Abstract
Background
Undifferentiated carcinoma with osteoclast-like giant cells (UCOGCs) is a rare subtype of pancreatic cancer (PC), and its clinicopathological characteristics are still unclear. Herein, we report a case of initially unresectable UCOGC that was successfully resected after FOLFIRINOX therapy.
Case presentation
A 63-year-old man was referred to us for evaluation of a pancreatic mass detected by computed tomography (CT) during a medical checkup. Computed tomography showed a 7.5-cm tumor located in the pancreatic head and body, which involved the common hepatic artery (CHA), gastroduodenal artery (GDA), and main portal vein (PV) with tumor thrombus. UCOGC was suspected by endoscopic ultrasonography-guided fine needle aspiration, and the patient was diagnosed with unresectable locally advanced pancreatic cancer. After ten cycles of FOLFIRINOX, the tumor size decreased to 3 cm and the tumor thrombus in the main portal trunk had disappeared in the follow-up CT scan. However, the patient experienced severe adverse drug reactions, including neutropenia and liver dysfunction. Therefore, we performed pancreatoduodenectomy with portal vein resection. The pathological diagnosis was UCOGC with a negative tumor margin. He was treated with FOLFIRINOX, and remains recurrence-free for 6 months after surgery.
Conclusions
We experienced a case undergoing conversion surgery for unresectable UCOGC, which resulted in R0 resection. FOLFIRINOX could be a possible regimen to achieve conversion surgery for UCOGC.
Keywords: Undifferentiated carcinoma with osteoclast-like giant cells, Conversion surgery, Pancreatic ductal adenocarcinoma, FOLFIRINOX
Background
Pancreatic cancer (PC) is a gastrointestinal cancer with a poor prognosis and limited potential for oncologic treatment. Undifferentiated carcinoma with osteoclast-like giant cells (UCOGCs) is a rare subtype of pancreatic ductal adenocarcinoma (PDAC), which accounts for less than 1% of non-endocrine pancreatic tumors [1]. Prognosis for patients with UCOGCis inconsistent relative to general PDAC [1–4], and the clinicopathological characteristics of UCOGC have not been elucidated. Herein, we experienced a case of initially unresectable UCOGC who underwent conversion surgery.
Case presentation
A 63-year-old man was admitted to our hospital for further evaluation of an asymptomatic pancreatic mass that was detected by computed tomography (CT) during a medical checkup. He had a medical history of hypertension and was treated with 20 mg of daily olmesartan medoxomil. Laboratory data showed a slightly elevated hemoglobin A1c of 6.8% and tumor markers were as follows: carcinoembryonic antigen (CEA), 3.7 ng/mL; cancer antigen-19–9 (CA19-9), 147 U/mL; cancer antigen-125, 42 U/mL; DUPAN-2, 120 U/mL; and Span-1, 72 U/m. A CT scan revealed a 7.5-cm mass located in the pancreatic head and body (Fig. 1A) that invaded the common hepatic artery (CHA), gastroduodenal artery (GDA), and proper hepatic artery (PHA). A tumor thrombus in the main portal vein (PV) was also detected. The celiac artery (CA) and superior mesenteric artery (SMA) were intact. Magnetic resonance cholangiopancreatography (MRCP) revealed main pancreatic duct (MPD) stenosis at the pancreatic head with distal dilatation of the MPD (Fig. 1B). Endoscopic ultrasonography-guided fine needle aspiration was performed, and UCOGC was suspected based on pathology. Therefore, the patient was diagnosed with unresectable locally advanced UCOGC and conventional FOLFIRINOX (oxaliplatin, 85 mg per square meter of body-surface area; irinotecan, 180 mg per square meter; leucovorin, 400 mg per square meter; and fluorouracil, 400 mg per square meter given as a bolus followed by 2,400 mg per square meter given as a 46-h continuous infusion, every 2 weeks) was administered. After 10 cycles (20 weeks) of FOLFIRINOX, contrast-enhanced CT showed a significant response to chemotherapy. Although the tumor was still in contact with the CHA and GDA, the tumor diameter shrank to 3 cm and the portal vein thrombus had disappeared (Fig. 1C). The tumor markers were reduced as follows: CEA, 2.3 ng/mL; CA 19–9, 18 U/mL; DUPAN-2, 25 U/mL. Severe adverse drug reactions including neutropenia and liver dysfunction were also observed, and it was difficult to continue FOLFIRINOX therapy. Therefore, a conversion surgery was performed.
