Abstract
Objective
To compare the effectiveness and safety of Super-Mini PCNL (SMP) and Retrograde Intrarenal Surgery (RIRS) in the management of renal calculi ≤ 2 cm.
Patients and methods
A prospective, inter-institutional, observational study of patients presenting with renal calculi ≤ 2 cm. Patients underwent either SMP (Group 1) or RIRS (Group 2) and were performed by 2 experienced high-volume surgeons.
Results
Between September 2018 and April 2019, 593 patients underwent PCNL and 239 patients had RIRS in two tertiary centers. Among them, 149 patients were included for the final analysis after propensity-score matching out of which 75 patients underwent SMP in one center and 74 patients underwent RIRS in the other. The stone-free rate (SFR) was statistically significantly higher in Group 1 on POD-1 (98.66% vs. 89.19%; p = 0.015), and was still higher in Group 1 on POD-30 (98.66% vs. 93.24%, p = 0.092) SFR on both POD-1 and POD-30 for lower pole calculi was higher in Group 1 (100 vs. 82.61%, p = 0.047 and 100 vs 92.61% p = 0.171). The mean (SD) operative time was significantly shorter in Group 1 at 36.43 min (14.07) vs 51.15 (17.95) mins (p < 0.0001). The mean hemoglobin drop was significantly less in Group 1 (0.31 vs 0.53 gm%; p = 0.020). There were more Clavien–Dindo complications in Group 2 (p = 0.021). The mean VAS pain score was significantly less in Group 2 at 6 and 12 h postoperatively (2.52 vs 3.67, 1.85 vs 2.40, respectively: p < 0.0001), whereas the mean VAS pain score was significantly less in Group 1 at 24 h postoperatively (0.31 vs 1.01, p < 0.0001). The mean hospital stay was significantly shorter in Group 1 (28.37 vs 45.70 h; p < 0.0001).
Conclusion
SMP has significantly lower operative times, complication rates, shorter hospital stay, with higher stone-free rates compared to RIRS. SMP is associated with more early post-operative pain though.
Keywords: RIRS, SMP, PCNL, Renal stones, Miniaturization, Flexible
Introduction
Nephrolithiasis is a common urologic disease and a major cause of morbidity spanning all age groups with a significant economic burden. The most prevalent are medium-sized renal stones (10–20 mm) while the best management option for these stones depends upon various factors including, stone size, location, density and calyceal anatomy [1, 2].
During the past decade, several treatment options have been introduced and matured including PCNL, SWL and RIRS. PCNL is superior to SWL for stone clearance and is not impaired by anatomical factors and stone density. The evolution of SMP in recent years has further optimized effectiveness by decreasing the complications and morbidity of PCNL [3]. With the new generation of flexible ureterorenoscopes, RIRS has emerged as a favored treatment option for lower-volume renal stones. However, its effectiveness is significantly dependent on calyceal anatomy and stone density [4].
This study was undertaken to bridge the void in high-level evidence that currently exists comparing these two minimally invasive treatment modalities in the treatment of renal calculi ≤ 2 cm. The primary objective was to compare short-term stone-clearance rates. The secondary objectives included outcomes in operating time, hemoglobin (Hb) decline, Clavien–Dindo complication rates, postoperative pain and hospital stay.
Patients and methods
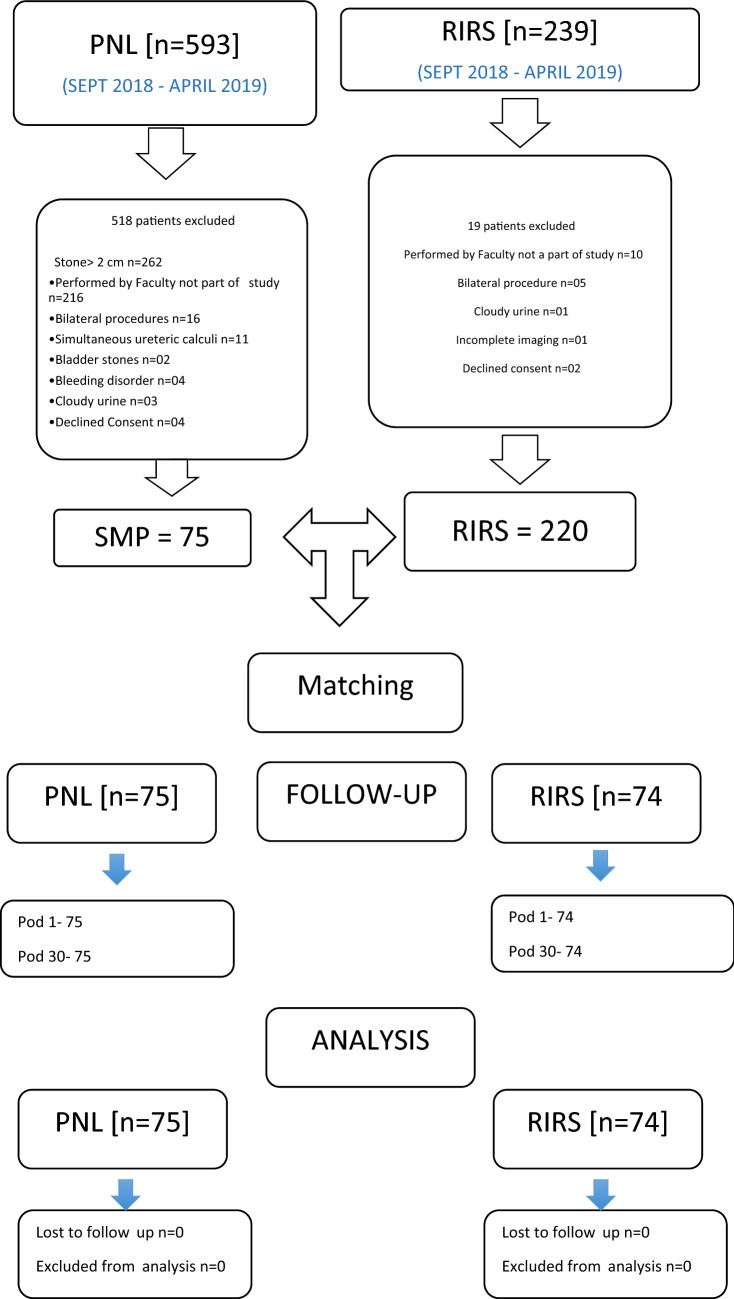
This prospective, inter-institutional, observational and propensity matched study was conducted from September 2018 to April 2019. The study was performed in two high-volume tertiary referral centers, performing > 2000 procedures for stone disease per year including PNLs, Ureteroscopy, and RIRS cases. In each team, two experienced faculty performed the procedures. From data of 593 patients with PCNL and 239 patients with RIRS, 149 patients were included. 75 patients undergoing SMPs for renal calculi of ≤ 2 cm (Group 1) were matched with 74 patients undergoing RIRS (Group 2). The matching was done for stone size, BMI, gender. The study was approved by the Institutional Review Board at both Institutions and informed patient consent was obtained from all study participants (IEC 532/2018 and IRB board approval number– EC/117/2010).
