Summary
Background
Age and gender specific prevalence rates for parkinsonism and Parkinson's disease (PD) are important to guide research, clinical practice, and public health planning; however, prevalence estimates in Latin America (LatAm) are limited. We aimed to estimate the prevalence of parkinsonism and PD and examine related risk factors in a cohort of elderly individuals from Latin America (LatAm).
Methods
Data from 11,613 adults (65+ years) who participated in a baseline assessment of the 10/66 study and lived in six LatAm countries were analyzed to estimate parkinsonism and PD prevalence. Crude and age-adjusted prevalence were determined by sex and country. Diagnosis of PD was established using the UK Parkinson's Disease Society Brain Bank's clinical criteria.
Findings
In this cohort, the prevalence of parkinsonism was 8.0% (95% CI 7.6%–8.5%), and the prevalence of PD was 2.0% (95% CI 1.7%–2.3%). PD prevalence increased with age from 1.0 to 3.5 (65–69vs. 80 years or older, p < 0.001). Age-adjusted prevalence rates were lower for women than for men. No significant differences were found across countries, except for lower prevalence in urban areas of Peru. PD was positively associated with depression (adjusted prevalence ratio [aPR] 2.06, 95% CI 1.40–3.01, I2 = 56.0%), dementia (aPR 1.57, 95% CI 1.07- 2.32, I2 = 0.0%) and educational level (aPR 1.14, 95% CI 1.01– 1.29, I2 = 58.6%).
Interpretation
The reported prevalence of PD in LatAm is similar to reports from high-income countries (HIC). A significant proportion of cases with PD did not have a previous diagnosis, nor did they seek any medical or neurological attention. These findings underscore the need to improve public health programs for populations currently undergoing rapid demographic aging and epidemiological transition.
Funding
The funding source had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Keywords: Parkinsonism, Parkinson's disease, Prevalence, Risk factors, Latin America
Research in context.
Evidence before this study
We searched Medline (Ovid), LILACS, and SciElo.org for articles investigating Parkinsonism and Parkinson Disease (PD) in Latin America, published from January 1990 to December 2020. The search strategy included terms related: (Parkinson Disease* OR Parkinsonism*) AND (epidemiology* OR prevalence* OR incidence* OR risk factors*) AND (‘Latin America*’ OR Argentin* OR Bolivia* OR Brazil OR Brasil OR Brazilian* OR Chile OR Chilean* OR Colombia* OR ‘Cost Rica*’ OR Cuba OR Cuban* OR Dominican* OR Quisqueyan* OR Ecuador* OR ‘French Guiana’ OR ‘French Guianese’ OR ‘Guayana Francesa’ OR Guianan* OR Guatemala* OR Haiti* OR Honduras OR Honduran* OR Mexico OR Mexican* OR Nicaragua* OR Panama* OR Paraguay* OR Guaraní OR Peru OR Peruvian* OR ‘Puerto Rico’ OR ‘Puerto Rican*’ OR Uruguay* OR Venezuela* OR Hispanic* OR Latino* OR Latina*). Previous data for prevalence and risk factors at a population level in Latin America are scarce, with six published studies on this topic (Uruguay, Argentina, Cuba, Bolivia, Brazil, and Colombia). Data available from LatAm also differs in protocols and study designs, making estimations of prevalence and comparisons across the region difficult and prone to bias.
Added value of this study
To the best of our knowledge, this is the first door-to-door survey with PD- related data in multiple Latin America countries using the same assessment methodology. This study adds population-based information for Parkinsonism and PD prevalence, and risk factors in a mixed rural-urban population from Latin America. We found no significant differences in Parkinsonism or PD prevalence within LatAm countries. Compared to HIC, we found a higher prevalence of Parkinsonism in LatAm countries, probably related to a higher frequency of vascular risk factors. This information is valuable for an unbiased estimation of the global burden of Parkinson Disease in Latin America.
Implications of all the available evidence
Our multi-national study provides current estimates for Parkinsonism and PD prevalence among individuals over the age of 65 years in six LatAm countries. Prevalence estimates in individual countries are generally similar, and the meta-analysis estimate confidence intervals are narrow, reflecting the large size per country. A significant proportion of cases with PD did not have a previous diagnosis, nor did they seek any medical or neurological attention. These findings underscore the need to improve public health programs for populations that are currently undergoing rapid demographic aging and epidemiological transition.
Alt-text: Unlabelled box
Introduction
The upcoming demographic shifts toward older populations have led efforts to estimate the expected healthcare burden for the coming decades, particularly for neurodegenerative diseases for which incidence rises considerably with age, such as Alzheimer's disease (AD) and Parkinson's disease (PD).1,2 Neurological disorders are now among the leading cause of disability globally.3 Among those, PD is the most common movement disorder, aside from essential tremor, and the second most common neurodegenerative disease after AD.4,5 Because of the global impact of PD, many epidemiological studies have been conducted worldwide over the past few decades.6 The overwhelming majority of the epidemiological studies in PD have been carried out in high-income countries (HIC). However, there is limited data from large population studies in low and middle-income countries (LMIC) available for consideration.7
Population aging is affecting LMIC countries at a faster rate than HIC, especially those in Latin America (LatAm). The demographic change in LatAm is accompanied by a health transition being driven by changes in habits and lifestyles, where non-communicable diseases, such as PD and AD, are becoming the primary cause of morbidity and disability. Therefore, determining PD prevalence and risk factors is particularly relevant for public health planning in LatAm.8
One of the great challenges in studying the epidemiology of PD is the relatively low frequency and the difficulty in establishing a diagnosis. In addition, prevalence estimates vary widely across studies and countries.9 Age-adjusted PD prevalence appears to be lower in Africa than in Europe and North America,10,11 whereas the prevalence in Asia is similar to that in Europe and North America,12,13 with relatively few population-based studies in LatAm. However, differences in methodology and diagnostic criteria hampers the interpretation of this geographic variation.
These factors, along with the absence of population-based disease registries, have significantly contributed to the gap in knowledge about the epidemiologic characteristics of PD in LatAm countries. This fact encouraged us to estimate the prevalence of parkinsonism and PD in older populations from Latin America.
To the best of our knowledge, studies on the prevalence of parkinsonism (a motor syndrome that manifests as rigidity, tremors, and bradykinesia) and PD (a primary degenerative disease of the brain) are scarce in Latin America, with only six published studies on this topic (Uruguay, Argentina, Cuba, Bolivia, Brazil, and Colombia).14, 15, 16, 17, 18, 19 Data available from LatAm also differs in protocols and study designs, making it difficult to establish comparisons across the region. These studies have employed diverse diagnostic criteria and methods, confounding meaningful comparisons due to significant variations on prevalence estimates by countries. Consequently, it remains unclear whether a true variation exists across LatAm.20 Recent studies from HIC suggest that community-based surveys on PD using the same methodology and diagnostic criteria are less likely to vary across countries and more suitable to drive cross-regional comparisons.20
The overarching goal of the present investigation was to assess the prevalence of parkinsonism, PD, and their risk factors in a cohort of older individuals from LatAm. The present study used data collected through the 10/66 population-based surveys. The 10/66 study is a large prospective cohort study established in 2003 to examine the frequency of dementia and evaluate possible risk factors for cognitive decline among the population over the age of 65 in LMIC. Details of the study have been previously published elsewhere.21 This study was not meant to identify participants with unrecognized PD symptoms but included self-reported PD status, a comprehensive neurological questionnaire, and a full neurological exam for all individuals.22 In this analysis, we present age and sex-specific figures for the prevalence of PD and its risk factors in LMIC. The present research features a regional, multicenter study using the same protocols and diagnosis assessments from six LatAm countries (Cuba, Dominican Republic (DR), Puerto Rico (PR), Mexico, Venezuela, and Peru).
Methods
Setting and study participants
The present investigation is part of the 10/66 research program, a population-based cohort study, comprising in principle all older residents aged 65 years and over, living in eight geographically- defined urban and rural catchment area sites in six LatAm countries.21,23 The primary analyses in this study utilized data from community-dwelling participants aged 65 years and over enrolled in the prevalence phase of the 10/66 study (N = 12,734).21,23 Urban sites were selected to comprise mixed socioeconomic status households. Urban sites were located in Cuba (one catchment area comprising sites in Havana and Matanzas, n = 2813), Dominican Republic (Santo Domingo, n = 2011), Puerto Rico (Bayamon, n = 2009), Venezuela (Caracas, n = 1965), Peru (Lima, n = 1381), and Mexico (Mexico City, n = 1003). Rural sites, remote from major population centers with low-density population and agriculture and related trades as the primary local employment, were located in Peru (Canete Province, n = 552) and Mexico (Morelos State, n = 1000). Site characteristics are summarized elsewhere.23
Written informed consent was obtained from all participants and their study partners. This project was approved by local institutional review boards and the King's College London Research Ethics Committee. The full protocol for the 10/66 population-based surveys is available in an open-access publication.23,24
Measures
The 10/66 research program was developed to address dementia prevalence, incidence, and impact across Latin American countries, China, and India using a validated and standard methodology. However, given that this was a population cohort, the scope of the research was much broader and entailed a comprehensive inquiry into health (common and burdensome chronic diseases, disability and health service utilization) and social aspects of aging (socioeconomic status, social protection, needs for care and care arrangements). 10/66 protocols also included a full neurological disease assessment and a physical and neurological exam. All interviewers and field examiners (4–9 per country) received uniform and standardized training, including (a) study protocol and procedures, (b) standard structured interview techniques, and (c) a two-day specific training for structured clinical assessment and neurological/physical examination. Field examiners (graduate physicians, primary care providers, geriatricians, specialists in internal medicine and registered nurses) were required to complete all training modules prior to the start of the study. Interviewers and field examiners were regularly monitored and supervised to ensure uniform data collection and procedures. Full details are available elsewhere.21,24 Here, we summarize the measures directly relevant to the analyses presented in this paper.
