Highlights
-
•
Recurrent metastatic choriocarcinoma has limited treatment protocols.
-
•
Molecular profiling of tumors can allow for targeted therapy.
-
•
Pembrolizumab can be used to retreat recurrence after complete response.
Keywords: Choriocarcinoma, Pembrolizumab, Retreatment, Recurrence
Abstract
Introduction
Recurrent metastatic choriocarcinoma is a rare disease with historically limited guidance in the literature regarding standardized treatment protocols. In this case report, we review the course of a patient with recurrent metastatic choriocarcinoma re-treated with single agent pembrolizumab.
Our patient was initially diagnosed with metastatic choriocarcinoma (FIGO Stage IV, WHO Score 13) after presenting for evaluation of amenorrhea. She received standard treatment with etoposide, methotrexate, actinomycin D, cyclophosphamide and vincristine (EMA-CO), with a complete response. However, she recurred one year later. Molecular profiling of a chest wall tumor demonstrated strong expression of programmed cell death ligand 1 (PD-L1), and she was started on treatment with single agent pembrolizumab achieving a complete response. Unfortunately, she recurred again 6 months following completion of treatment. She was re-treated with pembrolizumab for 2 years with complete response after 25 cycles and is currently without evidence of disease. She has been followed on surveillance, with no evidence of disease for more than 24 months following treatment.
Conclusion
This case represents the first to our knowledge to discuss re-treatment with pembrolizumab for relapsed choriocarcinoma after achieving a complete response.
1. Introduction
Gestational trophoblastic neoplasia (GTN) is a rare malignant transformation of placental trophoblastic cells that occurs weeks to years after any type of pregnancy. GTN encompasses invasive mole, placental-site trophoblastic tumor, epithelioid trophoblastic tumor, and gestational choriocarcinoma, the most aggressive histologic type characterized by early vascular invasion and widespread metastases. Programmed cell death ligand 1 signaling (PD1/PD-L1) likely plays an important role in the immunotolerance of normal pregnancy as well as in the development and persistence of GTN by protecting it from the maternal anti-tumor immune response. Current literature demonstrates diffuse PD-L1 expression in GTN, suggesting a role for anti-PD-1 blockade in chemotherapy-resistant tumors. To date, several case reports have demonstrated response with early treatment with checkpoint inhibitors (Huang et al., 2017, Clair et al., 2020, Paspalj, 2021). While the use of immune checkpoint inhibitors (ICIs) has revolutionized the oncologic landscape, there is limited data evaluating efficacy of re-treatment with ICIs (Yang, 2020). Furthermore, until recently, standard protocol guidelines regarding duration of treatment with checkpoint inhibitors were lacking, although evidence supports continuing for no more than 2 years (Weber, 2018). We present a case of recurrent metastatic choriocarcinoma re-treated with single agent pembrolizumab and review of available literature.
2. Case description
We present the case of a 44-year-old woman with a history of bilateral tubal ligation following a full-term delivery eight years prior, who visited her gynecologist for evaluation of a 1.5-year history of amenorrhea. Workup revealed an elevated human chorionic gonadotropin (hCG) of 2249 mIU/mL but no intrauterine pregnancy on ultrasound. The patient was subsequently managed with methotrexate for presumed ectopic pregnancy. However, hCG continued to rise to 3900 mIU/mL, and CT imaging demonstrated a 9 × 9 × 12 cm left lower lobe lung mass and a 1.5 × 1 cm vascular lesion in the body of the pancreas (FIGO Stage IV, WHO Score 13). CT guided biopsy of the lung mass showed metastatic trophoblastic tumor. The patient was subsequently referred to our institution for further management.
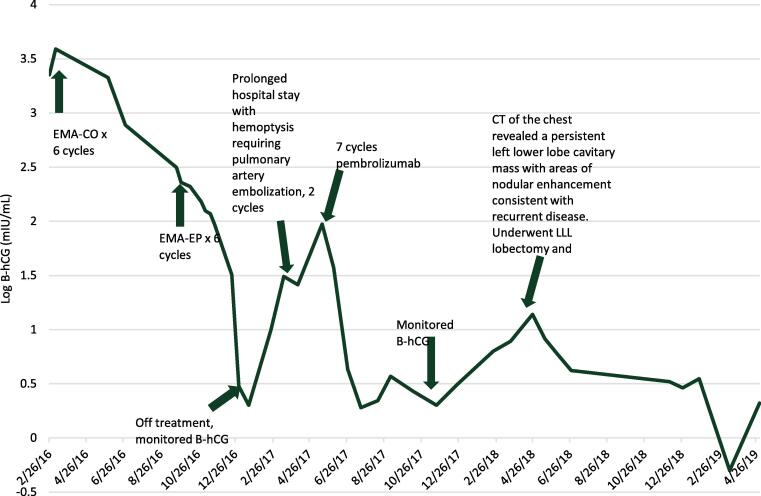
She received EMA-CO for six cycles before hCG levels plateaued (Fig. 1). CT imaging showed resolution of the pancreatic mass and decrease in size of the lung mass with no new lesions. Regimen was then changed to EMA on day 1, 2 followed by etoposide and cisplatin on day 8 (EP) with normalization of hCG after three cycles and she received another 3 cycles past normalization.
