Abstract
Simian immunodeficiency virus (SIV) infection of newborn rhesus macaques is a useful animal model of human immunodeficiency virus infection for the study of the emergence and clinical implications of drug-resistant viral mutants. We previously demonstrated that SIV-infected infant macaques receiving prolonged treatment with 9-[2-(phosphonomethoxy)propyl]adenine (PMPA) developed viral mutants with fivefold reduced susceptibility to PMPA in vitro and that the development of these mutants was associated with the development of a K65R mutation and additional compensatory mutations in reverse transcriptase (RT). To study directly the virulence and clinical implications of these SIV mutants, two uncloned SIVmac isolates with similar fivefold reduced in vitro susceptibilities to PMPA but distinct RT genotypes, SIVmac055 (K65R, N69T, R82K A158S, S211N) and SIVmac385 (K65R, N69S, I118V), were each inoculated intravenously into six newborn rhesus macaques; 3 weeks later, three animals of each group were started on PMPA treatment. All six untreated animals developed persistently high levels of viremia and fatal immunodeficiency within 4 months. In contrast, the six PMPA-treated animals, despite having persistently high virus levels, survived significantly longer: 5 to 9 months for the three SIVmac055-infected infants and ≥21 months for the three SIVmac385-infected infants. Virus from only one untreated animal demonstrated reversion to wild-type susceptibility and loss of the K65R mutation. In several other animals, additional RT mutations, including K64R and Y115F, were detected, but the biological role of these mutations is unclear since they did not affect the in vitro susceptibility of the virus to PMPA. In conclusion, this study demonstrates that although SIVmac mutants with the PMPA-selected K65R mutation in RT were highly virulent, PMPA treatment still offered strong therapeutic benefits. These results suggest that the potential emergence of HIV mutants with reduced susceptibility to PMPA in patients during prolonged PMPA therapy may not eliminate its therapeutic benefits.
During recent years, the discovery of novel potent antiretroviral drugs has led to significant advances in the clinical management of human immunodeficiency virus (HIV)-infected patients (4). In many patients, however, there is not the desired strong and persistent suppression of viral replication. Although many factors may contribute to reduced efficacy of antiviral drug therapy, a major limiting factor in sustaining durable benefits of antiviral drug therapy is the emergence of viral mutants with reduced in vitro susceptibility to antiviral drugs (6).
Although the emergence of drug-resistant viral mutants is a sign of incomplete virus suppression, the exact clinical implications on disease progression are often unclear. Therefore, many fundamental questions exist. Are drug-resistant mutants fully virulent and does their emergence contribute to disease progression? To what extent does the presence of drug-resistant mutants result in reduced drug efficacy and therefore require alteration of the drug treatment regimen? Unfortunately, attempts to demonstrate the virulence and long-term clinical implications of drug-resistant HIV mutants by conducting human clinical trials are extremely difficult and time-consuming (for a review, see reference 22).
Simian immunodeficiency virus (SIV) infection of newborn macaques is a practical animal model of HIV infection that allows researchers to gain insights into the emergence, virulence, and clinical implications of drug-resistant viral mutants. This animal model may allow the rapid identification of those potent antiviral drugs to which viral resistance emerges slowly and, if drug-resistant viral mutants do eventually emerge, whose therapeutic benefits would not be lost. We have previously validated this animal model by demonstrating that the benefits of prolonged zidovudine (AZT) therapy for SIV-infected macaques was significantly limited by the emergence of AZT-resistant SIV mutants, which proved to be fully virulent following subsequent inoculation into newborn macaques (30, 33).
A promising novel compound which inhibits viral reverse transcription is the acyclic nucleoside phosphonate 9-[2-(phosphonomethoxy)propyl]adenine (PMPA) (9). We previously demonstrated that PMPA treatment dramatically reduced virus levels and enhanced antiviral immune responses in infant macaques with established SIVmac251 infection (28). Although virus with an approximately fivefold reduced in vitro susceptibility to PMPA emerged within a few months of PMPA treatment, virus levels stayed low in most animals. This reduced susceptibility to PMPA was associated with the development of an arginine-to-lysine substitution at amino acid 65 (K65R) and additional mutations in reverse transcriptase (RT) (28). In the current study, we further investigated the virulence and clinical implications of these K65R SIV mutants by inoculating them into newborn macaques. We demonstrate that although these SIV mutants with reduced in vitro susceptibility to PMPA are highly virulent in the absence of PMPA therapy, PMPA administration significantly prolonged survival for infant macaques infected with these SIV isolates.
MATERIALS AND METHODS
Preparation of PMPA-resistant virus stocks SIVmac055 and SIVmac385.
As described previously, SIVmac with reduced in vitro susceptibility to PMPA was isolated from peripheral blood mononuclear cells (PBMCs) of SIVmac251-infected infant rhesus macaques (Macaca mulatta) receiving prolonged PMPA treatment; PBMCs were cocultivated with CEM×174 cells, and virus replication was monitored by p27 core antigen measurement (28). Virus-positive tissue culture supernatants were frozen at −70°C. Virus isolates from these animals (age, between 7 and 9 months) were thawed to prepare the virus stocks for the present study: on the basis of the RT genotypes (28), virus isolates from animals 29003, 29008, and 29045 were pooled and designated SIVmac385 (K65R, N69S, and I118V); virus isolates from animal 29055 were pooled into SIVmac055 (K65R, N69T, R82K, A158S, and S211N). Both virus stocks were divided into aliquots, frozen, and stored at −70°C; an aliquot was subsequently used to determine the 50% tissue culture infectious doses (TCID50s) by limiting dilution culture assay with CEM×174 cells in 24-well plates and subsequent p27 core antigen measurement during a 4-week period by previously described methods (32).
In vitro replication of SIVmac isolates in rhesus macaque PBMCs.
Rhesus macaque PBMCs derived from normal adult macaques were stimulated with Staphylococcus enterotoxin A (Toxin Technology, Sarasota, Fla.) and were propagated in RPMI 1640 medium supplemented with 10% heat-inactivated fetal calf serum (Gemini Bio-Products, Calabasas, Calif.), antibiotics, l-glutamine, and recombinant human interleukin-2 (Cetus Corporation, Emeryville, Calif.) by previously used methods (1). At day 3, the PBMC cultures were infected with the different SIVmac isolates at an approximate multiplicity of infection of 0.001 TCID50 per cell. Culture supernatants were monitored for the presence of p27 core antigen at 3- to 4-day intervals. To assess individual variability in infectivity of PBMCs, separate cultures were set up with PBMCs from four different donor animals.
Animals, virus inoculation, and PMPA administration.
Newborn rhesus macaques were from the type D retrovirus- and SIV-free colony at the California Regional Primate Research Center and were hand-reared in a primate nursery in accordance with American Association for Accreditation of Laboratory Animal Care Standards. We strictly adhered to the Guide for the Care and Use of Laboratory Animals, prepared by the Committee on the Care and Use of Laboratory Animals of the Institute of Laboratory Animal Resources, National Research Council (19a). When necessary, the animals were immobilized with ketamine HCl (Parke-Davis, Morris Plains, N.J.) at 10 mg/kg of body weight injected intramuscularly.
Between 1 and 3 days after birth, newborn macaques were inoculated intravenously with 0.5 ml of a 1:10 dilution in phosphate-buffered saline of SIVmac385 or SIVmac055, equivalent to approximately 1,000 TCID50s (the range, based on three or more independent determinations, was 500 to 2,000 TCID50s). To monitor the immune response to nonviral, nonreplicating antigens, all newborn rhesus macaques were immunized with 0.1 mg of the cholera toxin B subunit (List Biological Laboratories, Campbell, Calif.) subcutaneously, just before the virus inoculation. A booster immunization was given at 8 weeks of age. The cholera toxin-specific immunoglobulin G (IgG) enzyme-linked immunosorbent assay (ELISA) has been described previously (34).
Blood samples were collected regularly for the monitoring of viral and immunologic parameters as described previously (32, 33). Complete blood counts were performed with EDTA-anticoagulated blood samples from all animals. Samples were analyzed by using an automated electronic cell counter (Baker 9000; Serono Baker Diagnostics); differential cell counts were determined manually.
Three weeks after virus inoculation, half of the animals were started on prolonged PMPA treatment. PMPA (Gilead Sciences, Foster City, Calif.) was suspended in distilled water and was dissolved by the addition of NaOH to a final pH of 7.0 at a final concentration of 60 mg/ml and was filter sterilized (pore size, 0.2 μm; Nalgene, Rochester, N.Y.). PMPA was administered subcutaneously into the back of the animal at a dosage of 30 mg/kg of body weight once daily (25, 28). PMPA dosages were adjusted weekly according to body weight. The untreated control animals did not receive daily sham inoculations. Based on pharmacokinetic studies indicating reduced drug clearance in macaques following prolonged high-dose PMPA administration (29), the three longest-surviving animals (animals 29276, 29278, and 29279) were given a threefold PMPA dosage reduction starting at 14 to 17 months of age.
Quantitative virus isolation (cell-associated and cell-free virus).
