Abstract
Introduction
Seasonal human coronavirus (HCoV)-229E, -NL63, -OC43, and -HKU1 are seasonal coronaviruses that cause colds in humans. However, the clinical characteristics of pediatric inpatients infected with HCoVs are unclear. This study aimed to compare and clarify the epidemiological and clinical features of HCoVs and respiratory syncytial virus (RSV), which commonly causes severe respiratory infections in children.
Methods
Nasopharyngeal swabs were collected from all pediatric inpatients with respiratory symptoms at two secondary medical institutions in Fukushima, Japan. Eighteen respiratory viruses, including RSV and four HCoVs, were detected via reverse transcription-polymerase chain reaction.
Results
Of the 1757 specimens tested, viruses were detected in 1272 specimens (72.4%), with 789 single (44.9%) and 483 multiple virus detections (27.5%). RSV was detected in 639 patients (36.4%) with no difference in clinical characteristics between RSV-A and RSV-B. HCoV was detected in 84 patients (4.7%): OC43, NL63, HKU1, and 229E in 25 (1.4%), 26 (1.5%), 23 (1.3%), and 16 patients (0.9%), respectively. Patients with HCoV monoinfection (n = 35) had a significantly shorter period from onset to hospitalization (median [interquartile range] days, 2 [1–4.5] vs. 4 [2–5]), significantly shorter hospitalization stays (4 [3–5] vs. 5 [4–6]), and more cases of upper respiratory infections (37.1% vs. 3.9%) and croup (17.1% vs. 0.3%) but less cases of lower respiratory infection (54.3% vs. 94.8%) than patients with RSV monoinfection (n = 362).
Conclusion
Seasonal HCoV-infected patients account for approximately 5% of children hospitalized for respiratory tract infections and have fewer lower respiratory infections and shorter hospital stays than RSV-infected patients.
Keywords: Human coronavirus, Hospitalization, Children, Coronavirus, Respiratory syncytial virus, Croup
1. Introduction
Seasonal human coronaviruses (HCoVs) are enveloped RNA viruses with the largest genomes [1] and are known to cause zoonotic infections [2]. There are seven main HCoVs types; HCoV-229E and HCoV-NL63 belong to the genus alphacoronavirus, whereas HCoV-HKU1, HCoV-OC43, the Middle East respiratory syndrome (MERS)-CoV, severe acute respiratory syndrome (SARS)-CoV-1, and SARS-CoV-2 belong to the genus betacoronavirus [3]. HCoV-229E, -NL63, -OC43, and -HKU1 are seasonal coronaviruses that cause colds in humans [4,5]. Although seasonal HCoVs were first isolated and reported in the 1960s [[6], [7], [8]], their clinical features, virological features, and epidemic trends received little attention until MERS-CoV and SARS-CoV-1 epidemics.
The seasonal HCoV epidemic in children peaks from winter to spring [9], and most are infected by age 6 years [10]. Most respiratory tract infections with seasonal HCoV in children are mild. They are less important as causative viruses of hospitalization than respiratory syncytial virus (RSV), accounting for approximately 30% of cases of hospitalized children with respiratory tract infections [11]. However, pediatric immunosuppressed patients may develop severe lower respiratory tract inflammation due to seasonal HCoVs infection [12,13]. Nevertheless, the proportion and clinical characteristics of seasonal HCoV in pediatric inpatients are not well known. Moreover, seasonality may be very relevant to the SARS-CoV-2 epidemic in the future, when herd immunity is acquired through natural infection with SARS-CoV-2 or vaccination [[14], [15], [16]]. Therefore, understanding the epidemiological and clinical features of SARS-CoV-2 and pre-pandemic seasonal HCoV is important.
This study aimed to investigate the epidemic dynamics and clinical features of seasonal HCoV by identifying and comparing the RSV, which most commonly causes severe respiratory infections in children, and seasonal HCoV before the SARS-CoV-2 epidemic.
2. Materials and methods
2.1. Participants and sample collection
This study included all children aged <15 years who had respiratory symptoms at admission or were hospitalized for respiratory tract infections. Specifically, nasopharyngeal swabs (UTM™, COPAM Diagnostics Inc., Brescia, Italy) were collected from all pediatric inpatients with respiratory symptoms at two secondary medical facilities (Hospital A in Koriyama City and Hospital B in Fukushima City) in the Fukushima Prefecture between September 2017 and March 2020. Each of these medical institutions serves a city of about 300,000 people, with about 300–400 children admitted annually for respiratory infections in each hospital. The nasopharyngeal swab was transferred to a storage medium and cryopreserved at −80 °C until further analysis. Respiratory tract infections, including upper respiratory infection (URI), lower respiratory infection (LRI), croup, and tympanitis, were comprehensively judged based on clinical symptoms such as pharyngeal findings, cough, rhinorrhea, wheezing, fever, and imaging findings in the lung field. Moreover, we collected the following data at admission from patient charts: age; sex; C-reactive protein (CRP), and white blood cell count (WBC) at admission; PICU admission; dates of respiratory symptom onset, admission, and discharge; and clinical symptoms, including fever, cough, rhinorrhea, and wheezing.
The indications for admission were determined by experienced pediatric clinicians. The main reasons for admission included low oxygen saturation requiring oxygen support, poor oral intake, dehydration requiring fluids, a sick appearance, and bacterial infection requiring antimicrobial therapy.
2.2. PCR analysis for detection of virus
All swab specimens were tested for the following 18 types of respiratory viruses using a one- or two-step reverse transcription-polymerase chain reaction (RT-PCR) test: RSV-A and B; influenza virus (Flu) A, B, and C; HCoV -229E, -HKU1, -NL63, and -OC43; human metapneumovirus (hMPV); human parainfluenza virus (HPIV) -1, 2, 3, and 4; and human rhinovirus (HRV), and real-time PCR test: adenovirus (ADV) -2, 4; and human bocavirus (HBoV). Briefly, viral nucleic acids were extracted using one of the following kits: the QIAamp viral RNA mini kit (Qiagen, Hilden, Germany), QIAamp 96 Virus QIAcube HT kit, and Nucleospin 96 Virus kit (Macherey-Nagel, Düren, Germany). Moreover, AgPath-ID ™ One-Step RT-PCR (Thermo Fisher Scientific, Waltham, MA, USA) reagents were used for one-step RT-PCR. For two-step RT-PCR, cDNA was synthesized with random hexamer primers and Oligo(dT)12–18 Primer (Thermo Fisher Scientific) using SMART M-MLV Reverse Transcriptase (Takara Bio, Shiga, Japan). A two-step RT-PCR test was conducted using the LightCycler 480 Probes Master (Roche, Basel, Switzerland). HPIV -1, 2, 3, and 4, hMPV, HBoV, Flu C, HRV, and ADV -2 and 4 were detected using two-step PCR, and the remaining viruses were detected by one-step PCR. The setup of each primer, probe, and reaction conditions, based on previously described reports, is shown in Supplemental Table 1 [[17], [18], [19], [20], [21], [22], [23], [24]].