The inclusion criterion was a single renal stone of maximum diameter of ≤ 2 cm or multiple stones with a maximum cumulative diameter of ≤ 2 cm in the same calyx not needing another puncture. The stone size was defined as the maximum diameter as determined by non-contrast CT. Exclusion criteria were patients aged < 18 years, uncorrected coagulopathy, on antiplatelets or anticoagulants, and active urinary tract infections.
Preoperative evaluation included full blood count, serum creatinine and electrolytes, urine culture, coagulation profile, renal ultrasonography (US), X-ray of the kidneys, ureter and bladder (KUB), and non-contrast CT. All procedures were performed under general anesthesia. One dose of antibiotic on induction, followed by two doses postoperatively was given.
SMP
A 5-Fr ureteric catheter was placed retrograde, and patient positioned prone.
The selected calyx was accessed by the urologist under fluoroscopic guidance using an 18G needle and a 0.032-inch guide wire. A single-step metal dilator was then passed, over which the SMP sheath was advanced into the system.
The 14-Fr SMP sheath has an internal working channel caliber of 12.5 F and an oblique channel to which suction is attached (first-generation SMP; R.K. Medical devices, Mumbai, India). This oblique part consists of a pressure vent controlled by its degree of occlusion by the surgeon’s thumb. The irrigation fluid is connected to the side port of either the Nephroscope or SMP sheath. A 12-/7.5-F Nephroscope (Richard Wolf, Knittlingen, Germany) is used. The stone was either completely fragmented or dusted using a Holmium YAG laser. The resultant dust and tiny stone particles pass through the oblique channel into the suction collection bottle.
The larger hard fragments were extracted using a 3-Fr grasper. A JJ stent was inserted whenever indicated. The mean operative time was calculated as the time taken from the calyceal puncture to the completion of the procedure with a skin suture. Immediate stone clearance was confirmed by nephroscopy and fluoroscopy.
Retrograde intra-renal surgery
In lithotomy position, a ureteral guide wire was advanced followed by insertion of an 11/13 or 9/11 Fr ureteral access sheath (Cook Medicals, Bloomington, United states). Tight ureters were stented for a subsequent staged procedure. A Flex X2 ureterorenoscope (Karl Storz, Tuttlingen, Germany) was used in combination with a Holmium Yag Laser for stone fragmentation or dusting. Clear vision was maintained by continuous flow of normal saline irrigation at height of 40 cm attached to the flexible ureteroscope. During stone fragmentation, the flow was adjusted to keep excessive movement of stone as well as clear vision. The laser settings were dependent on the stone density and stone size. Indications for post-operative stenting included the presence of infection, pelvicalyceal system injury, access sheath-related ureteric mucosal injury, preoperative renal impairment, a solitary renal unit, a large stone burden and prolonged operative time. The mean operative time was calculated as the time taken from cystoscopy to stone clearance.
In either of the procedures, if only a JJ stent is placed, the procedure was considered “Tubeless” and when neither JJ stent nor PCN were placed the procedure was termed “Totally Tubeless”. In both groups, postoperative flank pain was assessed using the Visual Analog Scale (VAS) score. Parenteral Tramadol on demand was administered as per institutional protocol in first few hours and orally later when needed. The Clavien–Dindo classification was used to classify the complications. Plain X-ray KUB and renal US were performed by a radiologist to assess stone clearance on the first postoperative day and repeated at 1 month, at the proposed time of stent removal. Any visible hyperechoic area with posterior acoustic shadow on USG and/or any radiopacity on Xray KUB was considered as not stone-free.
Statistical analysis
The sample size was calculated using PASS software (NCSS, LLC, East Kaysville, UT, USA), Chi-square test based formula for Confidence Intervals for the difference between two proportions was used with a 80% power, 5% alpha and 95% level of confidence to demonstrate a difference within 12% in SFR (98% vs 85%) between SMP and RIRS after 3 month of surgery, the minimum sample size for each group was estimated to be 68. To account for patients lost to follow-up and study withdrawals, this number was increased to 75.
Propensity-score matching was done using XLSTAT 2020.3.1.1005 software utilizing Two-sample t test and z-test; and tests on contingency tables (Chi-square).
Results
75 patients underwent SMP (Group 1) and 74 patients underwent RIRS (Group 2). The patient demographics and stone characteristics of both groups were propensity matched (Table 1). The stone-free rate (SFR) was statistically significantly higher in Group 1 on POD-1 (98.66% vs 89.19%, p = 0.015), and higher but not statistically significant on POD-30 (98.66% vs 93.24%, p = 0.092) (Fig. 1). The mean (SD) operative time was significantly shorter in Group 1, at 36.43 (14.07) vs 51.15 (17.95) min (p < 0.0001). Total number of patients with totally tubeless procedure was significantly higher in Group 1 compared to Group 2 (28 vs 6, p < 0.0001).
Table 1.
