Abstract
Vernonia leopoldi (Sch. Bip. ex Walp.) Vatke (Asteraceae) is one of the widely used anti-cancer traditional medicinal plants in Ethiopia, despite the lack of data to support its therapeutic efficacy. Here we describe the isolation of compounds from the plant and the investigation of their cytotoxicity and other bioactivities. We identified the novel sesquiterpene lactone (SL) 11ß,13-dihydrovernodalol along with the three other SLs (vernomenin, vernolepin, and 11ß,13-dihydrovernodalin) and three flavonoids (apigenin, eriodyctiol, and luteolin) isolated from this plant for the first time. The structures of all the compounds were established based on extensive analysis of nuclear magnetic resonance spectroscopic data and confirmed by high-resolution electrospray ionization mass spectrometry. We then studied the biological activities of the SLs and found that all were cytotoxic at low μM ranges against MCF-7 and JIMT-1 breast cancer cells as well as against the normal-like MCF-10A breast epithelial cells evaluated in a spectrophotometric assay. All the SLs significantly reduced JIMT-1 cell migration after 72 h of treatment with 2 μM concentrations in a wound healing assay. Treatment with all SLs reduced the aldehyde dehydrogenase expressing cancer stem cell sub-population of the JIMT-1 cells significantly, evaluated by flow cytometry. Only 11ß,13-dihydrovernodalin resulted in a significant inhibition of tumor necrosis factor-α-induced translocation of nuclear factor κB to the cell nucleus. In addition, we show that the reporter fluorophore nitrobenzoxadiazole (NBD) can successfully be conjugated with an SL and that this SL-NBD conjugate is taken up efficiently in JIMT-1 cells. Therefore, the overall bioactivities of the SL compounds and specifically their effects against the stemness of breast cancer cells make them prime candidates for further in-depth investigation.
Abbreviations: ALDH, Aldehyde dehydrogenase; BSA, Bovine serum albumin; CFE, Colony forming efficiency; CSCs, Cancer stem cells; FBS, Fetal bovine serum; NBD, Nitrobenzoxadiazole; NF-κB, Nuclear factor-κB; PBS, Phosphate-buffered saline; SLs, Sesquiterpene lactones; TNF-α, Tumor necrosis factor-α
Keywords: Vernonia leopoldi, Traditional medicine, Isolation of anti-cancer bioactive compounds, Novel sesquiterpene lactone, Cancer stem cells
Graphical Abstract
Highlights
-
•
Vernonia leopoldi (Sch. Bip. ex Walp.) Vatke is a traditional anticancer medicinal plant in Ethiopia.
-
•
Sesquiterpene lactones (SLs) and flavonoids are isolated from V. leopoldi for the first time.
-
•
A novel SL, named 11ß,13-dihydrovernodalol, was discovered.
-
•
All the SLs reduce stemness and inhibit cell migration of cancer cells.
-
•
A novel fluorophore-conjugated SL was synthesized for the study of SL uptake and localization in cells.
1. Introduction
Vernonia leopoldi (Sch. Bip. ex Walp.) Vatke (Family: Asteraceae), which is found in Ethiopia, is a shrub or rarely woody herb, covered with soft hairs, that grows up to a height of 0.5–2.5 m [1], [2]. The genus Vernonia is represented by about 49 species excluding imperfectly known taxa [1] and encompasses herbs, shrubs, and small trees. The ecology of V. leopoldi encompasses forest margins, acacia wooded grasslands with scrub of Maytenus, Rosa abyssinica, and Carissa on shallow soils, often in ravines, roadside thickets, and wastelands found in the 1850 and 2850 m altitudinal range [1]. The traditional medicinal use of the plant includes treatment of malignancies [3], [4].
In addition to Ethiopia, V. leopoldi is found only in Yemen, where it is traditionally used for the treatment of cough, colic, and skin diseases [5], [6]. In 2016, a new lanostane-type triterpene (lanost-3β, 23S-dihydroxy-22(31)-ene) was isolated from the aerial parts of the plant from Yemen and cytotoxicity with IC50 values ranging between 25.7 and 58.8 µM were reported in various cell lines [6]. Understanding the composition of traditional medicinal plants is crucial both to increase an efficient use and reduce side effects [7]. Also, WHO has suggested the use of traditional medicine as a complement to national health care systems [8].
The aim of the present study was to isolate and chemically characterize compounds from the Ethiopian traditional anti-cancer medicinal plant V. leopoldi followed by investigation of the cytotoxicity in dose response and other bioactivity assays in breast-derived cell lines (cancer and normal-like) in agreement with our previous report [9]. We have investigated the effect of the SLs in inhibiting cancer cell migration, which is an important part of metastasis, the main cause of cancer death [10]. A problem causing cancer recurrence is the presence of treatment resistant cancer stem cells (CSCs), a small tumorigenic multi-potential sub-population of various tumors with self-renewal and differentiation properties [11] and thus we have investigated the effect of the SLs on this sub-population. An important aspect of new potential compounds for anti-cancer treatment is their up-take and cellular localization and thus we also synthesized a unique fluorescently labeled SL.
2. Materials and methods
2.1. Extraction and isolation of compounds
Powdered dry leaves of V. leopoldi (1 kg) were extracted by maceration at room temperature in 95% ethanol (EtOH) (5 L, 7 days). The filtered solvent was removed under reduced pressure to yield a semisolid residue (145.2 g). After being suspended in 1.2 L of a mixture of H2O:methanol (8:2, v/v), the solution was successively extracted with heptane (three times, 2:1, v/v) and ethyl acetate (three times, 1:1, v/v). The ethyl acetate extract (46.3 g) was subjected to vacuum liquid chromatography (VLC) on silica gel 60 (230–400 mesh, Merck, Darmstadt, Germany), using a step gradient of heptane:ethyl acetate (1:0–0:1, v/v), which afforded eighteen fractions (A-R). Fraction N (24.1 g) was subjected once more to VLC on silica gel, using a step gradient of heptane:ethyl acetate (8:2–0:1, v/v) to yield five sub-fractions (Na-Ne). The sub-fraction Nc (2.2 g) was subjected to Sephadex LH-20 chromatography, eluted with methanol, and four fractions (Nca-Ncd) were obtained. Compound 1 was obtained from fraction Nca. Fraction Ncc (60 mg) was separated by preparative thin layer chromatography using a preparative Silica gel 60 (F254, 20 ×20, 2 mm, glass, Merck) as a stationary phase and a chloroform/methanol 8:2 mobile phase to give compound 2 and a mixture of compound 2 and compound 3. Sub-fraction Nd (17.2 g) was subjected to VLC on silica gel and was eluted with heptane:ethyl acetate (8:2–0:1, v/v) to give 2 sub-fractions (Nda and Ndb). Fraction Ndb (11.5 g) was eluted with heptane:ethyl acetate (1:1, v/v) from which two fractions (Ndb1 and Ndb2) were obtained. The fraction Ndb1 was identified as compound 4. From Ndb2 (7.75 g), a portion (2.20 g) was purified using Sephadex LH-20 chromatography, eluted with methanol. The eluate was then subjected to silica gel column chromatography (CC) with heptane:ethyl acetate (4:6–0:100, v/v). From this, compounds 5, 6, and 7 were obtained.
