As of 10 December 2021, coronavirus disease 2019 (COVID‐19) caused by SARS‐CoV‐2 accounted for 267 million people with up to 5.3 million deaths worldwide (https://covid19.who.int). Since late 2019, much progress has been made in response to the COVID‐19 pandemic, including the rapid developments of effective vaccines and the treatment guidelines consisting of antiviral drugs, immunomodulators, and critical care support (https://covid19.who.int). However, SARS‐CoV‐2 evolves over time as its genome has a high mutation rate that leads to reasonable concerns of breakthrough infection due to immune escape and resistant strain emergence under antiviral pressure (Lipsitch et al., 2021; Szemiel et al., 2021). A newly emerging Omicron (B.1.1.529) variant rings alarms around the globe that, perhaps, the COVID‐19 war has just begun. Relentless efforts should be made to advance our knowledge and treatment regimens against COVID‐19. These included studies of mesenchymal stem cell (MSC) therapy that aimed to mitigate cytokine storm and promote tissue repair in severely ill patients with COVID‐19 pneumonia and acute respiratory distress syndrome (ARDS) (Hashemian et al., 2021; Meng et al., 2020; Zhu et al., 2021). Nevertheless, as extensively discussed in a recent review by Dr. Phillip W. Askenase of Yale University School of Medicine, the immunomodulatory and regenerative effects of MSC therapy are mediated through MSC‐derived extracellular vesicles (MSC‐EVs) (Askenase, 2020), while the use of MSC‐EVs has less safety concerns of thromboembolism, arrhythmia and malignant transformation. In this direction, MSC‐EV investigations for COVID‐19 treatment would be more appealing and undeniable if MSC‐EVs also exhibit anti‐SARS‐CoV‐2 effects. A previous study revealed that MSC‐EVs pertained antiviral activity against influenza virus in a preclinical model (Khatri et al., 2018). It is known that MSCs are highly resistant to viral infections (Wu et al., 2018), including SARS‐CoV‐2 (Avanzini et al., 2021). We, therefore, hypothesized that the EVs released from MSCs could inhibit SARS‐CoV‐2 infection.
Accordingly, we applied in vitro anti‐SARS‐CoV‐2 assays, which were previously developed by our group (Kanjanasirirat et al., 2020; Kongsomros et al., 2021; Sa‐Ngiamsuntorn et al., 2021), to determine the dose dependent anti‐SARS‐CoV‐2 effect of MSC‐EVs. In this study, MSC‐EV was referred to as the exosome (or small EV) subpopulation of extracellular vesicles. Full details of the methods used in this study were provided in Supplementary material.
MSC‐EVs were isolated from 100 ml culture media (supplemented with exosome‐depleted foetal bovine serum) of human umbilical cord‐derived MSCs (ATCC®PCS‐500‐010) by the combination of step‐wise centrifugation and 0.2 μm filtration (to remove microvesicles and apoptotic bodies), the 100‐kDa cutoff ultrafiltration (to concentrate the culture medium), and the Izon qEV size exclusion chromatography (to separate extracellular vesicles from soluble proteins) (Figure 1a). The final volume of the MSC‐EV isolate was 500 μl. Ten aliquots (50 μl each) were made before −80°C storage, and one freeze‐thaw cycle was allowable for each aliquot. The particle and protein evidence were validated to confirm MSC‐EV presence in the isolate as per the International Society of Extracellular Vesicles (ISEV) guideline (Théry et al., 2018), with the extended validation of functional evidence. Transmission electron microscopy with negative staining showed MSC‐EVs as cup‐shaped vesicles with approximately 100 nm in diameter (Figure 1b). Nanoparticle tracking analysis demonstrated the enrichment of small extracellular vesicles with the diameter of 96.7 ± 44.4 nm in the isolate, whereas the amount of large extracellular vesicles with the diameter > 200 nm was minute (Figure 1c). Western blot analysis confirmed the presence of exosome markers (i.e., Alix, Tsg101, Hsp70, CD9) and the MSC‐specific surface marker CD105, with the absence of EV negative control markers including GM130 (a Golgi marker), calnexin (an ER marker), and cytochrome C (Cyt‐C, a mitochondrial marker), in the MSC‐EV isolate compared to its original cell lysate (Figure 1d; the full‐length blot images were provided in Supplementary Figure 1). Since the regeneration and repair capabilities of MSC‐EVs are well documented (Hade et al., 2021), we then performed the wound healing assay to confirm that MSC‐EVs in the isolate retained bioactivities. At 24‐h, MSC‐EVs promoted HEK293 fibroblast cell migration to close the scratch wound in a dose‐dependent manner (Figure 1e,f). Validations of particle, protein, and functional evidence of MSC‐EVs supported the use of this MSC‐EV isolate to evaluate their potential activity against SARS‐CoV‐2 infection.
