Abstract
Dogs were experimentally inoculated with Rickettsia rickettsii (canine origin) in order to compare the efficacies of azithromycin and trovafloxacin to that of the current antibiotic standard, doxycycline, for the treatment of Rocky Mountain spotted fever. Clinicopathologic parameters, isolation of rickettsiae in tissue culture, and PCR amplification of rickettsial DNA were used to evaluate the response to therapy or duration of illness (untreated infection control group) in the four groups. Concentrations of the three antibiotics in plasma and blood cells were measured by high-performance liquid chromatography. Doxycycline and trovafloxacin treatments resulted in more-rapid defervescence, whereas all three antibiotics caused rapid improvement in attitudinal scores, blood platelet numbers, and the albumin/total-protein ratio. Based upon detection of retinal vascular lesions by fluorescein angiography, trovafloxacin and doxycycline substantially decreased rickettsia-induced vascular injury to the eye, whereas the number of ocular lesions in the azithromycin group did not differ from that in the infection control group. As assessed by tissue culture isolation, doxycycline resulted in the earliest apparent clearance of viable circulating rickettsiae; however, rickettsial DNA could still be detected in the blood of some dogs from all four groups on day 21 postinfection, despite our inability to isolate viable rickettsiae at that point. As administered in this study, trovafloxacin was as efficacious as doxycycline but azithromycin proved less efficacious, possibly due to the short duration of administration.
Rocky Mountain spotted fever (RMSF), which is caused by Rickettsia rickettsii, is an important human rickettsiosis in the United States. The disease induces hypoalbuminemia, thrombocytopenia, and vascular leakage and is frequently characterized by severe debilitating illness, accompanied by a 3 to 7% case fatality rate (7, 17). Postillness sequelae can include deafness, impaired vision, neurologic defects, amputation of gangrenous extremities, and intellectual deficits (1, 2, 16). Serologic surveys indicate that canine exposure to R. rickettsii is widespread in areas of endemicity (which include North Carolina), and reports of naturally occurring canine RMSF suggest that the disease in dogs is associated with significant morbidity and occasional mortality (3, 12, 18, 21). In regions of endemicity, dogs with acute febrile illness are routinely and appropriately treated by veterinarians with antimicrobial therapy directed against R. rickettsii, prior to or without serologic confirmation of RMSF.
Tetracycline and chloramphenicol are currently the only antibiotics recommended for treatment of human RMSF. Both have relative contraindications for use, particularly in young children, a population group predisposed to exposure to R. rickettsii (20). Tetracycline may discolor permanent teeth if given to children below the age of 7 years and is known to inhibit bone growth in neonates. Intravenous tetracycline has been associated with severe nephritis in dogs and with hepatitis when administered to pregnant women. Tetracycline is also not recommended if there is progressive or severe azotemia. In humans, chloramphenicol administration has been associated with a fatal aplastic anemia as well as with other hematologic abnormalities; the so-called gray syndrome of neonates, which is characterized by circulatory collapse, cyanosis, and hypothermia; and optic and peripheral neuritis. New antibiotics, which are safe for pregnant or lactating women and for children, are needed for the management of acute febrile illnesses in regions in which R. rickettsii is endemic in ticks.
In 1992, in vitro and in vivo studies conducted by our laboratory indicated that the fluoroquinolone enrofloxacin was as efficacious as chloramphenicol or tetracycline for the treatment of experimental canine RMSF but was not more effective (5). In a subsequent experimental-infection study, doxycycline administration resulted in a similarly favorable therapeutic outcome (6). Our clinical use of enrofloxacin in dogs has supported the efficacy of fluoroquinolones for the treatment of canine RMSF. However, doxycycline remains the current drug of choice because of the lack of efficacy data for fluoroquinolones in the treatment of canine ehrlichiosis, a rickettsial disease with clinical features similar to those of RMSF.
Azithromycin, a new azalide antibiotic, has been shown in clinical trials to be a safe, effective drug for children and adults against gram-positive organisms and some gram-negative organisms (24). Because the drug is concentrated inside neutrophils and mononuclear phagocytes, it is of potential utility for the treatment of intracellular pathogens. Studies have shown that azithromycin can be effective for respiratory-tract infections when used once a day for 3 days, compared to 5- to 10-day courses of other antibiotics, such as erythromycin (10).
Trovafloxacin belongs to a new generation of fluoroquinolones. In addition to activity against gram-negative bacteria, which is shared by other fluoroquinolones, such as ciprofloxacin, trovafloxacin also has activity against streptococci and some anaerobic bacteria. The clinical use of this drug has not been reported in dogs. Because it is a new drug, there are few clinical studies reported for humans. Although it is not recommended for use in children, initial data show it to be an effective broad-spectrum antibiotic (11). Because of the similarities in disease presentations, the use of a broad-spectrum antibiotic that also has efficacy for treating rickettsial disease would be advantageous for the treatment of fevers of undetermined origin in regions of endemicity for R. rickettsii. To our knowledge, no in vivo studies using either azithromycin or trovafloxacin against rickettsial organisms have been conducted. Therefore, in this study, we investigated the therapeutic efficacy of doxycycline, azithromycin, or trovafloxacin for the treatment of experimental RMSF in dogs.
MATERIALS AND METHODS
Inoculum preparation.
Because tissue culture growth of rickettsial organisms has been known to diminish the virulence of rickettsiae in animal hosts (15), Vero cell culture-grown R. rickettsii Domino was passaged by yolk sac injection of 4- to 5-day-old specific-pathogen-free and antibiotic-free embryonated eggs (Spafas, Inc., Norwich, Conn.). Embryos died by postinoculation day (PID) 5 and were harvested, pooled, homogenized, and diluted to 20% in sucrose phosphate glutamate solution following assessment of numbers of organisms by Gimenez staining of yolk sac smears. When the resulting 20% yolk sac stock was inoculated into Vero cell culture by serial 10-fold dilutions, 100 to 10−8 in triplicate, the 50% tissue culture infectious dose was determined to be 3.2 × 104 organisms.
To optimize the infectious dose to be used in the dog study, 12 young male Hartley strain guinea pigs were anesthetized and inoculated intraperitoneally in pairs with 0.5 ml of the yolk sac stock described above at dilutions of 100 to 10−5, representing inocula of 1.6 × 104 to <1 organism/animal. All guinea pigs receiving the three highest concentrations, which contained approximately 1.6 × 102 to 1.6 × 104 organisms, became febrile within 24 to 48 h after inoculation. Guinea pigs receiving dilutions containing 1.6 to 16 organisms became febrile by PID 4. The two guinea pigs that received the most-dilute suspension, containing an estimated <1 organism, did not become febrile at any time in the study, indicating the limiting infectious dose for this inoculum. All but one guinea pig, from the most-dilute-suspension pair, seroconverted to R. rickettsii antigens by PID 28. To ensure infectivity, but to minimize morbidity, an inoculum dose of 6.4 × 102 was selected to infect the dogs in this study.
