Abstract
The objective of this analysis was to determine if there are sex differences with esketamine for treatment-resistant depression (TRD). Post hoc analyses of three randomized, controlled studies of esketamine in patients with TRD (TRANSFORM-1, TRANSFORM-2 [18–64 years], TRANSFORM-3 [≥ 65 years]) were performed. In each 4-week study, adults with TRD were randomized to esketamine or placebo nasal spray, each with a newly initiated oral antidepressant. Change from baseline to day 28 in Montgomery-Åsberg Depression Rating Scale (MADRS) total score was assessed by sex in pooled data from TRANSFORM-1/TRANSFORM-2 and separately in data from TRANSFORM-3 using a mixed-effects model for repeated measures. Use of hormonal therapy was assessed in all women, and menopausal status was assessed in women in TRANSFORM-1/TRANSFORM-2. Altogether, 702 adults (464 women) received ≥ 1 dose of intranasal study drug and antidepressant. Mean MADRS total score (SD) decreased from baseline to day 28, more so among patients treated with esketamine/antidepressant vs. antidepressant/placebo in both women and men: TRANSFORM-1/TRANSFORM-2 women—esketamine/antidepressant -20.3 (13.19) vs. antidepressant/placebo -15.8 (14.67), men—esketamine/antidepressant -18.3 (14.08) vs. antidepressant/placebo -16.0 (14.30); TRANSFORM-3 women—esketamine/antidepressant -9.9 (13.34) vs. antidepressant/placebo -6.9 (9.65), men—esketamine/antidepressant -10.3 (11.96) vs. antidepressant/placebo -5.5 (7.64). There was no significant sex effect or treatment-by-sex interaction (p > 0.35). The most common adverse events in esketamine-treated patients were nausea, dissociation, dizziness, and vertigo, each reported at a rate higher in women than men. The analyses support antidepressant efficacy and overall safety of esketamine nasal spray are similar between women and men with TRD. The TRANSFORM studies are registered at clinicaltrials.gov (identifiers: NCT02417064 (first posted 15 April 2015; last updated 4 May 2020), NCT02418585 (first posted 16 April 2015; last updated 2 June 2020), and NCT02422186 (first posted 21 April 2015; last updated 29 September 2021)).
Keywords: Esketamine, s-Ketamine, Treatment-resistant depression, Sex
Introduction
Women compared to men exhibit a twofold higher risk of major depressive disorder (MDD) (Hasin et al. 2005), and differences in clinical presentation and comorbidities. In the Sequenced Treatment Alternatives to Relieve Depression (STAR*D) study of outpatients with nonpsychotic MDD, women had approximately 2 years earlier onset of their first major episode, greater severity of MDD, and approximately 5.6 months longer duration of depressive episodes, compared to the men (Marcus et al. 2005). Women were more likely to experience concurrent symptoms consistent with generalized anxiety disorder, somatoform disorder, bulimia, and atypical depression, and less likely to have concurrent obsessive compulsive, alcohol abuse, and drug abuse disorders (Marcus et al. 2005).
Likewise, response to antidepressants can vary between women and men, including differences in time to response, response rate, and adverse effects (Kornstein et al. 2000; Khan et al. 2005; Berlanga and Flores-Ramos 2006; Young et al. 2009; Sramek et al. 2016). Various factors have been suggested to explain disparity in antidepressant response between women and men, including differences in neuronal circuitry, hormone levels, and drug metabolism (Bigos et al. 2009; Dalla et al. 2010; Fernández-Guasti et al. 2012). For example, menopausal status and sex hormone therapy impacted treatment responses among women receiving selective serotonin reuptake inhibitor (SSRI) or a serotonin and norepinephrine reuptake inhibitor (SNRI) therapy in some studies (Kornstein et al. 2000; Grigoriadis et al. 2003; Thase et al. 2005; Pinto-Meza et al. 2006), but not in others (Quitkin et al. 2002; Cassano et al. 2005; Kornstein et al. 2013, 2014). A more recent study found no difference in efficacy for women compared to men with intravenous ketamine given as an acute treatment in patients with treatment-resistant depression (TRD) (Freeman et al. 2019). These mixed findings underscore the value of investigating if there are sex differences in response to newly approved antidepressants.
Esketamine (the S-enantiomer of ketamine racemate), a first-in-class glutamatergic N-methyl-D-aspartate (NMDA) receptor antagonist, has been approved by the US Food and Drug Administration, the European Medicines Agency, and multiple other health authorities for TRD, in conjunction with an oral antidepressant (Spravato Prescribing Information 2020; Spravato Summary of Product Characteristics 2020). The approval of esketamine nasal spray was based, in large part, on efficacy and safety findings from phase 2 and phase 3 studies in patients with TRD (Daly et al. 2018, 2019; Popova et al. 2019; Fedgchin et al. 2019; Wajs et al. 2020; Ochs-Ross et al. 2020). The database from these clinical development trials forms the basis for additional investigations that may facilitate clinical management of patients treated with esketamine nasal spray in real world clinical practice.
A post hoc analysis of data from three short-term phase 3 studies (Popova et al. 2019; Fedgchin et al. 2019; Ochs-Ross et al. 2020) was conducted to assess the effect of sex on the efficacy and safety of esketamine nasal spray in patients with TRD. The primary aims of these analyses are to determine whether there are differences between women and men based on improvement of depressive symptoms, comorbid anxiety, and response and remission rates with esketamine. Secondarily, among women, the aims were to determine if the aforementioned outcomes are affected by menopausal status or by use of hormonal therapy and if there are clinical factors that differentiated responders from non-responders, supporting data-informed decision-making for women.
Materials and methods
The methods of the TRANSFORM studies are published elsewhere (Popova et al. 2019; Fedgchin et al. 2019; Ochs-Ross et al. 2020). Study methods salient to the work reported here are summarized below.
Ethical practices
Institutional review boards/ethics committees approved the study protocols and their amendments, written consent was obtained from all patients before study participation, and the studies are registered at clinicaltrials.gov (identifiers: NCT02417064, NCT02418585, and NCT02422186).
Study design
The TRANSFORM trials were phase 3, double-blind, active-controlled, multicenter studies of esketamine nasal spray in patients with TRD. The trials comprised three phases: (1) 4-week screening/prospective observational phase assessing treatment response to the current antidepressant(s), (2) 4-week double-blind treatment phase with esketamine or placebo nasal spray combined with a newly initiated oral antidepressant, and (3) post-treatment follow-up phase assessing safety (TRANSFORM-1 and TRANSFORM-2: up to 24 weeks; TRANSFORM-3: 2 weeks). The studies were conducted between August 2015 and February 2018.
Patients
The studies enrolled outpatients (TRANSFORM-1 and TRANSFORM-2: aged 18–64 years; TRANSFORM-3: ≥ 65 years) with recurrent, moderate-to-severe MDD (DSM-5 diagnosis [APA 2013] without psychotic features, confirmed by the Mini International Neuropsychiatric Inventory (MINI) [Sheehan et al. 1998]). At randomization, participants had TRD, defined as non-response to two or more oral antidepressants, taken at an adequate dosage and for an adequate duration, during the current episode.
Key exclusion criteria included suicidal ideation with intent to act within the prior 6 months or suicidal behavior within the prior year; diagnosis of psychotic or bipolar disorders; recent history (within prior 6 months) of moderate or severe substance use disorder; and positive test result(s) for specified drugs of abuse. Full lists of the inclusion and exclusion criteria for each study are published (Popova et al. 2019; Fedgchin et al. 2019; Ochs-Ross et al. 2020).
Study drug dosing
Patients continued taking their current antidepressant during the 4-week screening/prospective observational phase. At the end of the screening phase, non-responders (≤ 25% improvement in Montgomery-Åsberg Depression Rating Scale [MADRS] total score from week 1 to week 4) discontinued all current antidepressant treatment(s) and were randomized to double-blind treatment, consisting of twice-weekly esketamine nasal spray or matching (appearance, taste, and packaging) placebo nasal spray, each combined with a newly initiated oral antidepressant (SSRI or SNRI) taken daily. The doses of esketamine were 56 mg (starting dose) and 84 mg in the TRANSFORM-1 (fixed dose) and TRANSFORM-2 (flexible dose) studies and 28 mg (starting dose and a dose option during the study), 56 mg, and 84 mg in TRANSFORM-3 (flexible dose).
Assessments
Improvement in symptoms of depression was assessed by the MADRS (Williams and Kobak 2008), which was administered by independent, blinded raters at baseline and subsequent visits during the double-blind treatment phase. In addition, patient-reported outcomes included an assessment of function using the Sheehan Disability Scale (SDS) (Leon et al. 1997), depressive symptoms using the Patient Health Questionnaire 9-item (PHQ-9) (Spitzer et al. 1999), and severity of anxiety using the Generalized Anxiety Disorder 7-item (GAD-7) Scale (GAD-7 in TRANSFORM-1 and TRANSFORM-2 only) (Spitzer et al. 2006).
