Highlights
-
•
Final results of a randomized controlled phase II trial.
-
•
OS and PFS data of neoadjuvant chemoradiation in pancreatic cancer.
-
•
Combination of cetuximab, gemcitabine and IMRT is safe and feasible.
-
•
Improvement of local tumor control and secondary resection rate by combined maintenance therapy with cetuximab and gemcitabine.
Keywords: Pancreatic cancer, Cetuximab, Locally advanced pancreatic cancer, Chemoradiation, Pancreatic ductal adenocarcinoma
Abstract
Purpose
Addressing the epidermal growth factor receptor (EGFR)-pathway by the competitive receptor ligand cetuximab is a promising strategy in pancreatic cancer. In the prospective randomized controlled phase II PARC-study (PARC: Pancreatic cancer treatment with radiotherapy (RT) and cetuximab), we evaluated safety and efficacy of a trimodal treatment scheme consisting of cetuximab, gemcitabine and RT in locally advanced pancreatic cancer (LAPC).
Methods
Between January 2005 and April 2007, 68 patients with inoperable pancreatic ductal adenocarcinoma were randomized in either trimodal therapy followed by gemcitabine maintenance (Arm A) or in trimodal therapy followed by gemcitabine plus cetuximab maintenance (Arm B). Intensity-modulated RT (IMRT) was performed with a total dose of 45 Gy in 25 fractions and with a simultaneous integrated boost to the gross tumor (54 Gy). Within the trimodal therapy, gemcitabine and cetuximab were administered weekly. Maintenance therapy consisted of gemcitabine only or gemcitabine plus cetuximab. Toxicity, overall survival (OS), secondary resection rate, local control and progression free survival (PFS) were evaluated.
Results
With a median followup time of 13 months (range: 2 – 184 months), one patient is still alive and one patient is lost to follow-up. Nausea and gastrointestinal hemorrhage were the most important higher-graded (>°II) acute and late non-hematological toxicity (13% and 7%). Median OS was 13.1 months without significant difference between both treatment arms (Arm A: 11.9 months; Arm B: 14.2 months). Compared to historical data, cetuximab did not improve OS. One- and two-year local control rates were 76.6% and 68.9%. Local tumor control and secondary resection rate (Arm A: 4%; Arm B: 16%) were significantly improved in Arm B. Median PFS was 6.8 months with distant metastasis as main treatment failure.
Conclusion
Trimodal therapy consisting of IMRT, gemcitabine and cetuximab can be considered safe and feasible. Compared to historical data, cetuximab does not improve treatment efficacy in LAPC patients treated with chemoradiation.
Introduction
Pancreatic cancer is correlated with a dismal prognosis. The five-year overall survival (OS) rate is at only 5–10% with pancreatic cancer being the seventh leading cause of tumor-related deaths worldwide [1]. Surgical resection is the only curative therapy option but the vast majority of all pancreatic cancers are deemed unresectable at time of diagnosis [2]. Unresectable patients present either with distant metastasis or with locally advanced disease. In case of distant metastasis, systemic therapy is the standard of care. In case of locally advanced pancreatic cancer (LAPC), the optimal management is still in discussion.
Several studies evaluated a potential benefit of combining chemotherapy with radiotherapy (RT) in LAPC patients. Whereas the Gastrointestinal Tumor Study Group (GITISG) trial 9283 [3] and the Eastern Cooperative Oncology Group (ECOG) trial E4201 [4] demonstrated a benefit of chemoradiation over chemotherapy alone, the more recent LAP07 trial could not confirm these findings [5]. Although chemoradiation was associated with improved local control in this study, there was no significant OS benefit of chemoradiation over chemotherapy alone. Altogether, data on optimal LAPC therapy remain inconclusive. Therefore, new treatment strategies need to be evolved.
As pancreatic cancer is known for epidermal growth factor receptor (EGFR) overexpression [6], [7], a promising treatment strategy is targeted therapy by EGFR inhibition. Furthermore, EGFR inhibition is supposed to enhance radiation sensitivity [8].
Erlotinib is a tyrosine kinase inhibitor that addresses the catalytic domain of EGFR and has been shown to improve overall survival in pancreatic cancer patients when added to gemcitabine [9], especially in patients who develop skin rash as immunological adverse effect [10].
Cetuximab is a monoclonal antibody that addresses the extracellular domain of the EGFR. A combination of cetuximab and RT has been demonstrated to significantly improve OS in advanced head and neck cancer patients [11]. In pancreatic cancer, convincing results of cetuximab therapy could be observed in preclinical studies [12], [13]. In a phase II study, Xiong et al. observed promising results combining cetuximab and gemcitabine in locally advanced or metastatic pancreatic cancer patients [14]. In contrast, later published large prospective phase II and III trials did not reveal any benefit of cetuximab in advanced pancreatic cancer patients [15], [16].
Nevertheless, the observed induced radiation sensitivity of cetuximab and the promising head and neck cancer results of Bonner et al. [11] emphasize the high potential of targeted therapy with cetuximab in pancreatic cancer, especially in combination with RT.
In the prospective phase II PARC trial, we evaluated safety and efficacy of two different trimodal treatment regimens consisting of cetuximab, gemcitabine and intensity-modulated RT (IMRT) in LAPC patients.
Methods
Study design
PARC (PAncreatic cancer treatment with Radiotherapy and Cetuximab) was a randomized controlled investigator initiated prospective phase II trial. Detailed information about the study design and the study procedures have been described previously [17]. The study was registered at ISRCTN (International Standard Randomized Controlled Trial Number) at May 11th, 2005 (ISCRTN 56652283). The primary objective of PARC was to evaluate feasibility and efficacy of two different trimodal treatment concepts consisting of gemcitabine, RT and cetuximab in LAPC patients. Secondary objectives were OS, response rate, time to progression and resection rate. Treatment consisted of trimodal therapy followed by maintenance treatment with gemcitabine (study arm A) or by combined maintenance therapy with gemcitabine and cetuximab (study arm B).
Patients
Primary inoperable locally advanced pancreatic adenocarcinoma patients without any sign of distant metastasis (cT4 cN0-1 cM0) could be included. The Karnofsky performance status (KPS) scale needed to be at least at 70% and chemotherapy needed to be applicable in these patients (hemoglobin > 10 g/dL, leucocytes > 3000/nL, platelets > 100/nL). Patients suffering from active infections, liver dysfunction, other severe systemic diseases, other severe malignancies, pregnancy, previous pancreatic cancer therapy (chemotherapy, RT or EGFR-targeted therapy), previous antibody therapy, previous experimental therapies (<4 weeks ago), allergy against CT contrast agents, known positive human antichimeric antibody and known allergy against extrinsical proteins could not participate in the study.