2.3. Statistical analysis
The chi-squared or Fisher's exact test was used to compare categorical variables such as age, sex, clinical symptoms, and diagnosis, whereas the Mann–Whitney U test was used to compare continuous variables, including age, period from onset to hospitalization, hospitalization period, WBC count, and CRP level. Moreover, the Kruskal–Wallis with Dunn's test was used to compare three or more groups of continuous variables. Statistical analyses were performed using the IBM SPSS version 26.0 (IBM Inc. Armonk, NY, USA), and significance was set at p < 0.05.
2.4. Ethics statement
The survey complied with the Declaration of Helsinki and was approved by the ethics review board of Fukushima Medical University (No. 29006). Informed consent was obtained from the parents of each patient included in this study.
3. Results
3.1. Characteristics of study participants
During the two-year and six-month periods between September 2017 and March 2020, 1757 specimens were included in the study analysis. The median age of patients admitted to the two institutions was 18 months (interquartile range [IQR], 10–39 months), and the proportion of male patients was 57.1%. Of the 1757 specimens targeted, viruses were identified in 1272 specimens (72.4%), with 789 single virus detections (44.9%) and 483 multiple virus detections (27.5%) (Table 1 ). RSV was the most detected pathogenic virus in hospitalized children, and single and multiple virus detection were identified in 639 specimens (639/1757, 36.3%). In contrast, single and multiple seasonal HCoV detections were observed in only 84 specimens (84/1757, 4.7%). There were five severe cases of admission to the PICU, namely three single virus detections (RSV, 2 cases; and hMPV, 1 case) and two multiple virus detections (RSV + HBoV, 2 cases). Thirty-two (32/1757, 1.8%) hospitalized children developed croup, of whom 27 (27/32, 84.4%) were observed to have the virus, 15 (15/32, 46.9%) were detected to have a single virus, and 12 (12/32, 37.5%) were detected to have multiple viruses (Table 2 ).
Table 1.
Clinical features of children with respiratory infections or respiratory symptoms on admission during the study period (N = 1757).
| Characteristics | Median/number | IQR/% |
|---|---|---|
| Age (months) | 18 | 10–39 |
| <2 months | 127 | 7.2 |
| 2–5 months | 170 | 9.7 |
| 6–11 months | 233 | 13.3 |
| 12–23 months | 506 | 28.8 |
| >24 months | 719 | 40.9 |
| Sex (male) | 1004 | 57.1 |
| Period from onset to hospitalization (day) | 3 | 2–5 |
| Hospitalization period (day) | 4 | 3–5 |
| Fever | 1570 | 89.4 |
| Rhinorrhea | 1189 | 67.7 |
| Cough | 1530 | 87.1 |
| Wheezing | 383 | 21.8 |
| WBC (/μL) | 10100 | 7300–13800 |
| CRP (mg/dL) | 1.3 | 0.5–3.3 |
| Intensive care in PICU |
5 |
0.29 |
| Diagnosis | ||
| Tympanitis | 34 | 1.9 |
| URI | 300 | 17.1 |
| Croup | 32 | 1.8 |
| LRI |
1359 |
77.3 |
| Virus detection | 1272 | 72.4 |
| Single | 789 | 44.9 |
| Double | 372 | 21.2 |
| Triple | 85 | 4.8 |
| Quadruple | 21 | 1.2 |
| Quintuple | 4 | 0.2 |
| Qextuple |
1 |
0.06 |
| RSV-A | 289 | 16.4 |
| RSV-B | 350 | 19.9 |
| Flu A | 60 | 3.4 |
| Flu B | 25 | 1.4 |
| Flu C | 9 | 0.5 |
| HCoV-HKU1 | 23 | 1.3 |
| HCoV-OC43 | 25 | 1.4 |
| HCoV-NL63 | 26 | 1.5 |
| HCoV-229E | 16 | 0.9 |
| hMPV | 160 | 9.1 |
| HPIV-1 | 33 | 1.9 |
| HPIV-2 | 12 | 0.7 |
| HPIV-3 | 104 | 5.9 |
| HPIV-4 | 27 | 1.5 |
| ADV-2 | 190 | 10.8 |
| ADV-4 | 79 | 4.5 |
| HBoV | 236 | 13.4 |
| HRV | 234 | 13.3 |
Data are shown as median (IQR) or number (%). The denominator for each percentage calculation is 1757, which is the total number of patients included in this study. The number of patients in whom viruses were detected included those with single and multiple virus detections.
IQR, interquartile range; WBC, white blood cell; CRP, C-reactive protein; PICU, pediatric intensive care unit; URI, upper respiratory infection; and LRI, lower respiratory infection; RSV, respiratory syncytial virus; Flu, influenza virus; HCoV, human coronavirus; hMPV, human metapneumovirus; human HPIV, parainfluenza virus; ADV, adenovirus; HBoV, human bocavirus; and HRV, human rhinovirus.
Table 2.
Causative virus detected in children with croup (n = 32).
| Croup | n (%) |
|---|---|
| Single virus detection | 15 (46.9) |
| HCoV-NL63 | 6 (18.8) |
| HPIV-1 | 3 (9.4) |
| Flu B | 2 (6.3) |
| RSV-B | 1 (3.2) |
| HPIV-2 | 1 (3.2) |
| HPIV-3 | 1 (3.2) |
| HRV |
1 (3.2) |
| Multiple virus detection | 12 (37.5) |
| HPIV-1+HBoV | 2 (6.3) |
| RSV-A + HPIV-2 | 1 (3.2) |
| RSV-B + HRV | 1 (3.2) |
| HCoV-NL63+HBoV | 1 (3.2) |
| hMPV + HBoV | 1 (3.2) |
| HPIV-2+ADV-2 | 1 (3.2) |
| HPIV-3+ADV-2 | 1 (3.2) |
| HPIV-3+ADV-4 | 1 (3.2) |
| HPIV-3+HBoV + HRV | 2 (6.3) |
| RSV-A + HPIV-2+ADV-2 | 1 (3.2) |
Data are shown as number (%). The denominator for each percentage calculation is 1757, which is the total number of patients included in this study.
