Graphical abstract
Keywords: Cashew apple, Carotenoid, Mixture design and response surface methodology, Ultrasound-assisted extraction, Conventional extraction
Abbreviations: ANOVA, Analysis of variance; CE, Conventional extraction; Df, Degree of freedom; FAO, Food and Agriculture Organization of the United Nations; LOF, Lack of fit; PE, Pure error; QM, Quadratic mean; QS, Quadratic sum; r, Residue; R, Regression; RSM, Response Surface Methodology; UAE, Ultrasonic-assisted extraction
Highlights
-
•
Two methods for carotenoids extraction from cashew samples were proposed.
-
•
Mixture and response surface design methodology was used.
-
•
UAE of carotenoids was faster, cheaper, and more effective than CE.
-
•
The suitable extraction condition for UAE was using acetone/methanol (44/56) and 153 mg of sample for 19 min.
Abstract
Carotenoids are an essential component of cashew and can be used in pharmaceuticals, cosmetics, natural pigment, food additives, among other applications. The present work focuses on optimizing and comparing conventional and ultrasound-assisted extraction methods. Every optimization step took place with a 1:1 (w:w) mixture of yellow and red cashew apples lyophilized and ground in a cryogenic mill. A Simplex-centroid design was applied for both methods, and the solvents acetone, methanol, ethanol, and petroleum ether were evaluated. After choosing the extractor solvent, a central composite design was applied to optimize the sample mass (59–201 mg) and extraction time (6–34 min). The optimum conditions for the extractor solvent were 38% acetone, 30% ethanol, and 32% petroleum ether for CE and a mixture of 44% acetone and 56% methanol for UAE. The best experimental conditions for UAE were a sonication time of 19 min and a sample mass of 153 mg, while the CE was 23 min and 136 mg. Comparing red and yellow cashews, red cashews showed a higher carotenoid content in both methodologies. The UAE methodology was ca. 21% faster, presented a more straightforward composition of extracting solution, showed an average yield of superior carotenoid content in all samples compared to CE. Therefore, UAE has demonstrated a simple, efficient, fast, low-cost adjustment methodology and a reliable alternative for other applications involving these bioactive compounds in the studied or similar matrix.
1. Introduction
The cashew apple is the pseudo-fruit of the cashew tree (Anacardium Occidentale L.), naturally occurring in three colors: orange, yellow, and red. There is a commercial preference for yellow and red cashews [1]. According to the Food and Agriculture Organization of the United Nations – FAO, in 2018, Brazil was the largest producer of cashew, ca. 1,541 tonnes representing approximately 90% of total world production [2]. Considering cashew nut production, Vietnam, India, and Côte d’Ivoire are the largest producers, while Brazil has the 9th largest production.
Although the cashew nut was the cashew product with the highest economic value, the cashew apple has ceased to be neglected and achieved relevance in the food industry in the form of products such as jam, sweets, drinks (e.g., cashew), and juice [3], [4]. The pseudo-fruit has a high nutritional diversity with nutrients such as polyphenols (flavonoids, carotenoids, anacardic acid, and tannins), minerals (copper, zinc, sodium, potassium, calcium, iron, phosphorus, and magnesium), sugars, organic acids, and vitamins [5], [6], [7], [8], [9].
Especially the carotenoids, the focus of this study, correspond to isoprenoids with forty atoms that can be synthesized by plants among other autotrophic organisms and form the natural pigments responsible for the expression of colors such as red, yellow, and orange [10], [11]. β-carotene, α-carotene, β-cryptoxanthin, violaxanthin, lutein, and zeaxanthin are the main carotenoids found in cashew apple [12], [13]. These compounds are bioactive, acting on the mechanism of radical scavenging, primarily reactive oxygen species (ROS). Some studies have demonstrated the biological activity for carotenoids such as antioxidant [14], anti-aging [15], cardiovascular [16], anticancer [17], diabetes, and pro-vitamin A [18], [19].
The extraction of these compounds consists of transferring the analyte present in the sample to a liquid phase. Traditionally, methods involving the extraction of carotenoids use several organic solvents, and the choice of solvent or extraction mixture is usually performed by a univariate method. Although it has been successful, this approach is not very robust because it disregards the possible interactions of the experimental parameters (extraction times, solid/solvent ratio, solvent volumes used, type, and composition of the extraction solvent) [20]. The extraction method is crucial to obtain reliable results. Therefore, the application of multivariate methodologies focused on improving sample preparation has been used to achieve the optimal conditions for several analytical procedures [11], [21], [22], [23], [24]. Multivariate approaches provide broad knowledge about the effects and interactions of the various factors in the experimental data set, achieving reliable results and robust experimental conditions [25].
Mixing modeling, factorial design, and surface response methodology are the most used multivariate optimization techniques to maximize the performance of the extraction method. Mixture modeling can contribute to selecting the optimal extraction phase composition, which is a critical factor for the analysis of carotenoids in biological matrices [26], [27], [28], [29]. Factorial design such as that of central compounds is interesting for studying the influence of system-independent variables (time, sample mass, extraction volume) on the response [30], [31]. The combined use of the described methodologies favors obtaining the certain optimum condition of the proposed extraction method.
Multivariate optimization has been applied to extract carotenoids in different matrices, such as peach palm [11], shrimp [19], annatto seeds(Yolmeh, Habibi Najafi e Farhoosh, 2014) [32], tomato (Strati e Oreopoulou, 2011)[33]. However, no studies have been developed for the extraction of carotenoids in the cashew apple.
The present study aims to optimize the method of carotenoid extraction from the cashew apple by using the modeling mixtures and central composite design and two extraction procedures, which were compared. The extraction evaluated strategies were (I) performed under mechanical agitation, considered as conventional extraction (CE), and (II) assisted by ultrasound radiation (UAE). The developed protocol must provide reliable results and high robustness to be applied in research or the industry involving the studied matrix carotenoids or similar.
2. Materials and methods
2.1. Sample and reagents preparation
All materials used to perform this work were decontaminated in a 10% HNO3 bath (v/v) for at least 24 h. The solutions were prepared using deionized water with ≥ 18.2 M Ω cm resistivity obtained in Milli-Q Direct purification system from Millipore® (Molsheim, France).
Cashew pseudo-fruits were collected in Valença do Piauí city (GPS S 06° 21′ 03.7″/W 41° 44′ 24.7″, Piauí, Brazil) in the final stage of maturation, ready for consumption. For the optimization of the extraction methods, all individuals were randomly selected, representing the population as much as possible. The colors red, yellow, and heir mixtures (Fig. 1) are the three acquired samples. The pseudo-fruits were cut into cubes (ca. 2 cm3) and freeze dryer (LIOTOP®, L101, São Carlos, SP, Brazil). After that, the pseudo-fruits were pulverized using a cryogenic mill (Marconi®, MA 775, Piracicaba, Brasil).