Radiotherapy
For radiation planning, patients were fixed by individual immobilization devices (stereotactic setting) in supine position. Patients underwent a contrast-enhanced computed tomography (CT) scan with a slice thickness of 3 mm. Four-dimensional CT scans were not performed on a regular basis. Radiation planning was performed using the inverse treatment planning system KonRad (Siemens Oncology Systems, Concorde USA) to generate step-and-shoot IMRT plans with seven coplanar beams and 50–65 segments.
The gross tumor volume (GTV) was defined as macroscopic tumor on imaging. The clinical target volume (CTV) included an expansion of 1 cm of the GTV as well as the regional lymphatic drainage. The CTV was irradiated with a total dose of 45 Gy in 25 fractions. For the GTV, the dose was mostly simultaneously increased to 54 Gy (boost radiation). The radiation dose was prescribed to the CTV/GTV without a separate PTV margin.
A maximum dose of 45 Gy was accepted in the gastrointestinal (GI) tract. The mean dose in the kidney should not exceed 10 Gy. Two thirds of the kidney should receive less than 20 Gy.
Chemotherapy
Gemcitabine was provided by our institution’s own pharmacy. Gemcitabine 300 mg/m2 body surface area (BSA) were administered weekly, starting the 5th day of RT (days 12, 19, 26, 33, 40). Maintenance chemotherapy was performed with 1000 mg/m2 BSA on days 47, 54 and 61.
Targeted therapy
Cetuximab was provided by Merck KGaA, Darmstadt. Cetuximab 400 mg/m2 BSA was administered intravenously one week prior to RT (day 1). During RT, five further treatments were applied weekly with cetuximab 250 mg/m2 BSA (days 8, 15, 22, 29, 36). In the experimental study arm B, patients received further 12 weekly doses of maintenance therapy with cetuximab 250 mg/m2 BSA, starting on day 43.
Outcome assessment
Follow-up was defined from the first day of radiation until last clinical evaluation or death. Follow-up was performed over a period of at least two years. Blood counts, blood chemistries and clinical examinations were done weekly during study treatment. Toxicity was assessed using the National Cancer Institute Common Toxicity Criteria Toxicity Criteria for Adverse Events (CTCAE) version 3.0. All observed and eventually treatment-related symptoms were included in the analysis. Follow-up imaging was performed every three months by contrast-enhanced CT scans and clinical evaluation. Response evaluation was done according to the RECIST-criteria [18]. Local progression was defined as tumor progression within the radiation area. Any other tumor recurrence was defined as distant tumor recurrence. In addition to the RECIST-criteria, any radiological described suspicion of local tumor progression was counted as progressive disease if it clinically influenced therapy decision.
The OS was defined as time from the start of RT until reported death due to any cause. Local control (LC) was defined from the start of RT until local progression or last imaging available. Freedom from distant metastasis (FFDM) was defined from the start of RT until first occurrence of distant metastasis or last imaging available. Progression free survival (PFS) was defined as time from the start of RT until any tumor progression or death or last imaging available.
Statistical considerations
A sample size of 66 patients was calculated based on the assumption that the one-year OS rate after gemcitabine-based therapy is expected to be at 42% [3], [19] and adding cetuximab increases the one-year OS rate to 67% [9], [11], [14]. Additionally, a drop-out rate of 15% was considered. To analyze the primary objective of the study – toxicity –methods of descriptive data analysis were used. As the sample size was calculated based on the one-year OS rate assumption, OS is the main secondary endpoint which was analyzed by non-parametric Kaplan-Meier estimates. Differences between both study arms were analyzed using the log-rank test. Concerning OS, multivariate Cox-regression was performed for sex, KPS-group, age and GTV. LC, FFDM and PFS were analyzed by non-parametric Kaplan-Meier estimates and log-rank tests, accordingly. Differences in the secondary resection rates of both study arms were analyzed using the Pearson-Chi-Square-test. Statistics and figures were performed with SPSS Statistics, version 27 (International Business Machines Corporation: IBM, New York, USA).
Ethics
The study was approved by the Ethics Committee of the University of Heidelberg, Germany (L-283/2004).
Results
Patient and treatment characteristics
Between January 2005 and May 2007, 68 patients were randomly assigned to study arm A (trimodal therapy with gemcitabine maintenance, n = 35) or study arm B (trimodal therapy with gemcitabine and cetuximab maintenance, n = 33). RT was performed as IMRT in all cases. Apart from two cases, all patients were irradiated with a total dose of 54 Gy to the tumor and with 45 Gy to the lymphatic drainage in 25 fractions. In one patient, RT was stopped after 24 fractions due to a gastrointestinal bleeding. The other patient denied the continuation of RT after 21 fractions. One patient developed an acute appendicitis during RT and underwent resection leading to an irradiation pause of six days.
In both study arms, patients were concomitantly treated with a median of six cycles targeted therapy with cetuximab 250 mg/m2 BSA after an induction treatment of 400 mg/m2 BSA one week prior to irradiation. Additionally, a median of five cycles chemotherapy with gemcitabine 300 mg/m2 BSA were applied. After RT, in study arm A, maintenance therapy consisted of a median of three weekly administrations of gemcitabine 1000 mg/m2 BSA. In study arm B, maintenance therapy additionally consisted of a median of 11 weekly administrations of targeted therapy with cetuximab 250 mg/m2 BSA.
During the treatment procedure, targeted therapy was interrupted in nine cases, mostly due to systemic infections. Chemotherapy was interrupted in 35 patients, because of systemic infections or leukopenia.
The treatment compliance was very good. All patients at least started RT and all patients underwent concomitant therapy. Ninety-six percent of the patients underwent maintenance chemotherapy and 97% of the patients of study arm B underwent maintenance cetuximab therapy.
Patient and treatment characteristics are demonstrated in Table 1, Table 2.
Table 1.
Patient and tumor characteristics.
| Arm A | Arm B | Total | (%) | |
|---|---|---|---|---|
| Number of patients | 35 | 33 | 68 | (1 0 0) |
| Sex | ||||
| Male | 21 | 17 | 38 | (56) |
| Female | 14 | 16 | 30 | (44) |
| Age at randomisation (median in years, range) | 61 (31–76) | 63 (47–80) | 62 (31–80) | |
| Karnofsky performance status | ||||
| 100 | 5 | 7 | 12 | (18) |
| 90 | 13 | 10 | 23 | (34) |
| 80 | 11 | 11 | 22 | (32) |
| 70 | 6 | 5 | 11 | (16) |
| Body mass index (median in kg/m2, range) | ||||
| Start of study | 24.8 (17.6–37.2) | 25.2 (19.1–34.1) | 25.1 (17.6–37.2) | |
| End of radiotherapy | 23.5 (15.6–37.2) | 24.1 (18.6–32.4) | 23.6 (15.6–37.2) | |
| Histology | ||||
| Ductal adenocarcinoma | 21 | 16 | 37 | (54) |
| Not performed | 14 | 17 | 31 | (46) |
| Localization of the tumor | ||||
| Pancreatic head | 20 | 21 | 41 | (60) |
| Pancreatic body | 14 | 11 | 25 | (37) |
| Pancreatic tail | 1 | 1 | 2 | (3) |
| Previous therapy | ||||
| Gemcitabine | 0 | 2 | 2 | (3) |
| None | 35 | 31 | 66 | (97) |
Table 2.
