Abstract
Cardiac arrhythmias are life-threatening events in which the heart develops an irregular rhythm. Mishandling of Ca2+ within the myocytes of the heart has been widely demonstrated to be an underlying mechanism of arrhythmogenesis. This includes altered function of the ryanodine receptor (RyR2)—the primary Ca2+ release channel located to the sarcoplasmic reticulum (SR). The spontaneous leak of SR Ca2+ via RyR2 is a well-established contributor in the development of arrhythmic contractions. This leak is associated with increased channel activity in response to changes in SR Ca2+ load. RyR2 activity can be regulated through several avenues, including interactions with numerous accessory proteins. One such protein is calsequestrin-2 (CSQ2), which is the primary Ca2+-buffering protein within the SR. The capacity of CSQ2 to buffer Ca2+ is tightly associated with the ability of the protein to polymerise in response to changing Ca2+ levels. CSQ2 can itself be regulated through phosphorylation and glycosylation modifications, which impact protein polymerisation and trafficking. Changes in CSQ2 modifications are implicated in cardiac pathologies, while mutations in CSQ2 have been identified in arrhythmic patients. Here, we review the role of CSQ2 in arrhythmogenesis including evidence for the indirect and direct regulation of RyR2 by CSQ2, and the consequences of a loss of functional CSQ2 in Ca2+ homeostasis and Ca2+-mediated arrhythmias.
Supplementary Information
The online version contains supplementary material available at 10.1007/s12551-021-00914-6.
Keywords: Calsequestrin-2, Ryanodine receptor, Calcium, Arrhythmia
Introduction
Cardiac function describes the process by which blood is pumped around the body, which forms an essential aspect of mammalian physiology. We rely on the regular contraction of the heart to achieve this function. In the healthy heart, this is initiated by electrical impulses generated by the cardiac pacemaker—the sinoatrial (SA) node. Action potentials originating from the SA node trigger a cascade of events, culminating in the physical contraction of the heart. This process is highly dependent on the tightly regulated movement of Ca2+ within the myocytes throughout the cardiac cycle. The dysregulation of Ca2+ has been linked to the generation of ectopic pacemaker activity, creating a disruption to the normal sinus rhythm of the heart. This is known as cardiac arrhythmia—a potentially life-threatening, abnormal heartbeat.
The intracellular store of the myocyte, the sarcoplasmic reticulum (SR), supplies the majority of Ca2+ for normal cardiac contraction. Paradoxically, this is also the primary source of the Ca2+ responsible for generating arrhythmic activity. Therefore, the regulation of SR Ca2+ is critical in maintaining cardiac function to both enable contraction, as well as prevent arrhythmogenesis. Within the SR, one prominently expressed protein is the Ca2+-buffering protein calsequestrin-2 (CSQ2), which has been heavily implicated in the regulation of SR Ca2+. The focus of this review is to highlight the role of CSQ2 in cardiac function, in both physiological and pathological settings. The impact of protein modifications on CSQ2 trafficking and function will be examined, together with the current hypotheses regarding mechanisms of CSQ2 regulation of Ca2+ handling within cardiac myocytes. Finally, evidence from patients and animal models will be discussed which implicate CSQ2 as a key player in Ca2+-mediated arrhythmias.
Calcium handling in cardiac function
Cardiac excitation–contraction coupling
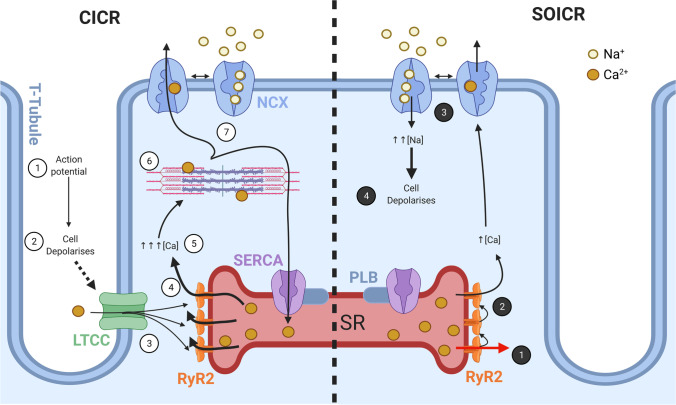
At the cellular level, the contraction of each cardiac myocyte is triggered by beat-to-beat fluctuations in intracellular Ca2+ levels. This highly precise and synchronised process is termed excitation–contraction (EC) coupling, which is summarised in Fig. 1. EC coupling is initiated when an action potential propagates into invaginations of the sarcolemmal membrane, known as transverse (t-)tubules. The increase in membrane voltage opens the voltage gated L-Type Ca2+ channel (LTCC; also termed dihydropyridine receptor, DHPR) embedded the t-tubule membrane (Bers 2002). The t-tubules form a close association with the junctional SR (SRJ), creating a specialised nanodomain known as the dyad. The small influx of extracellular Ca2+ from the LTCC (ICa) diffuses across the dyadic cleft and binds to the cytosolic region of the ryanodine receptor type 2 (RyR2), which is localised to the SRJ membrane (Soeller et al. 2007). This triggers RyR2 to open and release a large amount of stored Ca2+ from the SR and is termed Ca2+-induced Ca2+ release (CICR) (Fabiato 1983). The coordinated mass release of SR Ca2+ into the cytosol enables crossbridge formation at the myofilaments, and thus cardiac contraction to occur (Bers 2002).
Fig. 1.
Physiological and pathological Ca2+ signalling in cardiac myocytes. Schematic demonstrating the Ca2+ signalling within a cardiac myocyte. The left side represents physiological Ca2+ handling during excitation–contraction (EC) coupling: (1) an action potential originating from the sinoatrial (SA) node propagates down the transverse (t-)tubules which (2) depolarises the sarcolemma. This activates voltage-gated L-Type Ca2+ channels (LTCC), (3) mediating a small influx of Ca2+ which diffuses across the dyadic cleft and binds to the ryanodine receptor type 2 (RyR2). (4) RyR2 opens, triggering Ca2+-induced Ca2+ release from the sarcoplasmic reticulum (SR) and (5) a transient increase in cytosolic Ca2+. (6) This cytosolic Ca2+ binds to the myofilaments to facilitate crossbridge cycling and myocyte contraction, before (7) being pumped back into the SR via the sarco/endoplasmic reticulum Ca2+-ATPase (SERCA, which is regulated by phospholamban [PLB]) or extruded from the myocyte via the Na+/Ca2+ exchanger (NCX), enabling relaxation to occur. The right side demonstrates pathological Ca2+ activity: (1) RyR2 spontaneously open and leak Ca2+ from the SR due to store-overload induced Ca2+ release (SOICR). (2) This Ca2+ triggers nearby RyR2 to open, leading to a small increase in cytosolic Ca2+ which activates NCX to extrude Ca2+ and (3) bring Na+ into myocyte. (4) The increased intracellular Na+ depolarises the myocyte, which leads to the development of delayed after depolarisations (DADs). Created with Biorender.com
Relaxation of the cardiac muscle is an essential aspect of the cardiac cycle which requires Ca2+ to be removed from the cytosol. The bulk of the Ca2+ is returned to the SR by the ATP-dependent action of sarco/endoplasmic reticulum Ca2+-ATPase (SERCA), with a smaller portion extruded across the sacrolemmal membrane by the Na+/Ca2+-exchanger (NCX) and the plasma membrane Ca2+-ATPase (PMCA) (Bers 2002). Importantly, the amount of Ca2+ influx across the sarcolemmal and SR membranes during a cardiac cycle is equal to Ca2+ efflux (Delbridge et al. 1996; Bers 2002; Sankaranarayanan et al. 2017). This ensures cardiomyocyte Ca2+ levels do not become depleted or overloaded on a beat-to-beat basis. Therefore, the correct regulation of both Ca2+ handling proteins and the Ca2+ level within the SR store is critical to maintaining rhythmic cardiac contractions.
SR calcium leak and arrhythmia
The amount of Ca2+ stored in the SR is determined by the balance of Ca2+ release (RyR2 activity), Ca2+ reuptake by SERCA, and the expression Ca2+ buffering proteins within the SR, such as CSQ2. As described above, the physiological stimulus for RyR2 opening is the binding of Ca2+ to the cytosolic Ca2+ sensor region of the channel. However, RyR2 can also open in response to a high SR Ca2+ load (Jiang et al. 2004). This occurs when the amount of Ca2+ in the SR surpasses a certain threshold, causing RyR2 to spontaneously open in the absence of cytosolic Ca2+ binding (Fig. 1). Since these spontaneous Ca2+ release events increase in propensity as SR Ca2+ load is raised, they are commonly termed store overload induced Ca2+ release (SOICR), or SR Ca2+ leak events (Jones et al. 2008).
At the microscopic level, SOICR events are observed within single cardiac myocytes as the occurrence of Ca2+ sparks (Terentyev et al. 2003b; Chakraborty et al. 2019). Although the amount of SR Ca2+ released during an individual spark is not sufficient to activate crossbridge cycling in the myofilaments, it can lead to delayed after-depolarisations (DADs) and triggered arrhythmic contractions (Bers 2002). This occurs due to activation of the electrogenic NCX as a result of the increased cytosolic Ca2+ following a SOICR event. By extruding this excess Ca2+ from the myocyte, NCX generates an inward Na+ counter-current, which leads to depolarisation of the membrane (developed DAD). If a sufficient number of SOICR events, and thus Na+ influx occurs, then the membrane will reach the threshold to generate a DAD-triggered action potential, forming the basis of arrhythmic contractions (Bai et al. 2013).
This mechanism of arrhythmogenesis as a consequence of SR Ca2+ leak via RyR2 is a well-recognised occurrence in a multitude of cardiac pathologies, such as heart failure (HF), atrial fibrillation (AF) and inherited heart conditions (Jiang et al. 2010; Mohamed et al. 2018; Liu et al. 2020; Munro et al. 2021). Mutations in both RyR2 and other Ca2+-handling proteins can cause catecholaminergic polymorphic ventricular tachycardia (CPVT)—a severe and potentially fatal stress-induced arrhythmic disorder (Kim et al. 2007; Jiang et al. 2010; Roux-Buisson et al. 2011; Pölönen et al. 2020). Several mutations associated with CPVT have been demonstrated to increase the sensitivity of RyR2 to SR luminal Ca2+ levels (reduces the SR Ca2+ load threshold), thus increasing the occurrence of pro-arrhythmic SOICR events (Jones et al. 2008). Further to these gain-of-function mutations in Ca2+ handling proteins, cardiac arrhythmias have also been linked to additional mechanisms which regulate RyR2 luminal Ca2+ sensitivity (Jones et al. 2017).
Regulation of RyR2 and the macromolecular complex
The ryanodine receptor is a large (~ 2.2 MDa) tetrameric protein which gates the release of Ca2+ from the SR in striated muscle. Each monomer is almost 5,000 amino acids in length, with six transmembrane domains spanning the SR membrane (Peng et al. 2016; Gong et al. 2019). Within cardiac myocytes, the bulk of the RyR2 tetramer protrudes into the dyadic space, with only a small amount of the RyR2 structure accessible within the SR lumen. This luminal portion of RyR2 has been proposed to be sensitive to SR Ca2+ levels, with a luminal Ca2+ activation site leading to initial channel opening (Laver 2007). This then enables Ca2+ to bind to the cytosolic region and further activate the channel via a ‘feed-through’ mechanism (Xu and Meissner 1998; Laver 2007). However, this remains controversial, with others suggesting responses to luminal Ca2+ are mediated by residues within the channel pore (Jones et al. 2017).
Due to its large size, RyR2 can undergo extensive post-translational modification (PMT) and has numerous protein interaction partners. The most widely researched PMT is phosphorylation at sites S2808, S2814 and S2030 by protein kinase A (PKA) and Ca2+/calmodulin-dependent kinase II (CaMKII) (Wehrens et al. 2004; Huke and Bers 2008; Potenza et al. 2019). Phosphorylation of RyR2 at these sites causes an increase in open probability of RyR2, resulting in enhanced SR Ca2+ leak activity (Xiao et al. 2007; Carter et al. 2011). The exact mechanism of how phosphorylation confers hyper-excitability of RyR2 is debated and reviewed extensively by others (Gonano and Jones 2017; Dashwood et al. 2020).
As with phosphorylation, oxidation of RyR2 can also lead to a rapid increase in channel activity (Waddell et al. 2016). The capacity for RyR2 to become oxidised is extensive due to RyR2’s large size and number of accessible residues (Xu et al. 1998). However, the functional effect of oxidation is dependent on the type of redox modification (S-glutathionylation, S-nitrosylation, disulphide formation) (Donoso et al. 2011; Nikolaienko et al. 2018), as well as the duration of exposure to oxidants (Waddell et al. 2016). Both phosphorylation and oxidation of RyR2 have been demonstrated to become dysregulated in HF to promote arrhythmia by altering channel activity (Terentyev et al. 2008b; Respress et al. 2012).
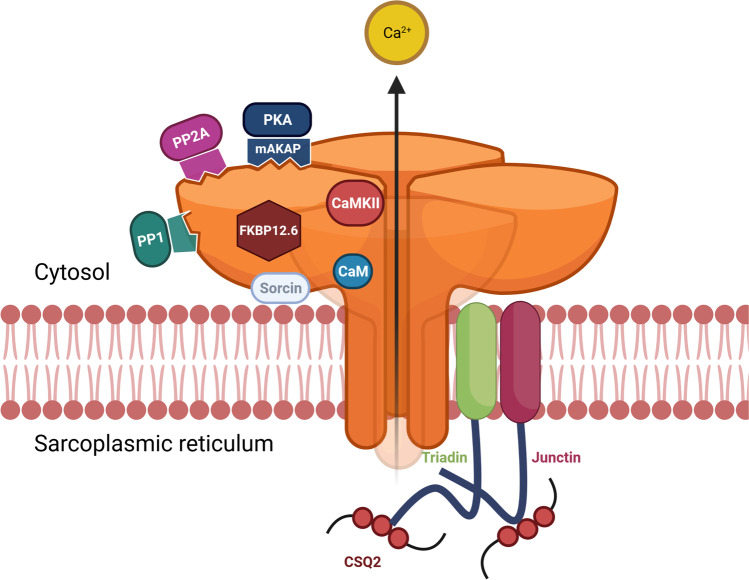
In addition to PMTs, RyR2 can also be regulated by numerous protein–protein interactions. The RyR2 macromolecular complex is made up of many accessory proteins, located both cytosolically and within the SRJ lumen (see Fig. 2). Key cytosolic accessory proteins include FK506-binding protein (FKBP) 12.6 (also known as calstabin 2), sorcin, calmodulin (CaM), CaMKII, PKA, as well as protein phosphatases PP1 and PP2A (Bers 2004). The interaction of these proteins with RyR2 may be direct or mediated via additional linker proteins (e.g. muscle-specific A-kinase anchoring protein (mAKAP)). FKBP regulation of RyR2 has been highly disputed in the literature. Some researchers show that these proteins stabilise RyR2 in a closed state and reduce the propensity of SR Ca2+ leak (Marx et al. 2000); however, others cannot replicate this result (Xiao et al. 2007; Gonano and Jones 2017). More recently, it has been shown that FKBPs reduce the amount of Ca2+ released by RyR2 by promoting early closure during a spontaneous release event (Zhang et al. 2016). CaM has been shown to have a similar effect to FKBP, by also promoting early cessation of RyR2 Ca2+ leak events (Tian et al. 2013). Additionally, CaM has a partial inhibitory role on RyR2 activation (Walweel et al. 2017).
Fig. 2.
The RyR2 macromolecular complex. Schematic of the ryanodine receptor type 2 (RyR2) Ca2+ channel and its associated proteins which together comprise the RyR2 macromolecular complex, localised to the junctional sarcoplasmic reticulum (SRJ) membrane. Cytosolic accessory proteins include calmodulin (CaM), Ca2+/CaM-dependent kinase II (CaMKII), FK506-binding protein (FKBP) 12.6, protein kinase A (PKA), protein phosphatases PP1 and PP2A, and sorcin. PKA interaction with RyR2 is facilitated via the muscle-specific A-kinase anchoring protein (mAKAP). Luminal accessory proteins include junctin and triadin which mediate the interaction of calsequestrin-2 (CSQ2). These three luminal proteins make the quaternary protein complex with RyR2. Created with BioRender.com
Proteins located within the SRJ also have important regulatory roles on RyR2 function. Triadin and junctin span the SR membrane and interact with RyR2 at its luminal interface (Dulhunty et al. 2012). Both proteins bind to CSQ2, the main Ca2+ buffer within the SR to form a quaternary protein complex with RyR2. Hence, triadin and junctin are thought to regulate RyR2 by both a direct interaction and by ensuring the co-localisation of RyR2 with CSQ2 (Chopra et al. 2009). It is currently unclear if CSQ2 binds directly to RyR2, a topic that is expanded on below. Irrespective of the mechanism of interaction, CSQ2 has been identified as being important for regulating RyR2 function, as it directly impacts the Ca2+ content stored in the SR and has been implicated in arrhythmogenesis (Terentyev et al. 2003a).
