Hydrostatic pressure is one of the main factors limiting the vertical range of marine organisms. However, little is known about the level of hydrostatic pressure that shallow-water fish can tolerate or how they respond to this potential stress. Here, we applied high-hydrostatic pressure treatment to wild-type AB line zebrafish (Danio rerio) and analyzed transcriptome data following treatment. Results showed that zebrafish died at 12.5 MPa exposure (close to the pressure at 1 250 m underwater), and the highest pressure at which fish survived was 18 MPa (close to the pressure at 1 800 m). Based on the transcriptome data, gene expression patterns were greatly altered in the treated samples, although differentially expressed genes differed across tissues. Among the four tissues investigated, the ovary was the most stable, while the brain was the most sensitive to high hydrostatic pressure. We also observed that gene expression stability decreased in the treated samples. Our research provides basic data for predicting the migration of fish to the deep sea and for understanding the adaptive mechanisms of deep-sea fish.
Hydrostatic pressure is the central factor limiting the vertical distribution of marine organisms (Young et al., 1997). For every 10 m of descent in the ocean, the hydrostatic pressure increases, on average, by about one atmosphere (atm, ~0.1 MPa). Thus, deep seas are a formidable challenge for human exploration as well as marine fish distribution (Young et al., 1997). Microbe-based studies have shown that high hydrostatic pressure can decrease membrane fluidity and increase protein chaperoning, resulting in compromised physiological function (Manisegaran et al., 2019). In addition, high hydrostatic pressure can strengthen hydrogen bonds, thereby impeding DNA replication, transcription, and translation (Yayanos & Pollard, 1969). Deep-sea organisms have evolved various mechanisms to adapt to these adverse factors (Feng et al., 2021; Wang et al., 2019), but adaptability in shallow-water fish remains unclear.
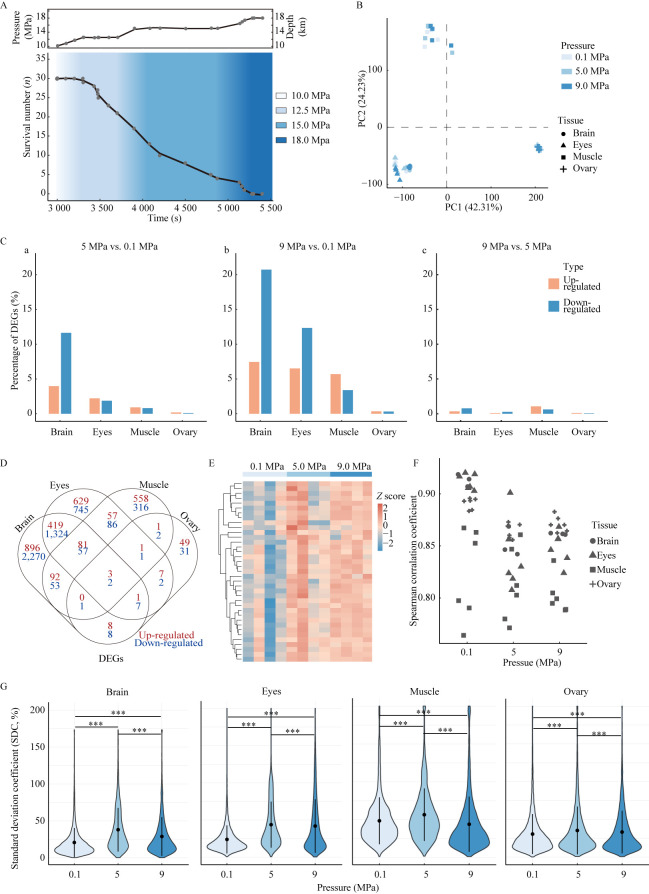
Zebrafish are a model species distributed in shallow waters (Beliaeva et al., 2010; Spence et al., 2006). Thus, we chose the AB zebrafish line to test responses to high hydrostatic pressure. To exclude potential effects of sex and maturity, we chose only female adults. The experiment was performed in a pressure vessel (FY-24-40, AILIPU Science and Technology, China), which provided stable and controllable high hydrostatic pressure. To adapt to the experimental environment, all wild-type zebrafish were maintained in zebrafish facilities at about 25 ℃ for 10 days. Experimental individuals were starved for 1 day before the pressurization experiment. We first placed 30 female individuals in the pressure vessel and treated them with gradually increasing hydrostatic pressure. No fish died until the pressure reached 12.5 MPa (close to the pressure at 1 250 m depth underwater). The pressure was held at this level for 10 min, during which time eight individuals died. The pressure was then increased to 15 MPa and held for 20 min, resulting in 18 deaths but the survival of three fish. The pressure was increased again, and the last individual died at 18 MPa (Figure 1A). Dissolved oxygen (DO) in the water was measured using a YSI Professional Plus (YSI, USA), and was 10.06±0.02 mg/L before the experiment and 5.70±0.02 mg/L after the experiment (Supplementary Figure S1A), indicating that the zebrafish did not die of hypoxia. Through the entire pressurization process, the temperature remained relatively constant (~25 ℃), as recorded by the pressure vessel. From the above results, we concluded that zebrafish easily tolerated pressures up to 10 MPa (100 atm, ~1 000 m depth underwater), but higher hydrostatic pressure led to eventual mortality, although the degree of tolerance varied among individuals.
