Abstract
Purpose.
Recently it has been suggested that a cellular pathway composed of integrin, integrin-linked kinase (ILK), rapamycin-insensitive companion of mTOR (RICTOR), and Akt may facilitate long-term structural and functional adaptations associated with exercise, independent of the mTORC1 pathway. Therefore, we examined changes in integrin-ILK-RICTOR-Akt protein in vastus lateralis (VL) before and after 8 weeks of eccentric cycling training (ECC) which was expected to increase muscle function and VL cross-sectional area (CSA).
Methods.
Eleven men (23±4 years) completed 24 sessions of ECC with progressive increases in intensity and duration, resulting in a 2-fold increase in work from the first three (75.4±14.1 kJ) to the last three sessions (150.7±28.4 kJ). Outcome measures included lower-limb lean mass, VL CSA, static strength, and peak and average cycling power output. These measures and VL samples were taken before and 4–5 days after the last training session.
Results.
Significant (P<0.05) increases in integrin-β1 (1.64-fold) and RICTOR (2.99-fold) protein as well as the phosphorylated-to-total ILK ratio (1.70-fold) were found, but integrin-α7 and Akt did not change. Increases in lower-limb, thigh, and trunk lean mass (2.8–5.3%, P<0.05) and CSA (13.3±9.0%, P<0.001) were observed. Static strength (18.1±10.8%) and both peak (8.6±10.5%) and average power output (7.4±8.3%) also increased (P<0.05). However, no significant correlations were found between the magnitude of increases in protein and the magnitude of increases in CSA, static strength, or power output.
Conclusion.
In addition to increased muscle mass, strength, and power, we demonstrate that eccentric cycling training increases integrin-β1 and RICTOR total protein and p-ILK/t-ILK, which may play a role in protection against muscle damage as well as anabolic signaling to induce muscle adaptations.
Keywords: eccentric exercise, integrin-β1, integrin-linked kinase, rapamycin-insensitive companion of mTOR, muscle cross-sectional area, lean mass
INTRODUCTION
Integrins are transmembrane glycoproteins that link the extracellular matrix (ECM) to the actin cytoskeleton to facilitate important cellular functions including migration, adhesion, and differentiation (1). Integrins are classified based on their β subunits, which form heterodimers with α subunits (2). The α7β1 integrin heterodimer is highly expressed within skeletal muscle and is essential for the maintenance of structural integrity, as the absence of the α7 integrin subunit in mice results in a progressive form of muscular dystrophy (3). The α7β1 integrin is also an important component of mechanosensing in skeletal muscle during contraction, relaying information across the membrane in a bidirectional manner (4).
Resistance exercise traditionally includes both shortening (concentric) and lengthening (eccentric) muscle actions. The mechanical force generated during unaccustomed eccentric muscle actions, while not metabolically demanding, causes ultrastructural disruption to sarcomeres and membrane damage that is frequently accompanied by immune cell infiltration, muscle functional loss and soreness for several days post-exercise (5–7). The myofiber initiates a stress response following damage, including upregulation of proteins necessary for repair and increased resilience to future mechanical stresses. While the entire repertoire of proteins responsible for adaptation has not been fully characterized, studies have reported consistent increases in α7β1 integrin mRNA and protein in both human and rodent skeletal muscle following acute eccentric exercise (1, 8–10). Mice genetically altered to overexpress the α7 integrin in skeletal muscle (MCK:α7BX2 integrin) are protected from mechanical-induced damage and demonstrate enhanced muscle hypertrophy following acute and repeated bouts of eccentric exercise (1, 11–13). While increased integrin protein at the membrane likely increases ECM adhesion in a manner that may or may not involve the integrin-linked kinase (ILK) (14, 15), the molecular mechanisms responsible for integrin-mediated myofiber remodeling and growth remain unknown.
It has been well established that mTOR complex 1 (mTORC1) plays a central role in mechanical load-induced skeletal muscle growth (16), but less is known about the role of mTOR complex 2 (mTORC2) (17). mTORC2 binds with the rapamycin-insensitive companion of mTOR (RICTOR), and this complex can activate Akt (protein kinase B) to regulate cytoskeletal organization and survival (18). Moreover, ILK can directly bind RICTOR and facilitate mTORC2-mediated Akt phosphorylation and cell survival in cancer cells, as well as cytoskeletal organization in HeLa cells (19). These findings suggest the possible existence of an integrin-ILK-RICTOR-Akt protein complex in mammalian cells. Interestingly, Ogasawara and Suginohara (20) demonstrated that long-term (>6 h) elevations in protein synthesis were insensitive to the administration of rapamycin, suggesting that a mTORC1-independent or rapamycin-insensitive pathway might exist to support myofiber growth in response to muscle contraction. When combined with the hypertrophic effects of increased integrin protein after eccentric exercise in mice (1, 11–13), the studies above suggest that eccentric exercise may promote the formation of the integrin-ILK-RICTOR-Akt protein complex at the fiber membrane, which could serve to facilitate long-term adaptations to training.
Large-volume eccentric exercise, including downhill running, has been used in previous studies to examine increases in integrin mRNA and protein in mouse skeletal muscle (1, 8). Although as few as 100 – 150 maximal eccentric muscle actions can induce increases in integrin-β1 gene expression at 3 – 24 h post-exercise (9, 10), the opportunity to introduce a greater number of eccentric muscle actions in a controlled manner exists. In eccentric cycling, the knee extensors perform only eccentric muscle actions, and the number of the actions within a 10-min cycling bout may exceed 500 (60 rpm × 10 min = 600). Eccentric cycling was used in the present study, because it was considered to be safer than downhill running due to reduced loading impact on lower extremity joints, and because it is easier to control exercise intensity, volume, and cadence. Moreover, eccentric cycling has been shown to be effective for increasing muscle mass (over 50% increase in myofiber cross-sectional area) in 24 – 33 sessions (21, 22). Thus, eccentric cycling appears to be a safe, well-controlled, and effective method to examine changes in both muscle mass and integrin-ILK-RICTOR-Akt complex proteins.
No previous studies to our knowledge have examined the effects of eccentric cycling on human muscle integrin or integrin-associated proteins. Given the important structural (protection against damage) and physiological (hypertrophic signaling) outcomes associated with integrin protein in muscle, and the lack of knowledge on the responses of the integrin-ILK-RICTOR-Akt signaling complex in response to eccentric exercise training, the purpose of this study was to investigate the extent to which eccentric cycling training could increase integrin-ILK-RICTOR-Akt complex protein. A secondary aim was to examine whether baseline, or changes in, integrin-ILK-RICTOR-Akt complex proteins would correlate with changes in muscle function or muscle cross-sectional area after the training.
METHODS
Participants and study design
Eleven healthy young men (age: 22.7 ± 4.0 years, body mass: 88.4 ± 20.5 kg, height: 179.5 ± 7.8 cm) took part in the study. They had not performed eccentric exercise nor partaken in lower-limb exercise routines in the past 6 months, were not under any medication, and reported no history of orthopedic lower limb injuries or neurological disorders. They were instructed to refrain from exercise, caffeine, and alcohol for 48 h before each testing session, and to keep a consistent diet throughout the study. All participants completed a written informed consent form and a medical questionnaire before participating in the study. Ethical approval from the institution’s human research ethics committee was sought before study commencement.