CSQ2 structure and function
CSQ2 structure
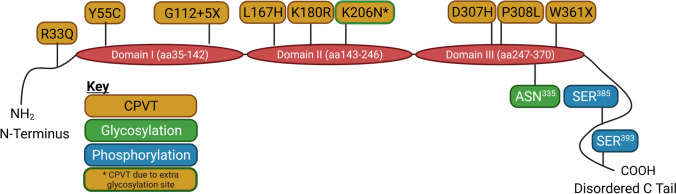
CSQ2 buffers the majority of Ca2+ within the SR of cardiac myocytes to maintain free Ca2+ levels at ~ 1 mM, with the maximum total SR Ca2+ capacity reported to be 19 mM (Shannon and Bers 1997; Shannon et al. 2003a). The abundant expression of CSQ2 in the SRJ, and its low affinity for Ca2+, allow CSQ2 to function as a Ca2+ reservoir in direct proximity to the site of SR Ca2+ release—RyR2 (Chopra et al. 2009). The CSQ2 gene (Casq2) is located on chromosome 1 at locus p13.3-p11 and consists of 11 exons (Otsu et al. 1993). Once transcribed, human CSQ2 is 399 amino acids long; however, the first 19 amino acids encode a signal peptide sequence which is likely cleaved prior to CSQ2 arriving its final destination—SRJ (Fliegel et al. 1987; Kim et al. 2007). The size of full-length CSQ2 can vary depending on species, although there is a high degree of homology (~ 89–96%) conserved across mammalian species (Lewis et al. 2016). The quaternary structure of CSQ2 protein includes five regions: a short N-terminus, three acidic thioredoxin repeats (termed domain I, II and III) and a disordered C-terminal tail (Kim et al. 2007) (Fig. 3). The structural integrity of each of these regions is important for CSQ2’s Ca2+ binding capacity and ability to polymerise (Park et al. 2003).
Fig. 3.
CSQ2 structure, mutations and regulatory sites. Representation of the structure and functional regions of human calsequestrin-2 (CSQ2), including the N-terminus, the three acidic thioredoxin repeat domains (I-III) involved in Ca2+ binding, and the disordered C-tail. The location of residues identified as regulating CSQ2 function via post-translational modifications (PMTs) is indicated for glycosylation (green boxes) and phosphorylation (blue boxes). Known CSQ2 mutations associated with catecholaminergic polymorphic ventricular tachycardia (CPVT), and sudden cardiac death are indicated in gold boxes. Created in Biorender.com
Ca2+ buffering and polymerisation of CSQ2
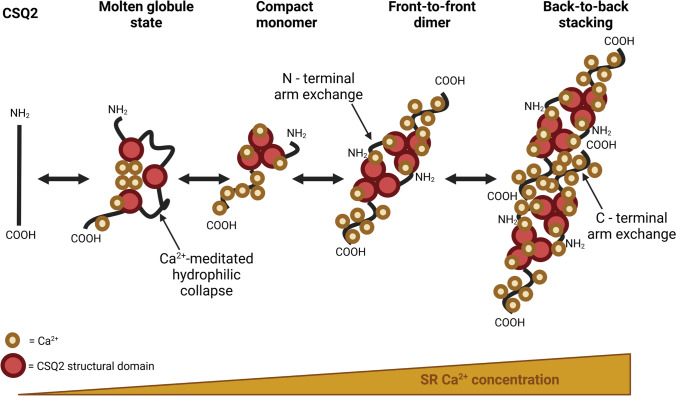
The ability for CSQ2 to buffer large amounts of Ca2+ is dependent on its folding and polymerisation (Park et al. 2003, 2004). At low levels of SR Ca2+, each CSQ2 protein (monomer) initially folds in an intermediate transitional ‘molten globule’ state, where the hydrophilic collapse attracts Ca2+ ions to negatively charged amino acids (aspartic acid and glutamic acid) within the acidic thioredoxin repeats (Fig. 4) (Wang et al. 1998; Kang et al. 2002). CSQ2 then folds into a compact monomer and binds Ca2+ at the negatively charged C-terminal tail. As the SR Ca2+ content increases, CSQ2 undergoes a further conformational change which results in dimerisation (Park et al. 2003; Kim et al. 2007). The CSQ2 homo-dimers initially form in a ‘front-to-front’ orientation, meaning that the N-terminus of each monomer interacts (Fig. 4). Dimers of CSQ2 can then form linear polymers by interactions of the C-terminus between dimers (‘back-to-back’) (Park et al. 2003). Polymerisation of CSQ2 into filaments creates pockets of negatively charged amino acid residues between dimers, which further attracts Ca2+ ions (Titus et al. 2020). Therefore, the process of polymerisation greatly increases the Ca2+ binding capacity of CSQ2, in comparison to single CSQ2 monomers (Park et al. 2004). This is a reciprocal relationship, with in vitro studies demonstrating that as Ca2+ concentration increases, the degree of CSQ2 polymerisation is enhanced, which would promote Ca2+ buffering capacity in the SR (Lewis et al. 2016). However, at physiological levels of free SR Ca2+, CSQ2 exists in a mixture of monomeric, dimeric and polymeric forms, with only ~ 30% localised to the SRJ (Wei et al. 2009; Lee et al. 2012).
Fig. 4.
Mechanism of Ca2+ binding and polymerisation of CSQ2. Demonstration of the proposed mechanism for the reversable binding of Ca2+ and polymerisation of calsequestrin-2 (CSQ2) in response to changes in sarcoplasmic reticulum (SR) Ca2+ concentration. At low concentrations, CSQ2 exists in an intermediate molten globule state, with Ca2+ initially binding at the three acidic thioredoxin domains of each CSQ2 monomer, creating a Ca2+-mediated hydrophilic collapse within the core region. As Ca2+ levels slightly increase, additional binding occurs within the C-tail and CSQ2 forms compact monomer. Further increases in Ca2+ concentration mediate a conformational change which promotes the formation of ‘front-to-front’ CSQ2 homo-dimers via the interaction of the N-termini. High SR Ca2+ levels lead to the ‘back-to-back’ stacking of homo-dimers and the formation of polymeric CSQ2. This polymerisation creates pockets of negatively charged residues, facilitating the binding of additional Ca2+ ions. Created with Biorender.com
Much of our understanding of CSQ2 Ca2+ binding and polymerisation comes from work performed studying the skeletal isoform of calsequestrin, CSQ1. CSQ1 has a high degree of structural similarity to CSQ2 (~ 61%), although this has some noteworthy differences. Since CSQ1 has a higher net negative charge and a shorter C-terminus tail, it can bind more Ca2+ ions per monomer compared to CSQ2 (Park et al. 2004). Cardiomyocytes are continuously cycling Ca2+ and release ~ 35–40% of the SR store when RyR2 opens (Bassani et al. 1995; Shannon et al. 2003b; Zima et al. 2008). As the concentration of Ca2+ in the SRJ is in constant flux, so too will be the polymerisation status of CSQ2. In contrast, skeletal muscle releases only 8% of SR Ca2+ following a single action potential and is capable of sustained tetanic contractions (Launikonis et al. 2006). Additionally, in vitro crosslinking experiments found that CSQ1 exists as a polymer at 1 mM Ca2+ (Wei et al. 2009), while the total SR content of skeletal muscle has been estimated to be 11–21 mM, depending on fibre type (Fryer and Stephenson 1996). These differences in polymerisation status between CSQ isoforms may reflect the differing SR Ca2+ cycling dynamics in cardiac and skeletal muscle, as well as differences in resting SR Ca2+ loads, although more evidence is needed to confirm this. Ultimately, the process of CSQ polymerisation in both cardiac and skeletal muscle is a highly organised, Ca2+-dependent process which plays a critical role in determining the size of the SR Ca2+ store.
The resting concentration of free SR Ca2+ is dictated by CSQ2 expression and the threshold for SOICR. It is thought that as Ca2+ buffering in the SRJ is reduced, the level of free Ca2+ is elevated and pro-arrhythmic Ca2+ release is more likely to occur. Hence, with a loss of CSQ2, the concentration of free Ca2+ near RyR2 will be drastically increased, and the threshold for a SOICR event will be met. With a higher propensity for SOICR, the SR can become depleted of Ca2+. This may have implications for contractile force in addition to the increased risk of arrhythmia (Belevych et al. 2007). In support of this, a knockdown of CSQ2 in rat cardiac myocytes results in an increased frequency of arrhythmogenic spontaneous Ca2+ sparks, despite an overall reduction in SR Ca2+ content (Terentyev et al. 2003a).
Regulation of CSQ2
The ability for CSQ2 to rapidly bind and release Ca2+ is essential for providing adequate Ca2+ for force generation. Moreover, the correct function of CSQ2 is critical to prevent spontaneous RyR2 Ca2+ release and arrhythmia development. During protein translation and trafficking, CSQ2 becomes both phosphorylated and glycosylated. Interestingly, increased CSQ2 glycosylation is detected in heart failure (Kiarash et al. 2004; Jacob et al. 2013) and a single point mutation, which adds an additional glycosylation site to CSQ2, causes stress induced arrhythmias, likely mediated by the impairment of Ca2+-buffering capacity (Kirchhefer et al. 2010) (discussed in detail later in review). Hence, PMTs appear to be a key regulator of CSQ2 function, acting to alter the affinity for Ca2+, and ultimately impact Ca2+ homeostasis.
Phosphorylation of CSQ2
The phosphorylation of CSQ2 by casein kinase 2 (CK2) was first described in the early 1990s by Cala and Jones using purified canine CSQ2 (Cala and Jones 1991). Since then, CK2 phosphorylation sites within the C-tail of CSQ2 have been confirmed and characterised. While there is an apparent conservation of these sites, due to differences in full-length CSQ2, the exact location of these sites is species-specific, with canine CSQ2 also containing an additional phospho-site (see Fig. 5) (Cala and Jones 1991; Sanchez et al. 2011; Lewis et al. 2016). Furthermore, there has been some disparity in the nomenclature of specific phospho-residues in human CSQ2 between studies, dependent on whether the 19 cleaved amino acids are included within the sequence alignment or not (S385 and S393 versus S366 and S374) (Sanchez et al. 2011; Lewis et al. 2016). Subsequently, work by Lewis et al. demonstrated that CSQ2 can exhibit different phosphorylation states, ranging from none to all the sites phosphorylated, and that these differing states can be present simultaneously within a population of CSQ2 proteins (Lewis et al. 2016). However, the physiological role of this PMT still remains largely unclear, with evidence to date implicating it in regulating protein trafficking and Ca2+ binding.
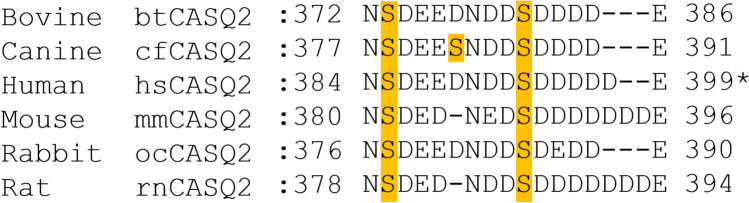
Fig. 5.
Species-specific location of CK2 phosphorylation sites on CSQ2. Alignment of known CK2 phosphorylation sites within the C-terminus of bovine, canine, human, mouse, rabbit and rat calsequestrin-2 (CSQ2). Two serine residues are highly conserved between species, with canine CSQ2 containing an additional serine phosphorylation site within this region. *Sequence alignment refers to full-length human CSQ2 without the cleavage of the first 19 residues; S385 and S393 correspond to residues S366 and S374 in cleaved hsCSQ2, respectively. Created with Biorender.com
The role of phosphorylation in CSQ2 trafficking and function
Since the discovery that CSQ2 can be modified by phosphorylation, the functional impact of this PMT has been studied by several groups over the past three decades. In 2011, Sanchez et al. demonstrated that a phospho-mimetic substitution at the two phosphorylation sites in human CSQ2 (S385 and S393) increased the Ca2+ binding capacity, as well as protein solubility by enabling the transition from a disordered coil to an ordered helical structure at the C-terminus (Sanchez et al. 2011). This latter effect supports the hypothesis of a phosphorylation-dependent trafficking mechanism, as newly synthesised CSQ2 protein already appears to be fully phosphorylated within the rough endoplasmic reticulum (ER) (Ram et al. 2004). This modification makes CSQ2 highly soluble and compact, and thus more suitable for trafficking through the secretory pathway. Therefore, an increase in CSQ2 solubility due to phosphorylation could facilitate its transport to the SRJ. However, this theory is confounded by the fact that CK2 resides exclusively in the nucleus and cytoplasm of cells, lacking an access sequence required to enter the ER lumen (McFarland et al. 2011), while CSQ2 is solely luminal in the SR. The emerging co-translocational hypothesis could help resolve this discrepancy in protein interaction. According to this hypothesis, the phosphorylation of CSQ2 by CK2 occurs directly after its translation by the cytoplasmic ribosome during the concurrent translocation through the translocon into the rough ER (McFarland et al. 2011). This is further supported by the fact that other CK2 substrates (e.g. phosphophoryns in osteoblasts-like cells (Sfeir and Veis 1995) and bile-salt-dependent lipase in pancreatic acinar cells (Pasqualini et al. 2000)) also exist in the lumen of the secretory pathway, and can be isolated with CK2 target sites already phosphorylated.
Despite the apparent reconciliation of these theories, the impact of CK2 phosphorylation on CSQ2 trafficking remains unclear. Additional evidence comes from studies by McFarland et al., using COS cells expressing phospho-deficient or -mimetic CSQ2 mutants. It was revealed that loss of CSQ2 phosphorylation enhanced anterograde trafficking, while phospho-mimetic mutations caused the retention of CSQ2 within the rough ER (McFarland et al. 2011). While this further supports a dependency of CK2-mediated phosphorylation for the correct trafficking of CSQ2, it suggests that reduced phosphorylation is required for SRJ targeting. This is supported by earlier reports of increased dephosphorylation observed for more distally localised CSQ2 (Ram et al. 2004).
Until recently, CK2 appeared to be CSQ2’s only known in vivo and in vitro kinase. However, a new kinase for CSQ2 has been recently proposed, Fam20C. Unlike CK2, Fam20C has a signal peptide which allows it to transit through the ER lumen, enabling phosphorylation via the secretory pathway (Tagliabracci et al. 2012). As the activity of FamC20 requires an S-x-E sequence, it has been proposed to target the same CSQ2 phosphorylation site as CK2 at S385, but not at S393 (Pollak et al. 2018). The phosphorylation by FamC20 is suggested to increase the polymeric structure of CSQ2 in SRJ and thus assist its Ca2+ binding abilities (Pollak et al. 2018), with this latter effect also proposed following CK2 phosphorylation. It has also been reported that at least three CSQ2 sites can be phosphorylated by protein kinase C in vitro (Rodriguez et al. 1999). Additionally, residue T299 of mouse CSQ2 (T282 in human) has been identified as a potential phospho-residue (Huttlin et al. 2010); however, whether this residue is phosphorylated in vivo, and by which kinase, remains to be confirmed. As CSQ2 mainly exists in the SRJ in its dephosphorylated form, the transition to the SRJ must be marked by dephosphorylation of CSQ2, presumably by an unidentified intraluminal phosphatase in secretory compartments (Kiarash et al. 2004).
CSQ2 phosphorylation in cardiac disease
To date, there have been limited studies assessing the role of altered CSQ2 phosphorylation in cardiac diseases. A lack of phosphorylation due to Fam20C knockout has been shown to result in fibrosis and the development of HF in mice (Pollak et al. 2018). This is coupled with significant changes in Ca2+ handling dynamics, which further supports the hypothesis that Fam20C phosphorylation of CSQ2 is important in SR Ca2+ regulation (Pollak et al. 2018). However, despite evidence of reduced CK2 activity in models of cardiac disease (Murtaza et al. 2008), hyperphosphorylation of CSQ2 has been observed in canine models of HF (Kiarash et al. 2004). Based on prior evidence, this would prevent the correct localisation of CSQ2 to the SRJ where it is required to exert its function (McFarland et al. 2011). It should be noted, however, that these alterations in trafficking were inferred from changes in mannose trimming. CSQ2 mannose content has been linked to the degree of ER retention (discussed below) and these findings suggest a co-dependency of phosphorylation and N-glycosylation in the regulation of CSQ2 trafficking (Fig. 6) (Houle et al. 2006; McFarland et al. 2011). It is clear that further studies are required to fully elucidate the role of these PMTs in CSQ2 regulation, particularly in cardiac pathologies.
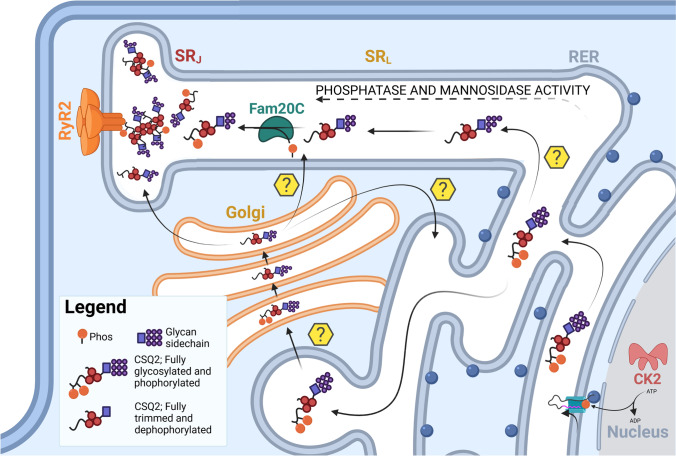
Fig. 6.