Fig. 1.
hCG trend throughout patient’s treatment course.
3. Diagnostic assessment
The patient initially did well; however, one year following completion of her treatment, she presented to an outside hospital with hemoptysis due to recurrent disease. hCG at that time was 10 mIU/mL, and chemotherapy was re-started after pulmonary artery embolization. Chemotherapy with carboplatin and paclitaxel was started for two cycles, at which point hCG level had risen to 31 mIU/mL. A month later patient again presented to the hospital with hemoptysis at which point a PET CT revealed that the lung mass had decreased in size but was thicker and more metabolically active with two new enhancing masses in the left lower lobe, and hypermetabolic paratrachial and subcarinal lymph nodes. hCG was 26. She was subsequently referred back to our institution for further management.
Molecular profiling of the chest wall tumor from the original CT-guided biopsy in 2016 showed strong expression (90%) of PD-L1. Single agent pembrolizumab 200 mg IV every 21 days was started based on strong expression of PD-L1. Prior to starting pembrolizumab, her hCG was 26, but her hCG levels normalized to less than 5 mIU/mL after two cycles and another five cycles were given prior to discontinuation of treatment with complete response based on hCG levels. Six months later, hCG levels increased to 13.8 mIU/mL, and CT imaging revealed a persistent left lower lobe cavitary mass with areas of nodular enhancement consistent with recurrent disease.
Single agent pembrolizumab was restarted with decreasing hCG to 4.2 mIU/mL prior to cycle 4. However, due to persistent hemoptysis she was evaluated at another hospital and a left lower lobe lobectomy after was performed following cycle six. Pathology revealed mostly necrotic (98%), nonviable metastatic trophoblastic tumor cells, consistent with choriocarcinoma with negative surgical margins. The patient was re-started on pembrolizumab after recovering from surgery (approximately 4 months postoperatively). At time of re-initiation of pembrolizumab, hCG was 3.3 mIU/mL. The patient tolerated single agent pembrolizumab with only mild arthralgias, and no thyroid abnormalities.
CT imaging prior to cycle 25 showed no evidence of disease. Throughout her treatment course, due to rarity of disease and lack of supporting literature, her case was reviewed at our institutional multidisciplinary conference. Specifically, discussion centered around duration of maintenance checkpoint inhibitor in relapsed choriocarcinoma. The decision was made to discontinue pembrolizumab with monthly hCG for twelve months and subsequent surveillance at increased intervals. She is currently on surveillance with no evidence of disease, and hCG levels have remained less than 5 mIU/mL in the last 24 months.
4. Discussion
Standard of care for advanced stage or recurrent high risk choriocarcinoma involves multi-agent, chemotherapy with EMA-CO (Lurain et al., 2010) or EMA-EP given on day 1–2 and 8 continuously (Lurain and Nejad, 2005). For patients who progress on first or second-line combination chemotherapy, there are few alternatives for effective treatment and no standard treatment options. Molecular profiling suggests that strong PD-L1 expression in GTN may make checkpoint inhibitors a viable treatment option (Bolze et al., 2017, Inaguma et al., 2016).
Programmed cell death 1 is a transmembrane glycoprotein of the Ig superfamily that acts as a T-cell co-inhibitory receptor. Binding of PD-1 to its ligand (PD-L1 or programmed cell death ligand 2) results in inhibition of T cell activation and function, which ultimately downregulates signaling from CD28 and T-cell co-receptor-CD3 complex (Sznol and Chen, 2013). In various solid tumors, upregulated expression of PD-L1 allows cancer to evade T-cell induced antitumor activity (Sznol and Chen, 2013). Pembrolizumab is a highly selective monoclonal IgG4 kappa isotype antibody against PD-1 which can disrupt PD-1 from its ligands and obstruct inhibitory signals in T cells (Reck et al., 2016). This restores recognition of tumor cells by cytotoxic T cells to elicit eradication (Reck et al., 2016).
