Abstract
Background
161Tb draws an increasing interest in nuclear medicine for therapeutic applications. More than 99% of the emitted gamma and X-rays of 161Tb have an energy below 100 keV. Consequently, precise activity measurement of 161Tb becomes inaccurate with radionuclide dose calibrators when using inappropriate containers or calibration factors to account for the attenuation of this low energy radiation. To evaluate the ionization chamber response, the sample activity must be well known. This can be performed using standards traceable to the Système International de Référence, which is briefly described as well as the method to standardize the radionuclides.
Methods
In this study, the response of an ionization chamber using different container types and volumes was assessed using 161Tb. The containers were filled with a standardized activity solution of 161Tb and measured with a dedicated ionization chamber, providing an accurate response. The results were compared with standardized solutions of high-energy gamma-emitting radionuclides such as 137Cs, 60Co, 133Ba and 57Co.
Results
For the glass vial type with an irregular glass thickness, the 161Tb measurements gave a deviation of 4.5% between two vials of the same type. The other glass vial types have a much more regular thickness and no discrepancy was observed in the response of the ionization chamber for these type of vials. Measurements with a plastic Eppendorf tube showed stable response, with greater sensitivity than the glass vials.
Conclusion
Ionization chamber measurements for low-energy gamma emitters (< 100 keV), show deviation depending on the container type used. Therefore, a careful selection of the container type must be done for activity assessment of 161Tb using radionuclide dose calibrators. In conclusion, it was highlighted that appropriate calibration factors must be used for each container geometry when measuring 161Tb and, more generally, for low-energy gamma emitters.
Keywords: 161Tb, Système International de Référence, Low-energy gamma, Activity measurement, Radionuclide dose calibrator
Introduction
Radionuclides are used extensively in nuclear medicine worldwide for diagnosis, using positron emission tomography (PET) or single-photon emission computed tomography (SPECT), and for therapy, using α-particles, β-particles or Auger electrons as locally-deposited energy to treat tumors and metastases using the locally deposited energy. The need for precise quantification of the radioactivity injected into the patient is becoming more important to optimize the image quality in diagnosis and to perform personalized therapy and accurate dosimetry.
In radionuclide production centers, preclinical research laboratories and nuclear medicine facilities, the activity of radionuclides is measured using radionuclide dose calibrators (RDCs). It is important to stress that such an instrument has to be calibrated with well-known samples in a similar physical or chemical form as used in the laboratory in question, using the same container type. Measurements with RDCs are sensitive to geometrical variations, such as the position of the container in the chamber well, the container type, syringe or vial and their shape, wall thickness, material components and container-filling volume. Most RDCs are usually calibrated with one calibration factor per radionuclide, usually in a "vial geometry" provided by the manufacturer. However, the container used for activity measurement has to be well selected, particularly for the measurement of low-energy gamma, X-ray and β-emitters, for which the attenuation will depend on the container type.
Several studies were carried out using different RDC types, with different radionuclides and geometries. Santos et al. reported the effects of geometry for 99mTc (main gamma emission is at 141 keV) [1]. This study showed that the volume of the solution must be placed in an area sufficiently deep in the well of the RDC chamber in question. Thus, if the syringe holder is too high or the syringe too full, large measurement errors of about 10% or more can occur. Cessna et al. [2] reported the calculation of a calibration factor for plastic syringes with 18F and compared it with the manufacturer's value given for a glass vial. The value obtained was approximately 12% higher for the two tested instruments, which meant that the response of the RDC was greater than that expected for the manufacturer's calibration factor. This is due to the fact that the manufacturer calibration factor value was obtain with a glass vial while, in the case of a syringe, the radiation is less attenuated. Moreover, the solution was not located in the same position in the RDC well as for the glass vial. The comparison of 111In activity measurements in a RDC with glass vials and plastic syringes showed that, for low-energy gamma-rays (23–26 keV of 111In), the measurement error can reach 35% even when using glass vials [3]. Olsovcova highlighted that the effect of low-energy gamma-rays of 123I (~ 30 keV) for plastic syringes can lead to an activity measurement error of 40% [4]. The effect of low energies (20–40 keV) for different container types were also discussed in [5]. An error of up to 25% for syringes with 111In was observed, confirming the results from [3, 4] described previously. A recent article [6] reported the activity measurements of 99mTc, 111In, 18F, and 68Ga with different syringes and vials using RDCs, showing discrepancies of up to 30% for 111In for syringes, due to the fact that only one calibration factor was available from the manufacturer and was calculated for glass vials. Significant differences were also reported for the other radionuclides depending on the geometries used. Vargas et al. performed a similar study to investigate the accuracy of RDCs for 99Tc, 111I, 123I, 124I, 131I 177Lu and 90Y [7]. An international multi-center investigation was reported using 32 RDCs from 8 hospitals located in The Netherlands, Belgium and Germany, respectively. It was concluded that, for 111I, 123I, 124I and 90Y, the response of RDCs was particularly sensitive to the sample and detector geometry.
This brief introduction to RDC measurements highlights the important requirements for the accurate use of these devices. For measurements of almost pure γ-sources like 57Co, 60Co, 99mTc or 131I, where electron emission energy is below 500 keV, the value of the calibration factors given by the manufacturer can generally be used for syringes and vials. On the other hand, for low-energy gamma emitters (below 100 keV) large deviations can be measured (up to 40%). In order to calibrate the RDC for each geometry, a well-known radioactive solution is needed. Such a standard, usually provided by Nuclear Metrology Institutes (NMIs), should be traceable to international comparisons. In practice, standards are provided by NMIs or Designated Institutes by the NMI in each country. To ensure good measurements practices by NMIs, the governments and the regulatory organizations worldwide have defined a comparison program at the General Conference on Weights and Measures (CGPM) to ensure the traceability of measurement standards. The Bureau of Weights and Measures (BIPM) produced a dedicated document, International Committee for Weights and Measures Mutual Recognition Agreement (CIPM MRA), initially written in 1999, revised in 2006 and signed by 106 institutes from 62 member states, 40 associated states of the CGPM and 4 international organizations [8]. The CIPM MRA is the framework through which NMIs demonstrate the international equivalence of their measurement standards and the calibration and measurement certificates. The outcomes of the arrangement are internationally recognized (peer-reviewed and approved).
Calibration and Measurement Capabilities (CMCs) describe the radionuclide measurement capabilities of the NMIs and then the realization of standards. This capability is evaluated with the measurement comparisons between NMIs. These results provide average values to validate the different standardization measurement techniques. The CIPM MRA established a program by which the results from one radionuclide metrology laboratory can be considered in the same context as a measurement of the same quantity at another NMI.
