Abstract
We have recently reported that nitrite reductase, a bifunctional enzyme located in the periplasmic space of Pseudomonas aeruginosa, could induce interleukin-8 (IL-8) generation in a variety of respiratory cells, including bronchial epithelial cells (K. Oishi et al. Infect. Immun. 65:2648–2655, 1997). In this report, we examined the mode of nitrite reductase (PNR) release from a serum-sensitive strain of live P. aeruginosa cells during in vitro treatment with four different antimicrobial agents or human complement. Bacterial killing of P. aeruginosa by antimicrobial agents induced PNR release and mediated IL-8 production in human bronchial epithelial (BET-1A) cells. Among these agents, imipenem demonstrated rapid killing of P. aeruginosa as well as rapid release of PNR and resulted in the highest IL-8 production. Complement-mediated killing of P. aeruginosa was also associated with PNR release and enhanced IL-8 production. The immunoprecipitates of the aliquots of bacterial culture containing imipenem or complement with anti-PNR immunoglobulin G (IgG) induced a twofold-higher IL-8 production than did the immunoprecipitates of the aliquots of bacterial culture with a control IgG. These pieces of evidence confirmed that PNR released in the aliquots of bacterial culture was responsible for IL-8 production in the BET-1A cells. Furthermore, the culture supernatants of the BET-1A cells stimulated with aliquots of bacterial culture containing antimicrobial agents or complement similarly mediated neutrophil migration in vitro. These data support the possibility that a potent inducer of IL-8, PNR, could be released from P. aeruginosa after exposure to antimicrobial agents or complement and contributes to neutrophil migration in the airways during bronchopulmonary infections with P. aeruginosa.
Pseudomonas aeruginosa (P. aeruginosa) is a virulent pathogen in immunocompromised hosts (32). Nosocomial pneumonia caused by P. aeruginosa is associated with a high rate of mortality, despite recent advances in antimicrobial chemotherapy (4, 30). Pseudomonas pneumonia is frequently associated with acute respiratory distress syndrome (43). Infections due to P. aeruginosa are also closely associated with the progression of chronic airway diseases, including cystic fibrosis, diffuse panbronchiolitis, and bronchiectasis (10, 28). Interleukin-8 (IL-8), a chemotactic and activating factor for neutrophils, participates in the generation of dense neutrophil accumulations in acute pneumonia and acute respiratory distress syndrome, as well as chronic airway diseases (5, 22, 28, 35).
The bronchial epithelium participates in the airway inflammation of asthma, cystic fibrosis, and diffuse panbronchiolitis. Recent studies have demonstrated that a nonprotein factor of less than 1 kDa in culture supernatant of P. aeruginosa could stimulate bronchial epithelial cells to produce IL-8 (19). Pilin-mediated adherence of P. aeruginosa and Pseudomonas autoinducer were reported to be potent stimuli for IL-8 production by bronchial epithelium (9). Through analysis of an inducer among Pseudomonas products for IL-8 production in human bronchial epithelial cells (BET-1A), we have further identified the nitrite reductase from P. aeruginosa as a potent IL-8 inducer in this cell line and other respiratory cells (27). The Pseudomonas nitrite reductase (PNR) with a molecular mass of 60,204 Da is recognized as a periplasmic component active in energy generation (38). The enzymatic activity of PNR is not essential for the IL-8-inducing activity of PNR, and direct stimulation of bronchial epithelial cells by the PNR is a possible mechanism for IL-8 gene induction. Our recent data indicated the involvement of NF-κB in activating the IL-8 gene in human pulmonary epithelial cells after stimulation with PNR (23). If the PNR is released from the periplasmic space of Pseudomonas cells in the lung, this protein probably induces IL-8 production and causes neutrophil accumulation. However, the mode of PNR release from live P. aeruginosa cells and its functional activities have not been explored.
This study was designed to elucidate how PNR could be released from P. aeruginosa and induce IL-8 production in human bronchial epithelial cells. We describe here the kinetics of PNR release from live P. aeruginosa cells by several antimicrobial agents and complement and the induction of IL-8 and neutrophil chemotactic factor (NCF) activity in the BET-1A cells.
MATERIALS AND METHODS
Purification of PNR.
A serum-sensitive strain with a mucoid phenotype, P. aeruginosa 5276, was isolated from a patient with diffuse panbronchiolitis (28). This strain was grown overnight in Mueller-Hinton broth (Difco Laboratories, Detroit, Mich.). Bacteria in the post-log phase were harvested in sterile normal saline. Harvested bacterial cells were sonicated 10 times with an ultrasonifier (cell disruptor 185; Branson Ultrasonics, Co., Danbury, Conn.) with 1-min intervals. The sonicated supernatant of P. aeruginosa was obtained following ultracentrifugation at 18,000 × g for 60 min at 4°C and filtration through a 0.45-μm-pore-diameter filter. PNR was purified as previously described (27). The purified PNR was stored at −80°C until use.
Cell culture.
BET-1A, which is a human bronchial epithelial cell line transformed by simian virus 40, was cultured in serum-free LHC-9 media (Biofluids, Rockville, Md.) with 25 μg of amphotericin B per ml, 25 U of penicillin per ml, and 25 μg of streptomycin per ml in a 24-well plate coated with fibronectin and collagen (24). The BET-1A cell line was employed for determination of non-lipopolysaccharide (LPS)-mediated IL-8 production because of its lack of responsiveness to LPS stimulation (27). After confluent cultures had been washed with HEPES-buffered saline, cells were incubated with purified PNR or the aliquots of bacterial culture with antimicrobial agents or absorbed normal human serum (AbsNHS), diluted with LHC-9 medium. Lactate dehydrogenase release was measured to assess cell viability by using an in vitro toxicology assay kit (Sigma Chemical Co. St. Louis, Mo.) and never exceeded 5% release under these conditions. The uniformity of the monolayer was also determined by quantifying the number of cells per well. Cell-free supernatants of culture media were harvested after incubation for the indicated times. All supernatants of culture media were stored at −80°C for less than a week until tested with the enzyme-linked immunosorbent assay (ELISA) for IL-8 and the NCF assay. Each value represents the mean ± standard deviation of three determinations.
IL-8 assay.
IL-8 levels were determined by an ELISA with a monoclonal antibody WS 4 as the capturing antibody and a polyclonal rabbit anti-IL-8 antibody as a secondary antibody, both of which were raised against human recombinant IL-8 as previously described (16). The detection limit of this assay was 31.1 pg of IL-8 per ml.
Bacterial killing by antimicrobial agents.
