Abstract
Salmonella typhimurium phage type DT104 has become an important emerging pathogen. Isolates of this phage type often possess resistance to ampicillin, chloramphenicol, streptomycin, sulfonamides, and tetracycline (ACSSuT resistance). The mechanism by which DT104 has accumulated resistance genes is of interest, since these genes interfere with treatment of DT104 infections and might be horizontally transferred to other bacteria, even to unrelated organisms. Previously, several laboratories have shown that the antibiotic resistance genes of DT104 are chromosomally encoded and involve integrons. The antibiotic resistance genes conferring the ACSSuT-resistant phenotype have been cloned and sequenced. These genes are grouped within two district integrons and intervening plasmid-derived sequences. This sequence is potentially useful for detection of multiresistant DT104.
The emergence of pathogens possessing multiple-antibiotic resistance genes has become a major concern in recent years. The routine use of antibiotics in medical and agricultural circles has resulted in widespread antibiotic resistance and in the development of genetic mechanisms efficient for the dissemination of antibiotic gene cassettes, especially within and between species of gram-negative organisms (3, 10). The frequency of isolation of Salmonella strains resistant to one or more antibiotics has risen in the United Kingdom (8) and the United States (4). A recent newcomer to the food safety pathogen list, Salmonella typhimurium phage type DT104 often possesses resistance to multiple antibiotics, including ampicillin, tetracycline, chloramphenicol, sulfamethoxazole, and streptomycin (1, 8, 11, 13). Among the multiresistant salmonellae, DT104 is one of the most prevalent phage types of S. typhimurium in the United Kingdom (13) and is rapidly becoming the most prominent phage type in the United States, since 39 of 43 multiresistant isolates were DT104 or a closely related phage type in a 1994 to 1996 study (4). DT104 is a common infectious agent in cattle and has been contracted by humans exposed to infected cattle (14). DT104 may have become multiresistant as a result of antibiotic use in livestock (15). Organisms have often accumulated antibiotic resistance genes by plasmid transfer or by transposon- or integron-mediated mechanisms and have routinely harbored these resistance genes on plasmids (10). Notably, class 1 integrons have been found in many multiresistant organisms and many gram-negative species. These genetic elements often contain a 5′ integrase gene and a 3′ sul1 gene (encoding sulfamethoxazole resistance) and resistance gene cassettes separated by 59-bp stretches which are involved in the incorporation of additional cassettes within an integron (10). While these integrons have been plasmid borne in many instances (6), they have become chromosomally integrated in S. typhimurium DT104 (13). Chromosomal integration has been speculated to allow resistance genes to persist even in the absence of antibiotic selection (13). In Danish isolates of DT104, streptomycin, sulfonamide, and ampicillin resistance genes have been found grouped into at least two class 1 integrons which do not include the tetracycline and chloramphenicol resistance genes (11). The relative arrangement of these integrons and the location of the other resistance genes have not been established. In the present study, the arrangement is described for all five genes encoding streptomycin, chloramphenicol, tetracycline, ampicillin, and sulfamethoxazole resistances (aadA2, a cmlA homologue, tetA, blaCARB-2, and sul1, respectively), and the implications of their arrangement are addressed.
MATERIALS AND METHODS
Isolates and preparation of DNA.
S. typhimurium non-DT104 isolates and DT104 isolates were obtained from the U.S. Department of Agriculture (USDA), National Veterinary Services Laboratories (Athens, Ga.), National Animal Disease Center (Ames, Iowa), Food Safety and Inspection Service (Athens, Ga.), the Washington State Public Health Laboratories (Seattle, Wash.), the Centers for Disease Control and Prevention (CDC) (Atlanta, Ga.), and the Public Health Laboratory Service (London, United Kingdom) (Table 1). Control isolates were obtained from Michael Haas, Agricultural Research Service, USDA (LT2 isolate), Victor Cook, Food Safety and Inspection Service (isolate F4797), and the CDC (ASSuT-resistant Salmonella hadar; FSIS no. MF60404). Genomic DNA was prepared with the GNOME kit (Bio 101, La Jolla, Calif.). Plasmid DNA was prepared with the Qiagen (Santa Clarita, Calif.) plasmid Mini kit. DNA prepared from agarose gels was purified with the QIAEX II DNA extraction kit (Qiagen). All restriction enzymes were obtained from Promega (Madison, Wis.) or from New England Biolabs (Beverly, Mass.). All transformations were performed with high-efficiency JM109 Escherichia coli (Promega).