When a NMI standardizes a radionuclide and wants to participate in a comparison study to obtain the CMC, it submits a sample of its standardized solution to the Système International de Référence (SIR). The SIR is the measurement system, based at BIPM, where the comparison measurement is performed with a dedicated ionization chamber and the comparison results are evaluated. Since the CIPM MRA was signed in 1999, the SIR has been designated as the method by which NMIs compare their results [9, 10]. Approved CMCs and supporting technical data are publicly available from the CIPM MRA database, the Key Comparison Data Base (KCDB) [11]. In practice, when a NMI standardizes a radionuclide with primary measurement techniques, an aliquot of the standardized solution is used to fill a dedicated ampoule with a specific geometry and a precisely-known mass. The sealed ampoule is sent to the BIPM for measurement with the SIR. The same standardized solution is used to fill dedicated ampoules at the NMI, which are measured in a dedicated ionization chamber.
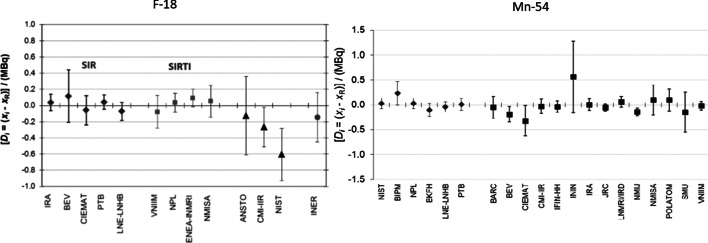
At the Institute of Radiation Physics (IRA; Lausanne, Switzerland) this dedicated ionization chamber is called Chambre d’Ionisation de Référence (CIR). It has been in operation since 1983 and is regularly maintained and checked to ensure its stability [12]. Using the known activity of the ampoules, a calibration factor, called equivalent activity (Ae), is calculated with an accuracy of around 0.5%. Once Ae is known, the CIR is used to calculate the unknown activity of any solution containing the radionuclide of interest. This chamber can therefore, be used to measure the activity of solutions and to produce standards that are traceable to the SIR. In Switzerland, IRA is the designated institute, by the Swiss Federal Office of Metrology (METAS), for radioactivity measurement. IRA has more than 20 CMCs and provides standards for the calibration of radioactivity measurement instruments. Figure 1 shows two examples of comparison measurements performed for 18F and 54Mn by IRA and other NMIs [13, 14]. As the radioactivity users have to establish a quality assurance system for activity measurement, the measurement devices must be calibrated using reference standards and also be regularly verified as defined in the Swiss regulation [15, 16]. According to this regulation, the activity of each dose unit must be determined before medical use. The measurement can be made by an instrument calibrated with nationally-recognized standards or manufacturer’s instructions. RDC is the device recognized by the authority to assay activity before administration to patients. The regulation in Switzerland allows a tolerance of ± 10% [15, 16] for the activity measured with a RDC, while the International Atomic Energy Agency (IAEA) recommends ± 5% [17]. Clearly, the use of quality standards for the calibration of RDCs is required. Providing certified standards ensures the accurate calibration of radioactivity measurement devices in the scientific community, allowing quality assurance for the end users, such as nuclear medicine services.
Fig. 1.
International comparison for 18F and 54Mn for IRA and other NMIs in the Système International de Référence. The vertical axis reports the difference between the reference activity (XR) and the measured activity (Xi)
The beta-emitting 161Tb (Eβav−: 154 keV (100%), T1/2 = 6.953 d [18]) is an attractive radionuclide for targeted radionuclide therapy. It has been considered as an alternative to the clinically-approved 177Lu [19]. Dose calculations have demonstrated that 161Tb can be more effective than 177Lu for small tumor lesions due to the emission of low-energy conversion and Auger electrons [20, 21]. Preclinical in-vivo and in-vitro studies performed using [161Tb]Tb-PSMA-617 have also shown very promising results compared to [177Lu]Lu-PSMA-617 [22, 23]. Moreover, 161Tb can be used for monitoring the activity distribution and dosimetry thanks to the emission of gamma-radiation. Marin et al. established a SPECT/CT protocol for imaging of 161Tb using an energy window centered at 74.6 keV [24]. Recently, the first-in-human application of [161Tb]Tb-DOTATOC demonstrated the 161Tb imaging properties using SPECT/CT [25]. The results showed high quality images, and it was even possible to visualize small metastases in the liver and bones. Moreover, the development of a protocol towards Good Manufacturing Practice (GMP)-compliant production of [161Tb]-DOTATOC is in progress [26].
As a result, at this stage of 161Tb research, the precision of 161Tb activity measurements is crucial to shorten the transition period of this potential therapeutic radionuclide to the clinics. More than 99% of the emitted gamma and X-rays of 161Tb have an energy below 100 keV [27, 28] (Table 1). An adequate container has to be used for the activity measurement using dose calibrators, as large attenuation could occur depending on the container characteristics. As a result, this study aims at evaluating the effect of sample container and source geometry on the RDC measurements of 161Tb.
Table 1.
Gamma-ray and X-ray intensities for 161Tb (only the main gamma-emission lines are shown)
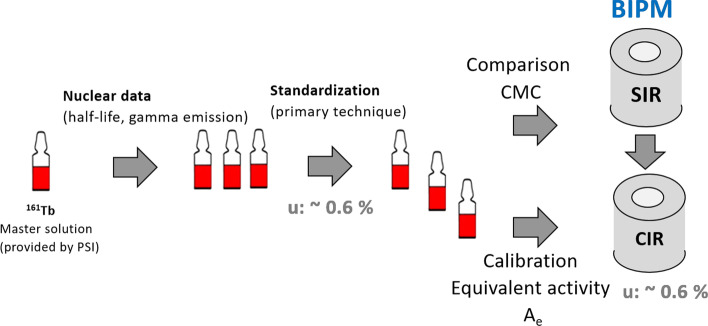
Precise nuclear data, such as half-life and decay emissions, play a major role towards the accuracy of the activity measurement. Recently-performed half-life, emission intensities measurements and standardization of 161Tb at IRA [18, 28, 29] enabled us to submit a 161Tb sample to the SIR as well as to calculate an Ae for the CIR, which is briefly summarized in the following section. Thus, IRA has the ability to produce 161Tb standards for instrument calibration (Fig. 2). Several standardized 161Tb solutions were used to study the response of an ionization chamber for different container types to pin down the attenuation effect for the low-energy gamma emissions, showing the necessity to have a dedicated factor for each container.