RSV, respiratory syncytial virus; Flu, influenza virus; HCoV, human coronavirus; hMPV, human metapneumovirus; HPIV, human parainfluenza virus; ADV, adenovirus; HBoV, human bocavirus; and HRV, human rhinovirus.
There was no difference between the two hospitals with respect to the detection rate of RSV (304/819, 37.1% vs. 335/938, 35.7%) and seasonal HCoV (37/819, 4.5% vs. 47/938, 5.0%). The clinical differences in single detection of RSV and seasonal HCoV between Hospitals A and B are shown in Supplemental Table 2 and Supplemental Table 3, respectively. For RSV, the length of hospital stay tended to be significantly longer in Hospital B than in Hospital A. However, there were no other clinically significant differences between Hospital A and Hospital B for RSV and HCoV infections. Regarding RSV infection, the hospitalized children were younger in Hospital B than in Hospital A, and this may have been a factor in the longer hospital stay.
3.2. Epidemic status and clinical characteristics of RSV subgroup
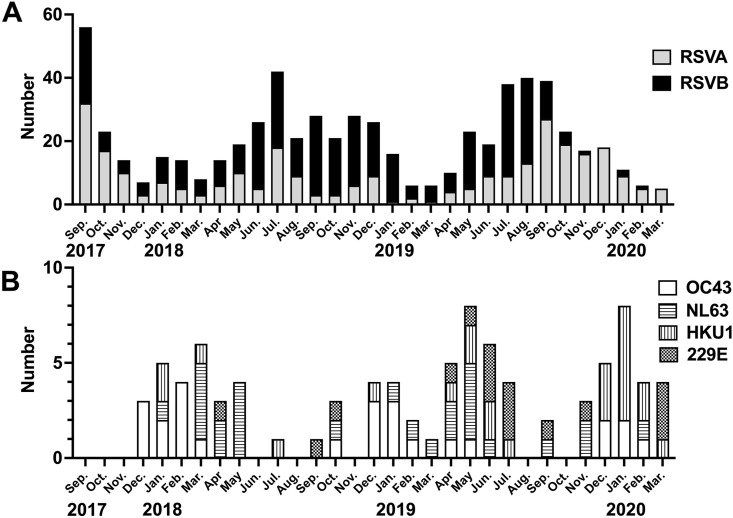
The epidemic characteristics of RSV subgroups A and B are shown in Fig. 1 A. During the study period, RSV was detected all year-round, with an epidemic peak from summer to autumn. Of the 639 RSV specimens, RSV-A and RSV-B were detected in 289 (289/639, 45.2%) and 350 (350/639, 54.7%) specimens, respectively. There was no clinically significant difference in any of the items (Table 3 ).
Fig. 1.
Trends in the number of patients with RSV and those with HCoV per month during the two-year and six-month survey period. A: RAV A and B; and B: HCoV-HKU1, -OC43, -NL63 and 229E.RSV, respiratory syncytial virus; and HCoV, human coronavirus.
Table 3.
Clinical features associated with different RSV subgroups (n = 639).
| Characteristics | RSV-A (n = 289) |
RSV-B (n = 350) |
|
|---|---|---|---|
| Median [IQR] /number (%) |
Median [IQR] /number (%) |
||
| Age (months) | 15 [5–29] | 14 [5–26] | 0.368 |
| <2 | 36 (12.5) | 45 (12.9) | |
| 2–5 | 39 (13.5) | 46 (13.1) | |
| 6–11 | 37 (12.8) | 38 (16.6) | |
| 12–23 | 81 (28.0) | 100 (28.6) | |
| >24 | 96 (33.2) | 101 (28.9) | |
| Sex (male) | 144 (49.8) | 204 (58.3) | 0.074 |
| Period from onset to hospitalization (day) | 4 [2–5] | 3 [2–5] | 0.938 |
| Hospitalization period (day) | 5 [4–6] | 5 [4–6] | 0.834 |
| Fever | 250 (86.5) | 304 (86.9) | 0.997 |
| Rhinorrhea | 222 (76.8) | 251 (71.7) | 0.092 |
| Cough | 269 (93.1) | 325 (92.9) | 0.544 |
| Wheezing | 78 (27.0) | 95 (27.1) | 0.960 |
| WBC (/μL) | 9400 [7100–12300] | 9600 [7200–13125] | 0.312 |
| CRP (mg/dL) | 1.1 [0.3–2.6] | 1.1 [0.2–3.7] | 0.831 |
| Admission of PICU |
3 (1.0) |
1 (0.3) |
0.225 |
| Diagnosis | |||
| Tympanitis | 2 (0.7) | 6 (1.7) | 0.254 |
| URI | 15 (5.2) | 29 (8.3) | 0.135 |
| Croup | 2 (0.7) | 2 (0.6) | 0.838 |
| LRI |
270 (93.4) |
315 (90.0) |
0.058 |
| Single virus detection | 174 (60.2) | 188 (53.7) | 0.077 |
| Multiple virus detection | 115 (39.8) | 162 (46.3) | |
| Flu (A, B, C) | 8 (2.8) | 8 (2.3) | 0.679 |
| HCoV (HKU1, OC43, 229E, and NL63) | 8 (2.8) | 13 (3.7) | 0.412 |
| hMPV | 11 (3.8) | 20 (5.7) | 0.310 |
| HPIV (1, 2, 3, 4) | 19 (6.6) | 30 (8.6) | 0.466 |
| ADV (2, 4) | 32 (11.1) | 52 (14.9) | 0.148 |
| HBoV | 40 (13.8) | 53 (15.1) | 0.550 |
| HRV | 30 (10.4) | 44 (12.6) | 0.263 |
Data are shown as median (IQR) or numbers (%).
RSV, respiratory syncytial virus; IQR, interquartile range; WBC, white blood cell; CRP, C-reactive protein; PICU, pediatric intensive care unit; URI, upper respiratory infection; LRI, lower respiratory infection; Flu, influenza virus; HCoV, human coronavirus; hMPV, human metapneumovirus; HPIV, human parainfluenza virus; ADV, adenovirus; HBoV, human bocavirus; and HRV, human rhinovirus.