Treatment characteristics.
| Arm A | Arm B | Total | (%) | |
|---|---|---|---|---|
| Radiotherapy | 35 | 33 | 68 | (100) |
| Time in days: diagnosis to radiotherapy (median, range) | 36 (17–147) | 34 (20–99) | 35 (17–147) | |
| Radiation technique: Intensity-modulated radiotherapy (IMRT) | 35 | 33 | 68 | (1 0 0) |
| Irradiation dose | ||||
| Tumor: 54 Gy / Lymphatic drainage: 45 Gy | 34 | 32 | 66 | (97) |
| Tumor: 51.8 Gy / Lymphatic drainage: 43.2 Gy | 1 | 0 | 1 | (1) |
| Tumor: 45.4 Gy / Lymphatic drainage: 37.8 Gy | 0 | 1 | 1 | (1) |
| Irradiation boost concept | ||||
| Simultaneous integrated boost radiation | 34 | 32 | 66 | (97) |
| Sequentially applicated boost radiation | 1 | 1 | 2 | (3) |
| Volume in ccm (median, range) | ||||
| Gross tumor volume: GTV (boost volume) | 203 (46–443) | 182 (83–294) | 191 (46–443) | |
| Clinical tumor volume: CTV (including lymphatic drainage) | 518 (210–1001) | 569 (215–957) | 541 (210–1001) | |
| Interruption of radiotherapy | 6 | 6 | 12 | (18) |
| 1 day | 4 | 3 | 7 | (10) |
| 2 days | 2 | 2 | 4 | (6) |
| > 2 days | 0 | 1 | 1 | (1) |
| Concomitant chemotherapy | 35 | 33 | 68 | (1 0 0) |
| Gemcitabine 300 mg/m2 body surface area (BSA) weekly | ||||
| Number of cycles (median, range) | 5 (3–6) | 5 (2–6) | 5 (2–6) | |
| Concomitant targeted therapy | 35 | 33 | 68 | (1 0 0) |
| Cetuximab 400 mg/m2 BSA one week prior to RT | ||||
| Cetuximab 250 mg/m2 BSA weekly | ||||
| Number of cycles (median, range) | 6 (3–7) | 6 (3–7) | 6 (3–7) | |
| Maintenance chemotherapy | 34 | 31 | 65 | (96) |
| Gemcitabine 1000 mg/m2 BSA weekly | ||||
| Number of cycles (median, range) | 3 (0–7) | 3 (0–4) | 3 (0–7) | |
| Maintenance targeted therapy | 0 | 32 | 32 | (47) |
| Cetuximab 250 mg/m2 BSA weekly | ||||
| Number of cycles (median, range) | 0 | 11 (2–15) | 11 (2–15) | |
| Secondary surgical intervention | 21 | 19 | 40 | (59) |
| Exploratory laparotomy | 18 | 8 | 26 | (38) |
| Tumor resection | 3 | 11 | 14 | (21) |
| R0 | 2 | 5 | 7 | (10) |
| R1 | 1 | 6 | 7 | (10) |
| Intraoperative radiotherapy | 5 | 8 | 13 | (19) |
| 12 Gy | 1 | 2 | 3 | (4) |
| 15 Gy | 4 | 6 | 10 | (15) |
| Post-study treatment | ||||
| Chemotherapy (different regimens) | 20 | 24 | 44 | (65) |
| No further treatment | 2 | 2 | 4 | (6) |
| Unknown | 13 | 7 | 20 | (29) |
Follow-up
Median follow-up time was 13.0 months (range: 2 – 184 months) with one patient being still alive and one patient lost to follow-up. Median time interval from the start of RT until the last imaging available was 8 months.
Overall survival (OS)
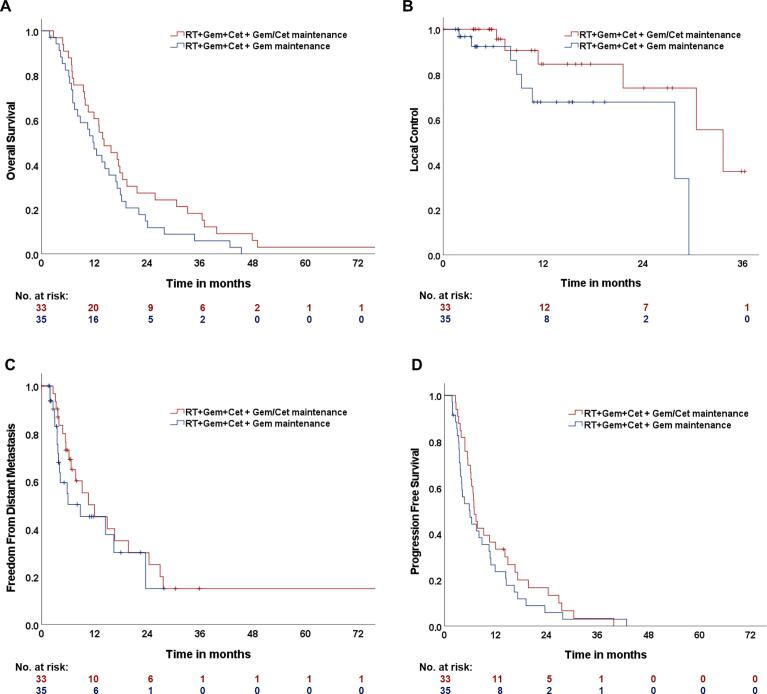
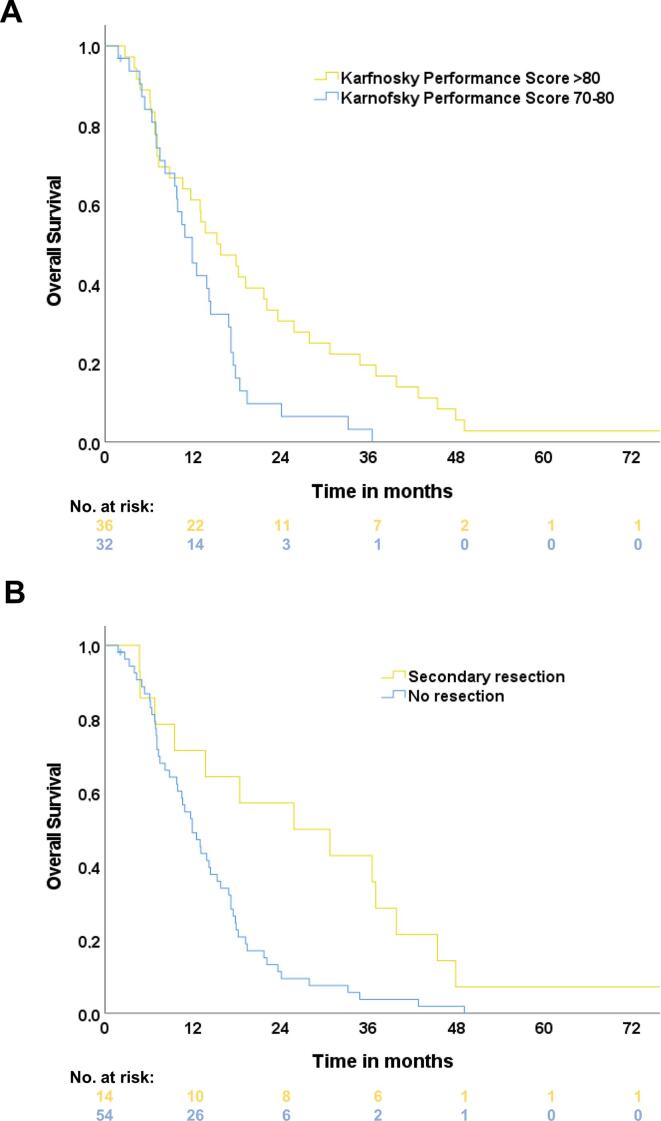
The estimated median OS of the study population was 13.1 months (95%-Confidence interval (CI): 10.6–15.6 months, one-year OS: 53.7%, two-year OS: 20.9%, Fig. 1A). The median OS of the participants treated in study arm A was 11.9 months (CI: 9.1 – 14.7 months). The patients treated with additional cetuximab maintenance (study arm B) achieved a median OS of 14.2 months (CI: 9.5 – 18.9 months). The log-rank test did not show any statistically significant difference between both treatment arms (p = 0.11). The estimated one-year OS rate was at 47.1% (study arm A) vs. 60.6% (study arm B) and the according two-year OS rate was at 14.7% (A) vs. 27.3% (B). Patients with an initial KPS of 90–100% achieved a median OS of 15.3 months (CI: 8.2 – 22.4 months) compared to 13.1 months (CI: 10.6 – 15.6) when presenting with an KPS of 70–80% (p = 0.01, log-rank, Fig. 2A). Multivariate Cox-regression analyzes of sex, age, body mass index at therapy start and tumor size (GTV) did not show any significant difference.
Fig. 1.
Kaplan-Meier estimates of A Overall survival (OS), B Local control (LC) C Freedom from distant metastasis (FFDM) and D Progression free survival (PFS) of the study population consisting of 68 patients. Study treatment consisted of radiotherapy (RT), gemcitabine (Gem) and cetuximab (Cet), followed by Gem maintenance therapy (study arm A, illustrated in blue) or followed by Gem/Cet maintenance therapy (study arm B, illustrated in red). (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