Cell-associated and cell-free virus levels in peripheral blood were determined regularly by limiting dilution culture assays (four replicates per dilution) of PBMCs and plasma, respectively, with CEM×174 cells in 24-well plates and subsequent p27 core antigen measurement by previously described methods (32, 33, 34). In addition, for animals with low or undetectable virus levels, 1 × 106 to 20 × 106 PBMCs were cocultivated for 8 weeks with CEM×174 cells in tissue culture flasks as described previously (32). Virus levels in fresh spleen, thymus, and lymph node were determined by a limiting dilution culture assay of single-cell suspensions by a method similar to that used for PBMCs.
Plasma viral RNA levels.
Quantitative assays for the measurement of SIV RNA were performed by a branched DNA signal amplification assay specific for SIV (7). This assay is similar to the Quantiplex HIV RNA assay (21) except that target probes were designed to hybridize with the pol region of the SIVmac group of strains including SIVmac251. SIV pol RNA in plasma samples was quantified by comparison with a standard curve produced with serial dilutions of cell-free SIV-infected tissue culture supernatant. The quantification of this standard curve was determined by comparison with purified, quantified, in vitro-transcribed SIVmac239 pol RNA. The lower quantification limit of this assay was 10,000 copies of SIV RNA per specimen. Due to the limited blood volume that can be collected from newborn macaques, plasma volumes of ≤50 μl were available during the early time points, which limited the sensitivity of this assay to ≥200,000 copies of SIV RNA per ml of plasma. Samples from several later time points were tested by an enhanced branched DNA assay with a quantification limit of 1,500 RNA copies per sample (30,000 SIV RNA copies/ml).
Anti-SIV class-specific antibody determination.
The anti-SIV IgG and IgM-specific antibody ELISAs have been described previously (20, 31). Immunoblotting was performed by the Chiron RIBA HIV-1/HIV-2 Strip Immunoblot Assay (Chiron Corporation, Emeryville, Calif.) according to the manufacturer’s instructions.
T-lymphocyte phenotyping.
T-lymphocyte antigens were detected by direct labeling of whole blood with fluorescein-conjugated anti-human CD8 (Leu-2a; Becton Dickinson Immunocytometry Inc., San Jose, Calif.) and phycoerythrin-conjugated anti-human CD4 (OKT4; Ortho Diagnostic Systems Inc., Raritan, N.J.). Erythrocytes were lysed, and the samples were fixed in paraformaldehyde with the Coulter Q-prep system (Coulter Corporation, Hialeah, Fla.). Lymphocytes were gated by forward and side light scatter and were then analyzed with a FACSCAN flow cytometer (Becton Dickinson).
Drug susceptibility assay.
The phenotypic drug susceptibilities of the SIVmac isolates were characterized by an assay which was previously used to detect SIV mutants with decreased susceptibilities to PMPA and other antiretroviral drugs (28, 33).
Sequencing of viral RT region.
CEM×174 cells infected with virus isolated from the SIV-infected animals were harvested as soon as culture supernatants were positive by antigen-capture ELISA (14). The genomic DNA preparation, PCR, and sequencing of the RT region were done by previously described methods (28). In brief, PCR was performed with each DNA sample in at least two separate reactions, and the PCR products were subjected directly to dideoxy-DNA sequencing reactions, followed by automated analysis with the ALFexpress DNA Analysis System (Pharmacia, Piscataway, N.J.). Sequence data were aligned by using the DNA STAR software program. The RT amino acid sequence was compared with that of uncloned SIVmac251; the RT sequence of uncloned SIVmac251 was identical to that reported for the molecular clone SIVmac251 (19), with the exception that position 11 contained an alanine instead of a threonine. This method can detect the presence of a 20% subpopulation in the PCR mixture. Mixtures of wild-type and mutant amino acids are indicated by a slash.
Necropsy collection and preparation of tissue samples.
The animals were euthanized when it became apparent that their condition was terminal (34). A complete necropsy examination was performed on all animals that died during the course of the study. Tissues collected at necropsy were fixed in 10% buffered formalin, embedded in paraffin, sectioned at 6 μm, stained with hematoxylin and eosin, and examined by light microscopy.
Statistical analysis.
Statistical analysis was used to compare PMPA-treated versus untreated SIV-infected animals with regard to survival. Survival was compared by the generalized Wilcoxon test (8). We have previously shown that this analysis can distinguish biologically relevant differences in survival (16, 33).
RESULTS
Emergence of SIVmac mutants with reduced susceptibility to PMPA in SIV-infected infant macaques during prolonged PMPA treatment: long-term clinical course.
We previously demonstrated that long-term PMPA treatment of four infant rhesus macaques which were inoculated at birth with drug-sensitive, virulent uncloned SIVmac251 resulted in the emergence of viral mutants with fivefold reduced in vitro susceptibility to PMPA after 5 to 15 weeks of PMPA treatment (28). This low-level resistance was associated with the development of a K65R mutation in RT for all four animals. Additional mutations, which were presumably compensatory (since they did not alter PMPA susceptibility), emerged in RT, and two patterns were observed: virus from one animal (animal 29055) developed K65R, N69T, R82K, A158S, and S211N mutations; virus isolates from each of the other three animals (animals 29003, 29008, and 29045) developed the same three mutations in RT, namely, K65R, N69S, and I118V, within 7 to 9 months of PMPA therapy (28). For animal 29055, the emergence of these K65R SIV mutants was associated with an increase in the level of viremia, while for the other three animals (animals 29003, 29008, and 29045), virus levels remained low. An update on the clinical course in these animals is summarized in Fig. 1. Animal 29055 continued to receive daily PMPA treatment, and despite having persistently high virus levels (viral RNA levels, >107 copies/ml of plasma), the animal survived until 20 months of age before developing CD4+ T-cell depletion (<200/μl) and succumbing to immunodeficiency. Virus isolated from animal 29055 at the time of death still had the same fivefold reduced susceptibility to PMPA but had acquired many additional amino acid changes in RT (K40T, K64R, R78K/R/T, F116W, I118V, W153G, A158A/S, H166R, and Q182L) (Table 1). The other three animals are alive and healthy at more than 3 years of age (Fig. 1). Animal 29003 had very low virus levels (<1 infected cell per 10 × 106 PBMCs; viral RNA level, <30,000 copies/ml of plasma) from 12 months of age onward, and the effect of withdrawal of PMPA treatment was studied at 22 months of age: virus levels increased gradually during the next month and reached a level of 1 infected PBMC per 104 to 105 PBMCs and 105 to 106 copies of viral RNA per ml of plasma; animal 29003 was healthy at 39 months of age, with normal CD4+ and CD8+ T-cell counts (>1,000/μl); virus isolated from animal 29003 only 11 days after PMPA treatment was withdrawn was PMPA sensitive, and the virus lost all RT mutations that were associated with reduced susceptibility to PMPA (Table 1). Animals 29008 and 29045 continued to receive daily PMPA treatment for more than 3 years (the daily PMPA dose was reduced from 30 to 10 mg/kg at 25 months of age). Animal 29008 maintained moderate viral levels (∼1 infected PBMC per 1 × 106 to 5 × 106 PBMCs; approximately 106 viral RNA copies/ml), has a CD4+/CD8+ T cell ratio of 0.5, has an absolute CD4+ T-cell count of approximately 400/μl, but is clinically healthy at more than 3 years of age. Virus isolated from animal 29008 at 30 months of age had acquired additional mutations in RT, including K40R, K64R, Y115F, F116W, V181I, and S211N, but this virus still had the same fivefold reduced in vitro susceptibility to PMPA (Table 1). Animal 29045 maintained very low virus levels in peripheral blood (<1 infected cell per 5 × 106 to 10 × 106 PBMCs, no detectable infectious plasma viremia, and plasma viral RNA levels of <1,500 copies/ml at 34 months of age), has normal CD4+ T-cell counts (>1,000/μl) and CD4+/CD8+ T cell ratios (>1.0), and is healthy at more than 3 years of age; virus isolated from animal 29045 when the animal was between 7 and 30 months of age showed little change in the amino acid sequence of RT (Table 1).
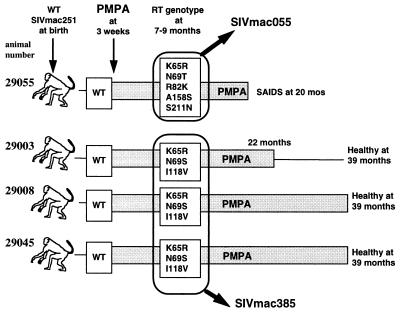
FIG. 1.
Summary of emergence of SIVmac mutants with reduced susceptibility to PMPA. As described previously (28), newborn macaques were inoculated with wild-type (WT) uncloned SIVmac251 and were started on prolonged PMPA treatment (shaded rectangle) 3 weeks later; virus with fivefold reduced susceptibility to PMPA was isolated after 5 to 15 weeks of PMPA treatment (28). PMPA treatment was withdrawn for animal 29003 at 22 months of age; the other three animals were maintained on PMPA treatment until the time of euthanasia because of simian AIDS (animal 29055) or indefinitely (>39 months; animals 29008 and 29045). Virus isolates obtained when the animals were between 7 and 9 months of age were pooled as indicated to prepare SIVmac055 and SIVmac385.