Histologic evidence demonstrates that gestational trophoblastic neoplasia expresses PD-L1 uniformly and ubiquitously. For example, Bolze et al. analyzed 83 GTN and gestational complete and partial hydatidiform moles samples in whole tissue sections from 76 patients and assessed immunohistochemistry using Allred total score (ATS), which combines intensity and proportion of expression on a 0- to 8-point scale (Bolze et al., 2017). They found that PD-L1 expression was ubiquitous and strong in all subtypes. In choriocarcinoma specimens specifically, ATS was 8 out of 8 in 80% of specimens (Bolze et al., 2017). Another study by Inaguma et al. performed a comprehensive immunohistochemical analysis in different types of cancer and found consistent and strong expression in placental trophoblasts including all (n = 8) choriocarcinoma samples analyzed (Inaguma et al., 2016). In our case, the tumor demonstrated strong PD-L1 expression, suggesting that PD-1/PD-L1 blockade may yield clinical benefit.
Pembrolizumab was started for chemotherapy refractory disease in our patient based on molecular data, prior experience, and lack of available standard therapy options (Huang et al., 2017). While our patient demonstrated an excellent response following two cycles of pembrolizumab, the duration of continued therapy in this rare disease is largely unknown. In GTN, chemotherapy is typically continued for three cycles after normalization of hCG and discontinued. Extrapolating from this treatment schema, our patient received a total of seven cycles of pembrolizumab rather than continuation of therapy as in other solid tumor malignancies (Jansen et al., 2019). Furthermore, in our experience with prior treatment, discontinuation after complete response did not result in recurrence. (Huang et al., 2017). While our patient tolerated treatment well, she was experiencing barriers to continuation of care (travel to appointments, time off work). Unfortunately, our patient did recur approximately six months after stopping pembrolizumab but based on known literature, decision was made to re-initiate therapy (Santini et al., 2018).
Our patient completed 25 cycles after re-initiation of pembrolizumab, which she tolerated well with only mild myalgias, arthralgias and fatigue. In comparison, the standard regimens of EMA-CO and EMA-EP both have significant toxicity profiles in the short term (alopecia, fatigue, myelosuppression, nausea, and vomiting) and long term (myelodysplastic disease, leukemia, infertility). Pembrolizumab is generally well tolerated, administered once every three weeks, and has an acceptable tolerability profile, with immune-related adverse events that are generally manageable or reversible: pneumonitis, dermatologic and mucosal toxicity, colitis, hypo/hyperthyroidism, and hepatotoxicity (Naidoo et al., 2015). On the other hand, treatment with checkpoint inhibitors is associated with higher treatment costs to the healthcare system, with an average of $9691 per cycle in the United States, and while many insurance plans cover the majority of the cost, medication necessity must be carefully weighed (Sarfaty et al., 2018).
Our case is the first to our knowledge to report complete response on re-treatment with pembrolizumab for relapsed choriocarcinoma after achieving a complete response with pembrolizumab. At the time of this manuscript, our patient with chemoresistant recurrent choriocarcinoma re-treated with pembrolizumab remains disease free (24 months). Other case reports have also suggested a promising role for pembrolizumab in the management of chemoresistant choriocarcinoma and GTN (Huang et al., 2017). Clinical trials utilizing checkpoint inhibitors for the management of choriocarcinoma are currently in progress. Future studies will need to include evaluation of duration of ICIs in GTN and correlate with outcomes.
5. Patient perspective
Investigators confirm that informed consent was obtained from the patient for creation and publication of this case report.
6. Statement of Consent
Investigators confirm that informed consent was obtained from the patient for creation and publication of this case report.