The CSQ2 trafficking pathway. Schematic of the proposed human calsequestrin-2 (CSQ2) trafficking pathway with regulatory post-translational modifications (PMTs). CSQ2 is immediately phosphorylated by casein kinase 2 (CK2) at two sites as it is translated in the rough endoplasmic reticulum (RER). Within the RER, CSQ2 is also maximally glycosylated to a mannose content of 8–9 residues. As CSQ2 moves out of the RER, phosphatase activity is proposed to dephosphorylate the protein. Progressive glycan trimming by mannosidase-I occurs as CSQ2 travels from the RER to the Golgi or through the longitudinal sarcoplasmic reticulum (SRL). Within this latter compartment, FamC20 may also phosphorylate a single residue CSQ2 before it is further trafficked to its final location with cardiac myocyte—the junctional SR (SRJ). Within the SRJ, CSQ2 is mainly dephosphorylated but glycosylated with ~ 4–6 mannose and exists as a mixture of monomers, dimers and polymers at physiological Ca2+ levels. Yellow question marks indicate the possible pathways CSQ2 may transit through to reach the SRJ. Created with BioRender.com
Glycosylation of CSQ2
CSQ2 is a glycoprotein which is immediately N-glycosylated as it is translated at the nuclear envelope/rough ER (Sleiman et al. 2015). Human CSQ2 has two known glycosylation sites, N212 and N335 (the latter was previously identified as site N316 due to disparities in sequence alignment (Lewis et al. 2016)). However, it is likely that CSQ2 is only glycosylated at N335, as this region is highly conserved between species and mass spectrometry results support only one site being maximally glycosylated to a mannose content of 8–9 (GlcNac2Man8-9) (O’Brian et al. 2002; Jacob et al. 2013). CSQ2 is trimmed sequentially as it travels to the SRJ (Fig. 6), with the protein responsible for this glycan trimming identified as mannosidase-I (O’Brian et al. 2002). CSQ2 that is present at the SRJ has a mannose content ~ 4–6 (GlcNac2Man4-6) and is the predominant form of CSQ2 (O’Brian et al. 2002; Ram et al. 2004). It should be noted that many studies have also examined CSQ1 glycosylation, and although both isoforms are maximally glycosylated at translation, CSQ1 undergoes more glycan trimming compared to CSQ2. The predominant form of CSQ1 is closer to a mannose content of 1 on the side chains (GlcNac2Man1), which is likely reflects it being more readily able to polymerise and having a slightly different target in the SRJ (O’Brian et al. 2002; Milstein et al. 2009; Sanchez et al. 2012).
The role in glycosylation in CSQ2 trafficking and polymerisation
Trafficking within the ER and SR of the heart is a somewhat elusive process, and this is highlighted by CSQ2’s journey from the rough ER to its final destination, the SRJ (Sleiman et al. 2015). How CSQ2 reaches the SRJ is somewhat controversial, with two main hypotheses being that either CSQ2 is retained within the ER/SR and travels through the lumen until it reaches the SRJ, or it is trafficked to the Golgi for further trimming by Golgi-associated mannosidases, before being trafficked back to the ER/SR. The evidence for an ER/SR retention is supported by in vitro non-muscle cell studies, where it is likely CSQ2 is retained within the ER and only undergoes glycan trimming by ER-associated mannosidases (α 1,2-mannosidase) to a mannose content of ~ 5–6 (Nori et al. 2001; O’Brian et al. 2002). An ER to SR pathway could be achieved by either trafficking transversely between separate ER/SR compartments or longitudinally through individual SR (Jacob et al. 2013; Sleiman et al. 2015). Others show a Golgi associated trafficking pathway (Houle et al. 2006). Unfortunately, in vivo models of trafficking within the heart highlight our lack of understanding of how trafficking of SR proteins occur (particularly CSQ2), with some evidence supporting ER/SR retention (Nori et al. 2001; O’Brian et al. 2002; Sleiman et al. 2015) and others supporting a Golgi-associated mechanism (Houle et al. 2006; Milstein et al. 2009).
Despite the uncertainty of exact trafficking pathways, it is clear that CSQ2 is translated in the rough ER and then eventually reaches the SRJ (McFarland et al. 2010; Sleiman et al. 2015). This trafficking is regulated by both the glycosylation and phosphorylation of CSQ2 (McFarland et al. 2011; Sanchez et al. 2011). A unifying theory for the relationship between CSQ2 trafficking and glycosylation can be demonstrated by in vitro studies showing CSQ2 polymerisation. In healthy cells, CSQ2 is found almost exclusively with a mannose content below 7 in the SRJ and may be present as dimers and polymers (McFarland et al. 2010; Jacob et al. 2013). In the more proximal compartments, CSQ2 exists in its monomeric form and is fully glycosylated (GlcNac2Man8-9) (McFarland et al. 2010; Jacob et al. 2013). This difference may reflect a relationship between the glycosylation and polymerisation state of CSQ2, and its localisation to the SRJ (Fig. 6).
Sanchez et al. (2012) demonstrated through in vitro methods that a high mannose content on the side chains is likely to cause a decrease in back-to-back polymerisation, but not necessarily a decrease in front-to-front formation. While these findings were based on CSQ1, they suggest that a high mannose content would reduce the polymerisation of CSQ into larger polymers but would not discourage the formation of dimers (Sanchez et al. 2012). By reducing the polymerisation of CSQ2 in areas of the ER/SR that are not the SRJ, it would prevent the aggregation of CSQ2 in the longitudinal SR (SRL) prior to it reaching its terminal destination (McFarland et al. 2010). CSQ2 found in the SRJ has a lower mannose content on its glycan side chains, with some groups proposing that these smaller side chains will act as ‘guard rails’ to hold CSQ2 back-to-back formations in a more stable position, and therefore encourage polymerisation (Lewis et al. 2016).
When trafficked to the SRJ, it is the first compartment of the ER/SR which has a high Ca2+ content, encouraging polymerisation (Milstein et al. 2009). Furthermore, the disruption of CSQ2 polymerisation has been demonstrated to result in the loss of CSQ2 retention from the ER in non-muscle cells (Houle et al. 2006). CSQ2 that is lost from the SRJ is likely degraded, but how CSQ2 is degraded is not fully understood (Valle et al. 2020). Evidence for polymerisation being involved in the loss of CSQ2 is that many mutant CSQ2 variants involved in cardiac disease have a reduced ability to polymerise, which causes an overall loss of protein (discussed later in review). In addition to role of glycosylation and polymerisation in trafficking, interaction with the RyR2 macromolecular complex is also believed to assist with the localisation of CSQ2 to the SRJ. As discussed below, CSQ2 may associate with RyR2 either indirectly via triadin and junctin as part of the quaternary complex, or directly via the first luminal loop of RyR2. It is important to note that there is a larger amount of CSQ2 relative to other quaternary complex proteins (RyR2, junctin and triadin) (Cala et al. 1990), so without polymerisation, some CSQ2 will not be bound and potentially be lost from the SRJ.
Altered CSQ2 glycosylation in HF
Within canine models, the trimming of the CSQ2 glycan side chain has been seen to change in HF, with an increased ratio of high mannose content to trimmed CSQ2. This was despite no change in total CSQ2 expression or mRNA (Kiarash et al. 2004; Jacob et al. 2013), which is similar to observations in HF patients (Münch et al. 1998). Rather than a change in expression, this altered glycosylation is proposed to result in a change in the localisation of CSQ2 (Kiarash et al. 2004; Jacob et al. 2013). Instead of being trafficked to the SRJ, CSQ2 is retained in the rough ER at a higher mannose content of 8–9 (GlcNac2Man8-9), with only a small amount of CSQ2 being trafficked to the SRJ (Jacob et al. 2013). It is not clear what mechanism leads to the retention of CSQ2 in the rough ER or its loss from the SRJ; however, one possible explanation is altered trafficking. This could be the result of impaired anterograde trafficking (perhaps due to ultrastructural changes in ER/SR membranes), whereby there is a loss of glycan trimming by mannosidase, which typically occurs in medial compartments as CSQ2 transits to the SRJ (Kiarash et al. 2004; Jacob et al. 2013). Interestingly, it has also been shown that CSQ2 which accumulates in the rough ER can cause an increase in SOICR, and lead to Ca2+ waves (Guo et al. 2012). Due to a loss of CSQ2 from the SRJ where RyR2 is present, it could lead to a loss of channel regulation (discussed further below) and may cause the increased SR Ca2+ leak and decreased contractility seen in HF (Lehnart et al. 2009).
Mechanisms of RyR2 regulation by CSQ2
As previously alluded to, CSQ2 is a major regulator of RyR2, achieving this regulation by being a multifaceted protein. CSQ2’s two main functional roles are Ca2+ buffering and forming part of the RyR2 macromolecular complex, with both functions allowing CSQ2 to regulate RyR2 (see Fig. 7).
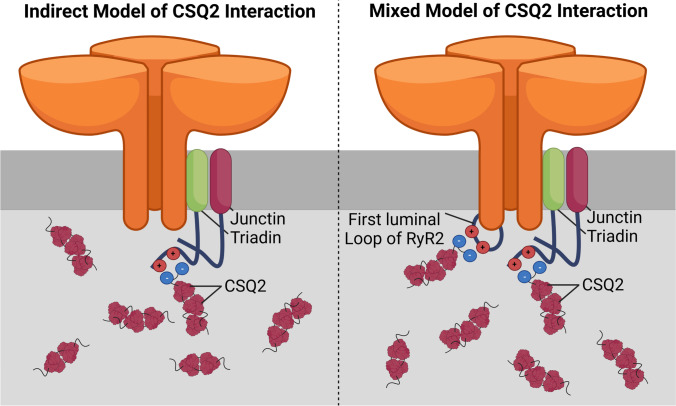
Fig. 7.
Proposed mechanisms of CSQ2 interaction with RyR2. Models representing the potential mechanisms by which calsequestrin-2 (CSQ2) interacts with the ryanodine receptor type 2 (RyR2). The indirect mechanism (left) proposes the association of CSQ2 with RyR2 is facilitated via interactions with junctin and triadin. A mixed model (right) suggests a combination of the previously described indirect mechanism and direct interaction of CSQ2 with the channel, mediated via charged residues within the first terminal loop of RyR2. Created with BioRender.com
Ca2+-buffering by CSQ2
As previously discussed, CSQ2’s primary function is to sequester Ca2+ and keep the concentration of free luminal Ca2+ in the SR at ~ 1 mM (Bers 2002) while keeping the total content of Ca2+ near RyR2 high. This process of Ca2+-buffering is tied to polymerisation, where Ca2+ binding will cause an increase in polymerisation, and in turn allow for more Ca2+ to bind (Fig. 4). Overexpression of CSQ2 is associated with increased SR Ca2+ content (Jones et al. 1998; Sato et al. 1998), while the loss of CSQ2 leads to a reduced Ca2+-binding capacity with a relatively unchanged SR Ca2+ load (Knollmann et al. 2006). A loss of buffering will cause a more rapid increase in free luminal Ca2+ during re-uptake by SERCA. This faster rise of free luminal Ca2+ can affect RyR2 by increasing the amount of releasable Ca2+, and give a faster recovery from refraction, increasing the chance of SOICR (Kornyeyev et al. 2012).
While Ca2+ buffering by CSQ2 is important for the regulation of RyR2, other measures can at least partially compensate for this mechanism following CSQ2 protein loss/dysfunction. This is supported by the fact that a complete ablation of CSQ2 in rat hearts only caused an ~ 11% decrease in overall SR Ca2+ content, indicating that there must be compensatory mechanisms if CSQ2 is the main Ca2+ buffering protein in the SR (Knollmann et al. 2006). While CSQ2 is the main determinant of the SR Ca2+ content (Terentyev et al. 2003a), the SR is able to offset the loss of CSQ2 buffering by increasing its volume, and thereby maintain overall SR Ca2+ content (Knollmann et al. 2006). Furthermore, CSQ2 is not the only protein within the SR that is capable of reversible Ca2+ buffering, with histidine rich Ca2+ binding protein (HRC) and calreticulin also providing some buffering. Interestingly, mutation of HRC is also associated with an increase in arrhythmogenic Ca2+ leak (Arvanitis et al. 2011; Zhang et al. 2014b), supporting the importance of SR Ca2+-buffering in regulating RyR2 activity. There is evidence that HRC and calreticulin expression can be increased to compensate when CSQ2 expression is decreased in knockout models (Song et al. 2007; Murphy et al. 2011), although these mechanisms are controversial and vary between models. The presence of these adaptive mechanisms to maintain SR Ca2+-buffering capability in the absence of CSQ2 highlights its importance in Ca2+ homeostasis. While Ca2+-buffering within the SR is an important regulator of RyR2 function, it is not the only mechanism by which CSQ2 is proposed to modulate channel activity.
Indirect regulation of RyR2
At present, the more popular theory for CSQ2 regulation of RyR2 is that it associates via triadin to confer a functional effect. A firm basis for this theory was established by in vitro bilayer studies, whereby triadin and junctin increased RyR2 activity, but this was subsequently decreased when CSQ2 was introduced (Györke et al. 2004). This led to the proposed model of triadin associating with RyR2 to destabilise the channel and increase SOICR, with CSQ2 association to triadin then disrupting this destabilisation. While this may suggest the mechanism involves a triadin/CSQ2 mediated conformational change in the channel to impact open probability, the involvement of altered Ca2+-buffering near the channel mouth cannot be ruled out. It seems that the effect of triadin sensitising RyR2 to luminal Ca2+ has only been shown in in vitro bilayer studies (Györke et al. 2004) and triadin overexpression studies in isolated rat myocytes (Terentyev et al. 2005). In more recent studies, these findings have become more controversial. In vivo studies suggest that triadin can stabilise RyR2 to reduce SOICR, with ablation of triadin observed to cause a dysregulation of SRJ proteins, including a decrease in expression of RyR2, CSQ2 and junctin (Chopra et al. 2009). This implicates triadin in supporting the structure and function of the RyR2 macromolecular complex. It is, however, possible that a major role of triadin in RyR2 regulation is to simply anchor CSQ2 close to the channel. This would help maintain CSQ2 localisation within the SRJ, allowing it to regulate RyR2 activity. Whether this channel regulation by CSQ2 is facilitated by providing Ca2+-buffering in close proximity to the pore, or through an interaction-mediated conformational change (Ikemoto et al. 1989) remains controversial.
CSQ2 as the luminal sensor for Ca2+
RyR2 will spontaneously open to release SR Ca2+ when the luminal concentration of Ca2+ is high enough, known as SOICR (Jiang et al. 2004), but how RyR2 is able to sense the concentration of luminal Ca2+ is not clear. A popular theory for a luminal Ca2+ sensor is CSQ2. The main evidence for this theory is that the association of CSQ2 with RyR2 will change depending on the Ca2+ level within the SR, which is driven by conformational changes after Ca2+ binds to CSQ2 (Györke et al. 2004; Wei et al. 2009). At lower SR Ca2+ concentrations, CSQ2 is associated with the macromolecular complex which reduces the activity of RyR2, but as luminal Ca2+ increases, CSQ2 will dissociate from the complex, and therefore, RyR2 activity will subsequently increase (Györke et al. 2004). For CSQ2 to bind to RyR2, a small amount of Ca2+ must be present for the CSQ2 to become ordered, due to Ca2+ causing a hydrophobic pocket to form (Kumar et al. 2013). At concentrations > 5 mM Ca2+, however, CSQ2 will undergo a further conformational change and then dissociate from RyR2 (Györke et al. 2004; Handhle et al. 2016). This association and dissociation of CSQ2 with changing Ca2+ levels may be how RyR2 is able to sense luminal Ca2+ concentrations. This theory is further supported by observations that mechanisms which disrupt CSQ2’s association with the macromolecular complex also blunt RyR2’s sensitivity to luminal Ca2+ (Hanna et al. 2017).
However, there is also growing contrary evidence which suggests that the luminal Ca2+ sensor may be endogenous to RyR2. Even in CSQ2-null mice, SR Ca2+ leak is still possible (Knollmann et al. 2006; Chopra et al. 2007), while purified RyR2 is also able to exhibit spontaneous Ca2+ leak (Jiang et al. 2004; Jones et al. 2008). This makes it likely that the luminal Ca2+ sensor is located within RyR2 itself and is instead modulated by CSQ2 (Knollmann et al. 2006; Handhle et al. 2016). While the mechanism of luminal activation of the channel remains disputed (Jones et al. 2017), studies examining RyR2 mutations provide some potential insights. Work by Chen et al. (2014) first characterised the E4872Q mutation, which is localised to the proposed gating/pore region of the channel (Chen et al. 2014). This residue is hypothesised be involved in forming a Ca2+-binding pocket which is only available when the channel is in an open state (Jones et al. 2017). Expression of the RyR2-E4872Q variant in mice resulted in a loss of luminal sensitivity, while retaining cytosolic activation properties (Chen et al. 2014). Furthermore, crossbreeding of these mice with a CSQ2-null strain resulted in reduced spontaneous Ca2+ activity and lower arrhythmic potential compared to CSQ2-null controls (Zhang et al. 2014a). This suggests that the luminal Ca2+ sensor may be within this region in wild-type RyR2, although how CSQ2 regulates this luminal Ca2+ sensitivity requires more investigation.