FIGURE 1.
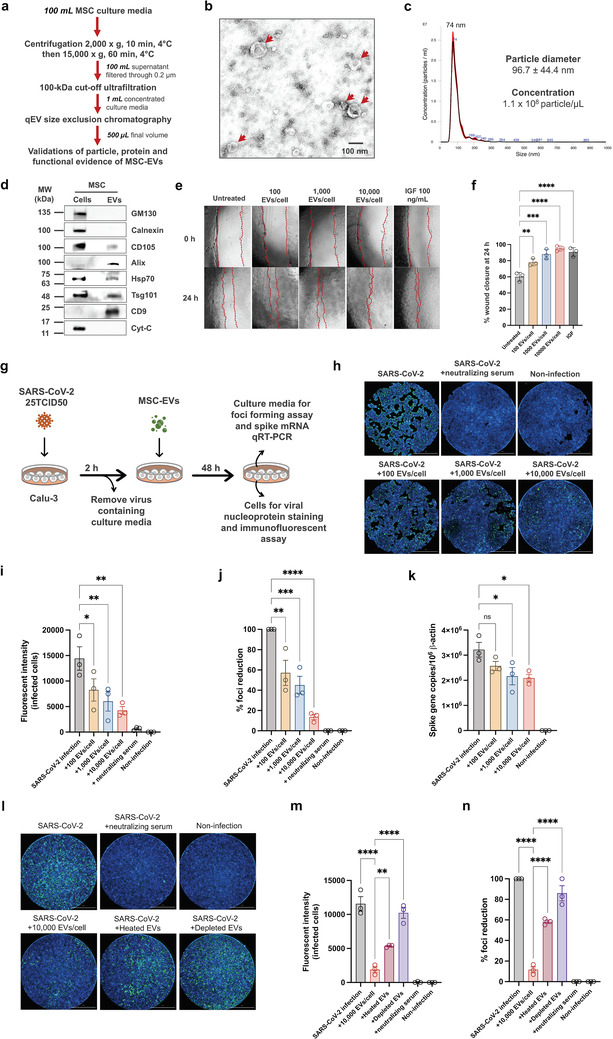
Anti‐SARS‐CoV‐2 effect of MSC‐EVs. (a) A workflow of MSC‐EV isolation and validation. (b) The single‐particle evidence of MSC‐EVs by transmission electron microscopy. Arrowheads indicated the cup‐shaped vesicles. (c) The size distribution of MSC‐EV particles by nanoparticle tracking analysis. (d) The protein evidence of MSC‐EVs by Western blot analysis (full‐length blot images were provided in Supplementary Figure 1). (e) The functional evidence of MSC‐EVs by wound healing assay (n = 3 biological replicates). (g) Study design for anti‐SARS‐CoV‐2 assays. Calu‐3 human lung epithelial cells were infected with SARS‐CoV‐2 at 25TCID50 and treated with different dosages of MSC‐EVs for 48 h. Neutralizing serum was served as the positive control. (h) The fluorescent images of SARS‐CoV‐2 infected Calu‐3 cells. Scale bar, 2 mm. (i) The fluorescent intensity of SARS‐CoV‐2 nucleoprotein‐positive cells (n = 3 biological replicates). The culture supernatant was subjected to viral output study and qRT‐PCR. (j) Viral output was determined by the percentage of foci reduction (n = 3 biological replicates). (k) The copy number of SARS‐CoV‐2 spike gene normalized to 106 copies of β‐actin was measured by qRT‐PCR (n = 3 biological replicates). (l) The fluorescent images of SARS‐CoV‐2 infected Calu‐3 cells (scale bar, 2 mm), (m) the fluorescent intensity of SARS‐CoV‐2 nucleoprotein‐positive cells (n = 3 biological replicates), and (n) the percentage of foci reduction by viral output study (n = 3 biological replicates) after treatments with heated MSC‐EVs (65°C for 2.5 h), depleted MSC‐EVs (100 kDa‐cutoff centrifugal filtration) or the untreated MSC‐EVs using the same starting dosage of 10,000 EVs per Calu‐3 cell. *, p < 0.05; **, p < 0.01; ***, p < 0.005; ****, p < 0.001; ns, not significant
Calu‐3 cells (ATCC®HTB‐55), a legitimate model of human lung epithelial cells for SARS‐CoV‐2 study (Hoffmann et al., 2020), were infected with live SARS‐CoV‐2 virus (SARS‐CoV‐2/01/human/Jan2020/Thailand; GenBank ID: QYZ85362.1) at 25TCID50 for 2 h and then treated with MSC‐EVs at different concentrations of 100, 1000 or 10,000 particles per cell for 48 h (Figure 1g; the detailed methods in Supplementary material). The inhibitory effect of MSC‐EVs against the viral replication was measured by reducing SARS‐CoV‐2 nucleoprotein expression. The Cytation 7 cell imaging system (Biotek) was applied to detect the immunofluorescent signals of SARS‐CoV‐2 nucleoprotein‐positive infected cells. Interestingly, the result showed that MSC‐EVs significantly suppressed SARS‐CoV‐2 replication in a dose‐dependent manner (Figure 1h,i). To confirm the anti‐SARS‐CoV‐2 activity of MSC‐EVs, the culture supernatant of SARS‐CoV‐2 infected cells was harvested to determine the levels of infectious virion by the foci forming assay (Figure 1j), and the copy numbers of SARS‐CoV‐2 spike gene by a quantitative RT‐PCR (Figure 1k). As expected, MSC‐EVs suppressed the release of SARS‐CoV‐2 infectious virions and their genetic materials with the dose‐response relationship. Heat treatment (65°C for 2.5 h; inactivating thermolabile molecules in the isolate) (Hettich et al., 2020) and the centrifugal filtration (100‐kDa cutoff; depleting EVs from the isolate) caused a significant reduction of anti‐SARS‐CoV‐2 effect of MSC‐EVs compared to the untreated condition (Figure 1l–n; the detailed methods in Supplementary material). This evidence suggested that anti‐SARS‐CoV‐2 activity was predominantly associated with MSC‐EVs rather than soluble mediators in the isolate.