CRediT authorship contribution statement
Adriana J. Wong: Writing – original draft. Lindsey Finch: Writing – review & editing. Joseph Matt Pearson: Conceptualization, Methodology, Writing – review & editing. Andre Pinto: Conceptualization, Methodology, Resources. Marilyn Huang: Conceptualization, Methodology, Writing – review & editing.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Bolze P.-A., Patrier S., Massardier J., Hajri T., Abbas F., Schott A.M., Allias F., Devouassoux-Shisheboran M., Freyer G., Golfier F., You B. PD-L1 Expression in Premalignant and Malignant Trophoblasts From Gestational Trophoblastic Diseases Is Ubiquitous and Independent of Clinical Outcomes. Int. J. Gynecol. Cancer. 2017;27(3):554–561. doi: 10.1097/IGC.0000000000000892. [DOI] [PubMed] [Google Scholar]
- Clair K.H., Gallegos N., Bristow R.E. Successful treatment of metastatic refractory gestational choriocarcinoma with pembrolizumab: A case for immune checkpoint salvage therapy in trophoblastic tumors. Gynecol. Oncol. Rep. 2020;34 doi: 10.1016/j.gore.2020.100625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang M., Pinto A., Castillo R.P., Slomovitz B.M. Complete Serologic Response to Pembrolizumab in a Woman With Chemoresistant Metastatic Choriocarcinoma. J. Clin. Oncol. 2017;35(27):3172–3174. doi: 10.1200/JCO.2017.74.4052. [DOI] [PubMed] [Google Scholar]
- Inaguma S., Wang Z., Lasota J., Sarlomo-Rikala M., McCue P.A., Ikeda H., Miettinen M. Comprehensive Immunohistochemical Study of Programmed Cell Death Ligand 1 (PD-L1): Analysis in 5536 Cases Revealed Consistent Expression in Trophoblastic Tumors. Am. J. Surg. Pathol.. 2016;40(8):1133–1142. doi: 10.1097/PAS.0000000000000653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jansen Y.J.L., Rozeman E.A., Mason R., Goldinger S.M., Geukes Foppen M.H., Hoejberg L., Schmidt H., van Thienen J.V., Haanen J.B.A.G., Tiainen L., Svane I.M., Mäkelä S., Seremet T., Arance A., Dummer R., Bastholt L., Nyakas M., Straume O., Menzies A.M., Long G.V., Atkinson V., Blank C.U., Neyns B. Discontinuation of anti-PD-1 antibody therapy in the absence of disease progression or treatment limiting toxicity: clinical outcomes in advanced melanoma. Ann. Oncol. 2019;30(7):1154–1161. doi: 10.1093/annonc/mdz110. [DOI] [PubMed] [Google Scholar]
- Lurain J.R., Nejad B. Secondary chemotherapy for high-risk gestational trophoblastic neoplasia. Gynecol. Oncol. 2005;97(2):618–623. doi: 10.1016/j.ygyno.2005.02.004. [DOI] [PubMed] [Google Scholar]
- Lurain J.R., Singh D.K., Schink J.C. Management of metastatic high-risk gestational trophoblastic neoplasia: FIGO stages II-IV: risk factor score > or = 7. J Reprod. Med. 2010;55(5–6):199–207. [PubMed] [Google Scholar]
- Naidoo J., Page D.B., Li B.T., Connell L.C., Schindler K., Lacouture M.E., Postow M.A., Wolchok J.D. Toxicities of the anti-PD-1 and anti-PD-L1 immune checkpoint antibodies. Ann. Oncol. 2015;26(12):2375–2391. doi: 10.1093/annonc/mdv383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paspalj V., et al. Long-term survival in multiresistant metastatic choriocarcinoma after pembrolizumab treatment: A case report. Gynecol. Oncol. Rep. 2021;37 doi: 10.1016/j.gore.2021.100817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reck M., Rodríguez-Abreu D., Robinson A.G., Hui R., Csőszi T., Fülöp A., Gottfried M., Peled N., Tafreshi A., Cuffe S., O’Brien M., Rao S., Hotta K., Leiby M.A., Lubiniecki G.M., Shentu Y., Rangwala R., Brahmer J.R. Pembrolizumab versus Chemotherapy for PD-L1-Positive Non-Small-Cell Lung Cancer. N. Engl. J. Med. 2016;375(19):1823–1833. doi: 10.1056/NEJMoa1606774. [DOI] [PubMed] [Google Scholar]
- Santini F.C., Rizvi H., Plodkowski A.J., Ni A., Lacouture M.E., Gambarin-Gelwan M., Wilkins O., Panora E., Halpenny D.F., Long N.M., Kris M.G., Rudin C.M., Chaft J.E., Hellmann M.D. Safety and Efficacy of Re-treating with Immunotherapy after Immune-Related Adverse Events in Patients with NSCLC. Cancer Immunol. Res. 2018;6(9):1093–1099. doi: 10.1158/2326-6066.CIR-17-0755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarfaty M., Hall P.S., Chan K.K.W., Virik K., Leshno M., Gordon N., Moore A., Neiman V., Rosenbaum E., Goldstein D.A. Cost-effectiveness of Pembrolizumab in Second-line Advanced Bladder Cancer. Eur. Urol. 2018;74(1):57–62. doi: 10.1016/j.eururo.2018.03.006. [DOI] [PubMed] [Google Scholar]
- Sznol M., Chen L. Antagonist antibodies to PD-1 and B7–H1 (PD-L1) in the treatment of advanced human cancer. Clin. Cancer Res. 2013;19(5):1021–1034. doi: 10.1158/1078-0432.CCR-12-2063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber J.S. 2018 ESMO Congress. 2018. The right time for discontinuing immunotherapy. [Google Scholar]
- Yang K., Li J., Sun Z., Zhao L., Bai C. Retreatment with immune checkpoint inhibitors in solid tumors: a systematic review. Therapeut. Adv. Med. Oncol. 2020 doi: 10.1177/1758835920975353. [DOI] [PMC free article] [PubMed] [Google Scholar]