Parkinsonism and PD
All participants underwent a comprehensive interview lasting three hours, including a structured interview, a physical and neurological examination, and an informant interview.24 The comprehensive questionnaire on self-reported non-communicable diseases (e.g., HTA, stroke, dementia) and neurological symptoms, together with the comprehensive neurological examination, permitted estimation of the prevalence of parkinsonism and PD. Based on the clinical interview and neurological exam available in 10/66 data, we generated a composite score to identify parkinsonism,22 and estimated PD based on the exclusion “negative” features (e.g., repeated strokes, supranuclear gaze palsy, cerebellar signs, cerebral tumor, and severe autonomic involvement, among others) that argue against a diagnosis of PD and “positive” features (supportive criteria, e.g., asymmetry, rest tremor, progressive disorder, etc.) that favor a PD diagnosis.22 Previous studies using the same data have shown the predictive validity of this composite score to identify Parkinsonism.22
We defined parkinsonism according to the Parkinson's Disease Society Brain Research centre of the United Kingdom criteria,25,26 requiring the presence of bradykinesia and at least one of the following: rest tremor, rigidity, or postural instability not caused by primary visual, vestibular, cerebellar, or proprioceptive dysfunction. Among subjects fulfilling these criteria, an attempt was made to identify etiologic subgroups. Subjects were diagnosed as having PD after excluding other possible causes of parkinsonism and following the UK Brain Bank criteria for PD.27 Clinically-probable PD was defined as having at least three supportive criteria and having no red flags (absolute exclusion criteria), including (1) no suggestion of a cause for another parkinsonian syndrome such as drugs, trauma, brain tumor, or stroke; and (2) no atypical features such as prominent oculomotor palsy, cerebellar signs, severe orthostatic hypotension, pyramidal signs, or limb apraxia, among others. This approach has been recommended for use in epidemiologic studies.28 In addition, to determine the algorithm's accuracy, the diagnostic algorithm was further validated in the Havana-Cuba sample using clinical diagnoses by trained neurologists as a reference standard. All cases of Parkinsonism or PD identified by the algorithm underwent a case confirmation procedure and were re-assessed by two neurologists with previous training in movement disorders (JJLl-G and JCLl-G) to confirm or exclude the presence of Parkinsonism or PD and determine if diagnostic criteria were met. Compared with the clinical assessment by a neurologist, the sensitivity and specificity of the diagnostic algorithm were 93.9% (95% CI; 88.9–96.5) and 96.7% (95% CI; 93.2–98.7) for parkinsonism and 85.5% (95% CI; 76.2–92.1) and 98.9% (95% CI; 95.9–99.8) for PD, respectively. Based on previous population-based studies,29 we considered this a valid and sensitive diagnostic algorithm.
Covariates
Age was ascertained using participant or informant reports, documented age, or an event calendar. Education level was ascertained and coded as no education, did not complete primary, completed primary, secondary, or tertiary education. Smoking status was self-reported and confirmed by the informant. Socioeconomic status was assessed according to the number of reported household assets (motor vehicles; television; refrigerator and/or freezer; water and electrical utilities; telephone; plumbed toilet; plumbed bathroom). We assessed physical, mental, and cognitive morbidity through measures of stroke, physical impairments, dementia, depression, and main contributors to disability and dependence.30,31 Dementia was diagnosed according to the cross-culturally developed, calibrated, and validated 10/66 dementia diagnosis algorithm.32 Stroke was self-reported (by participant or informant report), and/or all those with stroke suggestive neurological signs (asymmetric long tract signs, dysphasia, marked gait disturbance) were included.33 Physical multi-morbidity was defined as having three or more of nine self-reported, limiting physical impairments (arthritis; persistent cough; breathlessness, difficulty breathing or asthma; high blood pressure; heart trouble or angina; stomach or intestine problems; faints or blackouts; paralysis, limb weakness or loss; skin disorders such as pressure sores, leg ulcers or severe burns). International Classification of Diseases (ICD-10) depressive episode was diagnosed using a computerized algorithm applied to the Geriatric Mental Scale (GMS) structured clinical interview.34
Analysis
Prevalence rates were obtained for Parkinsonism and PD according to age and sex for each country, separately and combined. The age prevalence estimates are presented by five-year age groups and adjusted for household clustering with 95% confidence intervals (95% CI), calculated as described by Gardner and Altman.35 Direct standardization (for age, sex, and education) was used to compare Parkinsonism and PD prevalence among sites, with the whole sample serving as the standard population. For each site, we described associations between Parkinsonism/PD and age (per five-year band), sex (male versus female), education (per level), socioeconomic status (assets per quarter), and health-related factors (stroke, depressive episode, dementia, physical multi-morbidity). We used Poisson regression working models to calculate mutually adjusted prevalence ratios (aPRs) for the effects of age, sex and education on Parkinsonism and PD prevalence. We fitted the model separately for every site and then used a fixed effects meta-analysis to combine them, with Higgins' I2 to estimate the degree of heterogeneity with approximate 95% CIs. Higgins I2 estimates the proportion of between-site variability in the prevalence ratios accounted for by heterogeneity, as opposed to sampling error; up to 40% heterogeneity is conventionally considered negligible, while up to 60% may reflect moderate heterogeneity.36 Data analysis was performed using the Stata v. 16.0 software package (Stata Statistical Software; Stata Corporation, 2001, College Station, TX).
Role of the funding source
The 10/66 Dementia Research Group's research has been funded by the Welcome Trust Health Consequences of Population Change Program (GR066133–Prevalence phase in Cuba and Brazil; GR080002- Incidence phase in Peru, Mexico, Argentina, Cuba, Dominican Republic, Venezuela, and China). Secondary data analysis on parkinsonism and Parkinson disease in the 10/66 Latin American countries is supported by the Michael J. Fox Foundation (MJFF-020770). The content is solely the responsibility of the authors and does not represent the official views of WT or MJFF. The sponsors of the study had no role in study design, data collection, data analysis, data interpretation, writing of the report and decision to submit the manuscript for publication. All authors had full access to all the data in the study, and the corresponding author had final responsibility for the decision to submit for publication.
Results
Sample characteristics
The eligible population consisted of 12,734 participants surveyed in 8 sites from six countries, and 11,613 (91.2%) provided sufficient data to establish Parkinsonism/PD phenotype. The populations studied ranged in size from 546 in rural Peru to 2903 in urban Cuba. Mean ages varied between 72.5 and 76.3 years (Supplemental Table S1). Most participants (64.2%) were female. Education levels varied widely among sites; overall, 38.8% of the participants did not complete primary school. Educational levels and other health-related factors are detailed in Supplemental Table S1.
Parkinsonism and correlates
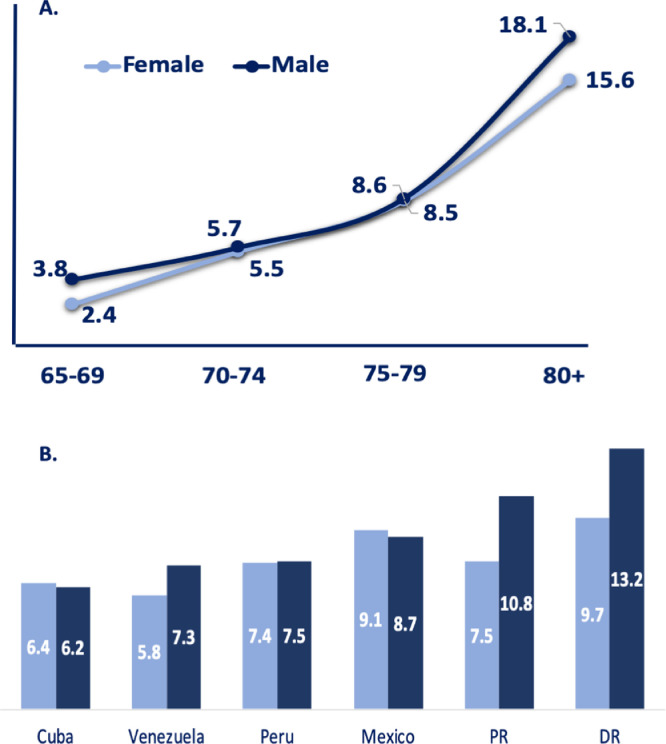
The global number of confirmed cases of parkinsonism was 932 (ranging from 88 to 101 per country), yielding a crude prevalence rate of 8.0% (95% CI, 7.6–8.5). The highest prevalence of parkinsonism was observed in the Dominican Republic, 10.9% (95% CI, 9.6–12.4), while the lowest was reported in Cuba, 6.3% (95% CI, 5.5–7.3) and Venezuela, 6.3% (95% CI, 5.2–7.7). Table 1 shows case number denominators and prevalence estimates for each country according to sex and age group. Among confirmed cases of parkinsonism, 366 were male and 566 were female, with higher prevalence estimates of 8.8% (95% CI, 8.0%−9.7%) in males compared to 7.6% (95% CI, 7.0–8.2, p < 0.001) in females. There was a clear trend in all sites for parkinsonism's prevalence to increase with age (for both men and women), ranging from 2.8% (95% CI, 2.3–3.4) in the 65–69 age group to 16.5 (95% CI, 15.1–17.9) in the 80 plus age group (p < 0.001). (Fig. 1).