TABLE 1.
Antibiotic resistance profiles of S. typhimurium isolates used in this worka
| Phage type | ACSSuT | ASSuT | Long PCR resultb |
|---|---|---|---|
| Non-DT104 | |||
| Wash. State isolates | |||
| S3447 (type 208, human) | x | − | |
| S3444 (type 771, bovine) | x | − | |
| S3426 (type 771, bovine) | x | − | |
| DT104 related | |||
| CDC isolates | |||
| G8430 (U302, human) | x | + | |
| G7601 (U302, human) | x | + | |
| DT104 | |||
| Wash. State bovine isolates | |||
| S3455 | x | + | |
| S2380 | x | + | |
| S3461 | x | − | |
| S3307 | x | + | |
| S2486 | x | + | |
| S2490 | x | + | |
| CDC human isolates | |||
| H3402 | x | − | |
| H3380 | x | + | |
| H2662 | x | + | |
| H3278 | x | + | |
| NADCc isolates | |||
| 13HP (swine) | x | + | |
| FDIU 2576 (human) | x | + | |
| DHEP 12362 (bovine) | x | + | |
| Untyped | |||
| FSIS-026 (FSIS, unknown) | x | + | |
| H3379 (untypeable, human) | x | − |
x, possession of resistance profile.
See Fig. 3 and Results and Discussion; +, presence of 10,036-bp band.
NADC, National Animal Disease Center.
Antibiotic disc diffusion testing of isolates.
All isolates were tested by Kirby-Bauer disc diffusion tests on Mueller-Hinton agar (Difco, Detroit, Mich.) according to the standard procedure outlined in National Committee for Clinical Laboratory Standards guidelines (9). Antibiotic discs (BBL Sensi-Discs [Becton Dickinson, Cockeysville, Md.] and Dispens-O-Discs [Difco]) were used for ampicillin (30 μg), tetracycline (30 μg), chloramphenicol (30 μg), sulfisoxazole (300 μg), trimethoprim (5 μg), streptomycin (10 μg), and sulfamethoxazole × trimethoprim (25 μg) testing, and results were interpreted by using charts supplied with the discs. Eighteen S. typhimurium DT104 and 7 non-DT104 and 2 untyped isolates were tested for their antibiotic resistance profiles. All isolates fell into one of two groups, one including isolates resistant to ampicillin, streptomycin, sulfonamides, tetracycline, and chloramphenicol (ACSSuT resistant) and the other including non-chloramphenicol-resistant organisms (ASSuT resistant) (Table 1).
PCR amplification and cloning.
Primers were synthesized by Gibco/BRL Life Technologies (Gaithersburg, Md.) or by the Nucleic Acid Facility, Thomas Jefferson Medical College (Philadelphia, Pa.). To expedite sequencing, amplifications of the Salmonella genes blaCARB-2, aadA2, and sul1 (GenBank accession no. Z18955, Y14748, and Y14748, respectively) were performed, resulting in the production of PCR products which were 639, 522, and 839 bp, respectively. PCR was performed by using the PCR reagent system (no. 10198-018; Life Technologies, Gaithersburg, Md.). Eighty nanograms of genomic DNA, 20 mM Tris-Cl (pH 8.4), 50 mM KCl, 1.5 mM MgCl2, 200 μM (each) deoxynucleoside triphosphate, 0.8 μM (each) primer, and 0.5 U of Taq polymerase in a 50-μl total reaction volume were subjected to PCR in a thermal cycler (model 9600; Perkin-Elmer, Foster City, Calif.) for 1 cycle at 95°C for 5 min, 30 cycles (95°C for 1 min, 60°C for 1 min, 72°C for 1 min), and 1 cycle at 72°C for 10 min and stored at 4°C. Primers had the following sequences: for blaCARB-2, caatggcaatcagcgcttcccgtt (forward) and cgctctgccattgaagcctgtgtt (reverse); for aadA2, gtacggctccgcagtggatggcgg (forward) and gcccagtcggcagcgaca tccttc (reverse); for sul1, atggtgacggtgttcggcattctg (forward) and gctaggcatgatctaaccctcgg (reverse). Long PCR of genomic DNA was performed as directed by the manufacturer of the rTth DNA polymerase XL kit (Perkin-Elmer). Genomic DNA (80 ng/100-μl reaction mixture) was amplified by using the aadA2 forward primer and the blaCARB-2 reverse primer and cycled at 1 cycle at 95°C for 5 min, 35 cycles (95°C for 1 min, 68°C for 1 min, and 72°C for 10 min), and 1 cycle at 72°C for 10 min. All PCR products were subjected to electrophoresis on 1% agarose gels in 1× TAE. Lambda HindIII/EcoRI markers (Promega) were used to estimate the sizes of the final products. A result was scored positive if a band of 10,036 bp was observed.