Direct regulation of RyR2
Although the prevailing theory for CSQ-RyR interaction has predominantly been via triadin (Györke et al. 2004), there have long been suggestions that skeletal CSQ1 and RyR1 directly interact (Murray and Ohlendieck 1998; Herzog et al. 2000). In a study by Beard and Dulhunty (2015), CSQ1 with the C-tail removed (which reportedly cannot associate with triadin/junctin) was observed to not only lose its ability to inhibit RyR1 activity, but to enhance channel open probability (Beard and Dulhunty 2015). Interestingly, while the deletion of the C-tail reduced the association of CSQ1 to RyR1, it did not completely ablate the interaction, suggesting that the interaction between CSQ and RyR may not be solely at the C-tail of CSQ (Beard and Dulhunty 2015). It should, however, be noted that some junctin binding was also still retained following the C-terminal deletion. Furthermore, this region is also responsible for Ca2+ binding, and subsequently, it is difficult to distinguish the contribution of Ca2+-buffering loss from the altered physical association with the channel (Beard and Dulhunty 2015). Although these findings were in different isoforms of CSQ and RyR, they share a large amount of homology, meaning that the mechanisms may be similar for both proteins. However, more recent work in cell models devoid of triadin and junctin expression have demonstrated a functional interaction between RyR2 and CSQ2, likely between the first luminal loop of RyR2 and the C-tail of CSQ2 (Fig. 7). This interaction was found to alter the Ca2+ release dynamics of RyR2, reducing the frequency of spontaneous Ca2+ release events (Handhle et al. 2016).
Despite these conflicting findings on how CSQ2 may confer its effect on RyR2, it remains clear that CSQ2 is a necessary and important regulator of RyR2 activity, although more work is needed to investigate the exact mechanisms of how this regulation occurs.
CSQ2 in arrhythmogenesis
Given the strong evidence surrounding the role of CSQ2 as a key regulator of Ca2+ homeostasis and RyR2 activity, it is not surprising that CSQ2 has been implicated in arrhythmia pathophysiology. This includes the involvement of CSQ2 mutations in the development of CPVT, as well as findings from CSQ2 expression studies in animal models of arrhythmia.
CSQ2 mutations and CPVT
CPVT is a class of arrhythmias induced by periods of stress, with patients often diagnosed during childhood due to exercise-induced syncope or sudden cardiac death, despite a structurally normal heart (Leenhardt et al. 1995). Patients with CPVT also demonstrate susceptibility to atrial arrhythmias (Sumitomo et al. 2007). CPVT is a familial form of arrhythmia, with several mutations in RyR2 characterised as underlying its inheritance. In addition to RyR2, mutations in the CASQ2 gene encoding CSQ2 have been established as the underlying basis in 2–5% of CPVT cases (Lieve et al. 2016). To date, there have been at least dozen missense, splicing and deletion CSQ2 mutations determined as being causative of CPVT (see Fig. 3 and Table 1), in addition to several newly identified CPVT-associated mutations (Online Resource 1). CSQ2-based CPVT is typically considered to be inherited in an autosomal recessive manner, although some recent pathological variants have been identified as dominant mutations (Gray et al. 2016; Titus et al. 2020). Two of the most extensively studied CSQ2 mutations are the R33Q and D307H missense substitutions, which have been proposed to confer their pathological effects through different mechanisms.
Table 1.
CSQ2 variants causative of CPVT
| Variant | Mutation type | Domain | Phenotype | Functional impact | References |
|---|---|---|---|---|---|
| L23fs + 14X (62delA) | Deletion | N-terminus | CPVT | Premature stop codon, absence of full-length CSQ2 | (Postma et al. 2002; Ng et al. 2020) |
| R33Q | Missense | N-terminus | CPVT |
Increased CICR gain, enhanced frequency of Ca2+ waves and sparks, reduced SR content, inability to inhibit RyR2, unaltered triadin binding; ~ 50% reduced expression Enhanced Ca2+-buffering, unaltered Ca2+-binding and polymerisation; reduced Ca2+-induced conformational changes with reduced Ca2+ binding and polymerisation; increased protein degradation |
(Terentyev et al. 2006, 2008a; Kim et al. 2007; Rizzi et al. 2008; Valle et al. 2008; Bal et al. 2010; Zhang et al. 2018; Ng et al. 2020; Wang et al. 2020) |
| R33X | Non-sense | CPVT | Premature stop codon, absence of full-length CSQ2 | (Postma et al. 2002; Li et al. 2019) | |
| Y55C | Missense | I | CPVT | Inability to dimerise | (de la Fuente et al. 2008; Ng et al. 2020) |
| G112 + 5X | Deletion | CPVT, cardiac arrest | Premature stop codon, absence of full-length CSQ2; does not bind Ca2+ | (di Barletta et al. 2006) | |
| L167H | Missense | II | CPVT | Increased sensitivity to cleavage, reduced Ca2+-dependent polymerisation | (di Barletta et al. 2006; Kim et al. 2007; Valle et al. 2008; Bal et al. 2011; Wang et al. 2020) |
| 532 + 1G > A | Incorrect splicing | CPVT, SD, cardiac arrest | Premature stop codon, absence of full-length CSQ2 | (Postma et al. 2002; Josephs et al. 2017; Li et al. 2019) | |
| K180R | Missense | II | CPVT, cardiac arrest | Maintained dimerisation but impaired polymerisation/filamentation | (Gray et al. 2016; Ng et al. 2020; Titus et al. 2020) |
| F189L | Missense | II | CPVT, SD | Possible reduced protein flexibility | (Liu et al. 2008; Eckey et al. 2010; Rajagopalan and Pollanen 2016) |
| K206N | Missense | II | Cardiac arrest |
Increased Ca2+ leak in basal and stimulated conditions, reduced SR Ca2+; unaltered triadin association Additional glycosylation site; reduced Ca2+ binding, altered polymerisation |
(Kirchhefer et al. 2010) |
| D307H | Missense | III | CPVT |
Structurally normal heart, CPVT sensitive; reduced SR content and impaired Ca2+ transients or unaltered SR content with enhanced Ca2+ leak under rest and stimulated conditions; 95% reduced expression Impaired Ca2+-buffering/binding and polymerisation; unaltered PMTs, reduced binding to triadin and junctin; correct localisation to SRJ; increased protein degradation; Ca2+ selectivity lost |
(Lahat et al. 2001; Houle et al. 2004; Viatchenko-Karpinski et al. 2004; Dirksen et al. 2007; Kim et al. 2007; Song et al. 2007; Kalyanasundaram et al. 2010, 2012; Bal et al. 2011; Valle et al. 2020; Wang et al. 2020) |
| P308L | Missense | III | CPVT, SD | Ability to dimerise but impaired polymerisation; Ca2+ selectivity lost | (de la Fuente et al. 2008; Bal et al. 2011; Hong et al. 2012; Ng et al. 2020) |
| W361X | Non-sense | CPVT, cardiac arrest | Not available | (Kawamura et al. 2013; Fujisawa et al. 2019) |
CICR, Ca2+-induced Ca2+ release; CPVT, catecholaminergic polymorphic ventricular tachycardia; CSQ2, calsequestrin-2; PMTs, post-translational modifications; RyR2, ryanodine receptor type 2; SD, sudden death; SR, sarcoplasmic reticulum; SRJ, junctional SR
R33Q mutant and impaired RyR2 regulation
Located in the N-tail adjacent to domain I, the R33Q mutation was first reported and characterised by Terentyev et al. in 2006. While overexpression of wildtype CSQ2 in isolated rat myocytes produces an enhanced Ca2+ store load, total SR load is unchanged following CSQ2-R33Q overexpression; however, the free Ca2+ concentration is reduced (Terentyev et al. 2006). R33Q overexpressing myocytes also demonstrate enhanced Ca2+ release activity, via both triggered and spontaneous mechanisms. This includes an amplified CICR gain and increased occurrence of Ca2+ waves and sparks, with an increased amplitude of these Ca2+ release events (Terentyev et al. 2006, 2008a). This led to the proposal of an enhanced Ca2+-buffering capacity of this mutant. Given these results were from overexpression of R33Q in the presence of wild-type CSQ2, it was proposed that this variant confers a dominant, gain-of-function effect. However, as the authors concede, these findings were not recapitulated when the mutant and wildtype CSQ2 were expressed at a 1:1 ratio, in agreement with heterozygous carriers of the mutation being asymptomatic (Terentyev et al. 2006). Homozygous CSQ2-R33Q knock-in mice demonstrate similar features to the aforementioned transgenic myocytes, with reduced SR content and adrenergically triggered electrical abnormalities (Rizzi et al. 2008). An increased incidence of Ca2+ sparks and waves is also reported in these animals, which is further exacerbated with an adrenergic agonist (Zhang et al. 2018). Intriguingly, many aspects of this pro-arrhythmic phenotype can be rescued with wildtype CSQ2 gene therapy (Denegri et al. 2014).
There have been conflicting findings regarding the Ca2+-binding and polymerisation capacity of this CSQ2 variant. Experiments by Terentyev et al. indicated that the Ca2+-binding of the R33Q mutant is unchanged compared to wildtype CSQ2, with fluorescence resonant energy transfer (FRET) analysis suggesting that its polymerisation is also unaltered (Terentyev et al. 2006, 2008a). However, others report improper dimer formation with impaired transition to a polymer form, resulting in a reduced capacity to bind Ca2+ and undergo Ca2+-dependent conformational changes (Kim et al. 2007; Bal et al. 2010; Ng et al. 2020; Wang et al. 2020). In search of the mechanistic link between this variant and arrhythmogenesis, it has been demonstrated that while wildtype CSQ2 inhibits RyR2 channel activity in lipid bilayer experiments, this effect is lost with the R33Q mutation (Terentyev et al. 2006). Subsequently, the popular hypothesis of how CSQ2-R33Q induces a CPVT phenotype is through altered regulation of RyR2 leading to enhanced channel activity, rather than impaired Ca2+-buffering. Interestingly, despite its proposed role in RyR2 activity modulation, it has been reported that CSQ2-R33Q does not exhibit altered binding to triadin (Terentyev et al. 2008a). This suggests either the potential impairment of a direct interaction with RyR2 or a conferment of an altered conformation of the mutant CSQ2 through the quaternary complex.
D307H mutant and impaired Ca2+ buffering
The D307H mutant was first described 20 years ago by Lahat et al. in Israeli families with an early-onset, autosomal recessive form of CPVT (Lahat et al. 2001). D307 is a residue highly conserved between species, located within an exposed loop on domain III of CSQ2 (Kim et al. 2007). It was initially hypothesised that the conversion of the negatively charged aspartic acid to a positively charged histidine would disrupt the ability of CSQ2 to bind Ca2+ ions (Lahat et al. 2001). This was later confirmed experimentally both in vitro and in vivo, with D307H mutant CSQ2 exhibiting reduced Ca2+ binding capacity and impaired polymerisation (Kim et al. 2007; Kalyanasundaram et al. 2010; Wang et al. 2020). Interestingly, phosphorylation and glycosylation of the D307H mutant are reportedly unchanged compared to wildtype (Houle et al. 2004), in agreement with the observation of correct trafficking and localisation to the SRJ (Dirksen et al. 2007; Kalyanasundaram et al. 2010).
However, there are some inconsistencies in the functional implications of CSQ2-D307H expression in myocytes, which appear to be model-specific. Homozygous knock-in CSQ2-D307H mice show reduced SR Ca2+ content with impaired Ca2+ transient properties and an increased propensity for Ca2+-mediated arrhythmias (Song et al. 2007). Similar findings are observed with virally-mediated overexpression of this mutant in isolated myocytes, resulting in decreased SR store content and a reduction in Ca2+ transient amplitude and duration in non-adrenergically challenged myocytes (Viatchenko-Karpinski et al. 2004). In contrast to the R33Q mutation, this suggests an impaired ability to store SR Ca2+, and subsequently release it during CICR in baseline conditions. Largely in agreement with these findings, mice overexpressing (2- to sixfold) CSQ2-D307H on a wild-type background were observed to maintain normal SR Ca2+ content, but with altered Ca2+ cycling dynamics in both the presence and absence of adrenergic stimulation (Dirksen et al. 2007). However, these results are contrary to observations in CPVT patients, who do not display altered cardiac dynamics at rest (apart from occasional mild bradycardia) (Postma et al. 2005). Further confounding the translatability of these latter models is that they express both wildtype and mutant CSQ2, making them representative of heterozygous gene carriers, who do not develop CPVT symptoms (Lahat et al. 2001).
Perhaps, more relevant to the development of autosomal recessive diseases is a mouse line with ~ twofold overexpression of the D307H mutant in CSQ2-null background mouse. These mice demonstrate unaltered Ca2+ cycling at rest with normal total SR Ca2+ content (Kalyanasundaram et al. 2012). A reduction in Ca2+ transient amplitude in response to adrenergic stimulation, an increased occurrence of spontaneous Ca2+ release events and enhanced susceptibility to CPVT were also observed (Kalyanasundaram et al. 2012). These latter findings are also consistently reported in the other D307H mouse models (Viatchenko-Karpinski et al. 2004; Dirksen et al. 2007; Song et al. 2007), highlighting the role of this mutation in CPVT arrhythmogenesis.
Combining the evidence from both in vitro and in vivo studies, it appears that the CSQ2-D307H mutant is able to maintain normal Ca2+ levels within the SR under resting conditions. However, following adrenergic stimulation, the increase in SR content surpasses the impaired Ca2+-buffering capacity of the mutant protein, resulting in enhanced Ca2+ leak to trigger ectopic contractions. Experimental work by Houle et al. also revealed changes in protein–protein interactions with the D307H mutant—specifically a reduction of binding to triadin and junctin (Houle et al. 2004). This was reportedly due to an impaired Ca2+-dependent conformational change which would normally facilitate this interaction (Houle et al. 2004). These studies further demonstrate the importance of dimerisation and polymerisation in the Ca2+-buffering capacity of CSQ2 and are an additional mechanism by which its mutations are functionally implicated in CPVT pathology.
Additional arrhythmogenic mechanisms
A more recently identified CSQ2 mutant associated with cardiac arrest, K206N, has been characterised which results in an additional N-glycosylation site being present within the protein (Kirchhefer et al. 2010). Hyper-glycosylation of this variant was confirmed, which correlated with reduced Ca2+-binding and altered protein aggregation (Kirchhefer et al. 2010). Due to its location within domain II, it was hypothesised that, rather than being directly involved in Ca2+ binding and polymerisation, the additional PMT may block the function of nearby Ca2+-binding residues. Within myocytes, CSQ2-K206N was associated with a blunted caffeine response, indicative of reduced SR Ca2+ load (Kirchhefer et al. 2010). Interestingly, the degree of interaction of the K206N mutant with triadin was unaltered. However, an enhanced open state of RyR2 was suggested, which was supported by an increased occurrence of Ca2+ leak events in both basal and stimulated conditions (Kirchhefer et al. 2010). These findings suggest the K206N mutation acts through a glycosylation-mediated mechanism to directly impact RyR2 regulation, although the exact nature of this mechanism remains to be fully elucidated.
In addition to the above described (and several other) missense mutations, CPVT-linked CSQ2 mutations can also result in truncations or frameshift deletions within the protein. These latter mutations have also been observed to alter Ca2+ release as a consequence of impaired Ca2+-buffering and polymerisation, as well as a reduced association with the RyR2 macromolecular complex (di Barletta et al. 2006; Song et al. 2007; Terentyev et al. 2008a). This reduced association maybe due to a physical inability to interact, or due to an altered polymerisation-mediated trafficking mechanism which results in the loss of mutant CSQ2 from the SRJ (Titus et al. 2020). A commonly reported feature of mutant CSQ2 knock-in mice is a reduction in CSQ2 protein expression levels. Both homozygous R33Q and D307H mutant knock-in animals demonstrate severely reduced CSQ2 protein expression (50% for R33Q and 95% for D307H), despite unchanged mRNA levels (Song et al. 2007; Rizzi et al. 2008). This suggests increased degradation of mutant CSQ2, which has been confirmed by enhanced susceptibility to proteolysis (Rizzi et al. 2008; Kalyanasundaram et al. 2010), although this is likely to occur via different mechanisms for each mutation (Valle et al. 2020).
Combined, evidence from the currently characterised mutants associated with CPVT reveal numerous and complex mechanisms through which CSQ2 is implicated in arrhythmogenesis. These include impaired polymerisation and Ca2+-buffering, altered regulation of RyR2, incorrect protein trafficking and increased protein degradation. Investigations to date indicate that any one of these mechanisms would be sufficient to generate a CPVT phenotype; however, it is likely that in many instances, a combination of these mechanisms is in play.