Since human umbilical cord‐derived MSCs naturally do not express surface ACE2 protein (Avanzini et al., 2021; Hernandez et al., 2021), MSC‐EVs should not have the receptor decoy mode of action (to prevent the viral entry) or the promoting effect of viral infectivity (through transferring ACE2 receptors to the recipient cells). To address this issue, SARS‐CoV‐2 at 25TCID50 was preincubated with various dosages of MSC‐EVs for 2 h, then adsorbed by Calu‐3 cells for 2 h, washing and replacing the culture with the fresh medium, and maintaining the culture for a further 48 h (Supplementary Figure 2a). As expected, MSC‐EVs exhibited neither the viral entry prevention nor the viral infectivity enhancement as shown by nonsignificant changes in the immunofluorescent signals of SARS‐CoV‐2 nucleoprotein‐positive cells (Supplementary Figure 2b,c) and the levels of infectious virions in the supernatant by the foci forming assay (Supplementary Figure 2d).
Taken together, we communicated that the anti‐SARS‐CoV‐2 effect of MSC‐EVs was mediated through EV‐cell interaction. MSC‐EVs induced SARS‐CoV‐2 infected lung epithelial cells to suppress viral replication and mitigate the production/release of infectious virions without the ability to modulate the viral entry process. For a mechanistic insight, we foresee that MSC‐EVs released the functional cargoes to induce the antiviral defence state of SARS‐CoV‐2 infected lung epithelial cells, at least in part, through the interferon‐stimulated genes (ISGs)‐related innate immune signalling pathways (Wu et al., 2018). From a clinical perspective, MSC‐EVs inhalation therapy would deliver the anti‐SARS‐CoV‐2 effect of MSC‐EVs directly to the infected respiratory epithelial cells, together with anti‐inflammatory and regenerative effects to ameliorate the outcomes in patients with COVID‐19 pneumonia and ARDS. It should be acknowledged that this study focuses on one source of EVs. Future research should be conducted to systematically examine the antiviral effects of EVs from other sources, e.g., human plasma, breastmilk, or ACE2‐expressed cells (Civra et al., 2021; Wang et al., 2021; Yao et al., 2018; Zhang et al., 2021). Molecular mechanisms behind the anti‐SARS‐CoV‐2 effects of EVs should also be determined. Preclinical and clinical studies, especially the ongoing trials of MSC‐EV treatment in COVID‐19 pneumonia and ARDS (ClinicalTrials.gov identifiers NCT04798716, NCT04602442), should include SARS‐CoV‐2 viral titters in addition to anti‐inflammation and tissue regeneration endpoints. This study provided evidence to support MSC‐EV investigations in the context of stem cell‐free therapy for COVID‐19.
