Abstract
Objective
Oesophageal squamous cell carcinoma (OSCC), like other squamous carcinomas, harbour highly recurrent cell cycle pathway alterations, especially hyperactivation of the CCND1/CDK4/6 axis, raising the potential for use of existing CDK4/6 inhibitors in these cancers. Although CDK4/6 inhibition has shown striking success when combined with endocrine therapy in oestrogen receptor positive breast cancer, CDK4/6 inhibitor palbociclib monotherapy has not revealed evidence of efficacy to date in OSCC clinical studies. Herein, we sought to elucidate the identification of key dependencies in OSCC as a foundation for the selection of targets whose blockade could be combined with CDK4/6 inhibition.
Design
We combined large-scale genomic dependency and pharmaceutical screening datasets with preclinical cell line models, to identified potential combination therapies in squamous cell cancer.
Results
We identified sensitivity to inhibitors to the ERBB family of receptor kinases, results clearly extending beyond the previously described minority of tumours with EGFR amplification/dependence, specifically finding a subset of OSCCs with dual dependence on ERBB3 and ERBB2. Subsequently. we demonstrated marked efficacy of combined pan-ERBB and CDK4/6 inhibition in vitro and in vivo. Furthermore, we demonstrated that squamous lineage transcription factor KLF5 facilitated activation of ERBBs in OSCC.
Conclusion
These results provide clear rationale for development of combined ERBB and CDK4/6 inhibition in these cancers and raises the potential for KLF5 expression as a candidate biomarker to guide the use of these agents. These data suggested that by combining existing Food and Drug Administration (FDA)-approved agents, we have the capacity to improve therapy for OSCC and other squamous cancer.
Keywords: oesophageal cancer, cell cycle control, pharmacogenomics, pharmacotherapy
Significance of this study.
What is already known on this subject?
CDK4/6 inhibition has shown striking success when combined with endocrine therapy in oestrogen receptor positive breast cancer; however, CDK4/6 inhibitor palbociclib monotherapy has not revealed evidence of efficacy to date in oesophageal squamous cell carcinoma (OSCC) clinical studies.
What are the new findings?
We first confirm the sensitivity of OSCC to CDK4/6 inhibitor palbociclib in cell line models. We then identified ERBB family as key dependencies in OSCC and pan-squamous cell carcinoma, and combination of ERBB inhibitor augments the efficacy of CDK4/6 inhibitor monotherapy in several squamous models both in vitro and in vivo. Finally, we found that squamous lineage transcription factor KLF5 facilitates activation of ERBBs in OSCC.
How might it impact on clinical practice in the foreseeable future?
Oesophageal squamous cancer is one of the most common causes of cancer death worldwide, while systemic therapy remains reliant on empirical chemotherapy. Although several genomic studies identified highly recurrent amplificated oncogenes targeted by existing, FDA-approved drugs, patients with OSCC are largely treated with cytotoxic and immune therapy. The combination strategy identified in our study suggested we have the capacity to improve therapy for OSCC and other squamous cancer with FDA-approved drugs.
Introduction
Oesophageal cancer is the sixth most common cause of cancer death worldwide1 with 5-year survival rates approximating 20%.2 The most common form is oesophageal squamous cell carcinoma (OSCC), comprising almost 90% of cases worldwide.1 For patients with metastatic disease, the mainstay of therapy remains cytotoxic therapy, now supplemented with immune checkpoint inhibitors.3 4 However, there are no approved molecularly targeted inhibitors for OSCC despite ample genomic data5–7 that suggest candidate therapeutic targets. There is a clear need for improved biomarker-driven treatment strategies for OSCC.
Several projects have mapped genomic aberrations in OSCC.5–7 Beyond mutations of TP53, nearly universally present, the most common family of genes somatically altered in OSCC relate to cell cycle regulation.8 In The Cancer Genome Atlas (TCGA), over 90% of patients with OSCC harboured cell cycle pathway alterations, most commonly amplification of CCND1 (cyclin D1) in 57% and deletion or mutation of CDKN2A in 76%.5 Analysis of somatic copy number alterations across squamous cell carcinoma (SCC) from multiple lineages noted CCND1 as the most significant focus of amplification.9 These data raise clear hypotheses regarding targeting cyclin D-CDK4/6 in SCC. There are three CDK4/6 inhibitors FDA approved for metastatic hormone positive breast cancer in combination with antioestrogen therapy.10 By directly blocking the cyclin D-CDK4/6 axis, these agents act to prevent progression from the G1 to the S phase. While genomic data suggest a role for these inhibitors in OSCC, a phase II trial in OSCC using palbociclib monotherapy (NCT01037790) revealed no objective responses.11
While the lack of apparent benefit to CDK4/6 inhibitors does not support their use in OSCC, these data do not necessarily indicate that these drugs do not have a role in therapy, especially with combination therapy. Indeed, in studies investigating modes of augmenting EGFR-directed therapy in epidermal growth factor receptor (EGFR)-amplified OSCC, we found that CDK4/6 inhibitors could overcome effects of adaptive resistance to EGFR blockade, demonstrating potential efficacy in combination therapy.12 Indeed, the experience with breast cancer cautions against dismissing CDK4/6 inhibitors based on lack of monotherapy responses. CDK4/6 inhibitors palbociclib, ribociclib and abemaciclib have had striking success when combined with the hormone receptor antagonists in oestrogen receptors (ORs) positive breast cancers.10 By contrast, monotherapy with palbociclib and ribociclib, the two inhibitors with greatest specificity for CDK4 and CDK6 have been disappointing in breast cancer (NCT02549430), with median progression-free survival (PFS) 6.5 months for palbociclib monotherapy compared with 10.8 months for palbociclib in combination with endocrine therapy.13 14
Given this background, we sought to identify targets to augment efficacy of CDK4/6 inhibition in OSCC. Building from the experience in breast cancer where addition of CDK4/6 agents potentiate a pre-existing sensitivity to antioestrogen therapy, we sought to define potent baseline dependencies in OSCC as a foundation for combination development. Through mining functional genomic and pharmacogenomic datasets, we identified sensitivity to inhibitors to the ERBB family of receptor kinases, results extending beyond tumours with EGFR amplification. We demonstrated marked efficacy of combined pan-ERBB and CDK4/6 inhibition in vitro and in vivo suggesting that by combining FDA-approved agents, we may improve therapy for this lethal malignancy.
