Abstract
We aimed to characterize NDM-5-producing Enterobacteriaceae from aquatic products in Guangzhou, China. A total of 196 intestinal samples of grass carp collected in 2019 were screened for carbapenemase genes. Characterization of blaNDM-5 positive isolates and plasmids was determined by antimicrobial susceptibility testing, conjugation experiments, Illumina HiSeq, and Nanopore sequencing. One Citrobacter freundii and six Escherichia coli strains recovered from seven intestinal samples were verified as blaNDM-5 carriers (3.57%, 7/196). TheblaNDM-5 genes were located on the IncX3 (n=5), IncHI2 (n=1), or IncHI2-IncF (n=1) plasmids. All blaNDM-5-bearing plasmids were transferred by conjugation at frequencies of ~10−4–10−6. Based on sequence analysis, the IncHI2 plasmid pHNBYF33-1 was similar to other blaNDM-5-carrying IncHI2 plasmids deposited in GenBank from Guangdong ducks. In all IncHI2 plasmids, blaNDM-5 was embedded in a novel transposon, Tn7051 (IS3000-ΔISAba125-IS5-ΔISAba125-blaNDM-5-bleMBL-trpF-tat-∆dct-IS26-∆umuD-∆ISKox3-IS3000), which was identical to the genetic structure surrounding blaNDM-5 found in some IncX3 plasmids. The IncHI2-IncF hybrid plasmid pHNTH9F11-1 was formed by homologous recombination of theblaNDM-5-carrying IncHI2 plasmid and a heavy-metal-resistant IncF plasmid through ∆Tn1721. To the best of our knowledge, this is the first report on the characterization of blaNDM-5-bearing plasmids in fish in China. The IncHI2 plasmid pHNBYF33-1 may be transmitted from ducks, considering the common duck-fish freshwater aquaculture system in Guangdong. Tn7051 is likely responsible for the transfer of blaNDM-5 from IncX3 to IncHI2 plasmids in Enterobacteriaceae, resulting in the expansion of transmission vectors of blaNDM-5.
Keywords: bla NDM-5 , Enterobacteriaceae, Plasmid, Fish, Carbapenemase
INTRODUCTION
Carbapenemase-producing Enterobacteriaceae (CPE), especially New Delhi metal-β-lactamase (NDM) producers, have been increasingly reported worldwide and pose a significant challenge to public health (Wu et al., 2019). Since the discovery of NDM-1 in 2008 (Yong et al., 2009), NDM-producing Enterobacteriaceae have spread globally. To date, 41 NDM enzyme variants (NDM-1–NDM-41) (https://www.ncbi.nlm.nih.gov/pathogens/refgene/#NDM) have been identified, with theblaNDM-1 and blaNDM-5 genes being the most prevalent (Wu et al., 2019).
In 2011, blaNDM-5 was first reported in an E. coli strain isolated from a patient in the UK (Hornsey et al., 2011). Since then, blaNDM-5 has been detected in more than 40 countries (https://www.ncbi.nlm.nih.gov/pathogens/microbigge/#blaNDM-5). NDM-5 is the most common NDM variant in E. coli, especially in China and Southeast Asia. Although blaNDM-5 is widespread due to diverse self-transferable plasmids such as IncX3 and IncF (FII, FIA, and FIB) (Wu et al., 2019), it is rarely reported in the IncHI2 plasmid, except in swine- and duck-origin E. coli from Guangdong Province, China (Ma et al., 2021; Zhao et al., 2021b).
In China, NDM-5-producing Enterobacteriaceae have been widely detected in humans (Tian et al., 2020), farm animals (Ma et al., 2021), companion animals (Wang et al., 2021a), wild animals (Wang et al., 2017), retail meats (Zhang et al., 2019), and the environment (Zhao et al., 2021a), but rarely in aquatic products. Aquatic products are also considered important reservoirs and transmission vectors of resistant bacteria (Xu et al., 2020). Of note, the integrated duck-fish freshwater aquaculture system is very common in Guangdong, and antimicrobial resistant bacteria can be transmitted between ducks and fish (Shen et al., 2020). Grass carp (Ctenopharyngodon idella) is the most popular freshwater fish in aquaculture and is cultivated in 32 provinces in China. According to the China Fishery Statistical Yearbook 2020 (https://data.cnki.net/trade/Yearbook/Single/N2021020168?z=Z009), the production of grass carp reached 5.5 million tons in 2019, accounting for 21.7% of the maximum annual production in freshwater fish. However, the occurrence of clinically important resistant bacteria, such as CPE, in grass carp has rarely been studied. Hence, we investigated the prevalence of CPE in intestinal samples of grass carp from wet markets in Guangzhou and characterized blaNDM-positive isolates and plasmids to understand the transmission mechanism of blaNDM5 in aquatic products.
MATERIALS AND METHODS
Sample collection, bacterial isolation, and detection of blaNDM
In January 2019, a total of 196 intestinal samples from grass carp were randomly collected from 24 wet markets located in seven districts of Guangzhou, China. We collected fish samples from different stalls, with three intestinal samples randomly collected from each sampling booth. Each sample was placed in a separate sterile sample bag and transported to the laboratory in a freezer box for processing within 12 h. The fish intestines were dissected with sterile surgical scissors, and 1 g of intestinal content was enriched in 2 mL of Luria-Bertani (LB) broth at 37 °C overnight with shaking. The overnight cultures were streaked onto MacConkey agar plates supplemented with 1 mg/L meropenem and incubated at 37 °C for 18–24 h. Enterobacteriaceae colonies with different morphologies were selected from the plates to screen for blaNDM-, blaKPC-, and blaOXA-48-positive isolates using polymerase chain reaction (PCR) with specific primers as described previously (Poirel et al., 2011).
Antimicrobial susceptibility testing
According to the recommendations of the Clinical and Laboratory Standards Institute (2017), the minimal inhibitory concentrations (MICs) of 18 antimicrobials against NDM-positive Enterobacteriaceae isolates were determined using the agar dilution or broth microdilution (colistin and tigecycline) methods. Escherichia coli ATCC 25922 was used as the control. The MICs were interpreted according to the criteria of CLSI (M100-S30) and EUCAST (http://www.eucast.org).
Conjugation experiments
In this study, streptomycin-resistant E. coli C600 was used as the recipient, and each blaNDM-positive isolate was used as the donor for conjugation by broth mating at 37 ℃ for 16–20 h. Transconjugants were selected on MacConkey agar plates supplemented with 3 000 mg/L streptomycin and 1 mg/L meropenem. Conjugation frequency was calculated following previously reported methods (Chen et al., 2007).