Pulsed-field gel electrophoresis and Southern blotting.
Pulsed-field electrophoresis, digestion with XbaI, and Southern blotting were performed by using standard protocols (5, 12) and instructions within the manual supplied with the Bio-Rad CHEF Mapper XA. Probes were synthesized by using either primers and the enhanced chemiluminescence 3′ oligolabeling and detection kit (Amersham Life Science, Buckinghamshire, England) or gel-purified blaCARB-2, aadA2, or sul1 PCR products and the DIG High Prime Labeling and Detection Starter kit (Boehringer Mannheim, Indianapolis, Ind.). After the hybridization of duplicate blots with individual antibiotic resistance gene probes to the blaCARB-2, aadA2 [also known as ant(3′′)-Ia], and sul1 genes, a 10-kb fragment was determined to contain a complete aadA2 gene at its 5′ end, an internal partial sul1 gene, and an incomplete 3′ blaCARB-2 gene, which extends beyond the 3′ XbaI site.
Cloning and sequencing of genomic DNA.
The 10-kb XbaI band which hybridized with antibiotic resistance gene probes was excised from a pulsed-field gel, purified, and cloned into the XbaI site of the SuperCos 1 vector (no. 251301; Stratagene, Cambridge, United Kingdom) to create p10Xba. The 10-kb insert was digested with PstI, and fragments of 4, 2.5, and 1 kb were cloned into PstI-digested pSp72 (Promega) and individually sequenced. A cloned 3-kb genomic fragment containing the entire blaCARB-2 ampicillin resistance gene was obtained by digesting genomic DNA with PstI and cloning into the PstI site of pGFP-1 (Clontech, Palo Alto, Calif.) and selecting for colony growth on Luria-Bertani plates containing 100 μg of ampicillin/ml. PCR products of the purified blaCARB-2, aadA2, or sul1 gene were cloned into the pGEM-T vector (Promega). All constructs were cycle sequenced in a Perkin-Elmer Cetus thermal cycler (model 9600) by using Sp6 and T7 primers or primers synthesized with the previous sequence information. Dideoxy sequencing was performed by the Nucleic Acid Facility at Thomas Jefferson Medical College with an Applied Biosystems model 373A or 377 DNA sequencing system (Perkin-Elmer). All sequences were verified by sequencing each region at least twice. All sequence information was obtained with DNA derived from the human clinical S. typhimurium DT104 isolate H3380. Sequences obtained were compared to those in the GenBank database (National Center for Biotechnology Information). The sequence information was employed to deduce the genomic arrangement of the antibiotic resistance genes.
Nucleotide sequence accession number.
The sequence described in this work has been deposited in GenBank under accession no. AF071555.
RESULTS AND DISCUSSION
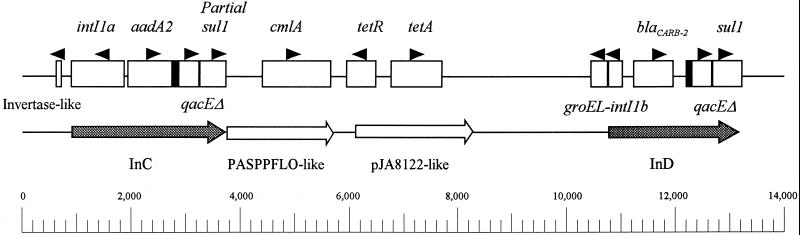
Arrangement of antibiotic resistance genes and mechanism of gene acquisition.