Fig. 2.
Scheme of the realization of a 161Tb standard (CMC Calibration and Measurement Capabilities, BIMP The Bureau of Weights and Measures, SIR Système International de Référence, CIR Chambre d’Ionisation de Référence)
A brief review of 161Tb precise activity measurement
Half-life determination
To realize a standard of 161Tb, its half-life was first measured at IRA using a chemically and radionuclidically purified sample provided by the Paul Scherrer Institute (PSI) [18]. Terbium‐161 was produced using the 160Gd(n,γ)161Gd → 161Tb nuclear reaction by neutron irradiation of enriched 160Gd targets (98.2%, Isoflex, San Francisco, CA, USA) at the SAFARI‐1 reactor (Necsa, Pelindaba, South Africa). After a chemical separation process, only 160Tb (T1/2 = 72.3 h) was identified as a radionuclidic impurity using High Purity Germanium (HPGe) detector with an 160Tb/161Tb activity ratio of 4.93(15).10–5. Its contribution was taken into account in the 161Tb half-life calculation. Three different independent systems (CIR, portable ionization chamber (TCIR) and a CeBr3 γ-emission detector with digital electronics) were used for the measurements performed over a period of more than two times the 161Tb half-life, giving an improved result with low uncertainty (0.028%) [18].
Emission intensity measurements
A total of 28 gamma-rays and 4 X-rays were measured using an HPGe detector and compared with previous 161Tb emission intensity measurements [28]. A large reduction of the uncertainties was obtained thanks to the high radionuclidic purity of the source (160Tb ≤ 0.007%), a highly precise half-life value of 161Tb [18], a rigorous Monte Carlo calculation for the coincidence summing correction and an accurate precise activity measurement [29].
Activity standardization
After the half-life determination, a 161Tb sample was standardized at IRA using the β–γ coincidence technique with analogue and digital acquisition systems, as well as with the Triple-to-Double Coincidence Ratio method (TDCR) [29]. The sample solution containing a 160Tb impurity with a 160Tb/161Tb activity ratio of 4.53(20).10−5 was also provided by PSI. Using the standardized solution, an equivalent activity value was calculated for the CIR chamber.
Precise activity measurement of 161Tb
The 161Tb half-life was measured as 6.953(2) days, corresponding to a relative uncertainty of 0.03% [18]. The coincidence measurements with analogue electronics and the TDCR method showed a good consistency and were compatible with the digital coincidence results within uncertainties. The final result gave an activity measurement with an uncertainty of 0.58%. A sample of this standardized solution was submitted to the BIPM to be measured by the SIR.
Two ampoules filled with this solution were measured in the CIR chamber in order to determine the equivalent activity for 161Tb (i.e. its calibration factor). Both gave consistent results and the obtained equivalent activity value is Ae = 219.05 ± 1.32 (0.602%) MBq [29]. These results give IRA the capability to produce 161Tb standards with a precision of 0.6%.
Materials and methods
161Tb activity measurement with ionization chamber using different vials
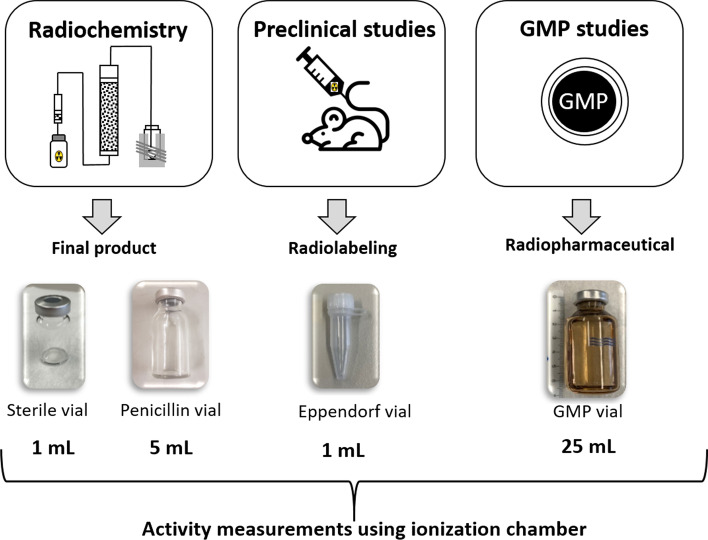
In this study, four different vials, used for the different stages of 161Tb research such as radiochemistry (radiochemical separation), preclinical studies and GMP studies (production) (Fig. 3), were chosen for the activity measurement of 161Tb using an ionization chamber whose characteristics are described in [30, 31].
Fig. 3.
Scheme of the different container types used to assess the activity at different stages of 161Tb research
Activity measurement in penicillin vials
During the standardization process at IRA [29], a solution of 161Tb consisting of 0.1 mol L−1 HCl solvent with a Tb3+-ion concentration of 25 μg g−1 was measured using different geometries. The density of the solution was 1.000(6) g cm−3. Two 10 mL penicillin glass vials, provided from Flaigg AG (Aesch, Switzerland) [32], with a diameter of 25.2 mm and a height of 52.8 mm, were filled with 5 g of the solution and their activity measured in the portable ionization chamber TCIR, which was able to accurately measure the current produced in the gas chamber [30, 31]. The mass of the solution deposited in each vial was accurately weighed in order to normalize the measured current and compare the two measurements.