The clinician-rated Massachusetts General Hospital Female Reproductive Lifecycle and Hormones Questionnaire (Freeman et al. 2013), Module 1, was used to assess and prospectively document reproductive lifecycle status (pre-menopausal, peri-menopausal, or post-menopausal), history of worsening mood during the luteal phase of the menstrual cycle, length and regularity of menstrual cycles, and use of exogenous hormones, including hormonal oral contraceptives (OC) and hormone replacement therapy (HRT). In reporting menopause status, the investigator selected the appropriate choice from the following: pre-menopausal; peri-menopausal (irregular periods and/or other symptoms of peri-menopause, such as hot flashes not explained by other reasons); post-menopausal non-surgical (> 12 months of amenorrhea); and post-menopausal surgical (status post bilateral oophorectomy).
Treatment-emergent adverse events were assessed throughout the study.
Statistical analyses
Efficacy was analyzed in a data set that included all randomized patients who received at least 1 dose of intranasal study drug (esketamine or placebo) and 1 dose of oral antidepressant, and adverse events were analyzed in a data set that included all patients that received at least 1 dose of either medication. Data from the TRANSFORM-1 and TRANSFORM-2 studies were pooled.
Baseline characteristics and psychiatric history were summarized by sex using descriptive statistics. The prevalence of comorbid anxiety at baseline was determined using one of the following: generalized anxiety disorder current, panic disorder current, social anxiety disorder current, post-traumatic stress disorder current, or obsessive–compulsive disorder current based on the MINI, or having screening and baseline GAD-7 total score ≥ 10 (for TRANSFORM-1/TRANSFORM-2 only).
The primary efficacy endpoint in the TRANSFORM studies—change from baseline to endpoint (day 28) in MADRS total score—was analyzed by sex using a mixed-effects model for repeated measures (MMRM). The model included baseline MADRS total score as a covariate, and treatment, study (TRANSFORM-1/TRANSFORM-2 only), region, oral antidepressant class (SNRI or SSRI), day, sex, day-by-treatment, treatment-by-sex, and day-by-treatment-by-sex interaction as fixed effects, and a random patient effect. Changes in SDS and PHQ-9 were analyzed using the MMRM model described for the primary efficacy endpoint, but using the respective baseline score (SDS, PHQ-9) as covariate. Change in GAD-7 was analyzed using analysis of covariance with baseline GAD-7 as a covariate and treatment, region, oral antidepressant class, sex, and treatment-by-sex as factors.
Response rate (defined as ≥ 50% decrease from baseline MADRS total score) and remission rate (defined as MADRS ≤ 12) at day 28 were analyzed by treatment group and sex using the generalized Cochran-Mantel–Haenszel (CMH) test. Response rate was also evaluated by menopausal status and by use of sex hormones ([i.e., HRT or OCs], yes or no) among women. In other analyses, frequency distributions were provided by treatment group and sex for adverse events as a measure of safety.
Results
Across the TRANSFORM trials, 711 patients were randomized to treatment, 6 of whom did not receive intranasal study drug and 3 additional patients did not receive either study drug. Thus, efficacy and safety were evaluated in 702 patients (464 [66.1%] women and 238 [33.9%] men) and 705 patients, respectively. Most randomized patients (women: 427/471, 90.7%; men: 207/240, 86.3%) completed double-blind treatment.
Within each of the TRANSFORM studies, women and men were similar, in general, with respect to demographic and baseline clinical characteristics (Table 1). Per protocol, patients in TRANSFORM-3 were older (≥ 65 years). Approximately half of women in the TRANSFORM-1 and TRANSFORM-2 studies reported being pre-menopausal (182/379, 48.0%). Of note, in the TRANSFORM-1/2 studies, the mean age at MDD diagnosis (32.8 and 31.2 years old, respectively) and mean duration of the current episode (161.5 and 181.4 weeks, respectively) were similar between women and men. In TRANSFORM-3, women received an MDD diagnosis at an earlier age (41.6 vs. 45.6 years old for men) and had a shorter current episode of MDD (188.6 vs. 260.3 weeks for men).
Table 1.
Demographic and baseline characteristics by sex in short-term randomized, controlled TRD trials
| TRANSFORM-1/TRANSFORM-2 | TRANSFORM-3 | |||||
|---|---|---|---|---|---|---|
| Characteristic | Women N = 379 |
Men N = 186 |
Total N = 565 |
Women N = 85 |
Men N = 52 |
Total N = 137 |
| Age, years | ||||||
| Mean (SD) | 46.6 (11.05) | 44.9 (12.22) | 46.1 (11.46) | 70.3 (4.9) | 69.5 (3.8) | 70.0 (4.52) |
| Range | (18; 64) | (18; 64) | (18; 64) | (65; 86) | (65; 79) | (65; 86) |
| Race, n (%) | ||||||
| American Indian or Alaskan Native | 1 (0.3) | 0 | 1 (0.2) | 0 | 0 | 0 |
| Asian | 5 (1.3) (1.2) | 2 (1.1) | 7 (1.2) | 0 | 0 | 0 |
| Black or African American | 23 (6.1) | 7 (3.8) | 30 (5.3) | 0 | 0 | 0 |
| White | 302 (79.7) | 168 (90.3) | 470 (83.2) | 81 (95.3) | 49 (94.2) | 130 (94.9) |
| Other | 25 (6.6) | 4 (2.2) | 29 (5.1) | 0 | 0 | 0 |
| Multiple | 1 (0.3) | 2 (1.1) | 3 (0.5) | 2 (2.4) | 2 (3.8) | 4 (2.9) |
| Not reported | 22 (5.8) | 3 (1.6) | 25 (4.4) | 1 (1.2) | 1 (1.9) | 2 (1.5) |
| Unknown | 0 | 0 | 0 | 1 (1.2) | 0 | 1 (0.7) |
| Body mass index (kg/m2) | ||||||
| Mean (SD) | 28.4 (6.60) | 28.7 (5.59) | 28.5 (6.28) | 28.9 (6.3) | 28.9 (4.5) | 28.9 (5.64) |
| Range | (17; 56) | (16; 56) | (16; 56) | (16; 45) | (22; 42) | (16; 45) |
| Menopause statusa, n (%) | ||||||
| Pre-menopausal | 182 (48.0) | NA | NA | NA | ||
| Peri-menopausal | 24 (6.3) | NA | NA | NA | ||
| Post-menopausal—non-surgical | 120 (31.7) | NA | NA | NA | ||
| Post-menopausal—surgical | 53 (14.0) | NA | NA | NA | ||
| Regular menstrual cycles, n (%) | N = 203 | |||||
| Yes | 160a (78.8) | NA | NA | NA | ||
| No | 43 (21.2) | NA | NA | NA | ||
| Median length of typical menstrual cycle (days) | 28.0 | NA | NA | NA | ||
| History of worsening luteal phase, n (%) | N = 203 | |||||
| Yes | 43 (21.2) | NA | NA | NA | ||
| No | 160a (78.8) | NA | NA | NA | ||
| Employment statusb, n (%) | ||||||
| Any type of employment | 218 (57.5) | 107 (57.5) | 325 (57.5) | 14 (16.5) | 10 (19.2) | 24 (17.5) |
| Any type of unemployment | 122 (32.2) | 65 (34.9) | 187 (33.1) | 4 (4.7) | 4 (7.7) | 8 (5.8) |
| Other | 39 (10.3) | 14 (7.5) | 53 (9.4) | 67 (78.8) | 38 (73.1) | 105 (76.6) |
| Region, n (%) | ||||||
| Europe | 1542 (37.5) | 77 (41.4) | 219 (38.8) | 40 (47.1) | 19 (36.5) | 59 (43.1) |
| North America | 151 (39.8) | 93 (50.0) | 244 (43.2) | 40 (47.1) | 30 (57.7) | 70 (51.1) |
| Age when diagnosed with MDD, years | ||||||
| Mean (SD) | 32.8 (12.69) | 31.2 (132.69) | 32.3 (12.70) | 41.6 (15.9) | 45.6 (16.5) | 43.1 (16.2) |
| Range | (9; 61) | (5; 64) | (5; 64) | (10; 75) | (11; 77) | (10; 77) |
| Duration of current episode, weeks | ||||||
| Mean (SD) | 161.5 (236.4) | 181.4 (276.5) | 168.1 (250.2) | 188.6 (279.5) | 260.3 (423.6) | 215.8 (341.7) |
| Range | (6; 2288) | (12; 2028) | (6; 2288) | (8; 1700) | (8; 2184) | (8; 2184) |
| No. of previous antidepressantsc,d, n (%) | ||||||
| 1 or 2 | 245 (65.0) | 110 (59.2) | 355(63.1) | 54 (63.5) | 30 (57.7) | 84 (61.3) |
| ≥ 3 | 132 (35.0) | 76 (40.8) | 208 (36.9) | 31 (36.4) | 22 (42.3) | 53 (38.7) |
| Class of oral antidepressante, n (%) | ||||||
| SNRI | 236 (63.3) | 112 (60.2) | 348 (61.6) | 34 (40.0) | 27 (51.9) | 61 (44.5) |
| SSRI | 143 (37.7) | 74 (39.8) | 217 (38.4) | 51 (60.0) | 25 (48.1) | 76 (55.5) |
| Oral antidepressant, n (%) | ||||||
| Duloxetine | 174 (45.9) | 83 (44.6) | 257 (45.5) | 25 (29.4) | 23 (44.2) | 48 (35.0) |
