Some 10 years ago, in early 2012, we started discussing the establishment of the National Research Facility for Phenotypic and Genetic Analysis of Model Animals (Primate Facility). Even though the Primate Facility is still under construction, the rapid passing of a decade is a good excuse to look back and reflect for a moment.
As the Director General of the host institution (Kunming Institute of Zoology (KIZ), Chinese Academy of Sciences (CAS)) and the General Manager of this ambitious construction project, I strongly recall the difficulties faced by the entire team as well as the great support we received from many different sides over the last 10 years. One of the most significant challenges we faced in establishing this high-throughput analysis and research network for living monkeys was the continuous collation and design of the entire facility and the pressure to turn the engineering drawings into reality, with consideration of all services, equipment, structures, and buildings required to not only meet the needs of the primates but also the needs of the researchers and staff. This was a herculean task, especially during the COVID-19 pandemic, given that our team members were mainly trained as molecular biologists, not engineers, and we lacked experts for these technically specific tasks. I still vividly recall my anxiety when presenting reports on the essentiality, importance, and feasibility of the design and construction of the Primate Facility to various review committees and leaders of CAS and related state departments. Nonetheless, I appreciate those tough times as we greatly benefited from the constructive comments, feedback, and encouragement. After each round of reviews, we refined the proposal and transformed many conceptual ideas into a well-defined design that gradually became reality. We were very fortunate to have received such great support and help in establishing the Primate Facility. As well as the inherent project difficulties, the entire construction team also had to overcome many unexpected complications caused by the COVID-19 pandemic, while maintaining good control of the budget. However, it is inspiring to look back on these challenges and our ultimate success.
During the initial proposal for the establishment of the Primate Facility, supported by the “12th Five-Year” National Major Scientific and Technological Infrastructure Projects of China, we conducted preliminary negotiations with another team from the China Agricultural University (CAU) in Beijing, who wished to establish a similar research facility dedicated to the study of porcines (Pig Facility) in Zhuozhou, Hebei Province, using the same financial support. These initial negotiations were difficult as we attempted to resolve whether the KIZ and CAU teams were competitors or collaborators in pursuing these ambitious projects on large animals. Fortunately, under the guidance and help of CAS leaders, with the dedication and hard work of both teams, and with the support of KIZ researchers and staff, we solved these issues such that both facilities could be constructed concurrently and independently by each side under the support of the same project. The project proposals, feasibility reports, and preliminary designs of the Pig and Primate facilities were approved by the National Development and Reform Commission in 2016, 2017, and 2018, respectively. Each official approval constituted extensive reviews, presentations, defenses, dialogues, and revisions of the proposals, as well as the social responsibilities of scientists and officials, thus reflecting the benefits of a nationwide system concentrating on research and resources for key national undertakings. After all essential preconditions were finally completed, we announced the commencement of infrastructure construction on 27 October 2019, with the Primate Facility scheduled for completion and operation in 2023.
I must say that my understanding of the importance of non-human primate (NHP) research has expanded and strengthened each time I have given a presentation on the essentialness of the facility based on accumulating evidence. Indeed, NHPs have many advantages over other experimental animals in advancing biomedical research, especially the modeling of neurodegenerative and infectious diseases, and in understanding human beings, given the high degree of similarity in respect to genetics, anatomy, physiology, behavior, emotion, and cognitive function (Capitanio & Emborg, 2008; Feng et al., 2020; Roelfsema & Treue, 2014; Phillips et al., 2014). To elucidate the puzzles of human mental disorders and infectious diseases, including COVID-19 (Bi et al., 2021; Muñoz-Fontela et al., 2020), and for vaccine evaluation and new drug development, NHPs constitute irreplaceable and in many ways superior models compared with common experimental animals such as rodents (Capitanio & Emborg, 2008; Feng et al., 2020; Roelfsema & Treue, 2014; Phillips et al., 2014). Using NHPs to clarify the mechanisms underpinning genotypes and phenotypes will undoubtedly improve our understanding of complex traits and human diseases, as well as the responses of biological processes to environmental factors. On this point, NHPs constitute the perfect living template for us humans to understand ourselves. The social, cultural, and ecological importance of NHPs is also well recognized in the field. Thus, there is no doubt that NHPs are important, not only for humans and our evolution but for the planet as a whole (Estrada et al., 2017).
