
| Year of birth | Age of death (Years) | Cause of death |
|---|---|---|
| 1945 | 57 | Cardiopulmonary arrest, pneumonia, COPD, cor pulmonale |
| 1946 | 14 | Encephalopathy, hepatitis, hepatic necrosis, adenovirus |
| 1947 | 18 | Chronic pulmonary infection |
| 1949 | 21 | Staphylococcus pericarditis, drug abuse |
| 1950 | 50 | NA |
| 1951 | 29 | Listeria meningitis, adenovirus encephalitis, bronchopneumonia, cirrhosis |
| 1953 | 10 | Gastrointestinal bleeding, acute liver disease, hepatitis |
| 1954 | 27 | Cardiorespiratory arrest, chronic lung disease |
| 1955 | 26 | Staphylococcus septicemia, chronic infection of prosthetic hip |
| 1957 | 23 | Cardiopulmonary arrest, COPD-hypoxemia, cor pulmonale, pneumonitis |
| 1959 | 19 | Cor pulmonale, COPD |
| 1962 | NA | NA |
| 1963 | 21 | Lung disease |
| 1964 | 16 | Viral encephalitis, seizures |
| 1976 | 4 | Aspiration, chronic CNS impairment |
| 1985 | 29 | Sepsis, hemorrhage, liver impairment, bronchiectasis, brain abscess, organ failure |
| 1988 | 30 | Malignancy, infection |
| 1991 | 16 | NA |
| 1997 | 3 | Cardiac arrhythmia related to acute viral myocarditis |
| 2004 | 6 | Renal failure, necrotizing VAP, ARDS, bacteremia, candidemia |
In all patients with a specified cause of death (N = 17), death was attributed partially or fully to acute infection in 12. Nine patients (bolded) had either acute lower respiratory tract infection (LRTI) or chronic lower respiratory condition (a consequence of repeated LRTI infection) listed as a cause of death
ARDS acute respiratory distress syndrome, COPD chronic obstructive pulmonary disease, CNS central nervous system, NA not available, VAP ventilator-associated pneumonia
To understand whether later diagnosis and treatment initiation was associated with increased mortality, we compared median age at diagnosis and treatment initiation among patient characteristics including living versus deceased status, those with and without LRTI, and those with and without family history of agammaglobulinemia (Table 6). We acknowledge that within the 231-patient cohort, 186 patients (81%) had the age at diagnosis recorded and 113 patients (49%) had age of treatment initiation data available for these analyses.
Table 6.
Comparison of age at diagnosis and age at treatment initiation for 231 patients with X-linked agammaglobulinemia in the USIDNET Registry
| Comparison groups | Age in years at diagnosis | p Value* | Age in years at treatment initiation | p Value* | ||
| Median (IQR) | N (%) with available data | Median (IQR) | N (%) with available data | |||
| Living (N = 148) | 1.8 (0.5–5.0) | 122 (82%) | 0.04 | 1.7 (0.5–4.9) | 83 (56%) | 0.05 |
| Versus deceased (N = 20) | 2.7 (1.6–6.0) | 18 (90%) | 3.4 (2.3–7.0) | 8 (40%) | ||
| Without LRTI (N = 102) | 1.5 (0.5–3.3) | 75 (74%) | 0.0026 | 1.0 (0.4–2.4) | 48 (47%) | 0.0006 |
| Versus with LRTI (N = 129) | 3.0 (1.0–5.0) | 111 (85%) | 2.8 (1.0–5.4) | 65 (50%) | ||
| With FH (N = 90) | 1.0 (0.2–4.0) | 72 (80%) | 0.0002 | 1.0 (0.2–3.5) | 47 (52%) | 0.0025 |
| Versus without FH (N = 141) | 2.6 (1.3–5.0) | 114 (80%) | 2.5 (1.0–5.0) | 66 (47%) | ||
| Comparison groups | Age in years at diagnosis | p Value* | Age in years at treatment initiation | p Value* | ||
| Odds ratio (95% CI) | N (%) with available data | Odds ratio (95% CI) | N (%) with available data | |||
| Living (N = 148) | 1.038 (0.929–1.160) | 122 (82%) | 0.51 | 1.020 (0.933–1.115) | 83 (56%) | 0.67 |
| Versus deceased (N = 20) | 18 (90%) | 8 (40%) | ||||
| Without LRTI (N = 1 02) | 1.099 (0.999–1.207) | 75 (74%) | 0.05 | 1.216 (1.048–1.411) | 48 (47%) | 0.01 |
| Versus with LRTI (N = 129) | 111 (85%) | 65 (50%) | ||||
| With FH (N = 90) | 0.892 (0.807–0.986) | 72 (80%) | 0.03 | 0.969 (0.902–1.041) | 47 (52%) | 0.39 |