Infection and treatment.
Sixteen 4- to 5-month-old female laboratory-raised beagles were randomly divided into four groups (n = 4 dogs/group). All four groups were inoculated with a sublethal dose (6.4 × 102) of R. rickettsii intradermally. Group I served as untreated controls. Groups II through IV received doxycycline hyclate (5 mg/kg of body weight given orally, at 12-h intervals), azithromycin (3 mg/kg orally, once daily), or trovafloxacin (5 mg/kg orally, at 12-h intervals), respectively. Antibiotic therapy was begun on PID 5, to mimic the clinical situation in which therapy would be initiated in the practice setting, and continued for 7 days for doxycycline and trovafloxacin, and for 3 days for azithromycin.
Clinicopathologic testing.
Complete physical examinations, including rectal-temperature measurements, were conducted twice daily at 12-h intervals during the project. Attitudinal changes were quantitated daily by using the following numerical scale: 5, alert, active, and eating; 4, alert, inactive, and eating; 3, depressed, inactive, and anorectic; 2, severely depressed and anorectic; 1, recumbent; 0, dead. Laboratory evaluation included complete blood counts and serum albumin/globulin ratio determinations, obtained preinoculation and every 3rd day during the study.
Vascular-permeability studies.
Fluorescein angiography (FA) of the ocular fundus was performed on PID 5 (immediately prior to the onset of treatment) and on PID 12, a time of peak retinal vascular injury as determined by a previous study (8). The number of surface lesions and sites of sodium fluorescein dye leakage from injured retinal vessels were quantitated visually and morphometrically.
Serology.
To assess the effect of antibiotic therapy on seroreactivity to R. rickettsii antigens, which could have relevance for the diagnosis of naturally occurring RMSF, serum antibody titers were determined preinoculation and every 3rd day thereafter by using a microimmunofluorescence methodology as described previously (5).
Rickettsial reisolation.
Blood, collected aseptically on PID 3, 5, 7, 10, 13, and 21 from the jugular veins of all 16 dogs, was used to inoculate Vero cell cultures as described previously (5). Whole heparinized blood diluted 100 to 10−2 in duplicate (0.1 ml/well) was overlaid on confluent monolayers in 24-well plates. After 14 days of culture, monolayers were resuspended, and slides were made from cell suspensions, stained by direct (rabbit anti-R. rickettsii fluorescein-conjugated antiserum; Centers for Disease Control and Prevention, Atlanta, Ga.) and indirect (canine anti-R. rickettsii antiserum followed by fluorescein isothiocyanate-conjugated goat anti-canine immunoglobulin G; Organon Teknika, West Chester, Pa.) immunofluorescence, and examined with a UV microscope.
DNA extraction and nested-PCR analysis.
With a commercially available QIAmp blood kit (Qiagen, Chatsworth, Calif.), DNA was extracted from 600 μl of stored EDTA–whole-blood samples that had been frozen at −70°C. Cell culture-grown R. rickettsii was used as a positive control. The sensitivity of a novel nested-PCR technique for amplification of rickettsial 16S ribosomal DNA was compared to that of isolation of R. rickettsii in tissue culture. To avoid PCR contamination, historically associated with the nested procedure, a single-tube nested-PCR technique was developed in which outer eubacterial primers were created and combined with an inner primer pair specific for the genus Rickettsia. Both reactions were performed in a single tube by incorporating annealing temperatures for each primer pair that were substantially different. Annealing at successive temperatures makes it unnecessary to open the tube during the amplification process in order to add other primers; therefore, no amplicons are released into the environment to cause potential contamination.
The specificity of this nested method was examined by attempted amplification of DNA from other rickettsial species and closely related genera that can also be transmitted by ticks or other vectors (Rickettsia canada, R. felis, R. typhi, R. rhipicephali, Bartonella vinsonii, B. henselae I [Houston], B. henselae II, B. clarridgeiae, B. quintana, Ehrlichia canis, E. chaffeensis, E. equi, E. ewingii, and E. risticii). Spotted-fever and typhus group rickettsial DNAs were detected, but Bartonella and Ehrlichia species were not. Sensitivity was determined by testing 200 μl of EDTA-blood spiked with 10-fold serial dilutions of rickettsial DNA (1 μg to 1 pg) and was found to be 100 pg.
Nested-PCR amplification was performed in a 50-μl reaction mixture containing 1 μg of DNA template; 200 μM (each) dATP, dTTP, dCTP, and dGTP; 0.2 pmol of the outer primers designated Rr-out1 (5′-GGCGTAAAGAGTGCGTAGGCGGTTTAGTAAG-3′) and Rr-out2 (5′-GGACTTAACCCAACATCTCACGACACGAG-3′); 10 pmol of the inner primers (26) designated Rr-prim3 (5′-GAAACCGAAAGAGAATCTTCCGAT-3′) and Rr-prim4 (5′-TCCTAGTGTAGAGGTGAAATTCTTA-3′); 4 mM MgCl2; and 2.5 U of Taq DNA polymerase (Promega, Madison, Wis.) in a 1× reaction buffer (50 mM KCl–10 mM Tris HCl [pH 8.3]). First-round amplification cycles included denaturation at 94°C for 1 min, annealing at 70°C for 1 min, and chain extension at 72°C for 1 min. The PCR cycle was repeated for 20 cycles. This was followed immediately by the second round of amplification, which included 40 cycles of denaturation at 94°C for 45 s, annealing at 45°C for 45 s (a temperature substantially lower than that in the first round), chain extension at 72°C for 1 min, and a final extension of 5 min at 72°C. The PCR products were electrophoresed through 1% agarose gels in Tris-boric acid-EDTA buffer, and the DNA fragments were visualized by ethidium bromide staining under UV fluorescence.
Drug measurement.