Whole-genome sequencing and bioinformatics analysis
Whole-genomic DNA of NDM-positive isolates was sequenced using the Illumina Hiseq X Ten and Oxford Nanopore MinIon platforms, and complete genomes were obtained by hybrid assembly using Unicycler v0.4.7 (Wick et al., 2017). MLST v2.19 (https://github.com/tseemann/mlst) was used to identify the sequence type (ST) of the blaNDM-positive strains. Plasmid replicons, antimicrobial resistance genes, and heavy metal resistance genes were analyzed using ABRicate v1.0 (https://github.com/tseemann/abricate) with the PlasmidFinder (Carattoli et al., 2014), ResFinder (Zankari et al., 2012), and AMRFinderPlus databases (https://github.com/ncbi/amr), respectively. Plasmid double-locus sequence typing (pDLST) for IncHI2 plasmids was identified using pMLST v2.0 (https://cge.cbs.dtu.dk/services/pMLST/). Insertion sequence (IS) elements were identified using ISfinder (https://isfinder.biotoul.fr/). Single nucleotide polymorphism (SNP) calling was performed using Snippy (https://github.com/tseemann/snippy). The blaNDM-carrying plasmids were further compared and analyzed using the BLAST ring image generator (Alikhan et al., 2011). The genetic context of blaNDM was analyzed by GalileoTM AMR (http://galileoamr.arcbio.com/mara/), Gene Construction Kit v4.5 software (Textco BioSoftware, USA), and Easyfig v2.2.5 (http://mjsull.github.io/Easyfig/files.html).
Nucleotide sequence accession numbers
The complete genome sequences of seven blaNDM-5-positive Enterobacteriaceae were deposited in GenBank under BioProject No. PRJNA636005.
RESULTS
Characterization of blaNDM-5-carrying isolates
A total of seven (3.57%) unduplicated carbapenem-resistant isolates, including six E. coli and one C. freundii, were obtained from the seven intestinal samples of grass carp (Table 1). All seven isolates were identified as blaNDM-5-positive by PCR and sequencing, while blaKPC and blaOXA-48 were not detected.
Table 1. Characterization of blaNDM-5-carrying Escherichia coli andCitrobacter freundii isolates .
| Isolates | Species | MLST | Farmers market (FM) | Other resistance genes | Heavy metal-resistant genes | Chromosomal mutations | Location of blaNDM-5 | Plasmid
size (bp) |
Conjugation frequencya |
| a: Average±Standard error (SE). N.D.: Not detected. Bold: blaNDM-5−carrying plasmids and other resistance genes. | |||||||||
| BY9F33M | E. coli | ST57 | FM4 | aac(3)-IV, aadA2b, aph(3'')-Ib, aph(3')-Ia, aph(6)-Id, aph(4)-Ia, blaTEM-1B, floR, sul3, cmlA1 aadA1 , aadA5, aph(3')-IIa, mdf(A), mph(A), qnrS1, arr-2, sul1, tet(A), dfrA14, dfrA27 | terDZW,merPRT, arsADR | GyrA (p.S83L, p.D87N)
ParC (p.S80I) |
IncHI2, IncFIB, IncX1, IncY | 238 926 | (4.93±0.91)×10−6 |
| BY9F36M | C. freundii | ST557 | FM4 | aac(3)-IId, aadA16,aac(6')-Ib-cr, aph(3'')-Ib, aph(6)-Id, blaTEM-1B, blaCMY-129, mph(A), floR, qnrB18, qnrB26, qnrB6, arr-3, sul1, sul2, tet(A), dfrA27 | arsADR, merCPTR | N.D. | IncX3, IncFIB (K) | 46 161 | (2.05±0.21)×10−4 |
| PY9F04M | E. coli | ST48 | FM21 | aadA1,aadA2, aph(3')-Ia, blaOXA-10, mdf(A), mph(A), erm(42), cmlA1, floR, oqxAB, arr-2, sul2, tet(A), tet(M), dfrA12, dfrA14 | pcoABCDESR,iucABCD, silABCEFPRS | GyrA (p.S83L, p.D87N)
ParC (p.S80I) |
IncX3, IncFII, IncFIB, IncFIA | 46 161 | (3.75±0.80)×10−5 |
| PY9F07M | E. coli | ST48 | FM21 | ant(3'')-Ia, aadA1,aadA2,aph(3')-Ia, blaOXA-10, mdf(A), mph(A), erm(42), cmlA1, floR, oqxAB, arr-2, sul2, tet(A), tet(M), dfrA12, dfrA14 | pcoABCDESR,iucABCD, silABCEFPRS | GyrA (p.S83L, p.D87N)
ParC (p.S80I) |
IncX3, IncFII, IncFIB, IncFIA | 46 161 | (2.88±1.41)×10−5 |
| PY9F09M | E. coli | ST155 | FM21 | aadA1,blaOXA-10, mdf(A), cmlA1, floR, qnrS1, arr-2, tet(A), dfrA14 | − | N.D. | IncX3, IncFIB | 46 161 | (4.87±0.25)×10−5 |
| TH9F11M | E. coli | ST101 | FM8 | aac(3)-IV, aadA2b, aph(3'')-Ib, aph(3')-Ia, aph(6)-Id, aph(4)-Ia, blaOXA-10,blaCTX-M-55, fosA3, cmlA1, floR, qnrS1, sul3, tet(A), dfrA1, dfrA14, arr-3,mdf(A), arr-2, sul2 |
iroBCDEN,
arsADR, terDZW |
GyrA (p.S83L, p.D87N)
ParC (p.S80I) ParE (p.S458A) |
IncHI2-IncF, IncFII, p0111 | 407 456 | (2.79±0.35)×10−5 |
| HZ9F01M | E. coli | ST9124 | FM12 | aadA22, aph(3')-Ia, aph(6)-Id, blaTEM-1B, mdf(A), mph(A), lnu(F), floR, qnrS1, arr-2, sul3, tet(A), dfrA14 | terDZW | GyrA (p.S83L, p.D87N)
ParC (p.S80I) |
IncX3, IncFII, IncHI2, IncI1 | 46 161 | (2.35±0.31)×10−5 |
The seven blaNDM-5-carrying isolates showed multidrug-resistant phenotypes and harbored multiple resistance genes (Tables 1, 2). Molecular typing results showed that the C. freundii strain belonged to ST557. Six of the NDM-5-positive E. coli strains belonged to five different STs, namely ST48, ST57, ST101, ST155, and ST9124. The two ST48 E. coli isolates (PY9F04M and PY9F07M) were recovered from the same market but from different sample booths (Table 1) and were related as they showed only 10 core-genome SNP (cgSNP) differences from each other (Schürch et al., 2018), although the resistance genes they carried were not the same (Table 1).
Table 2. Antibiotic susceptibility of blaNDM-5-carrying isolates and their transconjugants .