CSQ2 expression and arrhythmogenesis
As described above, one of the consequences of CPVT-associated CSQ2 mutations can include the loss of full-length protein. As the impact of this CSQ2 loss can be difficult to separate from the functional effects of the mutation itself, CSQ2-null mice were developed by Knollmann et al. Surprisingly, despite a complete lack of CSQ2, there is minimal change observed in the SR Ca2+ content or Ca2+ transient properties in these mice at rest, creating doubt as to the importance of this buffering protein in Ca2+ homeostasis (at least in basal conditions) (Knollmann et al. 2006). It has been proposed that the enlarged SR which is observed in these mice is able to, at least partially, compensate for the loss of Ca2+-buffering within the store (Knollmann et al. 2006; Kornyeyev et al. 2012). However, as expected, CSQ2-null mice demonstrate enhanced SOICR activity, particularly following exercise or adrenergic agonist exposure, creating an increased susceptibility to both CPVT and AF events (Knollmann et al. 2006; Faggioni et al. 2014; Glukhov et al. 2015). Interestingly, despite this enhanced propensity for SOICR-mediated arrhythmogenesis, CSQ2-null mice reportedly have a reduced susceptibility to Ca2+ alternans. It is hypothesised that the loss of SR Ca2+ buffering leads to faster filling of the SR during diastole, which results in shortened refractoriness of Ca2+ release (Kornyeyev et al. 2012). While these studies indicate that CSQ2 plays an important role in regulating RyR2 activity and SR Ca2+ release, it also suggests that it is not directly responsible for RyR2 luminal sensing, but rather modulates it, as leak can occur in the absence of CSQ2 (Knollmann et al. 2006). However, considering the size of RyR2 and the importance of SR Ca2+ release, it is highly likely that multiple mechanisms contribute to the sensitivity of the channel to SR Ca2+ content.
Similar to reports from CPVT patients, CSQ2-null mice demonstrate normal cardiac contractility at rest, albeit with the presence of bradycardia (Knollmann et al. 2006; Kornyeyev et al. 2012). This reduced resting heart rate has been noted as a perplexing finding given increasing Ca2+ leak from the SR would be indicative of increased AP generation in the SA node (Glukhov et al. 2015). Furthermore, knockout of CSQ2 is also associated with the development of atrial and ventricular hypertrophy, as well as interstitial fibrosis (Knollmann et al. 2006; Glukhov et al. 2015), which is not apparent in CPVT patients. While some animal models of CPVT indicate a near-absence of CSQ2 protein, such as the D307H mutant (Song et al. 2007), this is not recapitulated in all studies, with others suggesting either no change or only a reduction in protein expression (Rizzi et al. 2008; Kalyanasundaram et al. 2012). Furthermore, mice homozygous for the D307H mutation on a CSQ2-null background demonstrate a reduced susceptibility to arrhythmia compared to CSQ2-null mice, indicating that the presence of mutant CSQ2 confers a functional effect compared to the complete absence of CSQ2 (Kalyanasundaram et al. 2012). It should also be noted that, surprisingly, little is known about the expression of CSQ2 in CPVT patients. Therefore, perhaps, more relevant to patient arrhythmogenic mechanisms is the examination of reduced CSQ2 expression, rather than the complete absence of the protein. Heterozygous CSQ2 knockout mice demonstrate a 25% reduction in CSQ2 expression levels, without significant changes to other quaternary complex proteins (Knollmann et al. 2006; Chopra et al. 2007). In basal conditions, this modest reduction in expression leads to unaltered SR Ca2+ content and release properties, and is not associated with remodelling of the SR, unlike that observed with complete loss of CSQ2 (Chopra et al. 2007). However, these heterozygous mice demonstrate an increased occurrence of SR Ca2+ leak events and enhanced susceptibility to ventricular arrhythmias following adrenergic stimulation compared to wildtype mice, but lower than that observed in homozygous mice (Chopra et al. 2007). Furthermore, this Ca2+ leak becomes more prevalent as SR load is increased (Chopra et al. 2007).
On the other end of the scale, the functional impact of CSQ2 overexpression has also been extensively studied. Findings from cardiac-specific CSQ2 overexpression (~ 10- to 20-fold) in mice were first described in 1998, which was associated with the development of cardiac hypertrophy and impaired myocyte contractile function (Jones et al. 1998; Sato et al. 1998). Perhaps, unsurprisingly, these mice demonstrate an enhanced SR Ca2+ load capacity and altered Ca2+ release dynamics, with a reduction in the amplitude of CICR and diminished Ca2+ spark activity (Jones et al. 1998; Sato et al. 1998; Wang et al. 2000). A similar effect of enhanced SR store capacity but reduced EC coupling gain has also been observed with more modest CSQ2 overexpression (~ 60%) in isolated rabbit myocytes (Miller et al. 2005). It has been proposed that the reduced SR Ca2+ release in the CSQ2 mice results in impaired inhibition of LTCC, leading to prolonged ICa (Jones et al. 1998; Sato et al. 1998; Wang et al. 2000), which is likely to contribute to the significant electrical disturbances presented in these mice (Knollmann et al. 2000). Interestingly, sensitisation of RyR2 by low dose caffeine resulted in normalisation of Ca2+ activity (Wang et al. 2000), further supporting the role of CSQ2 as a modulator of RyR2 activity.
Maintaining the CSQ2:RyR2 balance
The heart is capable of remodelling in both physiological and pathological conditions, with the expression of proteins, such as CSQ2, often being part of this (Yang et al. 2021). While one study identified a reduction in RyR2, triadin and junctin expression levels in CSQ2-overexpressing mice (Jones et al. 1998), another reported no changes in these SR proteins (Sato et al. 1998). Interestingly, CSQ2-null mice show a reduction of both triadin and junctin protein levels, suggesting an interplay in the regulation of expression for these quaternary complex proteins (Knollmann et al. 2006). Some mice expressing CPVT-associated CSQ2 mutations demonstrate an upregulation of RyR2 as a mechanism to compensate for the reduced Ca2+ transient amplitude due to reduced SR Ca2+ content (Song et al. 2007). However, surprisingly, little is known about CSQ2 expression levels in CPVT patients. While some studies report no changes in CSQ2 mRNA or protein levels in persistent AF or HF patients (Münch et al. 1998; Lai et al. 1999), others suggest both protein and mRNA levels may be reduced in models of arrhythmia and HF (Yeh et al. 2008; Hu et al. 2011). Although CSQ2 loss may itself be important in arrhythmogenesis, considering the evidence to date, it is likely that it is the balance of proteins involved in the quaternary complex which is the key indicator of arrhythmic potential.
As detailed above, altering CSQ2 expression levels can result in perturbation of Ca2+ handling within the myocytes. In particular, the loss of functional CSQ2 results in severe arrhythmic consequences due to enhanced RyR2-mediated Ca2+ leak. This can also be viewed as a reduction in the ratio of CSQ2:RyR2 leading to a reduced capacity to modulate channel activity. While the research discussed above focussed on alterations in CSQ2 expression to highlight this functional impact, this consequence would hold true for changes in RyR2 expression. If the expression of CSQ2 (or other proteins of the RyR2 macromolecular complex responsible for the regulation of RyR2) remain constant, but RyR2 expression increases, then the relative capacity to regulate RyR2 will be reduced. Clinical evidence for this comes from studies investigating arrhythmogenic mechanisms in AF patients. Voigt et al. revealed an increase in RyR2 expression in paroxysmal AF patients which was associated with increased Ca2+ leak events and enhanced channel open probability (Voigt et al. 2014). Interestingly, this expression was normalised to CSQ2, although more recent evidence suggests that expression of CSQ2 itself may be altered in cardiac pathologies (Yang et al. 2021). Despite this caveat, this study clearly demonstrates a link between an altered CSQ2:RyR2 expression ratio and changes in RyR2 channel regulation.
It should be acknowledged that reduced RyR2 expression, or overexpression of CSQ2, can also alter this ratio. The functional consequences of increased CSQ2 expression have been described above, while a reduction in RyR2 expression has been associated with the development of arrhythmias or cardiac alternans (Yeh et al. 2008; Zhong et al. 2018). Additionally, loss of function mutations in RyR2 have been attributed to a recently described form of arrhythmia known as calcium release deficiency syndrome (CRDS) (Li et al. 2021; Sun et al. 2021). Interestingly, many studies examining the loss of RyR2 do not quantify CSQ2 expression, making it difficult to interpret the functional impact of altering the expression ratio of these proteins in this manner. Furthermore, considering the role of the quaternary complex (including RyR2) in anchoring CSQ2 within the SRJ, it is possible that reduced expression of RyR2 would also result in impaired localisation of CSQ2.
Considering all this evidence together, we propose that it is this balance between CSQ2 and RyR2 expression which is a key determinant of SR Ca2+ homeostasis (Fig. 8) and maintaining/restoring this balance would be beneficial in ameliorating cardiac dysfunction. Subsequently, assessing the expression of these two proteins in relation to each other would be prudent to determine novel insights as to mechanisms of Ca2+ disruption in cardiac pathology, particularly in the setting of arrhythmia. This could be considered akin to the importance of assessing relative changes in SERCA/PLB expression, and represents a new, yet important method for determining the relative contribution these of SR Ca2+ proteins to cardiac dysfunction.
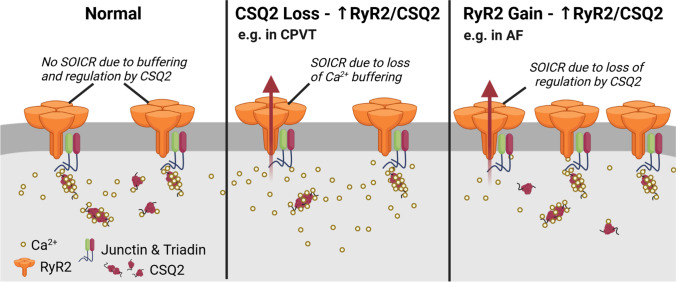
Fig. 8.
Arrhythmogenic mechanisms due to a misbalance in CSQ2:RyR2 expression. Schematic representing normal Ca2+ handling when calsequestrin-2 (CSQ2) and ryanodine receptor type 2 (RyR2) expression levels are maintained in balance with each other (left panel), with little or no store-overload induced Ca2+ release (SOICR) activity due to correct Ca2+-buffering and RyR2 channel regulation. This protein expression ratio may become misbalanced due to the loss of CSQ2 (middle panel) or an increase in RyR2 expression (right panel). The loss of functional CSQ2 relative to RyR2 expression would lead to a reduced Ca2+-buffering capability, loss of channel regulation and therefore enhanced SOICR activity, as observed in catecholaminergic polymorphic ventricular tachycardia (CPVT). An increase in RyR2 expression relative to CSQ2 levels would create a deficit in RyR2 channel regulation and increased SOICR propensity, such as that observed in atrial fibrillation (AF). Created with BioRender.com
Conclusions and perspectives
It is evident that maintaining Ca2+ homeostasis within cardiac myocytes is essential for the physiological role of RyR2 in cardiac function, as well as preventing arrhythmias. A central player in this is CSQ2 and its role in the regulation of SR Ca2+. While the importance of CSQ2 in Ca2+ homeostasis is uncontested, there is still much to be unravelled regarding the exact mechanisms of CSQ2 trafficking and function. The interplay of CSQ2 phosphorylation and glycosylation has been demonstrated to be essential in protein trafficking, although this is largely based on studies in non-cardiac derived cell lines. Therefore, determining how these modifications impact CSQ2 trafficking in cardiac myocytes is required, although difficult to achieve. Furthermore, clarifying the role of PMTs in CSQ2 polymerisation and Ca2+-buffering may identify this as a future mechanism to target in cardiac pathologies.
Perhaps more difficult to elucidate is the mechanism(s) by which CSQ2 confers its modulation of RyR2 activity. There is clear evidence in support of both a Ca2+-buffering mediated effect, and a protein–protein interaction role (whether via triadin/junctin or directly) which may confer a conformational change. It is highly likely that these two mechanisms are not mutually exclusive, with both required for correct regulation of the SR Ca2+ release channel. Evidence from CPVT patients and animal models clearly highlight the impact of CSQ2 in arrhythmogenesis, with both mutations and altered expression resulting in pathological Ca2+ handling. Additional studies, however, offer potential avenues for treatment, with virally mediated expression of wildtype CSQ2 reversing the arrhythmic phenotype in CPVT models (Denegri et al. 2014; Lodola et al. 2016). However, it remains essential that the balance of SR Ca2+ regulatory proteins is maintained. Super-resolution imaging has previously revealed that altering the association of RyR2 with other accessory proteins within the dyad impacts Ca2+ leak properties (Wang et al. 2014; Munro et al. 2016). Furthermore, the organisation of CSQ1 within skeletal muscle triads differs between fibre types, which may contribute to differences in Ca2+ handling dynamics (Jayasinghe et al. 2014), suggesting that super-resolution imaging could also elucidate the role of altered CSQ2 organisation in cardiac disease. It would therefore be of particular interest to determine how the association and expression ratio of CSQ2 and RyR2 within the dyad is altered in arrhythmia to impact Ca2+ handling dynamics.
Supplementary Information
Below is the link to the electronic supplementary material.
Funding
This work was supported by the Health Research Council of New Zealand [Emerging Researcher First Grant #20/625 to MLM; Project Grants #18/232 and #20/370 to PPJ], the Heart Foundation of New Zealand [Research Fellowship #1784 to MLM; Research Grant #1836 to PPJ] and the Marsden Fund from the Royal Society of New Zealand [Fast-Start Grant #UOO2009 to MLM].