Demographic and stone characteristics in the two study groups
| SMP | RIRS | p value | |
|---|---|---|---|
| No. of renal units | 75 | 74 | |
| Gender distribution (male: female) | 57: 18 | 51: 23 | = 0.333 |
| Mean age (years) | 48.36 (19–76) | 48.56 (23–76) | = 0.925 |
| Mean BMI (kg/m2) | 25.23 | 25.62 | = 0.544 |
| Comorbidities | |||
| Diabetes mellitus | 8 | 11 | = 0.442 |
| Hypertension | 16 | 27 | = 0.041 |
| Ischemic heart disease | 8 | 5 | = 0.383 |
| Mean size of stone (cm2) (SD) | 1.48 (0.78) | 1.41 (0.35) | = 0.477 |
| Mean Hounsfield units (SD) | 1247 (191) | 1012 (327) | < 0.001 |
| Laterality (right: left) | 38: 37 | 35: 39 | = 0.681 |
| Site of stone | = 0.954 | ||
| Pelvis | 29 | 26 | = 0.655 |
| Upper calyx | 9 | 10 | = 0.708 |
| Middle calyx | 16 | 15 | = 0.873 |
| Lower calyx | 21 | 23 | = 0.759 |
| Mean Pre-op serum creatinine (mg/dl) | 1.09 ± 0.44 | 1.13 ± 0.60 | = 0.624 |
Fig. 1.
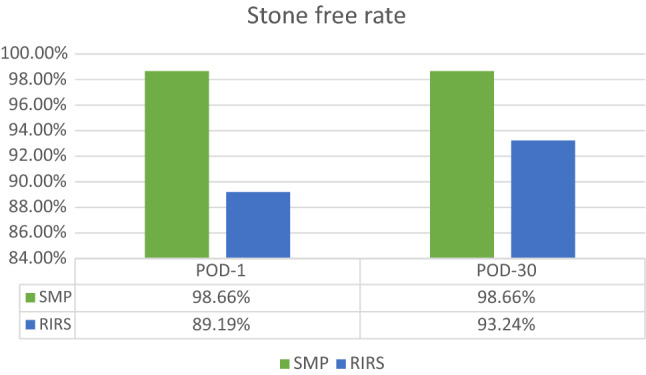
Comparison of stone-free rate in the two study groups
The mean hemoglobin drop was significantly less in Group 1, at 0.31 vs 0.53 gm% (p = 0.020). The mean increase in post-operative serum creatinine was similar in the two groups (p = 0.595).
There were more Clavien–Dindo complications in Group 2 as compared to Group 1 (Table 2). The mean VAS pain score was significantly less in Group 2 at 6 and 12 h postoperatively (2.51 vs 3.67, 1.85 vs 2.40, respectively; p < 0.0001), whereas mean VAS pain score was significantly less in Group 1 at 24 h postoperatively (0.31 vs 1.01, p < 0.0001). The mean hospital stay was also significantly shorter in Group 1 (28.37 vs 45.70 h, p < 0.0001).
Table 2.
Intra-operative and postoperative variables in the two study groups
| SMP | RIRS | p value | |
|---|---|---|---|
| Mean operative time minutes (mean ± SD) | 36.43 ± 14.07 | 51.15 ± 17.95 | < 0.0001 |
| Tubeless | 75 | 74 | |
| Totally tubeless | 28 | 6 | < 0.0001 |
| Postoperative complications | |||
| Clavien–Dindo Grade I | 1 | 1 | = 0.021 |
| Grade 2 | 0 | 7 | |
| Grade 3 | 0 | 2 | |
| Stone-free rate | |||
| POD–1 | 98.66% | 89.19% | = 0.015 |
| Pelvis | 96.55 (1) | 88.46 (4) | = 0.253 |
| Upper calyx | 100 | 100 | |
| Middle calyx | 100 | 93.33 (3) | = 0.301 |
| Lower calyx | 100 | 82.61 (4) | = 0.047 |
| POD-30 | 98.66% | 93.24% | = 0.092 |
| Pelvis | 96.55 (1) | 92.31 (2) | = 0.493 |
| Upper calyx | 100 | 100 | |
| Middle calyx | 100 | 93.33 (1) | = 0.301 |
| Lower calyx | 100 | 91.30 (2) | = 0.171 |
| Mean hemoglobin decline (g %) | 0.31 ± 0.49 | 0.53 ± 0.64 | = 0.020 |
| Mean post-op serum creatinine (mg/dl) | 1.10 ± 0.35 | 1.14 ± 0.61 | = 0.625 |
| Mean increase in serum creatinine (mg/dl) | 0.06 ± 0.09 | 0.07 ± 0.17 | = 0.595 |
| Mean pain visual analog score | |||
| 1 h | 3.67 (0.50) | 2.51 (0.97) | < 0.0001 |
| 6 h | 2.40 (0.49) | 1.85 (0.77) | < 0.0001 |
| 24 h | 0.31 (0.46) | 1.01 (0.77) | < 0.0001 |
| Hospital stay (h) | 28.37 ± 3.6 | 45.70 ± 16.7 | < 0.0001 |
Discussion
It is generally accepted that the best treatment for renal stones < 2 cm is ill-defined and undergoing continuous debate [2–11]. According to the European Association of Urology (EAU) guidelines, shockwave lithotripsy (SWL), percutaneous Nephrolithotomy (PNL), and retrograde intrarenal surgery (RIRS) are recommended treatment options for the treatment of renal calculi < 2 cm [5].
The present study shows that SMP in patients with renal stones of up to 2 cm has higher SFRs, in addition to lower complication rates, blood loss and hospital stay when compared to RIRS. This innovation provides good efficacy in a single session combined with reduced morbidity and most probably economy.
Parallel innovations in both miniaturization of PNL, in an attempt to decrease morbidity [3–9], and digital imaging alongside fiber-optic technology in FURS [4, 11], have all added to the urologist’s armamentarium, and are competing to achieve this much coveted position in the treatment algorithm of such stones.
The last decade has seen numerous studies comparing PNL to RIRS in the management of renal calculi < 2 cm (Table 3). While PNL and RIRS have extensively been analyzed in systematic reviews and meta-analysis [12–17], only one study to date has reviewed SMP versus RIRS for renal stones < 2 cm [18].
Table 3.