3.3. Epidemic status and clinical characteristics of four seasonal HCoVs
Fig. 1B shows the epidemic situation of HCoV-HKU1, -OC43, -NL63, and 229E. Of the 84 patients with seasonal HCoV, OC43, NL63, HKU1, and 229E were detected in 25 (29.8%), 26 (30.9%), 23 (27.3%), and 16 patients (19.0%), respectively. In total, 35 patients (35/84, 41.7%) were detected to have a single HCoV, and the remaining 49 (58.3%) had multiple viruses. There were five multiple detections of seasonal HCoVs (the combinations were HCoV-OC43 and -HKU1 in two, -OC43 and 229E in one, 229E and NL63 in one, and -OC43, -HKU1, and -NL63 in one case). The virus most detected with HCoV was RSV (n = 21, 25.0%). None of the HCoV-infected patients required intensive care in the PICU. Notably, among the HCoV-infected individuals, only those with HCoV-NL63 had croup, accounting for 26.9% (7/26) of the NL63 infections (Table 4 ).
Table 4.
Clinical features associated with four different HCoVs (n = 84).
| Total (n = 84) |
OC43 (n = 25) |
NL63 (n = 26) |
HKU1 (n = 23) |
229E (n = 16) |
|
|---|---|---|---|---|---|
| Median [IQR] /number (%) |
Median [IQR] /number (%) |
Median [IQR] /number (%) |
Median[IQR] /number (%) |
Median [IQR] /number (%) |
|
| Age (Months) | 16.5 [7.0–38.3] | 12.0 [5.0–29.0] | 16.5 [7.5–23.8] | 34 [9.5–46.5] | 18 [7.5–43.8] |
| <2 months | 8 (9.5) | 3 (12.0) | 1 (3.8) | 3 (13.0) | 3 (18.8) |
| 2–5 months | 10 (11.9) | 4 (16.0) | 4 (15.4) | 1 (4.3) | 1 (6.3) |
| 6–11 months | 11 (13.1) | 5 (20.0) | 4 (15.4) | 2 (8.7) | 1 (6.3) |
| 12–23 months | 23 (27.4) | 6 (24.0) | 10 (38.5) | 4 (17.4) | 4 (25.0) |
| >24 months | 32 (38.1) | 7 (28.0) | 7 (26.9) | 13 (56.5) | 7 (43.8) |
| Sex (male) | 52 (61.9) | 16 (64.0) | 18 (69.2) | 16 (69.6) | 6 (37.5) |
| Period from onset to hospitalization (day) | 3 [1–5] | 3 [1–4] | 2.5 [1.3–4] | 4 [1–5] | 2 [2–4] |
| Hospitalization period (day) | 4 [4–5] | 4 [4–5] | 4 [3–5] | 5 [4–6] | 4.5 [4–6.3] |
| Fever | 74 (88.1) | 18 (72.0) | 24 (92.3) | 21 (91.3) | 14 (87.5) |
| Rhinorrhea | 53 (63.1) | 20 (80.0) | 11 (42.3) | 18 (78.3) | 9 (56.3) |
| Cough | 77 (91.7) | 24 (96.0) | 25 (92.3) | 20 (87.0) | 14 (87.5) |
| Wheezing | 23 (27.4) | 4 (16.0) | 8 (30.8) | 7 (30.4) | 5 (31.3) |
| WBC (/μL) | 10400 [7350–14275] | 10400 [7800–12200] | 8650 [7225–13675] | 9100 [6700–15500] | 11150 [8650–14375] |
| CRP (mg/dL) |
1.1 [0.3–2.6] |
0.8 [0.3–1.7] |
1.0 [0.5–2.5] |
1.2 [0.3–2.2] |
0.9 [0.4–2.9] |
| Diagnosis | |||||
| Tympanitis | 4 (4.8) | 1 (4.0) | 1 (3.8) | 2 (8.7) | 0 (0.0) |
| URI | 19 (22.6) | 4 (16.0) | 10 (38.5) | 3 (13.0) | 3 (18.8) |
| Croup | 7 (8.3) | 0 (0.0) | 7 (26.9) | 0 (0.0) | 0 (0.0) |
| LRI |
59 (70.2) |
18 (72.0) |
15 (57.7) |
18 (78.3) |
11 (68.8) |
| Single virus detection | 35 (41.7) | 9 (36.0) | 11 (42.3) | 10 (43.5) | 2 (12.5) |
| Multiple virus detection |
49 (58.3) |
16 (64.0) |
15 (57.7) |
13 (56.6) |
14 (87.5) |
| RSV (A, B) | 21 (25.0) | 7 (28.0) | 8 (30.8) | 2 (8.7) | 5 (31.3) |
| Flu (A, B, C) | 3 (3.6) | 1 (4.0) | 1 (3.8) | 1 (4.3) | 0 (0.0) |
| HCoV (HKU1, OC43, 229E, NL63) | 6 (7.1) | 4 (16.0) | 2 (7.7) | 2 (8.7) | 3 (18.8) |
| hMPV | 10 (20.4) | 1 (4.0) | 2 (7.7) | 5 (21.7) | 2 (12.5) |
| HPIV (1, 2, 3, 4) | 3 (6.1) | 0 (0.0) | 0 (0.0) | 2 (8.7) | 1 (6.3) |
| ADV (2, 4) | 11 (13.1) | 4 (25.0) | 4 (15.4) | 1 (4.3) | 1 (6.3) |
| HboV | 12 (14.3) | 3 (12.0) | 4 (15.4) | 3 (13.0) | 2 (12.5) |
| HRV | 10 (11.9) | 2 (8.0) | 2 (7.7) | 3 (13.0) | 3 (18.8) |
Data are shown as median (IQR) or numbers (%).
IQR, interquartile range; WBC, white blood cell; CRP, C-reactive protein; PICU, pediatric intensive care unit; URI, upper respiratory infection; LRI, lower respiratory infection; RSV, respiratory syncytial virus; Flu, influenza virus; HCoV, hMPV, human metapneumovirus; HPIV, human parainfluenza virus; ADV, adenovirus; HBoV, human bocavirus; and HRV, human rhinovirus.
3.4. Comparison of clinical features of RSV and seasonal HCoV
To investigate the clinical features of less popular HCoVs, the specimens of patients with RSV monoinfection (n = 362), those with HCoV monoinfection (n = 35), and those with a co-infection of RSV and HCoV (n = 9), excluding other specimens with multiple viruses, were statistically compared. Patients with HCoV monoinfection had a significantly shorter period from onset to hospitalization (2 [1–4.5] days vs. 4 [1–4] days, P = 0.004) and a significantly shorter hospitalization period (4 [2–4] days vs. 5 [3–5] days, P = 0.008) than patients with RSV monoinfection. Additionally, patients with HCoV monoinfection had a significantly higher incidence of URI (13/35, 37.1% vs. 14/362, 3.9%), tympanitis (2/35, 5.7% vs. 2/362, 0.6%), and croup (6/35, 16.1% vs. 1/362, 0.3%), but a lower incidence of LRI (19/35, 54.3% vs. 343/362, 94.8%), than patients with RSV monoinfection (Table 5 ).