The MICs of imipenem (Banyu Pharmaceutical, Co., Ltd., Japan), ceftazidime (Glaxo, Tokyo, Japan), levofloxacin (Daiichi Pharmaceutical Co., Ltd., Japan), and gentamicin (Wako Pure Chemical Co., Ltd., Osaka, Japan) were determined by the standard method as previously described (6). The MICs of imipenem, ceftazidime, levofloxacin, and gentamicin were all 1.56 μg/ml. Levels of imipenem, ceftazidime, or gentamicin 10× the MIC or a level of levofloxacin 5× the MIC and P. aeruginosa 5276 at a concentration of 5 × 108 CFU/ml were employed to demonstrate bacterial killing and PNR release in vitro. Ten milliliters of bacterial suspension in chemically defined M9 medium was incubated in a sterile glass tube at 37°C for 1, 3, 5, and 12 h with continuous shaking (2). Aliquots were removed from triplicate samples for quantitative culture. The aliquots of bacterial culture containing antimicrobial agents were used for PNR detection by immunoblot analysis and immunoprecipitation after filter sterilization, in addition to stimulation of BET-1A cells after 1:10 dilution in LHC-9 medium.
Complement-mediated killing.
Veronal-buffered saline containing 0.1% (wt/vol) gelatin, 0.15 mM CaCl2, and 1.0 mM MgCl2 (GVB++) was used. AbsNHS was prepared as previously described (29). Briefly, separated sera were absorbed with P. aeruginosa 5276 by incubation in 1-ml aliquots with 5 × 108 CFU of bacteria per ml for 1 h at 0°C, removing bacteria by centrifugation at 2,000 × g for 10 min at 4°C, repeating the absorption, and filter sterilization of AbsNHS, the samples of which were stored in aliquots at −80°C before use. Complement activity was abolished by heating the serum at 56°C for 30 min. Fifty percent AbsNHS and P. aeruginosa 5276 at a concentration of 5 × 108 CFU/ml were employed to demonstrate bacterial killing and PNR release in vitro. Complement-mediated killing was determined by incubating 5 × 108 CFU of bacteria per ml with 50% AbsNHS or 50% heat-inactivated AbsNHS suspended in 1.0 ml of GVB++ with continuous shaking at 37°C for 1 h. Aliquots were removed from triplicate samples for quantitative culture. After separation of the supernatants of bacterial culture by centrifugation at 2,000 × g for 10 min at 4°C, the supernatants were filter sterilized and used for IL-8-inducing activity in BET-1A cell cultures and for detection of PNR by immunoblot analysis and immunoprecipitation. These supernatants, diluted 1:50 in LHC-9 medium, were tested with the BET-1A cells.
Immunoblotting.
The purified PNR or aliquots of bacterial culture containing antimicrobial agents or complement were separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (10% polyacrylamide), transferred to a polyvinylidene difluoride membrane, analyzed by immunoblotting with a polyclonal rabbit immunoglobulin G (IgG) against recombinant PNR at 1 μg/ml (27) and horseradish peroxidase-conjugated streptavidin (Amersham, Little Chalfont, United Kingdom), and subjected to detection by enhanced chemiluminescence (Amersham).
Immunoprecipitation.
Immunoprecipitation was done as previously described (27). In brief, a 400-μl volume of the 10-fold-concentrated aliquots of bacterial culture with imipenem at 5 h postincubation or 2-fold-concentrated aliquots of bacterial culture with AbsNHS at 1 h postincubation was incubated with a 100-μl volume of anti-PNR rabbit IgG (6 mg/ml) or the same volume of control rabbit IgG (6 mg/ml; Cappel, West Chester, Pa.) at 4°C overnight. A 100-μl volume of protein G-Sepharose (Pharmacia Biotech, Uppsala, Sweden) in 20 mM sodium phosphate (pH 7.0) was added to the reaction mixtures, which were then incubated at 35°C for 60 min. Immunoprecipitates were washed three times with 0.5 ml of phosphate-buffered saline and were incubated with 0.2 ml of glycine-HCl (pH 2.7) for 5 min. The reaction mixtures were centrifuged at 13,000 × g for 5 min, and the supernatants containing immunoprecipitates released from protein G-Sepharose were separated and dialyzed against phosphate-buffered saline at 4°C. A total of 0.2 ml of dialyzed samples was analyzed with the BET-1A cell culture and by immunoblotting.
NCF activity.
NCF activities in culture supernatants of the BET-1A cells were determined by using an assembly consisting of a 96-well chamber, polycarbonate filter membrane, and a 96-well microtiter plate (Neuro Probe, Inc., Gaithersburg, Md.) according to the manufacturer’s instructions (14). Neutrophils were purified from the peripheral blood of normal volunteers by dextran sedimentation and Ficoll-Hypaque density gradient centrifugation and suspended at a concentration of 2 × 107 cells per ml in RPMI-1640 containing 1 mM l-glutamine and 25 mM HEPES. Both the purity and the cell viability of neutrophils were determined to be >99%. Thirty microliters of the culture supernatant or 10 ng of recombinant human IL-8 (Genzyme, Cambridge, Mass.) per ml was placed in triplicate in the bottom wells of the chamber separated by a polycarbonate membrane filter with pores 3 μm in diameter. Twenty-five microliters of neutrophil suspension (5 × 105 cells/well) was added to the membrane filter. The chamber was incubated for 1 h at 37°C in humidified 95% air–5% CO2. After a 1-h incubation, the neutrophils that had migrated through the membrane filter were sedimented by centrifugation at 250 × g for 15 min at room temperature and lysed by adding 6 μl of 0.2% (vol/vol) of Triton X-100 (Feinbiochemical GmbH & Co., Heidelberg, Germany). Liberated peroxidase activity was measured by adding 24 μl of 0.34 mM O-dianisidine (3,3′-dimethoxybenzine, Sigma Chemical Co.) in 0.05 M phosphate-citrate buffer (pH 5.0), containing 0.02% (vol/vol) of a 30% H2O2 solution. After incubation for 15 min at room temperature, the optical density at 405 nm was measured with a microplate reader (Labsystems Multiscan, Needham Heights, Mass.). NCF activities were expressed as migrated neutrophil numbers per well as previously described (14). In neutralizing antibody studies, 45 μl of culture supernatants of BET-1A cells was incubated with 5 μl of anti-IL-8 polyclonal rabbit IgG (1 mg/ml; Endogen, Inc., Boston, Mass.) or control rabbit IgG (1 mg/ml; Cappel) for 1 h at 37°C and then employed in the NCF assay. Anti-IL-8 polyclonal rabbit IgG at 100 μg/ml completely neutralized neutrophil chemotaxis of recombinant IL-8, at least at the level of 10 ng/ml. The levels of IL-8 in the culture supernatants of the BET-1A cells were all less than 10 ng/ml. The percent reduction of NCF activity was determined as [1 − (NCF with anti-IL-8 IgG − baseline NCF with anti-IL-8 IgG/NCF with control IgG − baseline NCF with control IgG)] × 100.