| Escitalopram | 74 (19.5) | 37 (19.9) | 111 (19.6) | 36 (42.4) | 14 (26.9) | 50 (36.5) |
| Sertraline | 68 (17.9) | 37 (19.9) | 105 (18.6) | 14 (16.5) | 11 (21.2) | 25 (18.2) |
| Venlafaxine XR | 63 (16.6) | 29 (15.6) | 92 (16.3) | 10 (11.8) | 4 (7.7) | 14 (10.2) |
| CGI-S | ||||||
| Mean (SD) | 5.1 (0.67) | 5.1 (0.72) | 5.1 (0.68) | 5.0 (0.75) | 5.0 (0.85) | 5.0 (0.79) |
| MADRS total score | ||||||
| Mean (SD) | 37.7 (5.73) | 36.8 (5.21) | 37.4 (5.57) | 35.2 (6.41) | 35.2 (5.78) | 35.2 (6.16) |
| PHQ-9 total score | ||||||
| Mean (SD) | 20.6 (3.67) | 20.3 (3.91) | 20.5 (3.75) | 17.6 (5.53) | 17.4 (5.87) | 17.5 (5.65) |
| SDS total score | ||||||
| Mean (SD) | 24.5 (4.09) | 23.9 (4.40) | 24.3 (4.20) | 23.2 (5.12) | 21.3 (5.49) | 22.3 (5.36) |
| GAD-7 total score | ||||||
| Mean (SD) | 13.4 (5.23) | 12.9 (5.02) | 13.2 (5.16) | NA | NA | NA |
Abbreviations: CGI-S Clinical Global Impression–Severity; MDD major depressive disorder; NA not applicable or not available, not administered; PHQ Patient Health Questionnaire; SNRI serotonin and norepinephrine reuptake inhibitor; SSRI selective serotonin reuptake inhibitor; TRD treatment-resistant depression
aData from the Massachusetts General Hospital Female Reproductive Lifecycle and Hormones Questionnaire, Module I (Freeman et al. 2013), in the TRANSFORM-1 and TRANSFORM-2 studies
bAny type of employment includes any category containing “employed,” sheltered work, housewife or dependent husband, and student; any type of unemployment includes any category containing “unemployed”; other includes retired and no information available
cIn accordance with the trial protocols, patients entering the induction phase had non-response to at least 2 oral antidepressant medications prior to randomization. The data presented is the number of antidepressant medications with non-response (defined as ≤ 25% improvement) taken for at least 6 weeks during the current episode
dNs for the previous antidepressant medications in TRANSFORM-1/TRANSFORM-2 are 377, 186, and 563 for women, men, and total patients, respectively
eAssigned by the investigator at randomization
The majority (71.5%) of patients in the TRANSFORM-1/2 studies had comorbid anxiety symptoms at baseline, with no difference in prevalence between sexes (Table 2). Among the common medical comorbidities reported were hypertension, cardiovascular disease, diabetes, and thyroid disease. The prevalence of hypertension was balanced between sexes, whereas cardiovascular disease and diabetes were more common in older men than older women, and thyroid disease was more common in both older and younger women than men. Of note, when we refer to younger women, here and elsewhere, we are referring to those enrolled in the TRANSFORM-1/2 studies (who were 18–64 years) and older women were those enrolled in TRANSFORM-3 (who were 65 years and older). Usage of concomitant medications, which was in line with these comorbidities, was generally balanced between treatment groups among women and men, with the exception of higher levothyroxine usage by women (Table 3). In the TRANSFORM-1/2 studies, hormonal therapy (including HRT and OCs) was taken by 34.8% and 21.4% of pre-menopausal women in the esketamine/antidepressant and antidepressant/placebo groups respectively, 40% and 22.2% of peri-menopausal women, and 3.7% and 6.2% of post-menopausal women in the respective treatment groups. Hormonal therapy use by women in TRANSFORM-3 was uncommon (Table 3).
Table 2.
Comorbidities of study patients in short-term randomized, controlled TRD trials
| Number (%) of patients | ||||||
|---|---|---|---|---|---|---|
| TRANSFORM-1/TRANSFORM-2 | TRANSFORM-3 | |||||
| Comorbidities | Women N = 379 |
Men N = 186 |
Total N = 565 |
Women N = 85 |
Men N = 52 |
Total N = 137 |
| Anxietya | 272 (71.8%) | 132 (71.0%) | 404 (71.5%) | NA | NA | NA |
| Incidental surgery | 166 (43.8%) | 55 (29.6%) | 221 (39.1%) | 37 (43.5%) | 19 (36.5%) | 56 (40.9%) |
| Hypertension | 79 (20.8%) | 38 (20.4%) | 117 (20.7%) | 46 (54.1%) | 28 (53.8%) | 74 (54.0%) |
| Allergies | 69 (18.2%) | 31 (16.7%) | 100 (17.7%) | 11 (12.9%) | 4 (7.7%) | 15 (10.9%) |
| Thyroid disease | 57 (15.0%) | 10 (5.4%) | 67 (11.9%) | 23 (27.1%) | 6 (11.5%) | 29 (21.2%) |
| GERD | 48 (12.7%) | 15 (8.1%) | 63 (11.2%) | 13 (15.3%) | 8 (15.4%) | 21 (15.3%) |
| Trauma | 27 (7.1%) | 31 (16.7%) | 58 (10.3%) | 5 (5.9%) | 3 (5.8%) | 8 (5.8%) |
| Cardiovascular disease | 24 (6.3%) | 13 (7.0%) | 37 (6.5%) | 10 (11.8%) | 12 (23.1%) | 22 (16.1%) |
| Diabetes | 25 (6.6%) | 8 (4.3%) | 33 (5.8%) | 13 (15.3%) | 13 (25.0%) | 26 (19.0%) |
| Oncology | 13 (3.4%) | 5 (2.7%) | 18 (3.2%) | 16 (18.8%) | 11 (21.2%) | 27 (19.7%) |
| Skin disorder | 23 (6.1%) | 13 (7.0%) | 36 (6.4%) | 3 (3.5%) | 4 (7.7%) | 7 (5.1%) |
| Infection | 25 (6.6%) | 6 (3.2%) | 31 (5.5%) | 2 (2.4%) | 2 (3.8%) | 4 (2.9%) |
| Respiratory disease | 12 (3.2%) | 2 (1.1%) | 14 (2.5%) | 1 (1.2%) | 0 | 1 (0.7%) |
| Parathyroid disease | 2 (0.5%) | 1 (0.5%) | 3 (0.5%) | 0 | 1 (1.9%) | 1 (0.7%) |
Data presented in descending order of frequency for all patients
GERD gastroesophageal reflux disease; NA not available; TRD treatment-resistant depression
aComorbid anxiety defined by one of the following at screening: generalized anxiety disorder current, panic disorder current, social anxiety disorder current, post-traumatic stress disorder current, or obsessive–compulsive disorder current by MINI, or having GAD-7 total score ≥ 10 at screening and at baseline. GAD-7 was not conducted in the TRANSFORM-3 study
Table 3.
Concomitant medications most frequently used during double-blind treatment in short-term randomized, controlled TRD trials
| Women | Men | |||
|---|---|---|---|---|
| Specific or category of concomitant medication | Esketamine + antidepressant N = 282 |
Antidepressant + placebo N = 184 |
Esketamine + antidepressant N = 136 |
Antidepressant + placebo N = 103 |
| Benzodiazepine | 140 (49.6%) | 86 (46.7%) | 66 (48.5%) | 36 (35.0%) |
| Analgesic | 79 (28.0%) | 57 (31.0%) | 34 (25.0%) | 24 (23.3%) |
| Antihypertensive | 62 (22.0%) | 53 (28.8%) | 33 (24.3%) | 32 (31.1%) |
| Lipid-lowering agent | 45 (16.0%) | 36 (19.6%) | 32 (23.5%) | 28 (27.2%) |
| Proton pump inhibitor | 40 (14.2%) | 28 (15.2%) | 24 (17.6%) | 11 (10.7%) |
| Beta-blocker | 34 (12.1%) | 21 (11.4%) | 21 (15.4%) | 9 (8.7%) |
| Thyroid medications | 37 (13.1%) | 35 (19.0%) | 5 (3.7%) | 8 (7.8%) |
| Levothyroxine | 36 (12.8%) | 33 (17.9%) | 5 (3.7%) | 7 (6.8%) |
| Hormonal therapya | 49 (17.4%) | 24 (13.0%) | NA | NA |
| TRANSFORM-1/2 | ||||
| Pre-menopausal | 39/112 (34.8%) | 15/70 (21.4%) | ||
| Peri-menopausal | 6/15 (40.0%) | 2/9 (22.2%) | ||
| Post-menopausal | 4/108 (3.7%) | 4/65 (6.2%) | ||
| TRANSFORM-3 | 0/45 (0.0%) | 3/40 (7.5%) | ||
The table lists, in descending order of frequency for all patients, all specific or categories of concomitant medication with a usage rate during double-blind treatment of ≥ 10% in either treatment group, without regard to sex
aIncludes hormone replacement therapy and oral contraceptives
Mean MADRS total score decreased from baseline to day 28, with greater improvement among those treated with esketamine/antidepressant compared to antidepressant/placebo among both women and men. The mean MADRS change (SD) at day 28 for the esketamine/antidepressant and antidepressant/placebo groups were -20.3 (13.19) vs. -15.8 (14.67), respectively, among the women and -18.3 (14.08) vs. -16.0 (14.30), respectively, among the men in TRANSFORM-1/TRANSFORM-2; and -9.9 (13.34) vs. -6.9 (9.65), respectively, among the women and -10.3 (11.96) vs. -5.5 (7.64), respectively, among the men in TRANSFORM-3 (Table 4). The analysis failed to show any significant sex effect or treatment-by-sex interaction (p > 0.35).