Fig. 1.
Yellow and red cashew apple.
The solvents and reagents employed for extraction and quantification of carotenoids were: analytical standard of β-carotene, petroleum ether (Sigma-Aldrich®, Darmstadt, Germany), acetone, ethanol, and methanol (J. T. Baker®, Geel, Belgium).
2.2. Total carotenoid extraction
The total concentration of carotenoids was determined by UV–Vis, as described by [19.30], with minor modifications. The absorbance was measured at a wavelength of 454 nm using a spectrophotometer Drawell, DU – 8200 (Shanghai, China). The analytical curve was obtained using the β-carotene standard and acetone as an analytical blank. A stock solution of 1000 µg L-1 was prepared in acetone and diluted to the solutions from 0.25 to 5.0 µg L-1, utilized to construct the analytical curve. All analyzes were carried out in triplicate and protected from light.
2.3. Optimization of the extraction solution
The study optimized two methods: conventional extraction (CE) and another applying ultrasound-assisted extraction (UAE). Afterward, a comparison of their performance was carried out. The sample used was prepared at the ratio 1:1 (w:w) with the mixture of the yellow and red cashew apple pulverized so that a more significant number of carotenoid compounds were found in the cashews of the two colors is present in its composition. After that, the composition of the extraction solution was optimized by applying the simplex centroid design for pure solvents, acetone, ethanol, petroleum ether, methanol, and their respective binary, ternary and quarternary mixture (item 3.1, Table 2).
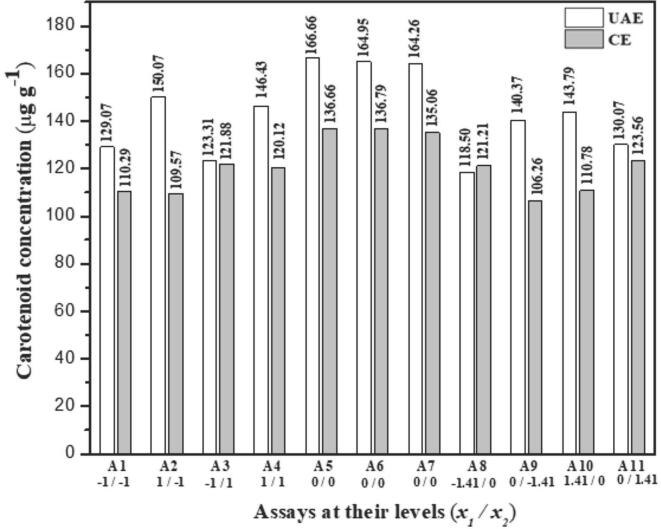
Table 2.
Total carotenoid content (µg g−1) found in the assays proposed by the simplex centroid mixture design using the UAE and CE methods.
| Assay | *a (%) | *e (%) | *p (%) | *m (%) | UAE | CE |
|---|---|---|---|---|---|---|
| A1 | 100 | 93.30 ± 2.72 | 102.19 ± 1.76 | |||
| A2 | 100 | 117.63 ± 0.39 | 112.50 ± 0.99 | |||
| A3 | 100 | 94.82 ± 3.92 | 105.44 ± 0.33 | |||
| A4 | 100 | 122.34 ± 3.08 | 118.99 ± 0.27 | |||
| A5 | 50 | 50 | 124.93 ± 3.00 | 123.15 ± 0.67 | ||
| A6 | 50 | 50 | 102.29 ± 6.54 | 125.57 ± 3.03 | ||
| A7 | 50 | 50 | 164.63 ± 5.50 | 133.68 ± 0.54 | ||
| A8 | 50 | 50 | 117.21 ± 5.13 | 110.90 ± 0.09 | ||
| A9 | 50 | 50 | 161.16 ± 0.00 | 122.03 ± 1.61 | ||
| A10 | 50 | 50 | 159.14 ± 4.35 | 132.00 ± 2.41 | ||
| A11 | 33.3 | 33.3 | 33.3 | 151.38 ± 1.66 | 140.03 ± 9.84 | |
| A12 | 33.3 | 33.3 | 33.3 | 161.06 ± 5.52 | 125.42 ± 3.31 | |
| A13 | 33.3 | 33.3 | 33.3 | 145.24 ± 1.64 | 135.87 ± 2.22 | |
| A14 | 33.3 | 33.3 | 33.3 | 130.99 ± 6.44 | 128.36 ± 5.52 | |
| A15 | 25 | 25 | 25 | 25 | 128.53 ± 1.20 | 137.77 ± 0.67 |
*Acetone (a), ethanol (e), petroleum ether (p) and methanol (m).
The proposed CE was based on the work developed by [1] with some modifications. For the applied protocol, ca. 90 mg of pulverized pseudo-fruit received the addition of 6.0 mL of extraction solution in a coated Falcon® tube to prevent the incidence of light. For extraction of the carotenoids, the mixture was kept under agitation for 20 min at 290 rpm in a mechanical shaker (Q225M, QUIMIS®, Diadema-SP, Brasil). The extract was centrifuged at 3500 rpm for 10 min (NI1812, Nova Instruments®, Piracicaba-SP, Brazil). The supernatant was collected and stored at −4 °C for subsequent analysis.
The ultrasound-assisted extraction was performed in an ultrasound bath (Sonitop 402-A, Soni-tech®, São Bernardo do Campo, SP, Brazil) operated at a frequency of 40 kHz and a power of 80 W a usable volume of 2.5 L (internal dimensions 15.0 × 13.7 × 15.0 cm). The experimental conditions such as sample mass, the volume of solution, and centrifugation step were the same as those applied in CE. For carotenoid extraction, the mixture was also maintained under the effect of ultrasound radiation for 20 min.
All assays (Table 2) were performed in duplicates and protected from light.
2.4. Experimental design to optimize extraction methodologies
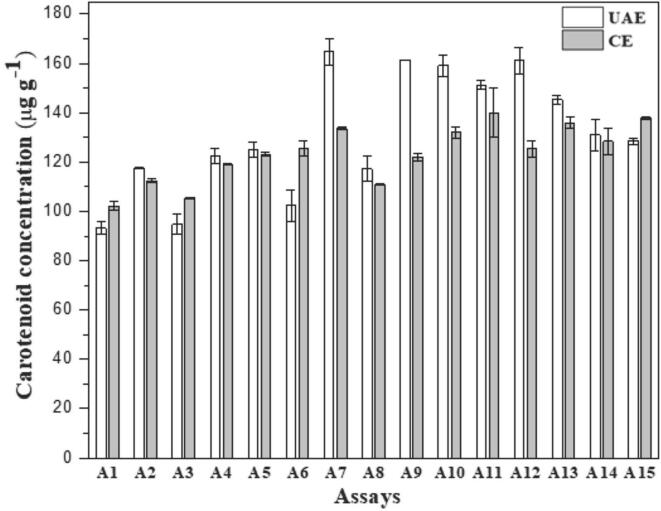
With the definition of the optimal extraction solvent, the optimization of other variables involved in the proposed methods was carried out. The independent variables, sample mass and time, have shown a high effect on carotenoid extraction in different biological matrices [19], [34] and so were studied here. The temperature during extraction was controlled in the range of 25–30 °C since undesirable reactions of carotenoid degradation can occur at elevated temperatures [33], [35]. The real and coded levels of the independent variables are shown in Table 1. The experimental arrangement applied corresponds to the factorial design 22 + central point + axial, consisting of 11 assays, including three repetitions at the central point, to obtain the experimental error (item 3.2, Fig. 4). The Response Surface Methodology (RSM) was applied for modeling to maximize the extraction yield and to establish the optimal working condition.