CONFLICT OF INTEREST
The authors report no conflict of interest.
AUTHOR CONTRIBUTIONS
Conceptualization, S.C.; methodology, S.C., S.K., N.P., L.K., A.T.; validation, S.C., K.P., S.H., A.T.; formal analysis, S.C., S.K., N.P., J.P., L.K., A.T.; investigation, S.K., N.P., J.P., L.K.; resources, S.C., K.P., S.H., A.T.; writing—original draft preparation, S.C.; writing—review and editing, S.K., N.P., J.P., L.K., K.P., S.H., A.T.; visualization, S.C., S.K., N.P., J.P.; supervision, S.C., K.P., S.H., A.T.; funding acquisition, S.C. and A.T. All authors have read and agreed to the published version of the manuscript.
Supporting information
Supplementary information
ACKNOWLEDGEMENTS
This work was funded by the Office of National Higher Education Science Research and Innovation Policy Council (PMU‐B), Thailand, grant numbers B17F640004 (S.C.), B05F630082 (S.C.), and B17F640005 (A.T.).
Contributor Information
Somchai Chutipongtanate, Email: schuti.rama@gmail.com, Email: chutipsi@ucmail.uc.edu.
Suradej Hongeng, Email: suradej.hon@mahidol.ac.th.
Arunee Thitithanyanont, Email: arunee.thi@mahidol.edu.
REFERENCES
- Askenase, P. W. (2020). COVID‐19 therapy with mesenchymal stromal cells (MSC) and convalescent plasma must consider exosome involvement: Do the exosomes in convalescent plasma antagonize the weak immune antibodies? Journal of Extracellular Vesicles, 10, e12004, 10.1002/jev2.12004[CrossRef] PMID: 33304473 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avanzini, M. A. , Mura, M. , Percivalle, E. , Bastaroli, F. , Croce, S. , Valsecchi, C. , Lenta, E. , Nykjaer, G. , Cassaniti, I. , Bagnarino, J. , Baldanti, F. , Zecca, M. , Comoli, P. , & Gnecchi, M. (2021). Human mesenchymal stromal cells do not express ACE2 and TMPRSS2 and are not permissive to SARS‐CoV‐2 infection. Stem Cells Translational Medicine, 10, 636–642, 10.1002/sctm.20-0385[CrossRef] PMID: 33188579 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Civra, A. , Francese, R. , Donalisio, M. , Tonetto, P. , Coscia, A. , Sottemano, S. , Balestrini, R. , Faccio, A. , Cavallarin, L. , Moro, G. E. , Bertino, E. , & Lembo, D. (2021). Human colostrum and derived extracellular vesicles prevent infection by human rotavirus and respiratory syncytial virus in vitro. Journal of Human Lactation, 37, 122–134, 10.1177/0890334420988239[CrossRef] PMID: 33534629 [DOI] [PubMed] [Google Scholar]
- Hade, M. D. , Suire, C. N. , & Suo, Z. (2021). Mesenchymal stem cell‐derived exosomes: Applications in regenerative medicine. Cells, 10, 1959. 10.3390/cells10081959[CrossRef] PMID: 34440728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashemian, S.‐.M. R. , Aliannejad, R. , Zarrabi, M. , Soleimani, M. , Vosough, M. , Hosseini, S.‐E. , Hossieni, H. , Keshel, S. H. , Naderpour, Z. , Hajizadeh‐Saffar, E. , Shajareh, E. , Jamaati, H. , Soufi‐Zomorrod, M. , Khavandgar, N. , Alemi, H. , Karimi, A. , Pak, N. , Rouzbahani, N. H. , Nouri, M. , … Baharvand, H. (2021). Mesenchymal stem cells derived from perinatal tissues for treatment of critically ill COVID‐19‐induced ARDS patients: A case series. Stem Cell Research & Therapy 12, 91, 10.1186/s13287-021-02165-4[CrossRef] PMID: 33514427 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernandez, J. J. , Beaty, D. E. , Fruhwirth, L. L. , Lopes Chaves, A. P. , & Riordan, N. H. (2021). Dodging COVID‐19 infection: Low expression and localization of ACE2 and TMPRSS2 in multiple donor‐derived lines of human umbilical cord‐derived mesenchymal stem cells. Journal of Translational Medicine, 19, 149, 