Table 4.
MADRS total score: change from baseline to day 28 of double-blind phase of short-term randomized, controlled TRD trials by sex and treatment group
| TRANSFORM-1/TRANSFORM-2 | TRANSFORM-3 | |||||||
|---|---|---|---|---|---|---|---|---|
| Women | Men | Women | Men | |||||
| Esketamine + antidepressant |
Antidepressant + placebo |
Esketamine + antidepressant |
Antidepressant + placebo |
Esketamine + antidepressant |
Antidepressant + placebo |
Esketamine + antidepressant |
Antidepressant + placebo |
|
| Baseline | ||||||||
| N | 235 | 144 | 108 | 78 | 45 | 40 | 27 | 25 |
| Mean (SD) | 37.7 (5.49) | 37.7 (6.11) | 36.9 (5.02) | 36.7 (5.50) | 35.7 (5.90) | 34.5 (6.97) | 35.2 (6.04) | 35.1 (5.60) |
| Change to day 28 | ||||||||
| N | 215 | 138 | 95 | 70 | 39 | 36 | 24 | 24 |
| Mean (SD) | -20.3 (13.19) | -15.8 (14.67) | -18.3 (14.08) | -16.0 (14.30) | -9.9 (13.34) | -6.9 (9.65) | -10.3 (11.96) | -5.5 (7.64) |
| MMRM analysisa | ||||||||
| Diff. of LS meansb (SE) | -4.5 (1.41) | -1.6 (2.04) | -3.4 (2.41) | -5.0 (3.05) | ||||
| 95% CI on difference | -7.26, − 1.70 | -5.60, 2.41 | -8.14, 1.41 | -11.05, 1.03 | ||||
MADRS total score ranges from 0 to 60; a higher score indicates a more severe condition. Negative change in score indicates improvement. Negative difference favors esketamine. The p-values were 0.6574 and 0.3993 for sex, and 0.3546 and 0.4937 for treatment-by-sex interaction in the TRANSFORM-1/2 and TRANSFORM-3 studies, respectively
CI confidence interval; LS least squares; MADRS Montgomery-Asberg Depression Rating Scale; TRD treatment-resistant depression
aMixed model for repeated measures (MMRM) analysis with change from baseline as the response variable and the fixed effect model terms for study number (pooled only), treatment (esketamine + antidepressant, antidepressant + placebo) day, region, class of antidepressant (SNRI or SSRI), sex, and treatment-by-day, treatment-by-sex, and treatment-by-day-by-sex, and baseline value as a covariate
bEsketamine + antidepressant minus antidepressant + placebo
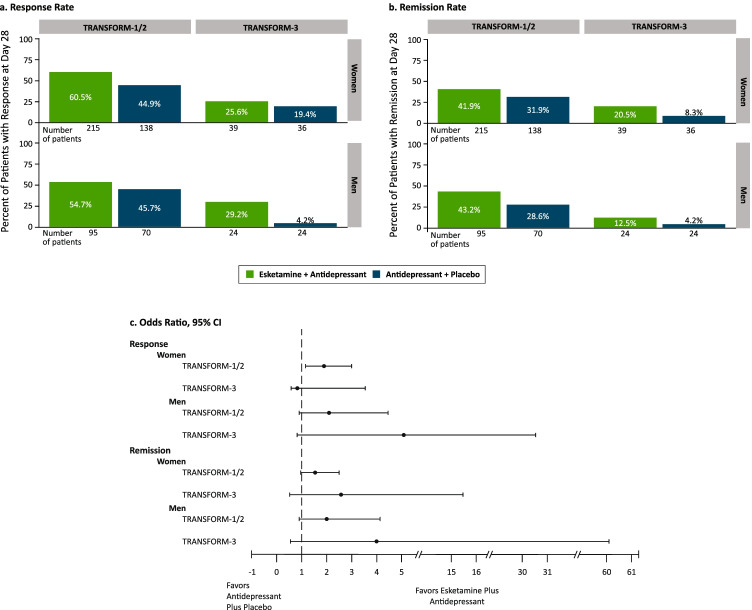
In the TRANSFORM trials, the proportions of patients who were responders at day 28 and the proportion of patients in remission at day 28 were numerically higher among both women and men treated with esketamine/antidepressant as compared to antidepressant/placebo (Fig. 1). In TRANSFORM-3, the remission rate, but not response rate, was numerically higher among the older women compared to their male counterparts. In TRANSFORM-1/TRANSFORM-2, pre-menopausal and post-menopausal women treated with esketamine achieved similar response rates, and the same between treatment group trend was observed for response rate among the pre-menopausal and post-menopausal women (Fig. 2). In the cohort of peri-menopausal women (n = 25), response rate with esketamine/antidepressant was numerically lower than among pre-menopausal and post-menopausal women. In the TRANSFORM-1/2 studies, use of hormonal therapy (HRT or OCs) had an impact on response rate at day 28 in the antidepressant/placebo arm (hormone users: 73.7% [14/19]; hormone non-users: 40.3% [48/119]), but not in the esketamine/antidepressant arm (hormone users: 54.2% [28/48]; hormone non-users: 62.3% [104/167]).
Fig. 1.
Response and remission rates by sex and treatment group in the TRANSFORM trials. CI = confidence interval. Notes: Response defined as ≥ 50% decrease from baseline Montgomery-Asberg Depression Rating Scale (MADRS) total score. Remission defined as MADRS total score ≤ 12. Odds ratio = odds of achieving response on esketamine + antidepressant divided by the odds of achieving response on antidepressant + placebo
Fig. 2.
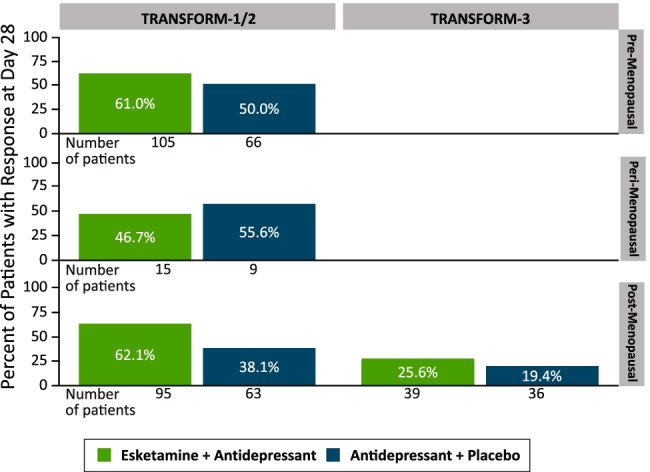
Response rates by menopausal status and treatment group in TRANSFORM trials. Notes: Response defined as ≥ 50% decrease from baseline Montgomery-Asberg Depression Rating Scale (MADRS) total score
Treatment benefit of esketamine was also observed in terms of functioning and self-reported depression for both women and men in the pooled TRANSFORM-1/TRANSFORM-2 trials (Table 5, Fig. 3). The analysis for SDS and PHQ-9 failed to show any significant sex effect or treatment-by sex-interaction (p > 0.20). For GAD-7, there was no treatment-by-sex interaction (p > 0.52); however, the sex effect trended towards significance (p = 0.07; i.e., women showed greater change from baseline than men, regardless of treatment).
Table 5.