Statistical analysis.
Differences in the bacterial numbers and levels of IL-8 production by the BET-1A cells among five groups were determined with a Kruskall-Wallis test and Bonferroni method for multiple comparisons. The differences in the NCF activities were analyzed by the paired Student’s t test. Data were considered statistically significant if P values were less than 0.05.
RESULTS
Killing of P. aeruginosa and PNR release by antimicrobial agents.
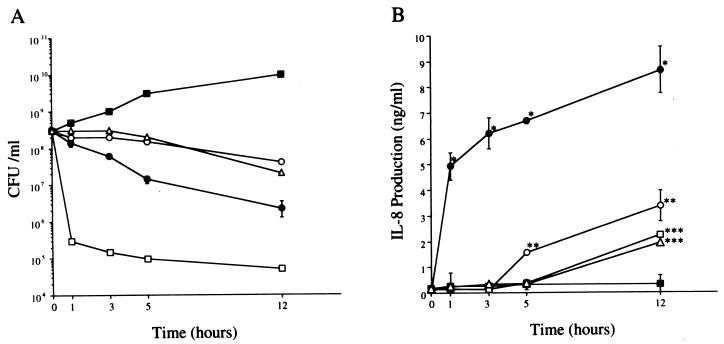
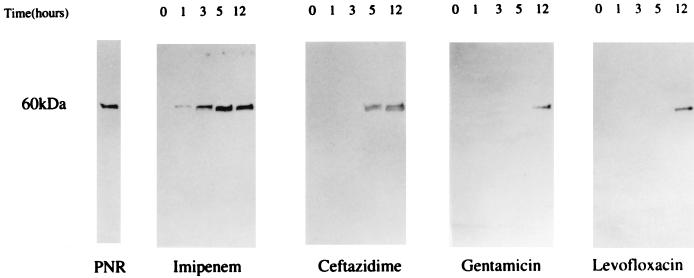
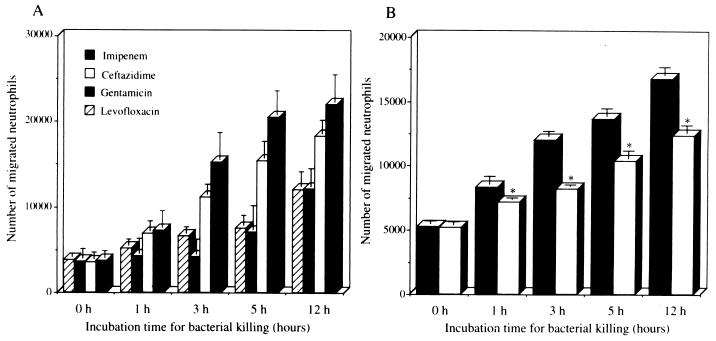
Among different antimicrobial agents, levofloxacin showed the most rapid and the highest level of killing at 1 h postincubation (Fig. 1A). Each antimicrobial agent significantly suppressed the bacterial numbers, compared with M9 medium alone at 1, 3, 5, and 12 h (P < 0.005). The rankings of bacterial killing by each antimicrobial agent were as follows: levofloxacin > imipenem > ceftazidime and gentamicin. There was no statistical difference between the numbers of P. aeruginosa cells treated with ceftazidime and gentamicin at each indicated time. The immunoblot analysis showed a faint band at 1 h and a broad band with a molecular mass of 60 kDa at 5 and 12 h after the treatment with imipenem (Fig. 2). The levels of PNR released by addition of imipenem appeared to be more than 1 μg/ml based on the detection limit of the immunoblot assay. Ceftazidime demonstrated PNR release at 5 and 12 h posttreatment, and levofloxacin and gentamicin showed PNR release only at 12 h posttreatment.
FIG. 1.
(A) Time course of change in bacterial numbers of P. aeruginosa 5276 after the indicated time during exposure to each antimicrobial agent. (B) Induction of IL-8 production by the BET-1A cells in response to the aliquots of bacterial culture with each antimicrobial agent. The aliquots of bacterial culture, harvested at the indicated time, were filtered and diluted 1:10 in LHC-9 medium and tested with the BET-1A cells for 24 h. IL-8 levels in the culture supernatants was measured as described above. ●, imipenem; ○, ceftazidime; □, levofloxacin; ▵, gentamicin; ■, control. Data represent the mean ± standard deviation of three experiments. ∗, P < 0.001 (compared with ceftazidime, levofloxacin, gentamicin, and control); ∗∗, P < 0.05 (compared with levofloxacin, gentamicin and control); ∗∗∗, P < 0.01 (compared with control).
FIG. 2.
Release of PNR associated with bacterial killing of P. aeruginosa 5276 by four different antimicrobial agents. The PNR in the aliquots of bacterial culture harvested at indicated time was detected by immunoblotting with anti-PNR IgG.
Induction of IL-8 production in BET-1A cells.
The aliquots of bacterial culture containing each antimicrobial agent were added to the culture of BET-1A cells, and imipenem was found to induce both the most rapid and the highest production of IL-8 (Fig. 1B), while imipenem, ceftazidime, gentamicin, or levofloxacin alone did not stimulate IL-8 production in the same cells. The levels of IL-8 production induced by imipenem were significantly higher than those induced by the other antimicrobial agents at each indicated time (P < 0.001). The levels of IL-8 production induced by ceftazidime were also significantly higher than those induced by gentamicin, levofloxacin, and the control at 5 and 12 h posttreatment (P < 0.05). Interestingly, the lowest and slowest production of IL-8 was induced by levofloxacin, which showed the highest bacterial killing, and gentamicin, which showed a lower level of bacterial killing. The potency of the induction of IL-8 by each antimicrobial agent was demonstrated in the following order: imipenem > ceftazidime > levofloxacin and gentamicin. The induction of IL-8 production in the BET-1A cells by each antimicrobial agent was correlated to the release of PNR from P. aeruginosa. These data indicate that bacterial killing by imipenem rapidly induced PNR release and IL-8 production in the BET-1A cell culture, while bacterial killing by ceftazidime, levofloxacin, or gentamicin resulted in slower PNR release and consequently delayed IL-8 production.
Immunoprecipitation of aliquots of bacterial culture containing imipenem.