Materials and methods
A detailed description of material and methods can be found in the online supplemental information.
gutjnl-2020-323276supp001.pdf (62.4KB, pdf)
Results
CDK4/6 inhibitors as a putative target for OSCC
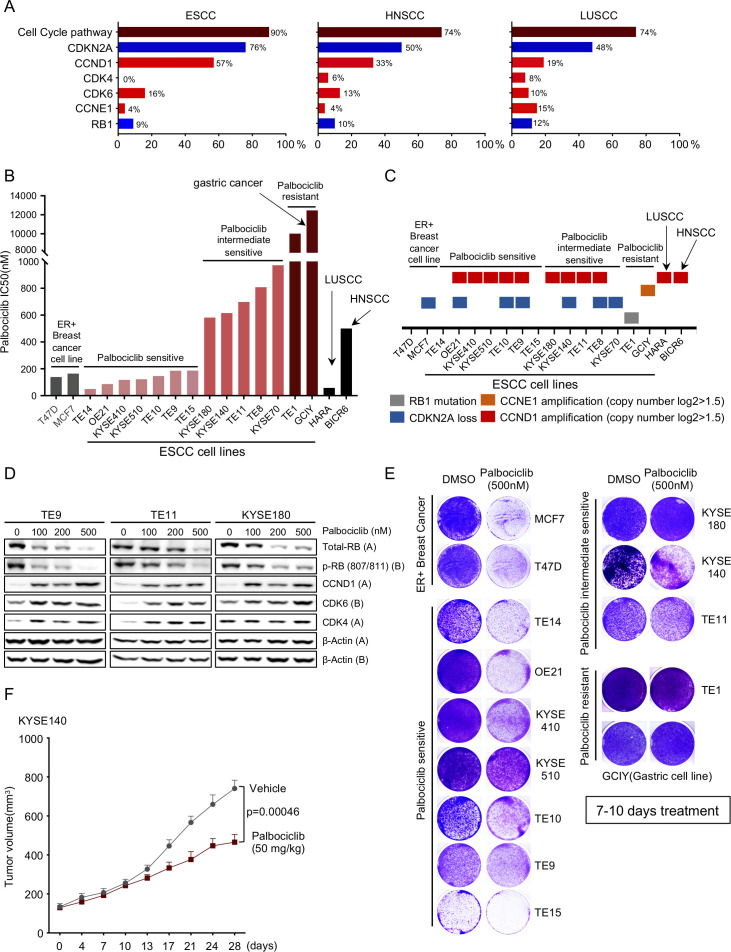
We reviewed data from TCGA studies of OSCC, head and neck SCC (HNSCC) and lung SCC (LUSCC). We systematically evaluated the cell cycle pathway alterations (specifically, CDKN2A, CCND1, CDK4, CDK6, CCNE1 and RB) in three major squamous types and observed 90% of OSCC, 74% HNSCC and 74% LUSCC harboured at least one key gene alteration. Amplification of CCND1 alone was observed in 57% of OSCC, 33% of HNSCC and 19% of LUSCC (figure 1A). We then evaluated the impact of CDK4/6 inhibition, using palbociclib, across a panel of OSCC cell lines. We used 13 OSCC cell lines, HNSCC line BICR6, LUSCC line HARA and two OR+ breast cancer cell lines, MCF7 and T47D, as positive controls,15 and a CCNE1 (Cyclin E1) amplified gastric cancer cell line (GCIY) as a negative control, as cyclin E1 is an established resistance mechanism for CDK4/6 inhibition.16 As shown in figure 1B and online supplemental figure S1A, 12 out of 13 OSCC lines showed a range of response to palbociclib, with an IC50 from 150 nM to 1 uM. Both BICR6 and HARA lines showed sensitivity to palbociclib, with IC50 of 740 nM and 58 nM. We defined seven OSCC cell lines with comparable IC50 as T47D and MCF7 as ‘palbociclib sensitive’ and five other lines as ‘palbociclib intermediate sensitive’. By contrast, only the TE1 OSCC line showed marked resistance.
Figure 1.