Blood was collected on PID 7 (2 days after the start of therapy) from treated dogs at 0, 1, 3, 8, and 12 h following antibiotic administration. Whole blood was centrifuged to separate plasma from blood cells. Plasma and blood cells were analyzed separately for concentrations of doxycycline, azithromycin, and trovafloxacin by high-performance liquid chromatography (HPLC) in the Clinical Pharmacology Research Laboratory at North Carolina State University. Because R. rickettsii-induced vascular permeability could influence drug distribution or the plasma drug clearance, analysis of drug concentrations during experimental infection was considered necessary to ensure accurate dosing for clinical cases.
The HPLC apparatus consisted of a Waters model 600 pump (Millipore Corp., Milford, Mass.); either a Hewlett-Packard (Palo Alto, Calif.) series 1050 Autosampler for doxycycline and trovafloxacin or a Waters model 712 WISP (Millipore Corp.) for azithromycin; and a computer for data collection and analysis (Hewlett-Packard HPLC2D ChemStation running in Windows 3.1 on a Hewlett-Packard 486/33N computer). Doxycycline and trovafloxacin were analyzed by UV detection (Hewlett-Packard series 1050 UV detector). Azithromycin was analyzed with electrochemical (EC) detection (Coulochem II; ESA Inc., Bedford, Mass.). For all analyses, the column was a Zorbax SB-C8 (4.6 mm by 15 cm), with a Zorbax RX-C18 guard column (4 mm by 1.25 cm) (both from MAC-MOD Analytical Inc., Chadds Ford, Pa.). For all analyses, the column was heated to 40°C.
HPLC conditions.
Azithromycin was eluted with a mobile phase consisting of 40% deionized water, 50% acetonitrile, and 10% methanol. To each liter of the mobile phase were added 0.3 g of ammonium acetate and 0.56 of sodium perchlorate. The pH was adjusted to 7.0 by adding a 2 M solution of acetic acid as needed after all components of the mobile phase were mixed. Doxycycline was eluted with a mobile phase consisting of 70% distilled water, 20% acetonitrile, and 10% methanol. To each liter of the mobile phase, 6.84 g of oxalic acid was added (0.1 M). Trovafloxacin was eluted with a mobile phase consisting of 81% distilled water, 19% acetonitrile, and 0.02% trifluoroacetic acid (TFA). All mobile phases were filtered and degassed prior to use.
The flow rate was 1.0 ml/min for each drug. Injection volumes were 25, 50, and 100 μl for doxycycline, trovafloxacin, and azithromycin, respectively. Doxycycline was detected at a wavelength of 350 nm, and trovafloxacin was detected at 279 nm. For azithromycin detection, the analytical electrode of the EC detector had a potential of +800 mV, the screening electrode had a potential of +700 mV, and the guard cell electrode had a potential of +850 mV. The retention times for elution were approximately 7.3 to 7.7 min for azithromycin, 3.5 to 3.7 min for trovafloxacin, 7.8 min for doxycycline in plasma, and 6.8 min for doxycycline in blood cells.
Preparation of calibration curve.
Pure reference standards of each drug were used to prepare stock solutions of 1 mg/ml. Azithromycin was dissolved in a 50:50 solution of deionized water and acetonitrile, doxycycline was dissolved in distilled water, and trovafloxacin was dissolved in a solution of 0.1% TFA. Stock solutions were kept refrigerated in tightly sealed vials until they were further diluted to obtain a series of spiking solutions. Spiking solutions for azithromycin and trovafloxacin were prepared in concentrations that allowed 10 μl to be added to 990 μl of blank (unfortified) plasma in order to obtain a range of seven calibration samples for each drug. For doxycycline, spiking solutions were prepared so that 10 μl was added to 90 μl of blank plasma to obtain a range of seven calibration samples. Blank plasma also was analyzed with each calibration curve. A new calibration curve was prepared for each day’s samples. We did not accept the calibration curve unless it was linear with an r2 value of at least 0.99. The lowest limit of quantitation (LOQ) was defined as the lowest concentration of the calibration samples that could be determined with an accuracy within 15% of the true value.
Preparation of plasma samples.
For azithromycin, plasma and extracts from blood cells were processed by using a solid-phase extraction cartridge (Waters Oasis HLB Extraction Cartridges; Millipore Corp.). The cartridges were conditioned with 1 ml of methanol followed by 1 ml of deionized water. The conditioning was followed by the addition of 500 μl of plasma, and the column was washed with 1 ml of a water-methanol solution (95:5). The volume after each of these steps was drawn through the cartridge with a vacuum manifold. Each of these eluates was discarded. Azithromycin was then eluted by drawing 1 ml of methanol through the cartridge. The eluate was collected in a clean tube and evaporated to dryness under a flow of nitrogen (20 lb/in2) at 45°C for 15 min. The residue in the tube was reconstituted with 200 μl of the mobile phase and transferred to an injection vial.
For trovafloxacin, plasma and extracts from blood cells were prepared by pipetting 300 μl of the sample into a clean glass tube. To this tube 5 ml of acetonitrile was added, and the mixture was centrifuged at 2,000 × g for 10 min at room temperature. The supernatant was transferred to another clean glass tube, and the contents were evaporated under a flow of nitrogen (20 lb/in2) at 40°C for 30 min. The residue in each tube was reconstituted with 200 μl of a mixture of methanol–0.1% TFA (15:85) and transferred to an HPLC injection vial.
For doxycycline, plasma and extracts from blood cells were prepared by mixing 100 μl of the sample with an equivalent volume of a protein releasing agent consisting of 20% acetonitrile, 2% phosphoric acid, and 78% distilled water. This admixture was vortexed for 10 s, pipetted into a 10,000-molecular-weight cutoff microcentrifuge tube (Ultrafree-MC; 10,000-nominal-molecular-weight-limit [NMWL] centrifugal filter unit; Millipore Corp.), and centrifuged for 30 min at 14,000 × g. The clear filtrate was transferred to an HPLC injection vial.
Preparation of blood cells.
Blood cells collected at each sampling point were analyzed for each of the three drugs. One milliliter of blood cells was resuspended with 1 ml of distilled water. The cells were lysed by sonication, and the tubes were centrifuged for 10 min at 5,000 × g. The clear supernatant was collected and processed in a manner similar to that for the plasma samples for each drug.
Pharmacokinetics.
The terminal points that formed a straight line on a semilogarithmic plot of drug concentration versus time were used to calculate an elimination rate constant (kel) using least-squares regression. The half-life of each drug (in hours) during the terminal phase of the curve was calculated as T1/2 = 0.693/kel, where 0.693 is derived from the natural logarithm of 1/2.
Statistics.