Fig. 1.
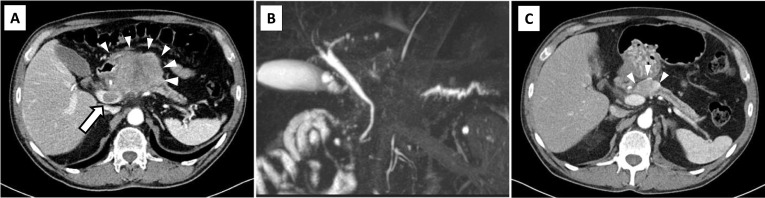
Computed tomography showed the pancreatic mass which was 7.5 cm in size at initial diagnosis (A, arrowhead). It revealed portal vein embolism (A, arrow), which was suggestive of tumor emboli at the main trunk and stenosis of the main pancreatic ducts with distal dilatation of the pancreatic duct (A). Magnetic resonance cholangiopancreatography exhibited the main pancreatic duct (MPD) stenosis at the pancreatic head with distal dilatation of MPD (B). After ten cycles of chemotherapy with FOLFIRINOX, the tumor shrunk to 3 cm (C, arrowhead), and the thrombi in the main portal vein could not be identified (C)
Intraoperative findings showed no peritoneal dissemination or liver metastasis. The tumor invaded the transverse mesocolon (Fig. 2A), but the SMA was not affected. Although tumor invasion to the neural plexus around the CHA and GDA was suspected (Fig. 2B), complete resection of these plexuses was achieved. After confirming no tumor invasion to the plexus by examining the intraoperative frozen section, the pancreatic parenchyma was dissected with a 3-cm tumor margin. Since PV invasion was strongly suspected, the PV/superior mesenteric vein (SMV) was resected 4 cm in length and reconstructed with end-to-end anastomosis. Finally, the pancreaticoduodenectomy with Child’s reconstruction was performed. The total operation time was 597 min, and the blood loss was 1,120 mL.
Fig. 2.

Intraoperative findings showed the tumor invading the transverse mesocolon. White arrows indicate tumor invasion to the transverse mesocolon (A). The tumor invasion was suspected at the neural plexus around the common hepatic artery and gastroduodenal artery. White arrows indicate tumor invasion to the neural plexus around the hepatic arteries (B). Complete tumor resection was achieved by resecting the neural plexuses and superior mesenteric vein (SMV) with 4 cm length. White arrows indicate remaining tumor cells surrounded by fibrosis (C)
The excised tumor was 3.5 × 3.1 × 2.4 cm in size and was located across the pancreatic head and body. Histopathological findings showed that the tumor cells were pleomorphic, irregularly shaped to spindle-shaped, and surrounded by osteoclast-like multinucleated giant cells, which was consistent with the diagnosis of UCOGC (Fig. 3A, B). Immunohistochemistry (IHC) showed positive tumor staining for programmed death-ligand 1 (PD-L1) (Fig. 3C). Some adenocarcinoma components were also identified. Tumor cell clusters were found in the portal vein. Tumor metastasis was found in one of the #8 lymph nodes in the anterosuperior region along the CHA, out of all 26 dissected nodes. The UICC TNM classification (8th edition) was defined as ypT2N1M0, stage IIB. No tumor exposure of the margins was observed; therefore, histological R0 was achieved. The postoperative course was uneventful, and the patient was discharged on the 16th postoperative day. He is now receiving adjuvant FOLFIRINOX without recurrence for more than 6 months after the operation.
Fig. 3.