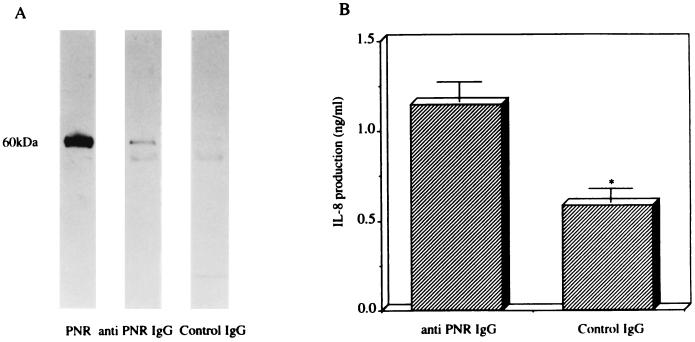
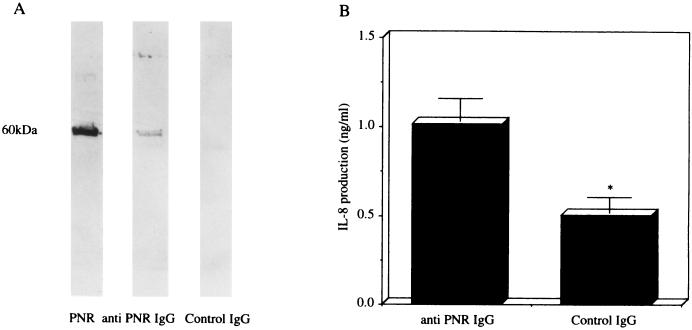
To confirm whether PNR release is responsible for IL-8 production-associated bacterial killing by imipenem in the BET-1A cells, immunoprecipitates of the aliquots of bacterial culture containing imipenem with anti-PNR IgG or control IgG were separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis and visualized by immunoblotting with anti-PNR IgG. The immunoblot analysis with anti-PNR IgG exhibited a broad band with a molecular mass of 60 kDa in the immunoprecipitates of the aliquots of bacterial culture containing imipenem with anti-PNR IgG, but exhibited no band with the same molecular mass in the aliquots of bacterial culture containing imipenem with control IgG (Fig. 3A). Furthermore, the level of induction of IL-8 production (1.20 ± 0.11 ng/ml) by the BET-1A cells in response to the dialyzed immunoprecipitates of the aliquots of bacterial culture containing imipenem with anti-PNR IgG was significantly higher than that in response to the immunoprecipitates of the aliquots of bacterial culture containing imipenem with control IgG (0.58 ± 0.08 ng/ml; P < 0.05) (Fig. 3B). These data confirmed that PNR released from P. aeruginosa by imipenem was responsible for induction of IL-8 production in the BET-1A cells.
FIG. 3.
(A) Immunoblot analysis with anti-PNR IgG for immunoprecipitates of the aliquots of bacterial culture containing imipenem with anti-PNR IgG or control IgG. (B) Induction of IL-8 production in the BET-1A cells in response to the dialyzed immunoprecipitates of the aliquots of bacterial culture containing imipenem with anti-PNR IgG or control IgG (B). The dialyzed samples (diluted 1:10 in LHC-9 medium) were tested with the BET-1A cells, and the levels of IL-8 production were measured. Data represent the mean ± standard deviation of three experiments. ∗, P < 0.05 (compared with anti-PNR IgG by Student’s t test).
Complement-mediated bacteriolysis of P. aeruginosa and PNR release and induction of IL-8 production in BET-1A cells.
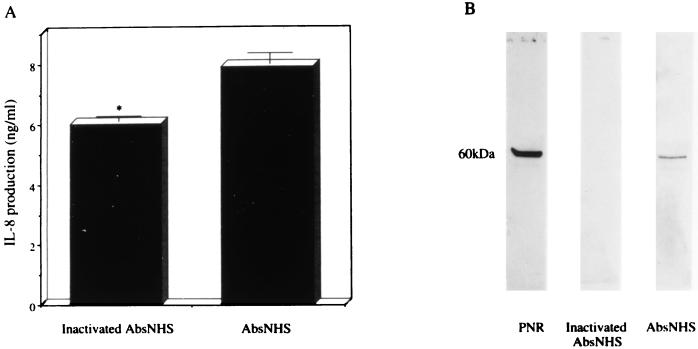
Treatment of P. aeruginosa 5276 at 5 × 108 CFU/ml with AbsNHS caused a significant decrease in the number of bacteria to 5 × 107 CFU/ml at 1 h postincubation, while heat-inactivated AbsNHS did not decrease the number of bacteria during the same incubation time. Complement-mediated bacteriolysis by AbsNHS demonstrated PNR release, while the heat-inactivated AbsNHS revealed no PNR release (Fig. 4B). AbsNHS was added to the culture of BET-1A cells, and AbsNHS at a final concentration of 1% induced high levels of IL-8 production (5.94 ± 0.41 ng/ml) in the BET-1A cells without causing cell damage, while the unstimulated cells induced negligible levels of IL-8 production. The IL-8 production in the BET-1A cells by serum factors may be responsible for the soluble form of CD14 (40). The aliquots of bacterial culture containing AbsNHS at 1 h postincubation were added to the culture of BET-1A cells, and the aliquots of bacterial culture containing AbsNHS at a final concentration of 1% induced a significantly higher level of IL-8 production in the BET-1A cells (7.89 ± 0.35 ng/ml; P < 0.05) (Fig. 4A) than those containing the heat-inactivated AbsNHS at the same concentration (6.01 ± 0.18 ng/ml). These data indicate that complement-mediated killing of P. aeruginosa released PNR and increased IL-8 production in the BET-1A cell culture.
FIG. 4.
(A) Induction of IL-8 production by the BET-1A cells in response to the aliquots of bacterial culture containing AbsNHS with or without heat inactivation. The aliquots of bacterial culture harvested at 1 h postincubation (diluted 1:50 in LHC-9 medium) were tested with the BET-1A cells, and the levels of IL-8 production were measured. Data represent the mean ± standard deviation of three experiments. ∗, P < 0.05 (compared with AbsNHS by Student’s t test). (B) Release of PNR associated with complement-mediated killing of P. aeruginosa 5276. The PNR in the aliquots of bacterial culture containing AbsNHS with or without heat inactivation was detected by immunoblotting with anti-PNR IgG.
Immunoprecipitation of aliquots of bacterial culture containing complement.
The immunoblot analysis with anti-PNR IgG exhibited a broad band with a molecular mass of 60 kDa in the immunoprecipitates of the aliquots of bacterial culture containing AbsNHS with anti-PNR IgG but exhibited no band with the same molecular mass in the immunoprecipitates of the aliquots of bacterial culture containing AbsNHS with a control IgG (Fig. 5A). Furthermore, induction of IL-8 production (1.02 ± 0.13 ng/ml) by the BET-1A cells in response to the dialyzed immunoprecipitates of the aliquots of bacterial culture containing AbsNHS with a polyclonal IgG against PNR was significantly higher (0.50 ± 0.09 ng/ml; P < 0.05) than that in response to the immunoprecipitates of the aliquots of bacterial culture containing AbsNHS with a control IgG (Fig. 5B). These data confirmed that PNR release associated with complement-mediated bacteriolysis was responsible for induction of IL-8 production in the BET-1A cells.