The aadA2, sul1, and blaCARB-2 probes each hybridized to the same 10-kb XbaI fragment of genomic DNA (Southern blot not shown). The arrangement of the antibiotic resistance genes found within the multiresistant S. typhimurium isolate H3380 is shown in Fig. 1. The 13,078-bp sequence contains four resistance genes which are highly similar to GenBank sequence no. Y14748 (aadA2, sul1), S52437 (tetA), and Z18955 (blaCARB-2) and which encode resistance to streptomycin, sulfonamides, tetracycline, and ampicillin, respectively (Table 2). The cmlA-like gene shows 97% identity (Fig. 2 and Table 2) with cmlA, which encodes an exporter nucleotide sequence found in Pseudomonas aeruginosa (GenBank accession no. M64556 [2]) and confers chloramphenicol resistance. The 13,078-bp fragment is composed of two class 1 integrons separated by an R plasmid sequence most closely related to plasmids of Pasteurella piscicida and Vibrio anguillarum (accession no. D37826 and S52437, respectively). The 5′ integron is contained within a larger sequence which differs from that of the IncG plasmid pCG4 of Corynebacterium glutamicum (accession no. Y14748) by only 1 nucleotide. Both class 1 integrons possess the classical structure, as evidenced by the presence of a 5′ integrase gene and a 3′ sul1 gene, separated by an antibiotic resistance gene and a disinfectant resistance gene, qacEΔ (accession no. Y14748). The aadA2 gene, the cmlA-like gene, tetA, and the blaCARB-2 gene are all present within the same reading frame. Interestingly, an approximately 350-bp fragment with 83 to 92% identity to the groEL gene of Chromatium vinosum (accession no. M99443) is found adjacent to the 5′ end of an integron described previously (InD) (11), is in the same reading frame as the integrase gene, which is only a partial gene, and is possibly expressed as a fusion protein with it. A region with 100% identity to an incomplete invertase gene (or a transposon resolvase) is found near the 5′ end of the multiple-resistance gene arrangement. Thus, both integrons and the intervening resistance plasmid could be part of a larger transposon. Confirmation of this hypothesis must await cloning and sequencing of the flanking regions to determine if characteristic repeated sequences, such as those found at the junctions of transposon insertions, are present. In order to investigate whether this multiple gene configuration is characteristic of multiresistant DT104 isolates, 10 additional ACSSuT-resistant DT104 isolates, two phage type U302 isolates (possibly closely related to DT104) with the ACSSuT profile, one non-DT104 ACSSuT-resistant isolate, two DT104 ASSuT-resistant isolates, and an ACSSuT-resistant S. hadar isolate were tested by using a long PCR technique. Primers were chosen so that all sequences between the aadA2 and blaCARB-2 genes would be included in the PCR product. All ACSSuT-resistant DT104 isolates possessed the expected 10-kb band, while those which lacked resistance to chloramphenicol or were multiresistant and non-DT104 (type 771 isolate) lacked this band (Table 1 and Fig. 3). This result was surprising for the ASSuT-resistant DT104 isolates, since these isolates possess the aadA2 gene, and long PCR with an aadA2 forward primer did not result in an amplification product. Cloning of flanking sequences is currently in progress. Sequencing of these regions may verify whether other integrons or gene arrangements exist and whether a larger transposon or phage-derived sequence or pathogenicity island (7) is the source of the resistance genes. It is not firmly established whether or not DT104 isolates typically possess increased virulence, a property which is independent of their multiple-antibiotic resistance. If genes for virulence were associated with those for antibiotic resistance, virulence would be selected for by antibiotic use. Additionally, the phage type U302 isolates tested in this study possessed additional resistance to kanamycin, gentamicin, and neomycin. One phage type U302 isolate possessed resistance to trimethoprim (data not shown). If the DT104 isolates possess an enhanced ability to incorporate plasmid-borne resistance genes into the antibiotic resistance gene cluster characterized in this work, a larger chromosomal multiresistance gene cluster may be generated. The transfer of such a multiresistance gene fragment to other pathogenic bacteria could result in a serious health concern. Furthermore, work needs to be done to determine if these multiresistant gene clusters are stable within bacterial genomes after antibiotics are withdrawn from the environment. An understanding of the antibiotic resistance gene arrangements in DT104 will probably have an appreciable impact on antibiotic use in agriculture and medicine.