Characterization of the penicillin vial
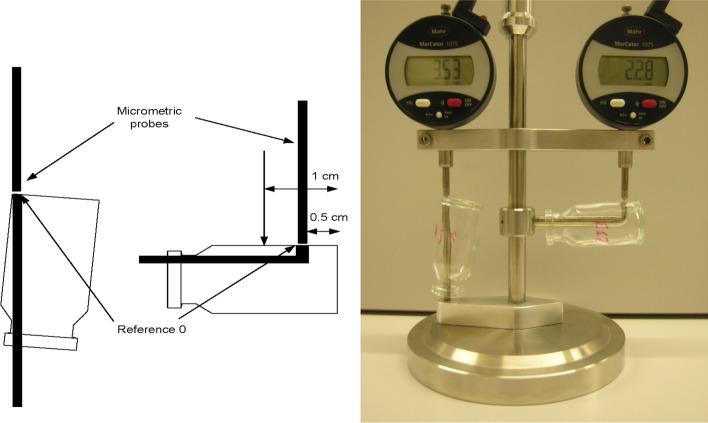
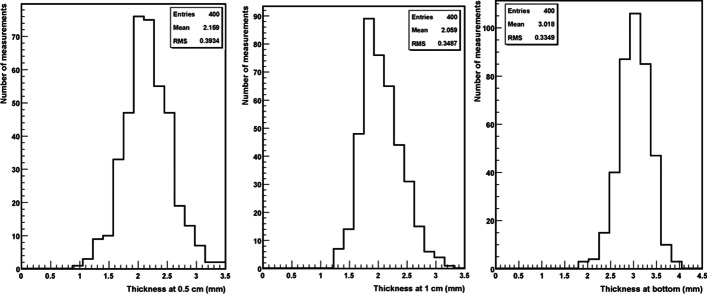
The penicillin vials were characterized by measuring their wall thickness with a dedicated tool made at IRA (Fig. 4). Ten points were measured over the circumference at two positions, 0.5 cm and 1 cm from the bottom. Ten other points over the circumference of the bottom thickness were also measured to check its regularity and to select the vials with an uniform thickness.
Fig. 4.
Left: scheme showing the locations of the probe for measurements at 0.5 and 1 cm with respect to the bottom of the vial and location of the probe for the bottom thickness measurement. For each position the vial is rotated manually to perform 10 measurements along its circumference. Right: picture of the measurement tool
Activity measurement in a sterile glass vial
A 5-mL glass vial, obtained from Infochroma AG (Goldau, Switzerland) [33], with an external diameter of 20 mm and a height of 38 mm was used in this work and is referred to as a sterile glass vial. The regularity of this vial was also characterized by measuring its wall thickness, as performed with the penicillin vial.
A 161Tb solution (1 g) was used to fill six sterile glass vials to assess their suitability for low-energy emission measurement. The solution consisted of 0.1 mol L−1 HCl solvent with a Tb3+-ion concentration of 25 µg g−1 with an activity concentration of 24.387 ± 0.146 (0.60%) MBq/g at the reference date. The concentration was measured at IRA with the CIR chamber, using the 161Tb equivalent activity as explained in the previous section. All the vials were weighed before and after filling to determine the precise mass of their content. Each vial was measured four times with the TCIR at different dates to check the consistency of the measurements over time.
Activity measurement in Eppendorf vial
To show the effect of the container geometry on low-energy gamma emissions with a lighter container, two plastic Eppendorf tubes were filled with 1 g of the same solution as the sterile glass vials to measure the difference in the response of the ionization chamber. The Eppendorf is a tube of 43.5 mm in height and 10 mm external diameter with a conical end. A dedicated holder was designed to place the Eppendorf tube at a similar position to the one of the sterile glass vial inside the well of the TCIR ionization chamber (Fig. 5).
Fig. 5.
Eppendorf tube (left), sterile glass vial (middle) and GMP vial (right), in their respective holders before insertion inside the well of the ionization chamber
Activity measurement in a GMP vial
The containers called GMP vials (evacuated vials 25 mL (E6-12701)) were provided by Curium/b.e. imaging AG (Schwyz, Switzerland). They are used for the pharmaceutical production before injection into the patient. Usually, this vial is filled with 20 mL solution. The GMP vial has a diameter of 32.5 mm a height of 57 mm. The regularity of this vial was also checked by measuring its wall thickness, as done for the penicillin and the sterile glass vials.
A 161Tb solution with the same chemical composition as the one used for the sterile glass vials was used to fill the four GMP vials at 20 mL. The activity concentration was measured with the CIR and was determined to be 7.307 ± 0.044 (0.60%) MBq/g at the reference date. Each vial was weighed before and after filling to determine the precise mass of its content. The GMP vial was inserted into the TCIR chamber using the same holder as used for the other vials (Fig. 5).
Results
161Tb activity measurement with ionization chamber using different vials
161Tb activity measurement in penicillin vial
Table 2 gives the normalized current at the same reference date, obtained for several measurements of two penicillin vials, showing a discrepancy of 4.5% between the two vials.
Table 2.
Normalized current produced in the ionization chamber for two penicillin vials filled with the same solution of 161Tb
| Vial # | Meas. date | Current (pA/MBq) | Uncertainty | Rel. uncertainty | Average (pA/MBq) | Stand. dev. |
|---|---|---|---|---|---|---|
| 1 | 23.08.2019 | 0.912 | 0.001 | 0.08% | 0.911 | 0.001 |
| 1 | 24.08.2019 | 0.910 | 0.001 | 0.08% | ||
| 1 | 28.08.2019 | 0.910 | 0.001 | 0.08% | ||
| 2 | 23.08.2019 | 0.869 | 0.001 | 0.08% | 0.869 | 0.001 |
| 2 | 24.08.2019 | 0.869 | 0.001 | 0.08% | ||
| 2 | 28.08.2019 | 0.869 | 0.001 | 0.08% | ||
| Diff: | 4.5% |
Characterization of the penicillin vials
Figure 6 shows the distribution of the values obtained for 40 vials for the three measurements considered, namely, thickness at 0.5 cm, thickness at 1 cm and bottom thickness (Fig. 4). The Root Mean Square (RMS) of the distributions (> 0.3 mm) shows the large spread of the distributions. However, these distributions are not useful to see how thickness can vary within the same vial. Therefore, the following quantities are defined to characterize the fluctuations in one vial:
Fig. 6.
Distributions of the thicknesses measured for the three positions of the probes for 40 vials
It was observed that, for some vials, the difference of the thickness was greater than 1 mm between two positions on the circumference. The bottom thickness was more regular, as the average difference and the standard deviation were 0.125 mm and 0.08 mm, respectively, compared with 0.27 mm and 0.16 mm for the side wall. These measurements showed that very few vials have regular thickness. In order to select regular vials, the following 6 criteria were used:
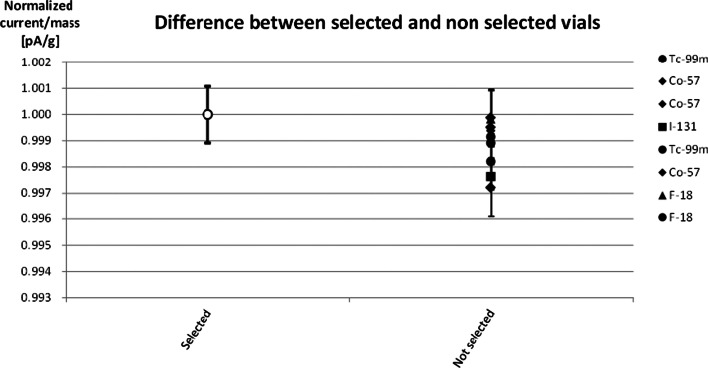
Fewer than 9% of the measured vials, over a sample of 223 vials, fulfilled these criteria. These selection criteria were validated using 99mTc, 57Co, 18F and 131I. Selected and non-selected vials were filled with the same solution and measured in the ionization chamber. The results show differences between those vials satisfying the criteria and those not, but they are still less than 0.5% (Fig. 7). As this test was performed with radionuclides having gamma emissions above 100 keV, we used a 161Tb solution to ascertain whether low-energy gamma emissions could be measured with this vial type.
