Abstract
Recent studies have revealed an increase in the incidence of serious infections caused by non-albicans Candida species. Candida lusitaniae is of special interest because of its sporadic resistance to amphotericin B (AmB). The present in vitro study demonstrated that, unlike other Candida species, C. lusitaniae isolates frequently generated AmB-resistant lineages form previously susceptible colonies. Cells switching from a resistant colony to a susceptible phenotype were also detected after treatment with either UV light, heat shock, or exposure to whole blood, all of which increased the frequency of switching. In some C. lusitaniae lineages, after a cell switched to a resistant phenotype, the resistant phenotype was stable; in other lineages, colonies were composed primarily of AmB-susceptible cells. Although resistant and susceptible lineages were identical in many aspects, their cellular morphologies were dramatically different. Switching mechanisms that involve exposure to antifungals may have an impact on antifungal therapeutic strategies as well as on standardized susceptibility testing of clinical yeast specimens.
The management of disseminated Candida lusitaniae infection is complicated because isolates are frequently resistant to amphotericin B (AmB). Moreover, many Candida lusitaniae isolates seemingly develop AmB resistance in vivo during therapy (21, 22, 41). The ability to transform from AmB susceptibility to resistance as well as the expression of phenotypic switching has not been previously described in C. lusitaniae isolates. Phenotypic or colony morphology switching in Candida albicans is, however, a well-described phenomenon. It involves high-frequency switching of either individual colonies or cells from one morphology or phenotypic state to another (46, 47). Switching may be a nonsexual mechanism which certain Candida species use to introduce variability to cells. Phenotypic changes appear to increase the chance that a daughter cell can survive immunological or other microenvironmental host defenses (60, 61).
There are several switching “systems” in C. albicans; all are believed to have an overlapping mechanism (57). Switching is characterized as reversible, pleiotropic, of high frequency (about one switch per 104 cells), occurring at different rates in different clinical isolates, and inducible (up to 200-fold) by low doses of UV light, temperature, or other environmental stimuli (23, 39, 54, 55, 59). At this rate, any individual colony of more than 106 cells will be heterogeneous. Switching systems have been studied primarily in C. albicans but are also known to exist in other Candida species, such as C. tropicalis (55, 56).
There is no evidence that gross chromosomal alterations are responsible for switching in C. albicans (56). Instead, recent molecular genetic analysis suggests that there are a number of genes that are transcribed in a colony-specific manner and are activated by type-specific transcriptional activators that act in a combinatorial fashion at conserved sites upstream of the affected genes. At least one gene is regulated by both yeast-to-hyphal phase transition and white-to-opaque switching (11, 56, 57).
In vitro identification of spontaneous mutants resistant to AmB in C. albicans is extremely rare. Similarly, clinical Candida isolates that have high-level resistance to AmB are not common (69). Despite this, there are numerous examples of clinical failure during AmB therapy which are thought to reflect drug failure only. AmB resistance is, however, described more frequently among the non-albicans Candida species than among C. albicans. In particular, C. lusitaniae has frequently been reported to be resistant to AmB (8, 21, 22, 38, 41, 43, 44).
In the present study, we characterized a new form of switching discovered in clinical C. lusitaniae isolates. The phenotypic switch, which occurs at a relatively high frequency, is from an AmB-susceptible phenotype to an AmB-resistant phenotype. In addition, the reverse switch from AmB resistance to susceptibility also occurs but at a lower frequency. The ability of susceptibility phenotypes to switch in C. lusitaniae, but not C. albicans, isolates is consistent with the description of clinical failure and in vitro resistance developing during AmB administration in C. lusitaniae-infected patients.
MATERIALS AND METHODS
Candida strains.
C. lusitaniae isolates were obtained from either the American Type Culture Collection (ATCC) or from hospitalized patients in Harper Hospital, Detroit, Mich., or from William Beaumont Hospital, Royal Oak, Mich. These isolates were recovered from multiple anatomic sites and at separate times over a 1-year period in individual patients. All isolates were identified by germ tube production and chlamydospore formation on cornmeal agar, the yeast API 20C method (Sherwood Medical, Plainview, N.Y.). In addition, yeast isolates were also verified by CHROMagar Candida plates (40, 42), randomly amplified polymorphic DNA (RAPD) fingerprinting (64), and electrophoretic karyotyping (68, 70). ATCC isolates were used as reference standards for the various Candida species.
Yeast cells were initially isolated on Sabouraud dextrose (SAB) agar, stored in a 1:1 mixture of brain heart infusion broth (Difco, Detroit, Mich.) and glycerol, and frozen at −70°C.
Determination of in vitro susceptibility.
AmB as a lyophilized cake of AmB and sodium deoxycholate was purchased from Gensia Laboratories (Irvine, Calif.). This was suspended in sterile water at 1 mg/ml and stored frozen in light-protected vials. In later experiments, AmB was purchased from Sigma (St. Louis, Mo.) and dissolved at 1 mg/ml in dimethyl sulfoxide.
Several methods for determining the in vitro susceptibility of Candida isolates to antifungals were evaluated and utilized. These in vitro assays included inoculating dilute cell suspensions into microtiter plates containing RPMI 1640 broth medium (American Biorganics, Inc., Niagara Falls, N.Y.), buffered to pH 7.0 with 0.165 M morpholinepropanesulfonic acid (MOPS) buffer without sodium bicarbonate (3). In microtiter plate assays, we used an alternate medium, phosphate-buffered synthetic dextrose medium (SD; yeast nitrogen base broth plus 1% dextrose [Difco]). Two additional in vitro susceptibility assays were also utilized and evaluated. In one assay, in vitro susceptibility was assessed by spreading yeast cell suspensions on the surface of SAB or SD (Difco) agar plates containing different concentrations of AmB. The most sensitive and reproducible method, however, was the embedded agar assay (EAA). In this assay, yeast cell suspensions were pipetted into empty 100-mm-diameter petri plates and 20 to 25 ml of phosphate-buffered SAB agar solution containing AmB was added (67). The cell-agar suspensions were mixed and rapidly cooled on the laboratory bench top before being incubated at 30 to 35°C for 3 days. The fungicidal effects of short-term exposures to AmB of the Candida isolates were determined by incubating actively growing cultures in increasing concentrations of AmB in buffered SD and then plating serial dilutions on SAB agar plates, as previously described (67).
AmB-susceptible isolates (C. albicans B311 and C. lusitaniae 3778; AmBMIC < 0.05 μg/ml) and an AmB-resistant control (C. lusitaniae 7227R6; MIC > 0.15 μg/ml) were utilized in all assays as controls. Standard in vitro susceptibility assays using the National Committee for Clinical Laboratory Standards guidelines were also performed as previously described (3).
In vitro susceptibility studies of the Candida strains to fluconazole and intraconazole were determined by the EAA method. Susceptibility to other compounds was determined by replica plating suspensions. The cell suspensions were made by suspending 1-day-old colonies in 100 μl of sterile water in microtiter plates and subcultured on a series of SD agar plates constituting a twofold serial dilution of the compound.
UV light treatment.
UV induction of switching was a modification of the method of Morrow et al. (39). One to ten 2- to 3 day-old colonies, grown on SAB agar, were resuspended in 30 ml of sterile distilled water, transferred into a 100-mm-diameter petri dish, and exposed with slow stirring to a ChromatoView model CC-20 short-UV light source (UV Products, Inc., San Gabriel, Calif.). Samples were withdrawn at intervals to determine the exposure time required to achieve 10% survival, typically 25 s. Afterwards, survivors were scored as CFU by plating serial dilutions onto SAB agar plates. the individual UV doses for each Candida species were determined separately.
Zymolyase incubations.
Candida cultures were initiated from single colonies in SD broth (1 ml), grown overnight at 35°C with shaking, and the harvested in 1.5-ml centrifuge tubes, washed once with 1 ml of 1 M sorbitol–10 mM (Tris (pH 7.5), and resuspended in 400 μl of the same buffer at 2 × 107 to 2 × 107 cells/ml. This volume was split into duplicate tubes, 200 μl each. Zymolyase (1.5 U) was added to one of the duplicates, and both tubes were incubated at 35°C for 5 h. To determine viability, samples from both tubes were serially diluted into sterile distilled water before and after incubation. Afterwards, 5 μl of each dilution was plated on SD agar without AmB. Aliquots were also plated on SD agar with 0.25 and 0.35 μg of AmB per ml to determine the numbers of resistant cells with or without prior exposure to zymolayse.
Processing of human blood.
Ten-milliliter samples of venous blood were drawn from healthy volunteers in Vacutainers with either EDTA or heparin as the anticoagulant. Blood samples were stored on ice or at 4°C and used immediately or within hours of sampling. Yeast cultures were stored at 4°C. Serum was prepared from some of the blood samples by centrifugation at 7,000 × g for 5 min.