When the participants reported to the laboratory for the first time, body mass and height, and a dual-energy X-ray absorptiometry (DEXA) scan for body composition were taken followed by a muscle biopsy. On the next two separate days, they were familiarized with eccentric cycling as well as static strength and maximal concentric cycling tests performed on an eccentric ergometer (Grucox Eccentric Trainer, Grucox, South Africa). At least 72 h later, a baseline testing session was completed in which a B-mode ultrasound scan was obtained for vastus lateralis cross-sectional area (CSA) measurement, and both static strength and maximal concentric cycling tests after a 2-min warm-up on the eccentric ergometer at 50 W were completed. After these tests, each participant performed the first eccentric cycling training session, as shown below. The training was performed 2–3 times a week for 24 sessions; all participants completed the sessions in 8 – 10 weeks, with some participants requiring additional rest days between sessions to recover from muscle soreness or fatigue. The post-training measures were taken 4 days after the last training session for the CSA and measures on the ergometer. On the following morning (5 days after the last training session), body composition assessment by DEXA and muscle biopsy sampling were conducted after an overnight fast.
Eccentric cycling
The participants performed 24 sessions of eccentric cycling in which they were instructed to consistently (smoothly) resist the backward rotations of the pedals while maintaining a target power output displayed on a computer screen set in front of the ergometer. The pedal straps were removed from the ergometer to ensure the participants only pushed against the pedals and did not pull when the pedals were moving away from their bodies to generate power using their knee and hip flexors (23, 24). In each session, participants completed 2 min of concentric cycling at 50 W as a warm-up before performing eccentric cycling.
As shown in Table 1, the intensity of the first three sessions was 33% of the average power output measured during a 10-s isokinetic concentric cycling sprint at 60 rpm. This intensity was determined based on our pilot studies in which the cycling effort was rated 3 – 4 out of 10 on the scale explained below. Eccentric cycling intensity was increased by 10% of the initial intensity at sessions 4, 10, 16, and 22 so that the intensity of the last three sessions (sessions 22–24) was 40% greater than that of the first three sessions. The cycling time was 1 min per set, and the number of sets was increased from 7 to 10, with an increment every 6 sessions. An interval training protocol was chosen as it has been shown that higher intensities of eccentric work can be performed with the interval than continuous eccentric cycling protocol (25).
Table 1.
Average ± SD (range) power, intensity and number of sets performed during eccentric cycling training protocol over the 24 sessions.
| Session | Power (W) | Intensity | Number of sets |
|---|---|---|---|
| 1 – 3 | 179.6 ± 33.7 (123 – 234) | 33% of APO | 7 |
| 4 – 6 | 197.6 ± 37.2 (135 – 258) | +10% of sessions 1–3 | 7 |
| 7 – 9 | 197.6 ± 37.2 (135 – 258) | 8 | |
| 10 – 12 | 215.4 ± 40.4 (147 – 281) | +20% of sessions 1–3 | 8 |
| 13 – 15 | 215.4 ± 40.4 (147 – 281) | 9 | |
| 16 – 18 | 233.1 ± 43.9 (159 – 304) | +30% of sessions 1–3 | 9 |
| 19 – 21 | 233.1 ± 43.9 (159 – 304) | 10 | |
| 22 – 24 | 251.2 ± 47.4 (171 – 328) | +40% of sessions 1–3 | 10 |
APO: Average power output during maximal concentric cycling at 60 rpm for 10 s.
The rate of perceived effort during the cycling was assessed using a modified version of Borg’s category-ratio scale (0 –10; 0: nothing at all, 10: maximal effort) (26), as it has been reported that measuring “effort” is more valid than “exertion” during eccentric cycling (27). At the end of each set, participants were asked to rank the physical effort required by their knee extensors to maintain the target power while cycling.
Peak and average power output during eccentric cycling
Using the eccentric cycle ergometer, the peak (PPO) and average (APO) power outputs during maximal concentric cycling in isokinetic mode at 60 rpm for 10 s were assessed. Power data were sampled at 40 Hz and extracted to a spreadsheet that included the power of the left and right legs separately during the cycling. The maximum and average powers achieved for each leg were calculated, and the average of the right and left legs was used for further analyses.
Static strength test
Each participant performed a static strength test on the eccentric ergometer, using the “Static Strength Test” mode provided in the ergometer software. In this test, the pedals of the ergometer pushed the foot of the participant upward and the force applied was incrementally increased while the participant was instructed to keep the shaft of the ergometer crank parallel to the floor, and to remain seated. The torque at which the participant failed to provide sufficient resistance (i.e., braking strength) was recorded. The left leg was tested before the right leg, and the average of the two legs was used for further analyses.
Body composition
Participants were asked to come to the laboratory in a rested, fasted, and euhydrated state at the same time in the morning, wearing the same clothing for each scan; a researcher checked that these instructions were followed. The built-in computer software (Version 12.4; QDR for Windows, Hologic, USA) of a DEXA (Horizon A, Hologic Inc., USA) displayed the lean tissue mass of the trunk and lower limbs (sum of both left and right legs). An additional segmental analysis of the right thigh (biopsy site) of each participant was performed following the method of Hart et al. (28).
Vastus lateralis cross-sectional area
VL CSA was measured using B-mode ultrasonography (Aloka SSD-alpha10, Aloka Co., Japan) with the extended field-of-view function, based on the method of Trezise et al. (29). Before the measurement, each participant was asked to rest for 20 min in a horizontal position to allow fluid shifts to stabilize. Water-soluble gel was placed on the probe to promote acoustic coupling. Each participant laid supine with the legs fully extended and leg muscles relaxed. A rolled towel was placed under the knee joint to remove muscle compression. A perpendicular line was drawn at 50% of the distance from the femoral lateral epicondyle to the greater trochanter, and the probe was moved transversely across the thigh while taking a continuous single view and applying consistent pressure throughout the motion. This ensured that CSA was obtained at the same muscle region as the muscle biopsy sample was taken, as described below. Three images were captured at each time point and analyzed three times per image using image analysis software (ImageJ, National Institute of Health, USA). The mean of the values was used for subsequent analyses.
Muscle sampling
Muscle samples were obtained from the right vastus lateralis (VL) of each participant at 7 – 10 days before the first exercise session to allow for adequate tissue recovery after the biopsy procedure, and 5 days after the last exercise session to minimize the effects of potential swelling on the DEXA and ultrasound scanning outcomes. Local anesthetic cream (Emla 5% Cream, AstraZeneca, UK) was applied around the sample site 40 min before taking the muscle biopsy. The pre-training biopsy sample was taken at 50% distance between the greater trochanter and lateral epicondyle using a disposable, spring-loaded microbiopsy system (14g × 10cm, MAX-CORE, Bard Biopsy Systems, USA). The post-training sample was taken 2 cm adjacent to the first one, randomly selected between participants to be either more proximal or distal. From each site, three or four muscle samples were extracted for a total of at least 50 mg of tissue. The tissue samples were frozen immediately in liquid nitrogen and stored at −80°C for later analyses.
Western blotting
Muscle biopsy samples were homogenized using a handheld tissue homogenizer (Fisher Scientific) in ice-cold homogenization buffer containing 50 mM Tris-HCl (pH = 7.4), 150mM NaCl, 1 mM EDTA, 1% NP-40, 0.5% Sodium Deoxycholate, and 0.1% SDS, supplemented with protease inhibitor (Roche Complete Mini™) and phosphatase inhibitor (Roche PhosSTOP™). Homogenates were incubated on an inverter at 4°C for 1 h and then centrifuged at 14,000g for 5 min at 4°C. The supernatant was collected and stored for further analyses. Total protein measured with Bradford assay using bovine serum albumin (BSA) for the standard curve.