CDK4/6 inhibitors as a putative target for oesophageal squamous cell carcinoma (OSCC). (A) Frequencies of cell-cycle gene alterations (CDKN2A, CCND1, CDK4, CDK6, CCNE1 and RB1) in OSCC/HNSCC/LUSCC from The Cancer Genome Atlas. Deletion is depicted in blue and amplification is depicted in red. (B) Palbociclib drug sensitivity half maximal inhibitory concentration (IC50) values (nm). Cell lines are coloured as follows: grey: OR positive breast cancer; light red: palbociclib sensitive OSCC; red: palbociclib intermediate sensitive OSCC; dark red: palbociclib resistant OSC line TE1 and gastric line GCIY; black: LUSCC line HARA and HNSCC line BICR6. (C) Annotations of cell-cycle gene alterations in cell lines shown in figure 1B. (D) Immunoblot analysis of genes involved in cell-cycle pathway in OSCC cell lines TE9, TE11 and KYSE180 cells treated with palbociclib (100 nM, 200 nM and 500 nM), or with water control. Protein lysates were collected after drug treatment for 24 hours. Immunoblots from one representative experiment (n=2) are shown. (E) Images showing crystal violet staining of cell lines listed in figure 1B, after 500 nM palbociclib treatment for 7–10 days. Data from one representative experiment are presented (n=3). (F) Growth curve for KYSE140 xenograft tumours (n=6–10) treated with palbociclib (50 mg/kg) by daily gavage. Data are mean±SEM, and p value was calculated by t–tests at 28 days. OR, oestrogen receptor.
gutjnl-2020-323276supp002.pdf (15.8MB, pdf)
We next investigated if differences in response are associated with genomic features. The resistant cell line TE1 harbours an RB1 deletion, explaining its resistance. On initial evaluation, our palbociclib sensitive and intermediate cell line models showed similar genomic features, including amplification of CCND1, often co-occurring with inactivation of CDKN2A (figure 1C). To further assess predictive biomarkers, we compared our pharmacological data with the results of CRISPR screening data from these cell lines from the Broad Institute Dependency Map effort. We found that palbociclib sensitivity is correlated strongly with CCND1 and CDK6 but not CDK4 dependency in OSCC (online supplemental figure S1B). A more detailed assessment of genomic copy number and expression of cell cycle regulators relative to palbociclib revealed correlation with CCND1 copy number (online supplemental figure S1C), but not with copy number of CDK4, CDK6, CDK2, CDKN2A or RB (online supplemental figure S1C). We did not find significant associations between expression of queried cell cycle factors with palbociclib sensitivity except for CCNE1, where higher expression correlates with resistance (online supplemental figure S1C). We also observed a trend towards greater sensitivity with higher CCND1 expression levels, suggesting the degree of expression may impact this dependency (online supplemental figure S1C).
We next evaluated signalling changes in cell line models with palbociclib. As shown in figure 1D, we found that although 100 nM and 200 nM palbociclib can attenuate phosphorylated retinoblastoma protein (pRB)RB and total retinoblastoma protein (RB), 500 nM palbociclib more potently blocks pRB and total RB. We further queried the longer term efficacy of palbociclib monotherapy in SCC lines in vitro. As opposed to the 96-hour studies demonstrated in figure 1B, we evaluated growth here with 7–10 days treatment of 500 nM palbociclib (with media and drug replenished every 3 days) (figure 1E, online supplemental figure S1D, E). Although there was more stable attenuation of growth of the breast cancer models, we observed substantial outgrowth in each intermediate sensitive OSCC and four of seven sensitive models. We also evaluated an intermediate sensitive cell line, KYSE140, following flank implantation in mice into with 50 mg/kg palbociclib. We observed attenuated growth, but there was clear progression of the tumour with palbociclib (figure 1F). While these data support a role for CDK4/6 inhibition in OSCC, they are consistent with our hypothesis that development of combination therapy is critical.
ERBB family of kinases emerge as strong dependencies across OSCC
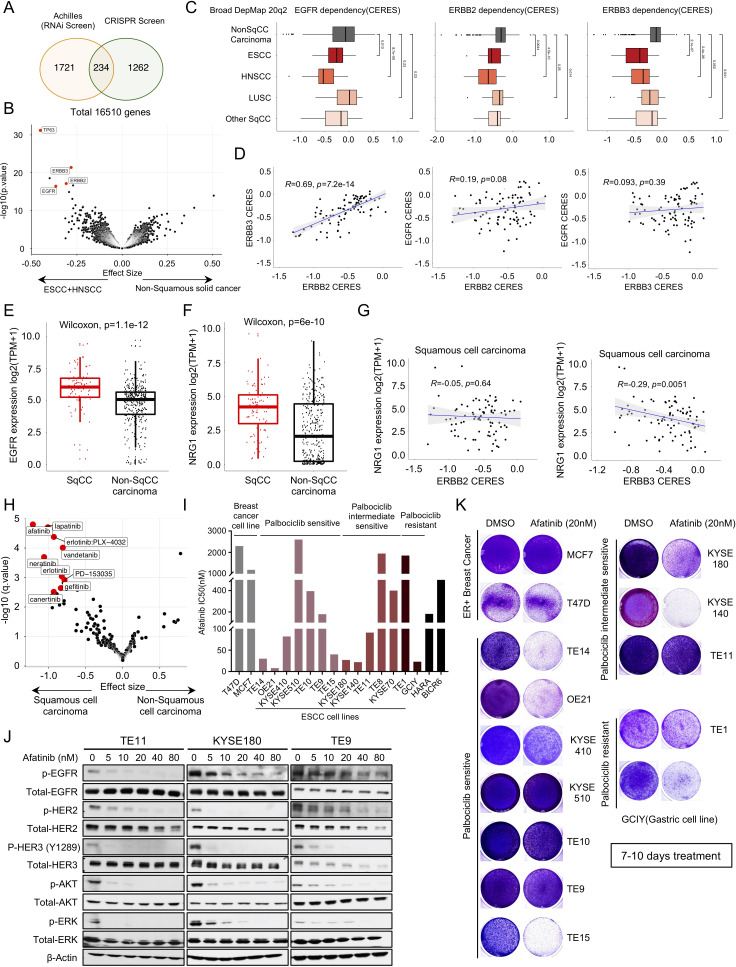
We next asked if identifying essential genes/targets within OSCC could enable combination strategies. We queried candidate vulnerabilities using two large-scale cancer dependency datasets with 673 cancer cell lines with a genome-scale CRISPR-Cas9 library and 647 cell lines with RNAi screening.17 18 To enhance statistical power, we pooled OSCC and HNSCC cell lines given the published similarity of these cancer types across multiple genomic features.5 9 We queried genes selectively essential in OSCC and HNSCC compared with other cancers. We found 234 genes that were consistently preferential dependencies in OSCC plus HNSCC compared with other non-squamous solid tumour independently across both functional genomic datasets (figure 2A). Among these genes were two members of the ERBB family of receptor tyrosine kinases: EGFR and ERBB3.