Table 5.
Comparison of clinical characteristics among patients with RSV monoinfection, HCoV monoinfection, and RSV and HCoV co-infection.
| RSV (n = 362) |
HCoV (n = 35) |
RSV + HCoV (n = 9) |
|||||
|---|---|---|---|---|---|---|---|
| Median [IQR]/number (%) | Median [IQR]/number (%) | Median [IQR]/number (%) | PA | PB | PC | PD | |
| Age (Months) | 14.0 [3.3–25.0] | 16.0 [8.0–40.0] | 7.0 [1.0–20.0] | 0.047 | 0.076 | 0.424 | 0.167 |
| <2 months | 58 (16.0) | 2 (5.7) | 3 (33.3) | ||||
| 2–5 months | 56 (15.5) | 5 (14.3) | 2 (11.1) | ||||
| 6–11 months | 46 (12.7) | 5 (14.3) | 3 (22.2) | ||||
| 12–23 months | 105 (29.0) | 9 (25.7) | 2 (22.2) | ||||
| >24 months | 98 (27.1) | 14 (40.0) | 1 (11.1) | ||||
| Sex (male) | 188 (51.9) | 25 (71.4) | 6 (66.7) | 0.067 | |||
| Period from onset to hospitalization (day) | 4 [2–5] | 2 [1–4.5] | 3 [2–3] | 0.003 | 0.004 | 0.540 | 1.0 |
| Hospitalization period (day) | 5 [4–6] | 4 [3–5] | 5 [4–5] | 0.007 | 0.008 | 0.897 | 1.0 |
| Fever | 308 (85.1) | 32 (91.4) | 8 (88.9) | 0.670 | |||
| Rhinorrhea | 280 (77.3) | 18 (51.4) | 8 (88.9) | 0.002 | |||
| Cough | 344 (95.0) | 32 (91.4) | 9 (100) | 0.510 | |||
| Wheezing | 95 (26.2) | 11 (31.4) | 1 (11.1) | 0.462 | |||
| WBC (/μL) | 9600 [7200–12850] | 11300 [7300–15300] | 8900 [7100–10300] | 0.207 | |||
| CRP (mg/dL) | 1.0 [0.2–2.7] | 1.2 [0.6–2.8] | 0.2 [0.1–0.8] | 0.138 | |||
| Admission of PICU |
2 (0.6) |
0 (0.0) |
0 (0.0) |
0.885 |
|||
| Diagnosis | |||||||
| Tympanitis | 2 (0.6) | 2 (5.7) | 0 (0.0) | 0.012 | |||
| URI | 14 (3.9) | 13 (37.1) | 0 (0.0) | <0.001 | |||
| Croup | 1 (0.3) | 6 (17.1) | 0 (0.0) | <0.001 | |||
| LRI | 343 (94.8) | 19 (54.3) | 9 (100) | <0.001 |
PA: Kruskal–Wallis test or Chi-squared test, PB, PC, PD: Dunn's multiple comparison tests.
PB, RSV vs. HCoV; PC, RSV vs. RSV + HCoV PD, HCoV vs. RSV + HCoV.
Data are shown as median (IQR) or number (%).
IQR, interquartile range; WBC, white blood cell; CRP, C-reactive protein; PICU, pediatric intensive care unit; URI, upper respiratory infection; and LRI, lower respiratory infection.
4. Discussion
This study revealed that the detection rates of RSV and seasonal HCoV in hospitalized children with airway symptoms in two secondary medical institutions in Japan for the two-year and six-month survey periods were 36.4% and 4.7%, respectively. Moreover, patients with seasonal HCoV monoinfection had a significantly shorter period from onset to hospitalization and period of hospitalization and a significantly higher incidence of URIs, tympanitis, and croup, but a lower incidence of LRI, than those with RSV monoinfection.
Although it depends on the virus detection methods, the virus detection rate by a PCR test in children hospitalized for respiratory tract infections was reported to be approximately 40–85% [[25], [26], [27], [28]]. The RSV and seasonal HCoV detection rates in children hospitalized for respiratory tract infections were between 10 and 40% [25,[27], [28], [29], [30], [31], [32]] and 2.5–9.0% [25,26,[28], [29], [30],[32], [33], [34], [35], [36]], respectively. Furthermore, previous reports have shown that the co-detection rate of seasonal HCoV and other respiratory viruses was 35–80% [25,26,[28], [29], [30],[33], [34], [35],37], of which the co-detection rate with RSV and seasonal HCoV was 10–49% [25,26,[28], [29], [30],[33], [34], [35],37]. In this study, the co-detection rate between seasonal HCoV and other respiratory viruses was 58.3%, of which the co-detection rate with RSV was 25.0%. The detection rate of multiple viruses during airway infection is reportedly higher in children than in adults [38]. Regarding the clinical severity of the detection of multiple or single viruses, a previous meta-analysis reported no clinically significant difference between the detection of multiple viruses and the detection of a single virus [39]. On the contrary, the detection of a single virus showed a significantly higher frequency of radiographic findings of alveolar pneumonia than the detection of multiple viruses [40]. The clinical severity of multiple virus detection remains unclear [41]. Although the number of overlapping detections of RSV and seasonal HCoV was small, our results do show that there was little difference in clinical severity compared to single detections.
Clinical differences have been reported among RSV subgroups. Several previous reports indicated that RSV-A infection is more severe than RSV-B infection [31,32]. In contrast, in children aged <6 months, RSV-B infection was associated with a significantly prolonged hospital stay and required respiratory support therapy [42]. There is no consensus on the differences in clinical symptoms and severity between subgroups [43]. Meanwhile, the BA genotype of RSV-B has been reported to cause more severe infections in patients than the ON1 genotype of RSV-A [44,45]. During this research period, the predominant genotypes of RSV-A and RSV-B worldwide were reportedly BA9 and ON1, respectively [[46], [47], [48]]. Nevertheless, this study showed no difference in severity, including the PICU admission rate and hospitalization period, between RSV-A and RSV-B.
The number of days from the onset of RSV to the peak of symptoms has been reported to be approximately 3–6 days [49,50]. The median time from onset to admission of patients with RSV monoinfection was 4 days (IQR 2–5 days), indicating that the time from onset to hospitalization was almost the same as that from onset to peak. Meanwhile, the median time from onset to admission of patients with seasonal HCoV monoinfection was shorter than that of patients with RSV monoinfection. To the best of our knowledge, there is limited knowledge on the time from onset to the peak of the HCoV infection; this is the first study to report that the time from onset to the peak of HCoV infection may be shorter than that of RSV infection.