Equal amounts of protein (50 μg for all other proteins apart from integrins which required 100 μg) were reduced with Laemmli sample buffer containing dithiothreitol at 95°C and separated in 8% acrylamide gels by SDS-PAGE. Proteins were transferred onto nitrocellulose membranes, and Ponceau S stain was used to verify equal loading of samples. Membranes were blocked in tris-buffered saline solutions containing 0.1% Tween20 (TBS-T) with 5% non-fat dry milk (for integrin blots) or 5% BSA (for other blots) for 1 h and then incubated with appropriate primary antibodies overnight at 4°C. After several washes in TBST, membranes were incubated with an HRP-conjugated secondary antibody for 1 h. The following antibodies were used in the current study: integrin-α7 (1:500, Abcam, Ab182941), integrin-β1D (1:1000, a kind gift from Woo Keun Song, Kwangju Institute of Science and Technology, Korea) (30), RICTOR (1:1000, Cell Signaling Technology, 2114), phospho ILK [Ser246], (1:1000, Sigma Aldrich (MilliporeSigma), AB1076), total ILK (1:1000, Cell Signaling Technology, 3862), phospho AKT [Ser473] (Cell Signaling Technology, 9271), total AKT (Cell Signaling Technology, 9272) and anti-Rabbit IgG HRP-conjugated antibody (1:2000, Cell Signaling Technology, 7074). Target proteins on the membrane were visualized using HRP Chemiluminescent Substrate (Thermo Scientific) and imaged with a Bio-Rad ChemiDoc imaging system. Quantitative analyses of the images were completed in Quantity One software (Bio-Rad).
Statistical analysis
The data were assessed for assumptions of normality using a Shapiro-Wilk test. Paired Student’s t-tests and Hedges’ gav effect sizes were used to assess changes in the criterion measures from pre- to post-training (31). For data that were non-normally distributed, a Wilcoxon signed-rank test was used. Pearson’s correlation coefficients (r) between baseline and changes in the molecular levels with changes in muscle CSA and function were calculated. As the data, even after log transformation, did not satisfy the assumption of normality, Spearman’s rank order correlation (ρ) coefficients were computed for non-normally distributed data. Using Cohen’s categories, we interpreted effect sizes ≥ |0.1| to |0.2| as trivial, from ≥ |0.2| to < |0.5| as small, from ≥ |0.5| to < |0.8| as moderate and scores of d ≥ |0.8| as a large effect (32). The significance level was set to P ≤ 0.05. All statistical testing was performed using Jamovi version 1.6.16 (Jamovi project, 2018). Data are presented as mean ± standard deviation (SD).
RESULTS
Eccentric cycling training
All participants completed the 24 sessions as planned, but the intensity varied between participants. Figure 1 shows the total mechanical work and effort for every 3 sessions over the 24 sessions. A significant (P < 0.01) increase in the effort was evident from the first three sessions (4.0 ± 1.7) to the last three training sessions (6.3 ± 1.0) with increases in average intensity from 180.0 ± 33.7 W to 251.0 ± 47.4 W (P < 0.001) and total mechanical work from 75.4 ± 14.1 to 150.7 ± 28.4 kJ (P < 0.001).
Figure 1.
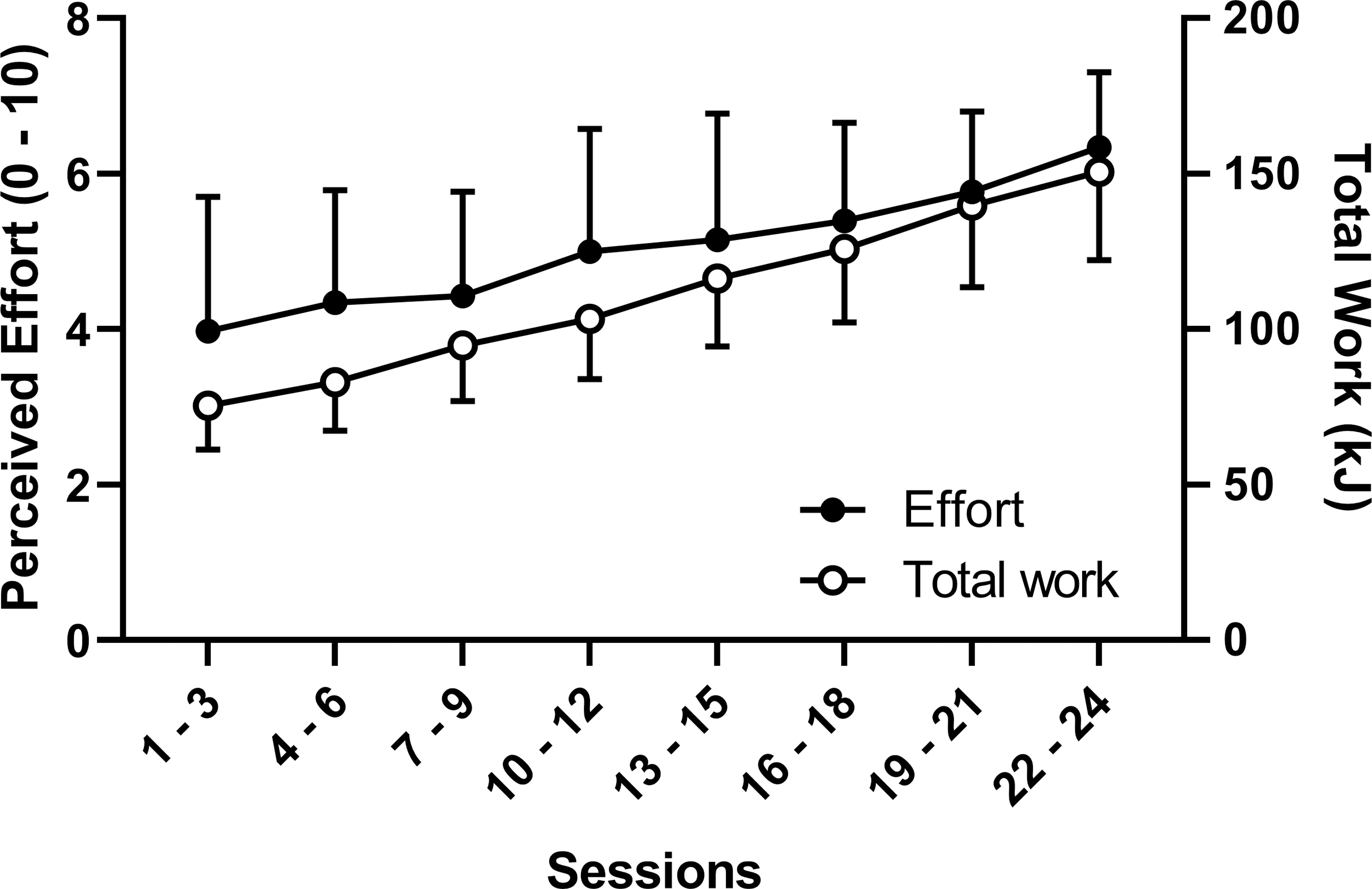
Three-session averages (mean and SD of 11 participants) of perceived effort (left Y-axis) and total mechanical work (right Y-axis) over 24 sessions of eccentric cycling training.
Lean mass
Right thigh lean mass increased significantly (P = 0.001, gav = 0.252) by 5.3 ± 4.3% from baseline (7,779 ± 1,416 g) to post-training (8,153 ± 1,332 g). Lower limb lean mass (left and right legs combined) increased significantly (P = 0.018, gav = 0.152) by 3.1 ± 3.6% from baseline (21,915 ± 3,730 g) to post-training (22,503 ± 3,399 g). Trunk lean mass also increased significantly (P = 0.006, gav = 0.114) by 2.8 ± 3.1% from baseline (29,535 ± 5,951 g) to post-training (30,238 ± 5,418 g).