| Isolates | Species | MIC (mg/L) | |||||||||||||||||
| AMP | FOX | CTX | CAZ | IPM | APR | STR | CIP | DOX | TET | TIG | AMI | GEN | NEO | CL | SXT | FLR | FOS | ||
| MIC, minimal inhibitory concentration. AMP, ampicillin; FOX, cefoxitin; CTX, cefotaxime; CAZ, ceftazidime; IPM, imipenem; APR, apramycin; STR, streptomycin; CIP, ciprofloxacin; DOX, doxycycline; TET, tetracycline; TIG, tigecycline; AMI, amikacin; GEN, gentamicin; NEO, neomycin; CL, colistin; SXT, sulfamethoxazole/trimethoprim; FLR, florfenicol; FOS, fosfomycin. | |||||||||||||||||||
| BY9F33M | E. coli | >128 | >128 | >128 | >128 | 8 | >128 | 128 | 32 | 64 | >128 | 0.5 | 1 | 32 | 128 | 0.25 | 32 | >128 | 16 |
| BY9F33M-1T | >128 | 128 | 32 | >128 | 4 | >128 | >256 | 0.25 | 4 | 16 | 0.25 | 1 | 8 | 128 | 0.125 | 16 | 64 | 16 | |
| BY9F36M | C. freundii | >128 | >128 | >128 | >128 | 8 | 8 | 128 | 8 | 32 | 128 | 0.5 | 1 | 32 | 1 | 0.25 | 32 | 128 | 16 |
| BY9F36M-1T | >128 | >128 | 64 | >128 | 4 | 8 | >256 | 0.008 | 1 | 1 | 0.25 | 1 | 0.5 | 1 | 0.125 | 0.25 | 2 | 16 | |
| PY9F04M | E. coli | >128 | >128 | 128 | >128 | 8 | 8 | 64 | 32 | 64 | 128 | 0.5 | 2 | 0.5 | 128 | 0.25 | 32 | 128 | 16 |
| PY9F04M-1T | >128 | >128 | 64 | >128 | 4 | 8 | >256 | 0.008 | 1 | 1 | 0.25 | 1 | 0.5 | 1 | 0.125 | 0.25 | 2 | 16 | |
| PY9F07M | E. coli | >128 | >128 | 128 | >128 | 8 | 8 | 64 | 32 | 64 | 128 | 0.5 | 2 | 0.5 | 128 | 0.25 | 32 | 128 | 16 |
| PY9F07M-1T | >128 | >128 | 128 | >128 | 4 | 8 | >256 | 0.004 | 8 | 1 | 0.25 | 2 | 0.5 | 1 | 0.125 | 0.25 | 2 | 16 | |
| PY9F09M | E. coli | >128 | >128 | 128 | >128 | 8 | 8 | 32 | 0.25 | 32 | 128 | 0.5 | 2 | 0.25 | 1 | 0.25 | 8 | 128 | 16 |
| PY9F09M-1T | >128 | >128 | 64 | >128 | 4 | 8 | >256 | 0.004 | 1 | 1 | 0.25 | 2 | 0.25 | 1 | 0.125 | 0.25 | 2 | 16 | |
| TH9F11-1M | E. coli | >128 | >128 | >128 | >128 | 8 | >128 | 256 | 128 | 64 | >128 | 1 | 1 | 32 | 128 | 0.125 | 32 | 128 | >256 |
| TH9F11-1M-1T | >128 | >128 | 128 | >128 | 4 | >128 | >256 | 0.25 | 16 | 128 | 0.5 | 1 | 8 | >128 | 0.125 | 16 | 64 | >256 | |
| HZ9F01M | E. coli | >128 | >128 | 128 | >128 | 8 | 8 | 256 | 32 | 64 | >128 | 1 | 2 | 0.5 | 64 | 0.25 | 32 | 128 | 16 |
| HZ9F01M-1T | >128 | >128 | 64 | >128 | 4 | 8 | >256 | 0.25 | 1 | 64 | 0.5 | 2 | 0.5 | 1 | 0.125 | 0.25 | 2 | 16 | |
Characterization of blaNDM-5-bearing plasmids
The conjugation experiments indicated that the seven blaNDM-5-carrying plasmids could be successfully transferred to the recipient E. coli C600 strain, and replicon typing results revealed that the blaNDM-5 genes were located on IncX3 (n=5), IncHI2 (n=1), and IncHI2-IncF (n=1). The conjugation frequencies of the IncX3-type plasmids varied from ~10−4 to 10−5 cells/donor, while the conjugation frequencies of the IncHI2-type and IncHI2-IncF-type plasmids were ~10−6 and ~10−5 cells/donor, respectively (Table 1).
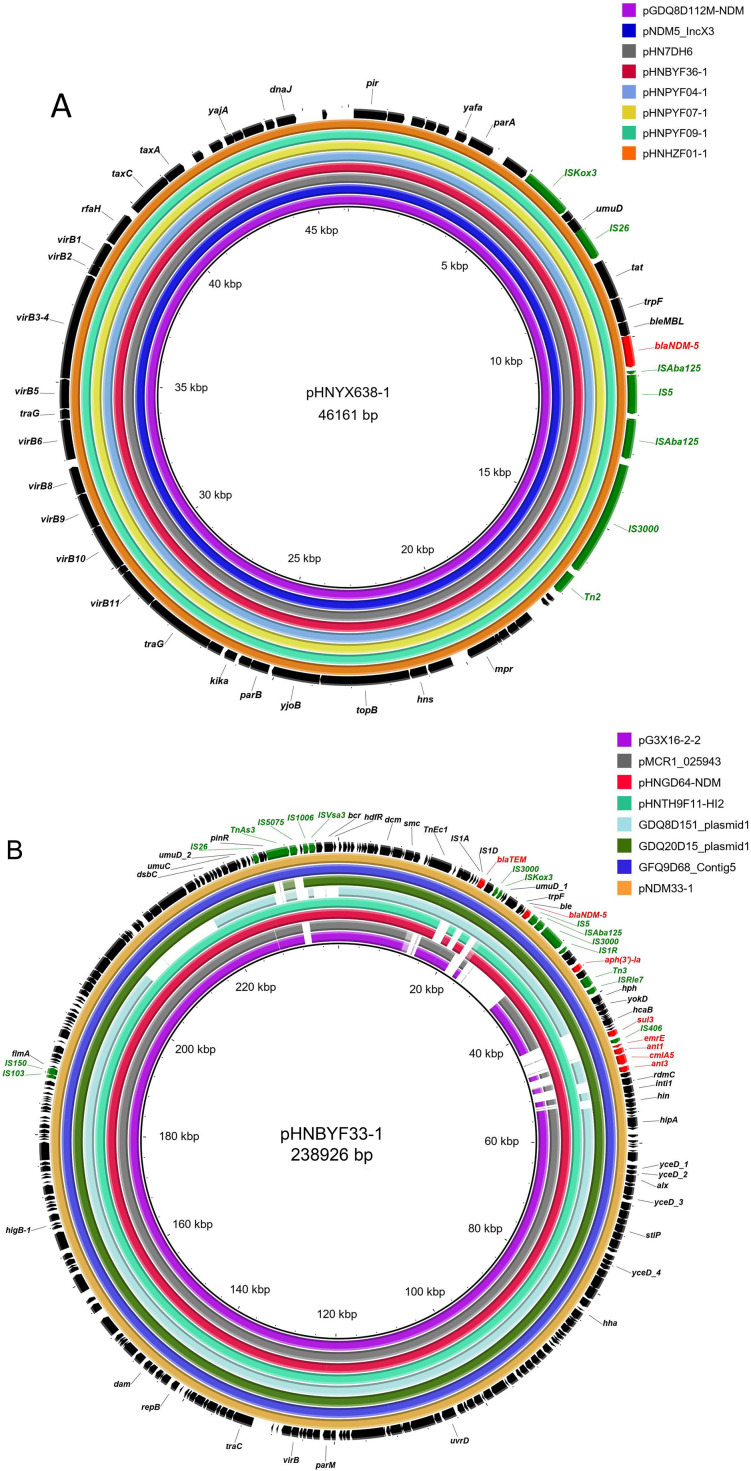
The complete sequences of all seven blaNDM-5-bearing plasmids were obtained using Illumina and Nanopore sequencing. The sequences of five IncX3 plasmids were similar to previously reported blaNDM-5-bearing IncX3 plasmids, including plasmids pGDQ8D112M-NDM (GenBank Accession No. MK628734, duck, China), pNDM5_IncX3 (KU761328.1, Homo sapiens, China), pHNYX638-1 (MK033577, pork, China), and pHN7DH6 (MN276080, dog, China) (Figure 1A).