Comparison studies of PNL versus RIRS
| Study | Design | Stone | Modality | Cases—N | Stone size (mm) | Months follow-up | SFR (%) | SFR definition | |
|---|---|---|---|---|---|---|---|---|---|
| Zhang [29] | RCT | LPS 1-2 cm |
UMP FURS SWL |
60 60 60 |
3 |
98 92 73 |
Fragment < 3 mm |
16.67 8.33 6.67 |
|
| Zeng [18] | RCT | LPS 1-2 cm |
RIRS SMP |
80 80 |
14.3 ± 3.4 15.0 ± 2.9 |
3 |
86.8 97.4 |
Fragment < 3 mm |
7 7 |
| Kandemir [30] | RCT | LPS ≤ 1.5 cm |
RIRS PNL-4.85Fr |
30 30 |
11.5 10.6 |
3 |
86.7 83.3 |
No residual stone |
16.7 20 |
| Bozzini [31] | RCT | LPS < 2 cm |
SWL RIRS PNL |
194 207 181 |
13.8 ± 3.1 14.8 ± 2.7 15.2 ± 3.3 |
3 |
61.8 82.1 87.3 |
Asymptomatic fragment < 3 mm |
6.7 14.5 19.3 |
| Fayad [32] | RCT | LPS < 2 cm |
RIRS PNL-16Fr |
60 60 |
14.1 ± 3.0 14.7 ± 3.0 |
3 |
84.3 92.7 |
Fragment < 2 mm |
3.3 5 |
| Akbulut [33] | CCT-R | LPS < 2 cm |
PNL-18Fr RIRS |
31 63 |
137.4–62.5 mm2 137.7–40.9 mm2 |
1 |
90.3 85.7 |
Asymptomatic fragment < 3 mm |
29 8 |
| Ozgor [34] | CCT-R | 1–2 cm |
PNL18-20Fr RIRS |
56 56 |
19.5 ± 3.9 18.3 ± 3.2 |
1–3 |
80.4 76.7 |
Fragment < 2 mm |
30.3 5.3 |
| Demirbas [35] | RCT | 1–2.5 cm |
PNL-14Fr RIRS |
30 43 |
185.86–88.29mm2 181.70–114.18mm2 |
1 |
80 74.4 |
Fragment < 3 mm |
23.3 13.9 |
| Kumar [36] | RCT | LPS 1–2 cm |
PNL-18Fr RIRS SWL |
41 42 43 |
13.3 ± 1.3 13.1 ± 1.1 13.2 ± 1.2 |
3 |
95.1 86.1 73.8 |
Fragment < 4 mm |
24.3 9.3 7.1 |
| Lee [37] | RCT | > 1 cm |
PNL-18Fr RIRS |
35 33 |
39.1 ± 30.7 28.9 ± 17.5 |
3 |
85.7 97 |
Fragment < 2 mm |
25.7 27 |
| Schoenthaler [38] | CCT-R-(match) | 1–2 cm |
PNL-14Fr RIRS |
30 30 |
15.1 14.4 |
– |
84 87 |
– |
7 7 |
| Ramon de Fata [39] | CCT | 1–3 cm |
PNL-4.85Fr RIRS |
8 12 |
1.9 cm2 1.3 cm2 |
87.5 91.7 |
12.5 8.3 |
||
| Kirac [40] | CCT-R | < 1.5 cm |
PNL ≤ 20Fr RIRS |
37 36 |
1.05 ± 0.22 1.02 ± 0.29 |
1–3 |
89 88.9 |
Fragment < 3 mm |
18.9 16.7 |
| Pan [41] | CCT-R | 2–3 cm |
PNL-18Fr RIRS |
59 56 |
22.37 ± 2.7 22.28 ± 2.6 |
1 |
96.6 71.4 |
Fragment < 2 mm |
11.9 16.1 |
| Kruck [42] | CCT-R |
PNL-18Fr RIRS SWL |
172 108 202 |
12.6 ± 9.5 6.8 ± 6.9 7.5 ± 5.1 |
3 |
79.6 77.8 58.4 |
No stones |
11.5 8.3 5 |
|
| Ozturk [43] | CCT-R | 1–2 cm |
PNL RIRS SWL |
144 38 221 |
1.74 ± 0.15 1.73 ± 0.15 1.70 ± 0.16 |
93.7 73.7 – |
13.2 5.3 3.2 |
||
| Sabnis [26] | RCT | < 1.5 cm |
PNL-4.85Fr RIRS |
35 35 |
1.1 1.04 |
3 |
97.1 94.3 |
No residual stone |
28.6 14.3 |
| Resorlu [44] | CCT-R | 1–2 cm |
PNL RIRS SWL |
140 46 251 |
17.3 ± 3.6 15.6 ± 3.4 14.9 ± 2.9 |
3 |
91.4 87 66.5 |
Fragment < 3 mm |
22.1 10.9 7.6 |
| Aboutaleb [45] | CCT-R | 1–2 cm |
PNL RIRS SWL |
19 13 24 |
1.73 ± 0.33 1.45 ± 0.32 1.56 ± 0.43 |
89.5 84.6 – |
31.6 46.2 41.7 |
||
| Bozkurt [46] | CCT-R | 1.5–2 cm |
PNL RIRS |
42 37 |
1.70 ± 0.12 1.65 ± 0.69 |
2 |
92.9 89.2 |
Fragment < 3 mm |
16.7 18.9 |
| Kuo [47] | RCT | LP < 2.5 cm |
PNL RIRS |
15 13 |
3 |
66.7 45.6 |
6.7 0 |
||
CCT-R case–control trial-retrospective
The unique irrigation-suction channel with the 14Fr sheath gives SMP a versatility unattained in the prior versions of PNL, as articulated by none other than Dr. Peter Alken, in his editorial, ‘based on my more than 40 years of experience with percutaneous stone removal and intense knowledge of the changes that were introduced I think it is justified to state the SMP technique is the most significant progress in this field and it will likely become the dominant method for percutaneous stone management in the future’ [6]. With miniaturized PCNL, from Micro-perc, Mini-perc to UMP, the reported SFRs have been in the range of 60–90% [3–9]. In comparison, the SFR in our present SMP group is higher at 98.6% [3–9]. This is attained by the dual modality of negative pressure suction, providing clear vision for effective fragmentation and extraction along with the use of a 3-F grasper to extract fragments from different calyces. Also, a lower intra-renal operating pressure ≤ 25 mmHg is better maintained [19].