VL cross-sectional area
As shown in Figure 2A, CSA increased significantly (P < 0.001, gav = 0.53) from baseline (2,755 ± 590 mm2) to post-training (3,100 ± 617 mm2) by 13.3 ± 9.0%. The range of increases varied between participants (3.5 – 34.0%), with five participants having an increase less than 10%, four between 11 – 20%, and two greater than 20%.
Figure 2.
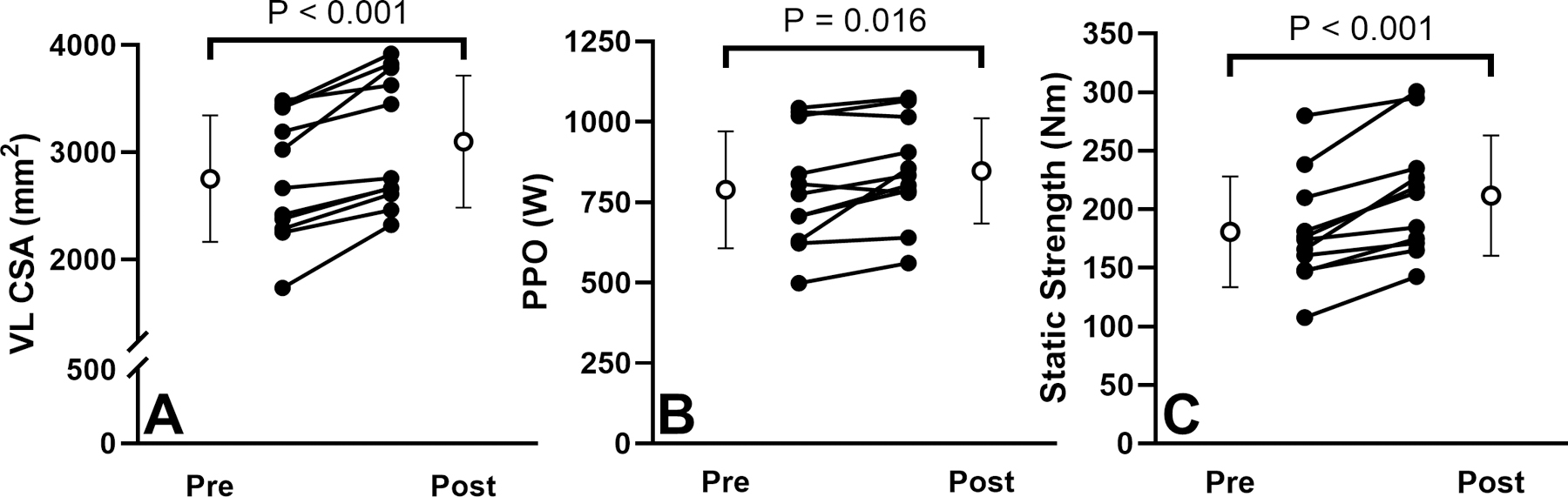
Changes in vastus lateralis cross-sectional area (A), peak power output during a 10-s maximal concentric cycling effort at 60 rpm (B) and static strength (C) from pre- to post-training for 11 participants (mean and standard deviation are shown in white circles).
Average (APO) and peak power output (PPO)
APO increased significantly (P = 0.008, gav = 0.275) from baseline (531.9 ± 116.6 W) to post-training (566.8 ±109.5 W) by 7.4 ± 8.3% and ranged −3.4% to 28.0%, with two participants showing a decrease, six participants showing an increase less than 10%, and three with more than 11% increase. Similarly, a significant increase (P = 0.016, gav = 0.313) was found for PPO from baseline (788.8 ± 181.5 W) to post-training (847.4 ± 163.4 W) by 8.6 ± 10.5%, with a range −3.4% to 35.0% (Figure 2B).
Static strength
A significant increase (P < 0.001, gav = 0.58) in lower-limb static strength by 18.1 ± 10.8% was observed from baseline (180.7 ± 47.2 Nm) to post-training (211.7 ± 51.4 Nm). The increase ranged from 5.4% to 31.4%, with three participants having an increase between 0 – 10%, four between 11 – 20%, and four greater than 20% (Figure 2C).
Integrin-ILK-RICTOR-Akt Protein
Figure 3 shows the blots of the tested proteins for each participant, and Figure 4 shows the changes in protein. Integrin-β1D protein increased 1.64-fold (P = 0.046, gav = 0.818), ranging 0.43 – 10.2-fold with three participants showing a decrease, four showing increases less than 2-fold, and four showing an increase greater than 2-fold. RICTOR protein increased 3.0-fold (P = 0.019, gav = 0.64) on average, ranging 0.7 – 20.4-fold with two participants showing a decrease, six showing an increase less than 4-fold, and three greater than 4-fold. The p-ILK/t-ILK ratio increased 1.7-fold in average (P = 0.023, gav = 0.83) and ranging 0.65 – 13.9-fold, with two participants showing a decrease, eight showing an increase less than 4-fold, and one greater than 4-fold. No statistically significant changes were observed in the levels of integrin-α7, p-Akt, t-Akt, p-Akt/t-Akt, p-ILK or t-ILK after the training.
Figure 3.
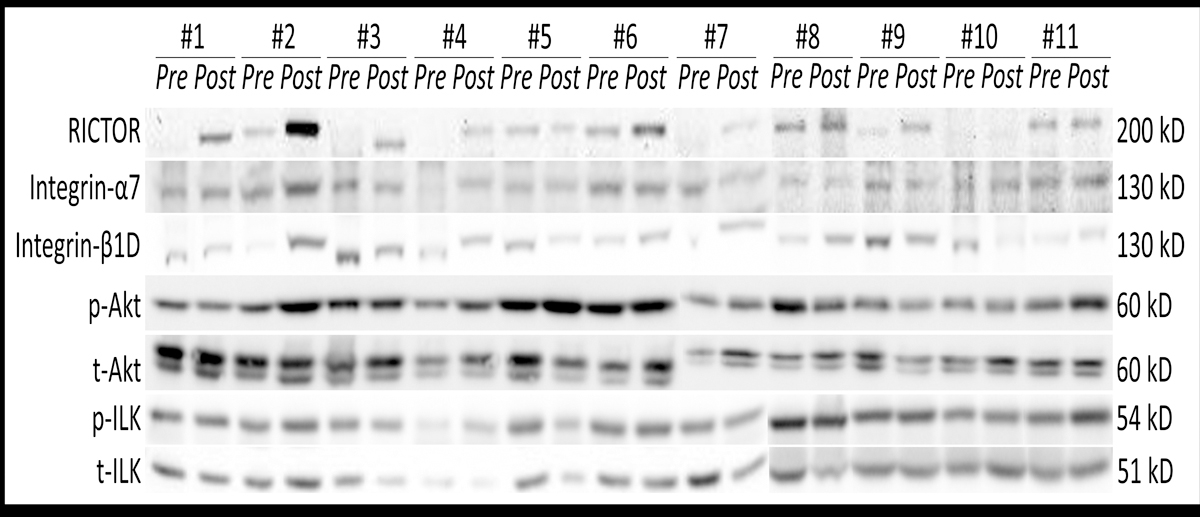
RICTOR, integrin-α7, integrin-β1D, phosphorylated Akt (AktSer473), total (t-Akt), phosphorylated (ILKSer246) and total (t-ILK) immunoblots from samples obtained pre- and post-training from the participants. The molecular weight for each protein is indicated on the right.
Figure 4.