Mean (SD) change from baseline to day 28 for SDS, PHQ-9, and GAD-7 total score by sex in pooled TRANSFORM-1/TRANSFORM-2 trials
| TRANSFORM-1/TRANSFORM-2 | ||||
|---|---|---|---|---|
| Women | Men | |||
| Esketamine + antidepressant |
Antidepressant + placebo |
Esketamine + antidepressant |
Antidepressant + placebo |
|
| SDS total score | ||||
| N | 177 | 115 | 84 | 60 |
| Mean (SD) | -12.5 (9.30) | -9.6 (9.50) | -10.6 (9.22) | -7.4 (8.12) |
| PHQ-9 total score | ||||
| N | 218 | 138 | 95 | 70 |
| Mean (SD) | -12.3 (7.39) | -10.1 (7.99) | -10.9 (7.62) | -8.6 (8.25) |
| GAD-7 total score | ||||
| N | 227 | 139 | 103 | 74 |
| Mean (SD) | -8.1 (5.90) | -6.9 (5.78) | -6.7 (5.84) | -5.4 (5.98) |
SDS total score ranges from 0 to 30; a higher score indicates greater impairment. PHQ-9 total score ranges from 0 to 27; a higher score indicates greater depression. GAD-7 total score ranges from 0 to 21; a higher score indicates more anxiety. Negative change in SDS total score, PHQ-9 total score, and GAD-7 total score indicates improvement for each
GAD-7 Generalized Anxiety Disorder 7-item; LS least squares; PHQ-9 Patient Health Questionnaire 9-item; SD standard deviation; SDS Sheehan Disability Scale
Fig. 3.
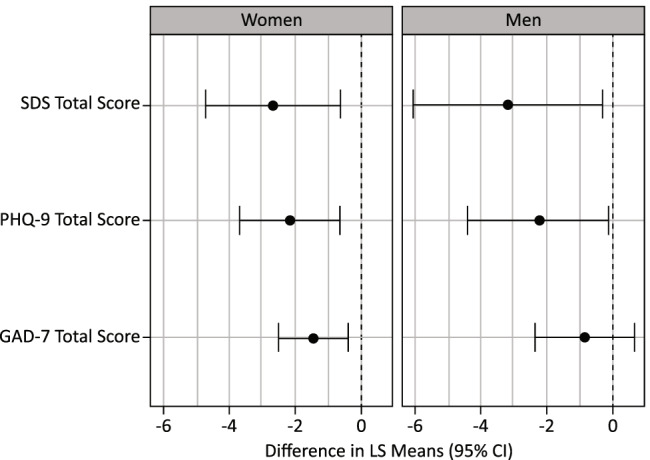
Difference in least square means for SDS, PHQ-9, and GAD-7 total score by sex in pooled TRANSFORM-1/TRANSFORM-2 trials. CI = confidence interval; GAD-7 = Generalized Anxiety Disorder 7-item; LS = least squares; PHQ-9 = Patient Health Questionnaire 9-item; SDS = Sheehan Disability Scale. Notes: SDS total score ranges from 0 to 30; a higher score indicates greater impairment. PHQ-9 total score ranges from 0 to 27; a higher score indicates greater depression. GAD-7 total score ranges from 0 to 21; a higher score indicates more anxiety. Negative change in SDS total score, PHQ-9 total score, and GAD-7 total score indicates improvement for each, and a negative difference favors esketamine
The most common adverse events (incidence > 20%) reported for esketamine/antidepressant were nausea, dissociation, dizziness, and vertigo (Table 6). Among esketamine-treated patients, the incidences of nausea and dissociation were higher among women than among men, regardless of age. The incidences of vertigo and dizziness were numerically higher and lower, respectively, among the younger women vs. younger men in the TRANSFORM-1/TRANSFORM-2 studies; the opposite by-sex trend for incidences of these events was observed among the older patients in TRANSFORM-3. While reported at a lower incidence overall (i.e., 9.3%), the incidence of increased blood pressure was numerically higher among younger men (12.8%) and older women (17.8%), than their counterparts. Overall, most adverse events occurred on nasal spray dosing days, were mild or moderate in severity, resolved the same day, and were generally not treatment limiting. With regard to dissociation events, their median duration ranged from 0.7 to 1 h across dosing sessions (within the post-dose observation period), a minority (3.1%) of events were classified as severe, and none were considered serious.
Table 6.
Most frequently reported treatment-emergent adverse events in the double-blind treatment phase of short-term randomized, controlled TRD trials by sex and treatment group
| TRANSFORM-1/TRANSFORM-2 | TRANSFORM-3 | |||||||
|---|---|---|---|---|---|---|---|---|
| Women | Men | Women | Men | |||||
| Esketamine + antidepressant N = 237 |
Antidepressant + placebo N = 144 |
Esketamine + antidepressant N = 109 |
Antidepressant + placebo N = 78 |
Esketamine + antidepressant N = 45 |
Antidepressant + placebo N = 40 |
Esketamine + antidepressant N = 27 |
Antidepressant + placebo N = 25 |
|
| Total with AEs | 209 (88.2%) | 96 (66.7%) | 92 (84.4%) | 47 (60.3%) | 34 (75.6%) | 23 (57.5%) | 17 (63.0%) | 16 (64.0%) |
| Nausea | 72 (30.4%) | 11 (7.6%) | 26 (23.9%) | 8 (10.3%) | 9 (20.0%) | 3 (7.5%) | 4 (14.8%) | 0 |
| Headache | 52 (21.9%) | 21 (14.6%) | 18 (16.5%) | 17 (21.8%) | 6 (13.3%) | 1 (2.5%) | 3 (11.1%) | 1 (4.0%) |
| Dizziness | 53 (22.4%) | 10 (6.9%) | 29 (26.6%) | 5 (6.4%) | 13 (28.9%) | 4 (10.0%) | 2 (7.4%) | 1 (4.0%) |
| Dissociation | 68 (28.7%) | 7 (4.9%) | 24 (22.2%) | 1 (1.3%) | 7 (15.6%) | 1 (2.5%) | 2 (7.4%) | 0 |
| Vertigo | 62 (26.2%) | 5 (3.5%) | 16 (14.7%) | 0 | 4 (8.9%) | 2 (5.0%) | 4 (14.8%) | 0 |
| Dysgeusia | 46 (19.4%) | 19 (13.2%) | 19 (17.4%) | 11 (14.1%) | 2 (4.4%) | 3 (7.5%) | 2 (7.4%) | 0 |
| Somnolence | 39 (16.5%) | 15 (10.4%) | 21 (19.3%) | 5 (6.4%) | 1 (2.2%) | 3 (7.5%) | 0 | 0 |
| Paresthesia | 31 (13.1%) | 2 (1.4%) | 12 (11.0%) | 2 (2.6%) | 2 (4.4%) | 2 (5.0%) | 2 (7.4%) | 0 |
| Anxiety | 19 (8.0%) | 10 (6.9%) | 12 (11.0%) | 2 (2.6%) | 2 (4.4%) | 2 (5.0%) | 0 | 3 (12.0%) |
| Fatigue | 19 (8.0%) | 9 (6.3%) | 6 (5.5%) | 2 (2.6%) | 7 (15.6%) | 4 (10.0%) | 2 (7.4%) | 1 (4.0%) |
| BP increased | 16 (6.8%) | 3 (2.1%) | 14 (12.8%) | 2 (2.6%) | 8 (17.8%) | 3 (7.5%) | 1 (3.7%) | 0 |
| Hypoesthesia | 25 (10.5%) | 1 (0.7%) | 13 (11.9%) | 2 (2.6%) | 3 (6.7%) | 1 (2.5%) | 1 (3.7%) | 0 |
| Hypoesthesia oral | 29 (12.2%) | 2 (1.4%) | 8 (7.3%) | 1 (1.3%) | 4 (8.9%) | 0 | 1 (3.7%) | 0 |
| Vomiting | 27 (11.4%) | 3 (2.1%) | 5 (4.6%) | 1 (1.3%) | 5 (11.1%) | 0 | 0 | 1 (4.0%) |
| UTI | 6 (2.5%) | 2 (1.4%) | 0 | 1 (1.3%) | 6 (13.3%) | 1 (2.5%) | 0 | 0 |
The table lists, in descending order of frequency for all patients, all adverse events with an incidence ≥ 10% in any esketamine group
AE adverse event, BP blood pressure, UTI urinary tract infection
A serious adverse event was reported during treatment with esketamine for two (0.7%) women (single events of depression and anxiety disorder) and four (2.9%) men (single events of headache, hip fracture, increased blood pressure, and multiple injuries/road traffic accident [the latter event occurred on day 16 and was considered doubtfully related to esketamine or to antidepressant; patient subsequently died on day 55]).
A minority of patients discontinued intranasal study drug due to adverse events in the TRANSFORM studies (esketamine: 20/415, 4.8%; placebo: 5/287, 1.7%). Among esketamine-treated patients, 12 women discontinued study drug prematurely, due to multiple events in some cases (5 events of increased systolic blood pressure and 1 event of anxiety disorder for 1 older patient each in TRANSFORM-3; 3 events each of dizziness and nausea, 2 events each of depression, headache, and vomiting, and single events of anxiety, disturbance in attention, drug intolerance, extrasystoles, feeling drunk, motion sickness, tachycardia, and vertigo for younger patients in TRANSFORM-1/TRANSFORM-2) and 8 men discontinued study drug prematurely (single events of hip fracture and increased blood pressure for 1 older patient each in TRANSFORM-3; 2 events of panic attack and single events of anxiety, depressive symptoms, mania, and multiple injuries/road traffic accident for younger patients in TRANSFORM-1/TRANSFORM-2).