The Primate Facility aims to be a world-class large-scale integrated research platform for the study of genetic and phenotypic patterns in NHPs, particularly rhesus (Macaca mulatta) and cynomolgus macaques (M. fascicularis). KIZ has several advantages for achieving this task. Notably, KIZ started to use NHPs for biomedical research in the 1960s and has a long tradition of research in primate behavior, cognition, brain science, animal models, and conservation genomics (Yao & Shen, 2019). KIZ researchers and collaborators have made impressive achievements using NHPs, including the creation of the first transgenic monkeys in China (Niu et al., 2010) and first transgenic monkey model with the human MCPH1 gene for studying neoteny (Shi et al., 2019); generation of the first monkey model showing drastic brain volume loss due to depletion of giant ANK2 (Qin et al, 2021); identification of the first cynomolgus monkey with naturally occurring Parkinson’s disease (Li et al., 2021a, 2021b); assembly of a high-quality reference genome for rhesus monkeys and elucidation of the evolutionary innovations during primate corticogenesis (He et al., 2019; Luo et al., 2021); description of a new species of Hoolock gibbon (Hoolock tianxing) (Fan et al., 2017); and establishment of a good example on primate conservation genomics (Yu et al., 2016). Researchers at KIZ have also pioneered studies on tree shrews, a close relative and promising alternative to NHPs (Yao, 2017), and created the first transgenic tree shrews (Li et al., 2017). Currently, KIZ houses more than 2600 NHPs belonging to seven species, including rhesus monkeys, cynomolgus monkeys, pig-tailed monkeys (M. leonina), and Yunnan snub-nosed monkeys (Rhinopithecus bieti). In 2008, the KIZ Primate Research Center received full accreditation from the International Association for the Assessment and Accreditation of Laboratory Animal Care (AAALAC), and in 2018 became the first experimental animal institution to be accredited by the China National Accreditation Committee (CNAS). Through endless effort over the past six decades, KIZ has strived to establish and maintain international standards and regulations related to the management of NHP breeding and veterinary and surgical practices, ensuring that all experimental protocols comply with the latest requirements in the field, especially animal welfare considerations and ethical issues. Furthermore, the natural climatic conditions and abundant NHP resources in Kunming and surrounding regions have not only helped ensure successful breeding and maintenance of NHPs but also their sustainable development. Nonetheless, despite this excellent foundation, there was no guarantee our team would be selected to carry out the Primate Facility project, with multiple competitors and organizations advancing NHP research in China and worldwide (Cyranoski, 2016). Thus, I was very honored that our group in Kunming was selected to establish the facility, with great input from different experts from KIZ and CAS.
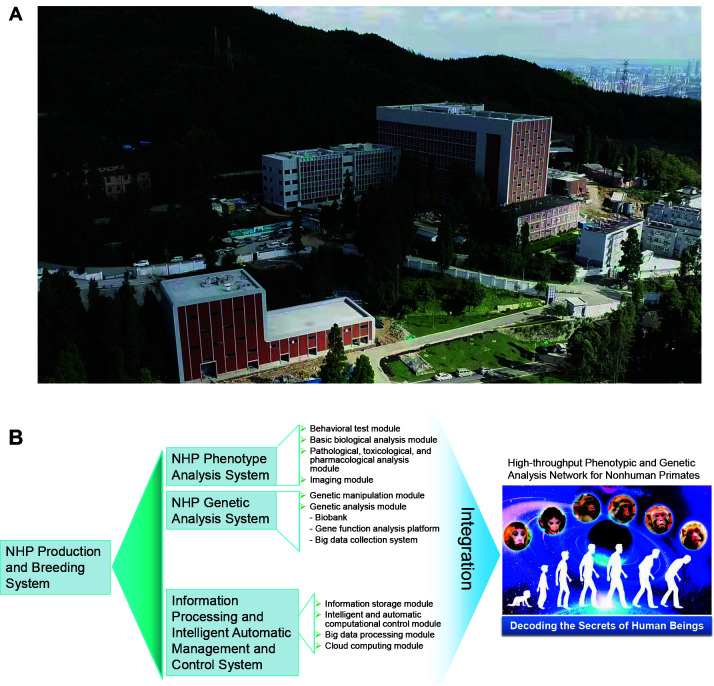
From the very beginning, the Primate Facility was designed to provide substantial technical support for both basic and advanced biomedical research using NHPs. It will carry out phenotypic and genetic analyses of NHPs and establish various analytical tools for using “big data” to solve key questions related to human health, including complex diseases and interactions between genetic and environmental factors during the development of life, as well as the evolution of human traits and behaviors. The Primate Facility will support comprehensive phenome, genome, epigenome, transcriptome, proteome, and metabolome study of NHPs, with a full-scale landscape from molecules to cells, tissues to whole bodies, and embryogenesis to adult behavior. To fulfill these tasks, the facility was designed to contain four systems: System I – NHP production and breeding system, which provides large-scale standardized production and breeding of NHPs with information management. System II – NHP phenotype analysis system, which collects data from biological, behavioral, pathological, toxicological, and pharmacological studies using NHPs, as well as data from molecular and anatomical imaging studies. System III – NHP genetic analysis system, which is composed of technical platforms for collecting comprehensive genetic data from NHPs and for genetic modification and manipulation of NHPs using cutting-edge gene-editing technology. System IV – Information processing and intelligent automatic management and control system, which supplies multiple functions for information storage, intelligent and automatic computational control, big data processing, and cloud computing services. Overall, the Primate Facility will provide a cutting-edge platform for the accurate and automatic collection and analysis of phenotypic and genetic data based on large-scale standardized production and breeding colonies of NHPs (Figure 1). These integrated systems will undoubtedly facilitate research on NHP models of human diseases and NHP biology and conservation.