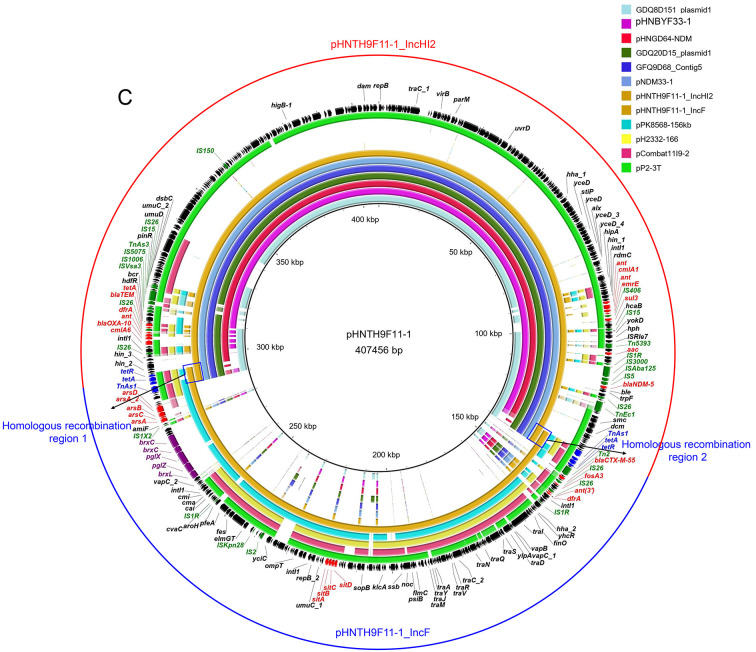
Figure 1.
Comparison of blaNDM-5-carrying plasmids
A: blaNDM-5-harboring IncX3 plasmids in this study with other similar plasmids. Plasmid pHNYX638-1 (MK033577) is reference plasmid. pGDQ8D112M-NDM (MK628734), pNDM5_IncX3 (CP065346), pHN7DH6 (MN276080), pHNBYF36-1, pHNPYF04-1, pHNPYF07-1, pHNPYF09-1, and pHNZF01-1. B: blaNDM-5-harboring IncHI2 plasmid pHNBYF33-1 in this study with other similar plasmids. pG3X16-2-2 (CP038139), pMCR1_025943 (CP027202), pHNGD64-NDM (MW296099), pHNTH9F11-HI2 (this study), GDQ8D151 plasmid1 (JAGFYD010000002), GDQ20D15 plasmid1 (JAGFYB010000003), GFQ9D68 Contig5 (JAGFYC010000005), and pNDM33-1 (MN915011). C: blaNDM-5-harboring IncHI2-IncF24:A-:B1 plasmid pHNTH9F11-1 in this study with other plasmids. GDQ8D151 plasmid1 (JAGFYD010000002), pHNBYF33-1 (this study), pHNGD64-NDM (MW296099), GDQ20D15 plasmid1 (JAGFYB010000003), GFQ9D68 Contig5 (JAGFYC010000005), pNDM33-1 (MN915011), pHNTH9F11-1_IncHI2, pHNTH9F11-1_IncF, pPK8568-156kb (CP080127), pH2332-166 (NC_025175), pCombat11I9-2 (CP021728), and pP2-3T (MG014722). All rings are represented from inside to outside. Red arrows, resistance genes; green arrows, mobile genetic elements; purple arrows, phage resistance system; blue arrows, homologous recombination regions. Red and blue semicircles in C represent pHNTH9F11-1_IncHI2 and pHNTH9F11-1_IncF, respectively.
Plasmid pHNBYF33-1, which belonged to IncHI2-ST3, was 238 926 bp in length with a GC content of 46.30% and carried 12 resistance genes. The BLASTn results indicated that plasmid pHNBYF33-1 exhibited high similarity (≥99.9% identity and ≥93.4% coverage) to four blaNDM-5-carrying IncHI2 plasmids deposited in GenBank, i.e., pNDM33-1 (MN915011) (Zhao et al., 2021b), GFQ9D68 Contig5 (JAGFYC010000005), GDQ8D151 plasmid1 (JAGFYD010000002), and GDQ20D15 plasmid1 (JAGFYB010000003) (Figure 1B). Interestingly, all four plasmids were carried by E. coli strains recovered from ducks in Guangdong, China.
Figure 1.
Plasmid pHNTH9F11-1 (IncHI2-IncF) was 407 456 bp in size and had an average GC content of 48.03%. pHNTH9F11-1 harbored three different replicons, including IncHI2, IncFII, and IncFIB. BLASTn analysis showed that pHNTH9F11-1 was a cointegrate plasmid comprised of sequences of IncHI2 (designated as pHNTH9F11-1_IncHI2), harboring blaNDM-5, and IncF24:A-:B1 (designated as pHNTH9F11-1_IncF) (Figure 1C). In addition, the hybrid plasmid pHNTH9F11-1 had 87% nucleotide sequence coverage of the IncHI2-IncFII plasmid pP2-3T (MG014722, swine, China). The sequence of plasmid pHNTH9F11-1_IncHI2 harboring blaNDM-5 was similar (≥99.99% identity and 100% coverage) to that of the blaNDM-5-carrying plasmid pHNGD64-NDM (MW296099) from a swine E. coli strain (Ma et al., 2021). Plasmid pHNTH9F11-1_IncF exhibited similarity (≥99.99% identity and ≥90% coverage) to IncF24:A-:B- plasmid pPK8568-156kb (CP080127, chicken, Pakistan), carrying multiple heavy metal resistance genes (arsABCD and sitABCD) and a phage resistance system (BREX, bacteriophage exclusion system) (Goldfarb et al., 2015). Further analysis revealed that pHNTH9F11-1_IncF and pHNTH9F11-1_IncHI2 were bound by two identical ∆Tn1721 transposons (hp-tetR-tet(A)-eamA) with a length of 5 492 bp, suggesting that the cointegrate plasmid pHNTH9F11-1 was formed by homologous recombination of these two plasmids through ∆Tn1721 (Figure 1C).