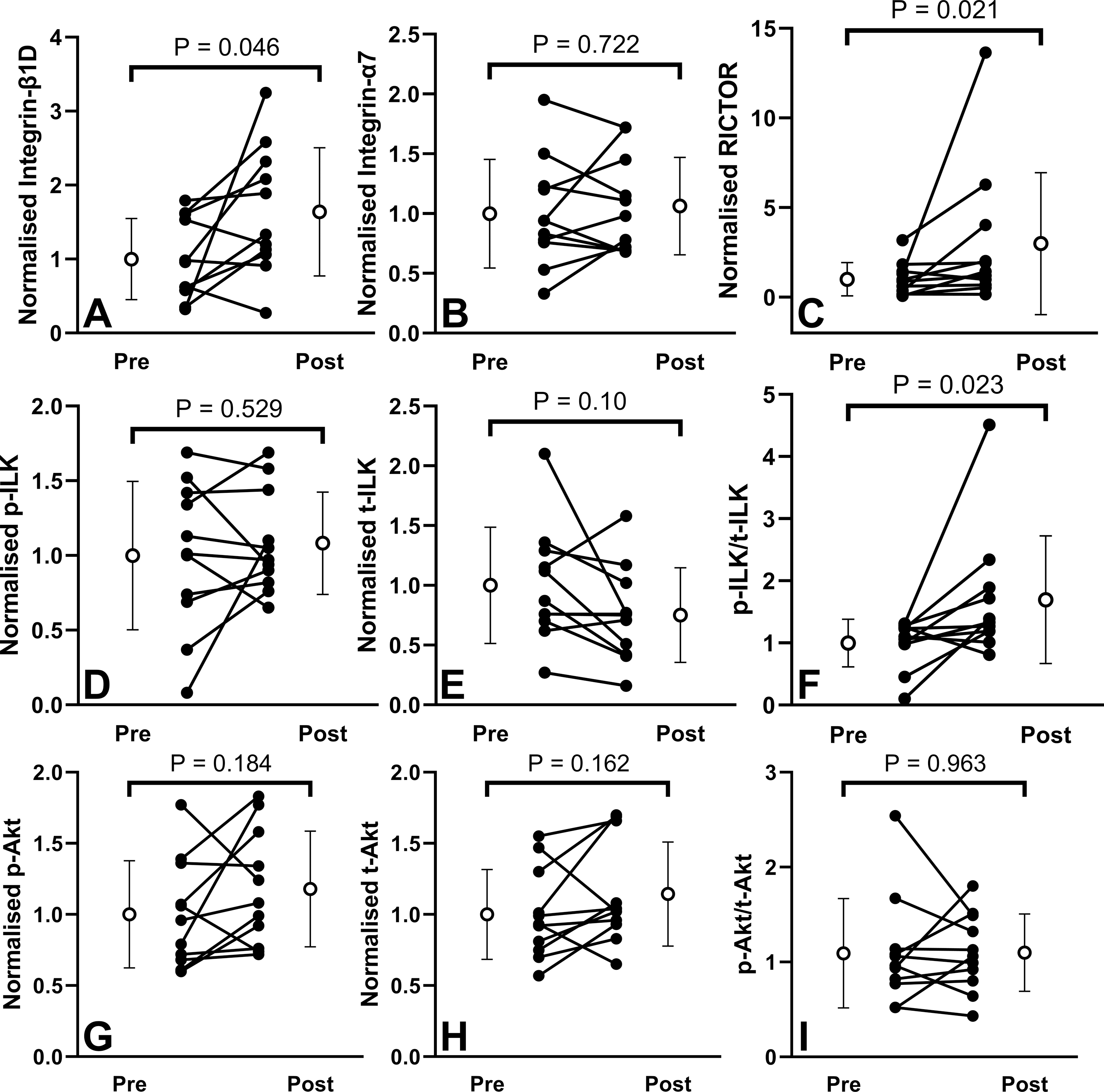
Changes in integrin-β1 (A), integrin-α7 (B), RICTOR (C), phosphorylated ILK (ILKSer246) (D), total (t-ILK) (E), p-ILK/t-ILK ratio (F), phosphorylated (AktSer473) (G), total (t-Akt) (H), and p-Akt /t-Akt ratio (I), normalized to Ponceau S staining. Values depict pre- to post-training for 11 participants (mean and standard deviation shown by white circles).
No significant correlations were evident between integrin-β1D and RICTOR (ρ = −0.018, P = 0.968), integrin-β1D and p-ILK/t-ILK (ρ = 0.328, P = 0.325), integrin-β1D and p-Akt (ρ = 0.445, P = 0.173), RICTOR and p-Akt (ρ = 0.364, P = 0.273), or RICTOR and p-ILK/t-ILK (ρ = −0.055, P = 0.873) before training. Similarly, there were no significant correlations between the changes in integrin-β1D and RICTOR (ρ = 0.300, P = 0.371), changes in integrin-β1D and p-Akt (ρ = 0.465, P = 0.150), changes in integrin-β1D and p-ILK/t-ILK (ρ = 0.118, P = 0.734), changes in RICTOR and p-Akt (ρ = 0.114, P = 0.739), or changes in RICTOR and p-ILK/t-ILK (ρ = 0.027, P = 0.946) before and after training. However, positive correlations in integrin-β1D and RICTOR (ρ = 0.627, P = 0.044) were observed post-training, although no correlations were observed between RICTOR and p-ILK/t-ILK (ρ = 0.182, P = 0.592) or integrin-β1D and p-ILK/t-ILK (ρ = 0.132, P = 0.699).
Correlations between protein and muscle CSA and functional changes
Figure 5 shows results for some of the correlation analyses between baseline and change scores for integrin and RICTOR total protein and CSA and muscle function changes. No significant correlations were observed between the changes in CSA and baseline integrin-β1D protein (r = −0.355, P = 0.284) or baseline RICTOR (ρ = −0.145. P = 0.673). No significant correlations were found between changes in static strength and either baseline integrin-β1D (r = 0.288, P = 0.501) or baseline RICTOR (ρ = −0.245. P = 0.468) protein. Similarly, no significant correlations were found between changes in PPO and baseline integrin-β1D (r = 0.051, P = 0.881) or baseline RICTOR (ρ = 0.164. P = 0.634).
Figure 5.