TABLE 1.
Genotypic and phenotypic evolution of SIV RT following inoculation of newborn macaques with wild-type or mutant SIV isolatesa
| Virus inoculum | PMPA treat-ment | Ani-mal no. | Time point | IC95 (μM [pheno-type]) | Genotype [mutation(s) in RT] | |||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| SIVmac251 | 30–50 (S) | |||||||||||||||||||||||||||
| + | 29055 | 7–9 mo | 220 (R) | K65R | N69T | R82K | A158S | S211N | ||||||||||||||||||||
| 20 mob | 240 (R) | K40T | K64R | K65R/K/I | N69T | R78K/R/T | R82K | F116W | I118V | W153G | A158S/A | H166R | Q182L | S211N | ||||||||||||||
| + | 29003 | 7–9 mo | 250 (R) | K65R | N69S | I118V | ||||||||||||||||||||||
| 30 moc | 40 (S) | |||||||||||||||||||||||||||
| + | 29008 | 7–9 mo | 220 (R) | K65R | N69S | I118V | ||||||||||||||||||||||
| 30 mo | 230 (R) | K40R | K64R | K65R | N69S | Y115F | F116W | I118V | V181I | S211N | ||||||||||||||||||
| + | 29045 | 7–9 mo | 200 (R) | K65R | N69S | I118V | ||||||||||||||||||||||
| 30 mo | 150 (R) | K65R | N69S | I118V | K173R | |||||||||||||||||||||||
| SIVmac055 | 200 (R) | K65R | N69T | R82K | A158S | S211N | ||||||||||||||||||||||
| − | 29668 | 15 wkb | 220 (R) | K64R | K65R | N69T | R78T/R | R82K | V148V/G | A158S | ||||||||||||||||||
| − | 29669 | 8 wkb | 200 (R) | K65R | N69T | R82K | A158S | S211N | ||||||||||||||||||||
| − | 29670 | 8 wkb | 190 (R) | K64R | K65R | N69T | R82K | A158S | S211N | |||||||||||||||||||
| + | 29283 | 39 wkb | 220 (R) | K64R | K65R | N69T | R82K | A158S | S211N | |||||||||||||||||||
| + | 29360 | 19 wkb | 220 (R) | K65R | N69T | R82K | A158S | S211N | ||||||||||||||||||||
| + | 29419 | 21 wkb | 200 (R) | K64R | K65R | N69T | R82K | A158S | S211N | |||||||||||||||||||
| SIVmac385 | 200 (R) | K65R | N69S | I118V | R172K | |||||||||||||||||||||||
| − | 29674 | 6 wk | 150 (R) | K65R | N69S | I118V | S211N | |||||||||||||||||||||
| 13 wkb | 50 (S) | N69S | V148V/G | |||||||||||||||||||||||||
| − | 29675 | 10 wkb | 250 (R) | K64R | K65R | N69S | I118V | |||||||||||||||||||||
| − | 29729 | 7 wkb | 190 (R) | K65R | N69S | I118V | ||||||||||||||||||||||
| + | 29276 | 8 wk | 200 (R) | K65R | N69S | I118V | S211N | |||||||||||||||||||||
| 16 wk | 220 (R) | K64R | K65R | N69S | I118V | S211N | ||||||||||||||||||||||
| 36 wk | 190 (R) | K64R | K65R | N69S | Y115F | I118V | F171Y | S211N | ||||||||||||||||||||
| 81 wk | 220 (R) | V17E | K43E | K64R | K65R | N69S | Y115F | I118V | F171Y | D185G | S211N | |||||||||||||||||
| + | 29278 | 8 wk | 190 (R) | K65R | N69S | I118V | S211N | |||||||||||||||||||||
| 16 wk | 230 (R) | K65R | N69S | Y115F | I118V | |||||||||||||||||||||||
| 36 wk | 200 (R) | K64R | K65R | N69S | Y115F | I118V | S211N | |||||||||||||||||||||
| 91 wkb | 220 (R) | V17D | K40Q | K64R | K65R | N69S | Y115F | I118V | V148V/G | H166R | Q182L | L196F | S211N | |||||||||||||||
| + | 29279 | 8 wk | 220 (R) | K64R | K65R | N69S | I118V | S211N | ||||||||||||||||||||
| 16 wk | 200 (R) | K65R | N69S | Y115F | I118V | S211N | ||||||||||||||||||||||
| 36 wk | 190 (R) | V17D | K64R | K65R | N69S | R105K | Y115F | I118V | S211N | |||||||||||||||||||
| 88 wk | 220 (R) | V17D | K40R | K64R | K65R | N69S | Y115F | I118V | F171Y | S211N | ||||||||||||||||||
| Conserved with HIV-1/ HIV-2d | N | N | C | C | C | N | C | N | N | C | C | N | C | C | N | N | C | N | N | N | C | C | N | N | ||||
Four newborn macaques were inoculated with wild-type SIVmac251 and were started on PMPA treatment at 3 weeks of age (28); virus isolates were obtained from these animals when the animals were 7 to 9 months of age and were pooled to prepare SIVmac055 and SIVmac385 (see Fig. 1). SIVmac055 and SIVmac385 were each inoculated into six newborn macaques, of which three animals were started on PMPA treatment 3 weeks later. Virus isolated from PBMCs at the indicated time points was used for phenotypic and genotypic analyses. Phenotypic in vitro susceptibility to PMPA is indicated by the 95% inhibitory concentration (IC95); 95% inhibitory concentrations of 30 to 50 and 150 to 250 μM indicate wild-type susceptibility (S) and fivefold reduced susceptibility (R), respectively. Virus isolated from the plasma of these animals at the time of euthanasia had a phenotype identical to that of virus isolated from PBMCs (data not shown). For the genotypic analysis, the RT amino acid sequence was compared with that of the uncloned SIVmac251 used as the inoculum. Amino acid substitutions in RT are given by their respective single-letter designations; mixtures are indicated by a slash between the amino acids. Boldface indicates the amino acid substitutions in the SIVmac055 and SIVmac385 inocula compared to the amino acids in the uncloned SIVmac251 inoculum and the presence of these mutations in virus isolates from infant macaques inoculated with SIVmac055 and SIVmac385.
Virus isolated at time of death or euthanasia with simian AIDS.
PMPA treatment for animal 29003 was withdrawn at 22 months of age.
Conservation of amino acids with HIV-1/HIV-2 is indicated by C (conserved) or N (notconserved).
Preparation and in vitro characterization of SIVmac055 and SIVmac385.
Two SIVmac stocks with reduced in vitro susceptibility to PMPA were prepared by pooling virus isolates, obtained from the animals described above when they were between 7 and 9 months of age. On the basis of the similar RT genotypes, virus isolates from animals 29003, 29008, and 29045 (with low levels of viremia) (28) were pooled and were called SIVmac385 (RT genotype, K65R, N69S, and I118V). Virus isolates obtained from animal 29055 (with a high level of viremia) were pooled into SIVmac055 (RT genotype, K65R, N69T, R82K, A158S, and S211N) (Fig. 1). Both virus stocks, SIVmac055 and SIVmac385, have similar fivefold reduced in vitro susceptibilities to PMPA (Table 1). The in vitro replication rates of SIVmac055 and SIVmac385 in rhesus macaque PBMCs were variable and depended upon the individual donor PBMCs, but SIVmac055 replicated as well as or better than the parental (wild-type) uncloned SIVmac251; SIVmac055 generally replicated better than SIVmac385 (data not shown).
Pathogenesis of SIVmac055 and SIVmac385 following inoculation of newborn macaques: virulence and response to PMPA therapy.
Within 72 h after birth, 12 newborn macaques were inoculated intravenously with SIVmac055 or SIVmac385 (six animals per virus strain; Table 2). For each group of six animals, three animals were used as untreated control animals (Table 2, groups A and B), while the other three infants were treated with PMPA (30 mg/kg subcutaneously once daily) beginning at 3 weeks of age (Table 2, groups C and D). To monitor the immune response to nonviral, nonreplicating antigens, all newborn rhesus macaques were immunized with cholera toxin B subunit subcutaneously, just before the virus inoculation, and a booster immunization was given at 8 weeks of age.
TABLE 2.