Figure 1.
Hydrostatic pressure exposure and transcriptome data analysis in zebrafish
A: Fitted curves of pressure and zebrafish survival number against experimental time. B: Cluster diagram of principal component analysis for all samples. C: Proportion of up- and down-regulated DEGs among all selected genes in each tissue. a: Proportion of DEGs in 5 MPa-treated group vs. 0.1 MPa-treated control; b: Proportion of DEGs in 9 MPa-treated group vs. 0.1 MPa-treated control; c: Proportion of DEGs in 9 MPa-treated group vs. 5 MPa-treated group. D: Venn diagram of up- and down-regulated DEGs in 5 MPa-treated group vs. 0.1 MPa-treated control and 9 MPa-treated group vs. 0.1 MPa-treated control. E: Heatmap of βγ-crystallin gene family expression in eyes. F: Distribution of Spearman correlation coefficients for samples within groups of each tissue. G: Violin diagram of standard deviation coefficient (SDC) distribution for each tissue (***: P<0.001, KS-test).
Subsequently, zebrafish were exposed to high pressure for 4 h (5 MPa and 9 MPa, separately) to test responses at the gene expression level. Dissolved oxygen content in the water before and after each treatment was measured to ensure the fish were not affected by hypoxia (Supplementary Figure S1A). For each fish, we collected four tissues (brain, muscle, eyes, and ovary), including three to four biological replicates per tissue per condition. We obtained a total of 45 samples and generated more than one million raw Illumina reads from RNA sequencing (RNA-seq) (Supplementary Table S1).
We used fastp, Scythe, and Sickle software to filter the raw data to remove low-quality reads and reads with adaptor sequences, resulting in at least 5.5 Gb of clean data per sample after filtering (Supplementary Table S1). We used HISAT2 to map the high-quality reads to the zebrafish reference genome (GRCz11, May 2017). Overall, ~93% (89%–95% for individual samples) of high-quality reads were mapped to the genome (Supplementary Figure S1B). StringTie software was used to quantify the expression of 25 428 protein-coding genes (Transcripts Per Kilobase Million (TPM)). To avoid the effect of low-expression genes on analyses, we only retained those genes with an average TPM>1 for each tissue. In total, 17 213, 17 356, 13 436, and 14 539 genes were retained for further analysis from the brain, eyes, muscle, and ovary, respectively.
Hierarchical clustering and principal component analysis (PCA) (Figure 1B; Supplementary Figure S2A) indicated that the samples were well clustered by tissue, suggesting that high hydrostatic pressure did not greatly alter their gene expression patterns. The PCA of single tissue samples showed that, except for the brain, the pressure-treated samples could not be well distinguished from the control sample (Supplementary Figure S2B). We next identified differentially expressed genes (DEGs) between the control and experimental groups using the R package DESeq2 with |log2(foldchange)|≥1 and corrected P<0.05 (Supplementary Tables S2-S4). Consistently, we identified many more DEGs in the brain than in the other tissues (Supplementary Table S5). Spearman correlation analysis within tissues also supported the above observations, i.e., the brain was most sensitive to hydrostatic pressure and the ovary was most stable (Supplementary Figure S3).