RAPD fingerprinting.
Individual colony lysates were prepared as previously described by using the toothpick method (64). Briefly, 1 μl of lysate was amplified in a Robocycler thermocyler (Stratagene, La Jolla, Calif.) with the following program: 5 cycles of 94°C for 30 s, 25°C for 2 min, and 72°C for 2 min; 45 cycles of 94°C for 30 s, 30°C for 30 s, and 72°C for 2 min; 45 cycles of 94°C for 30 s, 30°C for 30 s, and 72°C for 2 min; and 1 cycle of 72°C for 10 min. Typical reaction mixtures, in 10- to 25-μl volumes overlaid with mineral oil, consisted of 25 mM Tris-Hcl (pH 8.4), 50 mM KCl, 1.5 mM MgCl2, 100 μM deoxynucleoside triphosphates, 50 pmol of 10-mer primer, and 2.5 U of Taq DNA polymerase, as provided by Bethesda Research Laboratories (Bethesda, Md.). Reaction products were analyzed by ethidium bromide agarose gel electrophoresis (1.5% agarose or 1% Sepharide; Bethesda Research Laboratories) photographed, and scanned (Logitech Scanman II).
Primers.
The following sequences were used as primers at 50 pmol per 25-μl reaction volume: CX5, 5′ACACTGCTTC-3′ (64); REP, 5′-GAGGGTGGCGGTTCT-3′ (6, 53, 66); AP3, 5′-TCACGATGA-3′ (73); and PST, 5′-CAGTTCTGCAG-3′.
RESULTS
Isolation and characterization of AmB-resistant forms of C. lusitaniae 7227.
As an introduction to a description of switching of C. lusitaniae isolates from AmB susceptibility to resistance, we first describe assay systems used to define these phenotypes. A clonal derivative of clinical isolate 7227, called 7227R, was characterized as being less susceptible to AmB in one assay, the EAA (EEA concentration [minimal concentration of AmB which resulted in fewer than 5 colonies after incubation], >0.15 ug/ml), than the original clinical isolate. Isolate 7227R was recovered from 7227 after subculturing a single colony on SAB agar supplemented with 0.15 μg of AmB per ml. Subculturing of a single colony of 7227R resulted in the recovery of isolate 7227S, which was found to be susceptible to AmB (EEA concentration, <0.05 μg/ml). We used isolate 7227R6 (R = resistant; 6 = colony no. 6) and 7227S11 (S = susceptible; 11 = colony no. 11) for the majority of our experiments. Both isolates were derived from the parent C. lusitaniae 7227, a clinical isolate, as described previously.
In vitro susceptibilities of clonal derivatives of 7227 were evaluated by three different methods (Table 1). To obtain the MICs in these experiments, either SD or Difco antibiotic medium no. 3 (AM3) was used. After 24 h incubation of AM3, the MIC for the resistant isolate 7227R6 was about 30 times greater than the MIC for the susceptible strain 7227S11-3 (2.5 versus 0.08 μg/ml). In SD, the difference in MICs for the two isolates was only about eightfold (2.5 versus 0.31 μg/ml).
TABLE 1.
In vitro susceptibilities of C. lusitaniae and other Candida species to AmB
| Isolate | Results (μg of AmB/ml) in indicated medium after indicated h of exposure to druga
|
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| MIC
|
EAA
|
MFC
|
||||||||||
| SD
|
AM3
|
SD
|
AM3
|
SD
|
AM3
|
|||||||
| 24 | 48 | 24 | 48 | 48 | 72 | 48 | 72 | 24 | 48 | 24 | 48 | |
| Test | ||||||||||||
| C. lusitaniae 7227 | 0.02 | 0.04 | 0.04 | 0.04 | ||||||||
| C. lusitaniae 7227R6 | 2.5 | 5.0 | 2.5 | 2.5 | >20 | >20 | 1.25 | 2.5 | 5.0 | 5.0 | >20 | >20 |
| C. lusitaniae 7227S11-3 | 0.31 | 0.31 | 0.08 | 0.63 | 0.31 | 0.31 | 0.15 | 0.63 | 0.6 | 5.0* | 0.6 | 0.6 |
| C. guilliermondii 7207 | <0.02 | 10 | 1.25 | 2.5 | >20 | >20 | 1.25 | 2.5 | >20 | >20 | 2.5 | 5.0 |
| C. guilliermondii 7210 | <0.02 | 0.63 | 0.04 | 0.8 | 0.15 | 0.15 | 0.04 | 0.08 | 1.25 | 2.5 | 0.16 | 0.16 |
| Control | ||||||||||||
| C. albicans B311b | 0.15 | 0.31 | 0.15 | 0.31 | ||||||||
| C. guilliermondii ATCC 9390b | 0.08 | 0.08 | 0.08 | 0.08 | ||||||||
| C. krusei 7942 | 0.63 | 0.63 | 0.31 | 0.63 | ||||||||
| C. parapsilosis ATCC 90018b | 0.31 | 0.63 | 0.31 | 0.63 | ||||||||
| C. tropicalis ATCC 66029b | 0.31 | 0.31 | 0.15 | 0.15 | ||||||||
| C. glabrata 7650b | 0.31 | 0.63 | 0.31 | 0.63 | ||||||||
The MIC equals that concentration of AmB in the indicated medium that results in no visible turbidity after the indicated growth times at 35°C with shaking. For the EAA, 1,000 cells of each isolate were embedded in 20 ml of the indicated medium plus 1.5% agar. The concentrations given for this assay are the minimal concentrations of AmB which resulted in fewer than 5 colonies after the indicated incubation time at 35°C. The MFC equals that concentration of AmB in the indicated medium that results in fewer than 10 CFU/ml after the indicated incubation times. AmB concentrations that were tested ranged from 20 to 0.02 μg/ml in twofold increments, tested in 200-μl cultures in microtiter plates, inoculated with 100 cells per well. Aliquots were removed at the indicated times and serially diluted and plated onto SAB agar to determine the CFU after 2 more days at 35°C, for MFC determinations.
ATCC isolate. ATCC designation equivalents of some of the isolates: B311 = ATCC 32354; 7650 = ATCC 2001.
The EAA method also showed that 7227R6 was resistant relative to 7227S11-3. However, when this method was used with SD, the difference in MICs for the two strains was greater (about 60-fold), >20 and 0.31 μg/ml, respectively, than the difference in MICs when AM3 was used. This resulted from the ability of the resistant strain to grow even at the highest tested concentrations of AmB in SD. In addition, the EAA had a further advantage over standard MIC assays in that it maintained a clear cutoff for the minimum concentration, without slow residual growth of the susceptible isolate.
The minimal fungicidal concentration (MFC) was determined by using the cultures assayed for the MIC determinations, as described in Table 1, footnote a. The resistant phenotype in this assay was clear in both media after 24 h. The MFC for isolate 7227R6 was more than 30-fold higher than that for isolate 7227S11 in AM3 (>20 versus 0.6 μg/ml).
The levels of AmB resistance of 7227R6 by all assays were high and comparable to those of Candida guilliermondii 7207 (Table 1). This strain of C. guilliermondii was recovered from a patient who clinically failed to respond to AmB and who simultaneously demonstrated an in vitro increase in AmB MICs for this strain (69). Table 1 shows that the EAA confirmed that representative isolates of six different Candida species were susceptible to AmB. This supports the conclusion that the resistance observed in strains 7227R6 and 7207 was real. In general, the best media for determining AmB susceptibility depended on the assay utilized. For the standard MIC assay, AM3 was more discriminatory and provided better cutoffs. However, in the EAA method, SD provided greater discrimination between cutoffs. For the MFC assays, either medium was acceptable but was strain dependent.
Demonstration of switching of AmB susceptibility among cells from single colonies.
The susceptible isolate 7227S11 was derived from a resistant strain, 7227R6, as previously described. Repeated attempts to recover more susceptible isolates similar to 7227S11 from approximately 100 clonal derivatives of 7227R6 failed. However, new AmB-resistant variants derived from 7227S11 were recovered at relatively high frequencies, ranging from 1 in 10 to 1 in 104 cells in individual colonies. Thirty colonies derived from 7227S, designated strains 7227S1 to 7227S30, were plated at a density of approximately 106 cells per colony. From these strains, at least 100 colonies were recovered per plate supplemented with 0.15 μg of AmB per ml. Approximately 1 in 30 of these colonies was classified as AmB resistant because the isolates were able to generate the same number of colonies upon subculturing on medium with or without AmB. These strains were subsequently designed 7227SR, followed by a number to indicate the clonal origin. Differential plating of these variants is depicted in Fig. 1. Four clonal derivatives of isolate 7227 (7227R6, 7227S11, 7227RS9, and 7227SR5) all grew with the same efficiency on drug-free SAB agar (Fig. 1, lower plates). However, two resistant derivatives (7227R6 and 7227SR5) were able to grow with the same efficiency on agar plates with or without AmB. In contrast, two susceptible derivatives (7227S11 and 7227RS9) formed few to no colonies on SAB agar plates supplemented with AmB. Thus, the important observation was that a small percentage of cells derived from most susceptible C. lusitaniae colonies grew to colony size on AmB-supplemented plates.