Figure 2.
ERBB family of kinases emerge as strong dependencies across OSCC. (A) Venn diagram showing the overlap of genes that are significantly more essential in OSCC and HNSCC cell lines than other non-squamous solid tumour cell lines from Broad Institute Achilles RNAi screen and Broad Institute Achilles CRISPR screen datasets. (B) Broad Institute Achilles CRISPR screen data (DepMap 20q2 public) analysis showing selective dependency genes in OSCC and HNSCC versus non-squamous carcinoma cell lines, as illustrated in volcano plot. Each dot represents a gene, and the effect size explains the mean difference of gene dependency score between the two groups. (C) The dependency score (CERES) of EGFR, ERBB2 and ERBB3 in different squamous cell carcinomas subtypes and non-squamous carcinoma cells. Wilcoxon test was performed to compare CERES values in two groups. (D) Pearson correlation of EGFR, ERBB2 and ERBB3 gene dependency score in squamous cell carcinoma cell lines based on Broad Institute Achilles CRISPR screen data (DepMap 20Q2 public). (E) mRNA expression of EGFR in squamous carcinoma cell lines and non-squamous carcinoma cell lines based on Broad Institute Cancer Cell Line Encyclopedia data (DepMap 20Q2 public). Wilcoxon test was performed for two group comparison. (F) mRNA expression of NRG1 in squamous carcinoma cell lines and non-squamous carcinoma cell lines based on Broad Institute Cancer Cell Line Encyclopedia data (DepMap 20Q2 public). Wilcoxon test was performed for two group comparison. (G) Pearson correlation of NRG1 expression and ERBB2 (left) or ERBB3 (right) gene dependency score in squamous cell carcinoma cell lines. (H) CTRP CTD2drug sensitivity area under the curve (AUC) data mining showing selective drug sensitivity in genes in squamous carcinoma cell lines and non-squamous carcinoma cell lines. Each dot represents a drug, and the effect size explains the mean difference of drug sensitivity AUC between the two groups. (I) Afatinib drug sensitivity half maximal inhibitory concentration (IC50) values (nM). Cell lines are colour coded as shown in figure 1B. (J) Immunoblot analysis of genes involved in ERBBs and downstream pathway in TE9, TE11 and KYSE180 cells treated with afatinib (5 nM, 10 nM, 20 nM, 40 nM and 80 nM) or with DMSO control. Protein lysates were collected after drug treatment for 24 hours. Immunoblots from one representative experiment (n=2) are shown. (K) Images showing crystal violet staining of cell lines are listed in figure 2I, after 20 nM afatinib treatment for 7–10 days. Data from one representative experiment are presented (n=3). OSCC, oesophageal squamous cell carcinoma.
To investigate these targets, we focused on the Achilles genome wide CRISPR dataset given the greater size and the greater selectivity of CRISPR reagents. We found that the squamous transcription p63 was an outlier selective dependency in HNSCC and OSCCs (figure 2B). Furthermore, we identified EGFR, ERBB2 and ERBB3 as preferentially dependencies compared with non-squamous solid tumours (figure 2B). When we broadened our analysis to all SCCs, we also observed EGFR, ERBB2 and ERBB3 were highly preferentially dependencies in both SCCs compared with non-SCC (online supplemental figure S2A). We evaluated the dependency profiles of ERBBs in each SCC dataset and found ERBB3 dependence to be enriched in each SCC type (figure 2C). ERBB2 and EGFR showed the greatest overall dependence in HNSCC. We then used the CRISPR data across SCC models to explore the relationship between dependence on the different ERBB members. While we observed only modest correlations of EGFR with either ERBB2 or ERBB3, we found a strong (R=0.69, p<2.2e-16) correlation of ERBB2 and ERBB3 dependence (figure 2D). We then evaluated for correlates of ERBBs dependencies. As shown in online supplemental figure S2B, C, for each of the three ERBBs, higher mRNA expression is associated with greater dependence. None of the three receptors had dependence that correlated with their copy number.