Fig. 2.
Kaplan-Meier overall survival (OS) estimates separated by pre-defined and stratified Karnofsky Performance Score (KPS) status group (A). Group 1 (yellow) consisted of patients with a KPS > 80. Group 2 (blue) consisted of patients with a KPS 70–80. B OS estimates separated by patients undergoing secondary oncological resection (yellow) or no resection (blue). (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
Tumor response and secondary resection rate
During follow-up, 14 patients developed local tumor progression (study arm A: n = 8, study arm B: n = 6, Fig. 1B). The overall estimated one- and two-year local control (LC) rates were 76.6% and 68.9%. Local tumor control of the participants of study arm B was significantly increased compared to those of study arm A (p = 0,04, log-rank).
Fifty patients presented at least with stable disease, according to RECIST criteria. In 4 patients, tumor response could not be assessed due to missing adequate follow-up imaging. Forty of the patients underwent secondary surgical intervention (A: n = 21, B: n = 19). Oncological resection could be achieved in 14 patients (A: n = 3, B: n = 11), of whom 7 patients underwent R0-resection (A: n = 2, B: n = 5). In 13 of the 40 secondary resected patients, intraoperative RT with a median of 15 Gy was performed during resection. Patients that underwent secondary oncological resection achieved a median OS of 25.8 months (CI: 3.2 – 48.4 months) compared to 11.9 months without secondary resection (CI: 9.4 – 14.4, p = 0.004, log-rank, Fig. 2B).
The probability to undergo oncological resection was statistically significantly higher in study arm B compared to study arm A (16% vs. 4%, p = 0.01, Pearson-Chi-Square).
Freedom from distant metastasis (FFDM) and progression free survival (PFS)
Thirty-seven patients (54%) developed distant metastasis (Fig. 1C). The three most frequent locations of metastasis were peritoneum (n = 18), liver (n = 15) and lung (n = 6). The median time of FFDM was 10.6 months (CI: 3.5 – 17.7 months). Patients treated in study arm A demonstrated shorter FFDM compared to those of study arm B (8.8 vs 12.0 months, p = 0.38, log-rank).
The median PFS of the study population was 6.8 months (CI: 5.4 – 8.2 months, Fig. 1D). There was a trend towards an improved PFS for the patients of study arm B (7.0 vs 6.0 months, p = 0.29, log-rank). The overall one- and two-year PFS rates were at 28.4% and 11.0%.
Toxicity
The most severe observed hematological toxicity was leukopenia (°III-IV: 62% of all patients). The second most frequent severe hematological toxicity was anemia (°III-IV: 23%). However, there were no relevant differences between both study arms. Hematological toxicity is summarized in Table 3.
Table 3.
Hematological toxicity.
| Hematological toxicity (NCI CTCAE °II-IV) | Total, n (%) |
Arm A, n (%) |
Arm B, n (%) |
||||||
|---|---|---|---|---|---|---|---|---|---|
| °II | °III | °IV | °II | °III | °IV | °II | °III | °IV | |
| Alcaline phosphatase (↑) | 11 (16) | 6 (9) | 0 | 5 (7) | 2 (3) | 0 | 6 (9) | 4 (6) | 0 |
| Bilirubin (↑) | 8 (12) | 4 (6) | 1 (1) | 3 (4) | 2 (3) | 2 (3) | 5 (7) | 2 (3) | 0 |
| Aspartate AT* (↑) | 13 (19) | 7 (10) | 2 (3) | 5 (7) | 3 (4) | 3 (4) | 8 (12) | 4 (6) | 0 |
| Alanine AT* (↑) | 22 (32) | 12 (18) | 1 (1) | 12 (18) | 6 (9) | 1 (1) | 10 (15) | 6 (9) | 0 |
| Hemoglobin (↓) | 35 (51) | 13 (19) | 3 (4) | 17 (25) | 9 (13) | 1 (1) | 18 (26) | 4 (6) | 2 (3) |
| Leukocytes (↓) | 20 (29) | 30 (44) | 12 (18) | 7 (10) | 17 (25) | 8 (12) | 13 (19) | 13 (19) | 4 (6) |
| Platelets (↓) | 15 (22) | 5 (7) | 1 (1) | 8 (12) | 2 (3) | 1 (1) | 7 (10) | 3 (4) | 0 |
*Aminotransferase.