PIV, HCoV, RSV, Flu, and hMPV are causative viruses of croup [[51], [52], [53]]. In the 32 children requiring hospitalization who developed croup during the study period, HCoV-NL63 was the most frequently detected causative virus (n = 7, 21.8%), and among patients infected with HCoV, only those with HCoV-NL63 developed croup. Although receptors in each HCoV associated with the establishment of infection are different [54], the receptor for HCoV-NL63 is ACE2, similar to SARS-CoV-2 [54], and ACE2 is also present in the epithelium of the larynx [55]. SARS-CoV-2 can also cause croup in children [[56], [57], [58]]; however, croup symptoms, including barking cough and stridor, are not the main symptoms of the SARS-CoV-2 infection in children. Therefore, whether the presence of ACE2 is strongly involved in the development of croup remains unclear. Further studies on children infected with SARS-CoV-2 are needed to verify our results.
This study had a few limitations. Co-infection with bacteria, including Mycoplasma pneumoniae, was not considered. Co-infection with bacteria may affect the test values of CRP, WBC, and disease severity. It is also necessary to further investigate bacterial and viral infections.
In conclusion, this study showed that the detection rates of RSV and seasonal HCoV in hospitalized children up to the time immediately before the SARS-CoV-2 epidemic were 36.4% and 4.7%, respectively. Patients with seasonal HCoV monoinfection had less LRI and a shorter period from onset to hospitalization and hospital stay than those with RSV monoinfection. We believe that seasonal HCoV trends before the epidemic of SARS-CoV-2 will be helpful in understanding symptoms if SARS-CoV-2 becomes common in children after the pandemic.
Declaration of interest
None.
Author contributions
Y.K. and K.H. conceived and designed the research; Y. K. wrote the paper; Y.K., K.S., S.N., M.C., T.O., F.M., H.S., S.S., R.S. I.M., N.I., and H.T. contributed to data collection and analysis. K. H. edited the manuscript, and K.S., M.S., M.T., and M.H. critically revised the article for important intellectual content. All authors reviewed the results and approved the final version of the manuscript.
Authorship statement
All authors meet the ICMJE authorship criteria.
Funding
This work was supported by a Grants-in-Aid (grant number: fk0108119h0001) from the Japan Agency for Medical Research and Development.
Acknowledgments
We would like to thank Kyosuke Sasaki, Yuka Takeda, Hiroki Tsukada, Naoko Suzuki, Hirotaka Ichikawa, Junya Saito, Keishi Yamane, Hisao Okabe, Masayoshi Suzuki, and Mamiko Hosoya for their contributions to the collection of specimens.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jiac.2022.03.001.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Schoeman D., Fielding B.C. Coronavirus envelope protein: current knowledge. Virol J. 2019;16:69. doi: 10.1186/s12985-019-1182-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ye Z.W., Yuan S., Yuen K.S., Fung S.Y., Chan C.P., Jin D.Y. Zoonotic origins of human coronaviruses. Int J Biol Sci. 2020;16:1686–1697. doi: 10.7150/ijbs.45472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cui J., Li F., Shi Z.L. Origin and evolution of pathogenic coronaviruses. Nat Rev Microbiol. 2019;17:181–192. doi: 10.1038/s41579-018-0118-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Heikkinen T., Järvinen A. The common cold. Lancet. 2003;361:51–59. doi: 10.1016/S0140-6736(03)12162-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.De Wit E., Van Doremalen N., Falzarano D., Munster V.J. SARS and MERS: recent insights into emerging coronaviruses. Nat Rev Microbiol. 2016;14:523–534. doi: 10.1038/nrmicro.2016.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hamre D., Procknow J.J. A new virus isolated from the human respiratory tract. Proc Soc Exp Biol Med. 1966;121:190–193. doi: 10.3181/00379727-121-30734. [DOI] [PubMed] [Google Scholar]
- 7.McIntosh K., Dees J.H., Becker W.B., Kapikian A.Z., Chanock R.M. Recovery in tracheal organ cultures of novel viruses from patients with respiratory disease. Proc Natl Acad Sci U S A. 1967;57:933–940. doi: 10.1073/pnas.57.4.933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tyrrell D.A., Bynoe M.L. Cultivation of a novel type of common-cold virus in organ cultures. Br Med J. 1965;1:1467–1470. doi: 10.1136/bmj.1.5448.1467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Komabayashi K., Seto J., Matoba Y., Aoki Y., Tanaka S., Ikeda T., et al. Seasonality of human coronavirus OC43, NL63, HKU1, and 229E infection in Yamagata, Japan, 2010–2019. Jpn J Infect Dis. 2020;73:394–397. doi: 10.7883/yoken.JJID.2020.525. [DOI] [PubMed] [Google Scholar]
- 10.Dijkman R., Jebbink M.F., El Idrissi N.B., Pyrc K., Müller M.A., Kuijpers T.W., et al. Human coronavirus NL63 and 229E seroconversion in children. J Clin Microbiol. 2008;46:2368–2373. doi: 10.1128/JCM.00533-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rha B., Curns A.T., Lively J.Y., Campbell A.P., Englund J.A., Boom J.A., et al. Respiratory syncytial virus-associated hospitalizations among young children: 2015–2016. Pediatrics. 2020:146. doi: 10.1542/peds.2019-3611. [DOI] [PubMed] [Google Scholar]
- 12.Xia S., Xu W., Wang Q., Wang C., Hua C., Li W., et al. Peptide-based membrane fusion inhibitors targeting HCoV-229E spike protein HR1 and HR2 domains. Int J Mol Sci. 2018;19 doi: 10.3390/ijms19020487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chang T.Y., Du C.J., Chang C.C., Chen S.H., Chen C.J., Chiu C.Y., et al. Human coronavirus OC43 infection associated pneumonia in a girl with acute lymphoblastic leukemia: a case report. Med (Baltim) 2020;99 doi: 10.1097/MD.0000000000021520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Smith T.P., Flaxman S., Gallinat A.S., Kinosian S.P., Stemkovski M., Unwin H.J.T., et al. Temperature and population density influence SARS-CoV-2 transmission in the absence of nonpharmaceutical interventions. Proc Natl Acad Sci U S A. 2021;118 doi: 10.1073/pnas.2019284118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fontal A., Bouma M.J., San-José A., López L., Pascual M., Rodó X. Climatic signatures in the different COVID-19 pandemic waves across both hemispheres. Nat Comput Sci. 2021;1:655–665. doi: 10.1038/s43588-021-00136-6. [DOI] [PubMed] [Google Scholar]
- 16.Gavenčiak T., Teperowski Monrad J., Leech G., Sharma M., Mindermann S., Marcus Brauner J., et al. Seasonal variation in SARS-CoV-2 transmission in temperate climates. medRxiv. 2021 doi: 10.1101/2021.06.10.21258647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang L., Piedra P.A., Avadhanula V., Durigon E.L., Machablishvili A., López M.R., et al. Duplex real-time RT-PCR assay for detection and subgroup-specific identification of human respiratory syncytial virus. J Virol Methods. 2019;271:113676. doi: 10.1016/j.jviromet.2019.113676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Influenza diagnostic manual. Available at https://www.niid.go.jp/niid/images/lab-manual/influenza20190116.pdf.