In the brain, there were 465 up-regulated and 1 849 down-regulated DEGs shared in the 5 MPa-treated group vs. 0.1 MPa-treated control and the 9 MPa-treated group vs. 0.1 MPa-treated control (Supplementary Tables S5–S7). We performed functional enrichment analysis with the R package clusterProfiler and the online database (org.Dr.eg.db) for zebrafish. The R package GOSemSim was then used to reduce the redundancy of enriched Gene Ontology (GO) terms. The significantly enriched GO terms were related to transmembrane transport (e.g., “regulation of transport” and “metal ion transmembrane transporter activity”), activator activity (e.g., “GABA receptor activity” and “enzyme activator activity”), molecular binding (e.g., “GTPase binding” and “calmodulin binding”), synaptic signaling (e.g., “anterograde trans-synaptic signaling” and “gamma-aminobutyric acid signaling pathway”), and protein complex oligomerization (Supplementary Table S8). Moreover, 817 up-regulated and 1 720 down-regulated DEGs were induced in the 9 MPa-treated group compared with the 0.1 MPa-treated control. GO analysis showed that these DEGs were enriched in “response to temperature stimulus”, “structural constituent of ribosome”, and “ubiquitin-like protein ligase activity” (Supplementary Table S9). These results suggest that the response to hydrostatic pressure was similar to the response to temperature, although they have different effects on organisms (Mozhaev et al., 1996).
Among the 1 130 DEGs up-regulated in the eyes of the 9 MPa-treated group compared with the 0.1 MPa-treated control group, significantly enriched GO terms included “lens development in camera-type eye”, “visual perception”, “sensory perception of light stimulus”, and “regulatory region nucleic acid binding” (Supplementary Table S10). Crystallins play important roles in the structural integrity of eyes and in visual acuity. It has been shown that γ-crystallin is much more sensitive to pressure than the folded states of globular proteins (Cinar et al., 2019). However, abnormal crystallins can cause light scattering and lens opacity, which can affect vision. In the current study, many βγ-crystallin genes were up-regulated in the eye tissue under 9 MPa treatment (Figure 1E), implying that the weak eyesight of deep-sea fish is not only related to the lightless environment but may also be related to high hydrostatic pressure.
Interestingly, only five DEGs were shared by all four tissues and 149 DEGs were shared by three tissues in the high-pressure groups compared with the control (Figure 1D), suggesting that different tissues respond differently to hydrostatic pressure. We identified 80 up-regulated and 55 down-regulated DEGs shared by at least three tissues in the 9 MPa-treated group compared with the 0.1 MPa-treated control (Supplementary Figure S4A). These genes were only enriched in one GO term, i.e., “negative regulation of transcription, DNA-templated” (GO:0045892), implying that hydrostatic pressure may lead to the disruption of gene expression.
To test this hypothesis, we determined the stability of gene expression in each group. Results showed that the Spearman rank correlation coefficient in the control group was significantly higher than that in the hydrostatic pressure treatment group (Figure 1F), suggesting that the samples showed higher differential expression as pressure increased. Except for muscle, the Spearman distribution of tissues differed significantly (Student t-test P<0.05) between the control (0.1 MPa) and treatment groups (5 MPa and 9 MPa; Supplementary Figure S5). This coincides with the variation in the number of differential genes described above. A standard deviation coefficient was used to indicate differences in gene expression in samples within the experimental groups. Results showed significant differences in the 0.1 MPa-treated control vs. 5 MPa-treated group, 0.1 MPa-treated control vs. 9 MPa-treated group, and 5 MPa-treated group vs. 9 MPa-treated group for all four tissues based on the two-sample Kolmogorov-Smirnov test (P<0.001), with the trend 5 MPa>9 MPa>0.1 MPa (Figure 1G).
In the current study, we explored the ability of zebrafish to adapt to hydrostatic pressure and investigated their response at the transcriptional level. Results showed that zebrafish can adapt to hydrostatic stress at ~10 MPa (i.e., ~1 000 m depth underwater). However, differences were observed in different tissues, as manifested at the transcriptional level, coinciding with a decrease in transcriptional stability, suggesting that increased transcriptional stability may be an important step in the adaptation of fish to the deep-sea environment. However, our study has some limitations. Firstly, the species selected is not representative of all euryhaline fish taxa. Secondly, we selected only female adult fish, and it remains unknown whether sex or age will affect the results. Thirdly, our pressure incubation time was relatively short (4 h) due to the limitations of our equipment, i.e., small and too little oxygen to support longer treatment, and better equipment may be necessary for future studies. Finally, we did not examine the transcriptome response at higher hydrostatic pressures due to the mass mortality of individuals at 12 MPa. The transcriptional response at this stage may not be a true response to hydrostatic pressure, and thus the transcriptome response of zebrafish to extreme hydrostatic pressure remains unknown. Nevertheless, our study provides new data for understanding high-pressure adaptation in fish and their ability to migrate to deep waters. Our results suggest that hydrostatic pressure should not be a great barrier for fish descending to depths of up to 1 km.
DATA AVAILABILITY
Clean transcriptome sequence data are available from the Sequence Read Archive Database of the National Center for Biotechnology Information (BioProjectID PRJNA764015).