Non-hematological toxicity consisted mostly of an increase of GI symptoms during the treatment period such as diarrhea, nausea and vomiting (Table 4). These symptoms were observed to decrease after the end of study. Abdominal pain was described more frequently before the study treatment than during follow-up. Furthermore, 35% of all patients reported no symptoms or no new symptoms during follow-up.
Table 4.
Non-hematological toxicity.
| Symptoms+ (NCI CTCAE grades) |
Before RT*, n (%) |
Acute toxicity, n (%) |
Late toxicity, n (%) |
||||||
|---|---|---|---|---|---|---|---|---|---|
| Total | Arm A | Arm B | Total | Arm A | Arm B | Total | Arm A | Arm B | |
| Abdominal pain | |||||||||
| °I | 18 (26) | 10 (15) | 8 (12) | 0 | 0 | 0 | 5 (7) | 2 (3) | 3 (4) |
| °II | 21 (31) | 12 (18) | 9 (13) | 10 (15) | 8 (12) | 2 (3) | 9 (13) | 4 (6) | 5 (7) |
| °III | 1 (1) | 0 | 1 (1) | 0 | 0 | 0 | 0 | 0 | 0 |
| GI1 hemorrhage | |||||||||
| °I | 0 | 0 | 0 | 2 (3) | 0 | 2 (3) | 0 | 0 | 0 |
| °II | 1 (1) | 1 (1) | 0 | 0 | 0 | 0 | 2 (3) | 2 (3) | 0 |
| °III | 0 | 0 | 0 | 1 (1) | 1 (1) | 0 | 4 (6) | 3 (4) | 1 (1) |
| °V | 0 | 0 | 0 | 0 | 0 | 1 (1) | 0 | 0 | 0 |
| Diarrhea | |||||||||
| °I | 2 (3) | 1 (1) | 1 (0) | 3 (4) | 2 (3) | 1 (1) | 2 (3) | 2 (3) | 0 |
| °II | 3 (4) | 3 (4) | 0 | 7 (10) | 4 (6) | 3 (4) | 6 (9) | 6 (9) | 0 |
| °III | 1 (1) | 1 (1) | 0 | 2 (3) | 1 (1) | 1 (1) | 0 | 0 | 0 |
| Nausea | |||||||||
| °I | 4 (6) | 2 (3) | 2 (3) | 5 (7) | 2 (3) | 3 (4) | 4 (6) | 2 (3) | 2 (3) |
| °II | 3 (4) | 2 (3) | 1 (1) | 19 (28) | 12 (18) | 7 (10) | 1 (1) | 1 (1) | 0 |
| °III | 0 | 0 | 0 | 9 (13) | 3 (4) | 6 (9) | 0 | 0 | 0 |
| Vomiting | |||||||||
| °II | 2 (3) | 1 (1) | 1 (1) | 17 (25) | 8 (12) | 9 (13) | 1 (1) | 0 | 1 (1) |
| Constipation | |||||||||
| °I | 0 | 0 | 0 | 0 | 0 | 0 | 1 (1) | 0 | 1 (1) |
| Flatulence | |||||||||
| °I | 5 (7) | 4 (6) | 1 (1) | 0 | 0 | 0 | 1 (1) | 1 (1) | 0 |
| Ileus | |||||||||
| °III | 0 | 0 | 0 | 0 | 0 | 0 | 2 (3) | 0 | 2 (3) |
| Fatigue | |||||||||
| °I | 6 (9) | 2 (3) | 4 (6) | 6 (9) | 5 (7) | 1 (1) | 3 (4) | 2 (3) | 1 (1) |
| °II | 4 (6) | 1 (1) | 3 (4) | 4 (6) | 2 (3) | 2 (3) | 0 | 0 | 0 |
| Dizziness | |||||||||
| °I | 0 | 0 | 0 | 1 (1) | 1 (1) | 0 | 0 | 0 | 0 |
| Depression | |||||||||
| °II | 0 | 0 | 0 | 3 (4) | 1 (1) | 2 (3) | 0 | 0 | 0 |
| Cholangitis | |||||||||
| °III | 0 | 0 | 0 | 1 (1) | 0 | 1 (1) | 0 | 0 | 0 |
| Appendicitis | |||||||||
| °III | 0 | 0 | 0 | 1 (1) | 0 | 1 (1) | 0 | 0 | 0 |
| Allergic reaction | |||||||||
| °III | 0 | 0 | 0 | 4 (6) | 0 | 4 (6) | 0 | 0 | 0 |
| Skin rash | |||||||||
| °I | 0 | 0 | 0 | 30 (44) | 16 (24) | 14 (21) | 0 | 0 | 0 |
| °II | 0 | 0 | 0 | 10 (15) | 5 (7) | 5 (7) | 1 (1) | 1 (1) | 0 |
| Nail changes | |||||||||
| °I | 0 | 0 | 0 | 3 (4) | 0 | 3 (4) | 0 | 0 | 0 |
| °II | 0 | 0 | 0 | 3 (4) | 0 | 3 (4) | 0 | 0 | 0 |
| Radiation dermatitis | |||||||||
| °I | 0 | 0 | 0 | 2 (3) | 1 (1) | 1 (1) | 0 | 0 | 0 |
| No / no new symptoms | 7 (10) | 3 (4) | 4 (6) | 0 | 0 | 0 | 24 (35) | 11 (16) | 13 (19) |
*Radiotherapy.
including all possibly treatment-related symptoms during the study observation.
Gastrointestinal.
One patient developed biliary stent occlusion during the treatment period that led to cholangitis and bacteremia which needed intervention. In this case, study treatment was not influenced and could be continued as planned. The patient recovered well after the implantation of a new biliary stent.
Two patients of study arm B developed ileus °III. In one of these patients, the symptoms occurred 4 months after the end of RT and came along with a tumor marker increase which was observed several weeks later. Under conservative therapy, the symptoms decreased and chemotherapy was re-initiated due to the tumor marker increase. The second patient developed ileus °III 10 months after the end of RT. At this time, the patient was treated with chemotherapy due to peritoneal carcinomatosis. There was a stenosis of the duodenum leading to a secondary gastric ulcer that was treated conservatively. A gastric tube was installed. Several days after the first symptoms, local tumor progression was observed which is the most probable reason for the ileus.