- 19.Wong S., Pabbaraju K., Pang X.L., Lee B.E., Fox J.D. Detection of a broad range of human adenoviruses in respiratory tract samples using a sensitive multiplex real-time PCR assay. J Med Virol. 2008;80:856–865. doi: 10.1002/jmv.21136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Do D.H., Laus S., Leber A., Marcon M.J., Jordan J.A., Martin J.M., et al. A one-step, real-time PCR assay for rapid detection of rhinovirus. J Mol Diagn. 2010;12:102–108. doi: 10.2353/jmoldx.2010.090071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kaida A., Kubo H., Shiomi M., Kohdera U., Iritani N. Evaluation of real-time RT-PCR compared with conventional RT-PCR for detecting human Metapneumovirus RNA from clinical specimens. Jpn J Infect Dis. 2008;61:461–464. [PubMed] [Google Scholar]
- 22.Van De Pol A.C., Van Loon A.M., Wolfs T.F.W., Jansen N.J.G., Nijhuis M., Breteler E.K., et al. Increased detection of respiratory syncytial virus, influenza viruses, parainfluenza viruses, and adenoviruses with real-time pcr in samples from patients with respiratory symptoms. J Clin Microbiol. 2007;45:2260–2262. doi: 10.1128/JCM.00848-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kaida A., Kubo H., Takakura K.I., Iritani N. Detection and quantitative analysis of human bocavirus associated with respiratory tract infection in Osaka City, Japan. Microbiol Immunol. 2010;54:276–281. doi: 10.1111/j.1348-0421.2010.00207.x. [DOI] [PubMed] [Google Scholar]
- 24.Kaida A., Kubo H., Takakura K.I., Sekiguchi J.I., Yamamoto S.P., Kohdera U., et al. Associations between CO-detected respiratory viruses in children with acute respiratory infections. Jpn J Infect Dis. 2014;67:469–475. doi: 10.7883/yoken.67.469. [DOI] [PubMed] [Google Scholar]
- 25.Calvo C., Alcolea S., Casas I., Pozo F., Iglesias M., Gonzalez-Esguevillas M., et al. A 14-year prospective study of human coronavirus infections in hospitalized children: comparison with other respiratory viruses. Pediatr Infect Dis J. 2020;39:653–657. doi: 10.1097/INF.0000000000002760. [DOI] [PubMed] [Google Scholar]
- 26.Haddadin Z., Chappell J., McHenry R., Pulido C.G., Rahman H., Gu W., et al. Coronavirus surveillance in a pediatric population in Jordan from 2010 to 2013: a prospective viral surveillance study. Pediatr Infect Dis J. 2020;40:12–17. doi: 10.1097/INF.0000000000002965. [DOI] [PubMed] [Google Scholar]
- 27.Zhang Y., Su L., Chen Y., Yu S., Zhang D., Mao H., et al. Etiology and clinical characteristics of SARS-CoV-2 and other human coronaviruses among children in Zhejiang Province, China 2017–2019. Virol J. 2021;18:1–11. doi: 10.1186/s12985-021-01562-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tan J., Wu J., Jiang W., Huang L., Ji W., Yan Y., et al. Etiology, clinical characteristics and coinfection status of bronchiolitis in Suzhou. BMC Infect Dis. 2021;21:135. doi: 10.1186/s12879-021-05772-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhang S.F., Tuo J.L., Huang X.B., Zhu X., Zhang D.M., Zhou K., et al. Epidemiology characteristics of human coronaviruses in patients with respiratory infection symptoms and phylogenetic analysis of HCoV-OC43 during 2010-2015 in Guangzhou. PLoS One. 2018;13 doi: 10.1371/journal.pone.0191789. 1–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Heimdal I., Moe N., Krokstad S., Christensen A., Skanke L.H., Nordbø S.A., et al. Human coronavirus in hospitalized children with respiratory tract infections: a 9-year population-based study from Norway. J Infect Dis. 2019;219:1198–1206. doi: 10.1093/infdis/jiy646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ning G., Wang X., Wu D., Yin Z., Li Y., Wang H., et al. The etiology of community-acquired pneumonia among children under 5 years of age in mainland China, 2001–2015: a systematic review. Hum Vaccines Immunother. 2017;13:2742–2750. doi: 10.1080/21645515.2017.1371381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Huang X.B., Yuan L., Ye C.X., Zhu X., Lin C.J., Zhang D.M., et al. Epidemiological characteristics of respiratory viruses in patients with acute respiratory infections during 2009-2018 in southern China. Int J Infect Dis. 2020;98:21–32. doi: 10.1016/j.ijid.2020.06.051. [DOI] [PubMed] [Google Scholar]
- 33.Otieno G.P., Murunga N., Agoti C.N., Gallagher K.E., Awori J.O., Nokes D.J. Surveillance of endemic human coronaviruses (HCoV-NL63, OC43 and 229E) associated with childhood pneumonia in Kilifi, Kenya. Wellcome Open Res. 2020;5:150. doi: 10.12688/wellcomeopenres.16037.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zeng Z.Q., Chen D.H., Tan W.P., Qiu S.Y., Xu D., Liang H.X., et al. Epidemiology and clinical characteristics of human coronaviruses OC43, 229E, NL63, and HKU1: a study of hospitalized children with acute respiratory tract infection in Guangzhou, China. Eur J Clin Microbiol Infect Dis. 2018;37:363–369. doi: 10.1007/s10096-017-3144-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fischer N., Dauby N., Bossuyt N., Reynders M., Gérard M., Lacor P., et al. Monitoring of human coronaviruses in Belgian primary care and hospitals, 2015–20: a surveillance study. Lancet Microbe. 2021;(2) doi: 10.1016/S2666-5247(20)30221-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cabeça T.K., Granato C., Bellei N. Epidemiological and clinical features of human coronavirus infections among different subsets of patients. Influenza Other Respir Viruses. 2013;7:1040–1047. doi: 10.1111/irv.12101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kim T., Choi H., Shin T.R., Ko Y., Park Y.B., Kim HIl, et al. Epidemiology and clinical features of common community human coronavirus disease. J Thorac Dis. 2021;13:2288–2299. doi: 10.21037/jtd-20-3190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nascimento-Carvalho C.M., Ruuskanen O. Clinical significance of multiple respiratory virus detection. Pediatr Infect Dis J. 2016;35:338–339. doi: 10.1097/INF.0000000000001032. [DOI] [PubMed] [Google Scholar]