Table 1.
Prevalence of Parkinsonism by age and location for both sexes.
| Age groups years (n) | 65–69Rate (CI) | 70–74Rate (CI) | 75–79Rate (CI) | 80 +Rate (CI) | TotalRate (CI) |
|---|---|---|---|---|---|
| Cuba | N = 757 | N = 789 | N = 627 | N = 730 | N = 2903 |
| Female (n = 1 880) | 2.3 (1.3-4.1) | 4.1 (2.66.2) | 7.0 (4.9–10.0) | 12.1(9.5–15.3) | 6.4 (5.4−7.6) |
| Male (n = 1023) | 2.1 (1.0-4.8) | 3.1 (1.6–5.8) | 5.2 (3.0–9.0) | 15.9 (11.7 −21.3) | 6.2 (4.8–7.8) |
| Total (n = 2903) | 2.3 (1.4–3.6) | 3.7 (2.6–5.3) | 6.4 (4.7–8.6) | 13.3 (11.0–15.9) | 6.3 (5.5-7.3) |
| Dominican Republic | n = 516 | n = 494 | n = 368 | n = 457 | n = 1835 |
| Female (n = 1192) | 2.4 (1.2–4.7) | 9.1 (6.3–12.9) | 10.0 (6.8–14.4) | 18.1 (14.1–22.9) | 9.7 (8.1–11.5) |
| Male (n = 643) | 6.5 (3.4–11.2) | 10.1(6.5–15.4) | 14.2 (9.0–21.8) | 24.0 (17.9–31.4) | 13.2 (10.8–16.1) |
| Total (n = 1835) | 3.9 (2.5–5.9) | 9.5 (7.2–12.4) | 11.4 (8.5–15.1) | 20.1 (1.7–24.1) | 10.9 (9.6–12.4) |
| Puerto Rico | n = 339 | n = 376 | n = 402 | n = 487 | n = 1604 |
| Female (n = 1083) | 2.0 (0.8–4.6) | 1.6 (0.6–4.2) | 8.4 (5.7–12.4) | 16.0 (12.4–20.6) | 7.5 (6.1–9.2) |
| Male (n = 521) | 2.4 (0.6–9.2) | 6.3 (3.2–12.1) | 7.6 (4.1–13.6) | 20.4 (15.1–27.1) | 10.8 (8.4–13.8) |
| Total (n = 1604) | 2.1 (1.0–4.3) | 3.2 (1.8–5.5) | 8.2 (5.9–11.3) | 17.6 (14.5–21.3) | 8.6 (7.3–10.1) |
| Peru (urban) | n = 368 | n = 351 | n = 296 | n = 343 | n = 1358 |
| Female (n = 874) | 4.6 (2.6–7.9) | 5.9 (3.4–9.9) | 6.3 (3.6–10.8) | 11.2 (7.5–16.3) | 6.9 (5.4–8.7) |
| Male (n = 485) | 3.7 (1.4–9.5) | 3.0 (1.1–7.8) | 14.0 (8.6–22.0) | 18.1 (12.5–25.4) | 9.9 (7.5–12.9) |
| Total (n = 1359) | 4.3 (2.7–7.0) | 4.8 (3.0–7.) | 9.1 (6.3–13.0) | 14.0 (10.7–18.1) | 7.9 (6.6–9.5) |
| Peru (rural) | n = 179 | n = 140 | n = 99 | n = 128 | n = 546 |
| Female (n = 291) | 5.0 (2.1–11.5) | 7.3 (3.3–15.4) | 3.9 (1.0–14.5) | 17.3 (9.5–29.3) | 7.9 (5.311.6) |
| Male (n = 255) | 6.3 (2.6–14.4) | 5.2 (1.6–15.0) | 4.1 (1.0–15.4) | 4.2 (1.3–12.5) | 5.1(3.0–8.6) |
| Total (n = 546) | 5.6 (3.0–10.1) | 6.4 (3.4–11.9) | 4.0 (1.5–10.3) | 10.2 (6.0–16.6) | 6.6 (4.7–9.0) |
| Venezuela | n = 610 | n = 335 | n = 249 | n = 206 | n = 1400 |
| Female (n = 893) | 1.0 (0.4–2.7) | 4.2 (2.2–8.0) | 9.9 (6.0–15.7) | 16.6 (11.4–23.6) | 5.8 (4.4–7.6) |
| Male (n = 507) | 4.0 (2.1–7.5) | 8.8 (4.9–15.3) | 7.3 (3.5–14.7) | 16.5 (8.8–27.5) | 7.3 (5.3–9.9) |
| Total (n = 1400) | 2.1 (1.2–3.6) | 6.0 (3.9–9.1) | 8.9 (5.9–13.1) | 16.5 (12.0–22.2) | 6.3 (5.2–7.7) |
| Mexico (urban) | n = 243 | n = 326 | n = 202 | n = 211 | n = 982 |
| Female (n = 648) | 1.6 (0.5–4.9) | 10.1 (6.6 −15.0) | 13.8 (8.7–21.2) | 26.3 (19.5–34.4) | 11.7 (9.4–14.4) |
| Male (n = 334) | 3.3 (0.8–12.5) | 2.5 (0.8 - 7.6) | 9.0 (4.3–17.7) | 17.9 (10.8–28.1) | 7.7 (5.3–11.2) |
| Total (n = 982) | 2.1 (0.8–4.9) | 7.4 (5.0 −10.8) | 11.9 (8.1–17.2) | 23.2 (18.0–29.4) | 10.3 (8.6–12.5) |
| México (rural) | n = 299 | n = 251 | n = 217 | n = 217 | n = 984 |
| Female (n = 590) | 2.0 (0.7–5.3) | 2.7 (1.0–7.0) | 9.1 (5.2–15.3) | 15.9 (10.2–23.9) | 6.4 (4.7–8.7) |
| Male (n = 394) | 1.9 (0.5–7.5) | 7.7 (3.9–14.8) | 8.2 (3.9–16.3) | 20.2 (13.5–29.1) | 9.6 (7.1–13.0) |
| Total (n = 984) | 2.0 (0.9–4.4) | 4.8 (2.7–8.2) | 8.8 (5.6–13.3) | 18.0 (13.4–23.7) | 7.7 (6.2–9.5) |
| All centres combined | n = 3311 | n = 3062 | N = 2460 | n = 2780 | n = 11,613 |
| Female (n = 7451) | 2.4 (1.8–3.1) | 5.5 (4.5–6.6) | 8.5 (7.3–10.1) | 15.6 (13.9–17.3) | 7.6 (7.0–8.2) |
| Male (n = 4162) | 3.8 (2.8–5.1) | 5.7 (4.5–7.2) | 8.6 (7.0–10.7) | 18.1 (15.8–20.6) | 8.8 (8.0–9.7) |
| Total (n = 11,613) | 2.8 (2.3–3.4) | 5.5 (4.8–6.4) | 8.6 (7.5–9.8) | 16.5 (15.1–17.9) | 8.0 (7.6–8.5) |
The overall gender-specific prevalence rates are age adjusted to the whole sample as standard population. Numbers in parentheses indicates 95% Confidence Interval. CI=confidence interval.
Figure 1.
Prevalence of Parkinsonism by group age group, sex and country.
Panel A. shows prevalence of parkinsonism by sex and by five-year age groups. Panel B. shows prevalence of parkinsonism in six LatAm countries. Prevalence is expressed as the percentage of the population that is affected by the disease. DR=Dominican Republic, PR=Puerto Rico.
In the pooled adjusted prevalence analysis (Table 2), age was associated with Parkinsonism (aPR-per 5-year increment in age) = 1.06 (95% CI 1.05–1.07), with marked heterogeneity among sites (I2 = 77.4%). The pooled aPR for sex (M vs F) was 1.30, (95% CI 1.12–1.50, p < 0.001 [I2 = 9.9%]). Parkinsonism was positively associated with depression (aPR 2.02, 95% CI 1.68–2.42, p < 0.001, I2 = 51.2%) and dementia (aPR 1.37, 95% CI 1.22–1.55, p < 0.001, I2 = 70.6%). No associations were observed for socioeconomic status (aPR 0.98, 95% CI 0.93–1.04) and smoking status (aPR 0.86, 95% CI 0.74–1.00).
Table 2.