In total, four GI hemorrhages °III and one °V bleeding could be observed. The patient who died due to GI bleeding, developed the symptoms at the end of RT. Irradiation was stopped at 51.8 Gy (24 fractions) and the patient was successfully embolized. Four weeks later, the patient presented in the emergency department due to another GI bleeding episode and the patient was transferred to the intensive care unit. Several interventional attempts were made but the bleeding could not be stopped. Intraoperatively, the source of the bleeding was found to be the tumor in the pancreatic head that infiltrated the pancreaticoduodenal arteria. Also, the initial symptom that led to the diagnosis of pancreatic cancer had been GI bleeding in this case. Therefore, treatment-related toxicity is not very likely but it cannot be excluded, either. Two other patients presented with self-limiting GI bleedings, most probably due to overdosing of active anticoagulant therapy. Another patient developed a lower GI bleeding four years after the end of RT. The source of the bleeding could not be found but this patient needed red blood cell transfusions. At the time of the GI bleeding, the tumor had already metastasized in lung and liver and in imaging, progressive disease could be observed. Two months later, the patient deceased. Six months after RT, another patient developed upper GI bleeding that occurred along with local tumor progression. Due to the palliative overall situation, further diagnostics were not performed. As differential diagnosis to tumorous bleeding in this certain case, an intrahepatic pseudoaneurysm was suspected.
Cetuximab-induced skin rash was seen frequently (°I-II: 59%). Nail changes were less common (°I-II: 8%). Skin rash was successfully treated with topic antibiotic application of nadifloxacin or aureomycin. A systemic allergic reaction to cetuximab could be observed in 4 patients. Under pre-treatment with corticosteroids, all of them could continue the study treatment without further symptoms. Concerning skin rash, there was no difference between the study arms. Allergic reactions and nail changes were observed only in study arm B at the beginning of cetuximab therapy.
Discussion
The present PARC-study aimed to evaluate safety and efficacy of a trimodal treatment scheme consisting of IMRT, gemcitabine and cetuximab in LAPC patients. Final results were discussed at the annual meeting of the American Society of Clinical Oncology (ASCO) in 2008 [20]. Here, we present the first full paper demonstrating the final results of this randomized controlled trial.
The study demonstrates the safety and feasibility of the treatment approach with gemcitabine-based chemoradiation in combination with the EGFR-targeting antibody cetuximab. Skin rash, acne and allergic reactions are well-known adverse events in cetuximab therapy. In the present study, cetuximab-specific toxicity rates were comparable to those described in the S0205-study in which a combination of cetuximab and gemcitabine versus gemcitabine monotherapy in 745 advanced pancreatic ductal adenocarcinoma (PDAC) patients was evaluated [15]. Cetuximab therapy was tolerable and did not lead to treatment abortion or long-time toxicity in the present population. Furthermore, a prolongation of cetuximab therapy as dominant part of the maintenance treatment strategy (study arm B) did not increase toxicity.
Compared to the results of the large LAP07-trial [5], hematological liver toxicity was not increased in the present study. Anemia and leukopenia were observed more frequently in the present population than leukopenia (or neutrophil count) and anemia in the trials LAP07, E4201 and in S0205 [4], [5], [15]. This could partially be explained by the long observation period with weekly blood counts in the present study making it more probable to reach the definition of °III toxicity at one of the examined days.
The overall observed higher-graded (> °II) non-hematological toxicity was in most of the cases explicable by tumor progression or other confounding factors such as anticoagulant therapy. Therefore, toxicity rates should not be considered as purely treatment-related. Nevertheless, the observation of higher-graded GI toxicity induced by chemoradiation are in line with the data of the LAP07-trial [5]. Seven percent of the study population developed GI hemorrhage °III during follow-up which is comparable to the ECOG E4201 results published by Loehrer et al. [4]. However, in the E4201-trial, RT was limited to a total dose of 50.4 Gy, compared to 54.0 Gy in the present study. The safe application of the dose concept may be attributable to the fact that all patients received an IMRT as a very conformal radiation technique that was not yet standard of care at the time of the study.
Altogether, the PARC-study treatment was tolerated well and can be considered as safely applicable. The high rates of treatment completions demonstrate the feasibility of the protocol.
Twelve years after the end of study, all but one patient deceased and only one patient was lost to follow-up. This incident strengthens the scientific value of the observed OS results. Even though OS was not the primary endpoint of the study, the sample size calculation was based on OS rate assumptions highlighting the importance of the OS results. With a median overall OS of 13.1 months, the results of the present study are in line with comparable trials testing chemoradiation in LAPC patients reporting a median OS of 9.5 – 15.5 months [3], [4], [5], [21], [22], [23], [24], [25]. An OS improvement by adding cetuximab to chemoradiation could therefore not be hypothesized. However, there was a statistical trend towards an OS improvement by adding cetuximab to the gemcitabine maintenance therapy (study arm B), although a statistical significance could not be achieved.
The secondary resection rate and the local tumor control were significantly improved by adding cetuximab to the gemcitabine maintenance therapy but the study was not powered for these endpoints. However, the overall secondary resection rate of the population remains improvable. In LAPC patients, secondary resection rates of 20–30% are reported [26]. All unresectable cases were deemed suitable for study participation. Therefore, the study cohort retrospectively could have included nowadays defined as borderline resectable tumors.
As approximately one third of all PDAC associated deaths are due to local disease burden [27], local tumor control is of great importance in LAPC therapy. In the LAP07-trial, chemoradiation could be demonstrated to be significantly more effective than chemotherapy alone concerning local control [5]. The observed one- and two-year LC rates of the present study highlight the potential of chemoradiation to reduce local disease burden in LAPC. However, with a median PFS of 6.8 months, distant metastasis remains the main cause of tumor progression.
The treatment scheme of the present PARC-study is not part of recent first-line therapy decisions in LAPC patients, which is the major limitation of the data. In the last years, much more effective treatment regimens evolved such as FOLFIRINOX with reported median OS of 24.2 months and median PFS of 15 months, compared to 13.1 months and 6.8 months observed in the presented data [26]. Furthermore, RT has significantly improved since the end of the study and modern radiation techniques such as stereotactic body RT or carbon ion RT could probably be much more effective in LAPC therapy with described median OS of up to 18–20 months [28], [29]. Using these novel radiation techniques, it is possible to increase the radiation dose to the tumor while preserving the dose limits to the adjacent organs at risk [30], [31]. The latter is crucial to avoid higher-graded GI toxicity [32].