Inoculation of newborn macaques with SIVmac isolates with reduced susceptibility to PMPA: summary of experimental design and outcome
| Animal group | Animal no. | Virus inoculuma | PMPA at 3 wkb | Anti-SIV IgG response | Time of simian AIDS (euthanasia)c | Histopathology |
|---|---|---|---|---|---|---|
| A | 29668 | SIVmac055 | − | − | 15 wk | Lymphoid depletion, typhlocolitis, cystitis, nephritis, generalized cytomegalovirus infection |
| 29669 | SIVmac055 | − | − | 8 wk | Lymphoid depletion, enterocolitis, hypoproteinemia | |
| 29670 | SIVmac055 | − | − | 8 wk | Lymphoid depletion, colitis | |
| B | 29674 | SIVmac385 | − | − | 13 wk | Lymphoid depletion, cytomegalovirus infection, pancreatitis |
| 29675 | SIVmac385 | − | − | 10 wk | Lymphoid depletion, pancreatitis, hepatitis, meningitis, hypoproteinemia | |
| 29729 | SIVmac385 | − | − | 7 wk | Lymphoid depletion, pancreatitis, enteritis | |
| C | 29283 | SIVmac055 | + | − | 39 wk | Lymphoid depletion, Cryptosporidium-positive enteritis and cholangitis |
| 29360 | SIVmac055 | + | − | 19 wk | Lymphoid depletion, glomerulonephritis, necrotizing enterocolitis | |
| 29419 | SIVmac055 | + | − | 21 wk | Lymphoid depletion, necrotizing enterocolitis | |
| D | 29276 | SIVmac385 | + | + | Alive at 29 mo | NAd |
| 29278 | SIVmac385 | + | + | 21 mo | Granulomatous lymphadenitis, endocarditis, Cryptosporidium infection | |
| 29279 | SIVmac385 | + | + | 22 mo | Rhodococcus equi-positive granulomatous pneumonia, lymphadenitis, lymphoid depletion, cryptosporidium-positive enteritis |
Within 3 days of age, all animals were inoculated intravenously with 500 to 2,000 TCID50s of either SIVmac055 or SIVmac385.
Groups C and D were started on chronic PMPA treatment (30 mg/kg once daily) 3 weeks after virus inoculation.
Statistical analysis of survival showed significant benefit of PMPA treatment on clinical outcome (comparison groups C and D versus A and B, P = 0.002; group C versus group A, P = 0.025; group D versus group B, P = 0.025).
NA, not applicable.
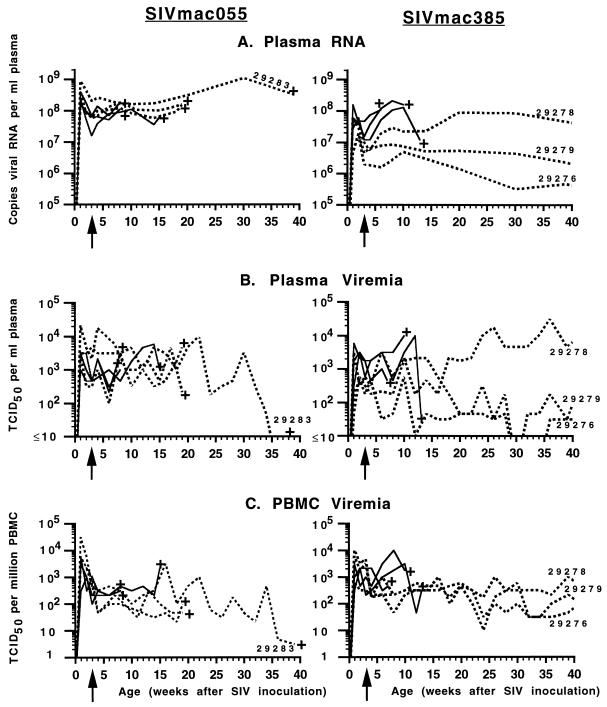
During the first 3 weeks of infection, the plasma of all 12 animals had high levels of viral RNA, and all animals had high levels of infectious PBMC-associated and plasma viremia (Fig. 2). The six untreated animals (Fig. 2, solid lines) maintained persistently high levels of viremia. Although these untreated animals made a detectable anti-SIV IgM response (data not shown), they made weak or no anti-SIV IgG responses as detected by ELISA (Table 3) or immunoblotting (data not shown). In addition, five of the six untreated animals made no detectable primary or secondary antibody responses following cholera toxin B subunit immunization, indicating rapid immunosuppression (Table 4). Four of the six untreated animals developed CD4+/CD8+ T-lymphocyte ratios of <1 (Table 5). These six untreated animals died at between 7 and 15 weeks of age, with widespread virus dissemination and with clinical signs and gross and microscopic pathologic changes consistent with immunodeficiency and terminal SIV infection (Table 2). Lymphoid tissues collected from these untreated animals at the time of euthanasia harbored high levels of infectious virus (Table 6). Infectious virus levels in the thymus were higher for the SIVmac385-infected animals than for the SIVmac055-infected infants (Table 6).
FIG. 2.
Time course of SIVmac055 and SIVmac385 infection of newborn macaques and the therapeutic effects of PMPA. Newborn macaques were inoculated intravenously with either SIVmac055 (left panels) or SIVmac385 (right panels) by 3 days of age. The untreated animals are represented by solid lines, while the animals that received prolonged PMPA treatment starting at 3 weeks of age (arrow) are represented by dashed lines. The longest-surviving animals are indicated by their numbers. The time of euthanasia because of simian AIDS is indicated (+). Plasma RNA levels (A) were measured by branched DNA assay; plasma (B) and PBMC-associated (C) virus levels were determined by limiting dilution culture of plasma and PBMCs, respectively.
TABLE 3.
Anti-SIV IgG responses in infant rhesus macaques following inoculation with SIVmac055 or SIVmac385
| Animal group | Animal no. | SIVa | PMPAb | Anti-SIV IgG titer at the following times (wk) after virus inoculationc:
|
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 0 | 2 | 3 | 4 | 6 | 7–8 | 10 | 12–13 | 14–16 | 18–21 | 30 | 39 | ||||
| A | 29668 | 055 | − | <100 | <100 | <100 | <100 | <100 | <100 | <100 | 400 | <100d | |||
| 29669 | 055 | − | <100 | <100 | <100 | <100 | <100 | <100d | |||||||
| 29670 | 055 | − | <100 | <100 | <100 | <100 | <100 | <100d | |||||||
| B | 29674 | 385 | − | <100 | <100 | <100 | <100 | <100 | <100 | <100 | <100d | ||||
| 29675 | 385 | − | <100 | <100 | <100 | <100 | <100 | <100 | <100d | ||||||
| 29729 | 385 | − | <100 | <100 | <100 | <100 | <100 | <100d | |||||||
| C | 29283 | 055 | + | <100 | <100 | <100 | <100 | <100 | <100 | <100 | <100 | <100 | <100 | <100 | <100d |
| 29360 | 055 | + | <100 | <100 | <100 | <100 | <100 | <100 | <100 | <100 | <100 | <100d | |||
| 29419 | 055 | + | <100 | <100 | <100 | <100 | <100 | <100 | <100 | <100 | <100 | <100d | |||
| D | 29276 | 385 | + | <100 | <100 | 100 | 1,600 | 25,600 | 25,600 | 25,600 | 25,600 | 25,600 | 102,400 | 102,400 | 102,400 |
| 29278 | 385 | + | <100 | <100 | 100 | 1,600 | 25,600 | 25,600 | 25,600 | 25,600 | 6,400 | 25,600 | 102,400 | 102,400 | |
| 29279 | 385 | + | <100 | <100 | <100 | <100 | 6,400 | 6,400 | 6,400 | 25,600 | 25,600 | 102,400 | 102,400 | 102,400 | |
Animals were inoculated at birth with SIVmac055 (055) or SIVmac385 (385).
Groups C and D were started on PMPA treatment 3 weeks after virus inoculation.
Anti-SIV IgG titers at the indicated times after virus inoculation were determined by ELISA and are expressed as the reciprocal of the highest of fourfold dilutions (starting from a 1/100 dilution with two replicates per dilution) which gave a positive optical density above the cutoff value. Significant titers (titer, ≥400) are indicated in boldface.
Time of death.
TABLE 4.
Anti-cholera toxin B subunit antibody responses in infant rhesus macaques following inoculation with SIVmac055 or SIVmac385
| Animal group | Animal no. | SIVa | PMPAb | Anti-cholera toxin B subunit IgG titer at the following times (wk) after virus inoculationc:
|
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 0 | 2 | 3 | 4 | 6 | 8 | 10 | 12 | 14–16 | ||||
| A | 29668 | 055 | − | <100 | <100 | 100 | 100 | 100 | 100 | 100 | <100 | <100d |
| 29669 | 055 | − | <100 | <100 | <100 | <100 | <100 | <100d | ||||
| 29670 | 055 | − | <100 | <100 | <100 | <100 | <100 | <100d | ||||
| B | 29674 | 385 | − | <100 | 400 | 1,600 | 6,400 | 1,600 | 6,400 | 6,400 | 1,600d | |
| 29675 | 385 | − | <100 | <100 | <100 | <100 | <100 | <100 | <100d | |||
| 29729 | 385 | − | <100 | <100 | <100 | 100 | <100d | |||||
| C | 29283 | 055 | + | <100 | <100 | 100 | 400 | 400 | 1,600 | 102,400 | 25,600 | 25,600 |
| 29360 | 055 | + | <100 | <100 | <100 | <100 | <100 | <100 | 100 | 100 | <100 | |
| 29419 | 055 | + | <100 | 100 | 100 | 400 | 100 | 100 | <100 | <100 | <100 | |
| D | 29276 | 385 | + | <100 | 100 | 100 | 100 | 400 | 400 | 25,600 | 25,600 | 6,400 |
| 29278 | 385 | + | <100 | <100 | 400 | 6,400 | 25,600 | 6,400 | 102,400 | 102,400 | 25,600 | |
| 29279 | 385 | + | <100 | <100 | <100 | <100 | 100 | 100 | 6,400 | 6,400 | 6,400 | |
Animals were inoculated at birth with SIVmac055 (055) or SIVmac385 (385).