All elution steps described above were carried out under an atmosphere of nitrogen in oven-dried glassware. Reagents and solvents were purchased from commercial suppliers and used as received. Nuclear magnetic resonance (NMR) spectra were recorded at room temperature on a Bruker Avance II 500 MHz spectrometer (Bruker, MA, USA). The spectra were recorded in deuterated chloroform (CDCl3) and the solvent signals (7.27/77.0) were used as references. VLC separation was carried out on Merck Silica gel 60G, while CC was performed using Silica gel 60 (230–400 mesh, Merck, Darmstadt, Germany) and gel permeation on Sephadex LH-20 (GE Healthcare, Chicago, IL, USA). Chromatograms were visualized under a UV lamp at 254 nm followed by spraying with vanillin heating. High-resolution electrospray ionization mass spectrometry (HR-ESI-MS) was performed in a Waters Q-TOF Micro system spectrometer (Waters, Wilmslow, UK) using H3PO4 for calibration and as internal standard.
2.2. Compound stock solutions
All compounds were dissolved to 100 mM stock solutions in 100% DMSO which were kept at − 20 °C. For dose response testing, serial dilutions from the 100 mM stock solutions were made with phosphate-buffered saline (PBS) to obtain solutions with 10 times higher concentrations than the desired concentrations in the 96-well plates (see Fig. 4, Fig. 5, Fig. 9). Twenty µL of these solutions were added to the wells of the 96-well plates with 180 µL medium resulting in a 10 times dilution (see below as well). When used at the highest concentration in dose response testing i.e. 100 μM (see Fig. 4, Fig. 9), the DMSO concentration was 0.1% in all wells. Control was then treated with 0.1% DMSO. We have confirmed that 0.1% DMSO did not affect the cells (not shown). When used in all other assays, the DMSO concentration was below 0.1% and control always received the appropriate DMSO concentration equivalent to that found in treated cultures. The treatment concentration used are found as data points in dose response curves (Fig. 4, Fig. 5, Fig. 9) and in figure legends.
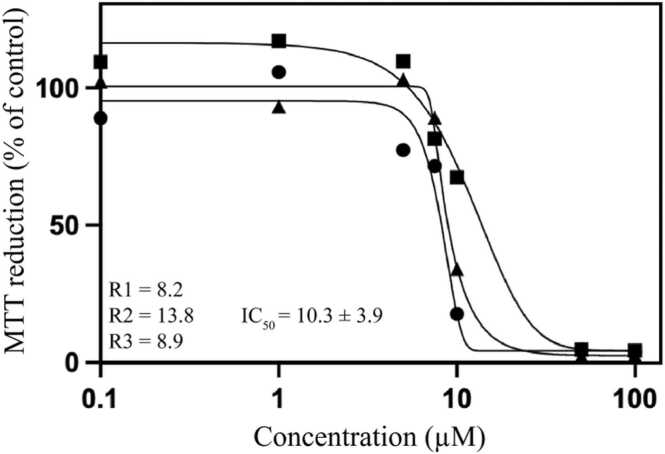
Fig. 4.
Dose response curves obtained after treatment of JIMT-1 and MCF-7 breast cancer cells and normal like MCF-10A breast epithelial cells with the SLs isolated from the ethyl acetate fraction of V. leopoldi. VN, Vernomenin (1). VP, Vernolepin (5). DV, 11ß,13-Dihydrovernodalin (6). 1a2-P, 11ß,13-Dihydrovernodalol (7). Each curve represents one experiment with the mean of 6 wells in each data point. The values in parenthesis show IC50 values of the different repeats (R1-R3).
Fig. 5.
Dose response curves obtained after treatment of JIMT-1 breast cancer cells (A) and normal like MCF-10A breast epithelial cells (B) with vernodalol. Each curve represents one experiment with the mean of 6 wells in each data point. The values to the left are the IC50 from the individual repeats R1 – R3 while the IC50 value to the right is the mean of the three repeats ± SD.
Fig. 9.
Dose response curves obtained after treatment of JIMT-1 cells for 72 h with the vernolepin-NDB conjugate. Each data point is the mean from six independent measurements. The values to the left are the IC50 from the individual repeats R1 – R3 while the IC50 value to the right is the mean of the three ± SD.
2.3. Cell lines and culturing conditions
For the bioactivity studies, the following cell lines were used: the human JIMT-1 and MCF-7 breast cancer cell lines and the human MCF-10A normal-like breast epithelial cell line. The cell lines MCF-7 (HTB-22) and MCF-10A (CRL-10317) were purchased from American Type Culture Collection (Manassas, VA, USA), whereas the JIMT-1 (ACC589) was purchased from the German Collection of Microorganisms and Cell Cultures (Braunschweig, Germany). The cell lines were routinely cultured and maintained as previously described [12]. The seeding densities were: at 1.5 × 104 cells/cm2 (JIMT-1), 2 × 104 cells/cm2 (MCF-7), and 104 cells/cm2 (MCF-10A) in tissue culture vessels of the appropriate size with the volume of medium about 0.2–0.3 mL per cm2.
2.4. Dose response assay
The dose response assays were performed as previously described [12]. Briefly, confluent cells were trypsinized using 0.05% Trypsin-EDTA and the cell number was determined by counting in a hemocytometer. Cells of the different cell lines were seeded at the recommended densities (described above) in 180 µL of medium into the wells of 96-well plates and then allowed to attach for 24 h before adding the test compounds in 20 µL aliquots to achieve the desired concentrations. After 72 h of incubation with the compounds, 20 µL of 3-(4, 5-dimethylthiazolyl-2)−2, 5-diphenyltetrazolium bromide (MTT) (Sigma-Aldrich Sweden AB, Stockholm, Sweden) solution (5 mg/mL in PBS) was added to the wells and the 96 well plates were wrapped with aluminum foil and returned to the CO2 incubator for 1 h. Then, dissolving the formazan precipitates and absorbance measurement were done as described earlier [12]. Three independent dose-response experiments were performed for each compound and the IC50 values were obtained using the GraphPad Prism software (San Diego, CA, USA) version 7.02. The results are presented as mean IC50 ± SD.