Furthermore, staging procedures of the present study are not consistent with the current diagnostic standard which aggravates the transfer of the study results to pancreatic cancer patients presenting today.
Another important limitation of the presented study, is the high rate of patients without pathological confirmation of the suspected pancreatic cancer. However, the majority of these patients developed distant metastasis in the course of the disease. Furthermore, the presented OS results of the study are in line with similar pancreatic patient cohorts assuming the correct diagnosis even in the cases in which histological confirmation was not achieved.
In conclusion, the presented PARC-trial provides reliable OS and toxicity data of LAPC patients treated with trimodal therapy consisting of IMRT, gemcitabine and cetuximab. The treatment scheme can be considered safe and feasible. Based on the presented data, in patients treated by this protocol, maintenance therapy should include gemcitabine and cetuximab.
Conflict of Interest Notification
Juergen Debus received grants from Merck Serono GmbH, The Clinical Research Institute GmbH (CRI), View Ray Inc., Accuray Incorporated, RaySearch Laboratories AB, Vision RT limited, Astellas Pharma GmbH, Astra Zeneca GmbH, Solution Akademie GmbH, Ergomed PLC Surrey Research Park, Siemens Healthcare GmbH, Quintiles GmbH, Pharmaceutical Research Associates GmbH, Boehringer Ingelheim Pharma GmbH Co, PTW-Freiburg Dr. Pychlau GmbH and Nanobiotix A.A. outside the submitted work. Amir Abdollahi reports grants from Merck and EMD, grants and from Fibrogen, BMS and Roche outside the submitted work. The other authors declare no conflict of interest.
Funding
Jakob Liermann is funded by the Physician-Scientist Program of Heidelberg University, Faculty of Medicine. Cetuximab was supplied by Merck KGaA, Frankfurter Str. 250, 64,293 Darmstadt, Germany.
References
- 1.Rawla P., Sunkara T., Gaduputi V. Epidemiology of pancreatic cancer: global trends, etiology and risk factors. World J Oncol. 2019;10(1):10–27. doi: 10.14740/wjon1166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ryan D.P., Hong T.S., Bardeesy N. Pancreatic adenocarcinoma. N Engl J Med. 2014;371(11):1039–1049. doi: 10.1056/NEJMra1404198. [DOI] [PubMed] [Google Scholar]
- 3.Treatment of locally unresectable carcinoma of the pancreas: comparison of combined-modality therapy (chemotherapy plus radiotherapy) to chemotherapy alone. Gastrointestinal Tumor Study Group. J Natl Cancer Inst. 1988;80(10):751-755. [PubMed]
- 4.Loehrer P.J., Feng Y., Cardenes H., Wagner L., Brell J.M., Cella D., et al. Gemcitabine alone versus gemcitabine plus radiotherapy in patients with locally advanced pancreatic cancer: an Eastern Cooperative Oncology Group trial. J Clin Oncol. 2011;29(31):4105–4112. doi: 10.1200/JCO.2011.34.8904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hammel P., Huguet F., van Laethem J.-L., Goldstein D., Glimelius B., Artru P., et al. Effect of chemoradiotherapy vs chemotherapy on survival in patients with locally advanced pancreatic cancer controlled after 4 months of gemcitabine with or without erlotinib: the LAP07 randomized clinical trial. JAMA. 2016;315(17):1844. doi: 10.1001/jama.2016.4324. [DOI] [PubMed] [Google Scholar]
- 6.Korc M., Chandrasekar B., Yamanaka Y., Friess H., Buchier M., Beger H.G. Overexpression of the epidermal growth factor receptor in human pancreatic cancer is associated with concomitant increases in the levels of epidermal growth factor and transforming growth factor alpha. J Clin Invest. 1992;90(4):1352–1360. doi: 10.1172/JCI116001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Xiong H.Q., Abbruzzese J.L. Epidermal growth factor receptor-targeted therapy for pancreatic cancer. Semin Oncol. 2002;29(5 Suppl 14):31–37. doi: 10.1053/sonc.2002.35645. [DOI] [PubMed] [Google Scholar]
- 8.Huang S.M., Harari P.M. Modulation of radiation response after epidermal growth factor receptor blockade in squamous cell carcinomas: inhibition of damage repair, cell cycle kinetics, and tumor angiogenesis. Clin Cancer Res. 2000;6(6):2166–2174. [PubMed] [Google Scholar]
- 9.Moore M.J., Goldstein D., Hamm J., Figer A., Hecht J.R., Gallinger S., et al. Erlotinib plus gemcitabine compared with gemcitabine alone in patients with advanced pancreatic cancer: a phase III trial of the National Cancer Institute of Canada Clinical Trials Group. J Clin Oncol. 2007;25(15):1960–1966. doi: 10.1200/JCO.2006.07.9525. [DOI] [PubMed] [Google Scholar]
- 10.Aranda E., Manzano J.L., Rivera F., Galán M., Valladares-Ayerbes M., Pericay C., et al. Phase II open-label study of erlotinib in combination with gemcitabine in unresectable and/or metastatic adenocarcinoma of the pancreas: relationship between skin rash and survival (Pantar study) Ann Oncol. 2012;23(7):1919–1925. doi: 10.1093/annonc/mdr560. [DOI] [PubMed] [Google Scholar]
- 11.Bonner J.A., Harari P.M., Giralt J., Azarnia N., Shin D.M., Cohen R.B., et al. Radiotherapy plus cetuximab for squamous-cell carcinoma of the head and neck. N Engl J Med. 2006;354(6):567–578. doi: 10.1056/NEJMoa053422. [DOI] [PubMed] [Google Scholar]
- 12.Tai C.-J., Wang H., Wang C.-K., Tai C.-J., Huang M.-T., Wu C.-H., et al. Bevacizumab and cetuximab with conventional chemotherapy reduced pancreatic tumor weight in mouse pancreatic cancer xenografts. Clin Exp Med. 2017;17(2):141–150. doi: 10.1007/s10238-016-0409-2. [DOI] [PubMed] [Google Scholar]
- 13.Larbouret C., Robert B., Bascoul-Mollevi C., Penault-Llorca F., Ho-Pun-Cheung A., Morisseau S., et al. Combined cetuximab and trastuzumab are superior to gemcitabine in the treatment of human pancreatic carcinoma xenografts. Ann Oncol. 2010;21(1):98–103. doi: 10.1093/annonc/mdp496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Xiong H.Q., Rosenberg A., LoBuglio A., Schmidt W., Wolff R.A., Deutsch J., et al. Cetuximab, a monoclonal antibody targeting the epidermal growth factor receptor, in combination with gemcitabine for advanced pancreatic cancer: a multicenter phase II Trial. J Clin Oncol. 2004;22(13):2610–2616. doi: 10.1200/JCO.2004.12.040. [DOI] [PubMed] [Google Scholar]