Rectal temperatures, attitudinal scores, neutrophil, and platelet counts, serum albumin/globulin ratio, and reciprocal titers were statistically compared among the four groups by analysis of variance (ANOVA). Treatment effects were analyzed by a multivariate repeated-measures ANOVA (13) using PROC GLM software (version 6.09) from SAS Inc. (SAS Institute, Cary, N.C.). A separate analysis was performed for each physiological parameter. A Student-Newman-Keuls multiple-comparisons procedure at an alpha level of 0.05 was carried out at each time point to differentiate significant effects among the groups. The analysis was done separately for times prior to treatment and times after treatments. Profile contrasts were used to detect significant differences in the shapes of the parameter response curves among the treatment groups. The nonparametric Kruskal-Wallis test was performed on attitudinal scores due to the nonnormality of the data. PROC NPAR1WAY software (version 6.09) from SAS Inc. was used.
RESULTS
Infection and treatment.
All dogs were febrile and positive for circulating rickettsiae in blood as detected by tissue culture and/or PCR by PID 5, at which time treatments were begun for the three treatment groups (Table 1). Treatment was administered orally for 3 days (azithromycin) or 7 days (doxycycline and trovafloxacin) as described in Materials and Methods.
TABLE 1.
Tissue culture and PCR results from dogs experimentally infected with R. rickettsiia
| Group | No. positive by tissue culture/no. positive by PCR on PID:
|
||||||
|---|---|---|---|---|---|---|---|
| 0 | 3 | 5 | 7 | 10 | 13 | 21 | |
| I (infection control) | 0/0 | 0/2 | 3/4 | 3/4 | 3/4 | 1/2 | 0/2 |
| II (doxycycline) | 0/0 | 0/2 | 0/4 | 1/1 | 2/4 | 0/1 | 0/2 |
| III (azithromycin) | 0/0 | 0/4 | 2/4 | 1/3 | 2/4 | 2/2 | 0/2 |
| IV (trovafloxacin) | 0/0 | 0/2 | 2/4 | 1/0 | 2/2 | 2/2 | 0/3 |
Each group consisted of four dogs. On PID 117, blood samples available from six dogs (one each from groups I and II and two each from groups III and IV) were PCR negative. Between days 0 and 13, there was concordance between tissue culture and PCR results for 55 of 96 samples. Of the discordant samples, 35 were PCR positive and tissue culture negative, and six were PCR negative and tissue culture positive.
Clinicopathologic findings.
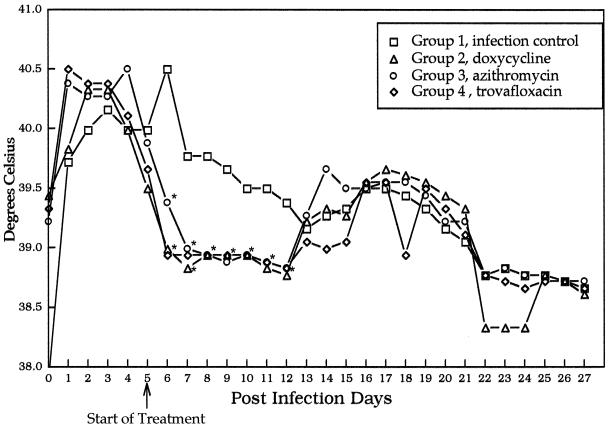
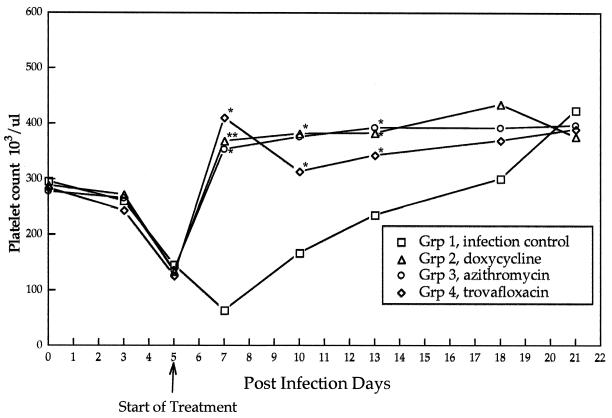
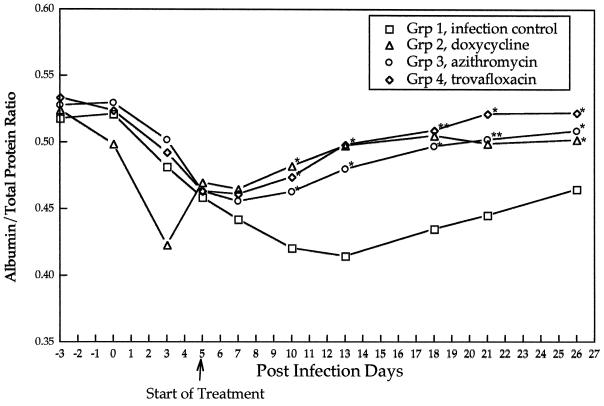
Prior to the initiation of treatment (PID 5), there were no statistically significant differences (P ≤ 0.05) in mean values for rectal temperature (P = 0.46), attitudinal score (P = 1.42), neutrophil count (P = 0.69), platelet number (P = 0.54), or the concentration of serum albumin as a percentage of total protein (P = 0.44) among the four groups of dogs. In the infection control group (group I), changes in attitudinal scores, mean daily rectal temperatures (Fig. 1), and platelet counts (Fig. 2) identified abnormalities indicative of R. rickettsii infection from PID 3 through PID 13, with a gradual return to preinoculation values through PID 27. Compared to the infection control group, treatment groups experienced a statistically significant improvement in attitudinal scores (P = 0.0003) by 48 h after the initiation of treatments. Differences persisted through PID 17. Following the initiation of antimicrobial therapy, rectal temperatures normalized after 12 h in groups II and IV and after 48 h in group III (Fig. 1). Throughout the treatment period, treatment groups differed significantly in mean temperatures from the infection control group. Platelet counts normalized in all three treatment groups within 48 h following initiation of antibiotics therapy, with no significant differences among treatment groups. After PID 7, the albumin/total protein ratio (Fig. 3) reflected attenuation of vascular leakage in all three treatment groups compared to the infection control group (P < 0.05). However, when animals were examined on PID 12, retinal vascular lesions (Table 2) were observed in all group I (4 of 4) and group III (4 of 4) dogs but were rarely visualized in group II (1 of 4) or group IV (0 of 4) dogs. The infection control group and all three treatment groups had elevated mean rectal temperatures again by PID 13 (groups II and III) or 16 (group IV), which persisted through PID 19 to 21.