Fig. 7.
Ratio between the normalized current produced in the ionization chamber by selected and non-selected vials for different radionuclides
The results show that it is not appropriate to use penicillin vials for 161Tb, since they satisfy the geometry selection criteria but give a difference of 4.5% in the response current. It was, therefore, decided to use a more regular vial, as presented in the next section.
161Tb activity measurement in sterile glass vials
The thickness of 20 sterile glass vials was measured to check their regularity. The results are shown in Fig. 8. The measurement of 10 points over the circumference showed very good regularity, the RMS of the distribution was less than 14 μm. The thickness was also thinner than for the penicillin vials: 1.3 mm compared to 2 mm. For all the 20 measured vials, the maximum fluctuation of the thickness was less than 50 μm. These vials were much more consistent than the penicillin ones and also have a thinner wall, which would attenuate the low –energy gamma rays less and produce more signal in the ionization chamber.
Fig. 8.
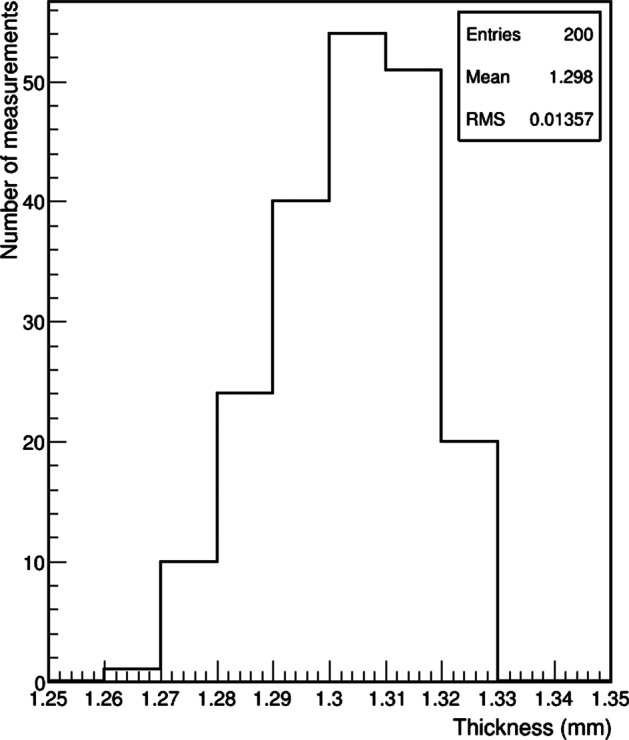
Distribution of the thickness measured for 10 points over the circumference of 20 sterile glass vials
Figure 9 shows the results obtained using six sterile glass vials. The maximum deviation between the 24 measurements was 0.4%.
Fig. 9.
Normalized current values for 6 sterile glass vials filled with 161Tb. Each vial was measured 4 times at regular intervals
161Tb activity measurement in the Eppendorf tube
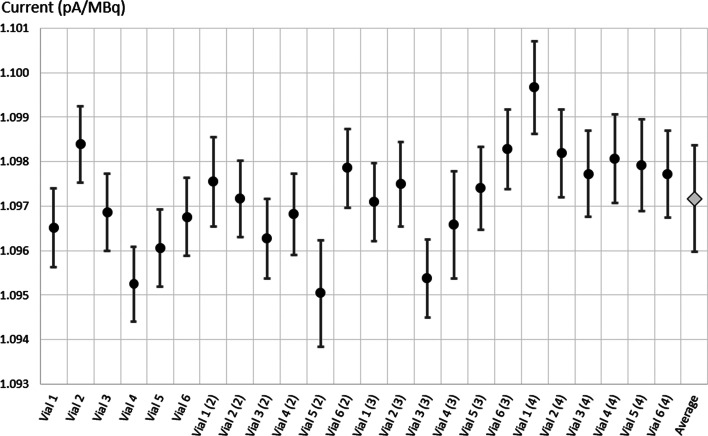
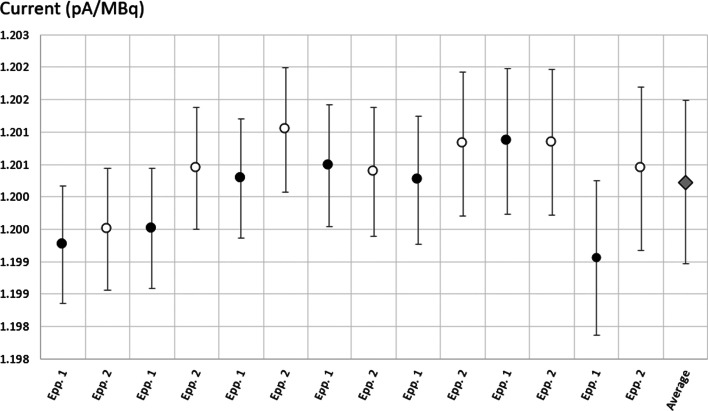
As shown in Fig. 10, the current of the measurements obtained with the TCIR gave consistent results for both Eppendorf tubes. The average normalized current is 1.2002 ± 0.0013 pA/MBq, which is larger than the one measured for the sterile glass vials (1.0972 ± 0.0012 pA/MBq) by more than 8.5%. This shows the importance of the container used, as plastic attenuates the low-energy gamma rays less than glass.
Fig. 10.