FIG. 5.
(A) Immunoblot analysis with anti-PNR IgG for immunoprecipitates of the aliquots of bacterial culture containing AbsNHS with anti-PNR IgG or control IgG. (B) Induction of IL-8 production in the BET-1A cells in response to the immunoprecipitates of the aliquots of bacterial culture containing AbsNHS with anti-PNR IgG or control IgG. These samples (diluted 1:10 in LHC-9 medium) were tested with the BET-1A cells, and the levels of IL-8 production were measured. Data represent the mean ± standard deviation of three experiments. ∗, P < 0.05 (compared with anti-PNR IgG by Student’s t test).
NCF activity in the culture supernatants of BET-1A cells.
We next examined NCF activity in the culture supernatants of the BET-1A cells stimulated with the aliquots of bacterial culture containing each antimicrobial agent and AbsNHS. The NCF activity mediated by the culture supernatants of the BET-1A cells stimulated with the aliquots of bacterial culture containing imipenem, ceftazidime, levofloxacin, or gentamicin is shown in Fig. 6A. The aliquots of bacterial culture containing imipenem harvested at various time points demonstrated the highest NCF activity among these antimicrobial agents. The aliquots of bacterial culture containing levofloxacin or gentamicin only at 12 h postincubation showed increased the NCF activities. We observed a ranking of potency in induction of NCF activity by the aliquots of bacterial culture containing each antimicrobial agent similar to that of the induction of IL-8 production in the BET-1A cells shown in Fig. 1B, but the levels of bacterial killing were not similar. We also performed neutralizing antibody experiments to determine how much IL-8 contributed to NCF activities of the aliquots of bacterial culture containing imipenem, by using antihuman IL-8 or control IgG. As shown in Fig. 6B, preincubation with anti-IL-8 IgG resulted in a significant reduction in the NCF activities of the aliquots of bacterial culture harvested at 1 (8,326 ± 71 versus 7,219 ± 71; P < 0.05), 3 (12,018 ± 309 versus 8,203 ± 142; P < 0.05), 5 (13,660 ± 539 versus 10,418 ± 554; P < 0.05) and 12 (16,776 ± 677 versus 12,387 ± 606; P < 0.05) h postincubation with imipenem. The mean percent reductions of NCF activities were 33% at 1 h, 56% at 3 h, 38% at 5 h, and 38% at 12 h, respectively.
FIG. 6.
Neutrophil migration induced by the culture supernatants of the BET-1A cells stimulated with aliquots of bacterial culture containing each antimicrobial agent harvested at the indicated time (A) and the inhibitory effects of antihuman IL-8 IgG on neutrophil migration induced by the culture supernatants of the BET-1A cells stimulated with aliquots of bacterial culture containing imipenem (B). The aliquots of bacterial culture were filtered and diluted 1:10 in LHC-9 medium and then tested with the BET-1A cells for 24 h. The culture supernatants were incubated with anti-IL-8 IgG (100 μg/ml; open columns) or control IgG (100 μg/ml; solid columns) for 1 h at 37°C. Data represent the mean ± standard deviation of three experiments. ∗, P < 0.05 (compared with control IgG by Student’s t test).
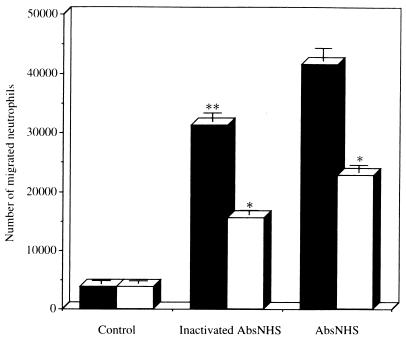
The NCF activity of the aliquots of bacterial culture containing AbsNHS was significantly higher than that of heat-inactivated AbsNHS (P < 0.01) (Fig. 7). A similar preincubation with anti-IL-8 IgG demonstrated a significant reduction of NCF activities of aliquots of bacterial culture containing heat-inactivated AbsNHS (31,320 ± 2,775 versus 15,627 ± 647; P < 0.05) and AbsNHS (41,578 ± 2,684 versus 22,885 + 1,205; P < 0.05). The mean percent reductions of NCF activities were 58% in heat-inactivated AbsNHS and 50% in AbsNHS. These data suggest there is significant participation of IL-8 in the NCF activity in the culture supernatants of BET-1A cells generated in response to aliquots of bacterial culture containing imipenem or serum factors.
FIG. 7.
Neutrophil migration induced by the culture supernatants of the BET-1A cells stimulated with aliquots of bacterial killing containing AbsNHS with or without heat inactivation and inhibitory effects of antihuman IL-8 IgG on them. The aliquots of bacterial culture were filtered and diluted 1:50 in LHC-9 medium and tested with the BET-1A cells for 24 h. The culture supernatants were incubated with anti-IL-8 IgG (100 μg/ml; open columns) or control IgG (100 μg/ml; solid columns) for 1 h at 37°C. Data represent the mean ± standard deviation of three experiments. ∗∗, P < 0.01 (compared with AbsNHS by Student’s t test); ∗, P < 0.05 (compared with control IgG by Student’s t test).
DISCUSSION
In this paper, PNR was found to be released from a serum-sensitive strain of P. aeruginosa, 5276, after exposure to different antimicrobial agents. Among these agents, treatment with imipenem, a carbapenem, at 10× the MIC caused the most rapid release of PNR during the killing of P. aeruginosa cells and in turn induced the highest production of IL-8 in BET-1A cell cultures. Imipenem at 5× the MIC also induced a rapid killing of P. aeruginosa 5276 and a high level of production of IL-8 in BET-1A cell cultures (data not shown). Treatment with ceftazidime, a cephalosporin, at 10× the MIC caused a slower release of PNR and a much lower level of IL-8 production. The concentrations of these β-lactam antibiotics used in this study have been reported to be clinically achievable in plasma and lung tissues (3, 20). We also confirmed a similar feature of PNR release and induction of IL-8 production in the BET-1A cells following bacterial killing of P. aeruginosa Fisher immunotype 1, a serum-resistant strain, by 4× the MIC of imipenem (data not shown).