FIG. 1.
Reversible phenotypic switching of C. lusitaniae 7227. Single colonies were diluted and subcultured on SD agar without AmB (−AmB) or with AmB at 0.15 μg/ml (+AmB). Representative plates inoculated with either 0.2 or 100 μl of the initial cell suspension are shown. The isolates are labeled as follows: designations that include “S” indicates isolates that are derived from a susceptible lineage; “R” indicates a resistant lineage; “SR” indicates isolates that switched from a susceptible to a resistant phenotype; and “RS” indicates isolates that switched from a resistant to a susceptible phenotype. Numbers following these labels indicate clonal lineages.
The C. lusitaniae strain 7227SR5, derived from one of the 7227S11 colonies that initially grew on AmB-supplemented agar (Fig. 1), was an example of a strain that switched from a susceptible lineage to a resistant lineage that grew with equal efficiency on plates with and without AmB. Conversely, strain 7227RS9, derived from strain 7227R6, represents a strain that switched from an AmB-resistant to an AmB-susceptible phenotype.
The AmB resistance switching phenotype was defined by the following observations. A subpopulation of cells form individual AmB-susceptible colonies grew into full-sized colonies in media supplemented with AmB at a concentration that inhibits the vast majority of cells. This was not observed with other Candida species, such as C. albicans B311 or C. albicans 3153A, despite previous demonstrations that strain 3153A can switch colony morphology at high frequencies (58). Notably, susceptible clonal derivatives of an AmB-resistant C. guilliermondii isolate recovered from a patient (69) did not demonstrate any in vitro switching. Thus, if the AmB-resistant C. guilliermondii isolates recovered from this patient were derived from susceptible strains, they may have become resistant via a mutation or by switching that had been induced under in vivo conditions and not reproduced by in vitro assays.
The degree to which individual colonies of C. lusitaniae were heterogeneous was examined in clonal lineages. As expected, resistant colonies (7227R6 and 7227SR5), whether derived from plates with or without AmB, were composed primarily of cells capable of generating new colonies on selective AmB agar media (Table 2). In contrast, susceptible colonies (7227S11 and 7227RS9), when grown on nonselective media, averaged about 1 in 10 cells to 1 in 102 cells that grew upon subculturing on selective media. For some individual colonies, this ratio was much lower (1 in 103 cells). Those rare colonies in susceptible lineages that were recovered from AmB-supplemented agar were composed of greater numbers of cells that could subsequently grown on AmB plates. The average frequency (resistant cell index [RI], defined as the ratio of cells per single colony that were capable of forming colonies on AmB plates to the total viable cells in that colony) increased about sevenfold, from 0.02 to 0.15, for 7227S11. The latter ratio, 0.15, was surprising, since this implies that 85% of the cells in these colonies, having grown on AmB plates, were not capable of growing into colonies when subcultured on the same selective media.
TABLE 2.
Frequency of resistant cells in individual colonies of C. lusitaniae 7227 derivatives
| Isolatea | Sourceb | RIc (mean ± SD) | No. of colonies |
|---|---|---|---|
| 7227R6 | −AmB | 0.8 ± 0.5 | 10 |
| 7227R6 | +AmB | 1.1 ± 0.7 | 7 |
| 7227SR5 | −AmB | 1.2 ± 0.4 | 9 |
| 7227SR5 | +AmB | 0.9 ± 0.5 | 7 |
| 7227S11 | −AmB | 0.02 ± 0.06 | 21 |
| 7227S11 | +AmB | 0.15 ± 0.4 | 18 |
| 7227RS9 | −AmB | 0.009 ± 0.02 | 18 |
| 7227RS9 | +AmB | 0.06 ± 0.06 | 15 |
7227 derivative names are explained in the legend to Fig. 1.
Individual colonies were sampled from SAB agar plates with or without 0.15 μg of AmB/ml (+ or −AmB, respectively).
The resistant cell index (RI) is defined as the ratio of cells per single colony capable of forming colonies on AmB plates to the total viable cells in that colony.
The data in Table 2 depict only the average ratios (± standard deviations). This is deceptive for the susceptible cell lineages since the ratios for individual colonies varied dramatically. The ratios of 75 colonies in an AmB-susceptible lineage, 7227S11-3, along with 30 resistant colonies were determined. About half of the susceptible colonies had fewer than 1 in 103 cells capable of growth on AmB-supplemented media. Approximately one-third of the colonies were composed of about 1% resistant cells, and others (five colonies) were composed entirely of resistant cells. The AmB resistance of individual 7227SR6 colonies was stable. In fact, most (>80%) of the individual cells in these colonies were able to initiate colonies on AmB-supplemented plates upon subsequent subculturing. This was true even after 10 serial subcultures on nonselective medium (SAB) grown at 30 or 37°C or at neutral pH.
We observed a wide range of switching frequencies from susceptible to resistant phenotypes among colonies analyzed at different times during the course of this study. The switching frequencies ranged from 1 per 102 to 1 per 104 cells (Table 2). This was a real variation within a lineage that was determined by unknown factors. The variation observed depended on when the strain was analyzed. We have not yet determined, for example, whether differences in colony age or cold storage, etc., reproducibly influence these frequency differences.
The identification of one AmB-resistant derivative among 102 to 104 susceptible parents was trivial because of the positive selection. However, the identification of a rare susceptible derivative was problematic. If the rate of this reverse switch was the same, each colony initiated by a resistant cell could have as few as 102 susceptible cells per 106 resistant cells. As expected, mass screening of approximately 2 × 104 colonies by using low-density replica plating methods did not detect any colonies that were entirely composed of AmB-susceptible revertants of 7227SR5. Similarly, no susceptible revertant cells were identified among 102 cells of a single 7227SR5 colony. The 102 cells were evaluated by growing each on nonselective medium (SAB) and then plating equal aliquots of dilute suspensions of each colony onto drug-free and AmB-supplemented plates. A colony arising from a susceptible revertant from the original 102 resistant cells would have generated a colony composed of susceptible cells. Subculturing of such a colony would have generated more colonies on drug-free than on AmB-supplemented plates, as seen in the susceptible lineages; however, none of the 102 colonies that were subcultured did so (Fig. 1). In fact, all sister platings of these colonies were identical. Therefore, the frequency of switching from AmB resistance to susceptibility was less than 0.01.
UV light induction of reversion in isolate 7227SR9.
To avoid searching among 104 colonies, we evaluated whether the switching frequency could be enhanced by exposure to UV light (39). Exposure of a single 7227SR5 colony to a UV light dose producing 10% survival of cells resulted in two revertant 7227RS colonies among the 60 tested colonies, a rate of 3 × 10−2. If we assume that the spontaneous rate of this reversion was the same as the switch from susceptible to resistant, i.e., 10−4, then the UV treatment resulted in a 300-fold enhancement of the switching frequency. This assumption is reasonable, given that no susceptible colonies were detected among 1 × 10 to 2 × 104 untreated switching colonies. A subculture of 7227RS9 cells on SD medium with 0.1 μm zinc (28), which is known to induce morphological switching of C. albicans (2, 5), had no effect on C. lusitaniae switching to an AmB-resistant phenotype.
Evaluation of the switching frequency from AmB susceptibility to resistance induced by heat shock and whole blood exposure.
After demonstrating that UV light induced AmB switching, we investigated whether any other physiological signals which may be encountered during a systemic fungal infection have the capability to induce switching. As shown in Fig. 2, a 30-min heat shock increased the number of resistant colonies by about 10-fold (Fig. 2, right plates). Exposing the same cell lineage to whole human blood increased resistant daughter colonies by about 100-fold (Fig. 2, left plates). In repeat experiments, the level of induction by blood was approximately 20-fold. Prolonged heat shocks of up to 5 h were required for optimal conversion in the white-to-opaque transition of C. albicans (49). In contrast, heat shock exposures longer than 30 min were suboptimal for C. lusitaniae switching. Blood-induced switching was similarly reproducible for C. lusitaniae 7227 but was ineffective in inducing switching in C. albicans B311 or C. guilliermondii 7210. Switching induction by exposure to whole blood did not require a 37°C heat shock and was not achieved if the blood was stored at 4°C for more than 3 days or if serum was substituted for whole blood (data not shown).