On the other hand, RIRS has the distinct advantage of not breaching the renal parenchyma. The ‘trade-off’ is however its dependence on the anatomical favorability of the ureter and pelvicalyceal system. The lower calyceal stones are a particular challenge, especially those with acute IPAs and narrow infundibula. Prolonged lasing time is yet another issue. The modern generation fURS with its enhanced maneuverability and vision affords a less invasive and safe option, but its effectiveness is tempered by the stone burden and density [20]. Studies in the management of “small renal stones”, show RIRS to have a stone-free rate of about 65–92% [4]. This is lesser in lower calyceal calculi, especially with unfavorable anatomy. Other drawbacks are the need for staged procedures in the case of hostile tight ureters, the higher costs of RIRS compared to PCNL, the risk of ureter injury, and the requirement of post-operative JJ stent [21, 22].
Stone-free rates of SMP range from 93.2% to 96.2% [3, 6, 18, 23]. This is in line with the present study and significantly higher than RIRS on POD-1 (98.66% vs 89.19%, p = 0.015). In SMP, small fragments and dust are removed by suction. Larger fragments were removed using 3F grasper. In RIRS, complete extraction of all fragments was not possible intraoperatively, thereby necessitating ureteral stenting. Some fragments may settle in the lower calyx and possibly act as a nidus for future stone growth. Upon subgroup analysis of stone-free rates in lower calyceal stones, SMP is superior to RIRS both on POD 1 and POD 30 (Table 2). Difficult angulation, narrow infundibula and prolonged lasing time make complete stone clearance challenging [24]. SMP bypasses these anatomical restrictions and allows for the effective removal of all stone fragments in a single treatment session.
At 1-month follow-up, SFR was still better in the SMP group, but was not statistically significant anymore (98.66% vs 93.24%, p = 0.092). This increased SFR in RIRS at 1 month, is explained by the spontaneous passage of the smaller stone fragments. SMP gives early stone-free rates while in RIRS, SFR keep on improving with time. SFR in RIRS on POD 30, though not statistically different were still lower than SMP. This difference would have been more significant had propensity matching included HU of stones. This highlights importance of patient counseling about postoperative fluid intake to let stone dust drain clear of urinary tract. There is no uniformity in published literature regarding standard time of evaluation for SFR in post RIRS patients.
The morbidity is on multiple aspects in the disadvantage of RIRS. The mean operative time was significantly longer in the RIRS group, the postoperative complications were significantly more common in the RIRS group. Interestingly, the Hb drop was higher, and hospital stay longer in the RIRS group.
The mean operative time was significantly longer in the RIRS group, 36.43 vs 51.15 min (p < 0.0001). This results from more time required to access, stabilize, fragment and extract the calculi in RIRS. In SMP, the suction helps to aggregate fragments at the opening of the sheath, thereby aiding in faster lithotripsy. Also, harder, bigger fragments can be retrieved using a grasper. Studies have already shown RIRS requiring longer operative times in stones of size > 1 cm [20].
Postoperative complications were significantly more common in the RIRS group (p = 0.021). 10 of the 75 patients in the RIRS group developed complications. 8 patients had postoperative fever managed conservatively. 1 patient required aspiration of urinoma detected intraoperatively. These were graded according to Clavien–Dindo classification. Grade 1 complication was noted in one patient in each group. Seven patients had grade 2 and two patients had grade 3 complications, which included a stent blockage that mandated an early stent removal on POD 3 and the other was a fluid collection in the abdomen that mandated an aspiration believed to be ureteric injury related to UAS insertion in the RIRS group. None of the patients had higher grade complications in SMP group.
Postoperative fever was the most common complication of the patients treated by RIRS. The increased renal pelvic pressures from irrigation may cause pyelovenous and pyelolymphatic backflow, leading to these infectious or non-infectious complications [25]. Conversely, SMP, with the continuous negative pressure aspiration maintains the intrarenal pressure consistently below 25 mmHg [19].
Mean increase in post-operative serum creatinine was similar in the two groups (p = 0.595). Mean hemoglobin drop was more in the RIRS group (p = 0.020). RIRS is associated with less Hemoglobin drop as compared standard PCNL. However, this comparison is not available with UMP, SMP. Micro-PNL has been found to be associated with reduced blood loss though not slighter than RIRS [26]. Mean hemoglobin drop in RIRS group could be due to continuous minimal ooze from access sheath associated Grade 1 mucosal injuries, mucosal injuries by laser fiber during stone pulverization. Prolonged time of fragmentation especially of lower calyceal stones with continuous irrigation often masks blood loss. Severe bleeding and sepsis have also been reported in other studies [27]. Straight tract in SMP with an easy maneuverability of the nephroscope in the collecting system, ease in laser fragmentation of stones in any calyx and stone fragments extraction collectively lead to reduced loss and decreased Hb drop in SMP. None of the patients in either groups had blood transfusion in our study.
Postoperative pain was significantly less in the RIRS group at 1 and 6 h (2.51 vs 3.67, 1.85 vs 2.40, respectively). The prolonged operative time with increased intra-renal pressures causing the renal capsule to stretch and potential extravasation can lead to persistent pain. However, at 24 h, pain was significantly less in the SMP group (0.31 vs 1.01). Postoperative pain is seen to be associated with nephrostomy tubes, tract size and intercostal nerve injury. All patients in our SMP group were ‘tubeless’. The SMP group had more ‘totally tubeless’ patients (28 versus 6 in the RIRS group), which could also explain the observed difference [21]. The absence of a stent also obviated another hospital visit for its removal.
Length of hospital stay was calculated in hours, after the surgery. The hospital stay was significantly shorter in the SMP group (28.37 vs 45.70 h) and was similar to that reported by Shah et al. [28] (28.37 vs 31.53 h). Reduced pain scores at 24 h, reduced complications and decreased need of any external drainage could all be contributory.
The ultimate goal of achieving ‘stone-free status’ in a single session with minimal invasiveness, minimal complications, short treatment time, decreased recurrence risk, and decreased costs is central in developing our treatment strategy for renal calculous disease, more so in countries with limited resources.