Discussion
Similar and robust improvement of depressive symptoms from baseline was observed with esketamine nasal spray compared to placebo nasal spray, in conjunction with an oral antidepressant, among both women and men with TRD. While the between-group difference observed with esketamine/antidepressant vs. antidepressant/placebo was numerically higher among women than men in TRANSFORM-1/TRANSFORM-2 (LS means (SE) -4.5 (1.41) 95% CI -7.26, -1.70 for women vs. -1.6 (2.04) 95% CI -5.60, 2.41 for men) and vice versa in TRANSFORM-3 (LS means (SE) -3.4 (2.41) 95% CI -8.14, 1.41 for women vs. -5.0 (3.05) 95% CI -11.05, 1.03 for men), the treatment-by-sex interaction was not statistically significant (p > 0.35) and the 95% CIs for the differences overlap. Furthermore, the between-group difference observed vs. antidepressant/placebo for both sex subgroups in TRANSFORM-1/TRANSFORM-2 and TRANSFORM-3 was in the range considered clinically meaningful (2-point to 3-point difference) (Montgomery and Möller 2009; Kim et al. 2019) and is consistent with that observed in clinical trials of the most recently approved biogenic amine antidepressants compared with only a placebo rather than an active comparator (Preskorn 2013).
The proportions of patients who were responders at day 28 and the proportion of patients in remission at day 28 were numerically higher among both women and men treated with esketamine/antidepressant as compared to antidepressant/placebo. While it is noted that the remission rate, but not response rate, was numerically higher among the older women, compared to the older men in TRANSFORM-3, the small cohort sizes limit a conclusion being made from the comparison. Additionally, as noted in the “Materials and methods” section, the TRANSFORM-3 study, unlike TRANSFORM-1 and 2, included a lower 28 mg dose and post hoc analyses of TRANSFORM-3 data revealed several factors that potentially contributed to its failure to achieve statistical significance on the primary endpoint (Ochs-Ross et al. 2020).
The treatment benefit of esketamine, regardless of sex, was also observed based on the patient-reported outcomes of functioning (SDS total score), severity of anxiety (GAD-7 total score), and depressive symptoms (PHQ-9 total). The absence of evidence of sex-based differences may be explained, in part, by the absence of differences in the pharmacokinetics of esketamine between male and female subjects (SpravatoTM Prescribing Information 2020).
Sex differences in efficacy outcomes have been reported for other antidepressants, although findings have been inconsistent (Sramek et al. 2016). In some studies, older men responded better to tricyclic antidepressants than women (Frank et al. 1988; Jacoby et al. 1993; Kornstein et al. 2000), and in other studies women responded better to SSRIs, and to a lesser extent SNRIs, than men (Khan et al. 2005; Kornstein et al. 2000; Berlanga and Flores-Ramos 2006; Young et al. 2009; Yang et al. 2011). Alternatively, other studies have reported no sex-based differences in antidepressant efficacy (Kornstein et al. 2006, 2010, 2018; Cuijpers et al. 2014). Differences in study design (prospective, retrospective, meta-analysis), patient selection criteria (e.g., age of patients, clinical presentation, severity/duration of depression), study drug (mechanism of action, dosage, duration of treatment), and response criteria may explain the inconsistency in these findings across studies.
As noted in the “Introduction” section, findings regarding the effect of menopausal status on response to antidepressants have also been mixed (Kornstein et al. 2000; Quitkin et al. 2002; Grigoriadis et al. 2003; Cassano et al. 2005; Thase et al. 2005; Pinto-Meza et al. 2006; Kornstein et al. 2014), with some research groups reporting greater response among women treated with an SSRI vs. a tricyclic antidepressant, driven by between-group differences in pre-menopausal women, and not in those who were post-menopausal (Kornstein et al. 2000). In a pooled analysis of data from 8 randomized, controlled trials, older women exhibited lower remission rates on SSRI than younger women, a trend that was reversed for those taking hormone replacement therapy; remission rates were higher for women treated with SNRI, irrespective of age and hormone replacement therapy (Thase et al. 2005). Although small sample size precluded analysis of efficacy by concomitant oral antidepressant class (SSRI, SNRI), by sex or menopausal status, oral antidepressant class was included in the MMRM as a fixed effect.
With esketamine, while the response rate with esketamine/antidepressant was numerically lower in the small sample of peri-menopausal women (n = 24), it appears that post-menopausal women with TRD achieve the same benefit that pre-menopausal women do. These findings are consistent with those of Freeman et al. (2019), who found no difference between women and men treated with intravenous ketamine for rapid reduction of depressive symptoms, with no difference observed based on menopause status, suggesting antidepressants with a glutamatergic mechanism of action, unlike biogenic amines, may not be impacted by the reproductive life cycle of women.
In the TRANSFORM-1/2 studies, use of hormonal therapy increased response rate at day 28 in the antidepressant/placebo arm, but not in the esketamine/antidepressant arm. Others have also observed that hormone therapy impacts response among women receiving SSRI or SNRI (Schneider et al 2001; Thase et al. 2005).
The most common adverse events experienced by esketamine-treated patients were dissociation, headache, nausea (each reported at a rate higher in women than men), dizziness (reported at a rate higher in older women than older men [in TRANSFORM-3]), and vertigo (reported at a rate higher in younger women than younger men [in TRANSFORM-1/TRANSFORM-2]). Increased blood pressure was reported most often among older women. This trend is in line with the higher risk for women having adverse drug reactions, in general (Anderson 2008), and for specific types of events during treatment with antidepressants (e.g., weight gain with SSRIs) (Noordam et al. 2015).
Limitations
The strengths of this post hoc analysis include the relatively large numbers of participants, the active-controlled design, validated diagnostic assessments for comorbid psychiatric disorders, and the systematic ascertainment of menopausal status and other sex-specific data.
Our findings are limited by the fact that patients were not stratified into the TRANSFORM studies based on sex, although the higher participation by women (66.1% overall) was similar across the TRANSFORM studies and is consistent with the well-known sex disparity in the prevalence of major depression worldwide (Seedat et al. 2009). We note that the results of analyses by menopausal status must be interpreted with caution given small sample sizes. Similarly, sub-analyses from TRANSFORM-3 (patients 65 years and older) should also be interpreted with caution due to the small sample size and differences in dosing regimen (including a lower 28 mg dose, in addition to 56 mg and 84 mg). Other limitations to the generalizability of findings from the current post hoc analysis include exclusion of patients with some common psychiatric and medical comorbidities and low participation by non-white patients.
Conclusion
These analyses support antidepressant efficacy and safety of esketamine nasal spray for women with TRD, without notable differential effects based on sex. These findings add to the existing literature and support data-informed decision-making for women with TRD.
Acknowledgements
We acknowledge Sandra Norris, PharmD of the Norris Communications Group LLC for medical writing assistance and Ellen Baum, PhD (Janssen Global Services, LLC) for additional editorial support.
Author contribution
Kimberly Cooper conducted the statistical analyses. The authors had full access to all of the data in the studies, were involved in interpretation of the results and writing and/or revising the manuscript, and had final responsibility for the decision to submit for publication. All authors meet ICMJE criteria and all those who fulfilled those criteria are listed as authors.
Funding
The TRANSFORM studies were funded by Janssen Research & Development, LLC, Titusville, NJ, USA.
Data availability
The data sharing policy of Janssen Pharmaceutical Companies of Johnson & Johnson is available at https://www.janssen.com/clinical-trials/transparency. As noted on this site, requests for access to the study data can be submitted through Yale Open Data Access (YODA) Project site at http://yoda.yale.edu.
Code availability
Not applicable.
Declarations
Ethics approval
The TRANSFORM studies were performed in line with the principles of the Declaration of Helsinki. Institutional review boards/ethics committees approved the study protocols and their amendments.
Consent to participate
Written consent was obtained from all patients before they participated in the TRANSFORM studies.
Consent for publication
Not applicable.