2.5. Breast CSC population estimation by the ALDH assay
The ALDH assay was performed as previously described [9] using JIMT-1 cells that had been treated for 72 h with a 2 μM concentration of the 4 SLs. The ALDEFLUOR™ kit (Stem Cell Technologies, Grenoble, France) procedures as given in the manufacturer’s protocol were followed. The BD Accuri C6 Flow cytometer (BD Biosciences, San Jose, CA, USA) was used to analyze the samples. DEAB-treated cells for each sample were used to set the ALDH+ region. The data was evaluated using the CFlow software.
2.6. Wound healing assay
The JIMT-1 cells were seeded at high density (~125,000 cells/cm2) in Petri dishes and incubated at 37˚C in a humidified incubator with 5% CO2 in air for 24 h to allow them to attach and form a confluent layer of cells. Then, the medium was removed and three parallel scratch wound areas were made in the cell layer using a sterile 200 µL pipette tip, following the previously established protocol [13]. Then it was washed twice with PBS and serum-free medium was added. The treatments were done at a final concentration of 2 μM, while the controls were treated with 0.005% DMSO. The zero hour (0 time) photographic images of the scratch wound area were taken immediately before the incubation at 37 °C in a CO2 incubator and then photographed 72 h later. To estimate the wound closure between time 0 and 72 h, the scratch areas were measured with ImageJ 1.47 v software (https://imagej.nih.gov) and calculated in percentage of the wound at time zero.
2.7. Nuclear factor-κB translocation assay
The inhibition of the compounds on TNF-α-induced translocation of NF-κB to the cell nucleus was evaluated. For this experiment, JIMT-1 cells were used and it was performed as reported in our previous study [9] and the compounds were added at a 10 μM concentration. An Olympus epifluorescence microscope (Olympus Optical Co. Ltd., Japan) equipped with a digital camera (Nikon Imaging Japan Inc., Japan) was used for the immunofluorescence imaging of the slides.
2.8. Synthesis of vernolepin-NBD conjugate
To a solution of nitrobenzoxadiazole-Cl (NBD-Cl) (310 mg, 1.6 mmol) in EtOH (3 mL), a solution of ethanolamine (0.175 mL, 2.17 mmol) in EtOH (2 mL) was added. The resulting solution was stirred at room temperature for 30 min. The NDB-linker formed as a yellow precipitate was filtered, dried in vacuum, and used in the next step without further purification. Phosgene 20% in toluene (300 µL, 0.65 mmol) was added dropwise over 3 min to a flask containing pyridine (1.3 mL, 16.3 mmol) at − 10 °C and a yellow suspension was formed immediately. The solution was stirred for 5 min, followed by addition of a solution of NBD-linker (129 mg, 0.543 mmol) in pyridine (0.87 mL, 10.87 mmol) which was added dropwise over 3 min. The resulting red suspension was stirred for 5 min, after which a solution of vernolepin (30 mg, 0.109 mmol) in pyridine (0.44 mL, 5.43 mmol) was added dropwise. The resulting mixture was stirred overnight and allowed to reach room temperature, before diluted with CH2Cl2 (5 mL) and washed with HCl (3 ×3 mL, 0.1 M aq.). The organic layer was separated, dried using a phase separator, and concentrated under reduced pressure. Purification by flash chromatography using CHCl3/MeOH (97:3, v/v) gave a yellow oil that was concentrated in vacuum affording 9 mg (15%) of the desired vernolepin-NBD (See Fig. 1). All conditions for the synthesis of vernolepin-NBD conjugate described above were carried out under an atmosphere of nitrogen in oven-dried glassware. 1H NMR (500 MHz, Chloroform-d) δ 8.44 (d, J = 8.9 Hz, 1H), 6.73 (dd, J =1.0, 0.8 Hz, 1H), 6.21 (d, J = 8.9 Hz, 1H), 6.19 (d, J = 3.1 Hz, 1H), 5.94 (t, J =1.0 Hz,1H), 5.70 (dd, J = 17.5, 10.9 Hz, 1H), 5.60 (d, J = 2.9 Hz, 1H), 5.33 (d, J = 10.9 Hz, 1H), 5.28 (d, J = 17.5 Hz, 1H), 4.88 (m, 1H), 4.66 – 4.59 (m, 1H), 4.51 (m, 3H), 4.42 (d, J = 12.3 Hz, 1H), 4.28 – 4.22 (dd, J =12.3, 0.8 Hz,1H), 3.99 (t, J = 11.3 Hz, 1H), 3.48 (s, 3H), 2.99 (d, J = 11.3 Hz, 1H), 2.87 (tt, J = 11.0, 4.6 Hz, 1H), 2.16 (dd, J = 14.2, 4.6 Hz, 1H), 1.58 (dd, J = 14.2, 10.2 Hz, 1H). 13C NMR (126 MHz, CDCl3) δ 168.14, 162.98, 153.82, 145.02, 144.57, 144.48, 139.28, 135.98, 135.23, 135.11, 129.65, 123.57, 121.69, 117.30, 101.95, 77.68, 72.55, 70.40, 65.94, 53.98, 50.10, 46.44, 41,67, 40.85, 38.72. HRMS calculated for C25H25O10N4 [M+H]: 541.1572, found: 541.1571 (see additional file: Fig. S1 and Fig. S2 for the 1D H1 and C13 NMR spectra).
Fig. 1.
Schematic representation of the synthesis of vernolepin-NBD conjugate.
2.9. Uptake and localization of vernolepin-NBD conjugate
The JIMT-1 cells were seeded (0.35 ×106 cells) in a Petri dish containing 2 mL regular medium and incubated for 48 h. Then, the vernolepin-NBD conjugate was added to a final concentration of 10 µM, under dark conditions whereby phase contrast and immunofluorescence images were taken by an inverted phase-contrast microscope equipped with a light source for fluorescence using the 40x objective at 1, 5, 10, 15, 30, 45, 60, and 120 min and at 24, 48, and 72 h after addition of the compound. The uptake and localization were qualitatively evaluated.
2.10. Statistical analysis
All results are shown as mean ± SD. The data were statistically treated by one-way analysis of variance (ANOVA) followed by post hoc Dunnett’s test. P < 0.05, CI = 95% was considered as statistically significant.
3. Results
3.1. The compounds
Seven compounds were isolated from the ethyl acetate fraction of V. leopoldi (Table 1 and Fig. 2). The relative proportion of the SLs was higher than that of the flavonoids (Table 1). The compound vernomenin (1), an SL, was isolated first in the successive extractions. The flavonoids apigenin (2) and eriodyctiol (3) were the next isolates. Another flavonoid, luteolin (4) was then obtained as a yellow precipitate and the final elution yielded the last three SLs: vernolepin (5), 11,13-dihydrovernodalin (6), and 7, the novel compound 4aR,5R,6S,7S,8aR)−5-hydroxy-6-(3-methoxy-3-oxoprop-1-en-2-yl)−4-methylene-3-oxo-8a-vinyloctahydro-1H-isochromen-7-yl 2-(hydroxymethyl)acrylate), that we named 11ß,13-dihydrovernodalol (Fig. 3). The three compounds (5, 6, and 7) were estimated to be present in quantities of 707 mg, 22 mg, and 32 mg, respectively, in the initial EtAc extract (Table 1). Vernomenin (1), vernolepin (5), and 11ß,13-dihydrovernodalin (6) have previously been isolated from other plant groups and here we report these compounds isolated from V. leopoldi for the first time.