Models from Poisson regression with robust 95% CI adjusted by household clustering for parkinsonism.
| Age | GenderM/F | Education | PM | SES | Depression ICD | Dementia | Stroke | Smoke | |
|---|---|---|---|---|---|---|---|---|---|
| Cuba (N = 2833) |
1.08 (1.06–1.09) |
1.07 (0.75 −1.49) |
1.21 (1.05 −1.40) |
1.32 (1.08 −1.62) |
1.04 (0.85–1.24) |
2.49 (1.63 −3.80) |
2.12 (1.53–2.94) |
1.79 (1.26–2.56) |
1.01 (0.72–1.39) |
| DR (N = 1813) | 1.05 (1.04–1.07) |
1.46 (1.11 −1.91) |
0.90 (0.77–1.05) |
1.49 (1.21–1.83) |
0.95 (0.87–1.04) |
1.81 (1.34–2.4) |
1.10 (0.93–1.29) |
2.51 (1.83–3.45) |
0.86 (0.65–1.13) |
| Puerto Rico (N = 1597) |
1.08 (1.05–1.10) |
1.39 (0.95 −2.04) |
1.02 (0.86 −1.20) |
1.23 (0.98–1.56) |
0.84 (0.67–1.05) |
3.61 (2.07–6.27) |
1.18 (0.76–1.85) |
2.17 (1.44–3.29) |
0.99 (0.66–1.48) |
| Peru Urban (N = 1343) |
1.02 (1.00–1.05) |
1.64 (1.10–2.42) |
0.79 (0.64 −0.95) |
1.44 (1.13–1.82) |
1.16 (0.82–1.63) |
2.13 (1.25 3.59) |
2.36 (1.53–3.65) |
2.02 (1.23–3.31) |
0.87 (0.54–1.44) |
| Peru rural (N = 537) |
1.03 (0.98–1.08) |
0.74 (0.34 −1.61) |
0.95 (0.64–1.41) |
1.59 (0.95–2.66) |
1.07 (0.88–1.31) |
1.12 (0.26–4.81) |
1.74 (0.71–4.21) |
3.96 (1.23–9.19) |
0.39 (0.10–1.48) |
| Venezuela (N = 1345) |
1.10 (1.06–1.12) |
1.53 (1.00–2.35) |
1.11 (0.88–1.42) |
1.00 (0.74–1.35) |
0.90 (0.76–1.05) |
2.97 (1.43–6.16) |
2.29 (1.26–4.15) |
2.41 (1.33–4.34) |
0.81 (0.53–1.25) |
| Mexico urban (N = 979) |
1.07 (1.05–1.10) |
0.96 (0.61–1.48) |
0.94 (0.79–1.12) |
1.28 (0.99–1.62) |
1.03 (0.86–1.22) |
0.92 (0.41–2.07) |
1.65 (1.02–2.68) |
1.04 (0.54–2.05) |
0.46 (028–0.75) |
| Mexico rural (N = 984) |
1.09 (1.06–1.12) |
1.21 (0.70–2.07) |
0.92 (0.69–1.23) |
1.24 (0.91–1.67) |
1.06 (0.92–1.22) |
0.99 (0.37–2.59) |
1.22 (0.67–2.21) |
1.04 (0.45–2.39) |
1.25 (0.72–2.17) |
| Pooled estimate | 1.06 (1.05–1.07) |
1.30 (1.12–1.50) |
0.99 (0.93–1.06) |
1.29 (1.20–1.40) |
0.98 (0.93–1.04) |
2.02 (1.68–2.42) |
1.37 (1.22–1.55) |
1.37 (1.22–1.55) |
0.86 (0.74–1.00) |
| I2 | 77.4 | 9.9 | 55.4 | 0.0 | 0.0 | 51.2 | 70.6 | 0.0 | 34.5 |
PM: Physical multimorbidity SES: Socioeconomic status. ICD: International Classification of Diseases DR Dominican Republic.
Parkinson's disease and risk factors
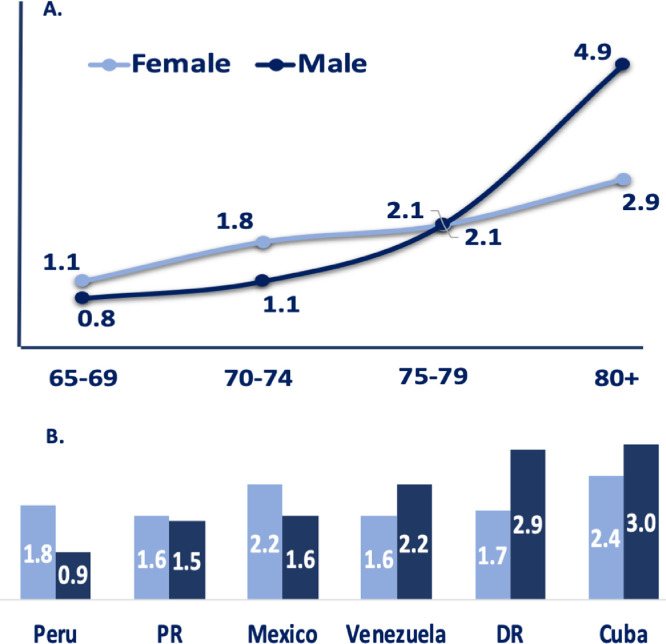
The number of PD cases identified by the diagnostic algorithm was 232; age and sex adjusted prevalence rate was 2.0% (95% CI, 1.7–2.3) across all the sites. The distribution by age and gender per country for PD cases and the population at risk is presented in Table 3. The lowest PD prevalence was reported in urban Peru (1.2, 95% CI, 0.8–2.1), while the highest prevalence was observed in Cuba at 2.6% (95% CI, 2.1–3.3). The men/women prevalence ratio for PD was 1.1 (54% men). For both men and women, the prevalence of PD increased with advancing age (p < 0.001). In men at older ages, the age-specific prevalence of PD was higher than in women (Fig. 2), except for women in rural communities (Table 3). Although limited by the sample size, we found no significant difference in prevalence estimates between rural and urban communities.
Table 3.
Prevalence of Parkinson's disease by age and location for both sexes.
| Age groups years (n) | 65–69Rate (CI) | 70–74Rate (CI) | 75–79Rate (CI) | 80 +Rate (CI) | TotalRate (CI) |
|---|---|---|---|---|---|
| Cuba | n = 757 | n = 789 | n = 627 | n = 730 | n = 2903 |
| Female (n = 1880) | 1.4 (0.7–3.0) | 1.8 (1.0–3.5) | 3.0 (1.7–5.2) | 3.4 (2.1–5.3) | 2.4 (1.8–3.2) |
| Male (n = 1023) | 0.7 (0.2–2.9) | 0.7 (0.2–2.7) | 2.2 (0.9–5.1) | 9.7 (6.5–14.3) | 3.0 (2.1–4.3) |
| Total (n = 2903) | 1.2 (0.6–2.2) | 1.4 (0.8–2.5) | 2.7 (1.71–4.3) | 5.3 (3.9–7.2) | 2.6 (2.1–3.3) |
| Dominican Republic | n = 516 | n = 494 | n = 368 | n = 457 | n = 1835 |
| Female (n = 1192) | 1.2 (0.5–3.1) | 1.3 (0.5–3.4) | 2.0 (0.8–04.7) | 2.3 (1.1–4.7) | 1.7 (1.1–2.6) |
| Male (n = 643) | 1.1 (0.3–4.3) | 1.6 (0.5–4.9) | 5.8 (2.8–11.8) | 4.5 (2.1–9.2) | 2.9 (1.9–4.6) |
| Total (n = 1835) | 1.2 (0.5–2.6) | 1.4 (0.7–2.9) | 3.3 (1.9–5.7) | 3.1 (1.82–5.1) | 2.1 (1.5–2.9) |
| Puerto Rico | n = 339 | n = 376 | n = 402 | n = 488 | n = 1, 605 |
| Female (n = 1084) | 1.2 (0.4–3.6) | 0.4 (0.1–2.8) | 2.6 (1.2–5.3) | 1.9 (0.8–4.2) | 1.6 (1.0–2.5) |
| Male (n = 521) | 0.0 | 1.6 (0.4–6.1) | 0.8 (0.1–5.2) | 2.8 (1.2–6.6) | 1.5 (0.8–3.1) |
| Total (n = 1605) | 0.9 (0.3–2.7) | 0.8 (0.3–2.4) | 2.0 (1.0–3.9) | 2.2 (1.3–4.0) | 1.6 (1.1–2.3) |
| Peru (urban) | n = 368 | n = 351 | n = 296 | n = 343 | n = 1358 |
| Female (n = 873) | 1.9 (0.8–4.5) | 1.4 (0.4–4.2) | 1.1 (0.3–4.1) | 1.0 (0.2–3.8) | 1.3 (0.7–2.4) |
| Male (n = 485) | 0.0 (0.0–0.0) | 0.8 (0.1–5.2) | 1.9 (0.5–7.2) | 2.2 (0.76.5) | 1.2 (0.5–2.7) |
| Total (n = 1358) | 1.4 (0.6–3.2) | 1.1 (0.4–3.0) | 1.4 (0.5–03.6) | 1.5 (0.6–3.4) | 1.2 (0.8–2.1) |
| Peru (rural) | n = 179 | n = 140 | n = 99 | n = 128 | n = 546 |
| Female (n = 291) | 1.0 (0.1–6.8) | 3.6 (1.2–10.8) | 0.0 (0.0–0.0) | 5.1 (1.6–15.0) | 2.4 (1.1–5.0) |
| Male (n = 255) | 1.3 (0.2–8.6) | 0.0 (0.0 – 0.0) | 0.0 (0.0 – 0.0) | 0.0 (0.0 – 0.0) | 0.4 (0.05–2.7) |
| Total (n = 546) | 1.1 (0.3–4.4) | 2.1 (0.7–6.5) | 0.0 (0.0 – 0.0) | 2.3 (0.7–7.1) | 1.4 (0.7–2.9) |