FIG. 1.
Average daily rectal temperatures after inoculation of R. rickettsii (day 0) and after initiation of antibiotic treatments (day 5). Data points reflect 24-h intervals. Asterisks indicate points at which treatment groups differ significantly from the infection control group.
FIG. 2.
Mean platelet counts of the infection control group and treatment groups after inoculation of R. rickettsii (day 0) and after initiation of antibiotic treatments (day 5). Asterisks indicate points at which treatment groups differ significantly from the infection control group.
FIG. 3.
Mean ratios of albumin to total protein for the infection control group and the treatment groups after inoculation with R. rickettsii (day 0) and after initiation of antibiotic treatments (day 5). Asterisks indicate points at which treatment groups differ significantly from the infection control group.
TABLE 2.
Retinal vascular lesions as detected by direct ophthalmoscopic examination or FA in dogs infected with R. rickettsii
| Groupa | Avg. no. of lesions/dog detected on:
|
|||
|---|---|---|---|---|
| PID 5
|
PID 12
|
|||
| By direct ophthalmoscopy | By FA | By direct ophthalmoscopy | By FA | |
| I (infection control) | 0.5 | 0 | 5.75 | 2.75 |
| II (doxycycline) | 0.25 | 0 | 0.75 | 0 |
| III (azithromycin) | 0.25 | 0.25 | 1.25 | 2.5 |
| IV (trovafloxacin) | 0 | 0 | 0 | 0 |
n = 4 dogs/group.
Drug concentrations.
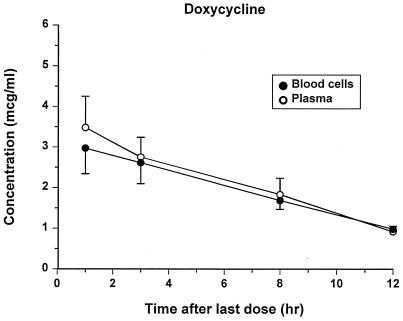
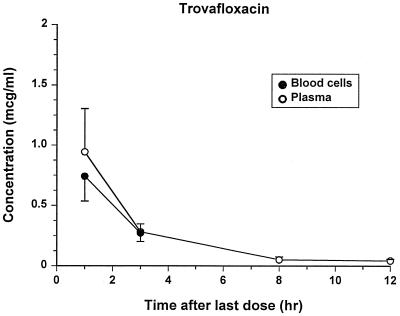
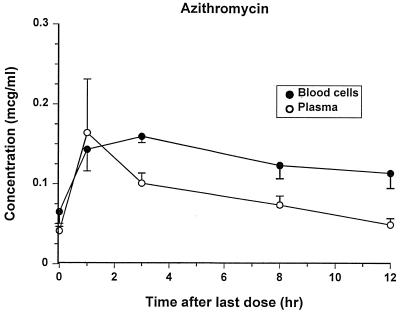
The assays for all drugs were specific and reliable. The lowest LOQs were 0.25, 0.03, and 0.05 μg/ml for doxycycline, azithromycin, and trovafloxacin, respectively. Concentrations of each antibiotic in plasma and blood after administration of the last oral dose are shown in Fig. 4 to 6. There was not an appreciable accumulation of any drug in blood cells, except after 3 h, when azithromycin concentrations were higher in the blood cells than in the plasma. Trovafloxacin was not detectable in blood cells after the 3-h sampling point. Extensive pharmacokinetic analysis was not performed for these drugs because data were collected at so few points, and the bioavailability of the drugs could not be assessed without an accompanying intravenous administration study. Estimates of the mean terminal half-lives were 5.92, 7.24, and 2.19 h for doxycycline, azithromycin, and trovafloxacin, respectively.
FIG. 4.
Concentrations of doxycycline (means ± standard errors) in plasma and blood cells after the last treatment.
FIG. 6.
Concentrations of trovafloxacin (means ± standard errors) in plasma and blood cells after the last treatment. (No trovafloxacin was detectable in blood cells at the 8- and 12-h sampling times.)
Vascular permeability studies.
Early in the course of infection (PID 5), there was minimal evidence of retinal vascular lesions as determined by direct ophthalmologic examination or by FA (Table 2). When animals were reexamined on PID 12, there was a reduction in retinal vascular lesions and vascular permeability in groups II, III, and IV; however, the number of retinal vascular lesions detected by FA in group III did not differ from that in the infection control group.
Serology.
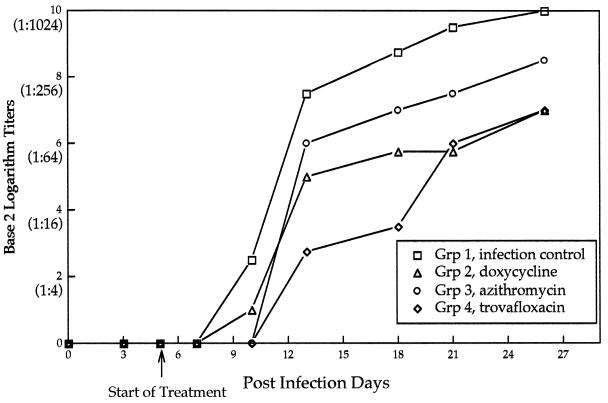
R. rickettsii-specific antibodies were detected in 15 of 16 dogs by PID 13. In group IV, seroconversion was delayed in dog 190 until PID 18, and the peak reciprocal titer for this dog was only 32. In general, the R. rickettsii antibody response was suppressed (reciprocal titers, <512) in groups II and IV as late as PID 26 (Fig. 7). The highest reciprocal antibody titers were detected in the untreated controls (≥512 by PID 18).
FIG. 7.
Mean microimmunofluorescence antibody titers for the infection control group and the antibiotic treatment groups.
Rickettsial reisolation.
Rickettsemia (Table 1) was not detected on PID 3, but Vero cell cultures of blood collected from 7 of 16 dogs on PID 5, before the beginning of treatment, were positive. Rickettsemia persisted in group II from PID 5 through PID 10 and in groups III and IV through PID 13. In one dog in group II (dog 188) and one in group IV (dog 183), it remained below levels detectable by tissue culture isolation throughout the study period. Both of these dogs developed the anticipated clinicopathologic abnormalities (fever and thrombocytopenia), seroconverted to R. rickettsii antigens, and were PCR positive at at least one data point.
PCR.
When DNAs from other rickettsial species or from organisms in closely related genera were amplified with the nested-PCR method described in this study to test for specificity, amplicons were obtained for spotted-fever and typhus group rickettsiae but not with Bartonella or Ehrlichia DNA. The sensitivity level was 100 pg of DNA.