Table 1.
The compounds isolated from the ethyl acetate fraction of V. leopoldi.
| Compound designation | Name of the compound | Chemical formula, MW |
|---|---|---|
| 1 | Vernomenin (220 mga), SLb | C15H16O5, 276.29 |
| 2 | Apigenin (5 mg), Fc | C15H10O5, 270.24 |
| 3 | Eriodyctiol (0.35 mg), F | C15H12O6, 288.26 |
| 4 | Luteolin (8 mg), F | C15H10O6, 286.24 |
| 5 | Vernolepin (707 mg), SL | C15H16O5, 276.29 |
| 6 | 11ß,13-Dihydrovernodalin (22 mg), SL | C19H22O7, 362.38 |
| 7 | 11ß,13-Dihydrovernodalol (32 mg), SL | C20H26O8, 394.42 |
aThe number denotes mg recovered from 46.3 g of acetate fraction; b SL, Sesquiterpene lactone; c F, Flavonoid.
Fig. 2.
Structures of the SLs (vernomenin (1), vernolepin (5), 11ß,13-dihydrovernodalin (6), and 11ß,13-dihydrovernodalol (7)) and the flavonoids (apigenin (2), eriodyctiol (3), and luteolin (4)) isolated from the ethyl acetate fraction of V. leopoldi.
Fig. 3.

Chemical structure of the novel compound 7, 4aR,5R,6S,7S,8aR)−5-hydroxy-6-(3-methoxy-3-oxoprop-1-en-2-yl)−4-methylene-3-oxo-8a-vinyloctahydro-1H-isochromen-7-yl 2-(hydroxymethyl)acrylate, named as 11ß,13-dihydrovernodalol.
Compound 7 was obtained as a clear oil. The [M + H]+ ion was observed at m/z 395.1710 which corresponds to C20H26O8 calculated 395.1706. This is in accordance with the 1D H1 and C13 NMR data (see additional file: Fig. S3 and Fig. S4). 1H NMR (500 MHz, Chloroform-d) δ 6.66 (d, J = 1.3 Hz, 1H), 6.15 (d, J = 1.2 Hz, 1H), 5.87 (dd, J = 1.4, 1.2 Hz, 1H), 5.78 (dd, J = 1.6, 0.8 Hz, 1H), 5.66 (dd, J = 17.3, 10.6 Hz, 1H), 5.24 (d, J = 4.1 Hz, 1H), 5.21 (m, 1H), 5.05 (td, J = 11.5, 4.7 Hz, 1H), 4.49 (d, J = 12.1 Hz, 1H), 4.35 (dd, J = 12.1, 2.1 Hz, 1H), 4.30–4.27 (m, 2H), 3.68 (s, 3H), 3.58 (td, J = 10.5, 4.1 Hz, 1H), 3.24 (qd, J = 7.2, 1.6 Hz, 1H), 2.52 (m, 1H), 2.50 (m, 1H), 1.99 (dd, J = 13.6, 4.7 Hz, 1H), 1.59 (dd, J = 13.6, 11.4 Hz, 2H), 1.12 (d, J = 7.1 Hz, 3H). 13C NMR (126 MHz, CDCl3) δ 176.01, 165.17, 163.58, 140.47, 139.23, 133.80, 132.52, 126.56, 116.02, 70.84, 67.37, 67.37, 62.14, 51.94, 51.84, 49.52, 39.34, 37.53, 35.89, 10.01.
3.2. The SLs were highly cytotoxic in dose response assays
The basal cytotoxicity of the SLs isolated from V. leopoldi was determined using an MTT dose response assay. The MTT assay was conducted using the JIMT-1, MCF-7, and MCF-10A cell lines. The four SLs were found to have similar cytotoxicity in the JIMT-1 and MCF-10A cell lines while the MCF-7 cell line was less sensitive (Table 2 and Fig. 4). The novel compound 11ß,13-dihydrovernodalol has the lowest IC50 value of 1.6 µM in JIMT-1 cells.
Table 2.
The IC50 (μM) values from dose response curves obtained after treatment of JIMT-1 and MCF-7 breast cancer cells and normal like MCF-10A breast epithelial cells with the SLs isolated from the ethyl acetate fraction of V. leopoldi.
| Cell lines | Vernomenin (1) | Vernolepin (5) | 11ß,13-Dihydrovernodalin (6) | 11ß,13-Dihydrovernodalol (7) |
|---|---|---|---|---|
| JIMT-1 | 2.2 ± 0.9 | 1.7 ± 0.3 | 2.2 ± 0.8 | 1.6 ± 0.05 |
| MCF-7 | 35.0 ± 16.5 | 15.9 ± 0.6 | 4.9 ± 0.3 | 3.9 ± 0.7 |
| MCF-10A | 1.6 ± 0.1 | 2.3 ± 0.2 | 2.0 ± 0.5 | 2.2 ± 0.9 |
The number of 96-well assays run for each compound is found in Fig. 4 as each assay generates one curve. The replicates are ≥ 3 96-well plate independent assays with n = 6 wells in each assay. The data are presented as mean ± SD.
Vernodalol, an SL with closely similar structure to the novel compound reported here, was found to be less cytotoxic against JIMT-1 (IC50 =11.13 +1.17) and MCF10A (IC50 =12.12 +1.52) cell lines (Fig. 5) when compared to the novel compound 11ß,13-dihydrovernodalol (IC50 =1.6 ± 0.05, JIMT-1 and IC50 =2.2 ± 0.9, MCF-10A) (Table 2).
3.3. Estimation of the CSCs sub-population using ALDH+ as a marker
The effect of treating JIMT-1 cells with a 2 μM concentration of the four SLs on the ALDH+ sub-population was evaluated following the ALDEFLUOR™ assay protocol. Treatment with vernomenin (1) highly reduced the ALDH+ CSC subpopulation (P < 0.005). Treatment with the other three SLs also resulted in a statistically significant reduction of the ALDH+ CSC subpopulation (P < 0.05) (Fig. 6A). Representative cytograms of the ALDEFLOUR™ assay are shown in Fig. 6B.
Fig. 6.