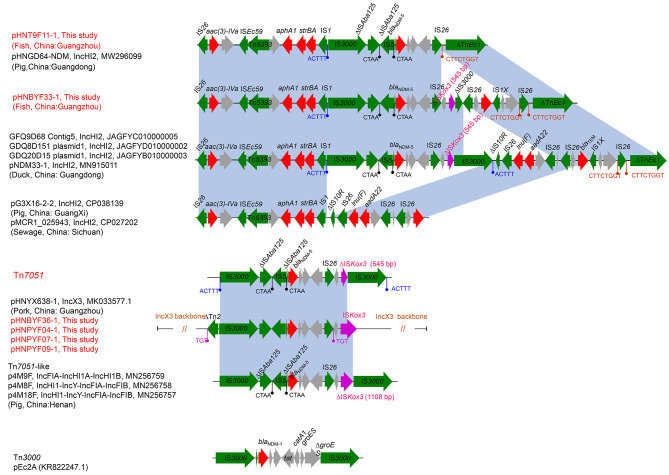
Genetic contexts of blaNDM-5 genes in IncX3 and IncHI2
All five blaNDM-5-carrying IncX3 plasmids showed identical genetic contexts (i.e., IS3000-ΔISAba125-IS5-ΔISAba125-blaNDM-5-bleMBL-trpF-tat-IS26-ΔumuD-ISKox3) (Figure 2), similar to that of the classical IncX3 plasmid pHNYX658-1 (Zhang et al., 2019). The genetic contexts of blaNDM-5 in pHNTH9F11-1_IncHI2 and pHNBYF33-1 (IncHI2) were similar to other blaNDM-5-carrying IncHI2 plasmids in GenBank, including pNDM33-1, GDQ8D151 plasmid1, GFQ9D68 Contig5, and GDQ20D15 plasmid1 (Figure 2). In these four IncHI2-type plasmids, theblaNDM-5 gene was identically embedded in a novel composite transposon (IS3000-ΔISAba125-IS5-ΔISAba125-blaNDM-5-bleMBL-trpF-tat-∆dct-IS26-∆umuD-∆ISKox3-IS3000) inserted between IS1 and IS10R of the multidrug resistance region of the IncHI2 plasmid with 5 bp target site duplications (TSDs) (ACTTT). Previous research has found that excision of this transposon from the plasmid pNDM33-1 forms a circular intermediate (Zhao et al., 2021b). Here, we renamed this novel 13 918 bp long transposon as Tn7051 (https://transposon.lstmed.ac.uk/). Comparative analysis demonstrated that Tn7051 shared 99.98% nucleotide sequence similarity (two SNP differences) with the genetic context ofblaNDM-5 in the IncX3 plasmid pHNYX638-1 (MK033577, pork, China), except that ISKox3 in Tn7051 was truncated by a copy of IS3000, creating only 545 bp remains (∆ISKox3) (Figure 2). Interestingly, when comparing the Tn7051 sequence, we found a Tn7051-like structure (14 482 bp) in three hybrid plasmids obtained from swine E. coli isolates in China (Yao et al., 2020), namely p4M9F (IncFIA-IncHI1A-IncHI1B, MN256759), p4M8F (IncHI1-IncY-IncFIA-IncFIB, MN256758), and p4M18F (IncHI1-IncY-IncFIA-IncFIB, MN256757). In the Tn7051-like structure, ISKox3 had more residues (1 108 bp) than that in Tn7051. Furthermore, the Tn7051-like structure exhibited 99.97%–100.00% nucleotide sequence identity (0–3 SNP differences) to the genetic context ofblaNDM-5 in the IncX3 plasmid pHNYX638-1. Given that Tn7051 and Tn7051-like transposons were similar to the genetic context of blaNDM-5 in the IncX3 plasmid pHNYX638-1, we speculated that both Tn7051 and Tn7051-like transposons were likely derived from the IncX3 plasmid.
Figure 2.
Genetic contexts of blaNDM-5 gene in IncX3 and IncHI2 plasmids
Regions with ≥99.0% nucleotide sequence identity are shaded blue. Open reading frames (ORFs) are depicted by arrows. Red, green, purple, and gray arrows represent resistance gene, insertion sequence, ISKox3, and hypothetical protein, respectively. Rods represent direct repeat sequences.
Although the IncHI2 plasmids pHNBYF33-1 and pNDM33-1 shared the same Tn7051 insertion site (Figure 2), compared with pNDM33-1, the plasmid pHNBYF33-1 lacked the ∆IS3000-∆IS10-IS26-lnu(F)-aadA2-hp-IS26 segment, which could be readily explained by a deletion event mediated by two copies of IS26 located in the same orientation (Harmer & Hall, 2016). The genetic context of blaNDM-5 in the hybrid plasmid pHNTH9F11-1 was the same as that in plasmid pHNGD64-NDM, and was very similar to pNDM33-1, except for the lack of the IS26-∆umuD-∆ISKox3-IS3000-∆IS10-IS26-lnu(F)-aadA2-hp-IS26-hp-IS26-blaTEM-IS1X unit (Figure 2). This may be due to the deletion of genes mediated by homologous recombination between two copies of IS26 in the same direction (i.e., IS26 in Tn7051 and IS26 upstream of ΔTnEc1), as IS26 located upstream of ΔTnEc1 had only an 8 bp TSD (CTTCTGGT) on one side (Figure 2).
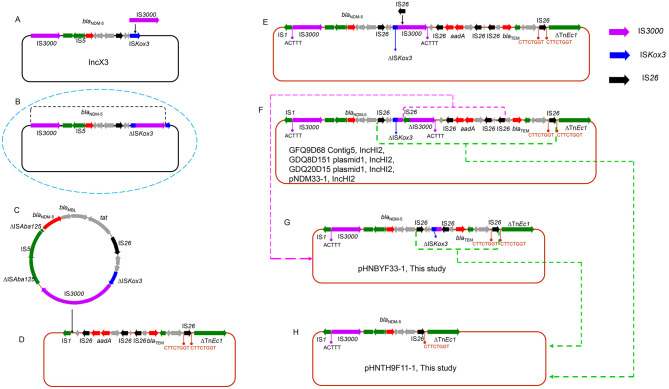
Proposed formation model of genetic contexts of blaNDM-5 in IncHI2 plasmids
Based on detailed sequence analysis, the co-integration mechanism of IS26 (Harmer & Hall, 2016), and the copy-out-paste-in mechanism of composite transposons (Piégu et al., 2015), we proposed a genetic environment formation model of blaNDM-5 in plasmids pHNBYF33-1 and pHNTH9F11-1, as shown in Figure 3. The assumed plasmid evolution process was as follows: IS3000 was inserted into ISKox3 of the IncX3 plasmid (Figure 3A), thus forming the IS3000-ΔISAba125-IS5-ΔISAba125-blaNDM-5-blaMBL-trpF-tat-∆dct-IS26-∆umuD-∆ISkox3-IS3000-∆ISkox3 unit (Figure 3B), hypothesized due to the absence of this unit in GenBank. The two same-orientated copies of IS3000, surrounding blaNDM-5, generated the circular intermediate Tn7051 (Figure 3C), which was further inserted into the region between IS1 and IS10 of the IncHI2 plasmid (Figure 3D) with 5 bp TSDs (ACTTT), resulting in the formation of the blaNDM-5-carrying IncHI2 plasmids (i.e., pNDM33-1, MN915011; GFQ9D68 Contig5, JAGFYC010000005; GDQ8D151 plasmid1, JAGFYD010000002; and GDQ20D15 plasmid1, JAGFYB010000003) (Figure 3E). IS3000, located downstream of ISKox3, was truncated by IS26 and, consequently, the IncHI2 plasmids evolved into the structure shown in Figure 3F (hypothesized due to the absence of a similar structure in GenBank). The IS26 that previously truncated IS3000 was recombined with the IS26 adjacent to blaTEM-1 (purple frame in Figure 3F), leading to the deletion of the ∆IS3000-∆IS10-IS26-lnu(F)-aadA2-hp-IS26 segment. As a result, the structure of the hypothetical plasmids (Figure 3F) entered the blaNDM-5-carrying plasmid pHNBYF33-1 (Figure 3G). Additionally, the two IS26 elements in Figure 3F and Figure 3G (light green frame) integrated, resulting in the formation of the blaNDM-5-carrying plasmid pHNTH9F11-1 (Figure 3H). Therefore, in summary, we speculated that Tn7051 may contribute to the transfer of blaNDM-5 from the IncX3 plasmids to the IncHI2 plasmids, and the genetic contexts of blaNDM-5 on the IncHI2 plasmids in fish were likely derived from plasmids carried by ducks in Guangdong, China.