| Versus without FH (N = 141) | 114 (80%) | 66 (47%) | ||||
Age at diagnosis was significantly lower among those who were alive, without LRTI, and with family history of agammaglobulinemia at the time of data entry. Age at treatment initiation was significantly lower in those without LRTI and with family history
CI confidence interval, FH family history of agammaglobulinemia, IQR interquartile ratio, LRTI lower respiratory tract infection
*Statistically significant p values are bolded. Calculated by Wilcoxon rank-sum (two-sided) and odds ratios
The median age at diagnosis was lower for patients reported as living (1.8 years, IQR 0.5–5.0) compared to patients reported as deceased (2.7 years, IQR 1.6–6.0) (p = 0.04). An odds ratio for age at diagnosis in these groups was calculated to be 1.038 (95% CI 0.929–1.160, p = 0.51). The median age at treatment initiation was somewhat lower for those reported as living (1.7 years, IQR 0.5–4.9) compared to those reported as deceased (3.4 years, IQR 2.3–7.0) (p = 0.05). An odds ratio for age at treatment initiation in these groups was calculated to be 1.020 (95% CI 0.933–1.115, p = 0.67).
Because LRTI was reported as a common infection and a common cause of mortality in our cohort, we compared age at diagnosis and treatment initiation among those with and without LRTI. The median age at diagnosis was lower in patients without LRTI (1.5 years, IQR 0.5–3.3) compared to those with LRTI (3.0 years, IQR 1.0–5.0) (p = 0.0026). An odds ratio for age at diagnosis in these groups was calculated to be 1.099 (95% CI 0.999–1.207, p = 0.05). Similarly, the median age at treatment initiation was found to be lower in patients without LRTI (1.0 years, IQR 0.4–2.4) compared to those with LRTI (2.8 years, IQR 1.0–5.4) (p = 0.0006). An odds ratio for age at treatment initiation in these groups was calculated to be 1.216 (95% CI 1.048–1.411, p = 0.01).
We hypothesized that family history of agammaglobulinemia could be associated with earlier age at diagnosis and treatment initiation due to heightened awareness in the patient, family, and provider. In our cohort of 231 patients, 90 patients (39%) had a positive family history of agammaglobulinemia. Patients with a family history of agammaglobulinemia had a lower median age at diagnosis (1.0 years, IQR 0.2–4.0) compared to patients without a family history of agammaglobulinemia (2.6 years, IQR 1.3–5.0) (p = 0.0002). An odds ratio for age at diagnosis in these groups was calculated to be 0.892 (95% CI 0.807–0.986, p = 0.03). Further, patients with a family history of agammaglobulinemia had a lower median age at treatment initiation (1.0 years, IQR 0.2–3.5) compared to those without a family history of agammaglobulinemia (2.5 years, IQR 1.0–5.0) (p = 0.0025). An odds ratio for age at treatment initiation in these groups was calculated to be 0.969 (95% CI 0.902–1.041, p = 0.39).
Comparison of Birth Year Among Infection Types and Mortality
Our 231-patient cohort was analyzed to better understand if there was an association between birth year and infection type or birth year and mortality. All patients in our 231-patient cohort had a birth year reported.
We compared birth year among patients with a LRTI (N = 129, 56%) and among patients without a LRTI (N = 102, 44%). We found that patients with a LRTI had an earlier median birth year (1976, IQR 1964–1998) compared to patients without a LRTI (1998, IQR 1979–2005) (p < 0.0001). We also compared birth year among patients with a URTI (N = 96, 42%) and without a URTI (N = 135, 58%). We found that patients with a URTI had a more recent median birth year (1995, IQR 1975–2004) compared to patients without a URTI (1976, IQR 1966–2000) (p = 0.0003).