Treatment with a 2 μM concentration of the SLs for 72 h significantly reduced the ALDH+ sub-population evaluated by flow cytometry. A. ALDH+ cells in % of control. The data are presented as mean ± SD for n = 3. *P < 0.05; **P < 0.005; CI: 95%; all the values were compared with control. B. Representative cytograms used to obtain the data shown in A. SSCA-A, side scatter. FL1-H, fluorescence signal from substrate cleaved by ALDH. The percentage in each figure represents the proportion of ALDH+ cells (found in the outlined red region) in relation to all cells. The red gate was set in samples that did not receive the substrate according to the manufacturer’s instructions. VN, Vernomenin (1). VP, Vernolepin (5). DV, 11ß,13-Dihydrovernodalin (6). 1a2-P, 11ß,13-Dihydrovernodalol (7).
3.4. Effect of the SLs on cell migration
Based on the results of the wound closure defined as 0% closure at 0 h for each sample, all the compounds inhibited cell migration after treatment with a 2 μM concentration for 72 h (P < 0.05). The maximum closure was attained by the treatment with vernomenin (1) (35.0%). In comparison to the closure attained by the control group (55.2%), this inhibition was also statistically significant (P < 0.05) (Fig. 7).
Fig. 7.
The effect of treating with 2 μM concentrations of the SLs on cell migration using a scratch wound healing assay. Wound closure at 72 h after the scratch (A). Representative images taken with an inverted phase contrast microscope (B). VN, Vernomenin (1). VP, Vernolepin (5). DV, 11ß,13-Dihydrovernodalin (6). 1a2-P, 11ß,13-Dihydrovernodalol (7). *P < 0.05; CI: 95%; all the values were compared against the control. Image bars = 100 µm.
3.5. Effects of the SLs on the nuclear translocation of NF-κB
Using JIMT-1 cells, the inhibitive effects of the compounds on TNF-α-induced NF-κB nuclear translocation was evaluated. Fresh solutions of the test compounds were prepared as 400 µM solutions and they were tested at 10 µM concentration. Only, the compound 11ß,13-dihydrovernodalin (6) resulted in a significant reduction of the NF-κB positive nuclei compared to control which was treated with TNF-α and the compound vehicle (P < 0.05) (Fig. 8).
Fig. 8.
Effects of treating JIMT-1 cells with the SLs on TNF-ɑ-induced translocation of NF-κB to the cell nucleus. Positive nuclei in % of total nuclei number (A). Representative images from independent experiments for each treatment (B). VN, Vernomenin (1). VP, Vernolepin (5). DV, 11ß,13-Dihydrovernodalin (6). 1a2-P, 11ß,13-Dihydrovernodalol (7). The control was treated with compound vehicle and TNF-α vehicle whereas TNF-α groups received compound vehicle for 60 min and then TNF-α. To visualize the NF-κB expression (green color), the cells were fixed and stained as described in the Materials and Methods section. The scale bars denote 20 µm. *P < 0.05; CI: 95%; all the values were compared against TNF-α treatment.
3.6. Uptake and localization of vernolepin-NBD conjugate
The vernolepin-NBD conjugate was found to be toxic in JIMT-1 cells, however, with an increased IC50 compared to vernolepin alone (IC50 = 10.3 µM and 1.7 µM, respectively) (Fig. 9).
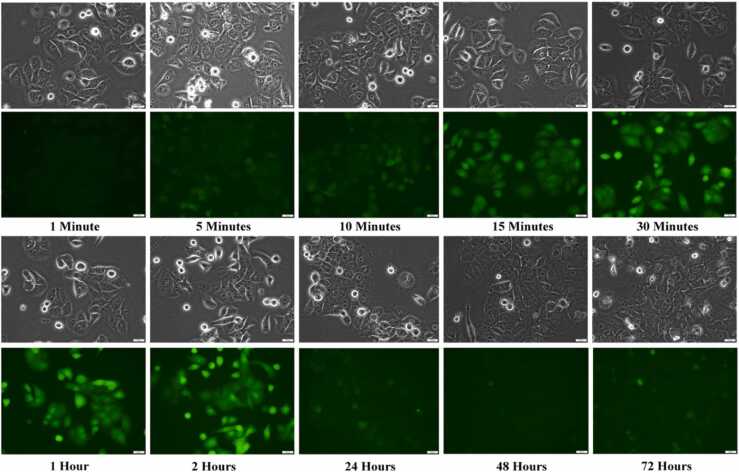
The uptake and localization study shows that the compound is taken up by JIMT-1 cells within 60 s and the intensity in the cells increases up to 2 h after addition of vernolepin-NBD to the cell culture medium (Fig. 10). The fluorescence is found in the entire cell. However, the intensity decreased after incubation with vernolepin-NBD for 24, 48, and 72 h (Fig. 10).
Fig. 10.
Uptake and localization of vernolepin-NBD conjugate in JIMT-1 cells. The times indicate time after addition of vernolepin-NDB. The upper panel shows the phase contrast image for the fluorescence image shown below. The scale bars denote 20 µm.
4. Discussion
Sesquiterpenoids are natural products with 15 carbons in the backbone and they are widely found in leaves, fruits, or roots of many plants [14]. The SLs are a subclass of sesquiterpenoids and they are typical secondary metabolites of several plant families including Acanthaceae, Anacardiaceae, Apiaceae, Euphorbiaceae, Lauraceae, Magnoliaceae, Menispermaceae, Rutaceae, and Winteraceae [15]. They are found in almost all genera of Asteraceae (also called Compositae), the sunflower family and notably in Vernonia, Tanacetum, Helenium, Ambrosia, Arnica, and Artemisia [16]. The compounds are bitter, colorless substances, with lipophilic character, and they are thermolabile and less volatile [17].
More than 5000 structurally different SLs are known so far with a proportionally high number (over 3000) isolated from Asteraceae [17], [18], [19], [20]. A wide variety of biological and pharmacological activities of the SLs have been described [17], [21]. The potential of many SLs for use in the treatment of cancer and cardiovascular disease have received large interest [18], [20], [22], [23] and anti-cancer activities have been reported [24]. The α-methylene-γ-lactone group found in SLs is correlated with many of their pharmacological actions [20], [25]. Studies involving structure-activity relationships indicate that rapid Michael type of addition takes place when cytotoxic SLs react with thiols, such as free sulfhydryl groups in mainly the amino acid cystine in proteins [17], [21]. According to these studies, selective alkylation of growth regulatory biological macromolecules (e.g. enzymes) results in the inhibition of tumor growth through cascades of reactions which leads to apoptosis. Factors including molecular geometry, lipophilicity, and chemical environment of the target sulfhydryl group affect their activities [21].