FIG. 2.
Induction of C. lusitaniae 7227RS9 switching by exposure to whole blood (left plates) and heat shock (right plates). A single colony was suspended and distributed to SD or fresh whole blood, and aliquots (0.2 or 20 μl) were plated on SD with or without AmB as indicated. After 30 min of incubation at 37°C, larger aliquots were plated the same way.
AmB-resistant and -susceptible isolates were clonal derivatives.
Several approaches were used to eliminate the possibility that resistant derivatives of susceptible lineages were random contaminants of a resistant species from the laboratory environment. First, rare laboratory contamination cannot explain the repeated, independent isolations of both resistant and susceptible derivatives from single, independent colonies of 7227S11 and 7227SR5. Second, all colonies were identical in colony morphology and color on CHROMagar Candida plates. Third, each colony was analyzed by colony filter hybridization, and all hybridized with a radioactive probe specific for C. lusitaniae (64a). Negative hybridization controls included colonies of 10 other Candida species. These data rule out the interpretation that the different phenotypes were due to colonies of other Candida species, notably C. krusei, which have different susceptibilities to AmB (44). Finally, all isolate 7227 derivatives were identical or nearly identical by RAPD fingerprinting. Individual colony lysates of the isolates were amplified by using different primers. Primer CX5 generates a single 2-kb product characteristic of C. lusitaniae (64). All 7227 lineage isolates generate this product, regardless of resistance phenotype. Primer REP hybridizes to a repetitive sequence element in Candida and so generates a large number of products (66). Neither REP nor any of 10 independent 10-mer primers generated different fingerprints for susceptible versus resistant lineages.
Comparison of AmB-resistant and -susceptible derivatives of isolate 7227.
Resistant strain 7227SR5 and switched susceptible strain 7227RS9 were compared to their parents for susceptibility to antifungals and other growth conditions. 7227SR5 was as resistant to AmB as parent 7227R, and both of these were more resistant than 7227S or 7227RS9. All forms of isolate 7227 were equally susceptible to fluconazole, clotrimazole, itraconazole, flucytosine, and terbinafine. All of the strains had the same temperature and pH limits and the same carbohydrate assimilation capacities as measured by the yeast API 20C identification method.
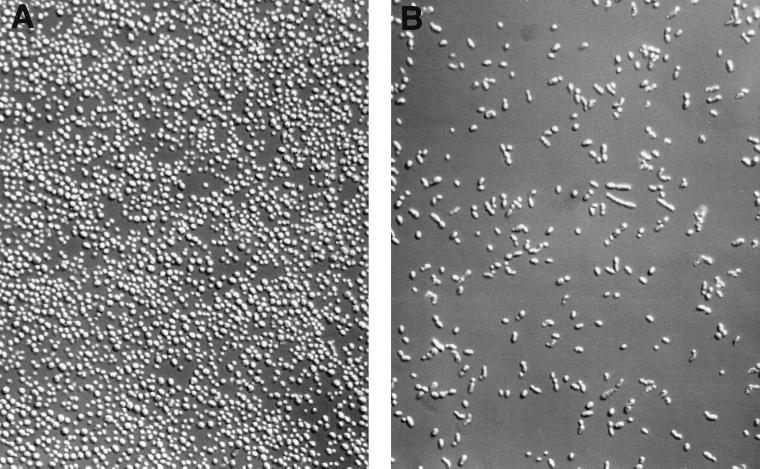
In contrast to these common features and identical colony morphologies, susceptible C. lusitaniae isolates had a cellular morphology that differed from that of resistant C. lusitaniae strains (Fig. 3). Susceptible cells were typically elongated and formed short linear chains. Resistant cells were round to ovoid, with typical budding daughter cells, and tended to cluster into small multicellular aggregates. The latter appearance was also a feature of Candida cells after exposure to fluconazole, which confers resistance to subsequent AmB exposure (67).
FIG. 3.
Cellular morphologies of resistant and susceptible derivatives of C. lusitaniae 7227. Cells were suspended in sterile water from single, fresh colonies grown on SAB agar and visualized by differential interface microscopy (160X Nikon E600 Research Microscope) and digitally imaged with the SPOT camera (Diagnostic Instruments Inc., Sterling Heights, Mich.). (A) Resistant cells from isolate 7227R6; (B) susceptible cells from isolate 7227S11.
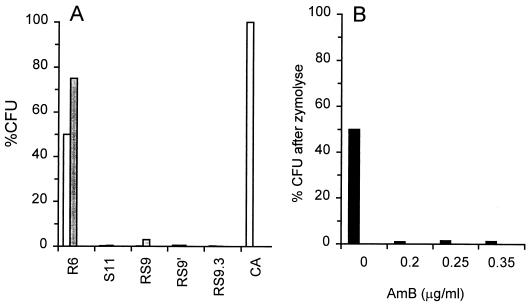
In an effort to evaluate differences in the cell wall between resistant and susceptible C. lusitaniae strains, both were exposed to zymolyase. Susceptible lineages of C. lusitaniae had increased sensitivity to digestion by zymolyase (Fig. 4). More than 95% of cells in the susceptible or switching lineages of isolate 7227 were lysed after 5 h of incubation at room temperature in 1 M sorbitol plus 1.5 U of zymolyase (ICN) per ml. Cells from the resistant isolate 7227R6 did not lyse in distilled water after the same exposures to zymolyase. This was also observed for the AmB-resistant strain 7227SR5 (data not shown). A control strain of C. albicans was also not rendered osmotically fragile under these conditions (Fig. 4A). We note that this strain, unlike 7227R6, was susceptible to AmB even though it was resistant to zymolyase-induced lysis. In addition, AmB-resistant C. guilliermondii 7207 cells were resistant to zymolyase-induced lysis; in comparison to the AmB-susceptible C. guilliermondii cells. Thus, both Candida species have a correlation between cell wall structure, reflected by zymolyase susceptibility, and AmB resistance despite the observation that C. guilliermondii cells do not switch from the susceptible to the resistant phenotype as do the C. lusitaniae cells.
FIG. 4.
Effect of zymolyase on viability and resistance to AmB. (A) The indicated Candida strains were digested with zymolyase as described in Materials and Methods. The percentages of CFU were calculated as the number of colonies per milliliter after zymolyase digestion, divided by colonies per milliliter of the same strain incubated without zymolyase (open bars). Less than 1% of cells from isolates, other than 7227R6 and C. albicans, survived zymolyase treatment and therefore are not evident in the figure. The same strains were also plated onto AmB agar without preincubations in zymolyase (closed bars) to verify their levels of susceptibility to AmB. (B) C. lusitaniae 7227R6 was incubated for 5 h in zymolyase as described in Materials and Methods and then plated on SD plus the indicated concentrations of AmB. %CFU values are percentage of the original CFU before zymolyase was added. Strain abbreviations: R6, 7227R6; S11, 7227S11; RS9, 7227RS9, a susceptible isolate derived from 7227R6; RS9′, a colony derived from the original 7227RS9; RS9.3, a variant derived from 7227RS9 that did not switch back from susceptibility to resistance; Ca, C. albicans B311.
Even though incubation of 7227R6 in zymolyase for 5 h did not lower cell viability, the incubation did have another dramatic effect. The majority of the surviving cells were now susceptible to even low concentrations of AmB (Fig. 4B). After incubation of zymolyase, C. lusitaniae 7227R6 cells were not stably converted to a susceptible phenotype. Instead, survivors that were grown into colonies on nonselective SD agar had the same resistant phenotype as control colonies not exposed to zymolyase.
Incubation with zymolyase therefore converted most of the population of AmB-resistant cells to an AmB-susceptible phenotype without making them osmotically fragile.
AmB switching in other Candida species.
We were unable to detect spontaneous AmB-resistant derivatives of susceptible Candida isolates in several Candida species, including C. glabrata, C. tropicalis, C. kefyr, and C. guilliermondii. In contrast, 30 of 30 independent clinical isolates of C. lusitaniae demonstrated the switching phenotype described for 7227S11 (Table 3). All tested C. lusitaniae isolates showed an incidence of at least 0.001 resistant cell per viable cell. From five randomly chosen isolates, five colonies from AmB plates were resuspended and replated on SD agar with and without AmB. All showed the resistant phenotype, i.e., equivalent numbers of CFU developed on both plates. These data suggest that AmB switching was a characteristic feature of many C. lusitaniae isolates which was not found among other Candida species.
TABLE 3.