Limitations
Our study utilized X-ray KUB and renal US to evaluate stone clearance. These minimized the radiation exposure and were economically viable in all our patients. However, non-contrast CT would have given a more accurate assessment. Another limitation was the prevalence of lower calyceal calculi as the second-commonest location after pelvic calculi in both study groups. This could, in part, account for the poorer results in the RIRS cohort. Another limitation of this study was that propensity matching did not include HU of stones, which may have changed results negatively for RIRS in terms of SFR on POD 30 and mean procedure time.
While institutional protocols were followed, some differences between both institutes may have influenced outcomes and complications. Although only high-volume surgeons have been involved in treating all patients, this may also not translate in generalizability of this study. Hence higher complications by a certain procedure may be reflected in the difference in techniques between operators. Obviously high volume does not automatically imply better outcomes per se. As for the evaluation, two different radiologists were involved in the study and different machines/equipment were used for evaluation. This may also result in differences in evaluation of SFR. Finally, VAS score was only for flank pain and USSQ stent-related questionnaire for stent-related symptoms was not used for patients with ureteral stents.
Conclusion
SMP provides early stone-free rates as compared or better to RIRS in a single session combined with reduced morbidity, in the management of renal calculus of size less than 2 cm. Although SMP is associated with more early post-operative pain, it has significantly lower operative times, complication rates and a shorter hospital stay.
Author contributions
SP—Manuscript writing/editing, AC—Manuscript writing/editing, JdlR—Protocol/project development, PL—Protocol/project development, RG—Data collection or management, SJR—Manuscript writing/editing, RS—Data analysis, AG—Protocol/project development, MD—Data analysis, AP—Manuscript writing/editing.
Funding
Open access funding provided by Manipal Academy of Higher Education, Manipal.
Code availability
Not applicable.
Declarations
Conflict of interest
Nil.
Ethics approval
Approve by institutional ethics committee (IEC 532/2018 and IRB board approval number—EC/117/2010
Consent for publication
All authors consent for the publication of the article.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Sunil Bhaskara Pillai, Email: sunil.pillai@manipal.edu.
Arun Chawla, Email: urologyarun@yahoo.com.
Jean de la Rosette, Email: j.j.delarosette@gmail.com.
Pilar Laguna, Email: m.p.laguna@gmail.com.
Rajsekhar Guddeti, Email: sekhar112raja@gmail.com.
Suraj Jayadeva Reddy, Email: drsuraj2012@gmail.com.
Ravindra Sabnis, Email: rbsabnis@gmail.com.
Arvind Ganpule, Email: doctorarvind1@gmail.com.
Mahesh Desai, Email: mrdesai@mpuh.org.
Aditya Parikh, Email: adityaparikh26@gmail.com.
References
- 1.Scales CD, Jr, Smith AC, Hanley JM, Saigal CS. Urologic diseases in America project prevalence of kidney stones in the United States. Eur Urol. 2012;62(1):160–165. doi: 10.1016/j.eururo.2012.03.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Raman JD, Pearle MS. Management options for lower pole renal calculi. Curr Opin Urol. 2008;18(2):214–219. doi: 10.1097/MOU.0b013e3282f517ea. [DOI] [PubMed] [Google Scholar]
- 3.Zeng G, Wan S, Zhao Z, et al. Super-mini percutaneous nephrolithotomy (SMP): a new concept in technique and instrumentation. BJU Int. 2016;117(4):655–661. doi: 10.1111/bju.13242. [DOI] [PubMed] [Google Scholar]
- 4.Kılıc O, Akand M, Van Cleynenbreugel B. Retrograde intrarenal surgery for renal stones e Part 2. Turk J Urol. 2017;43:252–260. doi: 10.5152/tud.2017.22697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Turk C, Knoll T, Petrik A et al (2020) Guideline on urolithiasis. European Association of Urology, pp 24–25. Available at http://uroweb.org/guideline/urolithiasis/2020
- 6.Alken P. Is super-mini-PCNL (SMP) a new way in the future? BJU Int. 2020;125:483–484. doi: 10.1111/bju.14969. [DOI] [PubMed] [Google Scholar]
- 7.Kukreja R, Desai M, Patel S, Bapat S, Desai M. Factors affecting blood loss during percutaneous nephrolithotomy: prospective study. J Endourol. 2004;18:715–722. doi: 10.1089/end.2004.18.715. [DOI] [PubMed] [Google Scholar]
- 8.Desai J, Solanki R. Ultra-mini percutaneous nephrolithotomy (UMP): one more armamentarium. BJU Int. 2013;112:1046–1049. doi: 10.1111/bju.12193. [DOI] [PubMed] [Google Scholar]
- 9.Desai MR, Sharma R, Mishra S, Sabnis RB, Stief C, Bader M. Single-step percutaneous nephrolithotomy (microperc): the initial clinical report. J Urol. 2011;186:140–145. doi: 10.1016/j.juro.2011.03.029. [DOI] [PubMed] [Google Scholar]
- 10.Jackman SV, Docimo SG, Cadeddu JA, Bishoff JT, Kavoussi LR, Jarrett T. The ‘mini-perc’ technique: a less invasive alternative to percutaneous nephrolithotomy. World J Urol. 1998;16:371–374. doi: 10.1007/s003450050083. [DOI] [PubMed] [Google Scholar]