10.1186/s12967-021-02813-6[CrossRef] PMID: 33853637 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hettich, B. F. , Ben‐Yehuda Greenwald, M. , Werner, S. , & Leroux, J. ‐. C. (2020). Exosomes for wound healing: Purification optimization and identification of bioactive components. Advanced Science, 7, 2002596, 10.1002/advs.202002596[CrossRef] PMID: 33304765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffmann, M. , Mösbauer, K. , Hofmann‐Winkler, H. , Kaul, A. , Kleine‐Weber, H. , Krüger, N. , Gassen, N. C. , Müller, M. A. , Drosten, C. , & Pöhlmann, S. (2020). Chloroquine does not inhibit infection of human lung cells with SARS‐CoV‐2. Nature, 585, 588–590, 10.1038/s41586-020-2575-3[CrossRef] PMID: 32698190 [DOI] [PubMed] [Google Scholar]
- Kanjanasirirat, P. , Suksatu, A. , Manopwisedjaroen, S. , Munyoo, B. , Tuchinda, P. , Jearawuttanakul, K. , Seemakhan, S. , Charoensutthivarakul, S. , Wongtrakoongate, P. , Rangkasenee, N. , Pitiporn, S. , Waranuch, N. , Chabang, N. , Khemawoot, P. , Sa‐Ngiamsuntorn, K. , Pewkliang, Y. , Thongsri, P. , Chutipongtanate, S. , Hongeng, S. , … Thitithanyanont, A. (2020). High‐content screening of Thai medicinal plants reveals Boesenbergia rotunda extract and its component Panduratin A as anti‐SARS‐CoV‐2 agents. Science Reports, 10, 19963, 10.1038/s41598-020-77003-3[CrossRef] PMID: 33203926 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khatri, M. , Richardson, L. A. , & Meulia, T. (2018). Mesenchymal stem cell‐derived extracellular vesicles attenuate influenza virus‐induced acute lung injury in a pig model. Stem Cell Research & Therapy, 9, 17, 10.1186/s13287-018-0774-8[CrossRef] PMID: 29378639 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kongsomros, S. , Suksatu, A. , Kanjanasirirat, P. , Manopwisedjaroen, S. , Prasongtanakij, S. , Jearawuttanakul, K. , Borwornpinyo, S. , Hongeng, S. , Thitithanyanont, A. , & Chutipongtanate, S. (2021). Anti‐SARS‐CoV‐2 activity of extracellular vesicle inhibitors: Screening, validation, and combination with remdesivir. Biomedicines, 9, 1230. 10.3390/biomedicines9091230[CrossRef] PMID: 34572416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipsitch M., Krammer F., Regev‐Yochay G., Lustig Y., Balicer R. D. (2022). SARS‐CoV‐2 breakthrough infections in vaccinated individuals: measurement, causes and impact. Nature Reviews Immunology, 22(1), 57–65. 10.1038/s41577-021-00662-4 PMID: 34876702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meng, F. , Xu, R. , Wang, S. , Xu, Z. , Zhang, C. , Li, Y. , Yang, T. , Shi, L. , Fu, J. , Jiang, T. , Huang, L. , Zhao, P. , Yuan, X. , Fan, X. , Zhang, Ji‐Y. , Song, J. , Zhang, D. , Jiao, Y. , Liu, L. , … Wang, Fu‐S. (2020). Human umbilical cord‐derived mesenchymal stem cell therapy in patients with COVID‐19: A phase 1 clinical trial. Signal Transduction and Targeted Therapy, 5, 172, 10.1038/s41392-020-00286-5[CrossRef] PMID: 32855385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sa‐Ngiamsuntorn, K. , Suksatu, A. , Pewkliang, Y. , Thongsri, P. , Kanjanasirirat, P. , Manopwisedjaroen, S. , Charoensutthivarakul, S. , Wongtrakoongate, P. , Pitiporn, S. , Chaopreecha, J. , Kongsomros, S. , Jearawuttanakul, K. , Wannalo, W. , Khemawoot, P. , Chutipongtanate, S. , Borwornpinyo, S. , Thitithanyanont, A. , & Hongeng, S. (2021). Anti‐SARS‐CoV‐2 activity of andrographis paniculata extract and its major component andrographolide in human lung epithelial cells and cytotoxicity evaluation in major organ cell representatives. Journal of Natural Products, 84, 1261–1270, 10.1021/acs.jnatprod.0c01324[CrossRef] PMID: 33844528 [DOI] [PubMed] [Google Scholar]