Switching frequencies among clinical isolates of C. lusitaniae
| Isolatea | RIb | MIC (μg/ml)c |
|---|---|---|
| 3778 | 0.02 | 0.10 |
| 2233 | 0.001 | 0.10 |
| 2263 | 0.02 | 0.20 |
| 2266 | 0.02 | 0.20 |
| 2278 | 0.05 | 0.10 |
| 2290 | 0.05 | 0.10 |
| 2291 | 0.04 | 0.10 |
| 2307 | 0.1 | 0.20 |
| 2328 | 0.02 | 0.20 |
| 2335 | 0.01 | NDd |
| 2350 | 0.008 | 0.20 |
| 2402 | 0.01 | 0.05 |
| 2407 | 0.01 | 0.05 |
| 2417 | 0.05 | 0.80 |
| 2423 | 0.008 | 0.05 |
| 2427 | 0.006 | 0.05 |
| 2430 | 0.02 | 0.40 |
| 2458 | 0.02 | 0.05 |
| 2477 | 0.01 | 0.20 |
| 2478 | 0.002 | 0.20 |
| 2662 | 0.005 | 0.05 |
| 2913 | 0.001 | 0.05 |
| 3049 | 0.03 | 0.10 |
| 3291 | 0.008 | 0.20 |
| 3511 | 0.09 | 0.10 |
| 3512 | 0.02 | 0.10 |
| 3784 | 0.1 | ND |
| 3785 | 0.07 | 0.20 |
| 7926 | 0.05 | ND |
| 7227R | 0.8 | 3.10 |
| 7227R | 0.8 | 0.75 |
| 7227SR | 1.2 | 0.75 |
| 7227SR | 0.5 | 0.75 |
| 7227S | 0.02 | 0.38 |
| 7227S | 0.002 | 0.40 |
| 3778 | 0.02 | 0.10 |
Isolates are listed more than once if independent duplicate assays were performed.
The RI was determined as described in Table 2, footnote c.
MICs were determined by the EAA.
ND, not determined.
Effects of high AmB concentrations on 7227R cells.
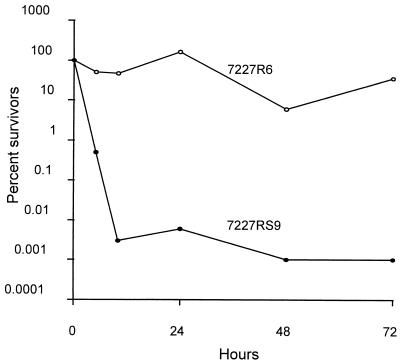
Table 3 also demonstrates that a standard MIC determination for a C. lusitaniae isolate did not reflect the dramatic switching phenotype of the minority population on agar plates. Indeed, even the MICs for 7227R6 were only slightly higher in these assays. To clarify this difference and to determine whether the switch to resistance on agar may have clinical significance, we compared the survival rates of 7227R6 and 7227RS9 after short-term exposures to high concentrations of AmB in SD broth (Fig. 5). The exposure of susceptible cultures to 2 μg of AmB per ml reduced viability by 5 orders of magnitude within 5 h. During the same interval, resistant cultures retained full viability and continued to survive for well over 24 h. Survival rates for both cultures exposed to 1 μg of AmB per ml were similar to those for cultures exposed to 2 μg/ml. In the absence of AmB, both cultures grew to confluency within 24 h (data not shown). Exposure of resistant cultures to even higher concentrations of AmB (5 to 20 μg/ml) was not highly fungicidal; in independent trials performed over more than a 1-year period, survival ranged between 10 and 50% after up to 24 h of exposure.
FIG. 5.
Resistance of C. lusitaniae 7227 cells to fungicidal effects of AmB. Single colonies of 7227R6 (○) or 7227RS9 (●) were inoculated into 5 ml of buffered SD in 50-ml tubes for overnight growth at 30°C. Twenty microliters of grown culture was transferred into 1 ml of buffered SD with or without AmB (2 μg/ml) for the indicated times. CFU were determined by plating four 10-fold serial dilutions of each sample onto SAB agar plates. Percent survivors were calculated from the ratios of these CFU to the CFU before AmB was added. Cultures without AmB grew to confluency in less than 24 h.
DISCUSSION
Switching in C. albicans has pleiotropic effects, beyond the initial colony and cellular morphologies, including surface antigenicity, cell wall structure, adhesiveness, susceptibility to polymorphonuclear leukocytes, virulence, and possibly drug susceptibility (27, 56). Presumably, the switching properties vary with the cell lineage and the individual cell (59, 61). Molecular epidemiological data have previously demonstrated that recurring C. albicans infections in a single individual are typically subtypes of the initial isolate (29, 34–36, 45, 59, 62). This has been interpreted as a longitudinal selection of variant phenotypes within the individual host to enhance survival in a particular environment (host, anatomical location, and commensal microorganisms). These variant phenotypes might be generated by a switching mechanism that is consistent with studies that correlate invasiveness of Candida isolates with a high switching mode and with a recent gene disruption study that links virulence of C. albicans to the ability to switch to hyphal growth (7, 23, 27).
We have observed the development of AmB resistance that involves rapid in vitro switching from AmB-susceptible to AmB-resistant phenotypes only in C. lusitaniae. The switch was reversible and occurred at a high and variable rate of about 1 per 10 to 10,000 cells. As a result, many cells in each colony (contains approximately 3 × 106 cells), switched phenotype from that of the cell that originated the colony. We have identified three different types of C. lusitaniae cells in standard colonies and have designated them types I, II, and III. Type I cells do not grow when replated on AmB-supplemented medium. Type II cells grow, but the resultant subculture of colonies on AmB-supplemented medium were still in a susceptible, switching mode. These cells generate only mixed-phenotype second-generation colonies, composed of mostly type II cells that produce an average of 1 in 50 daughter cells able to grow on AmB-supplemented agar. The ratio of type II to type I cells in a colony increases about seven- to eightfold in colonies recovered from AmB-supplemented agar versus those cells recovered from nonselective agar. Susceptible lineages derive from type I and type II cells and continue to switch at variable rates that range from 1 in 50 to 1 in 10,000 cells. Type III cells form colonies that have a stable resistant phenotype and grow with equal efficiency on media with and without AmB. These cells occur only once per 30 colonies subcultured on AmB-supplemented medium. The other 29 colonies are of the type II mixed-phenotype lineage. One might argue that there is no such thing as a purely resistant lineage. The “resistant” colony that arises once per 30 colonies from AmB plates may result from a switch to resistance early in the growth of that colony from its parent cell, especially if the frequency of switching from resistance to susceptibility was lower than the reverse. The other 29 type II colonies are explained by having switched to resistance late during the growth of each colony. However, resistant lineages retain a high RI even after many generations in the absence of selective pressure, and susceptible isolates can be recovered from these only after induction by UV. Present data cannot determine which of two models account for the resistant lineage. In one theoretical model, the persistently resistant lineage has turned off a switching mechanism that must be reactivated to restore switching. In a second model, resistant lineages start out with such a high percentage of resistant cells due to an early switch event. As long as the rate of switching from resistance to susceptibility is lower than the reverse switch, these colonies will appear to be composed of only resistant cells.
The resistance phenotype was specific for AmB, of all of the drugs tested, and was accompanied by changes in cellular morphology. Switching of this type seems commonplace among independent clinical isolates of C. lusitaniae but was not detected in other Candida species.
The resistant isolate 7227R6 was less vulnerable than susceptible derivatives of strain 7227 to zymolyase-mediated osmotic instability. However, 7227R6 was rendered susceptible to AmB after exposure to zymolyase. These data suggest that 7227R6 cells differ from susceptible lineages by having altered cell walls that were less readily digested by zymolyase and that the resistance may be conferred by a wall-mediated barrier that can be removed by zymolayse without decreasing cellular viability.
The level of AmB resistance in the switched isolates was sufficient to be clinically significant. In vivo, serum concentrations of AmB rarely exceed 2 to 3 μg/ml and decline steadily over the next 24 h (13). In vitro, 7227R6 was not affected by a 2- to 5-μg/ml concentration of AmB for at least 24 h. In addition, it is important to emphasize that this type of resistance, while easily measured by the EEA method or the MFC assay, would be completely missed by standard in vitro MIC protocols (Table 3). Standard MIC assays can only determine whether an isolate grows in the presence of the drug and so fail to differentiate among isolates that were or were not killed by the drug. This may offer one possible explanation for the frequently observed absence of documented in vitro AmB resistance in clinical C. lusitaniae isolates recovered from patients who were treated with AmB and clinically failed.