We also compared birth year, among the 168 patients in our 231-patient cohort who had living and deceased status entered into the database, to better understand an association between birth year and mortality. When comparing the patients identified as living (N = 148, 88%) or as deceased (N = 20, 12%), we found, not surprisingly, that the median birth year was more recent for patients identified as living (1993, IQR 1973–2002) compared to those identified as deceased (1958, IQR 1950–1980) (p < 0.0001).
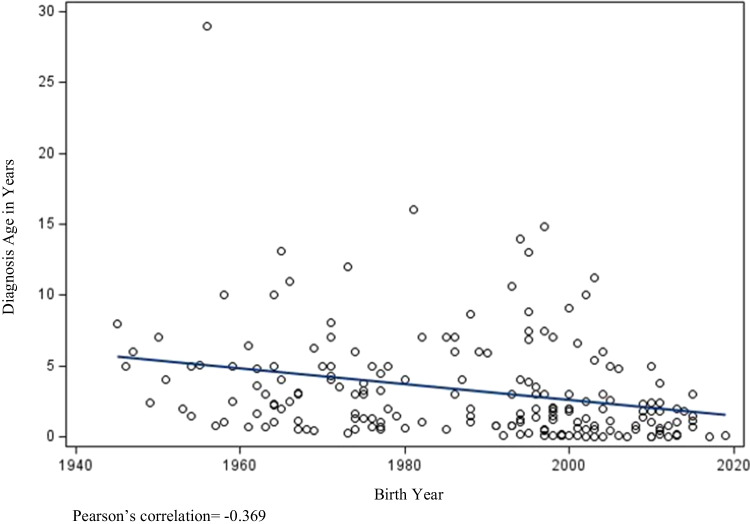
Further, as demonstrated in Fig. 2, we found a moderate negative correlation between birth year and age of diagnosis; as birth year increased, age of diagnosis decreased (p < 0.0001). Of note, our graph shows that patients with a more recent birth year contributed more data to the patient cohort, which could affect our correlative findings. However, as previously noted, the median age of death for patients identified as deceased was 21 years (IQR 15–28 years). By comparison, the median age of those identified as living was 16 years (IQR 9–25).
Fig. 2.
Scatter plot of birth year and diagnosis age among 231 patients with X-linked agammaglobulinemia in the USIDNET Registry
Discussion
Our study is the largest to-date describing infection frequencies and infection-related mortality in XLA from registry data. More recently, there have been other survey studies describing XLA, focused on morbidity and mortality as it relates to prompt diagnosis and treatment [5, 14–16]. As expected and similar to prior studies, respiratory infections, including pneumonia, sinusitis, and acute otitis media, were the most frequent types of infections [6]. XLA is most commonly associated with bacterial infections, a trend echoed in our study, where the most common organism identified was H. influenzae, followed by S. aureus and S. pneumoniae [6].
Based on data from the USIDNET Registry on 231 patients with XLA, we evaluated the hypothesis that earlier diagnosis, potentially with newborn screening, might be expected to alter disease course in XLA. Patients identified as living had a significantly earlier age at diagnosis compared to those identified as deceased. Further, we identified a significantly earlier age at diagnosis and treatment initiation among those without any LRTI compared to those with LRTIs. This suggests that earlier initiation of Ig replacement may prevent patients from experiencing LRTIs, which are a frequent cause of infection, morbidity, and mortality in XLA. This is particularly relevant because repeated LRTIs are associated with development of chronic lung disease, which is a common cause of mortality both in our patients (5 of 17 with reported cause; Table 5) and in previous reports [5, 6, 17]. Previous studies have demonstrated that acute and chronic lung disease can account for 41% of deaths in patients with XLA and that chronic lung disease is predictive of mortality in patients with XLA [5, 18].