Normalized current values for two Eppendorf tubes filled with 161Tb. Each point represents seven measurements of both Eppendorf tubes performed at regular intervals over a period of 2 weeks
Additional measurements were performed using other radionuclides to compare the response of the ionization chamber for Eppendorf tubes and sterile glass vials. Standardized samples of 137Cs, 60Co, 133Ba and 57Co were used to fill the two types of container with 1 g of solution. A chamber response difference of 0.23%, 0.21%, 0.42% and 0.60%, respectively, was determined (Table 3). The largest difference was observed for 57Co, which has two gamma emissions at 122.06 and 136.47 keV. It was concluded that for these four radionuclides, the same calibration factor could be used for Eppendorf tubes as for glass vials. It also showed that the attenuation of gamma rays above 100 keV could be neglected for these two vial types, assuming a fluctuation of lower than 1%.
Table 3.
Response difference comparison of the ionization chamber measurements of 161Tb, 137Cs, 60Co, 133Ba and 57Co solutions in Eppendorf tubes and sterile glass vials
| Radionuclides | Energy of gamma lines (keV) | Response difference (%) |
|---|---|---|
| 161Tb | 49–75 | 8.5 |
| 137Cs | 662 | 0.23 |
| 60Co | 1173–1332 | 0.21 |
| 133Ba | 276–384 | 0.42 |
| 57Co | 122–136 | 0.6 |
161Tb activity measurement in GMP vial
The wall thickness of five GMP vials was measured with 10 points over its circumference. The results showed very good regularity, similar to the sterile glass vials. The RMS of the distribution was 27 μm (Fig. 11) and the thickness 1.26 mm, much thinner than the penicillin vial. The maximum fluctuation of the thickness for the same vial was 90 μm.
Fig. 11.
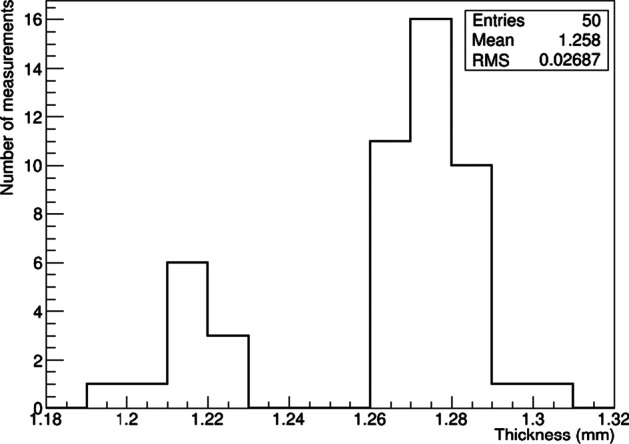
Distribution of the thickness measured for 10 points over the circumference of five GMP vials
Figure 12 gives the normalized current measured for the four GMP vials filled with 161Tb solution. The average value was 1.0319 ± 0.0008 pA/MBq, which was 6% and 14% smaller than for the sterile glass vial and Eppendorf tube, respectively. The difference between the two glass vials can be explained by the filling volume, which was 20 mL compared with 1 mL for the sterile glass vial and, therefore, the position of the radioactive solution inside the well was more distributed along the vertical axis, which changed the response of the chamber slightly.
Fig. 12.
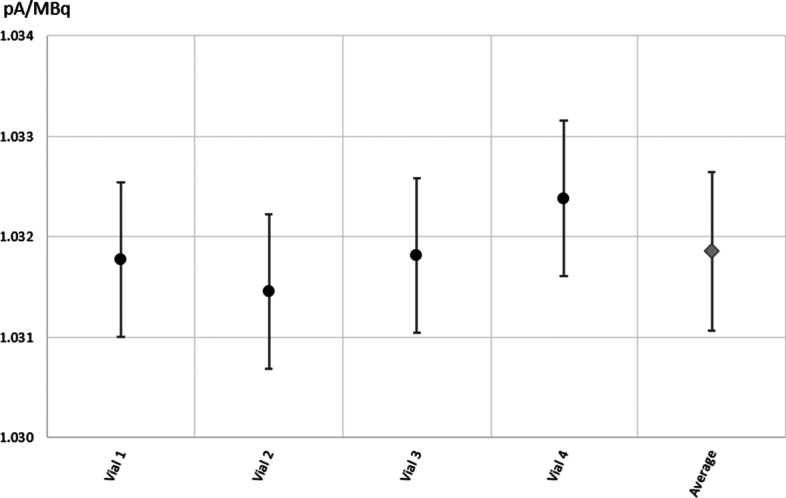
Normalized current values for the four GMP vials filled with 20 mL 161TbCl3 solution
In order to compare the effect of the filling level, a GMP vial was filled with 3.6 g of the 161Tb stock solution and gradually made up to 20 g using nonradioactive (“cold”) Tb solution. After each addition, the vial was shaken to ensure a homogenous solution, weighed and, finally, centrifuged. The vial was measured at the TCIR and the measured currents were normalized to the value obtained with 20 g filling level. As shown in Fig. 13, the measured current started stabilizing around 14 g, however, the difference between filling at 8.49 g and 20 g was around 0.5%. From 5 g and below, a significant decrease, larger than 1%, of the current was measured.
Fig. 13.
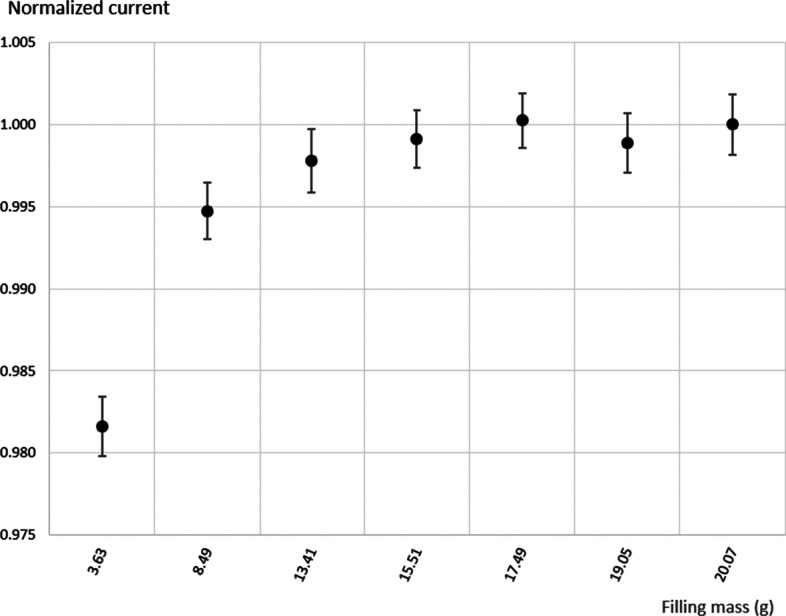
Normalized current values for different filling levels using a GMP vial
Discussion
The results obtained in this work demonstrate the importance of the solution volume, vial type, as well as the vial quality to ensure reproducible and accurate measurement of low-energy gamma-emitting radionuclides. Although some vial types can be suitable for measuring radionuclides with gamma-energy emissions above 100 keV, they are not necessarily suitable for the measurement of lower-energy gamma emissions.