To investigate further the preferential dependence on ERBB family members in SCCs, we first compared the mRNA expression between SCC and non-SCC cell lines. EGFR expression is higher in SCC compared with non-SCC (figure 2E). However, ERBB2 and ERBB3 expression are comparable (online supplemental figure S2D). Wilson et al 19 previously demonstrated that elevated expression of the ligand NRG1 activates ERBB3 and promotes pan-ERBB inhibitor afatinib sensitivity. Therefore, we evaluated NRG1 mRNA expression, finding significantly higher levels in SCC (figure 2F). Within SCC NRG1 expression was modestly correlated (R=−0.29, p=0.0051) with ERBB3 dependency but not with ERBB2 dependency (figure 2G).
We then queried two pharmacological screening screen datasets for agents with greater efficacy in SCC and found a strong enrichment of sensitivity to pan-ERBB inhibitors such as afatinib and lapatinib in SCCs (figure 2H and online supplemental figure S2E-G), consistent with functional genomic data. We next evaluated afatinib in OSCC cell lines and found that over half showed sensitivity to afatinib (figure 2I and online supplemental figure S2H). However, the sensitivity to afatinib is not associated with sensitivity to palbociclib. To assess predictors of sensitivity, we compared pharmacological data to CRISPR screening data. We found that afatinib sensitivity is correlated with EGFR and ERBB3 but not ERBB2 dependency in SCCs (online supplemental figure S2I). A more detailed assessment of genomic copy number status and expression of ERBB members relative to afatinib sensitivity revealed correlation with EGFR gene expression across the cell lines but not with copy number levels or gene expression levels of ERBB2 and ERBB3 (online supplemental figure S2I). We next evaluated the target engagement of afatinib in OSCC models and found that 20 nM afatinib can significantly block activity of pERBBs as well as downstream signalling pathways (figure 2J). We also evaluated a 7–10 day growth experiment and observed outgrowth in the majority of models (figure 2K, online supplemental figure S2J, K), arguing against pan-ERBB inhibitors as monotherapy.
Pan ERBB and CDK4/6 pathway dual inhibition demonstrates efficacy in OSCC
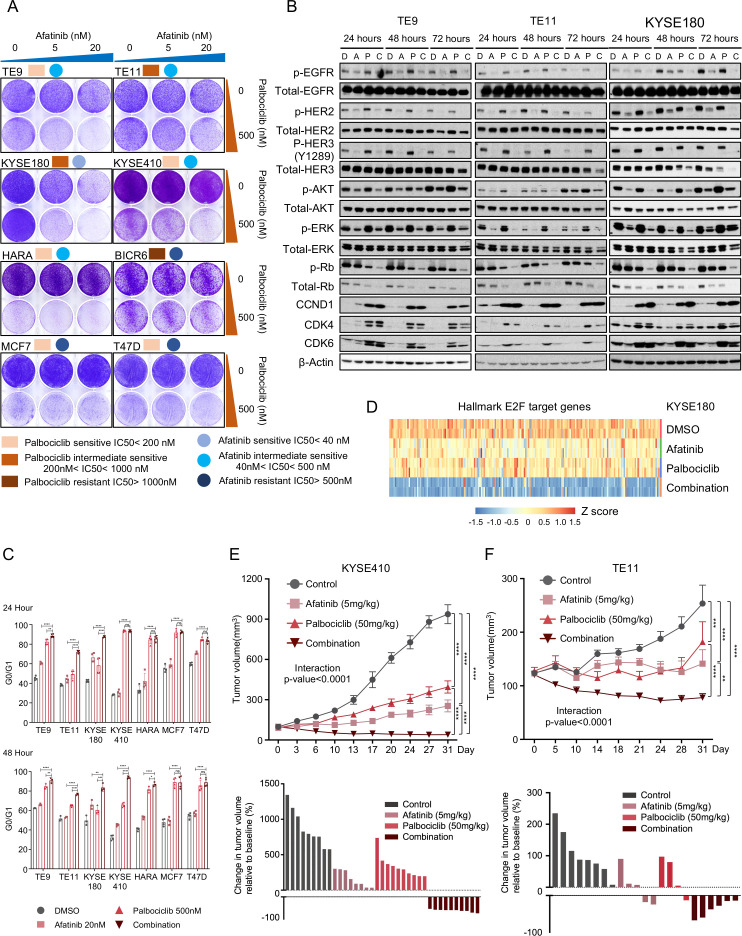
We next addressed our overarching hypotheses, that targeting a basal dependency, such as ERBB signalling, which act cooperatively with CDK4/6 inhibitors. We tested this hypothesis using afatinib, palbociclib and combination treatment in several OSCC models, one HNSCC and one LUSCC, with two OR+ breast lines as controls. We found that across SCC models with a range of sensitivity to monotherapy against either agent that combination therapy prolonged growth inhibition excepting models KYSE510 and TE1, the latter with RB1 loss. By contrast, no enhanced growth inhibition was observed in two OR+ breast lines (figure 3A, online supplemental figure S3A, B). We further tested more dose combinations by crystal violet staining and performed a synergy analysis based on the quantification of the signalling using Zero Interaction Potency (ZIP) model.20 As shown in online supplemental figure S3C, we found that in TE9, TE10 and TE11, the interaction between palbociclib and afatinib is likely to be synergistic (ZIP synergy score >10), whereas the interaction in KYSE140, KYSE180 and KYSE410 is likely to be additive (ZIP synergy score between −10 and 10). We next performed biochemical studies to confirm target engagement, finding that afatinib at 20 nM attenuated pERBB2, pEGFR and p-ERBB3 and reduced activation of the MAPK and PI3-K pathways, as measured by phosphorylation of AKT and ERK. While addition of 500 nM of palbociclib did not markedly impact the activity of PI3-K or MAPK markers, the addition of afatinib augmented the effects of palbocilib on inhibition of pRb and total RB (figure 3B). We also observed elevated protein levels of CDK4, CDK6 and cyclin D1 with palbociclib, perhaps indicating why blockade of mitogenic signalling enhances the ability of CDK4/6 therapy to block Rb phosphorylation. Also, in contrast to our earlier studies of EGFR inhibition in EGFR-amplified OSCC, we did not observe substantial adaptive reactivation of the MAPK pathway with sustained ERBB inhibition. The palbociclib and afatinib combination significantly induces G0/G1 arrest compared with either monotherapy in multiple SCC cell lines following both 24 and 48 hours of treatment (figure 3C). The sole exception was the KYSE410 model in which we did not observe a significant difference between the combination and palbociclib therapy at the 24-hour timepoint. However, we did not observe reproducible induction of apoptosis following combination therapy, suggesting that blockade of proliferation to be the primary effect (online supplemental figure S3D).