Figure 3.
Proposed formation mechanism of genetic environment of blaNDM-5 in plasmids pHNBYF33-1 and pHNTH9F11-1
Purple, blue, black, green, and red arrows represent IS3000, ISKox3, IS26, other insertion sequence, and resistance gene, respectively.
DISCUSSION
As the most common CPE, blaNDM-positive Enterobacteriaceae have been isolated from seafood and aquatic environments in several countries (Das et al., 2019; Köck et al., 2018). Moreover, blaNDM-positive Enterobacteriaceae have been detected in freshwater fish in Vietnam (Nakayama et al., 2022) and farmed fish in Egypt (Hamza et al., 2020). To the best of our knowledge, however, this is the first report of blaNDM in freshwater fish from China. Of concern, as fish intestines are consumed in Guangdong, NDM-5-positive Enterobacteriaceae in the intestines of retail fish products could spread to humans via the food chain.
In China, IncX3 plasmids are the most common type of plasmid carrying blaNDM-5 (Ma et al., 2020). NDM-5-producing IncX3 plasmids are widespread in environmental, animal, and clinical isolates (Ma et al., 2020), but are rarely reported in Enterobacteriaceae of freshwater fish origin. The similar IncX3 plasmids found in this study further highlight the importance of the epidemic IncX3 plasmid in the spread of theblaNDM-5 gene within the entire ecosystem. IncHI2/ST3 plasmids have been reported to mediate the transfer of various antibiotic resistance genes (ARGs), such as fosA3 (Wang et al., 2020), floR (Cao et al., 2020), blaCTX-M (Lü et al., 2020), and mcr (Long et al., 2019; Zhi et al., 2016), as well as various NDM-type carbapenemase genes, such as blaNDM-1, blaNDM-9, and blaNDM-4 (Liu et al., 2017; Oueslati et al., 2021). However, there are very few reports of blaNDM-5-carrying IncHI2 (Ma et al., 2021; Zhao et al., 2021b). Consequently, we downloaded all available complete genomes (n=5 974; as of 1 September 2021) of Enterobacteriaceae submitted to the NCBI assembly database (https://www.ncbi.nlm.nih.gov/assembly/) and found only fiveblaNDM-5-carrying IncHI2 plasmids (four from ducks and one from swine), all of which were from Guangdong, China. Although we could not trace the location of the grass carp farms and investigate the contamination source of the blaNDM-5-positive Enterobacteriaceae, it is worth noting that the detection rate of the blaNDM gene in duck samples from Guangdong is high (>30%) (Wang et al., 2021b) and integrated duck-fish farming is very common in Guangdong (Shen et al., 2020). In the duck-fish farm model, duck feces are discharged without treatment, and a large number of ARGs or residual agents can directly contaminate the fish ponds, promoting the transmission of ARGs between ducks and fish. Thus, considering the high similarity of the blaNDM-5-bearing IncHI2 plasmids in the fish and ducks, and that theblaNDM-5-bearing IncHI2 plasmid is currently only found in Guangdong, we speculate that the blaNDM-5-bearing IncHI2 plasmids found in Enterobacteriaceae from retail fish may have been derived from duck feces-contaminated fish ponds in Guangdong. As such, greater attention should be paid to the transfer risk of antimicrobial resistant bacteria in integrated duck-fish farming.
Here, pHNTH9F11-1 (IncHI2-IncF) was identified as a hybrid plasmid, formed by homologous recombination through ∆Tn1721. In gram-negative bacteria, the fusion of plasmids mediated by insertion sequences, such as IS26, is rather universal, leading to a plasmid that can encode multiple resistance and hypervirulence genes, thereby posing a considerable threat to human health; for example, the co-integration event mediated by IS26 between the blaNDM-5-bearing IncX3 plasmid and blaCMY-2-bearing IncA/C plasmid (Li et al., 2020). Moreover, the fusion of plasmids can expand the number of replicons and host range of plasmids, accelerating the dissemination of ARGs among various bacterial species (Dolejska et al., 2014; Wong et al., 2017). Of note, this fusion can also enable a non-conjugative plasmid to acquire conjugation ability, thereby facilitating the transmission of resistance genes, e.g., the recombination of non-conjugative mcr-1-carrying P7 phage-like plasmid pD72-mcr1 and conjugative F33:A-:B- plasmid pD72-F33 mediated by IS26, forming cointegrate plasmid pD72C with a conjugation frequency of 8×10-3 cells/donor (He et al., 2019). Hence, the cointegrate plasmid pHNTH9F11-1 with multidrug resistance, heavy metal resistance, and phage resistance system (ability to resist invasion of bacteriophages) may provide an advantage for the host to survive in the environment.
Composite transposons can mediate the jump of ARGs between different DNA molecules. The novel Tn7051 and Tn7051-like transposons can both be moved by a copy-out-paste-in mechanism utilizing a double-stranded circular DNA intermediate (Yao et al., 2020; Zhao et al., 2021b), thereby contributing to the transfer of theblaNDM-5 gene and expanding its transmission vectors. It has been widely reported that blaNDM-5 genes are mainly located on narrow-host-range plasmids (e.g., IncX3, IncF, and IncB/O) (Wu et al., 2019). However, the transfer of blaNDM-5 to the IncHI2 plasmid mediated by Tn7051 and to the IncHI1-IncY-IncFIA-IncFIB plasmid mediated by Tn7051-like suggested that these transposons may further accelerate the horizontal spread of the blaNDM-5 gene to various strains and plasmids, like Tn3000 and Tn125, which mediate the between-plasmid jumps of blaNDM-1 and accelerate the transfer of blaNDM-1 in different strains (Acman et al., 2021).
CONCLUSIONS
This study revealed the emergence ofblaNDM-5 in Enterobacteriaceae of fish origin in China. To the best of our knowledge, this is the first report of theblaNDM-5 gene, as well as blaNDM-5-bearing plasmids, in isolates from fish products in China. Our findings indicated that blaNDM-5 in the IncHI2 plasmids may originate from the IncX3 plasmid, transferred by the novel composite transposon Tn7051. Furthermore, the blaNDM-5-bearing IncHI2 plasmid may be transmitted from ducks, considering the common duck-fish freshwater aquaculture system in Guangdong. Based on the concept of “One health”, the surveillance of antibiotic resistance in aquatic products should be strengthened, and more measures should be taken to reduce the transfer of clinically important resistant bacteria, such as CPE, between food-producing animals and animal products.
DATA AVAILABILITY
The datasets in this study can be found in NCBI under BioProjectID PRJNA636005. The raw sequence data reported in this paper have been deposited in the Genome Sequence Archive (Genomics, Proteomics & Bioinformatics 2021) in the National Genomics Data Center (Nucleic Acids Res 2021), China National Center for Bioinformation/Beijing Institute of Genomics, Chinese Academy of Sciences (GSA: CRA005844), publicly accessible at https://ngdc.cncb.ac.cn/gsa.
COMPETING INTERESTS
The authors declare that they have no competing interests.