The switching of C. lusitaniae isolates from an AmB-susceptible to an AmB-resistant phenotype bears a number of similarities to colony morphology switching reported in C. albicans. Both occur at high frequencies relative to mutation rates, and both have phenotypes that switch in both directions. In addition, both increase switching rates after exposure to UV light, heat shock, or cellular components of blood (58). However, there are differences between the two switching systems. With C. lusitaniae AmB resistance phenotype switching, there was only a subtle change in colony morphology. Switching of C. lusitaniae is thus far limited to the in vitro AmB susceptibility phenotype switch, changes in cellular morphology, and alterations in fungal cell walls. In contrast, there were multiple switching changes seen in C. albicans. In C. lusitaniae, the least susceptible form was the elongate cellular form, in contrast to the situation observed in C. albicans (56).
C. albicans B311 does not switch to develop AmB resistance under the conditions described in this paper. Instead, C. albicans B311 and other Candida species may acquire a phenotypic resistance (AmBPR) when the cells are preexposed to azoles (14, 15, 52, 65, 67, 71) or when they age (17, 18, 71). In either circumstance, they undergo alterations in cell wall structures that may be functionally related to the resistance described previously (4, 9, 19). This resistance differs in several respects from switching. First, AmBPR C. albicans cells have only transient resistance; thus, they cannot be serially passaged on AmB-supplemented medium. Second, under optimal conditions, 10 to 99% of the cells in a C. lusitaniae population switch to resistance, which is much higher than the percentage of AmBPR cells (71). It remains possible, however, that the switching and phenotypic resistance are mediated through changes involving the fungal cell wall. One is mediated via physiological mechanisms and the other is mediated via genetic alterations, and a subset of wall changes may affect cellular and morphological changes. For C. lusitaniae switching, it remains to be determined whether wall and morphology changes are essential to the process of AmB resistance.
Few studies have evaluated whether there is a link between morphological switching of C. albicans and antifungal drug resistance. It was previously noted in the white-to-opaque transition of C. albicans that the AmB MICs for opaque cells were slightly higher than those for white cells and that the opaque cells showed increased resistance to the fungicidal effect of AmB (20). However, most of the previously published studies are confusing for several reasons. First, they are correlative studies with uncharacterized clinical isolates. Clinical isolates of C. albicans that were recovered from patients on azole therapy had switched colony morphologies, and some of these isolates were resistant to higher concentrations of azoles or in some cases to other nonclinical drugs (59, 61, 71). However, not all colonies with a shared morphology were resistant, and in some cases the resistant isolates were not recovered in a manner consistent with drug selection. In a second study, the authors noted that long-term exposure to inhibitory concentrations of heavy metals resulted in metal-resistant colonies with altered morphologies. These altered morphologies, however, were also seen among the susceptible colonies (30). These data suggest that switching produces alterations from a list of affected traits in individual cells, some combination of which may prove successful under the new environmental challenge.
What is known about the possible mechanism of AmB resistance? It is proposed that AmB kills Candida and other fungal cells by forming ion channels in the plasma membrane (10, 12). Among several clinical isolates of Candida, resistance has been correlated with decreased levels of ergosterol (16, 32, 33). In addition, resistance to AmB can also result from mutations in the sterol δ-5,6-desaturase. This defect results in the accumulation of 14α-methylfecosterol in the presence of fluconazole rather than 14α-methylergosta-8,24(28)-dien-3β,6α-diol (24, 25). Speculatively, alterations in the cell wall might directly affect the accessibility of AmB to membrane ergosterol or more indirectly change membrane composition, in turn affecting the ability of AmB to form ion channels. Conversely, changes in membrane composition mediated by fluconazole might result in cell wall structure alterations that affect accessibility of AmB and change cell morphology.
What potential clinical relevance might AmB switching have, both in the area of clinical specimen testing and in the progression of infection during therapy? First, that certain Candida species are inherently resistant, either to azoles (C. krusei and C. glabrata) (26, 31, 37, 63) or to AmB (C. lusitaniae and C. guilliermondii) (44), has been well described. Screening small numbers of clinical specimens from an immunocompromised patient for drug susceptibility could easily be misleading, if such samples were taken when the yeast was in an AmB-susceptible mode. Typically, samples in the laboratory were diluted and inoculated at about 300 cells per microtiter well (3). This small sample of cells would contain only a few to no resistant revertant cells. Even if a small number of resistant cells were present, they would not have time to grow to an extent that would generate sufficient turbidity to produce a higher MIC cutoff.
Finally, C. lusitaniae is similar to other Candida species in producing systemic or localized infections in compromised individuals (21, 22, 37, 50, 51, 72). However, this species is unique by virtue of its innate ability to switch antifungal susceptibility phenotype during growth. This ability is a reflection of its history of developing in vitro (1, 22, 43, 48) as well as in vivo (21, 22, 37) AmB resistance. During antifungal therapy, cells that switch to a resistant mode would be selected and the infection would potentially progress with the more resistant variants. Furthermore, since many in vitro conditions are known to stimulate morphological switching, it is reasonable to expect that localized conditions in vivo might also enhance morphological and AmB susceptibility switching, potentially changing other virulence traits. Our observation, that switching is efficiently induced by exposure to whole blood, reinforces this notion.
ACKNOWLEDGMENT
We thank Eileen Surma for excellent secretarial assistance.
REFERENCES
- 1.Ahearn D G, McGlohn M S. In vitro susceptibilities of sucrose-negative Candida tropicalis, Candida lusitaniae, and Candida norvegensis to amphotericin B, 5-fluorocytosine, miconazole, and ketoconazole. J Clin Microbiol. 1984;19:412–416. doi: 10.1128/jcm.19.3.412-416.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Anderson J M, Soll D R. Unique phenotype of opaque cells in the white-opaque transition of Candida albicans. J Bacteriol. 1987;169:5579–5588. doi: 10.1128/jb.169.12.5579-5588.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Barchiesi F, Colombo A L, McGough D A, Rinaldi M G. Comparative study of broth macrodilution and microdilution techniques for in vitro antifungal susceptibility testing of yeasts by using the National Committee for Clinical Laboratory Standards’ proposed standard. J Clin Microbiol. 1994;32:2494–2500. doi: 10.1128/jcm.32.10.2494-2500.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bastide M, Jouvert S, Bastide J M. A comparison of the effects of several antifungal imidazole derivatives and polyenes on Candida albicans: an ultrastructural study by scanning electron microscopy. Can J Microbiol. 1982;28:1119–1126. doi: 10.1139/m82-166. [DOI] [PubMed] [Google Scholar]
- 5.Bedell G W, Soll D R. Effects of low concentrations of zinc on the growth and dimorphism of Candida albicans: evidence for zinc-resistant and -sensitive pathways for mycelium formation. Infect Immun. 1979;26:348–354. doi: 10.1128/iai.26.1.348-354.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Berenguer J, Diaz-Guerra T M, Ruiz-Diez B, Bernaldo de Quiros J C, Rodriguez-Tudela J L, Martinez-Suarez J V. Genetic dissimilarity of two fluconazole-resistant Candida albicans strains causing meningitis and oral candidiasis in the same AIDS patient. J Clin Microbiol. 1996;34:1542–1545. doi: 10.1128/jcm.34.6.1542-1545.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bergen M S, Voss E, Soll D R. Switching at the cellular level in the white-opaque transition of Candida albicans. J Gen Microbiol. 1990;136:1925–1936. doi: 10.1099/00221287-136-10-1925. [DOI] [PubMed] [Google Scholar]