- 15.Philip P.A., Benedetti J., Corless C.L., Wong R., O'Reilly E.M., Flynn P.J., et al. Phase III study comparing gemcitabine plus cetuximab versus gemcitabine in patients with advanced pancreatic adenocarcinoma: Southwest Oncology Group-directed intergroup trial S0205. J Clin Oncol. 2010;28(22):3605–3610. doi: 10.1200/JCO.2009.25.7550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cascinu S., Berardi R., Labianca R., Siena S., Falcone A., Aitini E., et al. Cetuximab plus gemcitabine and cisplatin compared with gemcitabine and cisplatin alone in patients with advanced pancreatic cancer: a randomised, multicentre, phase II trial. Lancet Oncol. 2008;9(1):39–44. doi: 10.1016/S1470-2045(07)70383-2. [DOI] [PubMed] [Google Scholar]
- 17.Krempien R., Muenter M.W., Huber P.E., Nill S., Friess H., Timke C., et al. Randomized phase II–study evaluating EGFR targeting therapy with cetuximab in combination with radiotherapy and chemotherapy for patients with locally advanced pancreatic cancer–PARC: study protocol [ISRCTN56652283] BMC Cancer. 2005;5(1) doi: 10.1186/1471-2407-5-131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Therasse P., Arbuck S.G., Eisenhauer E.A., Wanders J., Kaplan R.S., Rubinstein L., et al. New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst. 2000;92(3):205–216. doi: 10.1093/jnci/92.3.205. [DOI] [PubMed] [Google Scholar]
- 19.Burris H.A., Moore M.J., Andersen J., Green M.R., Rothenberg M.L., Modiano M.R., et al. Improvements in survival and clinical benefit with gemcitabine as first-line therapy for patients with advanced pancreas cancer: a randomized trial. J Clin Oncol. 1997;15(6):2403–2413. doi: 10.1200/JCO.1997.15.6.2403. [DOI] [PubMed] [Google Scholar]
- 20.Munter M., Timke C., Abdollahi A., et al. Final results of a phase II trial [PARC-Study ISRCTN56652283] for patients with primary inoperable locally advanced pancreatic cancer combining intensity modulated radiotherapy (IMRT) with cetuximab and gemcitabine. J Clin Oncol. 2008;26(15) [Google Scholar]
- 21.Combs S.E., Habermehl D., Kessel K., Bergmann F., Werner J., Brecht I., et al. Intensity modulated radiotherapy as neoadjuvant chemoradiation for the treatment of patients with locally advanced pancreatic cancer. Outcome analysis and comparison with a 3D-treated patient cohort. Strahlenther Onkol. 2013;189(9):738–744. doi: 10.1007/s00066-013-0391-5. [DOI] [PubMed] [Google Scholar]
- 22.Huang W.K., Kuo Y.C., Tsang N.M., et al. Concurrent chemoradiotherapy with or without induction chemotherapy versus chemotherapy alone in patients with locally advanced pancreatic cancer. Anticancer Res. 2014;34(11):6755–6761. [PubMed] [Google Scholar]
- 23.Mattiucci G.C., Morganti A.G., Valentini V., Ippolito E., Alfieri S., Antinori A., et al. External beam radiotherapy plus 24-hour continuous infusion of gemcitabine in unresectable pancreatic carcinoma: long-term results of a phase II study. Int J Radiat Oncol Biol Phys. 2010;76(3):831–838. doi: 10.1016/j.ijrobp.2009.02.013. [DOI] [PubMed] [Google Scholar]
- 24.Cardenes H.R., Moore A.M., Johnson C.S., Yu M., Helft P., Chiorean E.G., et al. A phase II study of gemcitabine in combination with radiation therapy in patients with localized, unresectable, pancreatic cancer: a Hoosier Oncology Group study. Am J Clin Oncol. 2011;34(5):460–465. doi: 10.1097/COC.0b013e3181e9c103. [DOI] [PubMed] [Google Scholar]
- 25.Okusaka T., Ito Y., Ueno H., Ikeda M., Takezako Y., Morizane C., et al. Phase II study of radiotherapy combined with gemcitabine for locally advanced pancreatic cancer. Br J Cancer. 2004;91(4):673–677. doi: 10.1038/sj.bjc.6602001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Suker M., Beumer B.R., Sadot E., Marthey L., Faris J.E., Mellon E.A., et al. FOLFIRINOX for locally advanced pancreatic cancer: a systematic review and patient-level meta-analysis. Lancet Oncol. 2016;17(6):801–810. doi: 10.1016/S1470-2045(16)00172-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Iacobuzio-Donahue C.A., Fu B., Yachida S., Luo M., Abe H., Henderson C.M., et al. DPC4 gene status of the primary carcinoma correlates with patterns of failure in patients with pancreatic cancer. J Clin Oncol. 2009;27(11):1806–1813. doi: 10.1200/JCO.2008.17.7188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Teriaca M.A., Loi M., Suker M., Eskens F., van Eijck C.H.J., Nuyttens J.J. A phase II study of stereotactic radiotherapy after FOLFIRINOX for locally advanced pancreatic cancer (LAPC-1 trial): Long-term outcome. Radiother Oncol. 2021;155:232–236. doi: 10.1016/j.radonc.2020.11.006. [DOI] [PubMed] [Google Scholar]
- 29.Shinoto M., Yamada S., Terashima K., Yasuda S., Shioyama Y., Honda H., et al. Carbon ion radiation therapy with concurrent gemcitabine for patients with locally advanced pancreatic cancer. Int J Radiat Oncol Biol Phys. 2016;95(1):498–504. doi: 10.1016/j.ijrobp.2015.12.362. [DOI] [PubMed] [Google Scholar]
- 30.Bruynzeel A.M.E., Lagerwaard F.J. The role of biological dose-escalation for pancreatic cancer. Clin Transl Radiat Oncol. 2019;18:128–130. doi: 10.1016/j.ctro.2019.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Parisi S., Ferini G., Cacciola A., Lillo S., Tamburella C., Santacaterina A., et al. A non-surgical COMBO-therapy approach for locally advanced unresectable pancreatic adenocarcinoma: preliminary results of a prospective study. Radiol Med. 2022;127(2):214–219. doi: 10.1007/s11547-021-01441-w. [DOI] [PubMed] [Google Scholar]
- 32.Kelly P., Das P., Pinnix C.C., Beddar S., Briere T., Pham M., et al. Duodenal toxicity after fractionated chemoradiation for unresectable pancreatic cancer. Int J Radiat Oncol Biol Phys. 2013;85(3):e143–e149. doi: 10.1016/j.ijrobp.2012.09.035. [DOI] [PubMed] [Google Scholar]