Competing interests
Robyn R. Jones, Kimberly Cooper, Ella Daly, Carla M. Canuso, and Susan Nicholson are employees of Janssen Research & Development, LLC and hold company equity. Dr. Marlene Freeman reports Investigator-Initiated Trials/Research: JayMac, Sage; Advisory Boards: Eliem, Sage; Independent Data Safety and Monitoring Committee: Janssen (Johnson & Johnson), Novartis; Steering Committee for Educational Activities: Medscape; and Educational Activities: WebMD. Dr. Freeman is an employee of Massachusetts General Hospital, and works with the MGH National Pregnancy Registry [Current Registry Sponsors: Teva (2018-present), Alkermes, Inc. (2016-present); Otsuka America Pharmaceutical, Inc. (2008-present); Forest/Actavis (2016-present); Sunovion Pharmaceuticals, Inc. (2011-present)]. As an employee of MGH, Dr. Freeman works with the MGH CTNI, which has had research funding from multiple pharmaceutical companies and NIMH. Dr. Susan Kornstein reports Research Support: Pfizer, Allergan, Marinus, Takeda, Palatin, National Science Foundation; Consulting/Advisory Boards: Pfizer, Marinus, Sage, Acadia, Sunovion, Shire, Allergan, Palatin, AbbVie; and Royalties: Guilford Press.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- American Psychiatric Association . Diagnostic and statistical manual of mental disorders, 5th edition: DSM-5. Arlington, TX: American Psychiatric Publishing; 2013. [Google Scholar]
- Anderson GD. Gender differences in pharmacological response. Int Rev Neurobiol. 2008;83:1–10. doi: 10.1016/S0074-7742(08)00001-9. [DOI] [PubMed] [Google Scholar]
- Berlanga C, Flores-Ramos M (2006) Different gender response to serotonergic and noradrenergic antidepressants. A comparative study of the efficacy of citalopram and reboxetine. J Affect Disord 95(1–3):119–123. 10.1016/j.jad.2006.04.029 [DOI] [PubMed]
- Bigos KL, Pollock BG, Stankevich BA, Bies RR. Sex differences in the pharmacokinetics and pharmacodynamics of antidepressants: an updated review. Gend Med. 2009;6(4):522–543. doi: 10.1016/j.genm.2009.12.004. [DOI] [PubMed] [Google Scholar]
- Cassano P, Soares CN, Cusin C, Mascarini A, Cohen LS, Fava M. Antidepressant response and well-being in pre-, peri- and postmenopausal women with major depressive disorder treated with fluoxetine. Psychother Psychosom. 2005;74(6):362–365. doi: 10.1159/000087783. [DOI] [PubMed] [Google Scholar]
- Cuijpers P, Weitz E, Twisk J, Kuehner C, Cristea I, David D, DeRubeis RJ, Dimidjian S, Dunlop BW, Faramarzi M, Hegerl U, Jarrett RB, Kennedy SH, Kheirkhah F, Mergl R, Miranda J, Mohr DC, Segal ZV, Siddique J, Simons AD, Vittengl JR, Hollon SD. Gender as predictor and moderator of outcome in cognitive behavior therapy and pharmacotherapy for adult depression: an “individual patient data” meta-analysis. Depression Anxiety. 2014;31(11):941–951. doi: 10.1002/da.22328. [DOI] [PubMed] [Google Scholar]
- Dalla C, Pitychoutis PM, Kokras N, Papadopoulou-Daifoti Z. Sex differences in animal models of depression and antidepressant response. Basic Clin Pharmacol Toxicol. 2010;106(3):226–233. doi: 10.1111/j.1742-7843.2009.00516.x. [DOI] [PubMed] [Google Scholar]
- Daly EJ, Singh JB, Fedgchin M, Cooper K, Lim P, Shelton RC, Thase ME, Winokur A, Van Nueten L, Manji H, Drevets WC. Efficacy and safety of intranasal esketamine adjunctive to oral antidepressant therapy in treatment-resistant depression: a randomized clinical trial. JAMA Psychiat. 2018;75(2):139–148. doi: 10.1001/jamapsychiatry.2017.3739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daly EJ, Trivedi MH, Janik A, Li H, Zhang Y, Li X, Lane R, Lim P, Duca AR, Hough D, Thase ME, Zajecka J, Winokur A, Divacka I, Fagiolini A, Cubala WJ, Bitter I, Blier P, Shelton RC, Molero P, Drevets WC, Singh JB (2019) Efficacy of esketamine nasal spray plus oral antidepressant treatment for relapse prevention in patients with treatment-resistant depression: a randomized clinical trial. JAMA Psychiatry 76(9):893–903. Advance online publication. 10.1001/jamapsychiatry.2019.1189 [DOI] [PMC free article] [PubMed]
- Fedgchin M, Trivedi M, Daly EJ, Melkote R, Lane R, Lim P, Vitagliano D, Blier P, Fava M, Liebowitz M, Ravindran A, Gaillard R, Ameele H, Preskorn S, Manji H, Hough D, Drevets WC, Singh JB. Efficacy and safety of fixed-dose esketamine nasal spray combined with a new oral antidepressant in treatment-resistant depression: results of a randomized, double-blind, active-controlled study (TRANSFORM-1) Int J Neuropsychopharmacol. 2019;22(10):616–630. doi: 10.1093/ijnp/pyz039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernández-Guasti A, Fiedler JL, Herrera L, Handa RJ. Sex, stress, and mood disorders: at the intersection of adrenal and gonadal hormones. Horm Metab Res. 2012;44(8):607–618. doi: 10.1055/s-0032-1312592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank E, Carpenter LL, Kupfer DJ. Sex differences in recurrent depression: are there any that are significant? Am J Psychiatry. 1988;145(1):41–45. doi: 10.1176/ajp.145.1.41. [DOI] [PubMed] [Google Scholar]
- Freeman MP, Walker R, Laughren TP, Miller KK, Fava M. Female reproductive life cycle and hormones: methodology to improve clinical trials. J Clin Psychiatry. 2013;74(10):1018–1021. doi: 10.4088/JCP.13com08742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freeman MP, Papakostas GI, Hoeppner B, Mazzone E, Judge H, Cusin C, Mathew S, Sanacora G, Iosifescu D, DeBattista C, Trivedi MH, Fava M. Sex differences in response to ketamine as a rapidly acting intervention for treatment resistant depression. J Psychiatr Res. 2019;110:166–171. doi: 10.1016/j.jpsychires.2019.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grigoriadis S, Kennedy SH, Bagby RM. A comparison of antidepressant response in younger and older women. J Clin Psychopharmacol. 2003;23(4):405–407. doi: 10.1097/01.jcp.0000085415.08426.c6. [DOI] [PubMed] [Google Scholar]
- Hasin DS, Goodwin RD, Stinson FS, Grant BF. Epidemiology of major depressive disorder: results from the National Epidemiologic Survey on Alcoholism and Related Conditions. Arch Gen Psychiatry. 2005;62(10):1097–1106. doi: 10.1001/archpsyc.62.10.1097. [DOI] [PubMed] [Google Scholar]
- Jacoby R, Lunn A, Ardern M, Bergmann K, Conway J, Cooling N, Davies G, Fisk J, Gehlhaar E, Harrison-Read P, Hawley R, Jacoby R, Jones R, Levy R, MacDonald A, Naguib M, Oppenheimer C, Philpot M, Philpott R, Pitt B, Price M, Robinson J, Silverman M, Simons R, Slattery Z, Staley C, Taws E, Tym E, White M. How long should the elderly take antidepressants? A double-blind placebo-controlled study of continuation/prophylaxis therapy with dothiepin. Br J Psychiatry. 1993;162:175–182. doi: 10.1192/bjp.162.2.175. [DOI] [PubMed] [Google Scholar]
- Khan A, Brodhead AE, Schwartz KA, Kolts RL, Brown WA. Sex differences in antidepressant response in recent antidepressant clinical trials. J Clin Psychopharmacol. 2005;25(4):318–324. doi: 10.1097/01.jcp.0000168879.03169.ce. [DOI] [PubMed] [Google Scholar]
- Kim J, Farchione T, Potter A, Chen Q, Temple R. Esketamine for treatment-resistant depression—first FDA-approved antidepressant in a new class. N Engl J Med. 2019;381(1):1–4. doi: 10.1056/NEJMp1903305. [DOI] [PubMed] [Google Scholar]
- Kornstein SG, Schatzberg AF, Thase M, Yonkers KA, McCullough JP, Keitner GI, Gelenberg AJ, Davis SM, Harrison WM, Keller MB. Gender differences in treatment response to sertraline versus imipramine in chronic depression. Am J Psychiatry. 2000;157(9):1445–1452. doi: 10.1176/appi.ajp.157.9.1445. [DOI] [PubMed] [Google Scholar]
- Kornstein SG, Clayton AH, Soares CN, Padmanabhan SK, Guico-Pabia CJ. Analysis by age and sex of efficacy data from placebo-controlled trials of desvenlafaxine in outpatients with major depressive disorder. J Clin Psychopharmacol. 2010;30(3):294–299. doi: 10.1097/JCP.0b013e3181dcb594. [DOI] [PubMed] [Google Scholar]