Figure 3.
Pan ERBB and CDK4/6 pathway dual inhibition demonstrated efficacy in OSCC. (A) Images showing crystal violet staining of representative squamous carcinoma cell lines on treatment with afatinib (20 nM), palbociclib (500 nM), the combination or with DMSO control for 7–10 days. Data from one representative experiment are presented (n=2). (B) Immunoblot analysis of genes involved in ERBB signalling pathway and cell-cycle pathway in TE9, TE11 and KYSE180 cells treated with afatinib (20 nM), palbociclib (500 nM), the combination or with DMSO control. Protein lysates were collected after drug treatment for 24 hours, 48 hours and 72 hours. Immunoblots from one representative experiment (n=2) are shown. (C) The frequency of G0/G1 cells of squamous carcinoma cell lines on treatment with afatinib (20 nM), palbociclib (500 nM), the combination or with DMSO control for 24 hours (top) and 48 hours (bottom). Following treatment, the cells were harvested, stained with propidium iodide and assayed with flow cytometry. Data are shown as mean±SD and NS, *p<0.05, **p<0.01, ***p<0.001 and ****p<0.0001 as calculated by the two-way analysis of variance (ANOVA) test followed by post hoc test with Benjamini-Hochberg correction. (D) Heatmap showing the expression of common E2F target genes in KYSE180 on treatment of DMSO, 20 nM afatinib, 500 nM palbocilib or combination. (E) Top: growth curve for KYS410 xenograft tumours (n=6–10) treated with vehicle, afatinib (5 mg/kg), palbociclib (50 mg/kg) or the combination. Data are shown as mean±SEM and ****p<0.0001 as calculated by the two-way ANOVA test followed by post hoc test with Benjamini-Hochberg correction on day 31. Bottom: waterfall plot showing the tumour volume change (at day 31) relative to baseline volume (at day 1). Each bar represents one xenograft tumour. (F) Top: growth curve for TE11 xenograft tumours (n=6–10) treated with vehicle, afatinib (5 mg/kg), palbociclib (50 mg/kg) or the combination. Data are shown as mean±SEM and **p<0.01, ***p<0.001 and ****p<0.0001 as calculated by the two-way ANOVA test followed by post hoc test with Benjamini-Hochberg correction on day 31. Bottom: waterfall plot showing the tumour volume change (at day 31) relative to baseline volume (at day 1). Each bar represents one xenograft tumour. NS, not significant; OSCC, oesophageal squamous cell carcinoma.
To evaluate mechanisms of combination therapy, we performed gene expression analysis in KYSE180 following 20 nM afatinib and 500 nM palbociclib either alone or in combination for 72 hours. Gene Set Enrichment analysis (GSEA) identified that HALLMARK_E2F_TARGETS are consistently downregulated in both single agent and combination treatments (online supplemental figure S3E). In line with figure 3B, our gene expression analysis also demonstrated that the combination therapy resulted in stronger repression of the RB-E2F target genes than either mono-drug treatment, consistent with a role of cooperative cell cycle inhibition (figure 3D).
To further evaluate ERBBs and CDK4/6 blockade, we evaluated the single agent or combination therapies in vivo in nude mice harbouring xenografts of palbociclib sensitive cell line KYSE410 and palbociclib intermediate sensitive cell line TE11. Single agent afatinib (5 mg/kg) or palbociclib (50 mg/kg) delayed tumour growth, but progression still occurred. Only combination showed consistent reduction in tumour volume (figure 3D–E). Further analysis by two-way analysis of variance analysis showed there is a significant interaction between afatinib treatment and palbociclib treatment in vivo.