AUTHORS’ CONTRIBUTIONS
J.H.L. and L.C.L. conceived the research. X.G., Y.Y.L., M.Y.G., K.B.M., W.Y.H., and L.C.L. collected the data. L.C.L., J.H.L., Y.Y.L., X.G., and W.Y.H. analyzed and interpreted the data. Y.Y.L. and L.C.L. drafted the manuscript, J.H.L., W.Y.H., and X.G. revised the report. All authors read and approved the final version of the manuscript.
Funding Statement
This study was supported by the National Natural Science Foundation of China (31625026, 32141002) and Innovation Team Project of Guangdong University (2019KCXTD001)
References
- 1.Acman M, Wang RB, van Dorp L, Shaw LP, Wang Q, Luhmann N, et al. 2021. Role of the mobilome in the global dissemination of the carbapenem resistance gene blaNDM. bioRxiv,doi: 10.1101/2021.01.14.426698.
- 2.Alikhan NF, Petty NK, Ben Zakour NL, Beatson SA BLAST Ring Image Generator (BRIG): simple prokaryote genome comparisons. BMC Genomics. 2011;12:402. doi: 10.1186/1471-2164-12-402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cao YP, Lin QQ, He WY, Wang J, Yi MY, Lv LC, et al Co-selection may explain the unexpectedly high prevalence of plasmid-mediated colistin resistance gene mcr-1 in a Chinese broiler farm . Zoological Research. 2020;41(5):569–575. doi: 10.24272/j.issn.2095-8137.2020.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Carattoli A, Zankari E, García-Fernández A, Voldby Larsen M, Lund O, Villa L, et al In silico detection and typing of plasmids using PlasmidFinder and plasmid multilocus sequence typing . Antimicrobial Agents and Chemotherapy. 2014;58(7):3895–3903. doi: 10.1128/AAC.02412-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chen L, Chen ZL, Liu JH, Zeng ZL, Ma JY, Jiang HX Emergence of RmtB methylase-producing Escherichia coli and Enterobacter cloacae isolates from pigs in China . Journal of Antimicrobial Chemotherapy. 2007;59(5):880–885. doi: 10.1093/jac/dkm065. [DOI] [PubMed] [Google Scholar]
- 6.Clinical and Laboratory Standards Institute. 2017. Performance Standards for Antimicrobial Susceptibility Testing. 27th ed. Wayne: Clinical and Laboratory Standards Institute.
- 7.Das UN, Singh AS, Lekshmi M, Nayak BB, Kumar S Characterization of blaNDM-harboring, multidrug-resistant Enterobacteriaceae isolated from seafood . Environmental Science and Pollution Research. 2019;26(3):2455–2463. doi: 10.1007/s11356-018-3759-3. [DOI] [PubMed] [Google Scholar]
- 8.Dolejska M, Villa L, Minoia M, Guardabassi L, Carattoli A Complete sequences of IncHI1 plasmids carrying blaCTX-M-1 and qnrS1 in equine Escherichia coli provide new insights into plasmid evolution . Journal of Antimicrobial Chemotherapy. 2014;69(9):2388–2393. doi: 10.1093/jac/dku172. [DOI] [PubMed] [Google Scholar]
- 9.Goldfarb T, Sberro H, Weinstock E, Cohen O, Doron S, Charpak-Amikam Y, et al BREX is a novel phage resistance system widespread in microbial genomes. The EMBO Journal. 2015;34(2):169–183. doi: 10.15252/embj.201489455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hamza D, Dorgham S, Ismael E, El-Moez SIA, Elhariri M, Elhelw R, et al Emergence of β-lactamase- and carbapenemase- producing Enterobacteriaceae at integrated fish farms . Antimicrobial Resistance & Infection Control. 2020;9(1):67. doi: 10.1186/s13756-020-00736-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Harmer CJ, Hall RM IS26-mediated formation of transposons carrying antibiotic resistance genes . mSphere. 2016;1(2):e00038–16. doi: 10.1128/mSphere.00038-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.He DD, Zhu YY, Li RC, Pan YS, Liu JH, Yuan L, et al. 2019. Emergence of a hybrid plasmid derived from IncN1-F33: A-: B- and mcr-1-bearing plasmids mediated by IS26. Journal of Antimicrobial Chemotherapy, 74(11): 3184–3189.
- 13.Hornsey M, Phee L, Wareham DW A novel variant, NDM-5, of the New Delhi metallo-β-lactamase in a multidrug-resistant Escherichia coli ST648 isolate recovered from a patient in the United Kingdom . Antimicrobial Agents and Chemotherapy. 2011;55(12):5952–5954. doi: 10.1128/AAC.05108-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Köck R, Daniels-Haardt I, Becker K, Mellmann A, Friedrich AW, Mevius D, et al Carbapenem-resistant Enterobacteriaceae in wildlife, food-producing, and companion animals: a systematic review . Clinical Microbiology and Infection. 2018;24(12):1241–1250. doi: 10.1016/j.cmi.2018.04.004. [DOI] [PubMed] [Google Scholar]
- 15.Li RC, Xie MM, Liu LZ, Huang YL, Wu XY, Wang ZQ, et al Characterisation of a cointegrate plasmid harbouring blaNDM-1 in a clinical Salmonella Lomita strain . International Journal of Antimicrobial Agents. 2020;55(1):105817. doi: 10.1016/j.ijantimicag.2019.09.021. [DOI] [PubMed] [Google Scholar]
- 16.Liu BT, Song FJ, Zou M, Zhang QD, Shan H High incidence of Escherichia coli strains coharboring mcr-1 and blaNDM from chickens . Antimicrobial Agents and Chemotherapy. 2017;61(3):e02347–16. doi: 10.1128/AAC.02347-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Long HY, Feng Y, Ma K, Liu L, McNally A, Zong ZY. 2019. The co-transfer of plasmid-borne colistin-resistant genes mcr-1 and mcr-3.5, the carbapenemase gene blaNDM-5 and the 16S methylase gene rmtB from Escherichia coli. Scientific Reports, 9(1): 696.
- 18.Lü Y, Kang HQ, Fan JM A novel blaCTX-M-65-Harboring IncHI2 plasmid pE648CTX-M-65 isolated from a clinical extensively-drug-resistant Escherichia coli ST648 . Infection and Drug Resistance. 2020;13:3383–3391. doi: 10.2147/IDR.S269766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ma TF, Fu JN, Xie N, Ma SZ, Lei L, Zhai WS, et al. 2020. Fitness cost of blaNDM-5-carrying p3R-IncX3 plasmids in wild-type NDM-free Enterobacteriaceae. Microorganisms, 8(3): 377.