- 39.Asner S.A., Science M.E., Tran D., Smieja M., Merglen A., Mertz D. Clinical disease severity of respiratory viral co-infection versus single viral infection: a systematic review and meta-analysis. PLoS One. 2014;9 doi: 10.1371/journal.pone.0099392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Esposito S., Daleno C., Prunotto G., Scala A., Tagliabue C., Borzani I., et al. Impact of viral infections in children with community-acquired pneumonia: results of a study of 17 respiratory viruses. 2013. Influenza Other Respir Viruses. 7, 18-26. [DOI] [PMC free article] [PubMed]
- 41.Goka E.A., Vallely P.J., Mutton K.J., Klapper P.E. Single and multiple respiratory virus infections and severity of respiratory disease: a systematic review. Paediatr Respir Rev. 2014;15:363–370. doi: 10.1016/j.prrv.2013.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hornsleth A., Klug B., Nir M., Johansen J., Hansen K.S., Christensen L.S., et al. Severity of respiratory syncytial virus disease related to type and genotype of virus and to cytokine values in nasopharyngeal secretions. Pediatr Infect Dis J. 1998;17:1114–1121. doi: 10.1097/00006454-199812000-00003. [DOI] [PubMed] [Google Scholar]
- 43.Borchers A.T., Chang C., Gershwin M.E., Gershwin L.J. Respiratory syncytial virus--a comprehensive review. Clin Rev Allergy Immunol. 2013;45:331–379. doi: 10.1007/s12016-013-8368-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Esposito S., Piralla A., Zampiero A., Bianchini S., Di Pietro G., Scala A., et al. Characteristics and their clinical relevance of respiratory syncytial virus types and genotypes circulating in Northern Italy in five consecutive winter seasons. PLoS One. 2015;10 doi: 10.1371/journal.pone.0129369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Panayiotou C., Richter J., Koliou M., Kalogirou N., Georgiou E., Christodoulou C. Epidemiology of respiratory syncytial virus in children in Cyprus during three consecutive winter seasons (2010–2013): age distribution, seasonality and association between prevalent genotypes and disease severity. Epidemiol Infect. 2014;142:2406–2411. doi: 10.1017/S0950268814000028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tabor D.E., Fernandes F., Langedijk A.C., Wilkins D., Lebbink R.J., Tovchigrechko A., et al. Global molecular epidemiology of respiratory syncytial virus from the 2017–2018 INFORM-RSV study. J Clin Microbiol. 2020:59. doi: 10.1128/JCM.01828-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Chen X., Zhu Y., Wang W., Li C., An S., Lu G., et al. A multi-center study on molecular epidemiology of human respiratory syncytial virus from children with acute lower respiratory tract infections in the mainland of China between 2015 and 2019. Virol Sin. 2021;36:1475–1483. doi: 10.1007/s12250-021-00430-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Tavakoli F., Izadi A., Yavarian J., Sharifi-Zarchi A., Salimi V., Mokhtari-Azad T. Determination of genetic characterization and circulation pattern of respiratory syncytial virus (RSV) in children with a respiratory infection, Tehran, Iran, during 2018–2019. Virus Res. 2021;305:198564. doi: 10.1016/j.virusres.2021.198564. [DOI] [PubMed] [Google Scholar]
- 49.Barr R., Green C.A., Sande C.J., Drysdale S.B. Respiratory syncytial virus: diagnosis, prevention and management. Ther Adv Infect Dis. 2019;6 doi: 10.1177/2049936119865798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Xing Y., Proesmans M. New therapies for acute RSV infections: where are we? American Academy of Pediatrics. Eur J Pediatr. 2019;178:131–138. doi: 10.1007/s00431-018-03310-7. [DOI] [PubMed] [Google Scholar]
- 51.Sung J.Y., Lee H.J., Eun B.W., Kim S.H., Lee S.Y., Lee J.Y., et al. Role of human coronavirus NL63 in hospitalized children with croup. Pediatr Infect Dis J. 2010;29:822–826. doi: 10.1097/INF.0b013e3181e7c18d. [DOI] [PubMed] [Google Scholar]
- 52.Van Der Hoek L., Sure K., Ihorst G., Stang A., Pyrc K., Jebbink M.F., et al. Croup is associated with the novel coronavirus NL63. PLoS Med. 2005;2:e240. doi: 10.1371/journal.pmed.0020240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Bjornson C.L., Johnson D.W. Croup Lancet. 2008;371:329–339. doi: 10.1016/S0140-6736(08)60170-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Bosch B.J., Smits S.L., Haagmans B.L. Membrane ectopeptidases targeted by human coronaviruses. Curr Opin Virol. 2014;6:55–60. doi: 10.1016/j.coviro.2014.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Bertram S., Heurich A., Lavender H., Gierer S., Danisch S., Perin P., et al. Influenza and SARS-coronavirus activating proteases TMPRSS2 and HAT are expressed at multiple sites in human respiratory and gastrointestinal tracts. PLoS One. 2012;7 doi: 10.1371/journal.pone.0035876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Venn A.M.R., Schmidt J.M., Mullan P.C. Pediatric croup with COVID-19. Am J Emerg Med. 2021;43(287) doi: 10.1016/j.ajem.2020.09.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Lim C.C., Saniasiaya J., Kulasegarah J. Croup and COVID-19 in a child: a case report and literature review. BMJ Case Rep. 2021;14:1–4. doi: 10.1136/bcr-2021-244769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Tsoi K., Chan K.C., Chan L., Mok G., Li A.M., Lam H.S. A child with SARS-CoV2-induced croup. Pediatr Pulmonol. 2021;56:2377–2378. doi: 10.1002/ppul.25408. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.