Vernomenin (1), vernolepin (5), and 11ß,13-dihydrovernodalin (6), isolated from V. leopoldi, for the first time in the present study as well as the novel compound 11ß,13-dihydrovernodalol (7) were found to be cytotoxic to JIMT-1 and MCF-7 breast cancer cell lines at low doses, however, somewhat less toxic to MCF-7 cells compared to JIMT-1 cells. They were also toxic to the normal-like MCF-10A cells to a similar degree as to the JIMT-1 cell. We have previously studied the toxicity of other natural and synthesized SLs in the same cell lines and have then found a higher toxicity in JIMT-1 breast cancer cells compared to MCF-10A normal-like cells [22], [26]. Apparently, the compounds 1, 5, and 6, are not as selective between the cancerous JIMT-1 cells and non-cancerous MCF-10A cells compared to the SLs we used in previous studies [22]. However, as will be discussed below, all compounds decreased the CSC sub-population of the JIMT-1 cell line and this is a favorable property of these SLs.
Both vernomenin (1) and vernolepin (5) were first isolated from V. hymenolepis [27] and the spectra were used as a reference. A range of biological activities, including anti-trypanosomal [28], [29] and cytotoxicity against human pharyngeal carcinoma (KB) cells [27] were reported for vernomenin (1). It was also reported to be widely used in traditional Chinese medicine in various formulations [30].
In similarity with the present finding, vernolepin (5) was found to be cytotoxic to the normal L6 cell line [28], the normal VERO and LLC-PK1 cell lines [31], and to SK-MEL, KB, BT-549, and SK-OV-3 cancer cell lines [27], [31] at similar doses. Other biological activities reported for vernolepin (5) include anti-trypanosomal [28], anti-microbial [32], and platelet anti-aggregating [33] activities.
The compound 11ß,13-dihydrovernodalin (6) has been isolated from the leaf of V. amygdalina [34] and the spectrum was used as a reference. This compound is common in various plants in the family Asteraceae [23], [35], [36]. Strong anti-plasmodial [36], [37], anti-trypanosomal [28], and anti-feedant [34] activities were reported. In the present study, the compound 6 was found to be highly cytotoxic in all breast-derived cell lines studied, a finding which is corroborated by other reports [31], [34]. However, our finding that 11ß,13-dihydrovernodalin treatment significantly reduced the TNF-α-induced translocation of NF-κB to the cell nucleus has not been reported previously. The inhibition of NF-κB translocation testifies that the compound has mechanistic anti-inflammatory effects and also affects the maintenance of the CSC population [25], [31], [38].
The novel SL 11ß,13-dihydrovernodalol (7) isolated from V. leopoldi is closely related to a compound called vernodalol (C20H24O8; 392.404 g/mol). Vernodalol has a double bond at C1-C13 position whereas 11ß,13-dihydrovernodalol (7) is reduced at this position. In the present study we have shown that 11ß,13-dihydrovernodalol (7) is about 6.9 and 5.5 times more toxic to JIMT-1 and MCF-10A cells, respectively, in dose response experiments compared to vernodalol. Thus, such small difference in chemical composition can be exploited to increase the bioactivity of SLs of various kinds. The IC50 for vernodalol was elsewhere reported to be between 16.4 and 23.8 µM in NB4, GK-1a, and HL-60 cell lines derived from acute promyelocytic leukemia [39]. Vernodalol induced dose dependent G2/M growth arrest and apoptosis through the mitochondrial pathway and inhibited activation of the PI3K/Akt/mTOR signaling pathway [39].
The significant reduction of the ALDH+ CSC subpopulation of JIMT-1 cells by treatment with the SLs in the present study indicates a possible selective inhibition on CSCs at the treatment concentrations used. In addition, the significant inhibition of cell migration in a scratch wound healing assay is an indication that the compounds may have a role in reducing a metastatic burden of cancer. Since CSCs possess more mesenchymal features than bulk cancer cells, the inhibition observed here may signify the role of the SLs to affect invasive features of the CSCs which is associated with metastasis [22], [40].
Labeling a compound of interest with a fluorescence conjugate is a popular technique employed to study the intracellular accumulation and localization of xenobiotics [41]. To the best of our knowledge, the present study demonstrated for the first time that NBD can be conjugated with an SL for intracellular localization studies. The compound is found in the cytoplasm as well as in the nuclei. There is no clear accumulation in specific organelles as example has been found for salinomycin-NBD [42]. The decreasing intensity after 24 h could be due to degradation or excretion of the compound by xenobiotic export pump.
5. Conclusion
In conclusion, the IC50 values in low μM concentrations point to the anti-proliferative activity of the compounds. The SL compounds reported here have shown desired cytotoxicity and other bioactivities including reducing the stemness of JIMT-1 breast cancer cells. We isolated a novel SL, which despite a small difference in chemical structure compared to a related compound, was several fold more cytotoxic. Thus, a very small change in chemical structure resulted in a significant increase in toxicity which may be exploited to enhance the activity of SLs. The SLs, in general, are gaining attention for their potential applications in both cancer chemotherapy and chemoprevention. Wider bioassays are therefore recommended to obtain broader understanding of their specific anti-cancer effects and normal cell cytotoxicity. In addition, further well-designed studies are needed to clearly show the localization of vernolepin (5) and other SLs within the cancer cell. These would possibly include confocal microscopy imaging, differential labeling of organelles, and spectrophotometric determination of the level of NBD in the medium after incubating for 24–72 h.
Ethics approval and consent to participate
The study was approved by the Institutional Review Board of the College of Natural and Computational Science (CNS-IRB), Addis Ababa University (IRB/022/2016). Additionally, Armauer Hansen Research Institute/All Africa Leprosy Rehabilitation and Training Hospital (AHRI/ALERT) Ethics Review Committee granted an approval (Project Registration №: PO19/16). The material transfer agreement (MTA) was obtained from the Ethiopian Biodiversity Institute (EBI) to ship the genetic material and conduct scientific research overseas.
Funding
The study was sponsored by AHRI through the Biomedical Science Partnership Program (BSPP) funded from Swedish International Development Cooperation Agency (SIDA). The Graduate Studies of the Addis Ababa University has covered some costs incurred during the field work. This work was also funded by Carolina LePrince and the “Kalenderflickorna” through crowd funding.
Authors contribution
Conceptual framework: NT, DS, BP, SO. Plant material collection, identification, and extraction: NT, DS, EG, BP. Methodology: NT, DS, BP, SO, ZE, OS. Compound isolation, characterization: ZE, OS. Cell culturing experiments, spectrophotometry, data curation, and formal analysis: NT, SO. Supervision: DS, EG, BP, SO, OS. Writing – original draft: NT, SO, ZE. Writing – review & editing: NT, SO, DS, EG, BP, ZE, OS. All authors read and approved the final manuscript.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
Helena Fritz is acknowledged for her technical support in the laboratory at the Department of Biology. We thank Atena Malakpour-Permlid for her helping hands with some of the figures.
Consent for publication
Not applicable.