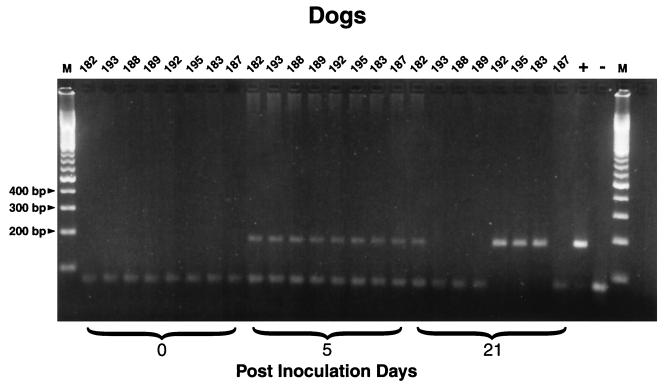
Rickettsial DNA was amplified from 21 of 24 EDTA-blood samples from untreated group I dogs, obtained between PID 3 and PID 21 (Table 1). With the exception of group IV dogs on PID 7, rickettsial DNA was amplified in all treatment groups from PID 3 through PID 21 (Table 1; Fig. 8). PCR was substantially more sensitive than tissue culture isolation during the period of expected rickettsemia (days 3 to 12). The enhanced sensitivity of PCR is best illustrated on PID 3, when 10 of 16 dogs were PCR positive but R. rickettsii was not isolated from any dog. In addition, the two dogs (dogs 183 and 188) from which rickettsial organisms were not isolated in tissue culture had detectable quantities of rickettsial DNA on more than one occasion. On PID 21, PCR amplicons were obtained from 14 of 16 dogs. The persistence of DNA through the last blood collection date of this study (PID 21) was unexpected. As six dogs were still available following the analysis phase of the study (PID 117), EDTA-blood samples were obtained for PCR analysis. Amplicons were not obtained, presumably reflecting clearance of rickettsial DNA.
FIG. 8.
PCR amplicons obtained with Rickettsia genus primers from EDTA-blood samples from two dogs in each study group on PIDs 0, 5, and 21. Dogs 182 and 193, group I (untreated infection controls), dogs 188 and 189, group II (doxycycline treated); dogs 192 and 195, group III (azithromycin treated); dogs 183 and 187, group IV (trovafloxacin treated); +, positive control (tissue culture-grown R. rickettsii); −, negative control (water).
DISCUSSION
The rapid therapeutic response observed with doxycycline or trovafloxacin in this study was similar to the response observed in two previous studies from our laboratory in which enrofloxacin, chloramphenicol, tetracycline hydrochloride, or doxycycline was administered (5, 6). Defervescence was followed by rapid improvement in attitudinal scores and hematologic abnormalities in all three treatment groups. Defervescence occurred within 12 h after the initiation of treatment with doxycycline or trovafloxacin. Based upon tissue culture isolation, doxycycline resulted in the earliest apparent clearance of viable circulating rickettsial organisms. Most importantly, prevention or resolution of vascular leakage, as evidenced by direct visualization of ocular lesions or following FA, was accomplished with equal efficacy by doxycycline or trovafloxacin. This observation is of potential clinical relevance, insomuch as vascular injury is of central importance to the pathogenesis of RMSF. Rapid attenuation of vascular injury should reduce morbidity or mortality associated with R. rickettsii infection and should decrease the incidence of posttreatment sequelae.
Defervescence was delayed in the azithromycin group. Although attitudinal scores, platelet counts, and the albumin/total protein ratio reflected improvement following azithromycin treatment, the degree of retinal vascular injury at PID 12, as detected by FA, was similar to that in the untreated infection control group. The short duration of azithromycin administration was used in this study because of the prolonged duration of intracellular concentrations reported after oral administration (19, 23). Plasma drug concentrations measured for azithromycin administration at the dosage used in this study (3 mg/kg every 24 h) have not been reported previously. Concentrations in plasma were lower than those reported for previous studies in which higher doses had been administered to dogs (0.27 μg/ml after one dose and 0.35 μg/ml after five doses of 10 mg/kg; and 4.2 μg/ml after one dose of 20 mg/kg) (23). In the study reported here, peak concentrations in plasma 1 h after dosing were 0.16 ± 0.07 μg/ml. MICs of azithromycin are 0.05 μg/ml for sensitive bacteria such as streptococci, but 2 μg/ml for staphylococci (19). A higher azithromycin dose or longer duration of administration may have resulted in an improved therapeutic response to this antibiotic. Because other doses were not tested, we could not assess if the low concentrations achieved in this study were responsible for the poor efficacy of azithromycin. The half-life in dogs was reported to be 4.2 h during the first 4 h after a dose, but longer (35 h) 24 to 72 h after the dose (23). The half-life of 7.2 h reported in this study probably represents the early-phase kinetics rather than the prolonged elimination phase.
Previous efforts to diagnose RMSF in human patients using PCR amplification from blood have been minimally successful, presumably due to the low numbers of circulating rickettsial organisms (22, 25). Drancourt and colleagues (9) used immunomagnetic beads to select circulating endothelial cells infected with Rickettsia conorii from blood samples in order to enhance the sensitivity of detection of organisms by direct staining of cells or to perform tissue culture isolation of spotted-fever group rickettsiae. Since no diagnostic modalities that enable a rapid acute-phase diagnosis of this potentially fatal disease are currently available, a highly sensitive PCR amplification procedure would be of substantial benefit for patient management. In this study, to facilitate detection of low levels of rickettsiae in EDTA-blood samples, PCR amplification using a single-tube nested system with two different annealing temperatures to prime the first- and the second-stage reactions was performed. This approach resulted in an increase in sensitivity without substantially increasing the risk of PCR contamination and represents an improvement over earlier attempts to develop PCR as an acute-phase diagnostic modality for RMSF (25, 26). The specificity of this method appears inclusive of known rickettsiae and exclusive of the closely related genera Bartonella and Ehrlichia. As there are few precedents for the persistence of spotted-fever group rickettsiae in dogs or humans following acute infection, detection of rickettsial DNA in 14 of 16 blood samples collected from dogs as late as PID 21 was unexpected but could be explained by residual DNA from nonviable organisms (rickettsemia was last detected by tissue culture on PID 13) or delayed immunotherapeutic clearance of rickettsiae that were too low in numbers to be detected by tissue culture isolation. As would be expected from previous R. rickettsii isolation and challenge studies, rickettsial DNA was not detected in blood samples obtained on PID 117. In previous experimental-infection studies, R. rickettsii was not isolated after 14 days of infection and humoral antibody titers to the organism decreased gradually over time, but immunity to subsequent infection was long-lived, at least 3 years in experimentally infected dogs (4). As described in Table 1, PCR was substantially more sensitive than tissue culture isolation during the early and convalescent stages of infection in these dogs.