Table 1.
Variables and levels of central composite design for Ultrasound-Assisted Extraction (UAE) and Conventional Extraction (CE).
| Variables | Symbol | Levels and code of variables |
||||
|---|---|---|---|---|---|---|
| −1 | 0 | +1 | ||||
| Mass (mg) | x1 | 59 | 80 | 130 | 180 | 201 |
| Time (min) | x2 | 6 | 10 | 20 | 30 | 34 |
Fig. 4.
Carotenoid concentration found (µg g−1) in the assays extracts proposed by the central composite design applied in the UAE and CE.
The assays were performed randomly on different days to avoid systematic errors. For the two proposed methodologies, the same procedure described in item 2.3 was used, modifying only the sample mass and extraction time factors using the optimal composition of the extraction solution obtained after the application of simplex centroid design for each methodology studied. The total carotenoid content was the analytical response used to proceed with multivariate optimization.
2.5. Data analysis
The statistical treatment of data and graphs was performed using MATLAB software (Version R2015a). Analysis of variance (ANOVA) with Tukey’s multiple comparison test was used to determine significant differences between assays responses at the 95% confidence level.
3. Results and discussion
3.1. Extraction solvent
In recent decades, there has been an increase in studies for the development of better methods of carotenoids extraction, and different organic solvents have been used in this process in different matrices [3], [10], [19], [26], [34], [36]. Generally, the choice of these solvents for a particular analytical matrix is not so trivial, depending on the chemical and physical properties, such as the polarity and solubility of the target carotenoids in each matrix.
In this sense, to find the best extraction solution, a multivariate approach was applied using simplex centroid design to evaluate four solvents acetone (a), ethanol (e), petroleum ether (p), and methanol (m). The solvents evaluated are the most utilized and accessible for extraction of carotenoids [1], [3], [12], [19], [33], [37]. In addition to solvents, their combinations in binary, ternary, and quaternary mixtures were evaluated, resulting in 15 experiments for the UAE and CE procedures. Table 2 presents the results obtained for the experiments proposed by the simplex centroid design of mixtures.
Considering the experimental window described in Table 2 for the optimization process of the extraction solution of carotenoids present in the cashew apple, that is noticed that the ultrasound-assisted extraction method showed a yield range from 93.30 to 164.63 µg g−1 and a total average of 131.64 µg g−1 of carotenoids extracted for the 15 assays performed in duplicate. The conventional extraction method found a yield range of 102.19 to 140.03 µg g−1 and total average content of 123.59 µg g−1 of carotenoids obtained from the cashew apple sample.
Fig. 2 shows the average yield for each assay using the proposed extraction methodologies. In comparison, it is observed that UAE presented for 73% of the tests as A2, A4, A5, A7 – A14 extraction yield superior to CE. It can also notice that the carotenoid content using UAE is higher with solvent mixtures compared to extracts obtained with pure solvents (Fig. 2). The assay extract using the binary acetone/methanol mixture was found to have the highest carotenoid content, ca. 164 µg g−1. Considering the EC, the results were similar. The content of carotenoids found in extracts using a mixture of solvents was higher than those with pure solvent. The ternary acetone/ethanol/petroleum ether mixture showed ca. 140.03 µg g−1. According to the results, it can highlighted that the combination of solvents as an extraction solution for both applied methods improves the extraction. In fact, the polarity of the extracting solvent plays a crucial role in the successful extraction of carotenoids from plant tissues. The mixture of solvents results in an extractor solution with intermediate polarity that increases the solute–solvent interaction, improving the dissolution and extraction of carotenoids. Heffernan et al. [47] achieved the greatest yield of carotenoid rich extracts from brown macroalgae using combination of solvent (hexane/acetone (70:30) in a solid–liquid extraction (SLE). Pure solvents are more effective for selective extraction of compounds as demonstrated by Mendes et al. [27].
Fig. 2.
Total carotenoid content in each assay proposed by simplex centroid design using UAE and CE extraction methods.
The carotenoid content obtained in each assay of the extraction methodologies was the analytical response used for statistical modeling of the data. The performance of the linear, quadratic, and special cubic model was assessed using analysis of variance (ANOVA). The special cubic model showed the best fit for the experimental domain of the proposed extraction methodologies. Table 3 shows ANOVA for the special cubic model with only the significant terms, with ten and eleven parameters for the model used in CE and UAE, respectively.
Table 3.
ANOVA for the special cubic model.
| Conventional extraction (CE) | |||||
|---|---|---|---|---|---|
| Variation source | Quadratic sum (QS) | Degrees of freedom (Df) | Quadratic mean (QM) | Fcalc (95%) | Ftab (95%) |
| Regression (R) | 3754.12 | 9 | 417.12 | 33.08 | 2.392 |
| Residue (r) | 252.16 | 20 | 12.61 | ||
| Lack of fit (LOF) | 85.63 | 5 | 17.13 | 1.54 | 2.901 |
| Pure error (PE) | 166.53 | 15 | 11.10 | ||
| R2 | 0.9371 | ||||
| R adjusted | 0.9087 | ||||
| Ultrasound-assisted extraction (UAE) | |||||
| Regression (R) | 16200.80 | 13 | 1246.22 | 24.47 | 2.397 |
| Residue (r) | 815.00 | 16 | 50.94 | ||
| Lack of fit (LOF) | 577.10 | 1 | 577.10 | 36.39 | 4.543 |
| Pure error (PE) | 237.90 | 15 | 15.86 | ||
| R2 | 0.9521 | ||||
| R adjusted | 0.9132 | ||||
The ANOVA for the CE procedure (Table 3) indicated that the model did not present a lack of fit, at the 95% confidence level, since the value of Fcalculated (1.54) found by the ratio between the quadratic mean of lack of fit and pure error was less than the Fcritical (2.901) for five degrees of freedom in the numerator and fifteen in the denominator. This fact shows no significant difference between the two quadratic means (MQFaj e MQEp). Besides that, the Fcalculated (33.08) for the ratio of the quadratic mean of the regression (MQR) and residue (MQr) was higher than the Fcritical (2.392) to nine degrees in the numerator and 20 in the denominator, demonstrating that the model is reliable for making predictions. The cubic model reached a coefficient of determination (R2) of 0.9371 explains 93.71% of the total data variance.