Groups C and D were started on PMPA treatment 3 weeks after virus inoculation.
Animals were immunized with cholera toxin B subunit at birth (just prior to the virus inoculations), and a booster immunization was given 8 weeks later. IgG titers at the indicated times after virus inoculation were determined by anti-cholera toxin B subunit IgG-specific ELISA and are expressed as the reciprocal of the highest of fourfold dilutions (starting from a 1/100 dilution with two replicates per dilution) which gave a positive optical density above the cutoff value. Positive antibody responses (titer, ≥400) are indicated in boldface. Four uninfected control newborn macaques from a different study (27) which received the same immunizations had IgG titers of ≥6,400 at 4 weeks of age and titers of ≥102,400 at 12 weeks of age (data not shown). Blank cells indicate that samples were not available (due to animal death prior to the time point).
Time point near the time of euthanasia.
TABLE 5.
CD4+/CD8+ T-lymphocyte ratio in peripheral blood of infant rhesus macaques following inoculation with SIVmac055 or SIVmac385
| Animal group | Animal no. | SIVa | PMPAb | CD4+/CD8+ T-cell ratio at the following times (wk) after virus inoculationc:
|
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 0 | 1 | 2 | 3 | 4 | 6–8 | 10 | 12–15 | 18–21 | 30 | 39 | ||||
| A | 29668 | 055 | − | 4.85 | 2.74 | 0.65 | 1.10 | 1.08 | 0.52 | 0.75 | 0.87d | |||
| 29669 | 055 | − | 3.63 | 3.18 | 1.13 | 1.27 | 0.88 | 0.82d | ||||||
| 29670 | 055 | − | 3.28 | 3.69 | 1.21 | 1.41 | 1.20 | 1.64d | ||||||
| B | 29674 | 385 | − | 3.05 | 4.23 | 1.48 | 1.74 | 1.82 | 1.28 | 1.36 | 0.54d | |||
| 29675 | 385 | − | 5.42 | 7.89 | 1.57 | 1.81 | 1.21 | 1.65 | 1.58d | |||||
| 29729 | 385 | − | 4 | 2.3 | 1.33 | 1.11 | 1.00 | 0.83d | ||||||
| C | 29283 | 055 | + | 3.82 | 9.33 | 2.14 | 2.31 | 2.58 | 1.95 | 2.02 | 1.43 | 1.83 | 1.81 | 1.94d |
| 29360 | 055 | + | 3.85 | 4.06 | 1.43 | 1.28 | 1.33 | 0.96 | 0.88 | 0.65 | 0.44d | |||
| 29419 | 055 | + | 5.69 | 9.67 | 3.59 | 3.41 | 2.45 | 2.55 | NAe | 1.47 | 1.63d | |||
| D | 29276 | 385 | + | 4.35 | 4.17 | 1.82 | 1.71 | 1.88 | 1.41 | 1.45 | 1.29 | 1.37 | 0.98 | 0.87 |
| 29278 | 385 | + | 5.53 | 8.32 | 2.04 | 2.49 | 2.00 | 2.09 | 2.16 | 1.54 | 1.75 | 1.87 | 1.13 | |
| 29279 | 385 | + | 3.73 | 4.04 | 1.64 | 1.61 | 1.76 | 1.29 | 1.68 | 1.00 | 1.24 | 1.22 | 1.23 | |
Animals were inoculated at birth with SIVmac055 (055) or SIVmac385 (385).
Groups C and D were started on PMPA treatment 3 weeks after virus inoculation.
Ratios of <1 are indicated in boldface.
Time of death.
NA, not available.
TABLE 6.
Infectious virus levels in peripheral blood and lymphoid tissues at time of simian AIDS
| Animal group | Animal no. | SIVa | PMPAb | Time of death | No. of TCID50sc
|
||||
|---|---|---|---|---|---|---|---|---|---|
| PBMC | Plasma | Spleen | Thymus | Lymph node | |||||
| A | 29668 | 055 | − | 15 wk | 2,200 | 1,000 | 3,200 | 46 | 320 |
| 29669 | 055 | − | 8 wk | 320 | 3,200 | 320 | 460 | 1,000 | |
| 29670 | 055 | − | 8 wk | 460 | 1,000 | 1,800 | 32 | 220 | |
| B | 29674 | 385 | − | 13 wk | 320 | 46 | 1,800 | 2,200 | 2,200 |
| 29675 | 385 | − | 10 wk | 1,800 | 10,000 | 10,000 | 4,600 | 320 | |
| 29729 | 385 | − | 7 wk | 460 | 460 | 320 | 2,200 | 180 | |
| C | 29283 | 055 | + | 39 wk | 3 | 5 | 5 | 3 | 5 |
| 29360 | 055 | + | 19 wk | 100 | 4,600 | 2,200 | 5 | 1,800 | |
| 29419d | 055 | + | 21 wk | 47 | 215 | NAe | NA | NA | |
| D | 29276f | 385 | + | NA | NA | NA | NA | NA | NA |
| 29278 | 385 | + | 21 mo | 220 | <3 | 460 | 32 | 100 | |
| 29279 | 385 | + | 22 mo | 3 | <3 | 32 | <0.3 | 100 | |
Animals were inoculated at birth intravenously with 500 to 2,000 TCID50s of SIVmac055 (055) or SIVmac385 (385).
Groups C and D were started on chronic PMPA treatment 3 weeks after virus inoculation.
Cell-associated and cell-free virus levels in peripheral blood and fresh lymphoid tissues collected at the time of euthanasia were determined by limiting dilution culture of single-cell suspensions or plasma and are expressed as the numbers of TCID50s per 106 cells or per milliliter of plasma.
Animal 29419 was found dead at 21 weeks of age, and no fresh tissues were available; values for PBMCs and plasma are those obtained at week 19.
NA, not available.
Animal 29276 was alive at 29 months of age.
In contrast to the six untreated animals, the six animals that were started on prolonged PMPA treatment at 3 weeks of age (Fig. 2, dashed lines) also had high virus levels but fared much better clinically. The three PMPA-treated, SIVmac055-infected infants (Table 2, group C) had persistently very high viremia levels similar to those for the untreated animals (plasma RNA levels were consistently between 5 × 107 and 1 × 109 RNA copies per ml; Fig. 2). Similar to the untreated control animals, these three PMPA-treated, SIVmac055-infected animals also had rapid immunosuppression: although they made a detectable anti-SIV IgM response (data not shown), none of the three animals made a detectable anti-SIV IgG response (Table 3). Only one of these three infants (animal 29283) made a significant anti-cholera toxin IgG response following booster immunization with this antigen at 8 weeks of age (Table 4). Yet, these three animals survived significantly longer than the untreated animals (P = 0.025): two animals survived until 19 to 21 weeks of age, while the third animal (animal 29283) was euthanized at 39 weeks (Table 2); absolute CD4+ T-cell counts for animals 29360 and 29283 at the time of euthanasia were 201 and 365 cells/μl, respectively. Infectious virus levels in lymphoid tissues of animal 29283 at the time of death were lower than those in the lymphoid tissues of the untreated animals (Table 6).
The three PMPA-treated, SIVmac385-infected animals (Table 2, group D) fared significantly better than the other animals (P < 0.05). These animals also had relatively high virus levels during the first 3 weeks of infection (Fig. 2). Compared to uninfected infant macaques, two of these three PMPA-treated SIVmac385-infected animals had poor primary antibody responses following cholera toxin subunit B immunization at birth (Table 4). Following PMPA treatment beginning at 3 weeks of age, however, the three SIVmac385-infected infants were able to make a strong anti-SIV IgG response (Table 3) and a secondary antibody response to cholera toxin subunit B following booster immunization with this antigen at 8 weeks of age (Table 4). Compared to the untreated animals and the PMPA-treated SIVmac055-infected animals, the PMPA-treated, SIVmac385-infected infants had more stabilized CD4+/CD8+ T-lymphocyte ratios (Table 5). One of these three animals, animal 29278, continued to have persistently high virus levels in peripheral blood (Fig. 2) and developed fatal immunodeficiency at 21 months of age (the plasma viral RNA titer at the time of euthanasia was 2.8 × 106 copies/ml, the CD4+ T-cell count was 354/μl, and the CD4+/CD8+ T-cell ratio was 0.97). The second PMPA-treated, SIVmac385-infected infant (animal 29279) died at 22 months of age (the plasma viral RNA titer near the time of death was 9 × 105 copies/ml, the CD4+ T-cell count was 136/μl, and the CD4+/CD8+ T-cell ratio was 0.41); this animal had widespread Rhodococcus equi infection (Table 2). Infectious virus levels in the lymphoid tissues of animals 29278 and 29279 were lower than those in the lymphoid tissues of untreated infants at the time of death (Table 6). The third PMPA-treated, SIVmac385-infected infant (animal 29276) is alive and AIDS-free at 29 months of age, with moderate viremia levels (9,840 copies of RNA/ml of plasma) and stable T-cell counts (CD4+/CD8+ T-cell ratio, ∼1; CD4+ T-cell counts, >500/μl).