- Szemiel, A. M. , Merits, A. , Orton, R. J. , Maclean, O. A. , Pinto, R. M. , Wickenhagen, A. , Lieber, G. , Turnbull, M. L. , Wang, S. , Furnon, W. , Suarez, N. M. , Mair, D. , Da Silva Filipe, A. , Willett, B. J. , Wilson, S. J. , Patel, A. H. , Thomson, E. C. , Palmarini, M. , Kohl, A. , & Stewart, M. E. (2021). In vitro selection of Remdesivir resistance suggests evolutionary predictability of SARS‐CoV‐2. Plos Pathogens, 17, e1009929, 10.1371/journal.ppat.1009929[CrossRef] PMID: 34534263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Théry, C. , Witwer, K. W. , Aikawa, E. , Alcaraz, M. J. , Anderson, J. D. , Andriantsitohaina, R. , Antoniou, A. , Arab, T. , Archer, F. , Atkin‐Smith, G. K. , Ayre, D. C. , Bach, J.‐M. , Bachurski, D. , Baharvand, H. , Balaj, L. , Baldacchino, S. , Bauer, N. N. , Baxter, A. A. , Bebawy, M. , … Zuba‐Surma, E. K. (2018). Minimal information for studies of extracellular vesicles 2018 (MISEV2018): A position statement of the International Society for Extracellular Vesicles and update of the MISEV2014 guidelines. Journal of Extracellular Vesicles, 7, 1535750, 10.1080/20013078.2018.1535750[CrossRef] PMID: 30637094 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, Y. , Zhu, X. , Jiang, X.‐M. , Guo, J. , Fu, Z. , Zhou, Z. , Yang, P. , Guo, H. , Guo, Xu , Liang, G. , Zeng, P. , Xiao, G. , Ma, J. , Yin, X. , Zhang, L‐Ke , Yan, C. , & Zhang, C‐Y. (2021). Decreased inhibition of exosomal miRNAs on SARS‐CoV‐2 replication underlies poor outcomes in elderly people and diabetic patients. Signal Transduction and Targeted Therapy, 6, 300, 10.1038/s41392-021-00716-y[CrossRef] PMID: 34381015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu, X. , Dao Thi, V. L. , Huang, Y. , Billerbeck, E. , Saha, D. , Hoffmann, H.‐H. , Wang, Y. , Silva, L. A. V. , Sarbanes, S. , Sun, T. , Andrus, L. , Yu, Y. , Quirk, C. , Li, M. , Macdonald, M. R. , Schneider, W. M. , An, X. , Rosenberg, B. R. , & Rice, C. M. (2018). Intrinsic immunity shapes viral resistance of stem cells. Cell, 172, 423–438.e25, 10.1016/j.cell.2017.11.018[CrossRef] PMID: 29249360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao, Z. , Qiao, Y. , Li, X. , Chen, J. , Ding, J. , Bai, Lu , Shen, F. , Shi, B. , Liu, J. , Peng, Lu , Li, J. , & Yuan, Z. (2018). Exosomes exploit the virus entry machinery and pathway to transmit alpha interferon‐induced antiviral activity. Journal of Virology, 92, e01578–18. 10.1128/JVI.01578-18[CrossRef] PMID: 30282711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, J. , Huang, F. , Xia, B. , Yuan, Y. , Yu, F. , Wang, G. , Chen, Q. , Wang, Q. , Li, Y. , Li, R. , Song, Z. , Pan, T. , Chen, J. , Lu, G. , & Zhang, H. (2021). The interferon‐stimulated exosomal hACE2 potently inhibits SARS‐CoV‐2 replication through competitively blocking the virus entry. Signal Transduction and Targeted Therapy, 6, 189, 10.1038/s41392-021-00604-5[CrossRef] PMID: 33980808 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu, R. , Yan, T. , Feng, Y. , Liu, Y. , Cao, H. , Peng, G. , Yang, Y. , Xu, Z. , Liu, J. , Hou, W. , Wang, X. , Li, Z. , Deng, L. , Wang, S. , Li, J. , Han, Q. , Li, H. , Shan, G. , Cao, Y. , … Zhao, R. C. (2021). Mesenchymal stem cell treatment improves outcome of COVID‐19 patients via multiple immunomodulatory mechanisms. Cell Research, 31, 1244–1262, 10.1038/s41422-021-00573-y[CrossRef] PMID: 34702946 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary information