- Kornstein SG, Toups M, Rush AJ, Wisniewski SR, Thase ME, Luther J, Warden D, Fava M, Trived MH. Do menopausal status and use of hormone therapy affect antidepressant treatment response? Findings from the Sequenced Treatment Alternatives to Relieve Depression (STAR*D) study. J Womens Health. 2013;22(2):121–131. doi: 10.1089/jwh.2012.3479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kornstein SG, Pedersen RD, Holland PJ, Nemeroff CB, Rothschild AJ, Thase ME, Trivedi MH, Ninan PT, Keller MB. Influence of sex and menopausal status on response, remission, and recurrence in patients with recurrent major depressive disorder treated with venlafaxine extended release or fluoxetine: analysis of data from the PREVENT study. J Clin Psychiatry. 2014;75(1):62–68. doi: 10.4088/JCP.12m07841. [DOI] [PubMed] [Google Scholar]
- Kornstein S, Chang CT, Gommoll CP, Edwards J. Vilazodone efficacy in subgroups of patients with major depressive disorder: a post-hoc analysis of four randomized, double-blind, placebo-controlled trials. Int Clin Psychopharmacol. 2018;33(4):217–223. doi: 10.1097/YIC.0000000000000217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kornstein SG, Wohlreich MM, Mallinckrodt CH, Watkin JG, Stewart DE (2006) Duloxetine efficacy for major depressive disorder in male vs. female patients: data from 7 randomized, double-blind, placebo-controlled trials. J Clin Psychiatry 67(5):761–770. 10.4088/jcp.v67n0510 [DOI] [PubMed]
- Leon AC, Olfson M, Portera L, Farber L, Sheehan DV. Assessing psychiatric impairment in primary care with the Sheehan Disability Scale. Int J Psychiatry Med. 1997;27(2):93–105. doi: 10.2190/T8EM-C8YH-373N-1UWD. [DOI] [PubMed] [Google Scholar]
- Marcus SM, Young EA, Kerber KB, Kornstein S, Farabaugh AH, Mitchell J, Wisniewski SR, Balasubramani GK, Trivedi MH, Rush AJ. Gender differences in depression: findings from the STAR*D study. J Affect Disord. 2005;87(2–3):141–150. doi: 10.1016/j.jad.2004.09.008. [DOI] [PubMed] [Google Scholar]
- Montgomery SA, Möller HJ. Is the significant superiority of escitalopram compared with other antidepressants clinically relevant? Int Clin Psychopharmacol. 2009;24(3):111–118. doi: 10.1097/YIC.0b013e32832a8eb2. [DOI] [PubMed] [Google Scholar]
- Noordam R, Aarts N, Tiemeier H, Hofman A, Stricker BH, Visser LE. Sex-specific association between antidepressant use and body weight in a population-based study in older adults. J Clin Psychiatry. 2015;76(6):e745–e751. doi: 10.4088/JCP.13m08896. [DOI] [PubMed] [Google Scholar]
- Ochs-Ross R, Daly EJ, Zhang Y, Lane R, Lim P, Morrison RL, Hough D, Manji H, Drevets WC, Sanacora G, Steffens DC, Adler C, McShane R, Gaillard R, Wilkinson ST, Singh JB. Efficacy and safety of esketamine nasal spray plus an oral antidepressant in elderly patients with treatment-resistant depression-TRANSFORM-3. Am J Geriatr Psychiatry. 2020;28(2):121–141. doi: 10.1016/j.jagp.2019.10.008. [DOI] [PubMed] [Google Scholar]
- Pinto-Meza A, Usall J, Serrano-Blanco A, Suárez D, Haro JM (2006) Gender differences in response to antidepressant treatment prescribed in primary care. Does menopause make a difference?. J Affect Disord 93(1–3):53–60. 10.1016/j.jad.2006.02.010 [DOI] [PubMed]
- Popova V, Daly EJ, Trivedi M, Cooper K, Lane R, Lim P, Mazzucco C, Hough D, Thase ME, Shelton RC, Molero P, Vieta E, Bajbouj M, Manji H, Drevets WC, Singh JB. Efficacy and safety of flexibly dosed esketamine nasal spray combined with a newly initiated oral antidepressant in treatment-resistant depression: a randomized double-blind active-controlled study. Am J Psychiatry. 2019;176(6):428–438. doi: 10.1176/appi.ajp.2019.19020172. [DOI] [PubMed] [Google Scholar]
- Preskorn SH. Declining differences in response rates with antidepressants versus placebo: a modest proposal for another contributing cause. J Psychiatr Pract. 2013;19(3):227–233. doi: 10.1097/01.pra.0000430506.37144.d1. [DOI] [PubMed] [Google Scholar]
- Quitkin FM, Stewart JW, McGrath PJ, Taylor BP, Tisminetzky MS, Petkova E, Chen Y, Ma G, Klein DF. Are there differences between women’s and men’s antidepressant responses? Am J Psychiatry. 2002;159(11):1848–1854. doi: 10.1176/appi.ajp.159.11.1848. [DOI] [PubMed] [Google Scholar]
- Schneider LS, Small GW, Clary CM. Estrogen replacement therapy and antidepressant response to sertraline in older depressed women. Am J Geriatr Psychiatry. 2001;9(4):393–399. doi: 10.1097/00019442-200111000-00007. [DOI] [PubMed] [Google Scholar]
- Seedat S, Scott KM, Angermeyer MC, Berglund P, Bromet EJ, Brugha TS, Demyttenaere K, de Girolamo G, Haro JM, Jin R, Karam EG, Kovess-Masfety V, Levinson D, Medina Mora ME, Ono Y, Ormel J, Pennell BE, Posada-Villa J, Sampson NA, Williams D, Kessler RC. Cross-national associations between gender and mental disorders in the World Health Organization World Mental Health Surveys. Arch Gen Psychiatry. 2009;66(7):785–795. doi: 10.1001/archgenpsychiatry.2009.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheehan DV, Lecrubier Y, Sheehan KH, Amorim P, Janavs J, Weiller E, Hergueta T, Baker R, Dunbar GC (1998) The Mini-International Neuropsychiatric Interview (M.I.N.I.): the development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. J Clin Psychiatry 59(suppl 20):22–33; quiz 34–57. PMID: 9881538 [PubMed]
- Spitzer RL, Kroenke K, Williams JB. Validation and utility of a self-report version of PRIME-MD: the PHQ primary care study. JAMA. 1999;282(18):1737–1744. doi: 10.1001/jama.282.18.1737. [DOI] [PubMed] [Google Scholar]
- Spitzer RL, Kroenke K, Williams JB, Löwe B. A brief measure for assessing generalized anxiety disorder: the GAD-7. Arch Intern Med. 2006;166(10):1092–1097. doi: 10.1001/archinte.166.10.1092. [DOI] [PubMed] [Google Scholar]
- Spravato (esketamine) Summary of product characteristics (2020) https://www.medicines.org.uk/emc/product/10977/smpc. Accessed 3 May 2021.
- SpravatoTM (esketamine) nasal spray Prescribing Information. © 2020 Janssen Pharmaceutical Companies.
- Sramek JJ, Murphy MF, Cutler NR (2016) Sex differences in the psychopharmacological treatment of depression. Dialogues Clin Neurosci 18(4):447–457. 10.31887/DCNS.2016.18.4/ncutler [DOI] [PMC free article] [PubMed]
- Thase ME, Entsua R, Cantillon M, Kornstein SG. Relative antidepressant efficacy of venlafaxine and SSRIs: sex-age interactions. J Womens Health (larchmt) (2002) 2005;14(7):609–616. doi: 10.1089/jwh.2005.14.609. [DOI] [PubMed] [Google Scholar]
- Wajs E, Aluisio L, Holder R, Daly EJ, Lane R, Lim P, George JE, Morrison RL, Sanacora G, Young, AH, Kasper S, Sulaiman AH, Li CT, Paik JW, Manji H, Hough D, Grunfeld J, Jeon HJ, Wilkinson ST, Drevets WC, Singh JB (2020) Esketamine nasal spray plus oral antidepressant in patients with treatment-resistant depression: assessment of long-term safety in a phase 3, open-label study (SUSTAIN-2). J Clin Psychiatry 81(3):19m12891. 10.4088/JCP.19m12891 [DOI] [PubMed]
- Williams JB, Kobak KA. Development and reliability of a structured interview guide for the Montgomery Asberg Depression Rating Scale (SIGMA) Br J Psychiatry. 2008;192(1):52–58. doi: 10.1192/bjp.bp.106.032532. [DOI] [PubMed] [Google Scholar]
- Yang SJ, Kim SY, Stewart R, Kim JM, Shin IS, Jung SW, Lee MS, Jeong SH, Jun TY. Gender differences in 12-week antidepressant treatment outcomes for a naturalistic secondary care cohort: the CRESCEND study. Psychiatry Res. 2011;189(1):82–90. doi: 10.1016/j.psychres.2010.12.027. [DOI] [PubMed] [Google Scholar]
- Young EA, Kornstein SG, Marcus SM, Harvey AT, Warden D, Wisniewski SR, Balasubramani GK, Fava M, Trivedi MH, Rush JA. Sex differences in response to citalopram: a STAR*D report. J Psychiatr Res. 2009;43(5):503–511. doi: 10.1016/j.jpsychires.2008.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data sharing policy of Janssen Pharmaceutical Companies of Johnson & Johnson is available at https://www.janssen.com/clinical-trials/transparency. As noted on this site, requests for access to the study data can be submitted through Yale Open Data Access (YODA) Project site at http://yoda.yale.edu.
Not applicable.