Genotypic and phenotypic analyses were performed with virus isolates from all 12 animals following inoculation with SIVmac055 or SIVmac385. Virus isolates from 11 of the 12 animals retained the K65R mutation; in addition, virus isolated from the PBMCs and plasma of these 11 animals still had the same fivefold reduced in vitro susceptibility to PMPA (Table 1). In viruses isolated from untreated, SIVmac385-infected animal 29674, the K65R mutation had become less predominant and was no longer detectable in PBMCs by conventional sequence analyses at the time of death; this virus also showed phenotypic reversion to wild-type susceptibility (Table 1); this reversion was first detectable in plasma at 8 weeks of age and then in PBMCs at 12 weeks of age; at the time of death (13 weeks of age), viruses from PBMCs, the spleen, and a peripheral lymph node had wild-type susceptibility, while the thymus still harbored virus with fivefold reduced susceptibility to PMPA (data not shown).
Most animals developed additional substitutions in RT during the course of the study (Table 1). Interestingly, viruses from 8 of the 11 infants which retained the K65R mutation developed the adjacent K64R mutation. Of these eight infants, four animals had been inoculated with SIVmac055 and four had been inoculated with SIVmac385; five animals received PMPA treatment, while the other three were untreated (Table 1). These virus isolates with the K64R and K65R mutations still had a similar fivefold level of reduced in vitro susceptibility to PMPA as the original SIVmac055 and SIVmac385 inocula. Viruses from the three PMPA-treated SIVmac385-infected animals all developed Y115F and S211N mutations in RT; the S211N mutation was not detected in the original SIVmac385 inoculum but was already present in the SIVmac055 inoculum. Other amino acid substitutions, which were found in viruses from at least two animals, include V17D/E, K40Q/R, V148G, and F171Y (Table 1). For all these K65R virus isolates, the acquisition of all these additional substitutions in RT did not alter the approximately fivefold reduced in vitro susceptibility to PMPA; there was no obvious correlation between the development of these additional mutations and changes in viral levels or disease progression.
DISCUSSION
The experiments described here demonstrate the usefulness of SIV-infected newborn rhesus macaques as an animal model for the study of the emergence, virulence, and clinical implications of drug-resistant viral mutants prior or complementary to the availability of such data from human studies.
We previously demonstrated strong therapeutic benefits of PMPA treatment for four infant macaques inoculated at birth with highly virulent wild-type SIVmac251 (28). While most untreated animals infected with SIVmac251 generally developed fatal immunodeficiency within 3 to 4 months (27, 31, 33, 34), PMPA treatment starting at 3 weeks of age resulted in reduced viremia levels, enhanced antiviral immune responses, and delayed disease progression (28). Although virus with fivefold reduced susceptibility to PMPA and a K65R substitution in RT emerged in these four animals within a few months of PMPA treatment, the four animals remained disease-free for a prolonged period of time (≥21 months). These studies suggested that reduced in vitro susceptibility to PMPA was different from that observed previously with AZT in the same animal model, because the emergence of AZT-resistant SIV was associated with the development of clinical disease (30, 33).
In the current study, we further investigated the clinical implications of low-level resistance to PMPA by inoculating two different SIV isolates, SIVmac055 and SIVmac385, into newborn macaques. Both SIVmac055 and SIVmac385 were uncloned virus isolates which had similar phenotypic resistance (fivefold reduced in vitro susceptibility) but distinct RT genotypes (SIVmac055, K65R, N69T, R82K, A158S, and S211N; SIVmac385, K65R, N69S, and I118V). We addressed three key questions: (i) are these K65R SIV isolates virulent for infant macaques, (ii) does low-level in vitro resistance to PMPA lead to a reduced efficacy of PMPA therapy in vivo, and (iii) how do these K65R mutant viruses evolve over time in the presence or absence of PMPA treatment?
Inoculation of SIVmac055 and SIVmac385 into newborn macaques demonstrated that in the absence of PMPA treatment, these viral mutants are highly virulent, because they replicated to high levels and induced rapid immunosuppression and fatal disease within 2 to 4 months. This fulminant course of SIVmac055 and SIVmac385 infection in untreated newborn macaques is indistinguishable from that seen following inoculation with the parental wild-type virus, uncloned SIVmac251 (16, 33). Thus, the RT mutations associated with low-level PMPA resistance did not attenuate the virus in this study. The rapid disease course observed in the untreated animals in this study was probably not related to the intravenous route of inoculation or the dose of the virus inoculum, because more recent studies with oral inoculation of newborn macaques with a minimal infectious dose of SIVmac055 also resulted in rapid disease progression in untreated animals (29). In addition, the virulence of the K65R SIV isolates in the current study is probably not due to the fact that newborn macaques were used for the virus inoculations, because we recently observed that oral SIVmac055 inoculation of juvenile macaques also resulted in high levels of virus replication and immunosuppression (29).
To determine whether in vitro resistance to PMPA affects the therapeutic efficacy of PMPA treatment in vivo, six newborn macaques were inoculated with SIVmac055 or SIVmac385 and were started on prolonged PMPA treatment 3 weeks later. PMPA treatment was withheld for 3 weeks because at that time these animals already had high levels of viremia and widespread systemic virus dissemination and demonstrated signs of immunosuppression (as indicated by poor IgG responses to SIV infection and to cholera toxin subunit B immunization). It is believed that drug treatment of established SIV infection is a more rigorous test of drug efficacy than treatment during the very early viremia stage (28, 32); accordingly, established infection of newborn macaques with high levels of PMPA-resistant mutants and the presence of immunosuppression can be considered a “worst-case scenario” for investigation of how reduced in vitro susceptibility to PMPA may affect the subsequent efficacy of PMPA therapy.
Interestingly, in contrast to the rapid disease course observed in the six untreated animals, the six infant macaques that were inoculated at birth with SIVmac385 or SIVmac055 but that received PMPA treatment showed a significantly delayed disease course. For the three SIVmac385-infected animals, PMPA treatment apparently reduced the immunosuppression, as indicated by their ability to mount better immune responses to viral antigens and to cholera toxin B subunit immunization. Similar to previous observations with drug-treated SIV-infected newborn macaques (28, 33), the development of antiviral immune responses likely contributed to a slower disease course in these animals, which, despite maintaining relatively high virus levels, survived for at least 21 months. For the three SIVmac055-inoculated animals, PMPA treatment did not reduce virus levels in peripheral blood; the inability of these three animals to mount an anti-SIV IgG response also indicated that PMPA therapy initiated at 3 weeks of age did not revert their severe immunosuppression; two of these animals also failed to make an antibody response to cholera toxin B subunit. Remarkably, however, these three PMPA-treated, SIVmac055-infected animals receiving PMPA therapy still survived significantly longer than the untreated control animals (P = 0.025). The observation that PMPA-treated, SIVmac055-infected infants were disease-free for long periods of time despite high levels of viremia with highly virulent virus and despite suppression of acquired immune responses was unexpected. To our knowledge, this paradox has not been seen previously in SIV-infected macaques. These data suggest that during PMPA treatment of SIV-infected macaques, virus levels and parameters related to acquired immunity are less reliable as predictors of disease progression. A possible explanation for this paradox is provided by the observation that PMPA and the related nucleotide analog 9-[2-(phosphonomethoxy)ethyl]adenine (PMEA) have been found to enhance natural killer cell activity in murine models (11, 26); PMPA also stimulated in vitro cytokine and nitric oxide production by murine macrophages (37). If PMPA has similar immunomodulatory effects in SIV-infected macaques, then this enhancement of natural immunity may explain how animals with deficiency of acquired immunity (which depends on CD4+ T-helper-cell activity) may still have some protection against infection by opportunistic pathogens and thus have a delayed disease course. Accordingly, future research will investigate the potential immunomodulatory effects of PMPA in macaques and will define the role of natural immunity on SIV and HIV disease progression.
Our study demonstrates that although SIVmac055 and SIVmac385 were both highly virulent in untreated newborn macaques, SIVmac055 replication was less affected by PMPA treatment than that of SIVmac385. This finding is consistent with the observation that, following emergence of PMPA-resistant virus, animals 29003, 29008, and 29045 (from which SIVmac385 was derived) had a slower clinical disease course than animal 29055 (which was the source of SIVmac055). In vitro, SIVmac055 replicated better in rhesus macaque PBMCs than SIVmac385, but both isolates had similar fivefold reduced susceptibilities to PMPA. Because SIVmac055 and SIVmac385 are uncloned virus isolates, it is unclear whether the difference in biological behavior between these two viruses is due to the different compensatory mutations in RT or to differences in other genes of the virus outside RT; further studies with site-directed mutagenesis of molecular clones are needed to clarify this distinction.