These data for PNR release from P. aeruginosa induced by β-lactam antibiotics differ from published data on endotoxin release by them. Ceftazidime, binding to PBP 3, released a larger amount of endotoxin from P. aeruginosa than imipenem, binding to PBP 2 (13). The endotoxin thus released has a variety of biological functions in vitro and in vivo. Nakano and Kirikae demonstrated that nanogram levels of ceftazidime-released endotoxin induced tumor necrosis factor, IL-6, and nitric oxide production in cultured peritoneal macrophages from LPS-responsive C3H/HeN and LPS-hyporesponsive C3H/HeJ mice, and lethal toxicity in d-(+)-galactosamine-sensitized mice (15, 25). Another recent paper demonstrated that PBP 2-specific imipenem-induced endotoxin release stimulated endothelial cells or whole blood cells to produce less IL-6 than did PBP 3-specific ceftriaxone and meropenem (1). Treatment of enterohemorrhagic Escherichia coli strains with imipenem similarly induced much lower levels of release of Shiga-like toxin, as well as endotoxin, than treatment with ceftazidime (41). Prins et al. reported that serum and urine cytokine levels increased by 10- to ∼40% after 4 h of ceftazidime treatment compared with no increase in imipenem-treated patients with gram-negative urosepsis (34). The lower levels of endotoxin release resulting from treatment with PBP 2 binding antibiotics may be explained by their rapid bactericidal actions, suppressing any increase in total cell mass. In addition, ciprofloxacin and gentamicin at levels of 16× the MIC have been reported to be potent inducers of endotoxin release (42).
On the other hand, the level of PNR release and IL-8 induction in the BET-1A cells after treatment with β-lactam antibiotics appears to be dependent on bactericidal effects, rather than total cell mass. Outer membrane damage associated with β-lactam antibiotic-induced bacterial killing may explain PNR release from the periplasmic space of P. aeruginosa, resulting in IL-8 induction in the BET-1A cells. In contrast, levofloxacin or gentamicin at levels higher than the MIC induced the slowest PNR release and the lowest IL-8 production in BET-1A cells, although the levels of these agents used in this study were higher than the clinically achievable levels in the airways (18, 26). Slower bacterial killing by gentamicin at a level over the MIC induced the slowest PNR release, because a mild bactericidal action of aminoglycoside could be caused by outer membrane damage due to incorporation of misread protein (8). The most rapid and the highest bactericidal action by levofloxacin, a fluoroquinolone, may involve minimal damage of the outer membrane, because the mode of bacterial killing by levofloxacin did not correlate with its mode of PNR release. In fact, our preliminary studies with electron microscopy confirmed minimal damage on the surfaces of P. aeruginosa cells after treatment with levofloxacin or gentamicin (data not shown). Levofloxacin, therefore, appeared to induce a rapid bacterial killing of P. aeruginosa without any serious damage to the cell surfaces. Although bacterial killing by quinolone agents appeared to require drug interaction with DNA gyrase, the molecular events in this phenomenon remain unknown. In contrast, serious damage to the outer membrane of imipenem-treated cells and a filamentous alteration of ceftazidime-treated cells were also observed by electron microscopy (data not shown). These pieces of evidence from our preliminary studies support minimal release of PNR by levofloxacin or gentamicin and maximum release of PNR by imipenem, as shown in Fig. 2. Consequently, the grade of PNR release by these antimicrobial agents well correlated with the levels of IL-8 production in the BET-1A cells.
The essential requirement of complement for clearance of microorganisms has been described previously (7). Complement activation can lead to microbial lysis, but it also plays an important role in phagocytosis and neutrophil recruitment. Up to 50- to 80% of strains of mucoid P. aeruginosa isolated from patients with cystic fibrosis or bronchiectasis and chronic bronchitis were shown to be serum sensitive (31, 39). Insertion of C5b-9 membrane attack complexes on the outer membrane induced killing of serum-sensitive strains of P. aeruginosa (37). We also reported that serum-sensitive strains were less virulent than serum-resistant strains in a murine model of pneumonia due to P. aeruginosa (39). In the present study, complement-mediated cell lysis of P. aeruginosa 5276 also released PNR and resulted in induction of IL-8 production in the BET-1A cells. These data may imply that increased levels of complement factors in the airways of patients with bronchopulmonary infections may cause complement-mediated cell lysis of serum-sensitive organisms, thereby inducing PNR release and IL-8 production (11).
Our present study confirmed that imipenem- or complement-stimulated release of PNR was responsible for induction of IL-8 production in the BET-1A cells. PNR released from live P. aeruginosa may also induce a high level of IL-8 by human alveolar macrophages and relatively low levels of IL-8 by neutrophils and pulmonary fibroblasts (27). However, treatment of P. aeruginosa with an antimicrobial agent or complement may also release nonpeptide IL-8-inducing factors with low molecular masses in bronchial epithelial cells (19, 27).
The intensity of NCF activity in the culture supernatants of the BET-1A cells induced by the aliquots of bacterial culture containing antimicrobial agents or complement factors was closely correlated with PNR release and induction of IL-8 production in the same cells. In addition, IL-8 was proved to be a major chemoattractant for neutrophils in the culture supernatants of the BET-1A cells, as shown by neutralizing antibody experiments with antihuman IL-8 IgG. A part of NCF activities in culture supernatants of the BET-1A cells may be due to other neutrophil chemotactic factors, such as epithelial cell-derived neutrophil-activating peptide (ENA-78) (17), leukotriene B4 (21), or platelet-activating factor (36), which are derived from pulmonary epithelial cells, or other chemotactic peptides from P. aeruginosa itself (12). However, it has not yet been determined whether PNR induces neutrophil chemoattractants other than IL-8 from bronchial epithelial cells.
These several lines of evidence suggest that PNR released from P. aeruginosa by antimicrobial agents or complement may induce IL-8 production and neutrophil migration in the airways of patients with bronchopulmonary infections. We have previously reported that the levels of IL-8 and the number of neutrophils in expectorated sputum from patients with bacterial bronchopulmonary infections have decreased after treatment with the appropriate antimicrobial agents (33). Therefore, antibiotic-induced PNR release and neutrophil migration through induction of IL-8 production in the airways may begin after initiation of therapy and gradually decrease during successful antimicrobial chemotherapy. In summary, PNR could be released from live P. aeruginosa cells after exposure to antimicrobial agents or serum complement factors, thereby inducing IL-8 production by bronchial epithelial cells and consequently neutrophil migration in vitro. Our present data may render new insight into a mechanism of neutrophil-mediated inflammations in bronchopulmonary infections with P. aeruginosa and support the idea that PNR-induced IL-8 production and neutrophil migration in the airways contribute to acute or chronic lung injuries associated with bronchopulmonary infections with P. aeruginosa.
ACKNOWLEDGMENTS
We are grateful to Mutsuyo Akiyose, Keiko Tagawa, and Yoko Terai for technical assistance; Christian Vessergarrd for reviewing the manuscript; and Keizo Matsumoto for critical comments on the manuscript.