- 8.Blumberg H M, Hendershot E F, Lott T J. Persistence of the same Candida albicans strain despite fluconazole therapy. Documentation by pulsed-field gel electrophoresis. Diagn Microbiol Infect Dis. 1992;15:545–547. doi: 10.1016/0732-8893(92)90106-4. [DOI] [PubMed] [Google Scholar]
- 9.Cassone A, Kerridge D, Gale E F. Ultrastructural changes in the cell wall of Candida albicans following cessation of growth and their possible relationship to the development of polyene resistance. J Gen Microbiol. 1979;110:339–349. doi: 10.1099/00221287-110-2-339. [DOI] [PubMed] [Google Scholar]
- 10.Cheron M, Cybulska B, Mazerski J, Grzybowska J, Czerwinski A, Borowski E. Quantitative structure-activity relationships in amphotericin B derivatives. Biochem Pharmacol. 1988;37:827–836. doi: 10.1016/0006-2952(88)90168-2. [DOI] [PubMed] [Google Scholar]
- 11.Chu W S, Rikkerink E H, Magee P T. Genetics of the white-opaque transition in Candida albicans: demonstration of switching recessivity and mapping of switching genes. J Bacteriol. 1992;174:2951–2957. doi: 10.1128/jb.174.9.2951-2957.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Clejan S, Bittman R. Rates of amphotericin B and filipin association with sterols. A study of changes in sterol structure and phospholipid composition of vesicles. J Biol Chem. 1985;260:2884–2889. [PubMed] [Google Scholar]
- 13.Daneshmend T K, Warnock D W. Clinical pharmacokinetics of systemic antifungal drugs. Clin Pharmacokinet. 1983;8:17–42. doi: 10.2165/00003088-198308010-00002. [DOI] [PubMed] [Google Scholar]
- 14.De Nollin S, Borgers M. Scanning electron microscopy of Candida albicans after in vitro treatment with miconazole. Antimicrob Agents Chemother. 1975;7:704–711. doi: 10.1128/aac.7.5.704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.De Nollin S, Borgers M. The ultrastructure of Candida albicans after in vitro treatment with miconazole. Subouraudia. 1974;12:341–351. [PubMed] [Google Scholar]
- 16.Dick J D, Merz W G, Saral R. Incidence of polyene-resistant yeasts recovered from clinical specimens. Antimicrob Agents Chemother. 1980;18:158–163. doi: 10.1128/aac.18.1.158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gale E F, Ingram J, Kerridge D, Notario V, Wayman F. Reduction of amphotericin resistance in stationary phase cultures of Candida albicans by treatment with enzymes. J Gen Microbiol. 1980;117:383–391. doi: 10.1099/00221287-117-2-383. [DOI] [PubMed] [Google Scholar]
- 18.Gale E F, Johnson A M, Kerridge D, Koh T Y. Factors affecting the changes in amphotericin sensitivity of Candida albicans during growth. J Gen Microbiol. 1975;87:20–36. doi: 10.1099/00221287-87-1-20. [DOI] [PubMed] [Google Scholar]
- 19.Gale E F, Johnson A M, Kerridge D, Waymen F. Phenotypic resistance to miconazole and amphotericin B in Candida albicans. J Gen Microbiol. 1980;117:535–538. doi: 10.1099/00221287-117-2-535. [DOI] [PubMed] [Google Scholar]
- 20.Ghannoum, M. A., I. Swairjo, and D. R. Soll. Variation in lipid and sterol contents in Candida albicans white and opaque phenotypes. J. Med. Vet. Mycol. 28:103–115. [PubMed]
- 21.Guinet R, Chanas J, Goullier A, Bonnefoy G, Ambroise-Thomas P. Fatal septicemia due to amphotericin B-resistant Candida lusitaniae. J Clin Microbiol. 1983;18:443–444. doi: 10.1128/jcm.18.2.443-444.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hadfield T L, Smith M B, Winn R E, Rinaldi M G, Guerra C. Mycoses caused by Candida lusitaniae. Rev Infect Dis. 1987;9:1006–1012. doi: 10.1093/clinids/9.5.1006. [DOI] [PubMed] [Google Scholar]
- 23.Jones S, White G, Hunter P R. Increased phenotypic switching in strains of Candida albicans associated with invasive infections. J Clin Microbiol. 1994;32:2869–2870. doi: 10.1128/jcm.32.11.2869-2870.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kelly S L, Lamb D C, Kelly D E, Loeffler J, Einsele H. Resistance to fluconazole and amphotericin in Candida albicans from AIDS patients. Lancet. 1996;348:1523–1524. doi: 10.1016/S0140-6736(05)65949-1. . (Letter.) [DOI] [PubMed] [Google Scholar]
- 25.Kelly S L, Lamb D C, Kelly D E, Manning N J, Loeffler J, Hebart H, Schumacher U, Einsele H. Resistance to fluconazole and cross-resistance to amphotericin B in Candida albicans from AIDS patients caused by defective sterol δ-5,6-desaturation. FEBS Lett. 1997;400:80–82. doi: 10.1016/s0014-5793(96)01360-9. [DOI] [PubMed] [Google Scholar]
- 26.Komshian S V, Uwaydah A K, Sobel J D, Crane L R. Fungemia caused by Candida species and Torulopsis glabrata in the hospitalized patient: frequency, characteristics, and evaluation of factors influencing outcome. Rev Infect Dis. 1989;11:379–390. doi: 10.1093/clinids/11.3.379. [DOI] [PubMed] [Google Scholar]
- 27.Leberer E, Ziegelbauer K, Schmidt A, Harcus D, Dignard D, Ash J, Johnson L, Thomas D Y. Virulence and hyphal formation of Candida albicans require the Ste20p-like protein kinase CaCla4p. Am J Clin Pathol. 1997;108:217–221. doi: 10.1016/s0960-9822(06)00252-1. [DOI] [PubMed] [Google Scholar]
- 28.Lee K L, Buckley H R, Campbell C C. An amino acid liquid synthetic medium for the development of mycelial and yeast forms of Candida albicans. Sabouraudia. 1975;13:148–153. doi: 10.1080/00362177585190271. [DOI] [PubMed] [Google Scholar]
- 29.Lockhart S R, Fritch J J, Meier A S, Schroppel K, Srikantha T, Galask R, Soll D R. Colonizing populations of Candida albicans are clonal in origin but undergo microevolution through C1 fragment reorganization as demonstrated by DNA fingerprinting and C1 sequencing. J Clin Microbiol. 1995;33:1501–1509. doi: 10.1128/jcm.33.6.1501-1509.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Malavasic M J, Cihlar R L. Growth response of several Candida albicans strains to inhibitory concentrations of heavy metals. J Med Vet Mycol. 1992;30:421–432. [PubMed] [Google Scholar]
- 31.Marichal P, Gorrens J, Coene M C, Le Jeune L, Vanden Bossche H. Origin of differences in susceptibility of Candida krusei to azole antifungal agents. Mycoses. 1995;38:111–117. doi: 10.1111/j.1439-0507.1995.tb00032.x. [DOI] [PubMed] [Google Scholar]
- 32.Mas J, Pina E. Candida resistant to nystatin becomes sensitive upon culture with ergosterol. Arch Investig Med. 1985;16:145–155. [PubMed] [Google Scholar]
- 33.Mas, J., and E. Pina. Disappearance of nystatin resistance in Candida mediated by ergosterol. J. Gen. Microbiol. 117:249–252. [DOI] [PubMed]
- 34.Mathaba L T, Davies G, Warmington J R. The genotypic relationship of Candida albicans strains isolated from the oral cavity of patients with denture stomatitis. J Med Microbiol. 1995;42:372–379. doi: 10.1099/00222615-42-5-372. [DOI] [PubMed] [Google Scholar]
- 35.McCullough M J, Ross B C, Dwyer B D, Reade P C. Genotype and phenotype of oral Candida albicans from patients infected with the human immunodeficiency virus. Microbiology. 1994;140:1195–1202. doi: 10.1099/13500872-140-5-1195. [DOI] [PubMed] [Google Scholar]
- 36.Mercure S, Poirier S, Lemay G, Auger P, Montplaisir S, de Repentigny L. Application of biotyping and DNA typing of Candida albicans to the epidemiology of recurrent vulvovaginal candidiasis. J Infect Dis. 1993;168:502–507. doi: 10.1093/infdis/168.2.502. [DOI] [PubMed] [Google Scholar]
- 37.Merz W G. Candida lusitaniae: frequency of recovery, colonization, infection, and amphotericin B resistance. J Clin Microbiol. 1984;20:1194–1195. doi: 10.1128/jcm.20.6.1194-1195.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Merz W G, Khazan U, Jabra-Rizk M A, Wu L C, Osterhout G J, Lehmann P F. Strain delineation and epidemiology of Candida (Clavispora) lusitaniae. J Clin Microbiol. 1992;30:449–454. doi: 10.1128/jcm.30.2.449-454.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Morrow B, Anderson J, Wilson J, Soll D R. Bidirectional stimulation of the white-opaque transition of Candida albicans by ultraviolet irradiation. J Gen Microbiol. 1989;135:1201–1208. doi: 10.1099/00221287-135-5-1201. [DOI] [PubMed] [Google Scholar]