Although ANOVA (Table 3) indicated a lack of fit (Fcalculated > Fcritical) at the 95% confidence level for the UAE, the special cubic model can be used to make predictions since the variance captured for regression differs from the variance of the residuals. The ratio of the square mean of the regression to the residue is ten times higher than the Fcritical (24.47 > 2.397), showing that the regression is significant. The model explained 92.65% of the total data variation. The value of R2 adj (0.9132) is similar to that R2 (Table 3), indicating that the proposed model is reliable because only 8.68% of the total variance has not been explained [38].
Polynomial Equations (1), (2) describe the special cubic model proposed for modeling the total carotenoid content obtained from the extraction methodologies CE e UAE, respectively.
| (1) |
| (2) |
Analyzing the significant coefficients of the model for CE (Eq. (1)), all effects show synergism. The interaction between water/ethanol/petroleum ether is the most important and highest value. The coefficients of the solvents were higher than those of binary mixtures, showing that the synergistic effect of the interactions slightly adds to the yield of the extraction methodology. Considering Eq. (2) for UAE, the effects for ternary mixtures between acetone/petroleum ether/methanol and ethanol/petroleum ether/methanol are antagonistic, and the other effects have synergism, that is, the response obtained with the two, three or four mixed components will always be greater than the individual sum of your answers.
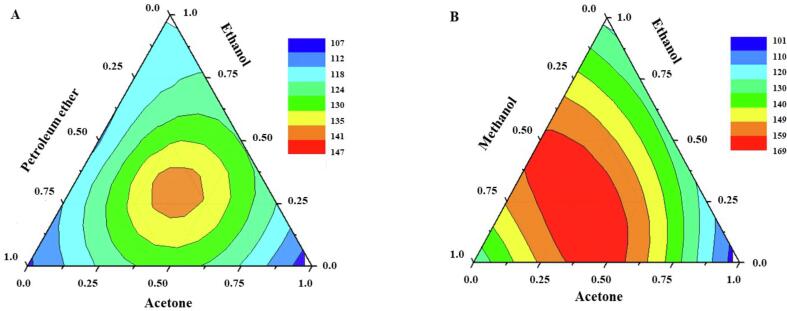
Fig. 3 shows the response surface graphs plotted to apply Eq. (1), (2), respectively. Analyzing Fig. 3A for CE, the best yield was achieved using the composition of the extraction solution with approximately equivalent proportions for the three solvents (acetone/ethanol/petroleum ether). As shown in Fig. 3B, the binary acetone/methanol mixture proves to be more favorable using UAE for the extraction of carotenoids from the cashew apple.
Fig. 3.
Contour plot plotted for Conventional Extraction (CE) (A) and Ultrasound-Assisted Extraction (UAE) (B) using the special cubic model.
The resolution of the polynomial represented by Eq.1 for the proposed regression model for CE indicates the ternary mixture containing 38% acetone, 30% ethanol, and 32% petroleum ether as the optimal extraction solution. For UAE, the optimal condition was the binary mixture composed of 44% acetone and 56% methanol, considered the resolution of the regression model represented by Eq.2. Then, the optimal extraction solution of each proposed methodology presents a different composition, indicating that the factors energy and composition of the extraction medium affect the extractability of carotenoids. Therefore, adapted methods can cause a loss of information about the system under study. It is essential to optimize the extraction solvent to expand the performance of the methods aiming at more reliable results in the study.
Saini e Keum [36] report the importance of methodologies for the effective extraction of carotenoids in complex matrices such as vegetables, in which applying a mixture composed of polar and non-polar solvents, for example, acetone/hexane or acetone/ethanol/hexane, are the most appropriate since they allow simultaneous extraction of polar and non-polar carotenoids. Such conditions are exactly obtained using multivariate optimization techniques. Univariate studies involving the extraction of carotenoids from cashew employed pure solvents such as acetone only [12], mixtures such as ethanol/hexane 3:4 v/v [1] and methanol/ethyl acetate/petroleum ether, 1:1:1 v/v/v [3].
3.2. Optimization of variables using a central composite design
The approach of our study consists of the multivariate sequential optimization of the cashew apple carotenoid extraction procedure. Then, variables such as sample mass (x1) and extraction time (x2) were optimized using central composite design for CE and UAE methodologies with the respective optimal extraction solution. Fig. 4 shows the carotenoid content found for the 11 assays proposed by the experimental design using the UAE and CE extraction methods.
As shown in Fig. 4, the performance of the tests applying UAE was superior to those of CE. The average global carotenoid content also increased by ca. 8.2% (143.41 µg g−1) compared to those in Fig. 2 (131.64 µg g−1), showing an improvement in the performance of the UAE. For the CE, the global average of extracted carotenoids slightly reduced from 123.59 to 121.10 µg g−1. The results shown in Fig. 4 were described using a quadratic model. Polynomials are represented by Equations (3), (4).
| (3) |
| (4) |
The fit quality of the models was assessed using ANOVA for only the significant terms (Table 4). For the UAE, the quadratic model did not present a lack of fit to the 95% confidence level since the Fcalculed value (7.99) was less than Fcritical (19.246). Also, the model is reliable for making predictions, Fcalculed > Fcritical (83.16 > 4.53). Regarding the results of ANOVA for the proposed model, the CE shows that there is no lack of fit, Fcalculed < Fcritical (7.99 < 19.246) as well as it can be used to make predictions, Fcalculed > F critical (58.16 > 4.533) [38]. Therefore, the proposed models for the CE and UAE extraction methods are adequate and reliable.
Table 4.