Handling Editor: Dr. Aristidis Tsatsakis
Footnotes
Supplementary data associated with this article can be found in the online version at doi:10.1016/j.toxrep.2022.02.011.
Contributor Information
Nigatu Tuasha, Email: ntuasha@gmail.com.
Zilma Escobar, Email: zilma.escobar@med.lu.se.
Daniel Seifu, Email: daniel.seifu@aau.edu.et.
Endalamaw Gadisa, Email: gadisa.endalamaw@gmail.com.
Beyene Petros, Email: abule2002@yahoo.com.
Olov Sterner, Email: olov.sterner@chem.lu.se.
Stina Oredsson, Email: stina.oredsson@biol.lu.se.
Appendix A. Supplementary material
Supplementary material
.
Data Availability
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
References
- 1.Hedberg I., Friis I., Edwards S. Addis Ababa, Ethiopia, and Department of Systematic Botany; Uppsala, Sweden: 2004. Flora of Ethiopia and Eritrea. Volume 4, part 2. Asteraceae. The National Herbarium. [Google Scholar]
- 2.Dagne E. Natural Database for Africa(NDA). CD-ROM Version 2.0. In. Addis Ababa, Ethiopia, 2011.
- 3.Abera B. Vol. 13. 2003. Medicinal plants used in traditional medicine in Jimma Zone, Oromiya, Ethiopia; pp. 85–94. (Ethiop. J. Health Dev,). [Google Scholar]
- 4.Wabe N.T., Mohammed M.A., Raju N.J. An ethnobotanical survey of medicinal plants in the Southeast Ethiopia used in traditional medicine. Spatula DD. 2011;1(3):153–158. doi: 10.5455/SPATULA.20110921101924. [DOI] [Google Scholar]
- 5.Schopen A. Traditionelle Heilmittel in Jemen: Wiesbaden.: Franz Steiner Verlag, 1983.
- 6.Marzouk A.M., Abd Elhalim O.B. A new lanostane-type triterpene and sesquiterpene lactones from Vernonia leopoldii and their in vitro cytotoxicity. Nat. Prod. Res. 2016;30(7):741–749. doi: 10.1080/14786419.2015.1062004. [DOI] [PubMed] [Google Scholar]
- 7.Ekor M. The growing use of herbal medicines: issues relating to adverse reactions and challenges in monitoring safety. Front. Pharmacol. 2014;4:177. doi: 10.3389/fphar.2013.00177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.WHO . World Health Organization; 2013. WHO Traditional Medicine Strategy: 2014-2023. [Google Scholar]
- 9.Tuasha N., Seifu D., Gadisa E., Petros B., Oredsson S. Solvent fractions of selected Ethiopian medicinal plants used in traditional breast cancer treatment inhibit cancer stem cells in a breast cancer cell line. BMC Complement. Med. Ther. 2020;20:366. doi: 10.1186/s12906-020-03154-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dillekås H., Rogers M.S., Straume O. Are 90% of deaths from cancer caused by metastases? Cancer Med. 2019;8(12):5574–5576. doi: 10.1002/cam4.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Al-Hajj M., Wicha M.S., Benito-Hernandez A., Morrison S.J., Clarke M.F. Prospective identification of tumorigenic breast cancer cells. Proc. Natl. Acad. Sci. USA. 2003;100(7):3983–3988. doi: 10.1073/pnas.0530291100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tuasha N., Seifu D., Gadisa E., Petros B., Oredsson S. Cytotoxicity of selected Ethiopian medicinal plants used in traditional breast cancer treatment against breast-derived cell lines. J. Med. Plants Res. 2019;13(9):188–198. doi: 10.5897/JMPR2019.6772. [DOI] [Google Scholar]
- 13.Rodriguez L., Wu X., Guan J. Wound-healing assay. Methods Mol. Biol. 2005;294:23. doi: 10.1385/1-59259-860-9:023. [DOI] [PubMed] [Google Scholar]
- 14.E.M. Davis, R. Croteau, Cyclization enzymes in the biosynthesis of monoterpenes, sesquiterpenes, and diterpenes, in: Leeper F.J., Vederas J.C. (eds) Biosynthesis. Topics in Current Chemistry, vol 209. Springer, Berlin, Heidelberg. 〈 10.1007/3-540-48146-X〉. [DOI]
- 15.Zhang S., Won Y.-K., Ong C.-N., Shen H.-M. Anti-cancer potential of sesquiterpene lactones: bioactivity and molecular mechanisms. Curr. Med. Chem. Anticancer Agents. 2005;5(3):239–249. doi: 10.2174/1568011053765976. [DOI] [PubMed] [Google Scholar]
- 16.Kreuger M.R.O., Grootjans S., Biavatti M.W., Vandenabeele P., D’Herde K. Sesquiterpene lactones as drugs with multiple targets in cancer treatment: focus on parthenolide. Anticancer Drugs. 2012;23(9):883–896. doi: 10.1097/CAD.0b013e328356cad9. [DOI] [PubMed] [Google Scholar]
- 17.Ivanescu B., Miron A., Corciova A. Sesquiterpene lactones from Artemisia genus: biological activities and methods of analysis. J. Anal. Methods Chem. 2015 doi: 10.1155/2015/247685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shoaib M., Shah I., Ali N., Adhikari A., Tahir M.N., Shah S.W.A., Ishtiaq S., Khan J., Khan S., Umer M.N. Sesquiterpene lactone! a promising antioxidant, anticancer and moderate antinociceptive agent from Artemisia macrocephala jacquem. BMC Complement. Altern. Med. 2017;17(1):27. doi: 10.1186/s12906-016-1517-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wu Q.-X., Shi Y.-P., Jia Z.-J. Eudesmane sesquiterpenoids from the Asteraceae family. Nat. Prod. Rep. 2006;23(5):699–734. doi: 10.1039/b606168k. [DOI] [PubMed] [Google Scholar]
- 20.Chadwick M., Trewin H., Gawthrop F., Wagstaff C. Sesquiterpenoids lactones: benefits to plants and people. Int. J. Mol. Sci. 2013;14(6):12780–12805. doi: 10.3390/ijms140612780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chaturvedi D. Sesquiterpene lactones: structural diversity and their biological activities, in-opportunity, challanges and scope of natural products in medicinal chemistry. ISBN: 978–81-308–0448-4. Res.earch Signpost, Trivandrum. 2011;313–34:22. [Google Scholar]