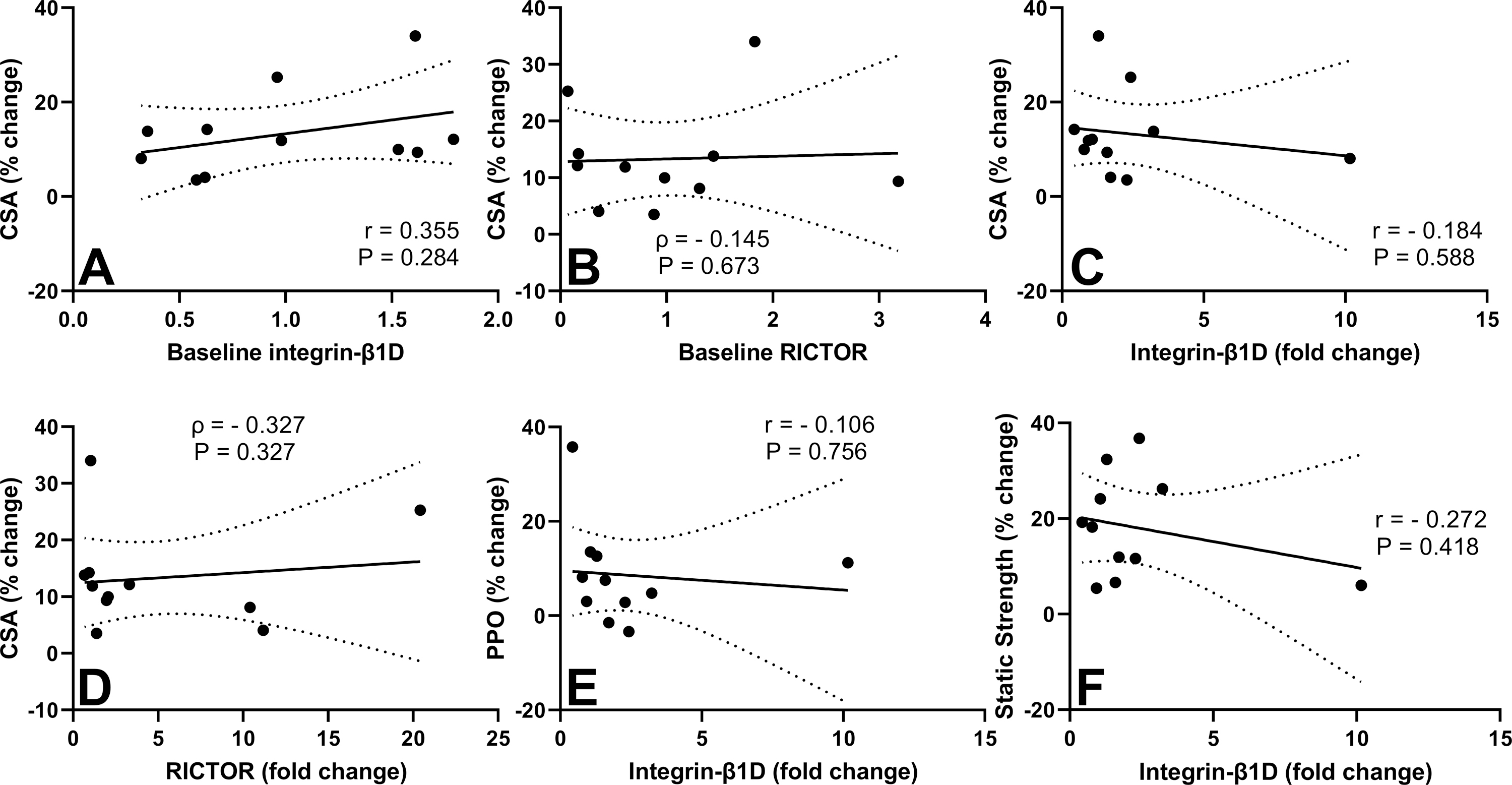
Scatterplots of relationships between baseline integrin-β1D and percent change in vastus lateralis cross-sectional area (VL CSA) (A), baseline RICTOR and percent change in VL CSA (B), fold-change in integrin-β1D and percent change in VL CSA (C), fold-change in RICTOR and percent change in VL CSA (D), fold-change in integrin-β1D and percent change in peak power output (PPO) (E), and fold-change in integrin-β1D and percent change in static strength (F). Pearson’s r, Spearman’s ρ and their respective P values are indicated in each graph. Dotted lines demonstrate 95% CIs for the linear regression line.
Regarding the changes from pre- to post-training, no significant correlations were found between CSA and integrin-β1D (r = −0.184, P = 0.588) or RICTOR (ρ = −0.327. P = 0.327) protein, or between static strength and integrin-β1D (r = −0.272, P = 0.418) or RICTOR (ρ = −0.055. P = 0.881) protein. No significant correlations were found between changes in PPO and integrin-β1D (r = −0.106, P = 0.756) or RICTOR (ρ = −0.391. P = 0.237) protein. The magnitude of increase in CSA was correlated with the magnitude of increase in static strength (r = 0.782, P = 0.004) but not PPO (r = 0.114, P = 0.739).
DISCUSSION
The present data show that the eccentric cycling training increased upper thigh (5.3%), whole leg (3.1%) and trunk (2.8%) lean mass, VL CSA (13%), average (7.4%) and peak (8.6%) concentric power outputs, and lower-limb static strength (18.1%) (Figure 2). Significant increases in integrin-β1D (1.64-fold), RICTOR (3-fold), and p-ILK/t-ILK (1.7-fold) were observed from pre- to post-eccentric cycling training (Figure 4). However, no significant correlations were observed between the magnitudes of increase in integrin-β1D, RICTOR, p-ILK/t-ILK and the magnitudes of increase in the muscle size, strength, and power parameters (Figure 5). These results suggest that eccentric cycling was a potent stimulus for changes in integrin, RICTOR, and ILK signaling, yet the role of these proteins in intrinsic or extrinsic muscle remodeling remains unknown.
As shown in Figure 1, the total work during the training period doubled from 75.4 ± 14.1 kJ from the first three to 150.7 ± 28.4 kJ to the last three training sessions. Although the perceived effort increased from 4.0 ± 1.7 to 6.3 ± 1.0 over the same time frame, it was not of a high magnitude, even in the highest intensity sessions. It should be noted that the exercise duration was short (10 min maximum) and the exercise intensity, which increased from ~180 to 250 W over the training period, was low when compared to the maximal possible eccentric cycling capacity, which could exceed 500 W (33). Despite this relatively lower-effort training, there were relatively larger muscle CSA and performance (strength and cycling power) increases than those reported in previous studies implementing eccentric exercise training (34).
The effects of eccentric cycling on lean mass, and muscle and myofiber CSA, have been shown in multiple studies (21, 22, 35, 36). For example, Julian et al. (36) reported a 1% (P < 0.01) increase in leg lean mass after 36 sessions of 10 – 30 min eccentric cycling at 30 – 70% of VO2peak in obese adolescents. In the present study, a much greater increase in leg lean mass (5.3%) was observed. Whilst LaStayo et al. (21) reported a 52% increase in VL fiber CSA after 28 sessions of 15-min eccentric cycling (54 – 65% of peak heart rate) in young healthy men, no previous study has reported changes in VL CSA after eccentric cycling training. Marzilger et al. (34) reported a 5.1% increase in VL CSA after 33 sessions of 15–100 isokinetic eccentric knee extensions at 100% of maximal voluntary isometric contraction strength. The intensity of eccentric muscle actions performed during the cycling in the present study was lower, however it induced a greater CSA increase (13.3%) than the maximal isokinetic eccentric exercise training (5.1%) reported by Marzilger et al. (34). It appears that the training protocol used in the present study was substantially effective for inducing muscle hypertrophy. This could potentially be due to the much greater number of eccentric contractions performed during training (420 – 600 per session) when compared with that (15 – 100) in the study of Marzilger et al. (34). Due to the capacity to increase muscle mass with brief exercise sessions (20 – 30 min weekly) performed at low effort levels (4 – 6 out of 10), eccentric cycling appears to provide clinical value for individuals who are frail and have limited exercise capacity. Future research should examine the optimal dosage and prescription of eccentric cycling to optimize outcomes for older adults and clinical populations.
The eccentric cycling training was also effective for increasing APO (7.4%), PPO (8.6%), and static strength (18.1%), as shown in Figure 2, although a large variation between participants existed. This increase is consistent with previous studies showing increases in cycling-specific performance parameters after eccentric cycling training (35, 37). For example, a 7% increase in PPO was reported after 21 sessions of 10 – 30 min eccentric cycling at 54 – 66% of maximum heart rate (37) and a 9% increase was reported after 16 sessions of 5 – 11 min of eccentric cycling at 20 – 55% of maximum concentric cycling power (35). Previous studies have also shown increases in maximal voluntary isometric knee extension torque after 36 sessions of 10 – 30 min eccentric cycling at 30 – 70% of VO2peak (28%) (36) as well as after 28 sessions of 15-min eccentric cycling at 54 – 65% of peak heart rate (36%) (21). Although the extent of muscle adaptation induced by the eccentric cycling training varied between participants in the present study, the majority showed increases in lean body mass, CSA, PPO, APO, and static strength (Figure 2). Interestingly, increases in muscle CSA were correlated with increases in static strength (r = 0.782, P = 0.004) but not PPO (r = 0.114, P = 0.739). Speculatively, this could suggest that some changes in muscle function after eccentric cycling training are influenced by increases in muscle size. Taken together, the eccentric cycling training protocol implemented in the current study clearly provided a potent stimulus to the knee extensors, resulting in increases in muscle function that were comparable to or greater than those reported in previous studies (21, 35–37).