This work was supported in part by a grant (06454273) from the Ministry of Education, Science and Culture, Japan.
REFERENCES
- 1.Arditi M, Zhou J. Differential antibiotic-induced endotoxin release and interleukin-6 production by human umbilical vein endothelial cells (HUVECs): amplification of the response by coincubation of HUVECs and blood cells. J Infect Dis. 1997;175:1255–1258. doi: 10.1086/593689. [DOI] [PubMed] [Google Scholar]
- 2.Atlas R M. M9 medium. In: Parks L C, editor. Handbook of microbiological media. Boca Raton, Fla: CRC Press; 1993. p. 529. [Google Scholar]
- 3.Benoni G, Cuzzolin L, Bertrand C, Pucchetti V, Velo G. Penetration of imipenem-cilastatin into the lung tissue and pericardial fluid of thoracotomized patients. Chemotherapia. 1987;6(Suppl. 1):259–260. [PubMed] [Google Scholar]
- 4.Bryan C S, Reynolds K L. Bacteremic nosocomial pneumonia. Am Rev Respir Dis. 1984;129:668–671. doi: 10.1164/arrd.1984.129.5.668. [DOI] [PubMed] [Google Scholar]
- 5.Chollet-Martin S, Jourdain B, Gibert C, Elbim C, Chastre J, Gougerot-Pocidalo M A. Interactions between neutrophils and cytokines in blood and alveolar spaces during ARDS. Am J Respir Crit Care Med. 1996;153:594–601. doi: 10.1164/ajrccm.154.3.8810592. [DOI] [PubMed] [Google Scholar]
- 6.Committee for the Susceptibility of Antimicrobial Agents. A report from the Committee for the Susceptibility of Antimicrobial Agents. Tokyo, Japan: Japanese Society of Chemotherapy; 1992. [Google Scholar]
- 7.Cooper N R, Nemerow G R. Complement and infectious agents: a tale of disguise and deception. Complement Inflamm. 1989;6:249–258. doi: 10.1159/000463100. [DOI] [PubMed] [Google Scholar]
- 8.Davis B D, Chen L, Tai P C. Misread protein creates membrane channels: an essential step in the bactericidal action of aminoglycosides. Proc Natl Acad Sci USA. 1986;83:6164–6168. doi: 10.1073/pnas.83.16.6164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.DiMango E, Zar H J, Byran R, Prince A. Diverse Pseudomonas aeruginosa gene products stimulate respiratory epithelial cells to produce interleukin-8. J Clin Investig. 1995;96:2204–2210. doi: 10.1172/JCI118275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fick R B. Pathogenesis of the Pseudomonas lung infection in cystic fibrosis. Clinical implication of basic research. Chest. 1989;96:158–164. doi: 10.1378/chest.96.1.158. [DOI] [PubMed] [Google Scholar]
- 11.Fick R B, Robbins R A, Squier S U, Schoderbe W E, Russ W D. Complement activation in cystic fibrosis respiratory fluids: in vivo and in vitro generation C5a and chemotactic activity. Pediatr Res. 1986;20:1258–1268. doi: 10.1203/00006450-198612000-00014. [DOI] [PubMed] [Google Scholar]
- 12.Fontán P A, Amura C R, Garcia V E, Cerquetti M C, Sordelli D O. Preliminary characterization of Pseudomonas aeruginosa peptide chemotactins for polymorphonuclear leukocytes. Infect Immun. 1992;60:2465–2469. doi: 10.1128/iai.60.6.2465-2469.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jackson J J, Kropp H. β-Lactam antibiotic-induced release of free endotoxin: in vitro comparison of penicillin-binding protein (PBP) 2-specific imipenem and PBP3-specific ceftazidime. J Infect Dis. 1992;165:1033–1041. doi: 10.1093/infdis/165.6.1033. [DOI] [PubMed] [Google Scholar]
- 14.Junger W G, Cardoza T A, Liu F C, Hoyt D B, Goodwin R. Improved rapid photometric assay for quantitative measurement of PMN migration. J Immunol Methods. 1993;160:73–79. doi: 10.1016/0022-1759(93)90010-5. [DOI] [PubMed] [Google Scholar]
- 15.Kirikae T, Kirikae F, Saito S, Tominaga K, Tamura H, Uemura Y, Yokochi T, Nakano M. Biological characterization of endotoxins released from antibiotic-treated Pseudomonas aeruginosa and Escherichia coli. Antimicrob Agents Chemother. 1998;42:1015–1021. doi: 10.1128/aac.42.5.1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ko Y, Mukaida N, Panyutich A, Voitenok N N, Matsushima K, Kawai T, Kasahara T. A sensitive enzyme-linked immunosorbent assay for human interleukin-8. J Immunol Methods. 1991;149:227–235. doi: 10.1016/0022-1759(92)90254-q. [DOI] [PubMed] [Google Scholar]
- 17.Kruger T, Baier J. Induction of neutrophil chemoattractant cytokines by Mycoplasma hominis in alveolar type II cells. Infect Immun. 1997;65:5131–5136. doi: 10.1128/iai.65.12.5131-5136.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Masaki H, Matsumoto K, Watanabe K, Mitarai S, Tao M, Oishi K, Terazono T, Yoshida T, Iwagaki A, Nagatake T. In vitro antibacterial activity, penetration into sputum, and clinical evaluation of levofloxacin in chronic respiratory tract infection. Chemotherapy. 1992;40(Suppl. 3):336–347. [Google Scholar]
- 19.Massion P P, Inoue H, Richman-Eisenstat J, Grunberger D, Jorens P G, Housset B, Pittet J-F, Wiener-Kronish J P, Nadel J A. Novel Pseudomonas product stimulates interleukin-8 production in airway epithelial cells in vitro. J Clin Investig. 1994;93:26–32. doi: 10.1172/JCI116954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Matsumoto K, Harada T, Shishido H, Uzuka Y, Nagatake T, Rikitomi N, Oishi K, Watanabe K. Laboratory and clinical evaluation on ceftazidime with special reference to respiratory infections caused by Pseudomonas aeruginosa. Chemotherapy. 1983;31(Suppl. 3):434–446. [Google Scholar]
- 21.McKinnon K P, Madden M C, Noah T L, Devlin R B. In vitro ozon exposure increases release of arachidonic acid products from a human bronchial epithelial cell line. Toxicol Appl Pharmacol. 1993;118:215–223. doi: 10.1006/taap.1993.1027. [DOI] [PubMed] [Google Scholar]
- 22.Miller E J, Cohen A B, Matthay M A. Increased interleukin-8 concentrations in the pulmonary edema fluid of patients with acute respiratory distress syndrome from sepsis. Crit Care Med. 1996;24:1448–1454. doi: 10.1097/00003246-199609000-00004. [DOI] [PubMed] [Google Scholar]
- 23.Mori, N., B. Sar, and K. Oishi. Unpublished data.