ANOVA for central composite design.
| Ultrasound-assisted extraction (UAE) | |||||
|---|---|---|---|---|---|
| Variation source | Quadratic sum (QS) | Degrees of freedom (Df) | Quadratic mean (QM) | Fcalc (95%) | Ftab (95%) |
| Regression (R) | 2858.25 | 4 | 714.56 | 83.16 | 4.533 |
| Residue (r) | 51.56 | 6 | 8.59 | ||
| Lack of fit (LOF) |
48.52 | 4 |
12.13 | 7.99 | 19.246 |
| Pure error (PE) | 3.04 | 2 | 1.52 | ||
| R2 | 0.983 | ||||
| R adjusted | 0.970 | ||||
| Conventional extraction (CE) | |||||
| Regression (R) | 1244.56 | 4 | 311.139 | 58.51 | 4.533 |
| Residue (r) | 21.83 | 6 | 3.639 | 5.14 | |
| Lack of fit (LOF) | 19.90 | 4 | 4.974 | ||
| Pure error (PE) | 1.94 | 2 | 0.968 | 19.246 | |
| R2 | 0.983 | ||||
| R adjusted | 0.971 | ||||
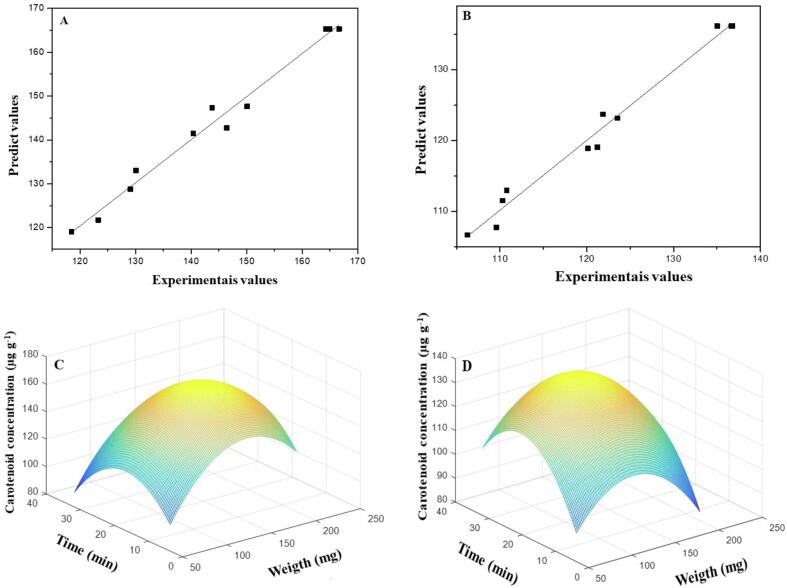
Fig. 5A and 5B show the predicted values versus the values obtained experimentally for the models proposed for the UAE and CE methods, respectively. Both models have a determination coefficient (R2) of 0.983 (Table 4), indicating a good correlation, and the 98.3% of the total data variance are explained by the proposed regression. In comparison to other studies reported in the literature, the adjustment of the proposed models is adequate. Poojary and Passamonti [39] obtained R2 90.1%. For the extraction of lycopene in tomato pulp residues, for watermelon pulp, the proposed model for extracting lycopene presented R2 of 98.6% [40]. Ordóñez-Santos, Pinzón-Zarate, and González-Salcedo [11] developed a method for extraction of total carotenoids in pupunha with a determination coefficient of 97.6%.
Fig. 5.
Response surface for experimental values versus predicted values and response surface plotted for mass and time of the UAE (A and C) and CE (B and D) methods.
Considering the good fit presented by the models, the response surfaces were constructed for the UAE and CE methods using Eq. (3), (4), respectively. Fig. 5C and 5D show the response surface plotted using the quadratic models of each extraction methodology. Looking at Fig. 5C, the optimal condition for extraction using UAE is near to central point for the time axis and slightly above the mass variable. For the CE methodology, the maximum extraction efficiency is above the central point on the time axis. In the region of the central point for the sample mass variable, it shows a positive sign (see Fig. 5D).
The optimization of the extraction time variable has been the focus of some studies reported in the literature. Ordóñez-Santos, Pinzón-Zarate, and González-Salcedo [11] evaluated the extraction of carotenoids in the 10–30 min time range in which the extracts obtained after 30 min was found to have the highest concentration of the analyte. Yolmeh, Najafi, and Farhoosh [32] investigated the sonication time of 2–10 min to extract carotenoids from annatto seeds. The best yield was obtained at 7.25 min. The extraction of pomegranate carotenoids was ca. 30 min to reach maximum performance, with the range being evaluated from 10 to 60 min [41].
Considering the response surfaces (Fig. 5C and 5D) and the proposed model (Eq. (3), (4)), the optimal condition for UAE is achieved with the sample mass of ca. 153 mg (0.300 level) in 19 min of extraction (- 0.100 level) using the optimized extraction solvent composed of 44% acetone and 56% methanol. The CE shows the maximum performance when applied using the extraction solution of 38% acetone/30% ethanol/32% petroleum ether, sample mass of 136 mg (level of −0.100) for 23 min (level of 0.274).
The predicted maximum yield values were 166.98 and 137.08 µg g−1 for the UAE and CE extraction procedures, respectively. Then, confirmatory experiments were carried out under the optimal conditions for each extraction methodology to ascertain whether the experimental results were close to those predicted by the model. Applying UAE, the total carotenoid content found was 165.47 ± 1.79 µg g−1 and 134.41 ± 1.40 µg g−1 in extracts obtained using the CE methodology. These values were not significantly different from those predicted by the proposed models, according to the Tukey test applied at the 95% confidence level. Therefore, the quality of fit for the proposed models was confirmed with the tests performed. Then, the methodologies in their optimum conditions were applied for the extraction of carotenoids from yellow and red cashews. The results found are shown in Table 5.
Table 5.
Extraction conditions for UAE and CE methods and the concentration of carotenoids (µg g−1) in different cashew peduncles.
| Ultrasound-Assisted Extraction (UAE) | |||||
|---|---|---|---|---|---|
| Solvente extrator | Sample mass | Extraction time | Carotenoid concentration (µg g−1) | ||
| 44% acetone/56% de methanol | 153 mg | 19 min | Yellow + red cashew | Red cashew | Yellow cashew |
| 165.47 ± 1.79A | 154.91 ± 2.17B | 144.67 ± 1.59C | |||
| Conventional extraction (CE) | |||||
| 38% acetone/30% ethanol/32% Petroleum ether | 136 mg | 23 min | 134.41 ± 1.40D | 115,55 ± 0.40E | 102.16 ± 1.19F |
Means that do not share the same letter are significantly different according to the Tukey test (P < 0.05).
3.3. Comparison between UAE and CE
In addition to proposing two optimized methodologies for extracting carotenoids from the cashew apple, this study made it possible to compare the parameters considered: yield, sample mass, extraction solvent, and extraction time. Table 5 shows the conditions for extraction methods and the concentration of carotenoids in different cashew apples. In terms of yield, the UAE was superior to CE for all studied cashew apple samples. The total carotenoid content found for yellow, red, and mixed cashew using UAE was higher ca. 42%, 34%, and 23% compared to CE. According to the Tukey test, the differences verified for the results of the UAE and CE methodologies are significant at 95% confidence. Red cashews had a higher carotenoid content than yellow cashews, while the combined sample showed a higher concentration than individual pseudo-fruits (yellow and red). Assunção and Mercadante found similar results in which the total carotenoid levels of red cashews were slightly higher when compared to those of yellow varieties [42].