- 40.Odds F C, Bernaerts R. CHROMagar Candida, a new differential isolation medium for presumptive identification of clinically important Candida species. J Clin Microbiol. 1994;32:1923–1929. doi: 10.1128/jcm.32.8.1923-1929.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pappagianis D, Collins M S, Hector R, Remington J. Development of resistance to amphotericin B in Candida lusitaniae infecting a human. Antimicrob Agents Chemother. 1979;16:123–126. doi: 10.1128/aac.16.2.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.faller M A, Houston A, Coffmann S. Application of CHROMagar Candida for rapid screening of clinical specimens for Candida albicans, Candida tropicalis, Candida krusei, and Candida (Torulopsis) glabrata. J Clin Microbiol. 1996;34:58–61. doi: 10.1128/jcm.34.1.58-61.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pfaller M A, Messer S A, Hollis R J. Strain delineation and antifungal susceptibilities of epidemiologically related and unrelated isolates of Candida lusitaniae. Diagn Micobiol Infect Dis. 1994;20:127–133. doi: 10.1016/0732-8893(94)90106-6. [DOI] [PubMed] [Google Scholar]
- 44.Powderly W G, Kobayashi G S, Herzig G P, Medoff G. Amphotericin B-resistant yeast infection in severely immunocompromised patients. Am J Med. 1988;84:826–832. doi: 10.1016/0002-9343(88)90059-9. [DOI] [PubMed] [Google Scholar]
- 45.Pujol C, Reynes J, Renaud F, Raymond M, Tibayrenc M, Ayala F J, Janbon F, Mallie M, Bastide J M. The yeast Candida albicans has a clonal mode of reproduction in a population of infected human immunodeficiency virus-positive patients. Proc Natl Acad Sci USA. 1993;90:9456–9459. doi: 10.1073/pnas.90.20.9456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Radford D R, Challcombe S J, Walter J D. Scanning electron microscopy of the development of structured aerial mycelia and satellite colonies of phenotypically switched Candida albicans. J Med Microbiol. 1997;46:326–332. doi: 10.1099/00222615-46-4-326. [DOI] [PubMed] [Google Scholar]
- 47.Radford D R, Challacombe S J, Walter J D. A scanning electronmicroscopy investigation of the structure of colonies of different morphologies produced by phenotypic switching of Candida albicans. J Med Microbiol. 1994;40:416–423. doi: 10.1099/00222615-40-6-416. [DOI] [PubMed] [Google Scholar]
- 48.Rex J H, Cooper C R, Jr, Merz W G, Galgiani J N, Anaissie E J. Detection of amphotericin B-resistant Candida isolates in a broth-based system. Antimicrob Agents Chemother. 1995;39:906–909. doi: 10.1128/aac.39.4.906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Rikkerink E H, Magee B B, Magee P T. Opaque-white phenotype transition: a programmed morphological transition in Candida albicans. J Bacteriol. 1988;170:895–899. doi: 10.1128/jb.170.2.895-899.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sanchez P J, Cooper B H. Candida lusitaniae: sepsis and meningitis in a neonate. Pediatr Infec Dis J. 1987;6:758–759. [PubMed] [Google Scholar]
- 51.Sanchez V, Vazquez J A, Barth-Jones D, Dembry L, Sobel J D, Zervos M J. Epidemiology of nosocomial acquisition of Candida lusitaniae. J Clin Microbiol. 1992;30:3005–3008. doi: 10.1128/jcm.30.11.3005-3008.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Scheven M, Schwegler F. Antagonistic interactions between azoles and amphotericin B with yeasts depend on azole lipophilia for special test conditions in vitro. Antimicrob Agents Chemother. 1995;39:1779–1783. doi: 10.1128/aac.39.8.1779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Schonian G, Meusel O, Tietz H J, Meyer W, Graser Y, Tausch I, Presber W, Mitchell T G. Identification of clinical strains of Candida albicans by DNA fingerprinting with the polymerase chain reaction. Mycoses. 1993;36:171–179. doi: 10.1111/j.1439-0507.1993.tb00746.x. [DOI] [PubMed] [Google Scholar]
- 54.Slutsky B, Buffo J, Soll D R. High-frequency switching of colony morphology in Candida albicans. Science. 1985;230:666–669. doi: 10.1126/science.3901258. [DOI] [PubMed] [Google Scholar]
- 55.Slutsky B, Staebell M, Anderson J, Risen L, Pfaller M, Soll D R. “White-opaque transition”: a second high-frequency switching system in Candida albicans. J Bacteriol. 1987;169:189–197. doi: 10.1128/jb.169.1.189-197.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Soll D R. The emerging molecular biology of switching in Candida albicans. ASM News. 1996;62:415–420. [Google Scholar]
- 57.Soll D R. Gene regulation during high-frequency switching in Candida albicans. Microbiology. 1997;143:279–288. doi: 10.1099/00221287-143-2-279. [DOI] [PubMed] [Google Scholar]
- 58.Soll D R. High-frequency switching in Candida albicans. Clin Microbiol Rev. 1992;5:183–203. doi: 10.1128/cmr.5.2.183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Soll D R, Galask R, Isley S, Rao T V, Stone D, Hicks J, Schmid J, Mac K, Hanna C. Switching of Candida albicans during successive episodes of recurrent vaginitis. J Clin Microbiol. 1989;27:681–690. doi: 10.1128/jcm.27.4.681-690.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Soll D R, Morrow B, Srikantha T. High-frequency phenotypic switching in Candida albicans. Trends Genet. 1993;9:61–65. doi: 10.1016/0168-9525(93)90189-O. [DOI] [PubMed] [Google Scholar]
- 61.Soll D R, Morrow B, Srikantha T, Vargas K, Wertz P. Developmental and molecular biology of switching in Candida albicans. Oral Surg Oral Med Oral Pathol. 1994;78:194–201. doi: 10.1016/0030-4220(94)90147-3. [DOI] [PubMed] [Google Scholar]
- 62.Soll D R, Staebell M, Langtimm C, Pfaller M, Hicks J, Rao T V. Multiple Candida strains in the course of a single systemic infection. J Clin Microbiol. 1988;26:1448–1459. doi: 10.1128/jcm.26.8.1448-1459.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Spinillo A, Capuzzo E, Egbe T O, Baltaro F, Nicola S, Piazzi G. Torulopsis glabrata vaginitis. Obstet Gynecol. 1995;85:993–998. doi: 10.1016/0029-7844(95)00047-U. [DOI] [PubMed] [Google Scholar]
- 64.Steffan P, Vazquez J A, Boikov D, Xu C, Sobel J D, Akins R A. Identification of Candida species by randomly amplified polymorphic DNA fingerprinting of colony lysates. J Clin Microbiol. 1997;35:2031–2039. doi: 10.1128/jcm.35.8.2031-2039.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64a.Steffan, P., J. A. Vazquez, and R. A. Akins. Unpublished data.
- 65.Sud I J, Feingold D S. Effect of ketoconazole on the fungicidal action of amphotericin B in Candida albicans. Antimicrob Agents Chemother. 1983;23:185–187. doi: 10.1128/aac.23.1.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Thanos M, Schonian G, Meyer W, Schweynoch C, Graser Y, Mitchell T G, Presber W, Tietz H J. Rapid identification of Candida species by DNA fingerprinting with PCR. J Clin Microbiol. 1996;34:615–621. doi: 10.1128/jcm.34.3.615-621.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Vazquez J A, Arganoza M T, Vaishampayan J K, Akins R A. In vitro interaction between amphotericin B and azoles in Candida albicans. Antimicrob Agents Chemother. 1996;40:2511–2516. doi: 10.1128/aac.40.11.2511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Vazquez J A, Beckley A, Donabedian S, Sobel J D, Zervos M J. Comparison of restriction enzyme analysis versus pulsed-field gradient gel electrophoresis as a typing system for Torulopsis glabrata and Candida species other than C. albicans. J Clin Microbiol. 1993;31:2021–2030. doi: 10.1128/jcm.31.8.2021-2030.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Vazquez J A, Lundstrom T, Dembry L, Chandrasekar P, Boikov D, Parri M B, Zervos M J. Invasive Candida guilliermondii infection: in vitro susceptibility studies and molecular analysis. Bone Marrow Transplant. 1995;16:849–853. [PubMed] [Google Scholar]
- 70.Vazquez J A, Sobel J D, Demitriou R, Vaishampayan J, Lynch M, Zervos M J. Karyotyping of Candida albicans isolates obtained longitudinally in women with recurrent vulvovaginal candidiasis. 1994. J. Infect. Dis. –170:1566–1569. [DOI] [PubMed] [Google Scholar]
- 71.Velegraki A. In vitro susceptibility to itraconazole and fluconazole of switch phenotypes of Candida albicans serotypes A and B, isolated from immunocompromised hosts. J Med Vet Mycol. 1995;33:83–85. [PubMed] [Google Scholar]
- 72.Wendt B, Haglund L, Razavi A, Rath R. Candida lusitaniae: an uncommon cause of prosthetic valve endocarditis. Clin Infect Dis. 1998;26:769–770. doi: 10.1086/517126. [DOI] [PubMed] [Google Scholar]
- 73.Williams J G, Kubelik A R, Livak K J, Rafalski JA, Tingey S V. DNA polymorphisms amplified by arbitrary primers are useful as genetic markers. Nucleic Acids Res. 1990;18:6531–6535. doi: 10.1093/nar/18.22.6531. [DOI] [PMC free article] [PubMed] [Google Scholar]