- 11.Alenezi H, Denstedt JD. Flexible ureteroscopy: technological advancements, current indications and outcomes in the treatment of urolithiasis. Asian J Urol. 2015;2(3):133–141. doi: 10.1016/j.ajur.2015.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Srisubat A, Potisat S, Lojanapiwat B, Setthawong V, Laopaiboon M (2009) Extracorporeal shock wave lithotripsy (ESWL) versus percutaneous nephrolithotomy (PCNL) or retrograde intrarenal surgery (RIRS) for kidney stones. Cochrane Database Syst Rev (4):CD007044 [DOI] [PMC free article] [PubMed]
- 13.Tsai SH, Chung HJ, Tseng PT, Wu YC, Tu YK, Hsu CW, Lei WT. Comparison of the efficacy and safety of shockwave lithotripsy, retrograde intrarenal surgery, percutaneous nephrolithotomy and minimally invasive percutaneous nephrolithotomy for lower-pole renal stones: A systematic review and network meta-analysis. Medicine. 2020;99:10(e19403). doi: 10.1097/MD.0000000000019403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gao X-S, Liao BH, Chen YT, et al. Different tract sizes of miniaturized percutaneous Nephrolithotomy versus retrograde intrarenal surgery: a systematic review and meta-analysis. J Endourol. 2017;31:1101–1110. doi: 10.1089/end.2017.0547. [DOI] [PubMed] [Google Scholar]
- 15.De S, et al. Percutaneous nephrolithotomy versus retrograde intrarenal surgery: a systematic review and meta-analysis. Eur Urol. 2015;67:125. doi: 10.1016/j.eururo.2014.07.003. [DOI] [PubMed] [Google Scholar]
- 16.Zhang W, et al. Retrograde intrarenal surgery versus percutaneous nephrolithotomy versus extracorporeal shockwave lithotripsy for treatment of lower pole renal stones: a meta-analysis and systematic review. J Endourol. 2015;29:745–759. doi: 10.1089/end.2014.0799. [DOI] [PubMed] [Google Scholar]
- 17.Donaldson JF, Lardas M, Scrimgeour D, et al. Systematic review and meta-analysis of the clinical effectiveness of shock wave lithotripsy, retrograde intrarenal surgery, and percutaneous nephrolithotomy for lower-pole renal stones. Eur Urol. 2015;67:612–616. doi: 10.1016/j.eururo.2014.09.054. [DOI] [PubMed] [Google Scholar]
- 18.Zeng G, Zhang T, Agrawal M, et al. Super-mini percutaneous nephrolithotomy (SMP) vs retrograde intrarenal surgery for the treatment of 1–2 cm lower-pole renal calculi: an international multicenter randomised controlled trial. BJU Int. 2018;122:1034–1040. doi: 10.1111/bju.14427. [DOI] [PubMed] [Google Scholar]
- 19.Alsmadi J, Fan J, Zhu W, Wen Z, Zeng G. The influence of super-mini percutaneous nephrolithotomy (SMPL) on renal pelvic pressure in vivo. J Endourol. 2018;32:819–823. doi: 10.1089/end.2018.0239. [DOI] [PubMed] [Google Scholar]
- 20.Sorokin I, Cardona-Grau DK, Rehfuss A, Birney A, Stavrakis C, Leinwand G, Herr A, Feustel PJ, White MD. Stone volume is best predictor of operative time required in retrograde intrarenal surgery for renal calculi: implications for surgical planning and quality improvement. Urolithiasis. 2016;44(6):545–550. doi: 10.1007/s00240-016-0875-8. [DOI] [PubMed] [Google Scholar]
- 21.Byrne RR, Auge BK, Kourambas J, Munver R, Delvecchio F, Preminger GM. Routine ureteral stenting is not necessary after ureteroscopy and ureteropyeloscopy: a randomized trial. J Endourol. 2002;16(1):9–13. doi: 10.1089/089277902753483646. [DOI] [PubMed] [Google Scholar]
- 22.Zeng G, Zhu W, Li J, Zhao Z, Zeng T, Liu C, Liu Y, Yuan J, Wan SP. The comparison of minimally invasive percutaneous nephrolithotomy and retrograde intrarenal surgery for stones larger than 2 cm in patients with a solitary kidney: a matched-pair analysis. World J Urol. 2015;33(8):1159–1164. doi: 10.1007/s00345-014-1420-4. [DOI] [PubMed] [Google Scholar]
- 23.Guddeti RS, Hegde P, Chawla A, de la Rosette JJ, Laguna Pes MP, Kapadia A. Super-mini percutaneous nephrolithotomy (PCNL) vs standard PCNL for the management of renal calculi of< 2 cm: a randomised controlled study. BJU Int. 2020;126(2):273–279. doi: 10.1111/bju.15144. [DOI] [PubMed] [Google Scholar]
- 24.Breda A, Ogunyemi O, Leppert JT, Schulam PG. Flexible ureteroscopy and laser lithotripsy for multiple unilateral intrarenal stones. Eur Urol. 2009;55:1190–1196. doi: 10.1016/j.eururo.2008.06.019. [DOI] [PubMed] [Google Scholar]
- 25.Suh LK, Rothberg MB, Landman J, Katsumi H, Gupta M. Intrarenal pressures generated during deployment of various antiretropulsion devices in an ex vivo porcine model. J Endourol. 2010;24(7):1165–1168. doi: 10.1089/end.2010.0118. [DOI] [PubMed] [Google Scholar]
- 26.Sabnis RB, Ganesamoni R, Doshi A, Ganpule AP, Jagtap J, Desai MR. Micropercutaneous nephrolithotomy (microperc) vs retrograde intrarenal surgery for the management of small renal calculi; a randomized controlled trial. BJU Int. 2013;112(3):355–361. doi: 10.1111/bju.12164. [DOI] [PubMed] [Google Scholar]
- 27.Xu Y, Min Z, Wan SP, Nie H, Duan G. Complications of retrograde intrarenal surgery classified by the modified Clavien grading system. Urolithiasis. 2018;46(2):197–202. doi: 10.1007/s00240-017-0961-6. [DOI] [PubMed] [Google Scholar]
- 28.Shah K, Agrawal MS, Mishra DK. Superperc: a new technique in minimally invasive percutaneous nephrolithotomy. Indian J Urol. 2017;33:48–52. doi: 10.4103/0970-1591.194784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhang H, Hong T, Li G, Jiang N, Hu C, Cui X, Chu C, Zhao J. Comparison of the efficacy of ultra-mini PCNL, flexible ureteroscopy, and shock wave lithotripsy on the treatment of 1–2 cm lower pole renal calculi. Urol Int. 2019;102:153–159. doi: 10.1159/000493508. [DOI] [PubMed] [Google Scholar]