The UAE procedure was faster ca. 21% concerning CE and presented an average yield of ca. 33% higher. The UAE combines high performance in less time. The application of this extraction procedure improves the analytical frequency and reduces costs for analyzing carotenoids in cashew apples. The reduction in time achieved by applying the UAE methodology (Table 5) is mainly related to the phenomenon of acoustic cavitation that causes the collapse of micro-bubbles on the cell wall surface, causing rupture, erosion, and fragmentation of its structure. This process increases extraction kinetics because it favors the interaction and release of the carotenoids present in the different cell structures with the extraction solvents [19], [34], [36], [43], [44].
The optimal extraction solution for the proposed extraction methodologies was also different (Table 5). For UAE, the binary mixture composed of acetone and methanol was the optimal extraction solution. The extraction medium optimized for CE was the ternary mixture formed of acetone, ethanol, and petroleum ether. This difference can be attributed to nature of the energy used in each extraction methodology [19]. The high energy supplied to the UAE system promotes an increase in interactions and mass transfer of the carotenoids present in the cell matrix to the extraction solvent. This synergistic effect between ultrasonic energy and the extraction medium improves the extractability of the compounds, simplifying the composition of the extraction phase used and, consequently, making the extraction method cheaper. [45]. The energy from the mechanical agitation applied in CE is smoother, which extraction of the carotenoid depends on the polarity requiring a more complex composition for the extraction solution, for example, the ternary mixture of acetone, ethanol, and petroleum ether [36], [46].
Although this study was carried out with a large sample, the CE used less cashew apple mass when compared to the UAE. However, as can be seen on the response surface plot for the UAE (Fig. 5C), reducing the sample mass from 153 mg to 136 mg causes a slight decrease in the extraction yield.
Compared with other reported studies, the proposed UAE methodology achieved similar or higher extraction yields. [1], [3], [12], [42]. Although the developed methodology has presented higher extraction yield, these differences in the concentration of carotenoids may be related to the edaphoclimatic conditions that the cashew tree was exposed to, such as light, temperature, humidity, and soil nutrients [42], [47].
4. Conclusions
Sequential multivariate optimization has been successfully applied, enabling the development of two reliable methods for extracting carotenoids from the cashew apple. The simplex and central composite centroid design showed satisfactory adjustment to the experimental data of both methods. Therefore, the optimal conditions found for both methods are reliable, repeatable, and exact. The application of high energy techniques for extraction of total cashew carotenoids is more effective to the conventional technique because the UAE showed higher yield, less time, and more straightforward extraction solution when compared to CE. The carotenoid content found in red cashew was higher than that of yellow cashew. In comparison, the results of our study applying the UAE are similar or higher to the carotenoid content presented in other works reported in the literature. Therefore, the UAE optimized for the extraction of carotenoids from cashews proved to be a simple, efficient, fast, low-cost adjustment methodology and a reliable alternative for other applications involving these bioactive compounds in the studied or similar matrix.
CRediT authorship contribution statement
Tiago Linus Silva Coelho: Formal analysis, Conceptualization, Visualization, Writing – original draft. Darlisson Slag Neri Silva: Formal analysis, Conceptualization, Visualization, Writing – original draft. Jedaias Marreiros dos Santos Junior: Formal analysis. Clecio Dantas: Conceptualization, Visualization. Ana Rita de Araujo Nogueira: Conceptualization, Visualization. Cícero Alves Lopes Junior: Funding acquisition, Project administration, Resources, Supervision, Visualization, Writing – review & editing. Edivan Carvalho Vieira: Funding acquisition, Project administration, Resources, Supervision, Visualization, Writing – review & editing.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
The authors thank the Piauí State Research Support Foundation (FAPEPI) (301269/2015-7), the National Council for Scientific and Technological Development (CNPq, Brasília, Brazil, 301550/2018-2; 140212/2020-5, 200275/2020-8), and the Coordination for the Improvement of Higher Education Personnel (CAPES, Brasília, Brazil) for financial support and fellowships. The authors also thank to Prof. Dr. Sherlan Lemos (Federal University of Paraiba – campus I) by the availability of the MATLAB software used for statistical analysis of the data. The authors acknowledge the financial support by the University of Graz.
Contributor Information
Cícero Alves Lopes Júnior, Email: cicero.lopes@uni-graz.at.
Edivan Carvalho Vieira, Email: edivanvieira@ufpi.edu.br.
References
- 1.Schweiggert R.M., Vargas E., Conrad J., Hempel J., Gras C.C., Ziegler J.U., Mayer A., Jiménez V., Esquivel P., Carle R. Carotenoids, carotenoid esters, and anthocyanins of yellow-, orange-, and red-peeled cashew apples (Anacardium occidentale L.) Food Chem. 2016;200:274–282. doi: 10.1016/j.foodchem.2016.01.038. [DOI] [PubMed] [Google Scholar]
- 2.FAO, Food and Agricultural Organisation der Vereinten Nationen. http://www.fao.org/, 2018 (accessed 10 March 2020).