- 20.Ma ZB, Zeng ZL, Liu J, Liu C, Pan Y, Zhang YA, et al Emergence of IncHI2 plasmid-harboring blaNDM-5 from porcine Escherichia coli isolates in Guangdong, China . Pathogens. 2021;10(8):954. doi: 10.3390/pathogens10080954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nakayama T, Hoa TTT, Huyen HM, Yamaguchi T, Jinnai M, Minh DTN, et al Isolation of carbapenem-resistant Enterobacteriaceae harbouring NDM-1, 4, 5, OXA48 and KPC from river fish in Vietnam . Food Control. 2022;133:108594. doi: 10.1016/j.foodcont.2021.108594. [DOI] [Google Scholar]
- 22.Oueslati S, Emeraud C, Grosperrin V, Levy M, Cotellon G, Creton E, et al Polyclonal dissemination of NDM-1- and NDM-9-producing Escherichia coli and Klebsiella pneumoniae in French Polynesia . Antimicrobial Agents and Chemotherapy. 2021;65(4):e02437–20. doi: 10.1128/AAC.02437-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Piégu B, Bire S, Arensburger P, Bigot Y A survey of transposable element classification systems-a call for a fundamental update to meet the challenge of their diversity and complexity. Molecular Phylogenetics and Evolution. 2015;86:90–109. doi: 10.1016/j.ympev.2015.03.009. [DOI] [PubMed] [Google Scholar]
- 24.Poirel L, Walsh TR, Cuvillier V, Nordmann P Multiplex PCR for detection of acquired carbapenemase genes. Diagnostic Microbiology and Infectious Disease. 2011;70(1):119–123. doi: 10.1016/j.diagmicrobio.2010.12.002. [DOI] [PubMed] [Google Scholar]
- 25.Schürch AC, Arredondo-Alonso S, Willems RJL, Goering RV Whole genome sequencing options for bacterial strain typing and epidemiologic analysis based on single nucleotide polymorphism versus gene-by-gene-based approaches. Clinical Microbiology and Infection. 2018;24(4):350–354. doi: 10.1016/j.cmi.2017.12.016. [DOI] [PubMed] [Google Scholar]
- 26.Shen YB, Zhang R, Schwarz S, Wu CM, Shen JZ, Walsh TR, et al Farm animals and aquaculture: significant reservoirs of mobile colistin resistance genes. Environmental Microbiology. 2020;22(7):2469–2484. doi: 10.1111/1462-2920.14961. [DOI] [PubMed] [Google Scholar]
- 27.Tian DX, Wang BJ, Zhang H, Pan F, Wang C, Shi YY, et al Dissemination of the blaNDM-5 gene via IncX3-type plasmid among Enterobacteriaceae in Children . mSphere. 2020;5(1):e00699–19. doi: 10.1128/mSphere.00699-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wang J, Ma ZB, Zeng ZL, Yang XW, Huang Y, Liu JH The role of wildlife (wild birds) in the global transmission of antimicrobial resistance genes. Zoological Research. 2017;38(2):55–80. doi: 10.24272/j.issn.2095-8137.2017.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wang J, Xia YB, Huang XY, Wang Y, Lv LC, Lin QQ, et al Emergence of blaNDM-5 in Enterobacteriaceae isolates from companion animals in Guangzhou, China . Microbial Drug Resistance. 2021a;27(6):809–815. doi: 10.1089/mdr.2020.0210. [DOI] [PubMed] [Google Scholar]
- 30.Wang MG, Yu Y, Wang D, Yang RS, Jia L, Cai DT, et al The emergence and molecular characteristics of New Delhi Metallo β-lactamase-producing Escherichia coli from ducks in Guangdong, China . Frontiers in Microbiology. 2021b;12:677633. doi: 10.3389/fmicb.2021.677633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wang ZY, Xu HY, Tang YY, Li QC, Jiao XA A multidrug-resistant monophasic Salmonella Typhimurium Co-harboring mcr-1, fosA3, blaCTX-M-14 in a transferable IncHI2 plasmid from a healthy catering worker in China . Infection and Drug Resistance. 2020;13:3569–3574. doi: 10.2147/IDR.S272272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wick RR, Judd LM, Gorrie CL, Holt KE Unicycler: resolving bacterial genome assemblies from short and long sequencing reads. PLoS Computational Biology. 2017;13(6):e1005595. doi: 10.1371/journal.pcbi.1005595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wong MHY, Chan EWC, Chen S. 2017. IS26-mediated formation of a virulence and resistance plasmid in Salmonella Enteritidis. Journal of Antimicrobial Chemotherapy, 72(10): 2750–2754.
- 34.Wu WJ, Feng Y, Tang GM, Qiao F, McNally A, Zong ZY NDM Metallo-β-lactamases and their bacterial producers in health care settings. Clinical Microbiology Reviews, 32(2): e00115-18. Xu CY, Lv ZQ, Shen YB, Liu DJ, Fu YL, Zhou L, et al. 2020. Metagenomic insights into differences in environmental resistome profiles between integrated and monoculture aquaculture farms in China . Environment International. 2019;144:106005. doi: 10.1016/j.envint.2020.106005. [DOI] [PubMed] [Google Scholar]
- 35.Yao H, Li AJ, Yu RH, Schwarz S, Dong HY, Du XD Multiple copies of blaNDM-5 located on conjugative megaplasmids from porcine Escherichia coli sequence type 218 isolates . Antimicrobial Agents and Chemotherapy. 2020;64(5):e02134–19. doi: 10.1128/AAC.02134-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yong D, Toleman MA, Giske CG, Cho HS, Sundman K, Lee K, et al Characterization of a new metallo-β-lactamase gene, blaNDM-1, and a novel erythromycin esterase gene carried on a unique genetic structure in Klebsiella pneumoniae sequence type 14 from India . Antimicrobial Agents and Chemotherapy. 2009;53(12):5046–5054. doi: 10.1128/AAC.00774-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zankari E, Hasman H, Cosentino S, Vestergaard M, Rasmussen S, Lund O, et al Identification of acquired antimicrobial resistance genes. Journal of Antimicrobial Chemotherapy. 2012;67(11):2640–2644. doi: 10.1093/jac/dks261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhang QH, Lv LC, Huang XY, Huang Y, Zhuang ZL, Lu JX, et al Rapid increase in carbapenemase-producing Enterobacteriaceae in retail meat driven by the spread of the blaNDM-5-carrying IncX3 plasmid in China from 2016 to 2018 . Antimicrobial Agents and Chemotherapy. 2019;63(8):e00573–19. doi: 10.1128/AAC.00573-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhao Q, Berglund B, Zou HY, Zhou ZY, Xia HY, Zhao L, et al Dissemination of blaNDM-5 via IncX3 plasmids in carbapenem-resistant Enterobacteriaceae among humans and in the environment in an intensive vegetable cultivation area in eastern China . Environmental Pollution. 2021a;273:116370. doi: 10.1016/j.envpol.2020.116370. [DOI] [PubMed] [Google Scholar]
- 40.Zhao QY, Zhu JH, Cai RM, Zheng XR, Zhang LJ, Chang MX, et al IS26 is responsible for the evolution and transmission of blaNDM-harboring plasmids in Escherichia coli of poultry origin in China . mSystems. 2021b;6(4):e0064621. doi: 10.1128/mSystems.00646-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhi CP, Lv LC, Yu LF, Doi Y, Liu JH Dissemination of the mcr-1 colistin resistance gene . The Lancet Infectious Diseases. 2016;16(3):292–293. doi: 10.1016/S1473-3099(16)00063-3. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets in this study can be found in NCBI under BioProjectID PRJNA636005. The raw sequence data reported in this paper have been deposited in the Genome Sequence Archive (Genomics, Proteomics & Bioinformatics 2021) in the National Genomics Data Center (Nucleic Acids Res 2021), China National Center for Bioinformation/Beijing Institute of Genomics, Chinese Academy of Sciences (GSA: CRA005844), publicly accessible at https://ngdc.cncb.ac.cn/gsa.