| Venezuela | n = 610 | n = 335 | n = 247 | n = 206 | n = 1398 |
| Female (n = 891) | 0.3 (0.04–1.8) | 1.9 (0.7–5.0) | 1.3 (0.7–4.9) | 4.9 (2.3–9.8) | 1.6 (0.9–2.6) |
| Male (n = 507) | 0.9 (0.2–3.5) | 2.4 (0.8–7.3) | 2.1 (0.5–8.1) | 6.5 (2.4–16.1) | 2.2 (1.2–3.9) |
| Total (n = 1398) | 0.5 (0.2–1.5) | 2.1 (1.0–4.3) | 1.6 (0.6–4.2) | 5.3 (3.0–9.4) | 1.8 (1.2–2.6) |
| Mexico(urban) | n = 243 | n = 326 | n = 203 | n = 211 | n = 981 |
| Female (n = 647) | 1.1 (0.3 - 4.3) | 3.8 (1.9–7.5) | 1.6 (0.4–6.3) | 3.8 (1.5–8.7) | 2.6 (1.6 - 4.2) |
| Male (n = 334) | 1.7 (0.2 −11.1) | 0.8 (0.2–5.8) | 1.3 (0.2–8.6) | 3.8 (1.2–11.3) | 1.8 (0.8 – 4.0) |
| Total (n = 981) | 1.2 (0.4–3.7) | 2.8 (1.4–5.2) | 1.5 (0.5–4.5) | 3.8 (1.9–7.4) | 2.3 (1.5 - 3.5) |
| México (rural) | n = 299 | n = 251 | n = 217 | n = 217 | n = 984 |
| Female (n = 590) | 1.0 (0.3–4.0) | 0.7 (0.09–4.6) | 2.3 (0.7–6.8) | 4.4 (01.8–10.2) | 1.9 (1.0–3.3) |
| Male (n = 394) | 1.0 (0.1–6.7) | 1.0 (0.1–6.6) | 1.2 (0.1–7.9) | 1.9 (0.5–7.4) | 1.3 (0.6–3.0) |
| Total (n = 984) | 1.0 (0.3–3.1) | 0.8 (0.2–3.1) | 1.8 (0.7–4.8) | 3.2 (1.5–6.6) | 1.6 (1.0–2.6) |
| All centres combined | n = 3308 | n = 3054 | n = 2459 | n = 2779 | n = 11, 610 |
| Female (n = 7444) | 1.1 (0.8–1.7) | 1.8 (1.2–2.4) | 2.1 (1.5–2.9) | 2.9 (2.2–3.8) | 1.9 (1.6–2.3) |
| Male (n = 4156) | 0.8 (0.4–1.6) | 1.1 (0.6–1.9) | 2.1 (1.4–3.3) | 4.6 (3.4–6.0) | 2.1 (1.7–2.6) |
| Total (n = 11,610) | 1.0 (0.7–1.4) | 1.5 (1.1–2.0) | 2.1 (1.6–2.7) | 3.5 (2.9–4.3) | 2.0 (1.7–2.3) |
The overall gender-specific prevalence rates are age adjusted to the whole sample as standard population. Numbers in parentheses indicates 95% Confidence Interval. CI=confidence interval.
Figure 2.
Prevalence of Parkinson Disease by age group, sex and country.
Panel A. shows prevalence of PD by sex and by five-year age groups. Panel B. shows prevalence of PD by six LatAm countries. Prevalence is expressed as the percentage of the population that is affected by the disease. DR=Dominican Republic, PR=Puerto Rico.
On pooled adjusted prevalence ratios (Table 4), PD was positively associated with depression (aPR 2.06, 95% CI 1.40–3.01, I2 = 56.0%), dementia (aPR 1.57, 95% CI 1.07- 2.32, I2 = 0.0%) and educational level (aPR 1.14, 95% CI 1.00-1.29, I2 = 58.6%).
Table 4.
Models from Poisson regression with robust 95% CI adjusted by household clustering for Parkinson.
| Age | Gender | Education | PM | Assets | Depression ICD | Dementia | Smoke | |
|---|---|---|---|---|---|---|---|---|
| Cuba (N = 2883) |
1.08 (1.04–1. 11) |
1.62 (0.93–2.80) |
1.23 (0.99–1.51) |
1.03 (0.72–1.48) |
1.07 (0.77- 1.48) |
2.71 (1.32- 5.52) |
1.54 (0.84–2.82) |
0.81 (0.47–1.41) |
| DR (N = 1833) | 1.04 (1.00–1.07) |
1.95 (1.02–3.73) |
0.89 (0.58–1.35) |
1.94 (1.18–3.18) |
0.96 (0.79 −1.16) |
1.08 (0.67–2.78) |
1.35 (0.50 −3.61) |
1.44 (0.74–2.80) |
| Puerto Rico (N = 1597) |
1.06 (1.01–1.13) |
0.85 (0.33–2.19) |
1.29 (0.81–2. 05) |
0.97 (0.56–1.70) |
0.85 (0.55–1.29) |
10.93 (3. 53–3.90) |
0.72 (0.18–2. 84) |
1.55 (0.59–4.06) |
| Peru Urban (N = 1343) |
0.98 (0.91–1.05) |
1.43 (0.51–4.04) |
0.66 (0.46–0.94) |
1.77 (1. 01–3.13) |
0.86 (0.49–1.52) |
1.25 (0.25–6.20) |
2.05 (0.50–8.27) |
0.28 (0.03–2.60) |
| Peru rural (N = 537) |
1.04 (0.91–1.18) |
0.20 (0.02–2.24) |
0.89 (0.35–2. 28) |
1.32 (0.53–3.25) |
1.81 (1. 15–2.86) |
# | # | |
| Venezuela (N = 1345) |
1.11 (1.06–1.17) |
1.58 (0.62–3.99) |
1.39 (0.91–2.14) |
1.19 (0.64- 2.19) |
0.83 (0.66–1.06) |
2.45 (0.49–12.2) |
2.36 (0.77–7.18) |
0.59 (0.24–1.43) |
| Mexico urban (N = 979) |
1.03 (0. 97–1.10) |
1.05 (0.39–2.80) |
1.46 (1.11–1.93) |
2.53 (1.51–4.24) |
0.80 (0. 56–1.15) |
1.56 (0.51–4.81) |
1.27 (0.33–4.86) |
0.50 (0.18–1. 36) |
| Mexico rural (N = 984) |
1.04 (0.97–1.10) |
0.84 (0. 19–3.66) |
0.64 (0. 32–1.27) |
1.81 (0.80–4.06) |
1.18 (0.85–1.64) |
0.89 (0.11–6.93) |
2.40 (0.71–8.16) |
0.70 (0.1–4.18) |
| Pooled estimate | 1.06 (1.04 −1.08) |
1.40 (1.04–1.90) |
1.14 (1.01- 1.29) |
1.42 (1.17–1.73) |
0.97 (0.87- 1.08) |
2.06 (1.40–3.01) |
1.57 (1.07–2.32) |
0.89 (0.64–1.23) |
| I2(%) | 39.7 | 0.0 | 58.6 | 42.9 | 42.8 | 56.0 | 0.0 | 73.7 |
PM: Physical multimorbidity SES: Socioeconomic status. ICD: International Classification of Diseases Dominican Republic, # estimates not computable due to low number of cases.
Discussion
Our multi-national study provides current estimates for Parkinsonism and PD prevalence among individuals over the age of 65 years from LatAm countries. Prevalence estimates in individual countries are generally similar, and the meta-analysis estimate confidence intervals are narrow, reflecting the large size per country. Despite the overall similarity, there is evidence for heterogeneity, particularly a higher estimated prevalence of Parkinsonism in the Dominican Republic and Mexico. Interestingly, we found that most Parkinsonism and/or PD cases had not been previously diagnosed, which might reflect relatively low access to health care and specialized services. To the best of our knowledge, this is the first door-to-door survey with PD-related data reported in multiple LatAm countries using the same assessment methodology.