FIG. 1.
Arrangement of antibiotic resistance genes of S. typhimurium DT104 isolate H3380 as deduced from the nucleic acid sequence described in this work. Black boxes represent 59-base elements.
TABLE 2.
Percent identity between S. typhimurium DT104 genes of isolate H3380 and entries within the GenBank database
| Nucleotide position | Sequence name | Closest similarity (nucleotide position) | Accession no. | % Identity |
|---|---|---|---|---|
| 0–597 | Unknown | No match | None | None |
| 598–665 complement | Invertase-like | paeR7IN (74–6) | S78872 | 100 |
| 666–3785 | InC-like plasmid | IncG (1–3120) | Y14748 | 99 |
| 802–1815 complement | intI1a | IncG (137–1150) | Y14748 | 100 |
| 1961–2752 | aadA2 | IncG (1296–2087) | Y14748 | 99 |
| 2754–2813 | 59-Base element | IncG (2088–2147) | Y14748 | 100 |
| 2916–3263 | qacΔE | IncG (2251–2598) | Y14748 | 100 |
| 3257–3785 | Partial sul1 | IncG (2592–3120) | Y14748 | 100 |
| 3783–5760 | Plasmid | PASPPFLO plasmid | D37826 | 94 |
| 4445–5659 | cmlA-like | ppflo (1071–2236) | D37826 | 97 |
| 6117–8328 | Plasmid | pJA8122 plasmid | S52437 | 98 |
| 5866–6492 complement | tetR | tetR (721–113) | S52438 | 98 |
| 6644–7771 | tetA | tetA (543–1667) | S52437 | 99 |
| 10433–10785 complement | Partial groEL | groEL (2004–1921) | M99443 | 90 |
| (1871–1807) | M99443 | 83 | ||
| (2119–2092) | M99443 | 92 | ||
| 10786–11086 complement | Partial intI1b | intI1 (850–1150) | Y14748 | 100 |
| 11292–12158 | blaCARB-2 | blaCARB-2 | Z18955 | 100 |
| 12210–12272 | 59-Base element | STCARB2A (1089–1150) | Z18955 | 100 |
| 12375–12722 | qacΔE | IncG (2251–2598) | Y14748 | 100 |
| 12716–13077 | Partial sul1 | IncG (2592–2951) | Y14748 | 100 |
FIG. 2.
Comparison of the chloramphenicol resistance gene product of an S. typhimurium DT104 isolate, H3380, to the most similar GenBank entry R10CMLA (PID: g151756); amino acids are indicated by their standard one-letter designations. The top row of sequence refers to the DT104 protein sequence. The bottom row is from a CmlA protein sequence of P. aeruginosa. The center line indicates amino acid identity when an amino acid is written, similarity when a plus symbol is present, and lack of similarity when nothing is written.
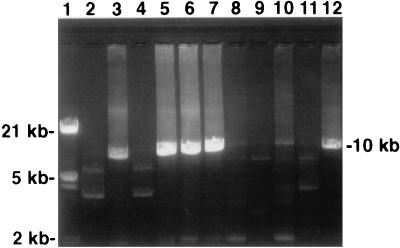
FIG. 3.
A representative result for the long PCR of S. typhimurium DT104 and non-DT104 isolates. Lane 1, MW markers; lanes 3, 5 to 7, and 12, (ACSSuT-resistant) DT104 (S3455, S2486, 13HP, H3380, and S2490, respectively); lanes 2, 4, 9, and 11, non-DT104 (F4797, S3426, S. hadar MF60404, and S3444, respectively); lanes 8 and 10, (ASSuT-resistant) DT104 (H3402 and S3461, respectively). A positive result is evidenced by the presence of a band of 10,036 bp.