Histopathological findings showed that the peritumoral area is highly fibrotic, which suggested that the tumor had shrunk by FOLFIRINOX. White arrows indicate remaining tumor cells surrounded by fibrosis (A) (HE × 20). The tumor cells were pleomorphic, irregularly shaped to spindle-shaped, and surrounded by osteoclast-like multinucleated giant cells, consistent with the diagnosis of UCOGC (B), (HE × 400). Both the cytoplasm of the cancer cells and osteoclast-like giant cells are positive for PD-L1 immunostaining (C) (PD-L1 immunostaining × 400)
Discussion
We successfully treated an unresectable locally advanced UCOGC case with conversion surgery after FOLFIRINOX therapy. PC is the most aggressive solid tumor in humans and has a very poor prognosis [5, 6]. Even if no metastasis is detected, it is often diagnosed as unresectable locally advanced pancreatic cancer (LAPC); the primary resectable PC is present in only 15–20% of all patients [7]. However, the number of cases of conversion surgery has been increasing due to the development of novel chemotherapeutic regimens. Of these regimens, FOLFIRINOX has been reported to improve the response rate of LAPC and the rate of conversion surgery considerably [7, 8]. To the best of our knowledge, there have been no reported cases of conversion surgery for UCOGC after FOLFIRINOX, and the current case is the first report.
UCOGC is extremely rare, with an incidence of 1.4% for invasive PCs and 0.4% for resected PCs [2]. The prognosis of UCOGC is inconsistent, because the disease is so rare that it is difficult to evaluate the prognosis by stage [1], and most UGOCG cases were found at the advanced stage and recurred early after resection [9]. Another reason is that UCOGC was not defined until 2010 when WHO integrated two subtypes, giant cell tumors (GCTs) with osteoclast-like cells and pleomorphic GCTs, as UCOGC [10]. In general, UCOGC is a rapidly growing tumor with abundant blood flow, often presenting as a large tumor with hemorrhage and necrosis [11, 12]. UCOGC is also an aggressive tumor, so composite resection with invaded adjacent organs such as the stomach, jejunum, colon, left kidney [13], diaphragm, and common hepatic artery is often needed [14]. Further investigations with large sample sizes are required to identify the molecular characteristics of UCOGC and establish a treatment strategy for UCOGC to achieve conversion surgery.
Conversion surgery may improve the prognosis of patients with PC. The number of cases with conversion therapy for PDAC has been increasing, owing to the recent advances in chemotherapy [6]. Although the efficacy of chemotherapy for UCOGC has not yet been fully evaluated, standard chemotherapy regimens for pancreatic cancer, including FOLFILINOX, have been selected because UCOGC is considered a variant of ductal adenocarcinoma of the pancreas [9]. In addition to FOLFIRINOX, there are reports that gemcitabine was effective against UCOGC [15]. Gemcitabine is often used in combination with nab-paclitaxel, capecitabine, or S-1. In addition to conventional chemotherapy, immune checkpoint inhibitors (ICIs) have recently attracted increasing attention for the treatment of various cancers, including PC [5]. IHC for PD-L1 was positive in 65% to 80% of UCOGC cases, and these cases showed poor prognosis [16, 17]. However, PD-L1-positive UCOGC showed a marked response to ICI, both in primary UCOGC tumors and metastatic diseases [18]. In the current case, IHC for PD-L1 was strongly positive in the surgically resected specimen, suggesting that ICIs may have been effective. Routine assessment of immune-related markers such as programmed death 1 (PD-1) and PD-L1 for tumor biopsy specimens at initial diagnosis could provide therapeutic options other than FOLFIRINOX to establish precision treatment for PC in the era of immunotherapy. Another important examination for patients with PDAC is germline genetic testing for BRCA pathogenic variant. For patients who have germline BRCA mutation, after at least 16 weeks of platinum-based chemotherapy such as FOLFIRINOX without disease progression, the PARP inhibitor olaparib is recommended [19]. Although olaparib is usually used in the second-line setting, we consider BRCA status of the patient in the current study is very important because the patient could be susceptible to tumor recurrence after conversion surgery. Since the patient underwent 20 weeks of preoperative FOLFIRINOX with favorable response, we plan to check BRCA status in the near future.
Conclusions
We encountered a case of conversion surgery for UCOGC of UR-LAPC, which resulted in R0 resection. Administering a FOLFIRINOX regimen can create more favorable conditions to perform conversion surgery for unresectable UCOGC. Moreover, routine assessment of immune-related markers may be useful for establishing precision medicine in patients with PC.
Acknowledgements
We would like to thank Editage (www.editage.com) for English language editing.