- 30.Kandemir A, Guven S, Balasar M, et al. A prospective randomized comparison of micropercutaneous nephrolithotomy (Microperc) and retrograde intrarenal surgery (RIRS) for the management of lower pole kidney stones. World J Urol. 2017;35:1771–1776. doi: 10.1007/s00345-017-2058-9. [DOI] [PubMed] [Google Scholar]
- 31.Bozzini G, Verze P, Arcaniolo D, et al. A prospective randomized comparison among SWL, PCNL and RIRS for lower calyceal stones less than 2 cm: a multicenter experience: a better understanding on the treatment options for lower pole stones. World J Urol. 2017;35:1967–1975. doi: 10.1007/s00345-017-2084-7. [DOI] [PubMed] [Google Scholar]
- 32.Fayad AS, Elsheikh MG, Ghoneima W. Tubeless mini-percutaneous nephrolithotomy versus retrograde intrarenal surgery for lower calyceal stones of 2 cm: a prospective randomised controlled study. Arab J Urol. 2017;15:36–41. doi: 10.1016/j.aju.2016.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Akbulut F, Kucuktopcu O, Kandemir E, et al. Comparison of flexible ureterorenoscopy and mini-percutaneous nephrolithotomy in treatment of lower calyceal stones smaller than 2 cm. Ren Fail. 2016;38:163–167. doi: 10.3109/0886022X.2015.1128792. [DOI] [PubMed] [Google Scholar]
- 34.Ozgor F, Tepeler A, Elbir F, et al. Comparison of miniaturized percutaneous nephrolithotomy and flexible ureterorenoscopy for the management of 10–20mm renal stones in obese patients. World J Urol. 2016;34:1169–1173. doi: 10.1007/s00345-015-1745-7. [DOI] [PubMed] [Google Scholar]
- 35.Demirbas A, Resorlu B, Sunay MM, et al. Which should be preferred for moderate-size kidney stones? Ultramini percutaneous nephrolithotomy or retrograde intrarenal surgery? J Endourol. 2016;30:1285–1289. doi: 10.1089/end.2016.0370. [DOI] [PubMed] [Google Scholar]
- 36.Kumar A, Kumar N, Vasudeva P, et al. A prospective, randomized comparison of shock wave lithotripsy, retrograde intrarenal surgery and miniperc for treatment of 1 to 2 cm radiolucent lower calyceal renal calculi: a single center experience. J Urol. 2015;193:1604. doi: 10.1016/j.juro.2014.07.088. [DOI] [PubMed] [Google Scholar]
- 37.Lee JW, Park J, Lee SB, et al. Mini-percutaneous Nephrolithotomy vs retrograde intrarenal surgery for renal stones larger than 10 mm: a prospective randomized controlled trial. Urology. 2015;86:873–877. doi: 10.1016/j.urology.2015.08.011. [DOI] [PubMed] [Google Scholar]
- 38.Schoenthaler M, Wilhelm K, Hein S, et al. Ultra-mini PCNL versus flexible ureteroscopy: a matched analysis of treatment costs (endoscopes and disposables) in patients with renal stones 10–20mm. World J Urol. 2015;33:1601–1605. doi: 10.1007/s00345-015-1489-4. [DOI] [PubMed] [Google Scholar]
- 39.Ramon de Fata F, Garcia-Tello A, Andres G, et al. Comparative study of retrograde intrarenal surgery and micropercutaneous nephrolithotomy in the treatment of intermediate-sized kidney stones. Actas Urol Esp. 2014;38:576–583. doi: 10.1016/j.acuro.2014.04.004. [DOI] [PubMed] [Google Scholar]
- 40.Kirac M, Bozkurt OF, Tunc L, et al. Comparison of retrograde intrarenal surgery and mini-percutaneous nephrolithotomy in management of lower-pole renal stones with a diameter of smaller than 15 mm. Urolithiasis. 2013;41:241–246. doi: 10.1007/s00240-013-0552-0. [DOI] [PubMed] [Google Scholar]
- 41.Pan J, Chen Q, Xue W, et al. RIRS versus mPCNL for single renal stone of 2–3 cm: clinical outcome and cost effective analysis in Chinese medical setting. Urolithiasis. 2013;41:73–78. doi: 10.1007/s00240-012-0533-8. [DOI] [PubMed] [Google Scholar]
- 42.Kruck S, Anastasiadis AG, Herrmann TR, et al. Minimally invasive percutaneous nephrolithotomy: an alternative to retrograde intrarenal surgery and shockwave lithotripsy. World J Urol. 2013;31:1555–1561. doi: 10.1007/s00345-012-0962-6. [DOI] [PubMed] [Google Scholar]
- 43.Ozturk U, Sener NC, Goktug HN, Nalbant I, Gucuk A, Imamoglu MA. Comparison of percutaneous nephrolithotomy, shock wave lithotripsy, and retrograde intrarenal surgery for lower pole renal calculi 10–20 mm. Urol Int. 2013;91:345–349. doi: 10.1159/000351136. [DOI] [PubMed] [Google Scholar]
- 44.Resorlu B, Unsal A, Ziypak T, et al. Comparison of retrograde intrarenal surgery, shockwave lithotripsy, and percutaneous nephrolithotomy for treatment of medium-sized radiolucent renal stones. World J Urol. 2013;31:1581–1586. doi: 10.1007/s00345-012-0991-1. [DOI] [PubMed] [Google Scholar]
- 45.Aboutaleb H, El-Shazly M, Badr EM. Lower pole midsize (1–2 cm) calyceal stones: outcome analysis of 56 cases. Urol Int. 2012;89:348–354. doi: 10.1159/000341557. [DOI] [PubMed] [Google Scholar]
- 46.Bozkurt OF, Resorlu B, Yildiz Y, Can CE, Unsal A. Retrograde intrarenal surgery versus percutaneous nephrolithotomy in the management of lower-pole renal stones with a diameter of 15 to 20 mm. J Endourol. 2011;25:1131–1135. doi: 10.1089/end.2010.0737. [DOI] [PubMed] [Google Scholar]
- 47.Kuo RL, Lingeman JE, Leveillee RJ, et al. Lower pole II: initial results from a comparison of shock wave lithotripsy (SWL), ureteroscopy (URS), and percutaneous nephrolithotomy (PNL) for lower pole nephrolithiasis. J Urol. 2003;169(Suppl):486. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.