Previous studies have shown differences in PD prevalence by region, country, age groups, and areas (rural vs. urban).1,20,37,38 In our study, after adjusting for the effect of age and sex using Poisson regression models, we found no significant differences in Parkinsonism and PD prevalence within LatAm countries. Compared to HIC, we found a higher prevalence of Parkinsonism in LatAm countries, probably related to a higher frequency of vascular risk factors. Vascular parkinsonism, a condition that is accompanied by the development of white matter lesions (WMLs) and lacunes in the brain, has been associated with vascular risk factors, such as stroke, hypertension, dyslipidaemia, diabetes mellitus, sleep apnea and smoking.39 As shown in this study and previous 10/66 studies,33,40,41 prevalence and incidence of vascular risk factors are high in Latin American countries which may contribute to increased prevalence of parkinsonism with a vascular etiology. The lower prevalence of vascular risk factors in some countries (e.g., Cuba) may also explain some of the observed differences in overall parkinsonism prevalence. Future cross-population HIC vs. LMIC studies will be needed to confirm this observation. Regarding PD prevalence, our study found a similar prevalence to studies in HIC using a similar case-finding approach. Overall, we found that our prevalence estimates are similar to the previous door-to-door population studies but higher than previous PD prevalence reports using medical registries or drug consumption.14, 15, 16, 17, 18, 19
Likely explanations for the difference in prevalence estimates between our study and previous surveys concerning both prevalence of parkinsonism and PD may be related to environmental factors, genetic factors or differences in methodology of the studies (e.g., variations in population selection, case-finding methods, etc.), as explained below. PD is thought to be caused by a complex interaction between aging, genetic and environmental exposure factors (chemicals, toxins, head trauma). The interactions between genes and the environment can be quite complex and may vary across populations. Previous studies have shown potential differences across ethnicities and increased prevalence of PD following regional distribution, suggesting differences in susceptibility to genetic risk factors and environmental exposures.37,42,43
Differences in environmental exposure to toxins (e.g., occupational exposure or pesticides), smoking, and dietary factors exist across populations and may explain some of the observed differences between HIC vs. LMIC in PD prevalence.42 Further investigations should also explore the impact of the environment in LatAm countries, as environmental toxicants can disproportionately affect LMIC. In addition, the frequency of susceptibility genes that affect the risk of developing sporadic (e.g., non-familial) PD44,45 may vary according to population admixture, explaining some of the observed differences. Therefore, the risk of PD will depend on genetic susceptibility and the presence or absence of pathogenic variants and/or common risk variants (individual variability), which will influence the susceptibility to environmental factors (population variability). Future studies will be needed to rule out the possibility of differences in the distribution by ethnicity of any susceptibility genes.
Finally, although environmental and genetic factors may explain some of the observed variability in PD prevalence, these are more likely to capture only a portion of the variance. Reasons for reported differences in prevalence might be related to screening methodology. PD cases may be identified through medical records; however, this approach may underestimate prevalence as it excludes individuals who have not sought medical attention for their symptoms, don't have access to health care, or those who have been seen by physicians but were misdiagnosed as not having the condition. In addition, studies that rely on the analysis of drug consumption data can be confounded by numerous other factors, including culturally determined treatment practices and differing access to medications that vary by country and region. The relative lack of population registries, combined with limitations and differences in access to health care from country to country, makes these approaches not suitable for LMIC, especially in LatAm.46 In theory, case ascertainment through door-to-door or population-based random sampling offers a more robust alternative, as it includes those patients who have not sought medical attention and those who have not had adequate access to medical care, and should, in theory, be more suitable for international comparisons.9,47
Our findings suggest a rise in the prevalence of parkinsonism and PD occurs with increasing age. Overall, the prevalence was greater with each increasing age group, consistent with PD being a result of an aging phenomenon, at least in part. The increasing prevalence of Parkinsonism and PD with age is a common finding and consistent with previous studies, as shown by several community studies.1,37,48 By contrast, studies based on existing medical records show a peak in prevalence followed by a decline among the oldest old.49, 50, 51 We believe that these differences might be related to PD under diagnosis among older subjects, especially in studies where PD cases are detected through medical records only. However, whether there is a progressive raise in late-life or a decline in incidence remains controversial.11,52,53
Similar to other studies,54, 55, 56 our data show significant gender differences with men having a higher prevalence of PD, but this relation varied by age. Interesting, women in our data show a sharp increase in prevalence after the age of 80, whereas in men, the increase in prevalence seems more or less linear with age. It is unclear whether the observed sharp increase in women after the 80s represents a true increase in the risk of PD or is simply a selection bias due to early mortality in men. Overall our findings with respect to age and gender are consistent with clinical observations, as well as with almost all studies of prevalence and incidence.8,52,53,56, 57, 58
It's worth noting that although some studies report that populations living in a rural environment are at a higher risk of developing PD compared to those living in urban cities,59, 60, 61, 62, 63 in our study, rural populations (rural Peru and rural Mexico) did not have a higher prevalence of PD compared to urban populations (La Habana, Santo Domingo, Puerto Rico, Lima, Caracas, and Mexico City). However, this finding should be interpreted with caution due to the limited sample size in rural areas.
Finally, the present study is not without limitations, and several aspects of our research need to be kept in mind when considering these results. First, PD is relatively uncommon, and even studies of large populations will find relatively few cases. Therefore, the potential error in any single study may be significant, especially in older population age groups where slowness and tremor may be misdiagnosed as one of several other common disorders affecting this age group (for example, arthritis, stroke, dementia). A second concern involves our reliance on survey data and that PD diagnoses were not made by a movement disorders specialist, which may create case underreporting, especially in those participants at earlier stages of the disease. As shown in previous studies, parkinsonism and PD diagnosis accuracy is higher when assessed by trained movement disorders physicians relative to non-movement disorders physicians.64,65 Furthermore, an unavoidable factor is the different training levels and clinical experience in screening personnel, which may create differences in case identification across countries. Finally, the use of survey methods combined with self-report measures is subject to underreporting, misclassification, and selection bias issues (e.g., gradients of non-response by important confounders such as economic status, education, etc.). Of note, the overall response rate in our study was high,66 but nonresponse may have led to a certain underestimation of the prevalence.
Despite previous limitations, there are several advantages to the current approach. First, population-based registries of PD are not standard in LatAm, and voluntary registries are not necessarily representative, due to a relative lack of access to health care. Therefore, the use of a population-based approach that involved direct contact with participants to assess disease status diminishes the possibility of prevalence underestimation. In addition, the use of the same assessment protocol is more suitable for cross-country comparisons and reduces the variation due to differences in access to and quality of medical care in various populations. Therefore, this study is particularly well-suited to estimate Parkinsonism and PD prevalence in LatAm populations. This is also the first large cross-country Parkinsonism and PD comparison including LatAm populations. Our study provides valuable data from which numerous hypotheses can be tested regarding the pathogenesis of Parkinson disease, setting the stage for future studies and new policy implementations.
It's worth mentioning that Latin American populations are not a monolithic group; a unique aspect of the Latino community is the diversity of its origins, which include people of the Caribbean, Mexican, Central American, and South American. LatAm countries are diverse in history, culture, and socioeconomic factors, therefore future studies should include other countries from the region that were not included in the 10/66 studies due to limited funding. In addition, future studies that aim to explore cross-population differences in PD prevalence and risk will be required, especially between HIC and LMIC. If differences are observed between these studies, we may find valuable clues about the determinants of PD.
Conclusions
The reported prevalence of PD in LatAm appears to be similar to the prevalence in HIC. A significant proportion of cases with probable PD did not have a previous diagnosis, nor did they seek any medical or neurological attention. These findings underscore the need to improve public health programs for populations that are currently undergoing rapid demographic aging and epidemiological transition. For LatAm countries, special focus should be on raising awareness of PD, improving health care personnel training opportunities and providing better access to required resources to facilitate diagnosis.
Data sharing statement
Individual participant data that underlie the results reported in this paper, after de-identification (text, tables, figures, and appendices) will be available (including data dictionaries in Spanish and English) immediately following publication indefinitely to anyone who wishes to access the data, for any purpose. Individuals who would like to access the data should contact the corresponding author.
Declaration of interests
Llibre-Guerra JJ, Prina M, Sosa AL, Acosta D, Guerra M, Jiménez-Velázquez I, Salas A, Llibre-Guerra JC, Valvuerdi A, Acosta I, Peeters G, Ziegemeier E, Tanner C, Juncos J and Llibre-Rodríguez J report no conflict of interest relevant to this manuscript.
Acknowledgments
This is a secondary analysis of data collected by the 10/66 Dementia Research Group (www.alz.co.uk/1066). The 10/66 Dementia Research Group's research has been funded by the Wellcome Trust Health Consequences of Population Change Programme (GR066133 – Prevalence phase in Cuba and Brazil; GR080002- Incidence phase in Peru, Mexico, Argentina, Cuba, Dominican Republic, Venezuela, and China), the World Health Organization (India, Dominican Republic, and China), the US Alzheimer's Association (IIRG – 04 – 1286 - Peru, Mexico, and Argentina), the Puerto Rico State Legislature (Puerto Rico), and FONACIT/ CDCH/ UCV (Venezuela). Secondary data analysis on parkinsonism and Parkinson disease in the 10/66 Latin American countries is supported by the Michael J. Fox Foundation (MJFF-020770).
Footnotes
Supplementary material associated with this article can be found in the online version at doi:10.1016/j.lana.2021.100136.