Abbreviations
- PC
Pancreatic cancer
- UCOGC
Undifferentiated carcinoma with osteoclast-like giant cells
- PDAC
Pancreatic ductal adenocarcinoma
- CT
Computed tomography
- CHA
Common hepatic artery
- GDA
Gastroduodenal artery
- PHA
Proper hepatic artery
- PV
Portal vein
- CA
Celiac artery
- SMA
Superior mesenteric artery
- MRCP
Magnetic resonance cholangiopancreatography
- MPD
Main pancreatic duct
- SMV
Superior mesenteric vein
- IHC
Immunohistochemistry
- PD-L1
Programmed death-ligand 1
- LAPC
Giant cell tumors
- GCTs
Locally advanced pancreatic cancer
- GEM
Gemcitabine
- nab-PTX
Nanoparticle albumin-bound paclitaxel
- ICI
Immune checkpoint inhibitor
- PD-1
Programmed death 1
Authors' contributions
YI, TT, and TG drafted the manuscript. TU, RH, YS, JY, KH, and KF for acquisition of data and critical revision. TI for final approval of the manuscript. All authors read and approved the final manuscript.
Funding
This research was supported by the Jikei University Research Fund, Grant Number 2021-021SR.
Availability of data and materials
Not applicable.
Declarations
Ethics approval and consent to participate
This retrospective study meeting the ethical standards of the World Medical Association Declaration of Helsinki was approved by The Jikei University School of Medicine [27-177(8062)].
Consent for publication
Informed consent was obtained from the patient for the publication of this case report.
Competing interests
All authors have no conflict of interest to declare.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Yosuke Igarashi, Email: igarashi-y@jikei.ac.jp.
Takeshi Gocho, Email: gocho@jikei.ac.jp.
Tomohiko Taniai, Email: t.taniai@jikei.ac.jp.
Tadashi Uwagawa, Email: uwatadashi@msn.com.
Ryoga Hamura, Email: rhamura@jikei.ac.jp.
Yoshihiro Shirai, Email: shirai@jikei.ac.jp.
Jungo Yasuda, Email: j.yasuda@jikei.ac.jp.
Koichiro Haruki, Email: haruki@jikei.ac.jp.
Kenei Furukawa, Email: k-furukawa@jikei.ac.jp.
Toru Ikegami, Email: toruikegamijikei@jikei.ac.jp.
References
- 1.Abid H, Gnanajothy R. Osteoclast giant cell tumor of pancreas: a case report and literature review. Cureus. 2019;11:e4710. doi: 10.7759/cureus.4710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Muraki T, Reid MD, Basturk O, Jang KT, Bedolla G, Bagci P, et al. Undifferentiated carcinoma with osteoclastic giant cells of the pancreas: clinicopathologic analysis of 38 cases highlights a more protracted clinical course than currently appreciated. Am J Surg Pathol. 2016;40:1203–1216. doi: 10.1097/PAS.0000000000000689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Luchini C, Pea A, Lionheart G, Mafficini A, Nottegar A, Veronese N, et al. Pancreatic undifferentiated carcinoma with osteoclast-like giant cells is genetically similar to, but clinically distinct from, conventional ductal adenocarcinoma. J Pathol. 2017;243:148–154. doi: 10.1002/path.4941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lukás Z, Dvorák K, Kroupová I, Valásková I, Habanec B. Immunohistochemical and genetic analysis of osteoclastic giant cell tumor of the pancreas. Pancreas. 2006;32:325–329. doi: 10.1097/01.mpa.0000202951.10612.fa. [DOI] [PubMed] [Google Scholar]
- 5.Schizas D, Charalampakis N, Kole C, Economopoulou P, Koustas E, Gkotsis E, et al. Immunotherapy for pancreatic cancer: a 2020 update. Cancer Treat Rev. 2020;86:102016. doi: 10.1016/j.ctrv.2020.102016. [DOI] [PubMed] [Google Scholar]
- 6.Yoshitomi H, Takano S, Furukawa K, Takayashiki T, Kuboki S, Ohtsuka M. Conversion surgery for initially unresectable pancreatic cancer: current status and unresolved issues. Surg Today. 2019;49:894–906. doi: 10.1007/s00595-019-01804-x. [DOI] [PubMed] [Google Scholar]