Figure 1.
National Research Facility for Phenotypic and Genetic Analysis of Model Animals (Primate Facility) in Kunming
A: A bird’s eye view of the facility buildings. B: Schematic of the large-scale integrated research platform for the study of genetic and phenotypic patterns in monkeys.
With the construction of the Primate Facility, it is also exciting to plan and consider projects that can be initiated by the facility ahead of time. As such, we are now ready to accept proposals and develop domestic and international collaborations, focusing on the key questions and frontiers of science, urgent needs at the regional, national, and international levels, and essential services for sustainable economic development, while ensuring collaborative equality and mutual benefits. Indeed, there are many interesting questions that remain to be answered. For instance, how to construct valid NHP animal models for Alzheimer’s and Parkinson’s disease, how to establish monkey spermatogonial stem cells in culture and create highly efficient gene-editing systems for the generation of genetically modified monkeys, how to understand neurobehavioral outcomes resulting from genotype-genotype and genotype-environment interactions, and how to simulate the aging process of humans using NHPs. Thus, NHPs are “a small but indispensable component of biomedical research……with far-reaching relevance that is irreplaceable for essential insights into cognitive functions, brain disease, and therapy” (Roelfsema & Treue, 2014). Recently, my colleagues and collaborators launched the ambitious Primate Genome Project (PGP) (Wu et al., 2022) with the Primate Facility, which aims to uncover the secrets of primate genomes. The launch of the PGP constitutes an excellent example of global collaboration, with experts working together to solve some of the biggest questions in the field. With your support, input, and expertise, we aim to build an open facility that is at the peak of the field. As such, we warmly welcome experts and friends from across the world. We are confident that the Primate Facility will become a high-profile and innovative center for basic and applied research and for profound international collaboration.
COMPETING INTERESTS
The author declares no competing interests.
AUTHOR'S CONTRIBUTION
Y.G.Y. wrote and approved the final version of the manuscript.
ACKNOWLEDGEMENTS
I would like to thank all KIZ researchers and staff, especially those from the Primate Facility Construction Office and Engineering Department, as well as experts from the Institute of Automation of CAS (Prof. Hong Qiao, Prof. Jie Tian, and their teams) for their input with the proposal and construction of the Primate Facility. I am also very grateful to the workers and managers of those companies responsible for the construction of the buildings and establishment of technical platforms. I am grateful to Dr. Christine Watts for language editing.