In further support of newborn screening for XLA, the median age at diagnosis and treatment initiation was 2 years and 1.8 years in our cohort, respectively. In addition, we found that patients with a family history of agammaglobulinemia had a statistically significant earlier age at diagnosis and treatment initiation compared to those without a family history of agammaglobulinemia, potentially supporting that earlier clinical suspicion could reduce age at diagnosis and treatment initiation. Therefore, we propose that identifying XLA patients in the first weeks of life through newborn screening has the potential to significantly reduce age at diagnosis and treatment initiation and therefore morbidity and mortality, particularly as it relates to infection and chronic lung disease.
Given the nature of our database, which includes data on patients from multiple decades, and the advances in diagnosis and treatment of XLA over time, we analyzed our patient cohort for an association between birth year and certain patient characteristics, including infection type and mortality. We found that deceased patients and those with LRTI were likely to be born in earlier decades, while patients with only URTI were born more recently. We further note that the median age of death in our cohort was young, supporting that improvement of therapy is related to better survival, rather than an older age of deceased patients born in earlier decades. The collected data again demonstrate that earlier diagnosis and treatment diminish more deleterious infections and reduce mortality among patients with XLA [6].
Our study is limited by the nature of the data collected in the USIDNET Registry. There are aspects of this cohort that could have been further investigated (i.e., the indication for HSCT, the relationship between organisms and infection type, Ig replacement therapy, and IgG trough levels as they relate to time of infection) that are not possible because of a lack of these data. We acknowledge that these areas are of interest to the primary immune deficiency community. We encourage future studies to investigate these issues and acknowledge past studies that have investigated them in the form of a retrospective cohort with available medical records [19].
Compared to prior similar studies, our dataset also did not allow us to connect identified organisms with infection types [6, 19]. While some studies have demonstrated a temporal relationship between infection and Ig replacement therapy and infection and IgG trough levels, we were unable to do so with this dataset, as timing of infections and their relationship to IgG trough levels are not recorded [19, 20]. As previously noted, while the reason for HSCT was not documented in our cohort, prior studies have indicated that patients with XLA undergo HCST to avoid significant infection, chronic lung disease, neurogenerative disease, or malignancy that develop despite appropriate therapy [13].
For many patients, living or deceased status was not specified. There was also missing data for a number of patients regarding age at diagnosis and treatment initiation; this could account for the fact that our age of diagnosis was slightly higher than our age of treatment initiation. It is possible that this missing data resulted in Type 2 errors, particularly for our finding that age at treatment initiation was not significantly different between patients identified as living compared to those identified as deceased. We were also limited by the cross-sectional nature of the dataset, leaving us unable to calculate prevalence; each patient’s cross section was also during a different time period given the nature of the database.
In summary, we reported on infections and infection-related mortality in 231 patients with XLA in the USIDNET Registry. Respiratory infections, particularly pulmonary infections including LRTIs (such as pneumonia), were identified as being among the most commonly reported infection types and a common cause of mortality in patients with XLA. Patients with LRTIs were identified as having a later age at diagnosis and treatment initiation. Our study findings support implementation of newborn screening for defects in B cell development, offering the opportunity to prevent pulmonary infections through earlier diagnosis and treatment initiation, which are a common cause of morbidity and mortality in XLA. We hope that our findings will help increase clinical suspicion for XLA in patients with recurrent infection, help providers anticipate infection types in patients with XLA, and advocate for the addition of profound defects in B cell development, such as XLA, to the panel of conditions to be included in newborn screening programs.
Abbreviations
- ARDS
Acute respiratory distress syndrome
- BTK
Bruton tyrosine kinase
- CI
Confidence interval
- CNS
Central nervous system
- COPD
Chronic obstructive pulmonary disease
- FH
Family history
- HSCT
Hematopoietic stem cell transplant
- IDF
Immune Deficiency Foundation
- Ig
Immunoglobulin
- IV
Intravenous
- IQR
Interquartile ratio
- LRTI
Lower respiratory tract infection
- NA
Not available
- NIAID
National Institute of Allergy and Infectious Diseases
- MRSA
Methicillin-resistant Staphylococcus aureus
- MSSA
Methicillin-sensitive Staphylococcus aureus
- SQ
Subcutaneous
- USIDNET
United States Immunodeficiency Network
- URI
Upper respiratory infection
- URTI
Upper respiratory tract infection
- VAP
Ventilator-associated pneumonia
- XLA
X-linked agammaglobulinemia
Author Contribution
All authors contributed to the study conception and design. Material preparation, data collection, and data analysis were performed by Dana O’Toole, M.D., Daniel Groth, M.D., Elizabeth Feuille, M.D., and Hannah Wright. The first draft of the manuscript was written by Dana O’Toole, M.D. and all authors commented on subsequent versions of the manuscript. All authors read and approved the final manuscript.