- 22.Sotillo W.S., Villagomez R., Smiljanic S., Huang X., Malakpour A., Kempengren S., Rodrigo G., Almanza G., Sterner O., Oredsson S. Anti-cancer stem cell activity of a sesquiterpene lactone isolated from Ambrosia arborescens and of a synthetic derivative. PLoS One. 2017;12(9) doi: 10.1371/journal.pone.0184304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chukwujekwu J., Lategan C., Smith P., Van Heerden F., Van Staden J. Antiplasmodial and cytotoxic activity of isolated sesquiterpene lactones from the acetone leaf extract of Vernonia colorata. S. Afr. J. Bot. 2009;75(1):176–179. doi: 10.1016/j.sajb.2008.10.001. [DOI] [Google Scholar]
- 24.Chaturvedi D., Dwivedi P.K., Mishra M. In: Bioactive Natural Products: Chemistry and Biology. Brahmachari G., editor. Wiley-VCH Verlag GmbH and Co.; KGaA: 2015. Sesquiterpene lactones: a versatile class of structurally diverse natural products and their semisynthetic analogs as potential anticancer agents; pp. 49–85. [DOI] [Google Scholar]
- 25.Burlec A.F., Cioanca O., Enache L.C., Hancianu M. Promising biological activities of sesquiterpene lactones. Rev. Med. Chir. Soc. Med. Nat. Iaşi. 2017;121(3):645–652. [Google Scholar]
- 26.Lozano M., Soria W., Almanza G.R., Manner S., Oredsson S., Villagomez R., Sterner O. Selective cytotoxicity of damsin derivatives in breast cancer cells. J. Adv. Pharm. Sci. Technol. 2019;2(1):23–37. doi: 10.14302/issn.2328-0182.japst-19-2759. [DOI] [Google Scholar]
- 27.Kupchan S.M., Hemingway R.J., Werner D., Karim A., McPhail A., Sim G. Tumor inhibitors. XXXI. Vernolepin, a novel elemanolide dilactone tumor inhibitor from Vernonia hymenolepis. J. Am. Chem. Soc. 1968;90(13):3596–3597. doi: 10.1021/ja01015a073. [DOI] [PubMed] [Google Scholar]
- 28.Kimani N.M., Matasyoh J.C., Kaiser M., Brun R., Schmidt T.J. Anti-trypanosomatid elemanolide sesquiterpene lactones from Vernonia lasiopus O. Hoffm. Molecules. 2017;22(4):597. doi: 10.3390/molecules22040597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Grieco P.A., Nishizawa M., Oguri T., Burke S.D., Marinovic N. Sesquiterpene lactones: total synthesis of (.+-.)-vernolepin and (.+-.)-vernomenin. J. Am. Chem. Soc. 1977;99(17):5773–5780. doi: 10.1021/ja00459a039. [DOI] [PubMed] [Google Scholar]
- 30.S. Lili, Y. Shubin, S. Chowing, Z. Qian, X. Hongmin, S. Dianhua, Medicament for treating breast cancer and preparation method thereof. China Patent CN 102000085, State Intellectual Property Office (SIPO) of the P.R.C., Beijing, PRC, 2011. 〈http://hdl.handle.net/10722/195802〉.
- 31.Ahmed S., Al-Rehaily A., Ahmad M., Yousaf M., Nur-e-Alam M., Thomas J., Khan S., Khan I. Cytotoxic and antiinflammatory activities of the chemical constituents isolated from Baccharoides schimperi DC. S. Afr. J. Bot. 2018;114:9–13. doi: 10.1016/j.sajb.2017.10.007. [DOI] [Google Scholar]
- 32.Jisaka M., Ohigashi H., Takegawa K., Huffman M.A., Koshimizu K. Antitumoral and antimicrobial activities of bitter sesquiterpene lactones of Vernonia amygdalina, a possible medicinal plant used by wild chimpanzees. Biosci. Biotechnol. Biochem. 1993;57(5):833–834. doi: 10.1271/bbb.57.833. [DOI] [PubMed] [Google Scholar]
- 33.Laekeman G., De Clerck F., Vlietinck A., Herman A. Vernolepin: an antiplatelet compound of natural origin. Naunyn Schmiedebergs Arch. Pharmacol. 1985;331(1):108–113. doi: 10.1007/BF00498859. [DOI] [PubMed] [Google Scholar]
- 34.Ganjian I., Kubo I., Fludzinski P. Insect antifeedant elemanolide lactones from Vernonia amygdalina. Phytochemistry. 1983;22:2525–2526. [Google Scholar]
- 35.Laekeman G., Mertens J., Totté J., Bult H., Vlietinck A., Herman A. Isolation and pharmacological characterization of vernolepin. J. Nat. Prod. 1983;46(2):161–169. doi: 10.1021/np50026a003. [DOI] [PubMed] [Google Scholar]
- 36.Kraft C., Jenett‐Siems K., Siems K., Jakupovic J., Mavi S., Bienzle U., Eich E. In vitro antiplasmodial evaluation of medicinal plants from Zimbabwe. Phytother. Res. 2003;17(2):123–128. doi: 10.1002/ptr.1066. [DOI] [PubMed] [Google Scholar]
- 37.Pedersen M.M., Chukwujekwu J.C., Lategan C.A., Van Staden J., Smith P.J., Staerk D. Antimalarial sesquiterpene lactones from Distephanus angulifolius. Phytochemistry. 2009;70(5):601–607. doi: 10.1016/j.phytochem.2009.02.005. [DOI] [PubMed] [Google Scholar]
- 38.Rinkenbaugh A., Baldwin A. The NF-κB pathway and cancer stem cells. Cells. 2016;5(2):16. doi: 10.3390/cells5020016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wu W., Han X., Wu C., Wei G., Zheng G., Li Y., Yang Y., Yang L., He D., Zhao Y. Vernodalol mediates antitumor effects in acute promyelocytic leukemia cells. Oncol. Lett. 2018;15(2):2227–2235. doi: 10.3892/ol.2017.7544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hale J.S., Li M., Lathia J.D. The malignant social network: cell-cell adhesion and communication in cancer stem cells. Cell. Adh. Migr. 2012;6(4):346–355. doi: 10.4161/cam.21294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Shi Z.-D., Karki R.G., Oishi S., Worthy K.M., Bindu L.K., Dharmawardana P.G., Nicklaus M.C., Bottaro D.P., Fisher R.J., Burke T.R., Jr. Utilization of a nitrobenzoxadiazole (NBD) fluorophore in the design of a Grb2 SH2 domain-binding peptide mimetic. Bioorg. Med. Chem. Lett. 2005;15(5):1385–1388. doi: 10.1016/j.bmcl.2005.01.017. [DOI] [PubMed] [Google Scholar]
- 42.Huang X., Borgström Br, Stegmayr J., Abassi Y., Kruszyk M., Leffler H., Persson L., Albinsson S., Massoumi R., Scheblykin I.G. The molecular basis for inhibition of stemlike cancer cells by salinomycin. ACS Cent. Sci. 2018;4(6):760–767. doi: 10.1021/acscentsci.8b00257. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material
Data Availability Statement
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.