References
- 1.Bi Z, Hong W, Yang J, Lu S, Peng X Animal models for SARS-CoV-2 infection and pathology. Medicine Communications. 2021;2(4):548–568. doi: 10.1002/mco2.98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Capitanio JP, Emborg ME Contributions of non-human primates to neuroscience research. Lancet. 2008;371(9618):1126–1135. doi: 10.1016/S0140-6736(08)60489-4. [DOI] [PubMed] [Google Scholar]
- 3.Cyranoski D Monkey kingdom. Nature. 2016;532(7599):300–302. doi: 10.1038/532300a. [DOI] [PubMed] [Google Scholar]
- 4.Estrada A, Garber PA, Rylands AB, Roos C, Fernandez-Duque E, Di Fiore A, et al Impending extinction crisis of the world's primates: Why primates matter. Science Advances. 2017;3(1):e1600946. doi: 10.1126/sciadv.1600946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fan PF, He K, Chen X, Ortiz A, Zhang B, Zhao C, et al Description of a new species of Hoolock gibbon (Primates: Hylobatidae) based on integrative taxonomy . American Journal of Primatology. 2017;79(5):e22631. doi: 10.1002/ajp.22631. [DOI] [PubMed] [Google Scholar]
- 6.Feng G, Jensen FE, Greely HT, Okano H, Treue S, Roberts AC, et al Opportunities and limitations of genetically modified nonhuman primate models for neuroscience research. Proceedings of the National Academy of Sciences of the United States of America. 2020;117(39):24022–24031. doi: 10.1073/pnas.2006515117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.He YX, Luo X, Zhou B, Hu T, Meng XY, Audano PA, et al Long-read assembly of the Chinese rhesus macaque genome and identification of ape-specific structural variants. Nature Communications. 2019;10(1):4233. doi: 10.1038/s41467-019-12174-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Li CH, Yan LZ, Ban WZ, Tu Q, Wu Y, Wang L, et al Long-term propagation of tree shrew spermatogonial stem cells in culture and successful generation of transgenic offspring. Cell Research. 2017;27(2):241–252. doi: 10.1038/cr.2016.156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Li H, Su LY, Yang LX, Li M, Liu QJ, Li ZH, et al A cynomolgus monkey with naturally occurring Parkinson's disease. National Science Review. 2021a;8(3):nwaa292. doi: 10.1093/nsr/nwaa292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Li H, Yao YG, Hu XT Biological implications and limitations of a cynomolgus monkey with naturally occurring Parkinson's disease. Zoological Research. 2021b;42(2):138–140. doi: 10.24272/j.issn.2095-8137.2021.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Luo X, Liu YT, Dang DC, Hu T, Hou YP, Meng XY, et al 3D genome of macaque fetal brain reveals evolutionary innovations during primate corticogenesis. Cell. 2021;184(3):723–740.E21. doi: 10.1016/j.cell.2021.01.001. [DOI] [PubMed] [Google Scholar]
- 12.Muñoz-Fontela C, Dowling WE, Funnell SGP, Gsell PS, Riveros-Balta AX, Albrecht RA, et al Animal models for COVID-19. Nature. 2020;586(7830):509–515. doi: 10.1038/s41586-020-2787-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Niu YY, Yu Y, Bernat A, Yang SH, He XC, Guo XY, et al Transgenic rhesus monkeys produced by gene transfer into early-cleavage-stage embryos using a simian immunodeficiency virus-based vector. Proceedings of the National Academy of Sciences of the United States of America. 2010;107(41):17663–17667. doi: 10.1073/pnas.1006563107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Phillips KA, Bales KL, Capitanio JP, Conley A, Czoty PW, 't Hart BA, et al Why primate models matter. American Journal of Primatology. 2014;76(9):801–827. doi: 10.1002/ajp.22281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Qin DD, Zhou JK, He XC, Shen XY, Li C, Chen HZ, et al Depletion of giant ANK2 in monkeys causes drastic brain volume loss. Cell Discovery. 2021;7(1):113. doi: 10.1038/s41421-021-00336-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Roelfsema PR, Treue S Basic neuroscience research with nonhuman primates: a small but indispensable component of biomedical research. Neuron. 2014;82(6):1200–1204. doi: 10.1016/j.neuron.2014.06.003. [DOI] [PubMed] [Google Scholar]
- 17.Shi L, Luo X, Jiang J, Chen YC, Liu CR, Hu T, et al Transgenic rhesus monkeys carrying the human MCPH1 gene copies show human-like neoteny of brain development . National Science Review. 2019;6(3):480–493. doi: 10.1093/nsr/nwz043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wu DD, Qi XG, Yu L, Li M, Liu ZJ, Yoder AD, et al Initiation of the Primate Genome Project. Zoological Research. 2022;43(2):147–149. doi: 10.24272/j.issn.2095-8137.2022.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yao YG Creating animal models, why not use the Chinese tree shrew (Tupaia belangeri chinensis) . Zoological Research. 2017;38(3):118–126. doi: 10.24272/j.issn.2095-8137.2017.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yao YG, Shen H From our roots, we grow. Zoological Research. 2019;40(6):471–475. doi: 10.24272/j.issn.2095-8137.2019.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yu L, Wang GD, Ruan J, Chen YB, Yang CP, Cao X, et al Genomic analysis of snub-nosed monkeys (Rhinopithecus) identifies genes and processes related to high-altitude adaptation . Nature Genetics. 2016;48(8):947–952. doi: 10.1038/ng.3615. [DOI] [PubMed] [Google Scholar]