Our studies with K65R SIVmac isolates provide evidence for the emergence of additional amino acid substitutions in RT due to ongoing virus replication in the presence or absence of PMPA therapy. There was no obvious correlation between the development of these additional RT substitutions and levels of virus replication or disease progression. Virus isolates with these additional mutations had the same fivefold reduced in vitro susceptibility to PMPA as virus with only the K65R substitution in RT.
The development of the K64R substitution in RT, immediately adjacent to K65R, in infant macaques inoculated with SIVmac055 or SIVmac385 is an interesting finding. The K64R substitution emerged in 8 of the 12 animals and was seen with both SIVmac385 and SIVmac055; there was no obvious correlation between the emergence of K64R and concomitant PMPA treatment. This K64R substitution eventually also emerged in viruses of two of the original PMPA-treated animals (animals 29008 and 29055), of which earlier virus isolates had been used to prepare SIVmac385 and SIVmac055 (Table 1). In wild-type virus, both lysine (K) residues at positions 64 and 65 are part of the IKKK motif, which is highly conserved for HIV and SIV (19); a similar conserved sequence is found in other retroviruses. Both Rous sarcoma virus and bovine leukemia virus already encode arginine (R) at position 64 (2, 3). The K64R substitution has also been described in conjunction with many known drug resistance mutations in multidrug-resistant HIV type 1 (HIV-1) isolated from a heavily treated patient (23). Another RT substitution, which emerged in the virus isolates from the three PMPA-treated, SIVmac385-infected animals and from animal 29008 was Y115F. The Y115F and K65R substitutions in HIV-1 are each associated with low-level resistance to (1R,4S)-4-[2-amino-6-(cyclopropyl-amino)-9H-purin-9-yl]-2-cyclo-pentene-1-methanol (1592U89; abacavir) (24); the mutant SIVmac isolates described here with only K65R or with both K65R and Y115F substitutions had approximately two- and fivefold reduced susceptibilities to 1592U89, respectively (data not shown). Phenylalanine (F) at position 115 is also found in several other retroviruses (2). Many other substitutions in RT were detected in this study (Table 1), but the biological significance of the accumulation of all these mutations is unclear. Because these substitutions did not further reduce the susceptibility of the virus to PMPA, they likely reflect compensatory mutations which emerged as a result of selection pressure toward increased replicative fitness. It is expected that a specific drug resistance mutation, such as the K65R mutation, impairs the in vivo replicative ability of the virus (5). The virus may accumulate additional substitutions in RT or other genes in an attempt to restore this replicative ability, and the genetic background of the virus may be critical in determining which evolutionary pathway it enters to reach maximal fitness under these conditions of drug treatment. Accordingly, the rather unpredictable and gradual accumulation of novel substitutions in RT likely reflects the very complex interaction between drug resistance mutations and viral fitness (15). It has also been demonstrated for HIV-1 that drug-resistant virus can take new evolutionary pathways by acquiring novel and unexpected amino acid substitutions (23, 36).
Recent data from phase I/II human trials have demonstrated very potent antiviral effects of PMPA for HIV-infected patients, and no emergence of PMPA-resistant HIV mutants was observed during up to 4 weeks of treatment (9, 10, 17). The K65R substitution in RT has, however, been described for HIV-1 selected for resistance to PMPA and PMEA in vitro and confers to HIV-1 a similar, approximately fivefold reduced susceptibility to PMPA (13, 35). The K65R mutation has not been observed in patients treated with PMEA for up to 18 months (18). If the K65R substitution emerges in HIV-infected patients receiving prolonged PMPA therapy, then the phenotypic and genotypic changes associated with reduced susceptibility to PMPA would be remarkably similar for SIV and HIV. In addition, the disease pathogenesis of SIV infection in macaques closely parallels that of HIV infection in people (12). Altogether, these similarities suggest that the clinical implications of the emergence of K65R viral mutants in vivo may also be very similar for both viruses.
In conclusion, this study demonstrated that although SIV isolates with reduced in vitro susceptibility to PMPA were virulent, PMPA monotherapy still offered strong therapeutic benefits for infant macaques infected with these PMPA-“resistant” viruses. The results obtained in this study with infant macaques suggest that if HIV mutants with reduced susceptibility to PMPA eventually emerge in patients during prolonged PMPA therapy, these mutants may partially reduce, but will not eliminate, the therapeutic benefits of PMPA administration.
ACKNOWLEDGMENTS
We thank E. Agatep, N. Aguirre, P. Allen, L. Antipa, V. Bakula, D. Bennett, C. Berardi, I. Bolton, L. Brignolo, B. Capuano, L. Carruthers, I. Cazares, S. Davis, Z. Dehqanzada, A. Enriquez, D. Florence, K. Gilardi, T. Greene, J. Greenier, H. Louie, E. Lee, C. Oxford, D. Robb, A. Spinner, S. Telm, C. Valverde, W. von Morgenland, T. Vogt, R. Walgenbach, C. Young, and Colony Services of the California Regional Primate Research Center for expert technical assistance; J. Booth for quantitation of plasma RNA; and T. North for useful discussions.
This research was supported by PHS grant RR-00169 to the California Regional Primate Research Center and E. Glaser Pediatric AIDS Foundation grant 50609-20-PG to K. Van Rompay; M. L. Marthas is an Elizabeth Glaser Scientist funded by the Pediatric AIDS Foundation.
REFERENCES
- 1.Banapour B, Marthas M L, Ramos R A, Lohman B L, Unger R E, Gardner M B, Pedersen N C, Luciw P A. Identification of viral determinants of macrophage tropism for simian immunodeficiency virus SIVmac. J Virol. 1991;65:5798–5805. doi: 10.1128/jvi.65.11.5798-5805.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Barber A M, Hizi A, Maizel J V, Hughes S H. HIV-1 reverse transcriptase: structure predictions for the polymerase domain. AIDS Res Hum Retroviruses. 1990;6:1061–1072. doi: 10.1089/aid.1990.6.1061. [DOI] [PubMed] [Google Scholar]
- 3.Boyer P L, Ferris A L, Hughes S H. Cassette mutagenesis of the reverse transcriptase of human immunodeficiency virus type 1. J Virol. 1992;66:1031–1039. doi: 10.1128/jvi.66.2.1031-1039.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Centers for Disease Control and Prevention. Update: trends in AIDS incidence—United States, 1996. Morbid Mortal Weekly Rep. 1997;46:862–867. [Google Scholar]
- 5.Coffin J M. HIV population dynamics in vivo: implications for genetic variation, pathogenesis, and therapy. Science. 1995;267:483–489. doi: 10.1126/science.7824947. [DOI] [PubMed] [Google Scholar]
- 6.Condra J H. Resisting resistance: maximizing the durability of antiretroviral therapy. Ann Intern Med. 1998;128:951–954. doi: 10.7326/0003-4819-128-11-199806010-00017. [DOI] [PubMed] [Google Scholar]
- 7.Dailey P J, Zamroud M, Kelso R, Kolberg J, Urdea M. Abstracts of the 13th Annual Symposium on Nonhuman Primate Models of AIDS. 1995. Quantitation of simian immunodeficiency virus (SIV) RNA in plasma of acute and chronically infected rhesus macaques using a branched DNA (bDNA) signal amplification assay, abstr. 99. [Google Scholar]
- 8.Dawson-Saunders B, Trapp R G. Basic and Clinical Biostatistics. Norwalk, Conn: Appleton & Lange; 1990. [Google Scholar]
- 9.Deeks S G, Barditch-Crovo P, Lietman P S, Collier A, Safrin S, Coleman R, Cundy K C, Kahn J O. Abstracts of the 5th Conference on Retroviruses and Opportunistic Infections. 1998. The safety and efficacy of PMPA prodrug monotherapy: preliminary results of a phase I/II dose-escalation study, abstr. 772. [Google Scholar]
- 10.Deeks S G, Barditch-Crovo P, Lietman P S, Hwang F, Cundy K C, Rooney J F, Hellmann N S, Safrin S, Kahn J. Safety, pharmacokinetics and antiretroviral activity of intravenous 9-[2-(R)-(phosphonomethoxy)propyl]adenine, a novel anti-human immunodeficiency virus (HIV) therapy, in HIV-infected adults. Antimicrob Agents Chemother. 1998;42:2380–2384. doi: 10.1128/aac.42.9.2380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Del Gobbo V, Foli A, Balzarini J, De Clercq E, Balestra E, Villani N, Marini S, Perno C F, Calio R. Immunomodulatory activity of 9-(2-phosphonylmethoxyethyl)adenine (PMEA), a potent anti-HIV nucleotide analogue, on in vivo murine models. Antivir Res. 1991;16:65–75. doi: 10.1016/0166-3542(91)90059-z. [DOI] [PubMed] [Google Scholar]