Declarations
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Conflict of interest
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Arvanitis DA, Vafiadaki E, Sanoudou D, Kranias EG. Histidine-rich calcium binding protein: the new regulator of sarcoplasmic reticulum calcium cycling. J Mol Cell Cardiol. 2011;50(1):43–49. doi: 10.1016/j.yjmcc.2010.08.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bai Y, Jones PP, Guo J, Zhong X, Clark RB, Zhou Q, Wang R, Vallmitjana A, Benitez R, Hove-Madsen L, Semeniuk L, Guo A, Song L-S, Duff HJ, Chen SRW. Phospholamban knockout breaks arrhythmogenic Ca2+ waves and suppresses catecholaminergic polymorphic ventricular tachycardia in mice. Circ Res. 2013;113(5):517–526. doi: 10.1161/CIRCRESAHA.113.301678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bal NC, Sharon A, Gupta SC, Jena N, Shaikh S, Gyorke S, Periasamy M. The catecholaminergic polymorphic ventricular tachycardia mutation R33Q disrupts the N-terminal structural motif that regulates reversible calsequestrin polymerization. J Biol Chem. 2010;285(22):17188–17196. doi: 10.1074/jbc.M109.096354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bal NC, Jena N, Sopariwala D, Balaraju T, Shaikh S, Bal C, Sharon A, Gyorke S, Periasamy M. Probing cationic selectivity of cardiac calsequestrin and its CPVT mutants. Biochem J. 2011;435(2):391–399. doi: 10.1042/BJ20101771. [DOI] [PubMed] [Google Scholar]
- Bassani JW, Yuan W, Bers DM. Fractional SR Ca release is regulated by trigger Ca and SR Ca content in cardiac myocytes. Am J Physiol. 1995;268(5 Pt 1):C1313–1319. doi: 10.1152/ajpcell.1995.268.5.C1313. [DOI] [PubMed] [Google Scholar]
- Beard NA, Dulhunty AF. C-terminal residues of skeletal muscle calsequestrin are essential for calcium binding and for skeletal ryanodine receptor inhibition. Skelet Muscle. 2015;5:6. doi: 10.1186/s13395-015-0029-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belevych A, Kubalova Z, Terentyev D, Hamlin RL, Carnes CA, Györke S. Enhanced ryanodine receptor-mediated calcium leak determines reduced sarcoplasmic reticulum calcium content in chronic canine heart failure. Biophys J. 2007;93(11):4083–4092. doi: 10.1529/biophysj.107.114546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bers DM. Cardiac excitation-contraction coupling. Nature. 2002;415(6868):198–205. doi: 10.1038/415198a. [DOI] [PubMed] [Google Scholar]
- Bers DM. Macromolecular complexes regulating cardiac ryanodine receptor function. J Mol Cell Cardiol. 2004;37(2):417–429. doi: 10.1016/j.yjmcc.2004.05.026. [DOI] [PubMed] [Google Scholar]
- Cala SE, Scott BT, Jones LR. Intralumenal sarcoplasmic reticulum Ca(2+)-binding proteins. Semin Cell Biol. 1990;1(4):265–275. [PubMed] [Google Scholar]
- Cala SE, Jones LR. Phosphorylation of cardiac and skeletal muscle calsequestrin isoforms by casein kinase II. Demonstration of a cluster of unique rapidly phosphorylated sites in cardiac calsequestrin. J Biol Chem. 1991;266(1):391–398. [PubMed] [Google Scholar]
- Carter S, Pitt SJ, Colyer J, Sitsapesan R. Ca2+-dependent phosphorylation of RyR2 can uncouple channel gating from direct cytosolic Ca2+ regulation. J Membr Biol. 2011;240(1):21–33. doi: 10.1007/s00232-011-9339-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chakraborty AD, Gonano LA, Munro ML, Smith LJ, Thekkedam C, Staudacher V, Gamble AB, Macquaide N, Dulhunty AF, Jones PP. Activation of RyR2 by class I kinase inhibitors. Br J Pharmacol. 2019;176(6):773–786. doi: 10.1111/bph.14562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen W, Wang R, Chen B, Zhong X, Kong H, Bai Y, Zhou Q, Xie C, Zhang J, Guo A, Tian X, Jones PP, O’Mara ML, Liu Y, Mi T, Zhang L, Bolstad J, Semeniuk L, Cheng H, Zhang J, Chen J, Tieleman DP, Gillis AM, Duff HJ, Fill M, Song L-S, Chen SRW. The ryanodine receptor store-sensing gate controls Ca2+ waves and Ca2+-triggered arrhythmias. Nat Med. 2014;20(2):184–192. doi: 10.1038/nm.3440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chopra N, Kannankeril PJ, Yang T, Hlaing T, Holinstat I, Ettensohn K, Pfeifer K, Akin B, Jones LR, Franzini-Armstrong C, Knollmann BC. Modest reductions of cardiac calsequestrin increase sarcoplasmic reticulum Ca 2+ leak independent of luminal Ca2+ and trigger ventricular arrhythmias in mice. Circ Res. 2007;101(6):617–626. doi: 10.1161/CIRCRESAHA.107.157552. [DOI] [PubMed] [Google Scholar]
- Chopra N, Yang T, Asghari P, Moore ED, Huke S, Akin B, Cattolica RA, Perez CF, Hlaing T, Knollmann-Ritschel BE, Jones LR, Pessah IN, Allen PD, Franzini-Armstrong C, Knollmann BC. Ablation of triadin causes loss of cardiac Ca2+ release units, impaired excitation-contraction coupling, and cardiac arrhythmias. Proc Natl Acad Sci U S A. 2009;106(18):7636–7641. doi: 10.1073/pnas.0902919106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dashwood A, Cheesman E, Beard N, Haqqani H, Wong YW, Molenaar P. Understanding how phosphorylation and redox modifications regulate cardiac ryanodine receptor type 2 activity to produce an arrhythmogenic phenotype in advanced heart failure. ACS Pharmacol Transl Sci. 2020;3(4):563–582. doi: 10.1021/acsptsci.0c00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de la Fuente S, Van Langen IM, Postma AV, Bikker H, Meijer A. A case of catecholaminergic polymorphic ventricular tachycardia caused by two calsequestrin 2 mutations. Pacing Clin Electrophysiol. 2008;31(7):916–919. doi: 10.1111/j.1540-8159.2008.01111.x. [DOI] [PubMed] [Google Scholar]
- Delbridge LM, MBassani JW, Bers DM. Steady-state twitch Ca2+ fluxes and cytosolic Ca2+ buffering in rabbit ventricular myocytes. Am J Physiol Cell Physiol. 1996;270(1 39 1):C192–C199. doi: 10.1152/ajpcell.1996.270.1.C192. [DOI] [PubMed] [Google Scholar]
- Denegri M, Bongianino R, Lodola F, Boncompagni S, De Giusti VC, Avelino-Cruz JE, Liu N, Persampieri S, Curcio A, Esposito F, Pietrangelo L, Marty I, Villani L, Moyaho A, Baiardi P, Auricchio A, Protasi F, Napolitano C, Priori SG. Single delivery of an adeno-associated viral construct to transfer the CASQ2 gene to knock-in mice affected by catecholaminergic polymorphic ventricular tachycardia is able to cure the disease from birth to advanced age. Circulation. 2014;129(25):2673–2681. doi: 10.1161/CIRCULATIONAHA.113.006901. [DOI] [PubMed] [Google Scholar]
- di Barletta MR, Viatchenko-Karpinski S, Nori A, Memmi M, Terentyev D, Turcato F, Valle G, Rizzi N, Napolitano C, Gyorke S, Volpe P, Priori SG. Clinical phenotype and functional characterization of CASQ2 mutations associated with catecholaminergic polymorphic ventricular tachycardia. Circulation. 2006;114(10):1012–1019. doi: 10.1161/CIRCULATIONAHA.106.623793. [DOI] [PubMed] [Google Scholar]
- Dirksen WP, Lacombe VA, Chi M, Kalyanasundaram A, Viatchenko-Karpinski S, Terentyev D, Zhou Z, Vedamoorthyrao S, Li N, Chiamvimonvat N, Carnes CA, Franzini-Armstrong C, Györke S, Periasamy M. A mutation in calsequestrin, CASQ2(D307H), impairs sarcoplasmic reticulum Ca(2+) handling and causes complex ventricular arrhythmias in mice. Cardiovasc Res. 2007;75(1):69–78. doi: 10.1016/j.cardiores.2007.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donoso P, Sanchez G, Bull R, Hidalgo C. Modulation of cardiac ryanodine receptor activity by ROS and RNS. Front Biosci (landmark Ed) 2011;16:553–567. doi: 10.2741/3705. [DOI] [PubMed] [Google Scholar]
- Dulhunty AF, Wium E, Li L, Hanna AD, Mirza S, Talukder S, Ghazali NA, Beard NA. Proteins within the intracellular calcium store determine cardiac RyR channel activity and cardiac output. Clin Exp Pharmacol Physiol. 2012;39(5):477–484. doi: 10.1111/j.1440-1681.2012.05704.x. [DOI] [PubMed] [Google Scholar]
- Eckey K, Strutz-Seebohm N, Katz G, Fuhrmann G, Henrion U, Pott L, Linke WA, Arad M, Lang F, Seebohm G. Modulation of human ether a gogo related channels by CASQ2 contributes to etiology of catecholaminergic polymorphic ventricular tachycardia (CPVT) Cell Physiol Biochem. 2010;26(4–5):503–512. doi: 10.1159/000322318. [DOI] [PubMed] [Google Scholar]
- Fabiato A. Calcium-induced release of calcium from the cardiac sarcoplasmic reticulum. Am J Phys Cell Physiol. 1983;14(1):C1–C14. doi: 10.1152/ajpcell.1983.245.1.C1. [DOI] [PubMed] [Google Scholar]
- Faggioni M, Savio-Galimberti E, Venkataraman R, Hwang HS, Kannankeril PJ, Darbar D, Knollmann BC. Suppression of spontaneous ca elevations prevents atrial fibrillation in calsequestrin 2-null hearts. Circ Arrhythm Electrophysiol. 2014;7(2):313–320. doi: 10.1161/CIRCEP.113.000994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fliegel L, Ohnishi M, Carpenter MR, Khanna VK, Reithmeier RA, MacLennan DH. Amino acid sequence of rabbit fast-twitch skeletal muscle calsequestrin deduced from cDNA and peptide sequencing. Proc Natl Acad Sci U S A. 1987;84(5):1167–1171. doi: 10.1073/pnas.84.5.1167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fryer MW, Stephenson DG. Total and sarcoplasmic reticulum calcium contents of skinned fibres from rat skeletal muscle. J Physiol Paris. 1996;493(Pt 2):357–370. doi: 10.1113/jphysiol.1996.sp021388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujisawa T, Aizawa Y, Katsumata Y, Udo A, Ito S, Hatakeyama K, Hirose M, Miyama H, Nakajima K, Nishiyama T, Kimura T, Nitta M, Misumi K, Takatsuki S, Kosaki K, Fukuda K. A homozygous CASQ2 mutation in a Japanese patient with catecholaminergic polymorphic ventricular tachycardia. Case Rep Genet. 2019;2019:9056596. doi: 10.1155/2019/9056596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glukhov AV, Kalyanasundaram A, Lou Q, Hage LT, Hansen BJ, Belevych AE, Mohler PJ, Knollmann BC, Periasamy M, Györke S, Fedorov VV. Calsequestrin 2 deletion causes sinoatrial node dysfunction and atrial arrhythmias associated with altered sarcoplasmic reticulum calcium cycling and degenerative fibrosis within the mouse atrial pacemaker complex1. Eur Heart J. 2015;36(11):686–697. doi: 10.1093/eurheartj/eht452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonano LA, Jones PP. FK506-binding proteins 12 and 12.6 (FKBPs) as regulators of cardiac Ryanodine Receptors: Insights from new functional and structural knowledge. Channels (austin) 2017;11(5):415–425. doi: 10.1080/19336950.2017.1344799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gong D, Chi X, Wei J, Zhou G, Huang G, Zhang L, Wang R, Lei J, Chen SRW, Yan N. Modulation of cardiac ryanodine receptor 2 by calmodulin. Nature. 2019;572(7769):347–351. doi: 10.1038/s41586-019-1377-y. [DOI] [PubMed] [Google Scholar]
- Gray B, Bagnall RD, Lam L, Ingles J, Turner C, Haan E, Davis A, Yang PC, Clancy CE, Sy RW, Semsarian C. A novel heterozygous mutation in cardiac calsequestrin causes autosomal dominant catecholaminergic polymorphic ventricular tachycardia. Heart Rhythm. 2016;13(8):1652–1660. doi: 10.1016/j.hrthm.2016.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo A, Cala SE, Song LS. Calsequestrin accumulation in rough endoplasmic reticulum promotes perinuclear Ca2+ release. J Biol Chem. 2012;287(20):16670–16680. doi: 10.1074/jbc.M112.340927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Györke I, Hester N, Jones LR, Györke S. The role of calsequestrin, triadin, and junctin in conferring cardiac ryanodine receptor responsiveness to luminal calcium. Biophys J . 2004;86(4):2121–2128. doi: 10.1016/S0006-3495(04)74271-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Handhle A, Ormonde CE, Thomas NL, Bralesford C, Williams AJ, Lai FA, Zissimopoulos S. Calsequestrin interacts directly with the cardiac ryanodine receptor luminal domain. J Cell Sci. 2016;129(21):3983–3988. doi: 10.1242/jcs.191643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanna AD, Lam A, Thekkedam C, Willemse H, Dulhunty AF, Beard NA. The anthracycline metabolite doxorubicinol abolishes RyR2 sensitivity to physiological changes in luminal Ca(2+) through an interaction with calsequestrin. Mol Pharmacol. 2017;92(5):576–587. doi: 10.1124/mol.117.108183. [DOI] [PubMed] [Google Scholar]
- Herzog A, Szegedi C, Jona I, Herberg FW, Varsanyi M. Surface plasmon resonance studies prove the interaction of skeletal muscle sarcoplasmic reticular Ca(2+) release channel/ryanodine receptor with calsequestrin. FEBS Lett. 2000;472(1):73–77. doi: 10.1016/s0014-5793(00)01431-9. [DOI] [PubMed] [Google Scholar]
- Hong RA, Rivera KK, Jittirat A, Choi JJ. Flecainide suppresses defibrillator-induced storming in catecholaminergic polymorphic ventricular tachycardia. Pacing Clin Electrophysiol. 2012;35(7):794–797. doi: 10.1111/j.1540-8159.2012.03421.x. [DOI] [PubMed] [Google Scholar]
- Houle TD, Ram ML, Cala SE. Calsequestrin mutant D307H exhibits depressed binding to its protein targets and a depressed response to calcium. Cardiovasc Res. 2004;64(2):227–233. doi: 10.1016/j.cardiores.2004.09.009. [DOI] [PubMed] [Google Scholar]
- Houle TD, Ram ML, McMurray WJ, Cala SE. Different endoplasmic reticulum trafficking and processing pathways for calsequestrin (CSQ) and epitope-tagged CSQ. Exp Cell Res. 2006;312(20):4150–4161. doi: 10.1016/j.yexcr.2006.09.010. [DOI] [PubMed] [Google Scholar]
- Hu ST, Liu GS, Shen YF, Wang YL, Tang Y, Yang YJ. Defective Ca(2+) handling proteins regulation during heart failure. Physiol Res. 2011;60(1):27–37. doi: 10.33549/physiolres.931948. [DOI] [PubMed] [Google Scholar]
- Huke S, Bers DM. Ryanodine receptor phosphorylation at Serine 2030, 2808 and 2814 in rat cardiomyocytes. Biochem Biophys Res Commun. 2008;376(1):80–85. doi: 10.1016/j.bbrc.2008.08.084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huttlin EL, Jedrychowski MP, Elias JE, Goswami T, Rad R, Beausoleil SA, Villén J, Haas W, Sowa ME, Gygi SP. A tissue-specific atlas of mouse protein phosphorylation and expression. Cell. 2010;143(7):1174–1189. doi: 10.1016/j.cell.2010.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikemoto N, Ronjat M, Mészáros LG, Koshita M. Postulated role of calsequestrin in the regulation of calcium release from sarcoplasmic reticulum. Biochemistry. 1989;28(16):6764–6771. doi: 10.1021/bi00442a033. [DOI] [PubMed] [Google Scholar]
- Jacob S, Sleiman NH, Kern S, Jones LR, Sala-Mercado JA, McFarland TP, Sabbah HH, Cala SE. Altered calsequestrin glycan processing is common to diverse models of canine heart failure. Mol Cell Biochem. 2013;377(1):11–21. doi: 10.1007/s11010-013-1560-7. [DOI] [PubMed] [Google Scholar]
- Jayasinghe I, Munro DM, Baddeley D, Launikonis BS, Soeller C. Observation of the molecular organization of calcium release sites in fast- and slow-twitch skeletal muscle with nanoscale imaging. J R Soc Interface. 2014;11(99):20140570. doi: 10.1098/rsif.2014.0570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang D, Xiao B, Yang D, Wang R, Choi P, Zhang L, Cheng H, Chen SRW. RyR2 mutations linked to ventricular tachycardia and sudden death reduce the threshold for store-overload-induced Ca2+ release (SOICR) Proc Natl Acad Sci USA. 2004;101(35):13062–13067. doi: 10.1073/pnas.0402388101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang D, Jones PP, Davis DR, Gow R, Green MS, Birnie DH, Chen SR, Gollob MH. Characterization of a novel mutation in the cardiac ryanodine receptor that results in catecholaminergic polymorphic ventricular tachycardia. Channels (austin) 2010;4(4):302–310. doi: 10.4161/chan.4.4.12666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones LR, Suzuki YJ, Wang W, Kobayashi YM, Ramesh V, Franzini-Armstrong C, Cleemann L, Morad M. Regulation of Ca2+ signaling in transgenic mouse cardiac myocytes overexpressing calsequestrin. J Clin Investig. 1998;101(7):1385–1393. doi: 10.1172/JCI1362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones PP, Jiang D, Bolstad J, Hunt DJ, Zhang L, Demaurex N, Chen SR. Endoplasmic reticulum Ca2+ measurements reveal that the cardiac ryanodine receptor mutations linked to cardiac arrhythmia and sudden death alter the threshold for store-overload-induced Ca2+ release. Biochem J. 2008;412(1):171–178. doi: 10.1042/BJ20071287. [DOI] [PubMed] [Google Scholar]