The main gamma-emission lines of 161Tb are below 75 keV (Table 1). A difference of 4.5% was observed between two penicillin vials of the same type using the same 161Tb solution. Vials with regular thicknesses are, therefore, needed for the measurement of low-energy gamma emitters. Measurements using sterile and GMP glass vials, which have good thickness regularity, demonstrated their ability to measure low-energy gamma emitters more accurately. However, the response of the ionization chamber showed a significant difference between the two vial types of around 6%. This necessitates the use of different calibration factors for each type of vial. In addition, measurements with plastic Eppendorf tubes gave a response 8.5% higher than for sterile glass vials and 14% higher than that for GMP vials when measuring 161Tb. However, for radionuclides with gamma energies above 100 keV, the response differences were similar (Table 3). This shows that gamma attenuation has to be taken into account below 100 keV and, consequently, it is necessary to have different calibration factors for different containers used for production, preclinical studies and GMP production, respectively (Fig. 3). The measurement results for GMP vials using different 161Tb solution volumes gave no difference if the vial was filled at 10 g or more. The chamber response slightly decreased, by approximately 2%, for low filling levels around 3 g. As a result, the calibration factor would have to be corrected according to the volumes used in practice. This results are in agreement with studies using Monte Carlo simulation [3, 4], which could be an alternative for calculation of calibration factors. However it would require a precise knowledge of the geometry of the dose calibrator (size of chamber, thickness and material of the inner walls…) as well as for the used container geometry.
These results demonstrate the importance of the container type for the measurement of 161Tb and more generally for low-energy gamma emitters with ionization chambers and radionuclide dose calibrators. It is necessary to have a dedicated calibration factor for each container type to account for geometry, material and filling level in order to achieve an accurate activity assessment for low-energy gamma emitters (< 100 keV).
Conclusion
The activity measurement of standardized 161Tb solution was performed using four different container types in an ionization chamber, showing significant discrepancies between vials that have insufficiently regular, or similar, geometry. A more regular vial type was chosen and standardized activity samples of 161Tb were measured, showing a stable response with the ionization chamber. It is important to highlight that, for glass vials, it would also be important to use even-shaped vials according to a specific standard in order to ensure consistent quality between the different production batches. Additionally, it was shown that Eppendorf tubes and glass vial containers have a similar response, within 1%, for gamma emitters above 100 keV, but their response can differ by 8.5% or 14% for 161Tb, which has 99% of its gamma emissions below 100 keV.
It is, therefore, very important to use the appropriate calibration factor according to the container, and also to take different filling volumes into account. Recalculating a calibration factor for each geometry condition (radionuclide, vial or syringe type, filling volume, etc.) is recommended for low-energy gamma emitters below 100 keV. For such radionuclides, using the wrong factor can lead to an assessment error of several percent of the activity in question. Ideally, in each practical case (radionuclide production, preclinical studies and radiopharmaceutical production), a calibration factor should be recalculated with a well-known source, traceable to a reference standard. It is worth mentioning that the results reported in this study will have particular importance for the precise activity measurement of the therapeutic radionuclide 161Tb for its clinical use.
Abbreviations
- PET
Positron emission tomography
- SPECT
Single-photon emission computed tomography
- RDC
Radionuclide dose calibrator
- NMIs
Nuclear Metrology Institutes
- CGPM
General Conference on Weights and Measures
- BIPM
The Bureau of Weights and Measures
- CIPM MRA
International Committee for Weights and Measures Mutual Recognition Agreement
- CMCs
Calibration and Measurement Capabilities
- SIR
Système International de Référence
- KCDB
Key Comparison Data Base
- CIR
Chamber d’ionization de référence
- IRA
Institute of Radiation Physics
- METAS
Swiss Federal Office of Metrology
- IAEA
International Atomic Energy Agency
- PSI
Paul Scherrer Institute
- HPGe
High Purity Germanium
- TDCR
Triple to Double Coincidence Ration method
- RMS
Root Mean square
Authors' contributions
All authors contributed to the study conception and design. Material preparation, data collection and analysis were performed by all authors. The first draft of the manuscript was written by FJ and ZT, and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Funding
The research was funded by the Swiss National Science Foundation (SNSF) (Grants: 200021_188495).
Availability of data and materials
The data that support the findings of this study are available from the corresponding author upon reasonable request and with permission of the institution where measurement data was acquired.
Declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Santos JAM, Carraso MF, Lencart J, Bastos AL. Syringe shape and positioning relative to efficiency volume inside dose calibrators and its role in nuclear medicine quality assurance programs. Appl Radiat Isot. 2009;67:1104–1109. doi: 10.1016/j.apradiso.2009.01.084. [DOI] [PubMed] [Google Scholar]
- 2.Cessna JT, Schultz MK, Leslie T, Bores N. Radionuclide calibrator measurement of 18F in a 3ml plastic syringe. Appl Radiat Isot. 2008;66:988–993. doi: 10.1016/j.apradiso.2008.02.046. [DOI] [PubMed] [Google Scholar]
- 3.Ceccatelli A, Benassi M, D’Andrea M, De Felice P, Fazio A, Nocentini S, Strigari L. Experimental determination of calibration settings of a commercially available radionuclide calibrator for various clinical measurement geometries and radionuclide. Appl Radiat Isot. 2007;65:120–125. doi: 10.1016/j.apradiso.2006.06.009. [DOI] [PubMed] [Google Scholar]
- 4.Olsovcova V. Monte Carlo simulation of activity measurement of 123I, 111I and 153Sm with a radionuclide calibrator. Appl Radiat Isot. 2010;68:1383–1387. doi: 10.1016/j.apradiso.2009.11.016. [DOI] [PubMed] [Google Scholar]
- 5.Bochud FO, Laederman JP, Baechler S, Kosinski M, Bailat C. Usefulness of specific calibrator coefficients for gamma-emitting sources measured by radionuclide calibrator in nuclear medicine. Med Phys. 2011;38:4073–4080. doi: 10.1118/1.3596528. [DOI] [PubMed] [Google Scholar]
- 6.Bauwens M, et al. A comparison of four radionuclide dose calibrators using various radionuclides and measurement geometries clinically used in nuclear medicine. Phys Med. 2019;60:14–21. doi: 10.1016/j.ejmp.2019.03.012. [DOI] [PubMed] [Google Scholar]
- 7.Vargas CS, et al. An international multi-center investigation on the accuracy of radionuclide calibrators in nuclear medicine theragnostics. EJNMMI Phys. 2020;7:1–18. doi: 10.1186/s40658-019-0269-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.CIPM Mutual Recognition Arrangement 1999 (revised 2003). http://www.bipm.fr/en/cipm-mra/.