- 3.de Abreu F.P., Dornier M., Dionisio A.P., Carail M., Caris-Veyrat C., Dhuique-Mayer C. Cashew apple (Anacardium occidentale L.) extract from by-product of juice processing: A focus on carotenoids. Food Chem. 2013;138(1):25–31. doi: 10.1016/j.foodchem.2012.10.028. [DOI] [PubMed] [Google Scholar]
- 4.Michodjehoun-Mestres L., Souquet J.-M., Fulcrand H., Bouchut C., Reynes M., Brillouet J.-M. Monomeric phenols of cashew apple (Anacardium occidentale L.) Food Chem. 2009;112(4):851–857. doi: 10.1016/j.foodchem.2008.06.056. [DOI] [Google Scholar]
- 5.Debrito E., Pessanhadearaujo M., Lin L., Harnly J. Determination of the flavonoid components of cashew apple (Anacardium occidentale) by LC-DAD-ESI/MS. Food Chem. 2007;105(3):1112–1118. doi: 10.1016/j.foodchem.2007.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Honorato T.L., Rodrigues S. Dextransucrase stability in cashew apple juice. Food Bioprocess Technol. 2010;3(1):105–110. doi: 10.1007/s11947-008-0053-2. [DOI] [Google Scholar]
- 7.Lima E.S., Da Silva E.G., Neto J.M.M., Moita G.C. Redução de vitamina C em suco de caju (Anacardium occidentale L.) industrializado e cajuína. Quim. Nova. 2007;30:1143–1146. doi: 10.1590/S0100-40422007000500017. [DOI] [Google Scholar]
- 8.Lowor S.T., Agyente-Ba C.K. Mineral and proximate composition of cashew apple (Anarcadium occidentale L.) juice from northern savannah, forest and coastal savannah regions in Ghana. Am. J. Food Technol. 2009;4(4):154–161. doi: 10.3923/ajft.2009.154.161. [DOI] [Google Scholar]
- 9.Trevisan M.T.S., Pfundstein B., Haubner R., Würtele G., Spiegelhalder B., Bartsch H., Owen R.W. Characterization of alkyl phenols in cashew (Anacardium occidentale) products and assay of their antioxidant capacity. Food Chem. Toxicol. 2006;44(2):188–197. doi: 10.1016/j.fct.2005.06.012. [DOI] [PubMed] [Google Scholar]
- 10.Dey S., Rathod V.K. Ultrasound assisted extraction of β-carotene from Spirulina platensis. Ultrason. Sonochem. 2013;20(1):271–276. doi: 10.1016/j.ultsonch.2012.05.010. [DOI] [PubMed] [Google Scholar]
- 11.Ordóñez-Santos L.E., Pinzón-Zarate L.X., González-Salcedo L.O. Optimization of ultrasonic-assisted extraction of total carotenoids from peach palm fruit (Bactris gasipaes) by-products with sunflower oil using response surface methodology. Ultrason. Sonochem. 2015;27:560–566. doi: 10.1016/j.ultsonch.2015.04.010. [DOI] [PubMed] [Google Scholar]
- 12.Assunção R.B., Mercadante A.Z. Carotenoids and ascorbic acid composition from commercial products of cashew apple (Anacardium occidentale L.) J. Food Compos. Anal. 2003;16(6):647–657. doi: 10.1016/S0889-1575(03)00098-X. [DOI] [Google Scholar]
- 13.Schweiggert R.M., Steingass C.B., Esquivel P., Carle R. Chemical and morphological characterization of Costa Rican papaya (Carica papaya L.) hybrids and lines with particular focus on their genuine carotenoid profiles. J. Agric. Food Chem. 2012;60(10):2577–2585. doi: 10.1021/jf2045069. [DOI] [PubMed] [Google Scholar]
- 14.Fiedor J., Science A.C. Potential Role of Carotenoids as Antioxidants in Human Health and Disease. Nutrients. 2014;6:466–488. doi: 10.3390/nu6020466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wojdyło A., Nowicka P., Bąbelewski P. Phenolic and carotenoid pro fi le of new goji cultivars and their anti- hyperglycemic, anti-aging and antioxidant properties. J. Funct. Food. 2018;48:632–642. doi: 10.1016/j.jff.2018.07.061. [DOI] [Google Scholar]
- 16.Voutilainen S., Nurmi T., Mursu J., Rissanen T.H. Carotenoids and cardiovascular health1 – 3. Am. J. Clin. Nutr. 2006;83:1265–1271. doi: 10.1093/ajcn/83.6.1265. [DOI] [PubMed] [Google Scholar]
- 17.Nara E., Hayashi H., Kotake M., Miyashita K., Nara E., Hayashi H., Kotake M., Miyashita K., Nagao A. Acyclic Carotenoids and Their Oxidation Mixtures Inhibit the Growth of HL-60 Human Promyelocytic Leukemia Cells. Nutrit. Canc. 2009;39(2):273–283. doi: 10.1207/S15327914nc392. [DOI] [PubMed] [Google Scholar]
- 18.Basto G.J., Carvalho C.W.P., Soares A.G., Costa H.T.G.B., Chávez D.W.H., Godoy R.L.d.O., Pacheco S. Physicochemical properties and carotenoid content of extruded and non-extruded corn and peach palm (Bactris gasipaes, Kunth) LWT - Food Sci. Technol. 2016;69:312–318. doi: 10.1016/j.lwt.2015.12.065. [DOI] [Google Scholar]
- 19.Tsiaka T., Zoumpoulakis P., Sinanoglou V.J., Makris C., Heropoulos G.A., Calokerinos A.C. Response surface methodology toward the optimization of high-energy carotenoid extraction from Aristeus antennatus shrimp. Anal. Chim. Acta. 2015;877:100–110. doi: 10.1016/j.aca.2015.03.051. [DOI] [PubMed] [Google Scholar]
- 20.Şahin S., Şamlı R. Optimization of olive leaf extract obtained by ultrasound-assisted extraction with response surface methodology. Ultrason. Sonochem. 2013;20(1):595–602. doi: 10.1016/j.ultsonch.2012.07.029. [DOI] [PubMed] [Google Scholar]
- 21.Coelho T.L.S., Braga F.M.S., Silva N.M.C., Dantas C., Lopes Júnior C.A., de Sousa S.A.A., Vieira E.C. Optimization of the protein extraction method of goat meat using factorial design and response surface methodology. Food Chem. 2019;281:63–70. doi: 10.1016/j.foodchem.2018.12.055. [DOI] [PubMed] [Google Scholar]
- 22.Paludo M.C., Colombo R.C., Filho J.T., Hermosín-Gutiérrez I., Balluse C.A., Godoy H.T. Optimizing the Extraction of Anthocyanins from the Skin and Phenolic Compounds from the Seed of Jabuticaba Fruits (Myrciaria jabuticaba(Vell.) O. Berg) with Ternary Mixture Experimental Designs. J. Braz. Chem. Soc. 2019;30:1506–1514. doi: 10.21577/0103-5053.20190047. [DOI] [Google Scholar]
- 23.Karvela E., Makris D.P., Kalogeropoulos N., Karathanos V.T. Deployment of response surface methodology to optimise recovery of grape (Vitis vinifera) stem polyphenols. Talanta. 2009;79(5):1311–1321. doi: 10.1016/j.talanta.2009.05.042. [DOI] [PubMed] [Google Scholar]
- 24.Khalajzadeh S., Ghasemi E., Amiri S., Sohrabi M.R., Shakeri A. Optimization of ultrasonic assisted extraction of fatty acids from Aesculus hippocastanum fruit by response surface methodology. Food Chem. 2018;271:762–766. doi: 10.1016/j.foodchem.2018.07.144. [DOI] [PubMed] [Google Scholar]