This is the first study to document changes in human muscle integrin-associated proteins in response to eccentric cycling training, although one human study previously reported changes in integrin-β1 protein after resistance training (38). Li et al. (38) reported a 1.4-fold increase in VL integrin-β1 protein after 27 sessions of 4 sets × 10 maximal concentric-eccentric flywheel knee extensions in men, but integrin-β1 was 3-fold higher at an earlier time point (after the 10th training session). As shown in Figure 4, a significant increase in integrin-β1D (1.64-fold, gav = 0.818) was observed after the eccentric-only cycling training. It should be noted that the intensity and number of eccentric muscle actions were progressively increased in the present study, even though the intensity of eccentric muscle actions was always submaximal, unlike the study of Li et al. (38). As no muscle biopsy samples were obtained at a mid-training time point (i.e., after the 12th session) in the present study, it cannot be discounted that integrin-β1D protein might have been higher at some time other than at the end of training. Interestingly, Li et al. (38) also found a significant (1.2-fold) increase in VL integrin-β1 protein after 8 and 34 days of bed rest. These findings may suggest that integrin-β1 may not strictly increase in response to mechanical loading, but rather increase in response to ECM and cytoskeleton remodeling. Regardless, eccentric-only exercise can stimulate long-term changes in integrin-β1D muscle protein that persist for at least 5 days after the last exercise session. Further studies are required to elucidate the functional advantages of increased integrin-β1 in the remodeling of myofibers and the ECM.
Significant changes in integrin-α7 protein were not detected five days after training in the present study (Figure 4B). Similarly, no changes in integrin-α7 protein were detected after 18 sessions of electrically simulated isometric contractions in rats (39). However, integrin-α7 protein was increased 24 h after maximal eccentric knee extensions in humans (10) and downhill running in mouse gastrocnemius-soleus (1). These findings suggest that increases in integrin-α7 content may be more associated with short-term ECM remodeling in response to eccentric exercise-induced muscle damage (9) rather than long-term changes in ECM structural integrity.
To the best of our knowledge, no previous study has examined changes in of RICTOR or ILK protein in response to exercise training. The eccentric cycling training imposed in the present study evoked significant increases in RICTOR protein and ILK phosphorylation (Figure 4), and a significant correlation between the post-training integrin-β1D and RICTOR (ρ = 0.627) was observed. ILK has been shown to play important roles in the regulation of muscle protein synthesis and fiber characteristics, such as fiber type (40). Moreover, ILK and RICTOR are both involved in long-term activation of Akt, thereby regulating cell proliferation, growth, survival, and metabolism (41). Increases in Akt phosphorylation were not observed in the current study, yet the time point (5 days after the last exercise session) was likely too late to detect any change in activation. Alternatively, both RICTOR and ILK are localized to the membrane may serve as an important scaffolding proteins necessary for muscle integrity (42, 43). Therefore, ILK and RICTOR upregulation may represent a mechanism necessary to protect muscle against damage, which is maximal by two weeks of training. Further studies are necessary to examine integrin-ILK-RICTOR complex formation during exercise training and the importance of this complex to muscular integrity and adaptation.
It was an unexpected finding that no relationships existed between baseline levels or changes in integrin-ILK-RICTOR- protein and changes in muscle CSA or function after the eccentric cycling training (Figure 5). Speculatively, an outlier with large increases in integrin-β1D, RICTOR, and p-ILK/t-ILK could have disproportionately influenced the results (Figure 4), however, reanalysis with the outlier removed resulted in the same outcome. Nonetheless, myofiber CSA was not directly measured in the current study, and no differentiation can be made between type I and II myofibers. Thus, the possibility exists that correlations may exist when examined at the fiber level.
In conclusion, 24 sessions of eccentric cycling training increased integrin-ILK-RICTOR protein, VL CSA, and lean mass of legs and trunk as well as muscle performance (strength and cycling power). The increases in protein were not directly correlated with muscle CSA or performance. However, the present data reveal, for the first time, increases in the integrin-ILK-RICTOR complex proteins in human muscle after exercise training, which may be necessary for protection, remodeling and/or adaptation.
ACKNOWLEDGMENTS
This work was supported by a travel grant by Edith Cowan University to GM to analyze the muscle tissue samples in the Beckman Institute for Advanced Science and Technology, Illinois, USA. This work was partially supported by National Institute of Arthritis and Musculoskeletal and Skin Diseases Grant R21 AR 065578 to MDB.
Footnotes
CONFLICT OF INTEREST
The authors declare that they have no conflicts of interest. The results of the present study do not constitute endorsement by ACSM. The results of the study are presented clearly, honestly, and without fabrication, falsification, or inappropriate data manipulation.
REFERENCES
- 1.Boppart MD, Burkin DJ, Kaufman SJ. Alpha7beta1-integrin regulates mechanotransduction and prevents skeletal muscle injury. Am J Physiol, Cell Physiol 2006;290(6):1660–5. [DOI] [PubMed] [Google Scholar]
- 2.Hynes RO. Integrins: versatility, modulation, and signaling in cell adhesion. Cell 1992;69(1):11–25. [DOI] [PubMed] [Google Scholar]
- 3.Mayer U, Saher G, Fässler R, et al. Absence of integrin alpha 7 causes a novel form of muscular dystrophy. Nat Genet 1997;17(3):318–23. [DOI] [PubMed] [Google Scholar]
- 4.Boppart MD, Mahmassani ZS. Integrin signaling: Linking mechanical stimulation to skeletal muscle hypertrophy. Am J Physiol, Cell Physiol 2019;317(4):C629–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fridén J, Sjöström M, Ekblom B. Myofibrillar damage following intense eccentric exercise in man. Int J Sports Med 1983;4(3):170–6. [DOI] [PubMed] [Google Scholar]
- 6.Armstrong RB. Initial events in exercise-induced muscular injury. Med Sci Sports Exerc 1990;22(4):429–35. [PubMed] [Google Scholar]
- 7.Nosaka K, Clarkson PM. Muscle damage following repeated bouts of high force eccentric exercise. Med Sci Sports Exerc 1995;27(9):1263–9. [PubMed] [Google Scholar]
- 8.Boppart MD, Volker SE, Alexander N, Burkin DJ, Kaufman SJ. Exercise promotes alpha7 integrin gene transcription and protection of skeletal muscle. Am J Physiol Regul Integr Comp Physiol 2008;295(5):1623–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hyldahl RD, Nelson B, Xin L, et al. Extracellular matrix remodeling and its contribution to protective adaptation following lengthening contractions in human muscle. FASEB J 2015;29(7):2894–904. [DOI] [PubMed] [Google Scholar]
- 10.De Lisio M, Farup J, Sukiennik RA, et al. The acute response of pericytes to muscle-damaging eccentric contraction and protein supplementation in human skeletal muscle. J Appl Physiol 2015;119(8):900–7. [DOI] [PubMed] [Google Scholar]