Compared to previous studies from our laboratory (5, 6), all four groups experienced an unprecedented relapse of fever. In the three treatment groups, rectal temperatures were suppressed during the period of antibiotic administration. Three to 6 days after administration of the last doses, rectal temperatures again became elevated in all three treatment groups, accompanied by a slight drop in attitudinal scores (not statistically significant) in trovafloxacin- and azithromycin-treated groups. Elevated temperatures persisted until PID 20. The relapse in rectal temperatures may have been related to nonrickettsial factors, such as concurrent infection with an unrecognized pathogen or a delayed-type hypersensitivity reaction to the yolk sac inoculum; however, there were no cytopathic effects, unusual inclusions, or other pathogenic organisms visualized in tissue culture, and previous studies using yolk sac inoculum have not induced a hypersensitivity reaction. In contrast to previous studies from our laboratory, passage through embryonated eggs may have enhanced the virulence of the R. rickettsii strain (Domino) used in this study. The presence of viable rickettsial organisms in the blood of 5 of 16 dogs on PID 13 may indicate that any attempt to shorten treatment regimens, although it might be desirable for patient management, cannot be recommended.
Seroreactivity to R. rickettsii antigen was suppressed by all three treatment regimens, as has been noted in previous experimental-infection studies when early treatment intervention is used. Consistent with other parameters indicating enhanced therapeutic efficacy, doxycycline and trovafloxacin suppressed R. rickettsii antibody production to a greater degree than azithromycin.
Plasma drug concentrations of doxycycline in this study were considerably lower than those in a previous study in dogs inoculated with R. rickettsii, in which we reported peak concentrations of 17.14 ± 0.85 μg/ml after approximately 5 mg/kg was given orally every 12 h for 6 days (6). In this study, we report peak concentrations of 3.48 ± 0.77 μg/ml 1 h after the oral dose, and concentrations are maintained to almost 1.0 μg/ml for the 12-h dosing interval. In another study, the peak concentrations in the plasma of dogs following tetracycline hydrochloride administration were similar to values reported here (5). As discussed in our previous paper, doxycycline half-lives and peak concentrations have varied considerably among other reported studies (6). The peak and trough concentrations in these dogs are similar to those reported for humans after administration of a therapeutic dose (14). Doxycycline concentrations in blood cells were approximately equal to concentrations in plasma, which suggests free diffusion of doxycycline into blood cells.
Azithromycin concentrations in blood cells have not been reported previously for dogs. Azithromycin accumulates extensively in leukocytes and attains concentrations many times higher than in plasma (19). In this study, azithromycin concentrations were measured in cells after centrifugation of whole blood; therefore, due to the relative proportions of erythrocytes versus leukocytes, the concentrations presented here represent primarily the concentrations in erythrocytes. Although azithromycin appears to diffuse freely into canine erythrocytes, there is no accumulation such as occurs in other cell types, such as macrophages and neutrophils.
Concentrations of trovafloxacin in plasma after 5 mg/kg was given orally every 12 h had a peak of 1.8 μg/ml 1 h after the oral dose and a terminal half-life of 2.2 h. Trovafloxacin pharmacokinetics have not previously been reported for dogs. In this study, trovafloxacin was eliminated faster and attained lower peak concentrations than other fluoroquinolones used in dogs. Trovafloxacin concentrations in blood cells were similar to those in plasma for the first 3 h but were undetectable in later samples. Other fluoroquinolones are known to accumulate in leukocytes, but concentrations in leukocytes have not been compared to concentrations in erythrocytes.
As administered in this study, trovafloxacin and doxycycline were equally efficacious for the treatment of experimental RMSF in dogs. On a comparative basis, azithromycin was not as efficacious and cannot be recommended as a first-line antibiotic for treatment of RMSF without additional study. On the basis of this study and a previous study demonstrating the efficacy of enrofloxacin for the treatment of experimental canine RMSF, fluoroquinolones such as trovafloxacin may represent an alternative to tetracycline derivatives for the treatment of RMSF and other spotted-fever group rickettsioses.
FIG. 5.
Concentrations of azithromycin (means ± standard errors) in plasma and blood cells after the last treatment.
ACKNOWLEDGMENTS
This work was supported by a grant from the U.S. Pharmaceuticals Group, Pfizer Inc.
We thank David Wilson for statistical analysis of the data, Julie Bradley for technical assistance, Delta Plummer for assistance in drug analysis, Dale Brown for careful monitoring of the dogs, and the Laboratory Animal Resources staff for the care of these dogs.