- Jones PP, Guo W, Chen SRW. Control of cardiac ryanodine receptor by sarcoplasmic reticulum luminal Ca(2+) J Gen Physiol. 2017;149(9):867–875. doi: 10.1085/jgp.201711805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Josephs K, Patel K, Janson CM, Montagna C, McDonald TV. Compound heterozygous CASQ2 mutations and long-term course of catecholaminergic polymorphic ventricular tachycardia. Mol Genet Genomic Med. 2017;5(6):788–794. doi: 10.1002/mgg3.323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalyanasundaram A, Bal NC, Franzini-Armstrong C, Knollmann BC, Periasamy M. The calsequestrin mutation CASQ2D307H does not affect protein stability and targeting to the junctional sarcoplasmic reticulum but compromises its dynamic regulation of calcium buffering. J Biol Chem. 2010;285(5):3076–3083. doi: 10.1074/jbc.M109.053892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalyanasundaram A, Viatchenko-Karpinski S, Belevych AE, Lacombe VA, Hwang HS, Knollmann BC, Gyorke S, Periasamy M. Functional consequences of stably expressing a mutant calsequestrin (CASQ2D307H) in the CASQ2 null background. American Journal of Physiology-Heart and Circulatory Physiology. 2012;302(1):H253–H261. doi: 10.1152/ajpheart.00578.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang C, Trumble WR, Dunker AK. Crystallization and structure-function of calsequestrin. Methods Mol Biol. 2002;172:281–294. doi: 10.1385/1-59259-183-3:281. [DOI] [PubMed] [Google Scholar]
- Kawamura M, Ohno S, Naiki N, Nagaoka I, Dochi K, Wang Q, Hasegawa K, Kimura H, Miyamoto A, Mizusawa Y, Itoh H, Makiyama T, Sumitomo N, Ushinohama H, Oyama K, Murakoshi N, Aonuma K, Horigome H, Honda T, Yoshinaga M, Ito M, Horie M. Genetic background of catecholaminergic polymorphic ventricular tachycardia in Japan. Circ J. 2013;77(7):1705–1713. doi: 10.1253/circj.cj-12-1460. [DOI] [PubMed] [Google Scholar]
- Kiarash A, Kelly CE, Phinney BS, Valdivia HH, Abrams J, Cala SE. Defective glycosylation of calsequestrin in heart failure. Cardiovasc Res. 2004;63(2):264–272. doi: 10.1016/j.cardiores.2004.04.001. [DOI] [PubMed] [Google Scholar]
- Kim E, Youn B, Kemper L, Campbell C, Milting H, Varsanyi M, Kang C. Characterization of human cardiac calsequestrin and its deleterious mutants. J Mol Biol. 2007;373(4):1047–1057. doi: 10.1016/j.jmb.2007.08.055. [DOI] [PubMed] [Google Scholar]
- Kirchhefer U, Wehrmeister D, Postma AV, Pohlentz G, Mormann M, Kucerova D, Müller FU, Schmitz W, Schulze-Bahr E, Wilde AA, Neumann J. The human CASQ2 mutation K206N is associated with hyperglycosylation and altered cellular calcium handling. J Mol Cell Cardiol. 2010;49(1):95–105. doi: 10.1016/j.yjmcc.2010.03.006. [DOI] [PubMed] [Google Scholar]
- Knollmann BC, Knollmann-Ritschel BE, Weissman NJ, Jones LR, Morad M. Remodelling of ionic currents in hypertrophied and failing hearts of transgenic mice overexpressing calsequestrin. J Physiol. 2000;525(Pt 2):483–498. doi: 10.1111/j.1469-7793.2000.t01-1-00483.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knollmann BC, Chopra N, Hlaing T, Akin B, Yang T, Ettensohn K, Knollmann BE, Horton KD, Weissman NJ, Holinstat I, Zhang W, Roden DM, Jones LR, Franzini-Armstrong C, Pfeifer K. Casq2 deletion causes sarcoplasmic reticulum volume increase, premature Ca2+ release, and catecholaminergic polymorphic ventricular tachycardia. J Clin Invest. 2006;116(9):2510–2520. doi: 10.1172/JCI29128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kornyeyev D, Petrosky AD, Zepeda B, Ferreiro M, Knollmann B, Escobar AL. Calsequestrin 2 deletion shortens the refractoriness of Ca2+ release and reduces rate-dependent Ca2+-alternans in intact mouse hearts. J Mol Cell Cardiol. 2012;52(1):21–31. doi: 10.1016/j.yjmcc.2011.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar A, Chakravarty H, Bal NC, Balaraju T, Jena N, Misra G, Bal C, Pieroni E, Periasamy M, Sharon A. Identification of calcium binding sites on calsequestrin 1 and their implications for polymerization. Mol Biosyst. 2013;9(7):1949–1957. doi: 10.1039/c3mb25588c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lahat H, Pras E, Olender T, Avidan N, Ben-Asher E, Man O, Levy-Nissenbaum E, Khoury A, Lorber A, Goldman B, Lancet D, Eldar M. A missense mutation in a highly conserved region of CASQ2 is associated with autosomal recessive catecholamine-induced polymorphic ventricular tachycardia in Bedouin families from Israel. Am J Hum Genet. 2001;69(6):1378–1384. doi: 10.1086/324565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai L-P, Su M-J, Lin J-L, Lin F-Y, Tsai C-H, Chen Y-S, Huang SKS, Tseng Y-Z, Lien W-P. Down-regulation of L-type calcium channel and sarcoplasmic reticular Ca2+-ATPase mRNA in human atrial fibrillation without significant change in the mRNA of ryanodine receptor, calsequestrin and phospholamban: an insight into the mechanism of atrial electrical remodeling. J Am Coll Cardiol. 1999;33(5):1231–1237. doi: 10.1016/s0735-1097(99)00008-x. [DOI] [PubMed] [Google Scholar]
- Launikonis BS, Zhou J, Royer L, Shannon TR, Brum G, Ríos E. Depletion “skraps” and dynamic buffering inside the cellular calcium store. Proc Natl Acad Sci U S A. 2006;103(8):2982–2987. doi: 10.1073/pnas.0511252103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laver DR. Ca2+ stores regulate ryanodine receptor Ca2+ release channels via luminal and cytosolic Ca2+ sites. Biophys J . 2007;92(10):3541–3555. doi: 10.1529/biophysj.106.099028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee KW, Maeng JS, Choi JY, Lee YR, Hwang CY, Park SS, Park HK, Chung BH, Lee SG, Kim YS, Jeon H, Eom SH, Kang C, Kim DH, Kwon KS. Role of Junctin protein interactions in cellular dynamics of calsequestrin polymer upon calcium perturbation. J Biol Chem. 2012;287(3):1679–1687. doi: 10.1074/jbc.M111.254045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leenhardt A, Lucet V, Denjoy I, Grau F, Ngoc DD, Coumel P. Catecholaminergic polymorphic ventricular tachycardia in children. A 7-year follow-up of 21 patients. Circulation. 1995;91(5):1512–1519. doi: 10.1161/01.cir.91.5.1512. [DOI] [PubMed] [Google Scholar]
- Lehnart SE, Maier LS, Hasenfuss G. Abnormalities of calcium metabolism and myocardial contractility depression in the failing heart. Heart Fail Rev. 2009;14(4):213–224. doi: 10.1007/s10741-009-9146-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis KM, Munske GR, Byrd SS, Kang J, Cho H-J, Ríos E, Kang C. Characterization of post-translational modifications to calsequestrins of cardiac and skeletal muscle. Int J Mol Sci. 2016;17(9):1539. doi: 10.3390/ijms17091539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Q, Guo R, Gao L, Cui L, Zhao Z, Yu X, Yuan Y, Xu X. CASQ2 variants in Chinese children with catecholaminergic polymorphic ventricular tachycardia. Mol Genet Genomic Med. 2019;7(11):e949–e949. doi: 10.1002/mgg3.949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Wei J, Guo W, Sun B, Estillore JP, Wang R, Yoruk A, Roston TM, Sanatani S, Wilde AAM, Gollob MH, Roberts JD, Tseng ZH, Jensen HK, Chen SRW. Human RyR2 (Ryanodine Receptor 2) Loss-of-function mutations: clinical phenotypes and in vitro characterization. Circ Arrhythm Electrophysiol. 2021;14(9):e010013. doi: 10.1161/CIRCEP.121.010013. [DOI] [PubMed] [Google Scholar]
- Lieve KV, van der Werf C, Wilde AA. Catecholaminergic polymorphic ventricular tachycardia. Circ J. 2016;80(6):1285–1291. doi: 10.1253/circj.CJ-16-0326. [DOI] [PubMed] [Google Scholar]
- Liu QQ, Oberti C, Zhang XQ, Ke T, Zhang T, Scheinman M, Hu DY, Wang QK. A Novel mutation of F189L in CASQ2 in families with catecholaminergic polymorphic ventricular tachycardia. Zhonghua Yi Xue Yi Chuan Xue Za Zhi. 2008;25(3):334–337. [PubMed] [Google Scholar]
- Liu T, Xiong F, Qi XY, Xiao J, Villeneuve L, Abu-Taha I, Dobrev D, Huang C, Nattel S. Altered calcium handling produces reentry-promoting action potential alternans in atrial fibrillation-remodeled hearts. JCI Insight. 2020;5(8):e133754. doi: 10.1172/jci.insight.133754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lodola F, Morone D, Denegri M, Bongianino R, Nakahama H, Rutigliano L, Gosetti R, Rizzo G, Vollero A, Buonocore M, Napolitano C, Condorelli G, Priori SG, Di Pasquale E. Adeno-associated virus-mediated CASQ2 delivery rescues phenotypic alterations in a patient-specific model of recessive catecholaminergic polymorphic ventricular tachycardia. Cell Death Dis. 2016;7(10):e2393. doi: 10.1038/cddis.2016.304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marx SO, Reiken S, Hisamatsu Y, Jayaraman T, Burkhoff D, Rosemblit N, Marks AR. PKA phosphorylation dissociates FKBP12.6 from the calcium release channel (ryanodine receptor): defective regulation in failing hearts. Cell. 2000;101(4):365–376. doi: 10.1016/s0092-8674(00)80847-8. [DOI] [PubMed] [Google Scholar]
- McFarland TP, Milstein ML, Cala SE. Rough endoplasmic reticulum to junctional sarcoplasmic reticulum trafficking of calsequestrin in adult cardiomyocytes. J Mol Cell Cardiol. 2010;49(4):556–564. doi: 10.1016/j.yjmcc.2010.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McFarland TP, Sleiman NH, Yaeger DB, Cala SE. The cytosolic protein kinase CK2 phosphorylates cardiac calsequestrin in intact cells. Mol Cell Biochem. 2011;353(1–2):81–91. doi: 10.1007/s11010-011-0777-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller SL, Currie S, Loughrey CM, Kettlewell S, Seidler T, Reynolds DF, Hasenfuss G, Smith GL. Effects of calsequestrin over-expression on excitation-contraction coupling in isolated rabbit cardiomyocytes. Cardiovasc Res. 2005;67(4):667–677. doi: 10.1016/j.cardiores.2005.04.023. [DOI] [PubMed] [Google Scholar]
- Milstein ML, Houle TD, Cala SE. Calsequestrin isoforms localize to different ER subcompartments: evidence for polymer and heteropolymer-dependent localization. Exp Cell Res. 2009;315(3):523–534. doi: 10.1016/j.yexcr.2008.11.006. [DOI] [PubMed] [Google Scholar]
- Mohamed BA, Hartmann N, Tirilomis P, Sekeres K, Li W, Neef S, Richter C, Zeisberg EM, Kattner L, Didié M, Guan K, Schmitto JD, Lehnart SE, Luther S, Voigt N, Seidler T, Sossalla S, Hasenfuss G, Toischer K. Sarcoplasmic reticulum calcium leak contributes to arrhythmia but not to heart failure progression. Sci Transl Med. 2018;10:458 eaan0724. doi: 10.1126/scitranslmed.aan0724. [DOI] [PubMed] [Google Scholar]
- Münch G, Bölck B, Hoischen S, Brixius K, Bloch W, Reuter H, Schwinger RH. Unchanged protein expression of sarcoplasmic reticulum Ca2+-ATPase, phospholamban, and calsequestrin in terminally failing human myocardium. J Mol Med (berl) 1998;76(6):434–441. doi: 10.1007/s001090050235. [DOI] [PubMed] [Google Scholar]
- Munro ML, Jayasinghe ID, Wang Q, Quick A, Wang W, Baddeley D, Wehrens XH, Soeller C. Junctophilin-2 in the nanoscale organisation and functional signalling of ryanodine receptor clusters in cardiomyocytes. J Cell Sci. 2016;129(23):4388–4398. doi: 10.1242/jcs.196873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munro ML, van Hout I, Aitken-Buck HM, Sugunesegran R, Bhagwat K, Davis PJ, Lamberts RR, Coffey S, Soeller C, Jones PP. Human atrial fibrillation is not associated with remodeling of ryanodine receptor clusters. Front. Cell Dev. Biol. 2021;9(394):633704. doi: 10.3389/fcell.2021.633704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy RM, Mollica JP, Beard NA, Knollmann BC, Lamb GD. Quantification of calsequestrin 2 (CSQ2) in sheep cardiac muscle and Ca2+-binding protein changes in CSQ2 knockout mice. Am J Physiol Heart Circ Physiol. 2011;300(2):H595–604. doi: 10.1152/ajpheart.00902.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray BE, Ohlendieck K. Complex formation between calsequestrin and the ryanodine receptor in fast- and slow-twitch rabbit skeletal muscle. FEBS Lett. 1998;429(3):317–322. doi: 10.1016/s0014-5793(98)00621-8. [DOI] [PubMed] [Google Scholar]
- Murtaza I, Wang HX, Feng X, Alenina N, Bader M, Prabhakar BS, Li PF. Down-regulation of catalase and oxidative modification of protein kinase CK2 lead to the failure of apoptosis repressor with caspase recruitment domain to inhibit cardiomyocyte hypertrophy. J Biol Chem. 2008;283(10):5996–6004. doi: 10.1074/jbc.M706466200. [DOI] [PubMed] [Google Scholar]
- Ng K, Titus EW, Lieve KV, Roston TM, Mazzanti A, Deiter FH, et al. An international multicenter evaluation of inheritance patterns, arrhythmic risks, and underlying mechanisms of CASQ2-catecholaminergic polymorphic ventricular tachycardia. Circulation. 2020;142(10):932–947. doi: 10.1161/CIRCULATIONAHA.120.045723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nikolaienko R, Bovo E, Zima AV. Redox dependent modifications of ryanodine receptor: basic mechanisms and implications in heart diseases. Front Physiol. 2018;9:1775. doi: 10.3389/fphys.2018.01775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nori A, Valle G, Massimino ML, Volpe P. Targeting of calsequestrin to the sarcoplasmic reticulum of skeletal muscle upon deletion of its glycosylation site. Exp Cell Res. 2001;265(1):104–113. doi: 10.1006/excr.2001.5172. [DOI] [PubMed] [Google Scholar]
- O’Brian JJ, Ram ML, Kiarash A, Cala SE. Mass spectrometry of cardiac calsequestrin characterizes microheterogeneity unique to heart and indicative of complex intracellular transit. J Biol Chem. 2002;277(40):37154–37160. doi: 10.1074/jbc.M204370200. [DOI] [PubMed] [Google Scholar]
- Otsu K, Fujii J, Periasamy M, Difilippantonio M, Uppender M, Ward DC, MacLennan DH. Chromosome mapping of five human cardiac and skeletal muscle sarcoplasmic reticulum protein genes. Genomics. 1993;17(2):507–509. doi: 10.1006/geno.1993.1357. [DOI] [PubMed] [Google Scholar]
- Park H, Wu S, Dunker AK, Kang C. Polymerization of calsequestrin: implications for Ca2+ regulation. J Biol Chem. 2003;278(18):16176–16182. doi: 10.1074/jbc.M300120200. [DOI] [PubMed] [Google Scholar]
- Park H, Park IY, Kim E, Youn B, Fields K, Dunker AK, Kang C. Comparing skeletal and cardiac calsequestrin structures and their calcium binding: a proposed mechanism for coupled calcium binding and protein polymerization. J Biol Chem. 2004;279(17):18026–18033. doi: 10.1074/jbc.M311553200. [DOI] [PubMed] [Google Scholar]
- Pasqualini E, Caillol N, Valette A, Lloubes R, Verine A, Lombardo D. Phosphorylation of the rat pancreatic bile-salt-dependent lipase by casein kinase II is essential for secretion. Biochem J. 2000;345(Pt 1):121–128. [PMC free article] [PubMed] [Google Scholar]
- Peng W, Shen H, Wu J, Guo W, Pan X, Wang R, Chen SR, Yan N. Structural basis for the gating mechanism of the type 2 ryanodine receptor RyR2. Science. 2016;354(6310):aah5324. doi: 10.1126/science.aah5324. [DOI] [PubMed] [Google Scholar]
- Pollak AJ, Liu C, Gudlur A, Mayfield JE, Dalton ND, Gu Y, Chen J, Heller Brown J, Hogan PG, Wiley SE, Peterson KL, Dixon JE. A secretory pathway kinase regulates sarcoplasmic reticulum Ca(2+) homeostasis and protects against heart failure. Elife. 2018;7:e41378. doi: 10.7554/eLife.41378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pölönen RP, Swan H, Aalto-Setälä K. Mutation-specific differences in arrhythmias and drug responses in CPVT patients: simultaneous patch clamp and video imaging of iPSC derived cardiomyocytes. Mol Biol Rep. 2020;47(2):1067–1077. doi: 10.1007/s11033-019-05201-y. [DOI] [PubMed] [Google Scholar]
- Postma AV, Denjoy I, Hoorntje TM, Lupoglazoff JM, Da Costa A, Sebillon P, Mannens MM, Wilde AA, Guicheney P. Absence of calsequestrin 2 causes severe forms of catecholaminergic polymorphic ventricular tachycardia. Circ Res. 2002;91(8):e21–26. doi: 10.1161/01.res.0000038886.18992.6b. [DOI] [PubMed] [Google Scholar]