- 7.Hank T, Strobel O. Conversion surgery for advanced pancreatic cancer. J Clin Med. 2019;8:1945. doi: 10.3390/jcm8111945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Klaiber U, Hackert T. Conversion surgery for pancreatic cancer-the impact of neoadjuvant treatment. Front Oncol. 2019;9:1501. doi: 10.3389/fonc.2019.01501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Demetter P, Marechal R, Puleo F, Delhaye M, Debroux S, Charara F, et al. Undifferentiated pancreatic carcinoma with osteoclast-like giant cells: what do we know so far? Front Oncol. 2021;11:630086. doi: 10.3389/fonc.2021.630086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hanayneh W, Parekh H, Fitzpatrick G, Feely M, George TJ, Starr JS. Two cases of rare pancreatic malignancies. J Pancreat Cancer. 2019;5:26–33. doi: 10.1089/pancan.2019.0007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sato K, Urakawa H, Sakamoto K, Ito E, Hamada Y, Yoshimitsu K. Undifferentiated carcinoma of the pancreas with osteoclast-like giant cells showing intraductal growth and intratumoral hemorrhage: MRI features. Radiol Case Rep. 2019;14:1283–1287. doi: 10.1016/j.radcr.2019.07.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhang L, Lee JM, Yoon JH, Joo I, Kang HJ, Han JK, et al. Huge and recurrent undifferentiated carcinoma with osteoclast-like giant cells of the pancreas. Quant Imaging Med Surg. 2018;8:457–460. doi: 10.21037/qims.2018.05.05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yazawa T, Watanabe A, Araki K, Segawa A, Hirai K, Kubo N, et al. Complete resection of a huge pancreatic undifferentiated carcinoma with osteoclast-like giant cells. Int Cancer Conf J. 2017;6:193–196. doi: 10.1007/s13691-017-0305-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shiozawa M, Imada T, Ishiwa N, Rino Y, Hasuo K, Takanashi Y, et al. Osteoclast-like giant cell tumor of the pancreas. Int J Clin Oncol. 2002;7:376–380. doi: 10.1007/s101470200059. [DOI] [PubMed] [Google Scholar]
- 15.Yoshioka M, Uchinami H, Watanabe G, Takahashi T, Nakagawa Y, Andoh H, et al. Effective use of gemcitabine in the treatment of undifferentiated carcinoma with osteoclast-like giant cells of the pancreas with portal vein tumor thrombus. Intern Med. 2012;51:2145–2150. doi: 10.2169/internalmedicine.51.7670. [DOI] [PubMed] [Google Scholar]
- 16.Hrudka J, Lawrie K, Waldauf P, Ciprová V, Moravcová J, Matěj R. Negative prognostic impact of PD-L1 expression in tumor cells of undifferentiated (anaplastic) carcinoma with osteoclast-like giant cells of the pancreas: study of 13 cases comparing ductal pancreatic carcinoma and review of the literature. Virchows Arch. 2020;477:687–696. doi: 10.1007/s00428-020-02830-8. [DOI] [PubMed] [Google Scholar]
- 17.Luchini C, Cros J, Pea A, Pilati C, Veronese N, Rusev B, et al. PD-1, PD-L1, and CD163 in pancreatic undifferentiated carcinoma with osteoclast-like giant cells: expression patterns and clinical implications. Hum Pathol. 2018;81:157–165. doi: 10.1016/j.humpath.2018.07.006. [DOI] [PubMed] [Google Scholar]
- 18.Besaw RJ, Terra AR, Malvar GL, Chapman TR, Hertan LM, Schlechter BL. Durable response to PD-1 blockade in a patient with metastatic pancreatic undifferentiated carcinoma with osteoclast-like giant cells. J Natl Compr Canc Netw. 2021;19:247–252. doi: 10.6004/jnccn.2021.7001. [DOI] [PubMed] [Google Scholar]
- 19.Golan T, Hammel P, Reni M, Van Cutsem E, Macarulla T, Hall MJ, et al. Maintenance olaparib for germline BRCA-mutated metastatic pancreatic cancer. N Engl J Med. 2019;381:317. doi: 10.1056/NEJMoa1903387. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.