- 11.Lueders TN, Zou K, Huntsman HD, et al. The α7β1-integrin accelerates fiber hypertrophy and myogenesis following a single bout of eccentric exercise. Am J Physiol Cell Physiol 2011;301(4):938–46. [DOI] [PubMed] [Google Scholar]
- 12.Zou K, Meador BM, Johnson B, et al. The α7β1-integrin increases muscle hypertrophy following multiple bouts of eccentric exercise. J Appl Physiol 2011;111(4):1134–41. [DOI] [PubMed] [Google Scholar]
- 13.Burkin DJ, Wallace GQ, Milner DJ, Chaney EJ, Mulligan JA, Kaufman SJ. Transgenic expression of α7β1 integrin maintains muscle integrity, increases regenerative capacity, promotes hypertrophy, and reduces cardiomyopathy in dystrophic mice. Am J Pathol 2005;166(1):253–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gheyara AL, Vallejo-Illarramendi A, Zang K, et al. Deletion of integrin-linked kinase from skeletal muscles of mice resembles muscular dystrophy due to α7β1-integrin deficiency. Am J Pathol 2007;171(6):1966–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang H-V, Chang L-W, Brixius K, et al. Integrin-linked kinase stabilizes myotendinous junctions and protects muscle from stress-induced damage. J Cell Biol 2008;180(5):1037–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Goodman CA, Frey JW, Mabrey DM, et al. The role of skeletal muscle mTOR in the regulation of mechanical load-induced growth. J Physiol 2011;589(Pt 22):5485–501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hodson N, McGlory C, Oikawa SY, et al. Differential localization and anabolic responsiveness of mTOR complexes in human skeletal muscle in response to feeding and exercise. Am J Physiol Cell Physiol 2017;313(6):C604–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Facchinetti V, Ouyang W, Wei H, et al. The mammalian target of rapamycin complex 2 controls folding and stability of Akt and protein kinase C. EMBO J 2008;27(14):1932–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sarbassov DD, Ali SM, Kim D-H, et al. Rictor, a novel binding partner of mTOR, defines a rapamycin-insensitive and raptor-independent pathway that regulates the cytoskeleton. Curr Biol 2004;14(14):1296–302. [DOI] [PubMed] [Google Scholar]
- 20.Ogasawara R, Suginohara T. Rapamycin-insensitive mechanistic target of rapamycin regulates basal and resistance exercise–induced muscle protein synthesis. FASEB J 2018;32(11):5824–34. [DOI] [PubMed] [Google Scholar]
- 21.LaStayo PC, Pierotti DJ, Pifer J, Hoppeler H, Lindstedt SL. Eccentric ergometry: increases in locomotor muscle size and strength at low training intensities. Am J Physiol Regul Integr Comp Physiol 2000;278(5):R1282–1288. [DOI] [PubMed] [Google Scholar]
- 22.LaStayo PC, Ewy GA, Pierotti DD, Johns RK, Lindstedt S. The positive effects of negative work: increased muscle strength and decreased fall risk in a frail elderly population. J Gerontol A Biol Sci Med Sci 2003;58(5):M419–24. [DOI] [PubMed] [Google Scholar]
- 23.Mavropalias G, Koeda T, Barley OR, et al. Comparison between high- and low-intensity eccentric cycling of equal mechanical work for muscle damage and the repeated bout effect. Eur J Appl Physiol 2020;120(5):1015–25. [DOI] [PubMed] [Google Scholar]
- 24.Mavropalias G, Calapre L, Morici M, et al. Changes in plasma hydroxyproline and plasma cell-free DNA concentrations after higher- versus lower-intensity eccentric cycling. Eur J Appl Physiol 2021;121(4):1087–97. [DOI] [PubMed] [Google Scholar]
- 25.Lipski M, Abbiss CR, Nosaka K. Oxygen consumption, rate of perceived exertion and enjoyment in high-intensity interval eccentric cycling. Eur J Sport Sci 2018;18(10):1390–7. [DOI] [PubMed] [Google Scholar]
- 26.Borg G Borg’s perceived exertion and pain scales Champaign, IL: Human Kinetics; 1998. [Google Scholar]
- 27.Peñailillo L, Mackay K, Abbiss CR. Rating of perceived exertion during concentric and eccentric cycling: are we measuring effort or exertion? Int J Sports Physiol Perform 2018;13(4):517–23. [DOI] [PubMed] [Google Scholar]
- 28.Hart NH, Nimphius S, Spiteri T, Cochrane JL, Newton RU. Segmental musculoskeletal examinations using dual-energy x-ray absorptiometry (DXA): positioning and analysis considerations. J Sports Sci Med 2015;14(3):620–6. [PMC free article] [PubMed] [Google Scholar]
- 29.Trezise J, Collier N, Blazevich AJ. Anatomical and neuromuscular variables strongly predict maximum knee extension torque in healthy men. Eur J Appl Physiol 2016;116(6):1159–77. [DOI] [PubMed] [Google Scholar]
- 30.Kim DJ, Park SH, Lim CS, Chun J-S, Kim J-K, Song WK. Cellular localization of integrin isoforms in phenylephrine-induced hypertrophic cardiac myocytes. Cell Biochemistry and Function 2003;21(1):41–8. [DOI] [PubMed] [Google Scholar]
- 31.Calculating Lakens D. and reporting effect sizes to facilitate cumulative science: a practical primer for t-tests and ANOVAs. Front Psychol 2013;4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cohen J Statistical power analysis for the behavioral sciences Routledge; 1988. 400 p. [Google Scholar]
- 33.Lipski M, Abbiss CR, Nosaka K. Cardio-pulmonary responses to incremental eccentric and concentric cycling tests to task failure. Eur J Appl Physiol 2018;118(5):947–57. [DOI] [PubMed] [Google Scholar]
- 34.Marzilger R, Bohm S, Mersmann F, Arampatzis A. Effects of lengthening velocity during eccentric training on vastus lateralis muscle hypertrophy. Front Physiol 2019;10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Leong CH, McDermott WJ, Elmer SJ, Martin JC. Chronic eccentric cycling improves quadriceps muscle structure and maximum cycling power. Int J Sports Med 2014;35(7):559–65. [DOI] [PubMed] [Google Scholar]
- 36.Julian V, Thivel D, Miguet M, et al. Eccentric cycling is more efficient in reducing fat mass than concentric cycling in adolescents with obesity. Scand J Med Sci Sports 2019;29(1):4–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Elmer S, Hahn S, McAllister P, Leong C, Martin J. Improvements in multi-joint leg function following chronic eccentric exercise. Scand J Med Sci Sports 2012;22(5):653–61. [DOI] [PubMed] [Google Scholar]
- 38.Li R, Narici MV, Erskine RM, et al. Costamere remodeling with muscle loading and unloading in healthy young men. J Anat 2013;223(5):525–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ogasawara R, Nakazato K, Sato K, Boppart MD, Fujita S. Resistance exercise increases active MMP and β1‐integrin protein expression in skeletal muscle. Physiol Rep 2014;2(11). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mathes S, Vanmunster M, Bloch W, Suhr F. Evidence for skeletal muscle fiber type-specific expressions of mechanosensors. Cell Mol Life Sci 2019;76(15):2987–3004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.McDonald PC, Oloumi A, Mills J, et al. Rictor and integrin-linked kinase interact and regulate Akt phosphorylation and cancer cell survival. Cancer Res 2008;68(6):1618–24. [DOI] [PubMed] [Google Scholar]
- 42.Mackinnon AC, Qadota H, Norman KR, Moerman DG, Williams BD. C. elegans PAT-4/ILK functions as an adaptor protein within integrin adhesion complexes. Curr Biol 2002;12(10):787–97. [DOI] [PubMed] [Google Scholar]
- 43.Ebner M, Sinkovics B, Szczygieł M, Ribeiro DW, Yudushkin I. Localization of mTORC2 activity inside cells. J Cell Biol 2017;216(2):343–53. [DOI] [PMC free article] [PubMed] [Google Scholar]