SUPPLEMENTARY DATA
The online version of this article contains supplementary material, which is available to authorized users.
COMPETING INTERESTS
The authors declare that they have no competing interests.
AUTHORS’ CONTRIBUTIONS
K.W., Q.Q., B.S.W., and C.G.F. designed the study. M.L.H., B.S.W., W.Q.L., and H.B.Z. collected the samples and performed the laboratory work. M.L.H., C.L.Z., W.J.X., and Y.Y. performed data analysis. M.L.H., C.G.F., B.S.W., and K.W. drafted the paper. H.B.Z., K.W., and C.G.F. revised the paper. All authors read and approved the final version of the manuscript.
ACKNOWLEDGMENTS
We thank Dr. Yang Zhou from the Institute of Deep-Sea Science and Engineering for expert support with data collection.
Funding Statement
This work was supported by the 1000 Talent Project of Shaanxi Province (to K.W.), National Natural Science Foundation of China (31801089 to K.W.), Research Funds for Interdisciplinary Subject of Northwestern Polytechnical University (19SH030408 to K.W.), China Postdoctoral Science Foundation (2021M693342 to C.G.F.), and Hubei Postdoctoral Innovation Post Project (to C.G.F.)
Contributor Information
Hai-Bin Zhang, Email: hzhang@idsse.ac.cn.
Kun Wang, Email: wangkun@nwpu.edu.cn.
Chen-Guang Feng, Email: fcg1989@126.com.
References
- 1.Beliaeva NF, Kashirtseva VN, Medvedeva NV, Khudoklinova II, Ipatova OM, Archakov AI. 2010. Zebrafish as a model organism for biomedical studies. Biomeditsinskaia Khimiia, 56(1): 120–131. (in Russian)
- 2.Cinar S, Cinar H, Chan HS, Winter R Pressure-sensitive and osmolyte-modulated liquid-liquid phase separation of eye-lens γ-crystallins. Journal of the American Chemical Society. 2019;141(18):7347–7354. doi: 10.1021/jacs.8b13636. [DOI] [PubMed] [Google Scholar]
- 3.Feng CG, Liu RY, Xu WJ, Zhou Y, Zhu CL, Liu J, et al The genome of a new anemone species (Actiniaria: Hormathiidae) provides insights into deep-sea adaptation. Deep Sea Research Part I:Oceanographic Research Papers. 2021;170:103492. doi: 10.1016/j.dsr.2021.103492. [DOI] [Google Scholar]
- 4.Manisegaran M, Bornemann S, Kiesel I, Winter R Effects of the deep-sea osmolyte TMAO on the temperature and pressure dependent structure and phase behavior of lipid membranes. Physical Chemistry Chemical Physics. 2019;21(34):18533–18540. doi: 10.1039/C9CP03812D. [DOI] [PubMed] [Google Scholar]
- 5.Mozhaev VV, Heremans K, Frank J, Masson P, Balny C High pressure effects on protein structure and function. Proteins. 1996;24(1):81–91. doi: 10.1002/(SICI)1097-0134(199601)24:1<81::AID-PROT6>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- 6.Spence R, Fatema MK, Reichard M, Huq KA, Wahab MA, Ahmed ZF, et al The distribution and habitat preferences of the zebrafish in Bangladesh. Journal of Fish Biology. 2006;69(5):1435–1448. doi: 10.1111/j.1095-8649.2006.01206.x. [DOI] [Google Scholar]
- 7.Wang K, Shen YJ, Yang YZ, Gan XN, Liu GC, Hu K, et al Morphology and genome of a snailfish from the Mariana Trench provide insights into deep-sea adaptation. Nature Ecology & Evolution. 2019;3(5):823–833. doi: 10.1038/s41559-019-0864-8. [DOI] [PubMed] [Google Scholar]
- 8.Yayanos AA, Pollard EC A study of the effects of hydrostatic pressure on macromolecular synthesis in escherichia coli. Biophysical Journal. 1969;9(12):1464–1482. doi: 10.1016/S0006-3495(69)86466-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Young CM, Tyler PA, Fenaux L Potential for deep sea invasion by Mediterranean shallow water echinoids: pressure and temperature as stage-specific dispersal barriers. Marine Ecology Progress Series. 1997;154:197–209. doi: 10.3354/meps154197. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The online version of this article contains supplementary material, which is available to authorized users.
Data Availability Statement
Clean transcriptome sequence data are available from the Sequence Read Archive Database of the National Center for Biotechnology Information (BioProjectID PRJNA764015).