- 25.Liyanapathirana C., Shahidi F. Optimization of extraction of phenolic compounds from wheat using response surface methodology. Food Chem. 2005;93(1):47–56. doi: 10.1016/j.foodchem.2004.08.050. [DOI] [Google Scholar]
- 26.Castro I.A., Moraes Barros S.B., Lanfer Marquez U.M., Motizuki M., Higashi Sawada T.C. Optimization of the antioxidant capacity of a mixture of carotenoids and α-tocopherol in the development of a nutritional supplement. Food Res. Int. 2005;38(8-9):861–866. doi: 10.1016/j.foodres.2005.02.010. [DOI] [Google Scholar]
- 27.Mendes M.K.d.A., Oliveira C.B.D.S., Veras M.D.A., Araújo B.Q., Dantas C., Chaves M.H., Lopes Júnior C.A., Vieira E.C. Application of multivariate optimization for the selective extraction of phenolic compounds in cashew nuts (Anacardium occidentale L.) Talanta. 2019;205:120100. doi: 10.1016/j.talanta.2019.06.100. [DOI] [PubMed] [Google Scholar]
- 28.Soares D.X., Scarminio I.S., Bruns R.E. Mixture designs for exploring class diversity and metabolite fingerprinting: An efficient column chromatographic strategy. Anal. Chim. Acta. 2011;702(2):288–294. doi: 10.1016/j.aca.2011.06.056. [DOI] [PubMed] [Google Scholar]
- 29.Tormena C.D., Marcheafave G.G., Rakocevic M., Bruns R.E., Scarminio I.S. Sequential mixture design optimization for divergent metabolite analysis: Enriched carbon dioxide effects on Coffea arabica L. leaves and buds. Talanta. 2019;191:382–389. doi: 10.1016/j.talanta.2018.09.002. [DOI] [PubMed] [Google Scholar]
- 30.Li Y., Fabiano-Tixier A.S., Tomao V., Cravotto G., Chemat F. Green ultrasound-assisted extraction of carotenoids based on the bio-refinery concept using sunflower oil as an alternative solvent. Ultrason. Sonochem. 2013;20(1):12–18. doi: 10.1016/j.ultsonch.2012.07.005. [DOI] [PubMed] [Google Scholar]
- 31.Wang X., Wu Y., Chen G., Yue W., Liang Q., Wu Q. Optimisation of ultrasound assisted extraction of phenolic compounds from Sparganii rhizoma with response surface methodology. Ultrason. Sonochem. 2013;20(3):846–854. doi: 10.1016/j.ultsonch.2012.11.007. [DOI] [PubMed] [Google Scholar]
- 32.Yolmeh M., Habibi Najafi M.B., Farhoosh R. Optimisation of ultrasound-assisted extraction of natural pigment from annatto seeds by response surface methodology (RSM) Food Chem. 2014;155:319–324. doi: 10.1016/j.foodchem.2014.01.059. [DOI] [PubMed] [Google Scholar]
- 33.Strati I.F., Oreopoulou V. Process optimisation for recovery of carotenoids from tomato waste. Food Chem. 2011;129(3):747–752. doi: 10.1016/j.foodchem.2011.05.015. [DOI] [PubMed] [Google Scholar]
- 34.Lianfu Z., Zelong L. Optimization and comparison of ultrasound/microwave assisted extraction (UMAE) and ultrasonic assisted extraction (UAE) of lycopene from tomatoes. Ultrason. Sonochem. 2008;15(5):731–737. doi: 10.1016/j.ultsonch.2007.12.001. [DOI] [PubMed] [Google Scholar]
- 35.Xu Y., Pan S. Effects of various factors of ultrasonic treatment on the extraction yield of all-trans-lycopene from red grapefruit (Citrus paradise Macf.) Ultrason. Sonochem. 2013;20(4):1026–1032. doi: 10.1016/j.ultsonch.2013.01.006. [DOI] [PubMed] [Google Scholar]
- 36.Saini R.K., Keum Y.S. Carotenoid extraction methods: A review of recent developments. Food Chem. 2018;240:90–103. doi: 10.1016/j.foodchem.2017.07.099. [DOI] [PubMed] [Google Scholar]
- 37.Song J., Yang Q., Huang W., Xiao Y., Li D., Liu C. Optimization of trans lutein from pumpkin (Cucurbita moschata) peel by ultrasound-assisted extraction. Food Bioprod. Process. 2018;107:104–112. doi: 10.1016/j.fbp.2017.10.008. [DOI] [Google Scholar]
- 38.B. Barros Neto, I.S. Scarmino, R.E. Bruns, Como fazer experimentos: Pesquisa e desenvolvimento na indústria, fourth ed., Bookman, Porto Alegre – RS, 2010.
- 39.Poojary M.M., Passamonti P. Optimization of extraction of high purity all-trans-lycopene from tomato pulp waste. Food Chem. 2015;188:84–91. doi: 10.1016/j.foodchem.2015.04.133. [DOI] [PubMed] [Google Scholar]
- 40.Oberoi D.P.S., Sogi D.S. Utilization of watermelon pulp for lycopene extraction by response surface methodology. Food Chem. 2017;232:316–321. doi: 10.1016/j.foodchem.2017.04.038. [DOI] [PubMed] [Google Scholar]
- 41.Goula A.M., Ververi M., Adamopoulou A., Kaderides K. Green ultrasound-assisted extraction of carotenoids from pomegranate wastes using vegetable oils. Ultrason. Sonochem. 2017;34:821–830. doi: 10.1016/j.ultsonch.2016.07.022. [DOI] [PubMed] [Google Scholar]
- 42.Assunção R.B., Mercadante A.Z. Carotenoids and ascorbic acid from cashew apple (Anacardium occidentaleL.): variety and geographic effects. Food Chem. 2003;81(4):495–502. doi: 10.1016/S0308-8146(02)00477-6. [DOI] [Google Scholar]
- 43.Pico Y. Ultrasound-assisted extraction for food and environmental samples. Tren. Analyt. Chem. 2013;43:84–99. doi: 10.1016/j.trac.2012.12.005. [DOI] [Google Scholar]
- 44.Chemat F., Rombaut N., Sicaire A., Meullemiestre A., Abert-vian M. Ultrasound assisted extraction of food and natural products. Mechanisms, techniques, combinations, protocols and applications. Ultrason. Sonochem. 2017:540–560. doi: 10.1016/j.ultsonch.2016.06.035. [DOI] [PubMed] [Google Scholar]
- 45.Romero I., Martínez-Patiño J.C., Brnčić M., Ruiz E., Žlabur J.Š., Castro E., Gullón B. Optimization of ultrasound-assisted extraction of biomass from olive trees using response surface methodology. Ultrason. Sonochem. 2019;51:487–495. doi: 10.1016/j.ultsonch.2018.05.031. [DOI] [PubMed] [Google Scholar]
- 46.Wang E., Dong C., Park R.F., Roberts T.H. Carotenoid pigments in rust fungi: Extraction, separation, quantification and characterisation, Fungal. Biol. Rev. 2018;32(3):166–180. doi: 10.1016/j.fbr.2018.02.002. [DOI] [Google Scholar]
- 47.Heffernan N., Smyth T.J., FitzGerald R.J., Vila-Soler A., Mendiola J., Ibáñez E., Brunton N.P. Comparison of extraction methods for selected carotenoids from macroalgae and the assessment of their seasonal/spatial variation. Innov. Food Sci. Emerg. Technol. 2016;37:221–228. doi: 10.1016/j.ifset.2016.06.004. [DOI] [Google Scholar]