Funding
The current study is unfunded. The U.S. Immunodeficiency Network (USIDNET), a program of the Immune Deficiency Foundation (IDF), has been supported by a cooperative agreement, U24AI86837, from the National Institute of Allergy and Infectious Diseases (NIAID).
Data Availability
The datasets used and analyzed for the current study are available from the corresponding author upon reasonable request.
Code Availability
The statistical analyses performed, using the mentioned statistical software SAS, are available from the corresponding author and statistician upon reasonable request.
Declarations
Ethics Approval
USIDNET has developed a clinical protocol and associated informed consent documents that have been reviewed and approved by Advarra IRB.
Consent to Participate
Not applicable.
Consent for Publication
Not applicable.
Conflict of Interest
Dr. Kathleen E. Sullivan is a consultant for the Immune Deficiency Foundation and Enzyvant and is an editor for Elsevier and Uptodate. The remaining authors declare they have no conflicts of interest.
Footnotes
Dana O’Toole and Daniel Groth are co-first authors
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Bruton OC. Agammaglobulinemia. Pediatrics. 1952;9(6):722–728. doi: 10.1542/peds.9.6.722. [DOI] [PubMed] [Google Scholar]
- 2.Conley ME, Brown P, Pickard AR, Buckley RH, Miller DS, Raskind WH, et al. Expression of the gene defect in X-linked agammaglobulinemia. N Engl J Med. 1986;315(9):564–567. doi: 10.1056/NEJM198608283150907. [DOI] [PubMed] [Google Scholar]
- 3.Conley ME, Mathias D, Treadaway J, Minegishi Y, Rohrer J. Mutations in BTK in patients with presumed X-linked agammaglobulinemia. Am J Hum Genet. 1998;62(5):1034–1043. doi: 10.1086/301828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ochs HD, Smith CI. X-linked agammaglobulinemia. A clinical and molecular analysis. Medicine (Baltimore) 1996;75(6):287–299. doi: 10.1097/00005792-199611000-00001. [DOI] [PubMed] [Google Scholar]
- 5.El-Sayed ZA, Abramova I, Aldave JC, Al-Herz W, Bezrodnik L, Boukari R, et al. X-linked agammaglobulinemia (XLA): phenotype, diagnosis, and therapeutic challenges around the world. World Allergy Organ J. 2019;12(3):100018. doi: 10.1016/j.waojou.2019.100018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Winkelstein JA, Marino MC, Lederman HM, Jones SM, Sullivan K, Burks AW, et al. X-linked agammaglobulinemia: report on a United States registry of 201 patients. Medicine (Baltimore) 2006;85(4):193–202. doi: 10.1097/01.md.0000229482.27398.ad. [DOI] [PubMed] [Google Scholar]
- 7.Perez EE, Orange JS, Bonilla F, Chinen J, Chinn IK, Dorsey M, et al. Update on the use of immunoglobulin in human disease: a review of evidence. J Allergy Clin Immunol. 2017;139(3S):S1–S46. doi: 10.1016/j.jaci.2016.09.023. [DOI] [PubMed] [Google Scholar]
- 8.Quartier P, Debre M, De Blic J, de Sauverzac R, Sayegh N, Jabado N, et al. Early and prolonged intravenous immunoglobulin replacement therapy in childhood agammaglobulinemia: a retrospective survey of 31 patients. J Pediatr. 1999;134(5):589–596. doi: 10.1016/S0022-3476(99)70246-5. [DOI] [PubMed] [Google Scholar]
- 9.Condino-Neto A, Espinosa-Rosales FJ. Changing the lives of people with primary immunodeficiencies (PI) with early testing and diagnosis. Front Immunol. 2018;9:1439. doi: 10.3389/fimmu.2018.01439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Soresina A, Nacinovich R, Bomba M, Cassani M, Molinaro A, Sciotto A, et al. The quality of life of children and adolescents with X-linked agammaglobulinemia. J Clin Immunol. 2009;29(4):501–507. doi: 10.1007/s10875-008-9270-8. [DOI] [PubMed] [Google Scholar]