- Postma AV, Denjoy I, Kamblock J, Alders M, Lupoglazoff JM, Vaksmann G, Dubosq-Bidot L, Sebillon P, Mannens MM, Guicheney P, Wilde AA. Catecholaminergic polymorphic ventricular tachycardia: RYR2 mutations, bradycardia, and follow up of the patients. J Med Genet. 2005;42(11):863–870. doi: 10.1136/jmg.2004.028993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potenza DM, Janicek R, Fernandez-Tenorio M, Camors E, Ramos-Mondragón R, Valdivia HH, Niggli E. Phosphorylation of the ryanodine receptor 2 at serine 2030 is required for a complete β-adrenergic response. J Gen Physiol. 2019;151(2):131–145. doi: 10.1085/jgp.201812155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajagopalan A, Pollanen MS. Sudden death during struggle in the setting of heterozygosity for a mutation in calsequesterin 2. Forensic Sci Med Pathol. 2016;12(1):86–89. doi: 10.1007/s12024-015-9733-1. [DOI] [PubMed] [Google Scholar]
- Ram ML, Kiarash A, Marsh JD, Cala SE. Phosphorylation and dephosphorylation of calsequestrin on CK2-sensitive sites in heart. Mol Cell Biochem. 2004;266(1–2):209–217. doi: 10.1023/b:mcbi.0000049164.28580.56. [DOI] [PubMed] [Google Scholar]
- Respress JL, Van Oort RJ, Li N, Rolim N, Dixit SS, Dealmeida A, Voigt N, Lawrence WS, Skapura DG, Skårdal K, Wisløff U, Wieland T, Ai X, Pogwizd SM, Dobrev D, Wehrens XHT. Role of RyR2 phosphorylation at S2814 during heart failure progression. Circ Res. 2012;110(11):1474–1483. doi: 10.1161/CIRCRESAHA.112.268094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rizzi N, Liu N, Napolitano C, Nori A, Turcato F, Colombi B, Bicciato S, Arcelli D, Spedito A, Scelsi M, Villani L, Esposito G, Boncompagni S, Protasi F, Volpe P, Priori SG. Unexpected structural and functional consequences of the R33Q homozygous mutation in cardiac calsequestrin: a complex arrhythmogenic cascade in a knock in mouse model. Circ Res. 2008;103(3):298–306. doi: 10.1161/CIRCRESAHA.108.171660. [DOI] [PubMed] [Google Scholar]
- Rodriguez MM, Chen CH, Smith BL, Mochly-Rosen D. Characterization of the binding and phosphorylation of cardiac calsequestrin by epsilon protein kinase C. FEBS Lett. 1999;454(3):240–246. doi: 10.1016/s0014-5793(99)00697-3. [DOI] [PubMed] [Google Scholar]
- Roux-Buisson N, Rendu J, Denjoy I, Guicheney P, Goldenberg A, David N, Faivre L, Barthez O, Danieli GA, Marty I, Lunardi J, Fauré J. Functional analysis reveals splicing mutations of the CASQ2 gene in patients with CPVT: implication for genetic counselling and clinical management. Hum Mutat. 2011;32(9):995–999. doi: 10.1002/humu.21537. [DOI] [PubMed] [Google Scholar]
- Sanchez EJ, Munske GR, Criswell A, Milting H, Dunker AK, Kang C. Phosphorylation of human calsequestrin: implications for calcium regulation. Mol Cell Biochem. 2011;353(1–2):195–204. doi: 10.1007/s11010-011-0787-4. [DOI] [PubMed] [Google Scholar]
- Sanchez EJ, Lewis KM, Munske GR, Nissen MS, Kang C. Glycosylation of skeletal calsequestrin: implications for its function. J Biol Chem. 2012;287(5):3042–3050. doi: 10.1074/jbc.M111.326363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sankaranarayanan R, Kistamás K, Greensmith DJ, Venetucci LA, Eisner DA. Systolic [Ca(2+) ](i) regulates diastolic levels in rat ventricular myocytes. J Physiol. 2017;595(16):5545–5555. doi: 10.1113/JP274366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato Y, Ferguson DG, Sako H, Dorn GW, 2nd, Kadambi VJ, Yatani A, Hoit BD, Walsh RA, Kranias EG. Cardiac-specific overexpression of mouse cardiac calsequestrin is associated with depressed cardiovascular function and hypertrophy in transgenic mice. J Biol Chem. 1998;273(43):28470–28477. doi: 10.1074/jbc.273.43.28470. [DOI] [PubMed] [Google Scholar]
- Sfeir C, Veis A. Casein kinase localization in the endoplasmic reticulum of the ROS 17/2.8 cell line. J Bone Miner Res. 1995;10(4):607–615. doi: 10.1002/jbmr.5650100414. [DOI] [PubMed] [Google Scholar]
- Shannon TR, Bers DM. Assessment of intra-SR free [Ca] and buffering in rat heart. Biophys J. 1997;73(3):1524–1531. doi: 10.1016/S0006-3495(97)78184-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shannon TR, Guo T, Bers DM. Ca2+ scraps: local depletions of free [Ca2+] in cardiac sarcoplasmic reticulum during contractions leave substantial Ca2+ reserve. Circ Res. 2003;93(1):40–45. doi: 10.1161/01.RES.0000079967.11815.19. [DOI] [PubMed] [Google Scholar]
- Shannon TR, Pogwizd SM, Bers DM. Elevated sarcoplasmic reticulum Ca2+ leak in intact ventricular myocytes from rabbits in heart failure. Circ Res. 2003;93(7):592–594. doi: 10.1161/01.RES.0000093399.11734.B3. [DOI] [PubMed] [Google Scholar]
- Sleiman NH, McFarland TP, Jones LR, Cala SE. Transitions of protein traffic from cardiac ER to junctional SR. J Mol Cell Cardiol. 2015;81:34–45. doi: 10.1016/j.yjmcc.2014.12.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soeller C, Crossman D, Gilbert R, Cannell MB. Analysis of ryanodine receptor clusters in rat and human cardiac myocytes. Proc Natl Acad Sci USA. 2007;104(38):14958–14963. doi: 10.1073/pnas.0703016104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song L, Alcalai R, Arad M, Wolf CM, Toka O, Conner DA, Berul CI, Eldar M, Seidman CE, Seidman JG. Calsequestrin 2 (CASQ2) mutations increase expression of calreticulin and ryanodine receptors, causing catecholaminergic polymorphic ventricular tachycardia. J Clin Invest. 2007;117(7):1814–1823. doi: 10.1172/JCI31080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sumitomo N, Sakurada H, Taniguchi K, Matsumura M, Abe O, Miyashita M, Kanamaru H, Karasawa K, Ayusawa M, Fukamizu S, Nagaoka I, Horie M, Harada K, Hiraoka M. Association of atrial arrhythmia and sinus node dysfunction in patients with catecholaminergic polymorphic ventricular tachycardia. Circ J. 2007;71(10):1606–1609. doi: 10.1253/circj.71.1606. [DOI] [PubMed] [Google Scholar]
- Sun B, Yao J, Ni M, Wei J, Zhong X, Guo W, Zhang L, Wang R, Belke D, Chen YX, Lieve KVV, Broendberg AK, Roston TM, Blankoff I, Kammeraad JA, von Alvensleben JC, Lazarte J, Vallmitjana A, Bohne LJ, Rose RA, Benitez R, Hove-Madsen L, Napolitano C, Hegele RA, Fill M, Sanatani S, Wilde AAM, Roberts JD, Priori SG, Jensen HK, Chen SRW. Cardiac ryanodine receptor calcium release deficiency syndrome. Sci Transl Med. 2021;13(579):eaba7287. doi: 10.1126/scitranslmed.aba7287. [DOI] [PubMed] [Google Scholar]
- Tagliabracci VS, Engel JL, Wen J, Wiley SE, Worby CA, Kinch LN, Xiao J, Grishin NV, Dixon JE. Secreted kinase phosphorylates extracellular proteins that regulate biomineralization. Science. 2012;336(6085):1150–1153. doi: 10.1126/science.1217817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terentyev D, Viatchenko-Karpinski S, Györke I, Volpe P, Williams SC, Györke S. Calsequestrin determines the functional size and stability of cardiac intracellular calcium stores: Mechanism for hereditary arrhythmia. Proc Natl Acad Sci U S A. 2003;100(20):11759–11764. doi: 10.1073/pnas.1932318100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terentyev D, Viatchenko-Karpinski S, Gyorke I, Terentyeva R, Gyorke S. Protein phosphatases decrease sarcoplasmic reticulum calcium content by stimulating calcium release in cardiac myocytes. J Physiol. 2003;552(Pt 1):109–118. doi: 10.1113/jphysiol.2003.046367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terentyev D, Cala SE, Houle TD, Viatchenko-Karpinski S, Gyorke I, Terentyeva R, Williams SC, Gyorke S. Triadin overexpression stimulates excitation-contraction coupling and increases predisposition to cellular arrhythmia in cardiac myocytes. Circ Res. 2005;96(6):651–658. doi: 10.1161/01.RES.0000160609.98948.25. [DOI] [PubMed] [Google Scholar]
- Terentyev D, Nori A, Santoro M, Viatchenko-Karpinski S, Kubalova Z, Gyorke I, Terentyeva R, Vedamoorthyrao S, Blom NA, Valle G, Napolitano C, Williams SC, Volpe P, Priori SG, Gyorke S. Abnormal interactions of calsequestrin with the ryanodine receptor calcium release channel complex linked to exercise-induced sudden cardiac death. Circ Res. 2006;98(9):1151–1158. doi: 10.1161/01.RES.0000220647.93982.08. [DOI] [PubMed] [Google Scholar]
- Terentyev D, Kubalova Z, Valle G, Nori A, Vedamoorthyrao S, Terentyeva R, Viatchenko-Karpinski S, Bers DM, Williams SC, Volpe P, Gyorke S. Modulation of SR Ca release by luminal Ca and calsequestrin in cardiac myocytes: effects of CASQ2 mutations linked to sudden cardiac death. Biophys J. 2008;95(4):2037–2048. doi: 10.1529/biophysj.107.128249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terentyev D, Györke I, Belevych AE, Terentyeva R, Sridhar A, Nishijima Y, de Blanco EC, Khanna S, Sen CK, Cardounel AJ, Carnes CA, Györke S. Redox modification of ryanodine receptors contributes to sarcoplasmic reticulum Ca2+ leak in chronic heart failure. Circ Res. 2008;103(12):1466–1472. doi: 10.1161/CIRCRESAHA.108.184457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian X, Tang Y, Liu Y, Wang R, Chen SR. Calmodulin modulates the termination threshold for cardiac ryanodine receptor-mediated Ca2+ release. Biochem J. 2013;455(3):367–375. doi: 10.1042/BJ20130805. [DOI] [PubMed] [Google Scholar]
- Titus EW, Deiter FH, Shi C, Wojciak J, Scheinman M, Jura N, Deo RC. The structure of a calsequestrin filament reveals mechanisms of familial arrhythmia. Nat Struct Mol Biol. 2020;27(12):1142–1151. doi: 10.1038/s41594-020-0510-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valle G, Galla D, Nori A, Priori SG, Gyorke S, de Filippis V, Volpe P. Catecholaminergic polymorphic ventricular tachycardia-related mutations R33Q and L167H alter calcium sensitivity of human cardiac calsequestrin. Biochem J. 2008;413(2):291–303. doi: 10.1042/BJ20080163. [DOI] [PubMed] [Google Scholar]
- Valle G, Arad M, Volpe P. Molecular adaptation to calsequestrin 2 (CASQ2) point mutations leading to catecholaminergic polymorphic ventricular tachycardia (CPVT): comparative analysis of R33Q and D307H mutants. J Muscle Res Cell Motil. 2020;41(2–3):251–258. doi: 10.1007/s10974-020-09587-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viatchenko-Karpinski S, Terentyev D, Györke I, Terentyeva R, Volpe P, Priori SG, Napolitano C, Nori A, Williams SC, Györke S. Abnormal calcium signaling and sudden cardiac death associated with mutation of calsequestrin. Circ Res. 2004;94(4):471–477. doi: 10.1161/01.RES.0000115944.10681.EB. [DOI] [PubMed] [Google Scholar]
- Voigt N, Heijman J, Wang Q, Chiang DY, Li N, Karck M, Wehrens XHT, Nattel S, Dobrev D. Cellular and molecular mechanisms of atrial arrhythmogenesis in patients with paroxysmal atrial fibrillation. Circulation. 2014;129(2):145–156. doi: 10.1161/CIRCULATIONAHA.113.006641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waddell HMM, Zhang JZ, Hoeksema KJ, McLachlan JJ, McLay JC, Jones PP. Oxidation of RyR2 has a biphasic effect on the threshold for store overload-induced calcium release. Biophys J. 2016;110(11):2386–2396. doi: 10.1016/j.bpj.2016.04.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walweel K, Oo YW, Laver DR. The emerging role of calmodulin regulation of RyR2 in controlling heart rhythm, the progression of heart failure and the antiarrhythmic action of dantrolene. Clin Exp Pharmacol Physiol. 2017;44(1):135–142. doi: 10.1111/1440-1681.12669. [DOI] [PubMed] [Google Scholar]
- Wang Q, Paskevicius T, Filbert A, Qin W, Kim HJ, Chen X-Z, Tang J, Dacks JB, Agellon LB, Michalak M. Phylogenetic and biochemical analysis of calsequestrin structure and association of its variants with cardiac disorders. Sci Rep. 2020;10(1):18115–18115. doi: 10.1038/s41598-020-75097-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang S, Trumble WR, Liao H, Wesson CR, Dunker AK, Kang CH. Crystal structure of calsequestrin from rabbit skeletal muscle sarcoplasmic reticulum. Nat Struct Biol. 1998;5(6):476–483. doi: 10.1038/nsb0698-476. [DOI] [PubMed] [Google Scholar]
- Wang W, Cleemann L, Jones LR, Morad M. Modulation of focal and global Ca2+ release in calsequestrin-overexpressing mouse cardiomyocytes. J Physiol. 2000;524(Pt 2):399–414. doi: 10.1111/j.1469-7793.2000.00399.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang W, Landstrom AP, Wang Q, Munro ML, Beavers D, Ackerman MJ, Soeller C, Wehrens XH. Reduced junctional Na+/Ca2+-exchanger activity contributes to sarcoplasmic reticulum Ca2+ leak in junctophilin-2-deficient mice. Am J Physiol Heart Circ Physiol. 2014;307(9):H1317–1326. doi: 10.1152/ajpheart.00413.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wehrens XH, Lehnart SE, Reiken SR, Marks AR. Ca2+/calmodulin-dependent protein kinase II phosphorylation regulates the cardiac ryanodine receptor. Circ Res. 2004;94(6):e61–70. doi: 10.1161/01.RES.0000125626.33738.E2. [DOI] [PubMed] [Google Scholar]
- Wei L, Hanna AD, Beard NA, Dulhunty AF. Unique isoform-specific properties of calsequestrin in the heart and skeletal muscle. Cell Calcium. 2009;45(5):474–484. doi: 10.1016/j.ceca.2009.03.006. [DOI] [PubMed] [Google Scholar]
- Xiao B, Tian X, Xie W, Jones PP, Cai S, Wang X, Jiang D, Kong H, Zhang L, Chen K, Walsh MP, Cheng H, Chen SRW. Functional consequence of protein kinase A-dependent phosphorylation of the cardiac ryanodine receptor: Sensitization of store overload-induced Ca 2+ release. J Biol Chem. 2007;282(41):30256–30264. doi: 10.1074/jbc.M703510200. [DOI] [PubMed] [Google Scholar]
- Xu L, Meissner G. Regulation of cardiac muscle Ca2+ release channel by sarcoplasmic reticulum lumenal Ca2+ Biophys J. 1998;75(5):2302–2312. doi: 10.1016/S0006-3495(98)77674-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu L, Eu JP, Meissner G, Stamler JS. Activation of the cardiac calcium release channel (ryanodine receptor) by poly-S-nitrosylation. Science. 1998;279(5348):234–237. doi: 10.1126/science.279.5348.234. [DOI] [PubMed] [Google Scholar]
- Yang M, Yan J, Wu A, Zhao W, Qin J, Pogwizd SM, Wu X, Yuan S, Ai X. Pflugers Arch. 2021;4733(351):362. doi: 10.1007/s00424-021-02538-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeh YH, Wakili R, Qi XY, Chartier D, Boknik P, Kääb S, Ravens U, Coutu P, Dobrev D, Nattel S. Calcium-handling abnormalities underlying atrial arrhythmogenesis and contractile dysfunction in dogs with congestive heart failure. Circulation. Arrhythmia and Electrophysiology. 2008;1(2):93–102. doi: 10.1161/CIRCEP.107.754788. [DOI] [PubMed] [Google Scholar]
- Zhang J, Chen B, Zhong X, Mi T, Guo A, Zhou Q, Tan Z, Wu G, Chen AW, Fill M, Song L-S, Chen SRW. The cardiac ryanodine receptor luminal Ca(2+) sensor governs Ca(2+) waves, ventricular tachyarrhythmias, and cardiac hypertrophy in calsequestrin null mice. Biochem J. 2014;461(1):99–106. doi: 10.1042/BJ20140126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang JC, Wu HL, Chen Q, Xie XT, Zou T, Zhu C, Dong Y, Xiang GJ, Ye L, Li Y, Zhu PL. Calcium-mediated oscillation in membrane potentials and atrial-triggered activity in atrial cells of Casq2(R33Q/R33Q) mutation mice. Front Physiol. 2018;9:1447. doi: 10.3389/fphys.2018.01447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang JZ, McLay JC, Jones PP. The arrhythmogenic human HRC point mutation S96A leads to spontaneous Ca2+ release due to an impaired ability to buffer store Ca2+ J Mol Cell Cardiol. 2014;74:22–31. doi: 10.1016/j.yjmcc.2014.04.019. [DOI] [PubMed] [Google Scholar]
- Zhang JZ, Waddell HM, Wu E, Dholakia J, Okolo CA, McLay JC, Jones PP. FKBPs facilitate the termination of spontaneous Ca2+ release in wild-type RyR2 but not CPVT mutant RyR2. Biochem J. 2016;473(14):2049–2060. doi: 10.1042/BCJ20160389. [DOI] [PubMed] [Google Scholar]
- Zhong X, Vallmitjana A, Sun B, Xiao Z, Guo W, Wei J, Ni M, Chen Y, O’Brien ER, Gillis AM, Hoshijima M, Takeshima H, Hove-Madsen L, Benitez R, Belke D, Wayne Chen SR. Reduced expression of cardiac ryanodine receptor protects against stress-induced ventricular tachyarrhythmia, but increases the susceptibility to cardiac alternans. Biochem J. 2018;475(1):169–183. doi: 10.1042/BCJ20170631. [DOI] [PubMed] [Google Scholar]
- Zima AV, Picht E, Bers DM, Blatter LA. Termination of cardiac Ca2+ sparks: role of intra-SR [Ca2+], release flux, and intra-SR Ca2+ diffusion. Circ Res. 2008;103(8):e105–115. doi: 10.1161/CIRCRESAHA.107.183236. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.