ACKNOWLEDGMENTS
We thank Hannes Alder (Nucleic Acid Facility at Thomas Jefferson Medical College, Philadelphia, Pa.) for providing technical advice, James Smith and Lance Bolton for helpful discussions, and Mike Haas and Ching-Hsing Liao for reviewing the manuscript.
REFERENCES
- 1.Angulo F. Multidrug-resistant Salmonella typhimurium definitive type 104. Emerg Infect Dis. 1997;3:414. [Google Scholar]
- 2.Bissonnette L, Champetier S, Buisson J P, Roy P H. Characterization of the nonenzymatic chloramphenicol resistance (cmlA) gene of the In4 integron of Tn1696: similarity of the product to transmembrane transport proteins. J Bacteriol. 1991;173:4493–4502. doi: 10.1128/jb.173.14.4493-4502.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ferber D. New hunt for the roots of resistance. Science. 1998;280:27. doi: 10.1126/science.280.5360.27. [DOI] [PubMed] [Google Scholar]
- 4.Glynn M K, Bopp C, Dewitt W, Dabney P, Molktar M, Angulo F J. Emergence of multidrug-resistant Salmonella enterica serotype typhimurium DT104 infections in the United States. N Engl J Med. 1998;338:1333–1338. doi: 10.1056/NEJM199805073381901. [DOI] [PubMed] [Google Scholar]
- 5.Gunderson K, Chu G. Pulsed-field electrophoresis of megabase-sized DNA. Mol Cell Biol. 1991;11:3348–3354. doi: 10.1128/mcb.11.6.3348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kazama H, Kizu K, Iwasaki M, Hamashima H, Sasatsu M, Arai T. Isolation and structure of a new integron that includes a streptomycin resistance gene from the R plasmid of Pseudomonas aeruginosa. FEMS Microbiol Lett. 1995;134:137–141. doi: 10.1111/j.1574-6968.1995.tb07927.x. [DOI] [PubMed] [Google Scholar]
- 7.Lee C. Pathogenicity islands and the evolution of bacterial pathogens. Infect Agents Dis. 1996;5:1–7. [PubMed] [Google Scholar]
- 8.Low J C, Angus M, Hopkins G, Munro D, Rankin S C. Antimicrobial resistance of Salmonella enterica typhimurium DT104 isolates and investigation of strains with transferable apramycin resistance. Epidemiol Infect. 1997;118:97–103. doi: 10.1017/s0950268896007339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.National Committee for Clinical Laboratory Standards. Performance standards for Antimicrobial Disk Susceptibility Tests, 6th ed. Approved Standard NCCLS document M2-A6. Villanova, Pa: National Committee for Clinical Laboratory Standards; 1997. [Google Scholar]
- 10.Recchia G D, Hall R M. Gene cassettes: a new class of mobile element. Microbiology. 1995;141:3015–3027. doi: 10.1099/13500872-141-12-3015. [DOI] [PubMed] [Google Scholar]
- 11.Sandvang D, Aarestrup F M, Jensen L B. Characterization of integrons and antibiotic resistance genes in Danish multiresistant Salmonella enterica typhimurium DT104. FEMS Microbiol Lett. 1998;160:37–41. doi: 10.1111/j.1574-6968.1998.tb12887.x. [DOI] [PubMed] [Google Scholar]
- 12.Southern E M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975;98:503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- 13.Threlfall E J, Frost J A, Ward L R, Rowe B. Epidemic in cattle and humans of Salmonella typhimurium DT104 with chromosomally integrated multiple drug resistance. Vet Rec. 1994;134:577. doi: 10.1136/vr.134.22.577. [DOI] [PubMed] [Google Scholar]
- 14.Wall P G, Morgan D, Lamden K, Griffin M, Threlfall E J, Ward L R, Rowe B. Transmission of multi-resistant Salmonella typhimurium from cattle to man. Vet Rec. 1995;136:591–592. doi: 10.1136/vr.136.23.591. [DOI] [PubMed] [Google Scholar]
- 15.Williams R J, Heymann D L. Containment of antibiotic resistance. Science. 1998;279:1153–1154. doi: 10.1126/science.279.5354.1153. [DOI] [PubMed] [Google Scholar]