- 11.Collins CJ, Yi F, Dayuha R, Whiteaker JR, Ochs HD, Freeman A, et al. Multiplexed proteomic analysis for diagnosis and screening of five primary immunodeficiency disorders from dried blood spots. Front Immunol. 2020;11:464. doi: 10.3389/fimmu.2020.00464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nakagawa N, Imai K, Kanegane H, Sato H, Yamada M, Kondoh K, et al. Quantification of kappa-deleting recombination excision circles in Guthrie cards for the identification of early B-cell maturation defects. J Allergy Clin Immunol. 2011;128(1):223–5 e2. doi: 10.1016/j.jaci.2011.01.052. [DOI] [PubMed] [Google Scholar]
- 13.Abu-Arja RF, Chernin LR, Abusin G, Auletta J, Cabral L, Egler R, et al. Successful hematopoietic cell transplantation in a patient with X-linked agammaglobulinemia and acute myeloid leukemia. Pediatr Blood Cancer. 2015;62(9):1674–1676. doi: 10.1002/pbc.25554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Aadam Z, Kechout N, Barakat A, Chan KW, Ben-Ali M, Ben-Mustapha I, et al. X-linked agammagobulinemia in a large series of North African patients: frequency, clinical features and novel BTK mutations. J Clin Immunol. 2016;36(3):187–194. doi: 10.1007/s10875-016-0251-z. [DOI] [PubMed] [Google Scholar]
- 15.Moin M, Aghamohammadi A, Farhoudi A, Pourpak Z, Rezaei N, Movahedi M, et al. X-linked agammaglobulinemia: a survey of 33 Iranian patients. Immunol Invest. 2004;33(1):81–93. doi: 10.1081/IMM-120027687. [DOI] [PubMed] [Google Scholar]
- 16.Singh S, Rawat A, Suri D, Gupta A, Garg R, Saikia B, et al. X-linked agammaglobulinemia: twenty years of single-center experience from North West India. Ann Allergy Asthma Immunol. 2016;117(4):405–411. doi: 10.1016/j.anai.2016.07.044. [DOI] [PubMed] [Google Scholar]
- 17.Chan JY, Stern DA, Guerra S, Wright AL, Morgan WJ, Martinez FD. Pneumonia in childhood and impaired lung function in adults: a longitudinal study. Pediatrics. 2015;135(4):607–616. doi: 10.1542/peds.2014-3060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lougaris V, Soresina A, Baronio M, Montin D, Martino S, Signa S, et al. Long-term follow-up of 168 patients with X-linked agammaglobulinemia reveals increased morbidity and mortality. J Allergy Clin Immunol. 2020;146(2):429–437. doi: 10.1016/j.jaci.2020.03.001. [DOI] [PubMed] [Google Scholar]
- 19.Paccoud O, Mahlaoui N, Moshous D, Aguilar C, Neven B, Lanternier F, Suarez F, Picard C, Fischer A, Blanche S, Lecuit M, Hermine O, Lortholary O; CEREDIH network. Current Spectrum of Infections in Patients with X-Linked Agammaglobulinemia. J Clin Immunol. 2021;41(6):1266–71. [DOI] [PubMed]
- 20.Gill PK, Betschel SD. Timing of infections in patients with primary immunodeficiencies treated with intravenous immunoglobulin (IVIg) Allergy Asthma Clin Immunol. 2018;14:35. doi: 10.1186/s13223-018-0247-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and analyzed for the current study are available from the corresponding author upon reasonable request.
The statistical analyses performed, using the mentioned statistical software SAS, are available from the corresponding author and statistician upon reasonable request.