- 24.Nakamura H, Yoshimura K, Jaffe H A, Crystal R G. Interleukin-8 gene expression in human bronchial epithelial cells. J Biol Chem. 1991;266:19611–19617. [PubMed] [Google Scholar]
- 25.Nakano M, Kirikae T. Biological characterization of Pseudomonas aeruginosa endotoxin release by antibiotic treatment in vitro. J Endotoxin Res. 1996;3:195–200. [Google Scholar]
- 26.Odio W, Van Laer E, Klastersky J. Concentrations of gentamicin in bronchial secretions after intramuscular and endotracheal administration. J Clin Pharmacol. 1975;15:518–524. doi: 10.1002/j.1552-4604.1975.tb01474.x. [DOI] [PubMed] [Google Scholar]
- 27.Oishi K, Sar B, Wada A, Hidaka Y, Matsumoto S, Amano H, Sonoda F, Kobayashi S, Hirayama T, Nagatake T, Matsushima K. Nitrite reductase from Pseudomonas aeruginosa induces inflammatory cytokines in cultured respiratory cells. Infect Immun. 1997;65:2648–2655. doi: 10.1128/iai.65.7.2648-2655.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Oishi K, Sonoda F, Kobayashi S, Iwagaki A, Nagatake T, Matsushima K, Matsumoto K. Role of interleukin-8 (IL-8) and an inhibitory effect of erythromycin on IL-8 release in the airways of patients with chronic airway diseases. Infect Immun. 1994;62:4145–4152. doi: 10.1128/iai.62.10.4145-4152.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Oishi K, Nancy K L, Guelde G, Pollack M. Antibacterial and protective properties of monoclonal antibodies reactive with Escherichia coli O111:B4 lipopolysaccharide: relation to antibody isotype and complement-fixing activity. J Infect Dis. 1992;165:34–45. doi: 10.1093/infdis/165.1.34. [DOI] [PubMed] [Google Scholar]
- 30.Pennington J E, Reynolds H Y, Carbone P P. Pseudomonas pneumonia. A retrospective study of 36 cases. Am J Med. 1973;55:155–160. doi: 10.1016/0002-9343(73)90163-0. [DOI] [PubMed] [Google Scholar]
- 31.Pier G B, Ames P. Mediation of the killing of rough, mucoid isolates of Pseudomonas aeruginosa from patients with cystic fibrosis by alternative pathway of complement. J Infect Dis. 1984;150:223–228. doi: 10.1093/infdis/150.2.223. [DOI] [PubMed] [Google Scholar]
- 32.Pollack M. The virulence of Pseudomonas aeruginosa. Rev Infect Dis. 1984;6:S617–S626. doi: 10.1093/clinids/6.supplement_3.s617. [DOI] [PubMed] [Google Scholar]
- 33.Ponglertnapagorn P, Oishi K, Iwagaki A, Sonoda F, Watanabe K, Nagtake T, Matsumoto K. Airway interleukin-8 in elderly patients with bacterial lower respiratory tract infections. Microbiol Immunol. 1996;40:177–182. doi: 10.1111/j.1348-0421.1996.tb03322.x. [DOI] [PubMed] [Google Scholar]
- 34.Prins J M, van Agtmael M A, Kuijper E J, van Deventer S J H, Speelman P. Antibiotic-induced endotoxin release in patients with gram-negative urosepsis: a double-blind study comparing imipenem and ceftazidime. J Infect Dis. 1995;172:886–891. doi: 10.1093/infdis/172.3.886. [DOI] [PubMed] [Google Scholar]
- 35.Rodriguez J L, Miller C G, DeForge L E, Kelty L, Shanley C J, Bartlett R H, Remick D G. Local production of interleukin-8 is associated with nosocomial pneumonia. J Trauma. 1992;33:74–82. doi: 10.1097/00005373-199207000-00015. [DOI] [PubMed] [Google Scholar]
- 36.Samet J M, Madden M C. Characterization of a secretory phospholipase A2 in human bronchoalveolar lavage fluid. Exp Lung Res. 1996;22:299–315. doi: 10.3109/01902149609031777. [DOI] [PubMed] [Google Scholar]
- 37.Schiller N L, Joiner K A. Interaction of complement with serum-sensitive and serum-resistant strains of Pseudomonas aeruginosa. Infect Immun. 1986;54:689–694. doi: 10.1128/iai.54.3.689-694.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Silvestrini M C, Galeotti C L, Gervais M, Schinina E, Barra D, Bossa F, Brunori M. Nitrite reductase from Pseudomonas aeruginosa: sequence of the gene and the protein. FEBS Lett. 1989;254:33–38. doi: 10.1016/0014-5793(89)81004-x. [DOI] [PubMed] [Google Scholar]
- 39.Sonoda F, Oishi K, Tanaka H, Miwa H, Kobayashi S, Nagatake T, Matsumoto K. Serum sensitivity of Pseudomonas aeruginosa isolated from sputum as a virulence factor in the lower respiratory tract. Jpn J Thorac Dis. 1991;29:703–709. [PubMed] [Google Scholar]
- 40.Striz I, Mio T, Adachi Y, Bazil V, Rennard S. The CD14 molecule participates in regulation of IL-8 and IL-6 release by bronchial epithelial cells. Immunol Lett. 1998;62:177–181. doi: 10.1016/s0165-2478(98)00046-7. [DOI] [PubMed] [Google Scholar]
- 41.Takahashi K, Narita K, Kato Y, Sugiyama T, Koide N, Yoshida T, Yokochi T. Low-level release of Shiga-like toxin (verocytotoxin) and endotoxin from enterohemorrhagic Escherichia coli treated with imipenem. Antimicrob Agents Chemother. 1997;41:2295–2296. doi: 10.1128/aac.41.10.2295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Van den Berg C, De Neeling A J, Schot C S, Hustinx W N M, Wemer J, De Wildt D J. Delayed antibiotic-induced lysis of Escherichia coli in vitro is correlated with enhancement of LPS release. Scand J Infect Dis. 1992;24:619–627. doi: 10.3109/00365549209054648. [DOI] [PubMed] [Google Scholar]
- 43.Winer-Muram H T, Jennings S G, Wunderink R G, Jones C B, Leeper K V., Jr Ventilator-associated Pseudomonas aeruginosa pneumonia: radiographic findings. Radiology. 1995;195:247–252. doi: 10.1148/radiology.195.1.7892480. [DOI] [PubMed] [Google Scholar]