- 12.Desrosiers R C. The simian immunodeficiency viruses. Annu Rev Immunol. 1990;8:557–578. doi: 10.1146/annurev.iy.08.040190.003013. [DOI] [PubMed] [Google Scholar]
- 13.Foli A, Sogocio K M, Anderson B, Kavlick M, Saville M W, Wainberg M A, Gu X, Cherrington J M, Mitsuya H, Yarchoan R. In vitro selection and molecular characterization of human immunodeficiency virus type 1 with reduced sensitivity to 9-(2-phosphonylmethoxyethyl)adenine. Antivir Res. 1996;32:91–98. doi: 10.1016/0166-3542(95)00985-x. [DOI] [PubMed] [Google Scholar]
- 14.Lohman B L, Higgins J, Marthas M L, Marx P A, Pedersen N C. Development of simian immunodeficiency virus isolation, titration, and neutralization assays which use whole blood from rhesus monkeys and an antigen capture enzyme-linked immunosorbent assay. J Clin Microbiol. 1991;29:2187–2192. doi: 10.1128/jcm.29.10.2187-2192.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Maeda Y, Venzon D J, Mitsuya H. Altered drug sensitivity, fitness, and evolution of human immunodeficiency virus type 1 with pol gene mutations conferring multi-dideoxynucleoside resistance. J Infect Dis. 1998;177:1207–1213. doi: 10.1086/515282. [DOI] [PubMed] [Google Scholar]
- 16.Marthas M L, Van Rompay K K A, Otsyula M, Miller C J, Canfield D, Pedersen N C, McChesney M B. Viral factors determine progression to AIDS in simian immunodeficiency virus-infected newborn rhesus macaques. J Virol. 1995;69:4198–4205. doi: 10.1128/jvi.69.7.4198-4205.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Miller M, Cherrington J M, Lamy P D, Mulato A S, Anton K E. Abstracts of the 12th World AIDS Conference. 1998. Genotypic changes in HIV RT which develop during Preveon (adefovir dipivoxil) therapy do not decrease susceptibility to PMPA, abstr. 41218. [Google Scholar]
- 18.Mulato A S, Lamy P D, Miller M D, Li W-X, Anton K E, Hellmann N S, Cherrington J M. Genotypic and phenotypic characterization of human immunodeficiency virus type 1 variants isolated from AIDS patients after prolonged adefovir dipivoxil therapy. Antimicrob Agents Chemother. 1998;42:1620–1628. doi: 10.1128/aac.42.7.1620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Myers G, Berzofsky J A, Korber B, Smith R F, Pavlakis G N. Human Retroviruses and AIDS. A compilation and analysis of nucleic acid and amino acid sequences. Los Alamos, N.M: Los Alamos National Laboratory; 1991. [Google Scholar]
- 19a.National Research Council. Guide for the care and use of laboratory animals. Washington, D.C: National Academy Press; 1996. [Google Scholar]
- 20.Otsyula M G, Miller C J, Marthas M L, Van Rompay K K A, Collins J R, Pedersen N C, McChesney M B. Virus-induced immunosuppression is linked to rapidly fatal disease in infant rhesus macaques infected with simian immunodeficiency virus. Pediatr Res. 1996;39:630–635. doi: 10.1203/00006450-199604000-00012. [DOI] [PubMed] [Google Scholar]
- 21.Pachl C, Todd J A, Kern D G, Sheridan P J, Fong S-J, Stempein M, Hoo B, Besemer D, Yeghiazarian T, Irvine B, Kolberg J, Kokka R, Neuwald P, Urdea M S. Rapid and precise quantification of HIV-1 RNA in plasma using a branched DNA signal amplification assay. J Acquir Immune Defic Syndr Hum Retrovirol. 1995;8:446–454. doi: 10.1097/00042560-199504120-00003. [DOI] [PubMed] [Google Scholar]
- 22.Richman D D. Resistance of clinical isolates of human immunodeficiency virus to antiretroviral agents. Antimicrob Agents Chemother. 1993;37:1207–1213. doi: 10.1128/aac.37.6.1207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shafer R W, Winters M A, Palmer S, Merigan T C. Multiple concurrent reverse transcriptase and protease mutations and multidrug resistance of HIV-1 isolates from heavily treated patients. Ann Intern Med. 1998;128:906–911. doi: 10.7326/0003-4819-128-11-199806010-00008. [DOI] [PubMed] [Google Scholar]
- 24.Tisdale M, Alnadaf T, Cousens D. Combination of mutations in human immunodeficiency virus type 1 reverse transcriptase required for resistance to the carbocyclic nucleoside 1592U89. Antimicrob Agents Chemother. 1997;41:1094–1098. doi: 10.1128/aac.41.5.1094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tsai C-C, Follis K E, Beck T W, Sabo A, Grant R F, Bischofberger N, Benveniste R E. Prevention of simian immunodeficiency virus infection in macaques by 9-(2-phosphonylmethoxyethyl)adenine (PMPA) Science. 1995;270:1197–1199. doi: 10.1126/science.270.5239.1197. [DOI] [PubMed] [Google Scholar]
- 26.Ussery M A, Wood O L, Kunder S C, Bacho M A, Broud D D, Papermaster S F, Hall B E, Nielsen C J, Chen M S, Cherrington J M, Black P L. Abstracts of the Tenth International Conference on Antiviral Research. 1997. PMPA prevents infection of HuPBMC SCID mice by HIV-1 (late breaker). In. [Google Scholar]
- 27.Van Rompay K K A, Berardi C J, Aguirre N L, Bischofberger N, Lietman P S, Pedersen N C, Marthas M L. Two doses of PMPA protect newborn macaques against oral simian immunodeficiency virus infection. AIDS. 1998;12:F79–F83. doi: 10.1097/00002030-199809000-00001. [DOI] [PubMed] [Google Scholar]
- 28.Van Rompay K K A, Cherrington J M, Marthas M L, Berardi C J, Mulato A S, Spinner A, Tarara R P, Canfield D R, Telm S, Bischofberger N, Pedersen N C. 9-[2-(Phosphonylmethoxyethyl)propyl]adenine therapy of established simian immunodeficiency virus infection in infant rhesus macaques. Antimicrob Agents Chemother. 1996;40:2586–2591. doi: 10.1128/aac.40.11.2586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Van Rompay, K. K. A., et al. Unpublished data.
- 30.Van Rompay K K A, Greenier J L, Marthas M L, Otsyula M G, Tarara R P, Miller C J, Pedersen N C. A zidovudine-resistant simian immunodeficiency virus mutant with a Q151M mutation in reverse transcriptase causes AIDS in newborn macaques. Antimicrob Agents Chemother. 1997;41:278–283. doi: 10.1128/aac.41.2.278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Van Rompay K K A, Marthas M L, Lifson J D, Berardi C J, Vasquez G M, Agatep E, Dehqanzada Z A, Cundy K C, Bischofberger N, Pedersen N C. Administration of 9-[2-(phosphonomethoxy)propyl]adenine (PMPA) for prevention of perinatal simian immunodeficiency virus infection in rhesus macaques. AIDS Res Hum Retroviruses. 1998;14:761–773. doi: 10.1089/aid.1998.14.761. [DOI] [PubMed] [Google Scholar]
- 32.Van Rompay K K A, Marthas M L, Ramos R A, Mandell C P, McGowan E K, Joye S M, Pedersen N C. Simian immunodeficiency virus (SIV) infection of infant rhesus macaques as a model to test antiretroviral drug prophylaxis and therapy: oral 3′-azido-3′-deoxythymidine prevents SIV infection. Antimicrob Agents Chemother. 1992;36:2381–2386. doi: 10.1128/aac.36.11.2381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Van Rompay K K A, Otsyula M G, Marthas M L, Miller C J, McChesney M B, Pedersen N C. Immediate zidovudine treatment protects simian immunodeficiency virus-infected newborn macaques against rapid onset of AIDS. Antimicrob Agents Chemother. 1995;39:125–131. doi: 10.1128/aac.39.1.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Van Rompay K K A, Otsyula M G, Tarara R P, Canfield D R, Berardi C J, McChesney M B, Marthas M L. Vaccination of pregnant macaques protects newborns against mucosal simian immunodeficiency virus infection. J Infect Dis. 1996;173:1327–1335. doi: 10.1093/infdis/173.6.1327. [DOI] [PubMed] [Google Scholar]
- 35.Wainberg M A, Quan Y, Salomon H, Cherrington J. Abstracts of the 5th Conference on Retroviruses and Opportunistic Infections. 1998. The M184V substitution in reverse transcriptase increases sensitivity of both HIV-1 and SIV to PMPA, abstr. 680. [Google Scholar]
- 36.Yerly S, Rakik A, Kinloch de Loes S, Hirschel B, Descamps D, Brun-Vézinet F, Perrin L. Switch to unusual amino acids at codon 215 of the human immunodeficiency virus type 1 reverse transcriptase gene in seroconverters infected with zidovudine-resistant variants. J Virol. 1998;72:3520–3523. doi: 10.1128/jvi.72.5.3520-3523.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zídek Z, Holy A, Franková D. Antiretroviral agent (R)-9-(2-phosphonomethoxypropyl)adenine stimulates cytokine and nitric oxide production. Eur J Pharmacol. 1997;331:245–252. doi: 10.1016/s0014-2999(97)01004-2. [DOI] [PubMed] [Google Scholar]