KLF5 facilitates ERBB activation and promotes ERBB dependency
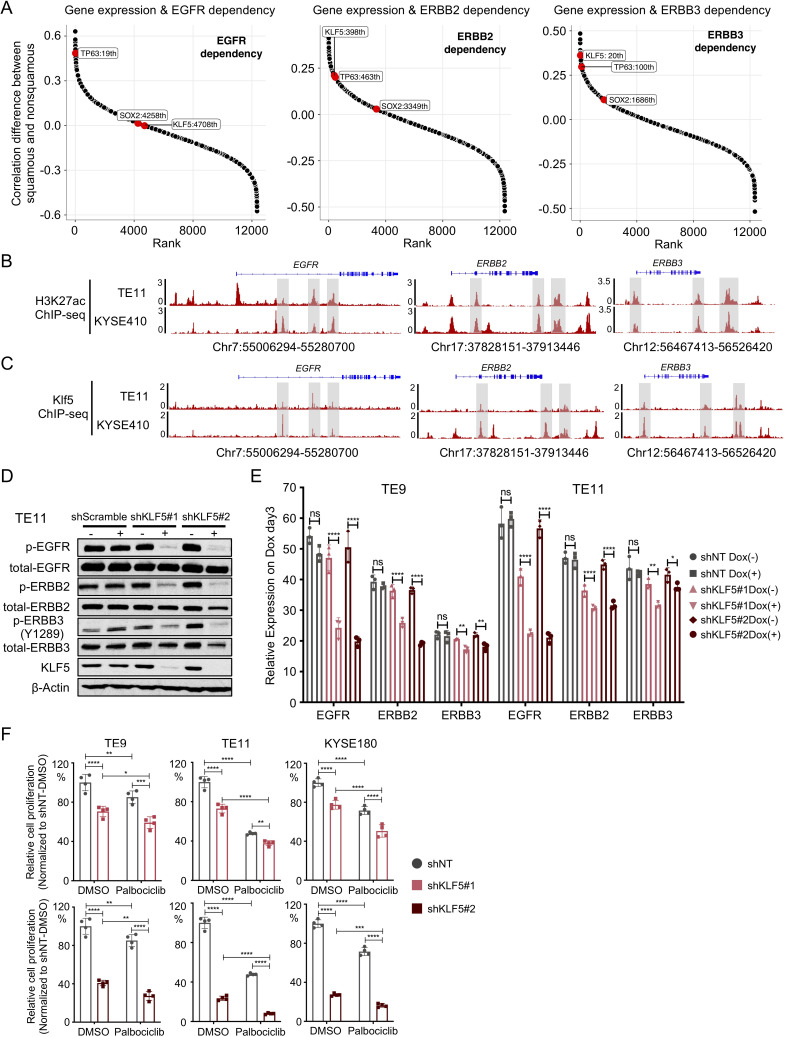
We next evaluated the aetiology of dependence on distinct ERBBs within SCC, especially the selective importance of ERBB3 within SCCs. A previous study21 revealed that squamous lineage transcription factors TP63 and SOX2 regulate EGFR expression by binding to the super-enhancers of EGFR, thereby promoting expression. We hypothesised that not only EGFR but perhaps also ERBB2 and ERBB3 may be regulated by a squamous transcription factor. We therefore evaluated ERBB2 and ERBB3 dependence relative to the expression of TP63 and SOX2 and KLF5, the latter recently identified to act as part of a complex with the other squamous factors.22 We first performed an unbiased analysis of genes whose expression level predicts ERBB gene dependencies in 89 SCC and 367 non-SCC cell lines. Interestingly, we observed that among the three squamous transcription factors, KLF5 expression is more correlated with ERBB3 gene dependency in SCC (R=−0.34, p=0.0012) than in non-SCC (R=0.024, p=0.65). We also found a correlation between KLF5 gene expression and ERBB2 gene dependency (R=−0.39, p=0.00017) in SCC and, to a less extent, in non-SCC models (R=−0.18, p=0.00063). KLF5 expression was correlated with EGFR gene dependency in both SCC (R=−0.36, p=0.00055) and non-SCC (R=−0.36, p=1e-12) (figure 4A, online supplemental figure S4A). In addition, KLF5 gene expression was also shown to strongly correlates with ERBB3 but not ERBB2 mRNA expression in SCC lines, further suggesting that KLF5 may be selectively important in ERBB3 regulation (online supplemental figure S4B). To further identify the gene dependency between squamous transcription factors and ERBBs, we confirmed that TP63 and EGFR were codependent in SCC (online supplemental figure S4C). However, no significant correlation was observed with TP63 and ERBB2/3 dependency or with SOX2 and EGFR/ERBB2/ERBB3 dependency. Intriguingly, KLF5 was modestly codependent with EGFR, ERBB2 and ERBB3 in SCC (online supplemental figure S4C).
Figure 4.