Appendix. Supplementary materials
References
- 1.Pringsheim T., Jette N., Frolkis A., Steeves T.D.L. The prevalence of Parkinson's disease: a systematic review and meta-analysis. Mov Disord. 2014;29:1583–1590. doi: 10.1002/mds.25945. [DOI] [PubMed] [Google Scholar]
- 2.Dorsey E.R., Constantinescu R., Thompson J.P., et al. Projected number of people with Parkinson disease in the most populous nations, 2005 through 2030. Neurology. 2007;68:384–386. doi: 10.1212/01.wnl.0000247740.47667.03. [DOI] [PubMed] [Google Scholar]
- 3.GBD 2016 Parkinson's Disease Collaborators ER. Elbaz A., Nichols E., et al. Global, regional, and national burden of Parkinson's disease, 1990-2016: a systematic analysis for the global burden of disease study 2016. Lancet Neurol. 2018;17:939–953. doi: 10.1016/S1474-4422(18)30295-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Alves G., Forsaa E.B., Pedersen K.F., Dreetz Gjerstad M., Larsen J.P. Epidemiology of Parkinson's disease. J Neurol. 2008;255:18–32. doi: 10.1007/s00415-008-5004-3. [DOI] [PubMed] [Google Scholar]
- 5.Ascherio A., Schwarzschild M.A. The epidemiology of Parkinson's disease: risk factors and prevention. Lancet Neurol. 2016;15:1257–1272. doi: 10.1016/S1474-4422(16)30230-7. [DOI] [PubMed] [Google Scholar]
- 6.Bower J.H., Maraganore D.M., McDonnell S.K., Rocca W.A. Influence of strict, intermediate, and broad diagnostic criteria on the age- and sex-specific incidence of Parkinson's disease. Mov Disord. 2000;15:819–825. doi: 10.1002/1531-8257(200009)15:5<819::aid-mds1009>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- 7.Troiano A.R., Micheli F.E., Alarcón F., Teive H.A.G. Movement disorders in Latin America. Park Relat Disord. 2006;12:125–138. doi: 10.1016/j.parkreldis.2005.08.009. [DOI] [PubMed] [Google Scholar]
- 8.Marras C., Beck J.C., Bower J.H., et al. Prevalence of Parkinson's disease across North America. npj Park Dis. 2018;4:21. doi: 10.1038/s41531-018-0058-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kasten M., Chade A., Tanner C.M. Epidemiology of Parkinson's disease. Handb Clin Neurol. 2007;83:129–151. doi: 10.1016/S0072-9752(07)83006-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Okubadejo N.U., Bower J.H., Rocca W.A., Maraganore D.M. Parkinson's disease in Africa: a systematic review of epidemiologic and genetic studies. Mov Disord. 2006;21:2150–2156. doi: 10.1002/mds.21153. [DOI] [PubMed] [Google Scholar]
- 11.Mayeux R., Marder K., Cote L.J., et al. The frequency of idiopathic Parkinson's disease by age, ethnic group, and sex in northern Manhattan, 1988-1993. Am J Epidemiol. 1995;142:820–827. doi: 10.1093/oxfordjournals.aje.a117721. [DOI] [PubMed] [Google Scholar]
- 12.Muangpaisan W., Hori H., Brayne C. Systematic review of the prevalence and incidence of Parkinson's Disease in Asia. J Epidemiol. 2009;19:281–293. doi: 10.2188/jea.JE20081034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhang Z.X., Roman G.C., Hong Z., et al. Parkinson's disease in China: prevalence in Beijing, Xian, and Shanghai. Lancet. 2005;365:595–597. doi: 10.1016/S0140-6736(05)17909-4. [DOI] [PubMed] [Google Scholar]
- 14.Barbosa M.T., Caramelli P., Maia D.P., et al. Parkinsonism and Parkinson's disease in the elderly: a community-based survey in Brazil (the Bambuí study) Mov Disord. 2006;21:800–808. doi: 10.1002/mds.20806. [DOI] [PubMed] [Google Scholar]
- 15.Nicoletti A., Sofia V., Bartoloni A., et al. Prevalence of Parkinson's disease: a door-to-door survey in rural Bolivia. Park Relat Disord. 2003;10:19–21. doi: 10.1016/s1353-8020(03)00066-x. [DOI] [PubMed] [Google Scholar]
- 16.Melcon M.O., Anderson D.W., Vergara R.H., Rocca W.A. Prevalence of Parkinson's disease in Junin, Buenos Aires Province, Argentina. Mov Disord. 1997;12:197–205. doi: 10.1002/mds.870120210. [DOI] [PubMed] [Google Scholar]
- 17.Sánchez J.L., Buriticá O., Pineda D., Uribe C.S., Palacio L.G. Prevalence of Parkinson's disease and Parkinsonism in a Colombian population using the capture-recapture method. Int J Neurosci. 2004;114:175–182. doi: 10.1080/00207450490269444. [DOI] [PubMed] [Google Scholar]
- 18.Chouza C., Ketzoian C., Caamaño J.L., et al. Prevalence of Parkinson's disease in a population of Uruguay. Adv Neurol. 1996;69:13–17. Preliminary results. [PubMed] [Google Scholar]
- 19.Giroud Benítez J.L., Collado-Mesa F., Esteban E.M. Prevalencia de la enfermedad de parkinson en un área urbana de la provincia Ciudad de La Habana, Cuba. Estudio poblacional ‘puerta a puerta. Neurologia. 2000;15:269–273. [PubMed] [Google Scholar]
- 20.de Rijk M.C., Tzourio C., Breteler M.M., et al. Prevalence of parkinsonism and Parkinson's disease in Europe: the EUROPARKINSON collaborative study. European community concerted action on the epidemiology of Parkinson's disease. J Neurol Neurosurg Psychiatry. 1997;62:10–15. doi: 10.1136/jnnp.62.1.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Prina A.M., Mayston R., Wu Y.T., Prince M. A review of the 10/66 dementia research group. Soc Psychiatry Psychiatr Epidemiol. 2018;54:1–10. doi: 10.1007/s00127-018-1626-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pasquini L., Llibre Guerra J., Prince M., Chua K.C., Prina A.M. Neurological signs as early determinants of dementia and predictors of mortality among older adults in Latin America: a 10/66 study using the NEUROEX assessment. BMC Neurol. 2018;18:163. doi: 10.1186/s12883-018-1167-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Prina A.M., Acosta D., Acostas I., et al. Cohort profile: the 10/66 study. Int J Epidemiol. 2016;46 doi: 10.1093/ije/dyw056. :406-406i. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Prince M., Ferri C.P., Acosta D., et al. The protocols for the 10/66 dementia research group population-based research programme. BMC Public Health. 2007;7:165. doi: 10.1186/1471-2458-7-165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Quinn N. Fortnightly review: parkinsonism—recognition and differential diagnosis. BMJ. 1995;310:447. doi: 10.1136/bmj.310.6977.447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hughes A.J., Daniel S.E., Kilford L., Lees A.J. Accuracy of clinical diagnosis of idiopathic Parkinson’s disease : a clinico-pathological study of 100 cases. J Neurol Neurosurg Psychiatry. 1992;55:181–184. doi: 10.1136/jnnp.55.3.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hughes A.J., Daniel S.E., Kilford L., Lees A.J. Accuracy of clinical diagnosis of idiopathic Parkinson's disease: a clinico-pathological study of 100 cases. J Neurol Neurosurg Psychiatry. 1992;55:181–184. doi: 10.1136/jnnp.55.3.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.De Rijk M.C., Rocca W.A., Anderson D.W., Melcon M.O., Breteler M.M.B., Maraganore D.M. A population perspective on diagnostic criteria for Parkinson's disease. Neurology. 1997;48:1277–1281. doi: 10.1212/wnl.48.5.1277. [DOI] [PubMed] [Google Scholar]
- 29.Dahodwala N., Siderowf A., Baumgarten M., Abrams A., Karlawish J. Screening questionnaires for parkinsonism: a systematic review. Park Relat Disord. 2012;18:216–224. doi: 10.1016/j.parkreldis.2011.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sousa R.M., Ferri C.P., Acosta D., et al. Contribution of chronic diseases to disability in elderly people in countries with low and middle incomes: a 10/66 Dementia Research Group population-based survey. Lancet. 2009;374:1821–1830. doi: 10.1016/S0140-6736(09)61829-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sousa R.M., Ferri C.P., Acosta D., et al. The contribution of chronic diseases to the prevalence of dependence among older people in Latin America, China and India: a 10/66 Dementia Research Group population-based survey. BMC Geriatr. 2010;10:53. doi: 10.1186/1471-2318-10-53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Prince M., Acosta D., Chiu H., Scazufca M., Varghese M. Dementia diagnosis in developing countries: a cross-cultural validation study. Lancet. 2003;361:909–917. doi: 10.1016/S0140-6736(03)12772-9. [DOI] [PubMed] [Google Scholar]