REFERENCES
- 1.Archibald L K, Sexton D J. Long-term sequelae of Rocky Mountain spotted fever. Clin Infect Dis. 1995;20:1122–1125. doi: 10.1093/clinids/20.5.1122. [DOI] [PubMed] [Google Scholar]
- 2.Bergeron J W, Braddom R L, Kaelin D L. Persisting impairment following Rocky Mountain spotted fever: a case report. Arch Phys Med Rehabil. 1997;78:1277–1280. doi: 10.1016/s0003-9993(97)90345-2. [DOI] [PubMed] [Google Scholar]
- 3.Breitschwerdt E B, Meuten D J, Walker D H, Levy M G, Kennedy K, King M, Curtis B C. Canine Rocky Mountain spotted fever: a kennel epizootic. Am J Vet Res. 1985;46:2124–2128. [PubMed] [Google Scholar]
- 4.Breitschwerdt E B, Levy M G, Davidson M G, Walker D H, Burgdorfer W, Curtis B C, Babineau C A. Kinetics of IgM and IgG responses to experimental and naturally acquired Rickettsia rickettsii infection in dogs. Am J Vet Res. 1990;51:1312–1316. [PubMed] [Google Scholar]
- 5.Breitschwerdt E B, Davidson M G, Aucoin D P, Levy M G, Szabados N S, Hegarty B C, Kuehne A L, James R L. Efficacy of chloramphenicol, enrofloxacin, and tetracycline for treatment of experimental Rocky Mountain spotted fever in dogs. Antimicrob Agents Chemother. 1991;35:2375–2381. doi: 10.1128/aac.35.11.2375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Breitschwerdt E B, Davidson M G, Hegarty B C, Papich M G, Grindem C B. Prednisolone at anti-inflammatory or immunosuppressive dosages in conjunction with doxycycline does not potentiate the severity of Rickettsia rickettsii infection in dogs. Antimicrob Agents Chemother. 1997;41:141–147. doi: 10.1128/aac.41.1.141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dalton M J, Clarke M J, Holman R C, Krebs J W, Fishbein D B, Olsen J G, Childs J E. National surveillance for Rocky Mountain spotted fever, 1981–1992: epidemiologic summary and evaluation of risk factors for fatal outcome. Am J Trop Med Hyg. 1995;52:405–413. doi: 10.4269/ajtmh.1995.52.405. [DOI] [PubMed] [Google Scholar]
- 8.Davidson M G, Breitschwerdt E B, Walker D H, Levy M G, Carlson C S, Hardie E M, Grindem C A, Nasisse M P. Vascular permeability and coagulation during Rickettsia rickettsii infection in dogs. Am J Vet Res. 1990;51:165–170. [PubMed] [Google Scholar]
- 9.Drancourt M, George F, Brouqui P, Sampol J, Raoult D. Diagnosis of Mediterranean spotted fever by indirect immunofluorescence of Rickettsia conorii in circulating endothelial cells isolated by monoclonal coated immunomagnetic beads. J Infect Dis. 1992;166:660–663. doi: 10.1093/infdis/166.3.660. [DOI] [PubMed] [Google Scholar]
- 10.Dunn C J, Barradell L B. Azithromycin. A review of its pharmacological properties and use as 3-day therapy in respiratory tract infections. Drugs. 1996;51:483–505. doi: 10.2165/00003495-199651030-00013. [DOI] [PubMed] [Google Scholar]
- 11.Felmingham D, Robbins M J, Ingley K, Mathias I, Bhogal H, Leakey A, Ridgway G L, Gruneberg R N. In-vitro activity of trovafloxacin, a new fluoroquinolone, against recent clinical isolates. J Antimicrob Chemother. 1997;39(Suppl. B):43–49. doi: 10.1093/jac/39.suppl_2.43. [DOI] [PubMed] [Google Scholar]
- 12.Greene C E, Burgdorfer W, Cavagnolo R, Philip R N, Peacock M G. Rocky Mountain spotted fever in dogs and its differentiation from canine ehrlichiosis. J Am Vet Med Assoc. 1985;186:465–472. [PubMed] [Google Scholar]
- 13.Johnson T A, Wichern D W. Applied multivariate statistical analysis. 2nd ed. Englewood Cliffs, N.J: Prentice-Hall, Inc.; 1988. p. 607. [Google Scholar]
- 14.Kapusnik-Uner J E, Sande M A, Chambers H F. Tetracyclines, chloramphenicol, erythromycin, and miscellaneous antibacterial agents. In: Hardman J G, Limbird L E, Molinoff P B, Ruddon R W, Gilman A G, editors. Goodman and Gilman’s The pharmacological basis of therapeutics. 9th ed. New York, N.Y: McGraw-Hill; 1996. [Google Scholar]
- 15.Kenyon R H, Acree W M, Wright G G, Melchior F W., Jr Preparation of vaccines for Rocky Mountain spotted fever from rickettsiae propagated in cell culture. J Infect Dis. 1972;125:146–152. doi: 10.1093/infdis/125.2.146. [DOI] [PubMed] [Google Scholar]
- 16.Kirkland K B, Marcom P K, Sexton D J, Dumler J S, Walker D H. Rocky Mountain spotted fever complicated by gangrene: report of six cases and review. Clin Infect Dis. 1993;16:629–634. doi: 10.1093/clind/16.5.629. [DOI] [PubMed] [Google Scholar]
- 17.Kirkland K B, Wilkinson W E, Sexton D J. Therapeutic delay and mortality in cases of Rocky Mountain spotted fever. Clin Infect Dis. 1995;20:1118–1121. doi: 10.1093/clinids/20.5.1118. [DOI] [PubMed] [Google Scholar]
- 18.Lissman B A, Benach J L. Rocky Mountain spotted fever in dogs. J Am Vet Med Assoc. 1980;176:994–995. [PubMed] [Google Scholar]
- 19.Lode H, Borner K, Koeppe P, Schaberg T. Azithromycin—review of key chemical pharmacokinetic and microbiologic features. J Antimicrob Chemother. 1996;37(Suppl. C):1–8. doi: 10.1093/jac/37.suppl_c.1. [DOI] [PubMed] [Google Scholar]
- 20.McKoy G K, editor. Drug information. 1998. p. 242. , 404–406. American Society of Health-System Pharmacists, Inc. Bethesda, Md. [Google Scholar]
- 21.Rutgers C, Kowalski J, Cole C R, Scherding R G, Chew D J, Davenport D, O’Grady M, Murtaugh R J. Severe Rocky Mountain spotted fever in five dogs. J Am Anim Hosp Assoc. 1985;21:361–369. [Google Scholar]
- 22.Sexton D J, Kanj S S, Wilson K H, Corey G R, Hegarty B C, Levy M G, Breitschwerdt E B. The use of a polymerase chain reaction as a diagnostic test for Rocky Mountain spotted fever. Am J Trop Med Hyg. 1994;50:59–63. [PubMed] [Google Scholar]
- 23.Shepard R M, Faulkner F C. Pharmacokinetics of azithromycin in rats and dogs. J Antimicrob Chemother. 1990;25(Suppl. A):49–60. doi: 10.1093/jac/25.suppl_a.49. [DOI] [PubMed] [Google Scholar]
- 24.Treadway G, Pontani D. Paediatric safety of azithromycin: worldwide experience. J Antimicrob Chemother. 1996;37(Suppl. C):143–149. doi: 10.1093/jac/37.suppl_c.143. [DOI] [PubMed] [Google Scholar]
- 25.Tzianabos T, Anderson B E, McDade J E. Detection of Rickettsia rickettsii DNA in clinical specimens using polymerase chain reaction technology. J Clin Microbiol. 1989;27:2866–2868. doi: 10.1128/jcm.27.12.2866-2868.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wilson K H, Blitchington R, Shah P, McDonald G, Gilmore R D, Mallavia L P. Probe directed at a segment of Rickettsia rickettsii rRNA amplified with polymerase chain reaction. J Clin Microbiol. 1989;27:2692–2696. doi: 10.1128/jcm.27.12.2692-2696.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]