- 9.Rytz A. Coherence of activity measurements. Environ Int. 1978;1:15–18. doi: 10.1016/S0160-4120(78)80071-3. [DOI] [Google Scholar]
- 10.Rytz A. The international reference system for activity measurements of γ-ray emitting nuclides. Appl Radiat Isot. 1983;34:1047–1056. doi: 10.1016/0020-708X(83)90170-9. [DOI] [Google Scholar]
- 11.KCDB, Key Comparison Data Base. https://www.bipm.org/kcdb/.
- 12.Juget F, et al. Determination of 137Cs half-life with an ionization chamber. Appl Radiat Isot. 2016;118:215–220. doi: 10.1016/j.apradiso.2016.09.002. [DOI] [PubMed] [Google Scholar]
- 13.Michotte C, et al. Activity measurements of the radionuclides 18F and 99mTc for the NMISA, South Africa in the ongoing comparisons BIPM.RI(II)-K4.F-18 and BIPM.RI(II)-K4.Tc-99m. Metrologia. 2017;54:06001. doi: 10.1088/0026-1394/54/1A/06001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Michotte C, et al. Update of the BIPM comparison BIPM.RI(II)-K1.Mn-54 of activity measurements of the radionuclide 54Mn to include the 2017 result of the PTB (Germany) and the linked results from the CCRI(II)-K2.Mn-54 comparison. Metrologia. 2020;57:06006. doi: 10.1088/0026-1394/57/1A/06006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ordonnance du DFJP sur les instruments de mesure des rayonnements ionisants. https://www.admin.ch/opc/fr/classified-compilation/20121129/index.html.
- 16.Directive L-09-01, Assurance qualité des activimètres. https://www.bag.admin.ch/dam/bag/fr/dokumente/str/str-wegleitungen/technische-qs/l-09-01.pdf.download.pdf/L-09-01_FR.pdf.
- 17.Quality Assurance for Radioactivity Measurements in Nuclear Medicine. Técnica Reports Series N° 454. Vienna: International Atomic Energy Agency (IAEA); 2006.
- 18.Duran MT, et al. Determination of 161Tb half-life by three measurement methods. Appl Radiat Isot. 2020;159:109085. doi: 10.1016/j.apradiso.2020.109085. [DOI] [PubMed] [Google Scholar]
- 19.Talip Z, Favaretto C, Geistlich S, van der Meulen N. A step by step guide for the novel radiometal production for medical applications: case studies with 68Ga, 44Sc, 177Lu and 161Tb. Molecules. 2020;25:966. doi: 10.3390/molecules25040966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Champion C, Quinto MA, Morgat C, Zanotti-Fregonara P, Hindie E. Comparison between three promising β−-emitting radionuclides, 67Cu, 47Sc and 161Tb, with emphasis on doses delivered to minimal residual disease. Theranostics. 2016;6:1611–1618. doi: 10.7150/thno.15132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Müller C, Reber J, Haller S, et al. Folate receptor targeted alpha-therapy using terbium-149. Pharmaceuticals. 2014;7:353–365. doi: 10.3390/ph7030353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Müller C, Umbricht CA, Gracheva N, et al. Terbium-161 for PSMA-targeted radionuclide therapy of prostate cancer. Eur J Nucl Med Mol Imaging. 2019;46:1919–1930. doi: 10.1007/s00259-019-04345-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Borgna F, Barritt P, Grundler PV, Talip Z, Cohrs S, Zeevaart JR, Köster U, Schibli R, van der Meulen NP, Müller C. Simultaneous visualization of 161Tb- and 177Lu-labeled somatostatin analogues using dual-isotope SPECT imaging. Pharmaceutics. 2021;13(4):536. doi: 10.3390/pharmaceutics13040536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Marin I, Ryden T, Van Essen M, et al. Establishment of a clinical SPECT/CT protocol for imaging of 161Tb. EJNMMI Phys. 2020;7:1–16. doi: 10.1186/s40658-020-00314-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Baum RP, Singh A, Kulkarni HR, et al. First-in-human application of Terbium-161: a feasibility study using 161Tb-DOTATOC. J Nucl Med. 2021 doi: 10.2967/jnumed.120.258376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Favaretto C, et al. The production of 161Tb and its introduction to the clinic through the Good Manufacturing Practice-compliant radiolabeling of [161Tb]Tb-DOTATOC. Nucl Med Biol. 2021;96–97:SP052. [Google Scholar]
- 27.ENSDF. 2020. https://www.nndc.bnl.gov/ensdf/.
- 28.Juget F, et al. Determination of the gamma and X-ray emission probabilities of 161Tb. Appl Radiat Isot. 2021;174:109770. doi: 10.1016/j.apradiso.2021.109770. [DOI] [PubMed] [Google Scholar]
- 29.Nedjadi Y, et al. Activity standardisation of 161Tb. Appl Radiat Isot. 2020;166:109411. doi: 10.1016/j.apradiso.2020.109411. [DOI] [PubMed] [Google Scholar]
- 30.Juget F, Nedjadi Y, Buchillier T, Duran T, Bochud F, Kottler C, Bailat C. A portable precision ionization chamber: the transfer ionization reference chamber. Appl Radiat Isot. 2018;134:95–99. doi: 10.1016/j.apradiso.2017.10.022. [DOI] [PubMed] [Google Scholar]
- 31.Juget F, Nedjadi Y, Buchillier T, Durán T, Bochud F, Bailat C. Efficiency curve of an ionization chamber and its application for short-lived isotope measurement in hospitals. ICRM technical series on radionuclide metrology. ISSN 2522–4328. 2020; p. 18–22. https://physics.nist.gov/ICRM/ICRM_technicalseries_2.pdf.
- 32.https://flaigg.ch/produkt/injektionsflasche-h-glas-klar/.
- 33.Infochroma. https://www.infochroma.ch.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request and with permission of the institution where measurement data was acquired.