- 33.Ferri C.P., Schoenborn C., Kalra L., et al. Prevalence of stroke and related burden among older people living in Latin America, India and China. J Neurol Neurosurg Psychiatry. 2011;82:1074–1082. doi: 10.1136/jnnp.2010.234153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Copeland J.R., Dewey M.E., Griffiths-Jones H.M. A computerized psychiatric diagnostic system and case nomenclature for elderly subjects: GMS and AGECAT. Psychol Med. 1986;16:89–99. doi: 10.1017/s0033291700057779. [DOI] [PubMed] [Google Scholar]
- 35.Gardner M.J., Altman D.G. Confidence intervals rather than P values: estimation rather than hypothesis testing. Br Med J (Clin Res Ed) 1986;292:746–750. doi: 10.1136/bmj.292.6522.746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Higgins J.P.T., Thompson S.G. Quantifying heterogeneity in a meta-analysis. Stat Med. 2002;21:1539–1558. doi: 10.1002/sim.1186. [DOI] [PubMed] [Google Scholar]
- 37.Wright Willis A., Evanoff B.A., Lian M., Criswell S.R., Racette B.A. Geographic and ethnic variation in Parkinson disease: a population-based study of us medicare beneficiaries. Neuroepidemiology. 2010;34:143–151. doi: 10.1159/000275491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ray Dorsey E., Elbaz A., Nichols E., et al. Global, regional, and national burden of Parkinson's disease, 1990–2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet Neurol. 2018;17:939–953. doi: 10.1016/S1474-4422(18)30295-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Korczyn A.D. Vascular parkinsonism—characteristics, pathogenesis and treatment. Nat Rev Neurol. 2015;11:319–326. doi: 10.1038/nrneurol.2015.61. 2015 116. [DOI] [PubMed] [Google Scholar]
- 40.Sousa R.M., Ferri C.P., Acosta D., et al. The contribution of chronic diseases to the prevalence of dependence among older people in Latin America, China and India: a 10/66 Dementia Research Group population-based survey. BMC Geriatr. 2010;10 doi: 10.1186/1471-2318-10-53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Prince M.J., Ebrahim S., Acosta D., et al. Hypertension prevalence, awareness, treatment and control among older people in Latin America, India and China: a 10/66 cross-sectional population-based survey. J Hypertens. 2012;30:177–187. doi: 10.1097/HJH.0b013e32834d9eda. [DOI] [PubMed] [Google Scholar]
- 42.Ben-Joseph A., Marshall C.R., Lees A.J., Noyce A.J. Ethnic variation in the manifestation of Parkinson's disease: a narrative review. J Park Dis. 2020;10:31. doi: 10.3233/JPD-191763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Van Den Eeden S.K., Tanner C.M., Bernstein A.L., Fross R.D., Bloch D.A., Nelson L.M. Incidence of Parkinson's disease: variation by age, gender, and race/ethnicity. Am J Epidemiol. 2003;157:1015–1022. doi: 10.1093/aje/kwg068. [DOI] [PubMed] [Google Scholar]
- 44.Billingsley K.J., Bandres-Ciga S., Saez-Atienzar S., Singleton A.B. Genetic risk factors in Parkinson's disease. Cell Tissue Res. 2018;373:9–20. doi: 10.1007/s00441-018-2817-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kia D.A., Zhang D., Guelfi S., et al. Identification of candidate Parkinson disease genes by integrating genome-wide association study, expression, and epigenetic data sets. JAMA Neurol. 2021;78:464–472. doi: 10.1001/jamaneurol.2020.5257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Báscolo E., Houghton N., Del Riego A. Types of health systems reforms in Latin America and results in health access and coverage*. Pan Am J Public Health. 2018 doi: 10.26633/RPSP.2018.126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Pringsheim T., Jette N., Frolkis A., Steeves T.D.L. The prevalence of Parkinson's disease: a systematic review and meta-analysis. Mov Disord. 2014;29:1583–1590. doi: 10.1002/mds.25945. [DOI] [PubMed] [Google Scholar]
- 48.Beghi E., Monticelli M.L., Sessa A., Simone P. The prevalence of parkinsonism in Italy: an epidemiological survey of the disease in general practice. Mov Disord. 1994;9:403–408. doi: 10.1002/mds.870090405. [DOI] [PubMed] [Google Scholar]
- 49.Rosati G., Granieri E., Pinna L., et al. The risk of parkinson disease in mediterranean people. Neurology. 1980;30:250–255. doi: 10.1212/wnl.30.3.250. [DOI] [PubMed] [Google Scholar]
- 50.D'Alessandro R., Gamberini G., Granieri E., Benassi G., Naccarato S., Manzaroli D. Prevalence of Parkinson's disease in the Republic of San Marino. Neurology. 1987;37:1679–1682. doi: 10.1212/wnl.37.10.1679. [DOI] [PubMed] [Google Scholar]
- 51.Okada K., Kobayashi S., Tsunemalsu T. Prevalence of parkinson's disease in Izumo City, Japan. Gerontology. 1990;36:340–344. doi: 10.1159/000213219. [DOI] [PubMed] [Google Scholar]
- 52.Hofman A., Collette H.J.A., Bartelds A.I.M. Incidence and risk factors of Parkinson's disease in The Netherlands. Neuroepidemiology. 1989;8:296–299. doi: 10.1159/000110197. [DOI] [PubMed] [Google Scholar]
- 53.Morens D.M., Davis J.W., Grandinetti A., Ross G.W., Popper J.S., White L.R. Epidemiologic observations on Parkinson's disease: incidence and mortality in a prospective study of middle-aged men. Neurology. 1996;46:1044–1050. doi: 10.1212/wnl.46.4.1044. [DOI] [PubMed] [Google Scholar]
- 54.Benito-León J., Bermejo-Pareja F., Rodríguez J., Molina J.A., Gabriel R., Morales J.M. Prevalence of PD and other types of parkinsonism in three elderly populations of central Spain. Mov Disord. 2003;18:267–274. doi: 10.1002/mds.10362. [DOI] [PubMed] [Google Scholar]
- 55.Wirdefeldt K., Gatz M., Bakaysa S.L., et al. Complete ascertainment of Parkinson disease in the Swedish Twin Registry. Neurobiol Aging. 2008;29:1765–1773. doi: 10.1016/j.neurobiolaging.2007.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Bower J.H., Maraganore D.M., McDonnell S.K., Rocca W.A. Incidence and distribution of parkinsonism in Olmsted County, Minnesota, 1976-1990. Neurology. 1999;52:1214–1220. doi: 10.1212/wnl.52.6.1214. [DOI] [PubMed] [Google Scholar]
- 57.Van Den Eeden S.K. Incidence of Parkinson's disease: variation by age, gender, and race/ethnicity. Am J Epidemiol. 2003;157:1015–1022. doi: 10.1093/aje/kwg068. [DOI] [PubMed] [Google Scholar]
- 58.Fall P.A., Axelson O., Fredriksson M., et al. Age-standardized incidence and prevalence of Parkinson's disease in a Swedish community. J Clin Epidemiol. 1996;49:637–641. doi: 10.1016/0895-4356(96)00003-0. [DOI] [PubMed] [Google Scholar]
- 59.Ho S.C., Woo J., Lee C.M. Epidemiologic study of Parkinson's disease in hong kong. Neurology. 1989;39:1314–1318. doi: 10.1212/wnl.39.10.1314. [DOI] [PubMed] [Google Scholar]
- 60.Schoenberg B.S., Osuntokun B.O., Adeuja A.O.G., et al. Comparison of the prevalence of Parkinson's disease in black populations in the rural United States and in rural Nigeria: door-to-door community studies. Neurology. 1988;38:645–646. doi: 10.1212/wnl.38.4.645. [DOI] [PubMed] [Google Scholar]
- 61.Lux W.E., Kurtzke J.F. Is Parkinson's disease acquired? Evidence from a geographic comparison with multiple sclerosis. Neurology. 1987;37:467–471. doi: 10.1212/wnl.37.3.467. [DOI] [PubMed] [Google Scholar]
- 62.Kurtzke J.F., Goldberg I.D. Parkinsonism death rates by race, sex, and geography. Neurology. 1988;38:1558–1561. doi: 10.1212/wnl.38.10.1558. [DOI] [PubMed] [Google Scholar]
- 63.Koller W., Vetere-Overfield B., Gray C., et al. Environmental risk factors in Parkinson's disease. Neurology. 1990;40:1218–1221. doi: 10.1212/wnl.40.8.1218. [DOI] [PubMed] [Google Scholar]
- 64.Juho J., Maria G., Matias R., Valtteri K. Diagnostic accuracy of parkinsonism syndromes by general neurologists. Park Relat Disord. 2014;20:840–844. doi: 10.1016/j.parkreldis.2014.04.019. [DOI] [PubMed] [Google Scholar]
- 65.Andrew J.H., Susan E.D., Yoav B.S., Andrew J.L. The accuracy of diagnosis of parkinsonian syndromes in a specialist movement disorder service. Brain. 2002;125:861–870. doi: 10.1093/brain/awf080. [DOI] [PubMed] [Google Scholar]
- 66.Rodriguez J.J.L., Ferri C.P., Acosta D., et al. Prevalence of dementia in Latin America, India, and China: a population-based cross-sectional survey. Lancet. 2008;372:464–474. doi: 10.1016/S0140-6736(08)61002-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.