KLF5 facilitated ERBBs activation and promoted ERBBs dependency. (A) Modified hockey-stick plot representing the rank of Pearson correlation coefficient difference between EGFR (left), ERBB2 (middle), ERBB3 (right) gene dependencies and gene expression in squamous carcinoma cell lines (n=89) and non-squamous carcinoma cell lines (n=367). Pearson correlation was performed between target gene dependency score and gene expression. Correlation coefficient difference was calculated by subtracting Pearson R of squamous carcinoma group from Pearson R of non-squamous carcinoma group. Bigger value represents that a gene whose expression level correlates with gene dependency more strongly in squamous cell carcinoma than non-squamous cell carcinoma. Smaller value represents that a gene whose expression level correlates with gene dependency more strongly in non-squamous cell carcinoma than squamous cell carcinoma. (B) Representative H3K27ac ChIP-Seq tracks showing enhancer elements at EGFR, ERBB2 and ERBB3 locus in OSCC cell lines TE11 and KYSE410. (C) Representative KLF5 ChIP-Seq tracks showing KLF5 binding sites at EGFR, ERBB2 and ERBB3 locus in OSCC cell lines TE11 and KYSE410. (D) Immunoblot analysis of protein levels of phospho-EGFR, total-EGFR, phospho-ERBB2, total-ERBB2, phospho-ERBB3, total-ERBB3 and KLF5 in OSCC cell line TE11 on shRNA-mediated KLF5 knockdown. Immunoblots from one representative experiment (n=2) are shown. (E) mRNA expression of EGFR, ERBB2 and ERBB3 in OSCC cell lines TE9, TE11 and KYSE180 on shRNA-mediated KLF5 knockdown. Data are presented as mean±SD of three technical replicates per group. *P<0.05, **p<0.01, ***p<0.001 and ****p<0.0001 as calculated by the one-way analysis of variance followed by post hoc test with Benjamini-Hochberg correction. NS indicates non-significant. (F) Cell viability of OSCC cell lines TE9, TE11 and KYSE180 was assessed by ATP bioluminescence 5 days after control or KLF5 knockdown with shRNA with/without palbociclib (500 nM) treatment. Two independent biological replicates were performed for each cell line. ATP bioluminescence values were normalised to the value of day 0 and shNT. Data are shown as mean±SD and *p<0.05, **p<0.01, ***p<0.001 and ****p<0.0001 as calculated by the two-way analysis of variance followed by post hoc test with Benjamini-Hochberg correction. NS, not significant; OSCC, oesophageal squamous cell carcinoma.
To further evaluate the function of KLF5 in ERBB regulation, we performed H3K27ac ChIP-seq (figure 4B) and KLF5 ChIP-seq (figure 4C) in order to identify active enhancers and sites of KLF5 binding in OSCC models with KLF5 and ERBB3 codependency (TE11 and KYSE180). We identified multiple KLF5 binding sites with transcriptional regulatory activity, as defined by H3K27AC signal at the EGFR, ERBB2 and ERBB3 loci, suggesting KLF5 directly promotes ERBB activation (figure 4B, C). Furthermore, silencing KLF5 attenuated p-EGFR, p-ERBB2 and p-ERBB3 protein expression (figure 4D) and EGFR, ERBB2 and ERBB3 total mRNA expression (figure 4E), as well as reduced cell proliferation (figure 4F). We also found silencing KLF5 can significantly add to the antiproliferative effect of palbociclib in OSCC cell lines (figure 4F). These data support KLF5-mediated regulation of ERBBs function in OSCC, nominating KLF5 expression as a candidate biomarker to guide the use of these agents in conjunction with markers that guide the use of CDK4/6 inhibitors (eg, CCND1 amplification and lack of RB1 inactivation).
Discussion
Despite our understanding of genomic features of SCCs, we lack effective targeted therapy. While genomic data point to the potential to introduce CDK4/6 inhibition, single agent therapy lacks efficacy, suggesting that combination therapy will be critical. Here, we demonstrate the potential for CDK4/6 inhibitors with ERBB family inhibitors as a combination strategy in SCCs based on data from unbiased RNAi and CRISPR genome-wide loss-of-function screens and pharmacological profiling.
We and others have observed efficacy with CDK4/6 inhibition in SCCs preclinical models,12 especially with short-term in vitro proliferation assays. However, translation has been limited, especially in OSCC. A phase II clinical trial demonstrated limited single-agent activity of palbociclib in five OSCC patients with intact nuclear RB expression, with median PFS of 1.8 months and median overall survival (OS) of 3 months (NCT:01037790).11 Similar results were also observed in another phase II trial (NCT02154490) in lung SCC.23 Trials to evaluate these drugs in HNSCC are in progress (NCT03088059).24 Other than retention of Rb1 expression, clear biomarkers of response to palbociclib treatment have yet to be identified, and neither amplification of CCND1 or loss of p16 were definitively linked to response in breast cancer.25 26 One paper predicted CDKN2a or 2B loss as a potential biomarker for CDK4/6 inhibitor sensitivity.27 Our data suggested a possible correlation of CCND1 amplification and palbociclib sensitivity. However, despite these correlations, our results in vivo and longer term in vitro growth caution against the potential of monotherapy. We also observed a decrease expression of total RB after palbociclib treatment in our OSCC cell lines in both dose-dependent and time-dependent manner. These results mirror results in other studies.15 The mechanism of CDK4/6-induced modulation of Rb protein levels requires further investigation.
Cancer cells harbour specific features that render them vulnerable to particular agents. Especially, when these targets promote the cell cycle, blockade may augment efficacy of CDK4/6 therapy. For example, in OR-positive breast cancer, OR blockade inhibits activation of CCND1. Preclinical studies and clinical trials have confirmed that addition of CDK4/6 inhibition to endocrine therapy in OR+/HER2− breast cancer has marked efficacy.25 28 Mechanistically, CDK4/6 inhibitors have been most effective when used with agents that promote growth arrest or cell death.29 Here, we took advantage of the power of large-scale cancer profiling efforts and advanced clinical sequencing technologies to uncover SCC vulnerabilities and biomarkers. We identified ERBB family members, EGFR, ERBB2 and ERBB3 as preferentially dependencies and ERBB family kinase inhibitors as having efficacy across SCC models. ERBB family members directly activate MAPK and PI3K pathways, thus feeding directly into the downstream cyclin D1/CDK4/6 axis, making pharmacological blockade of this axis an attractive therapeutic strategy to complement cell cycle therapy in OSCC.
Anti-EGFR antibodies such as cetuximab and panitumumab have been evaluated in OSCC, either in combination with radiotherapy or chemotherapy. So far, most studies demonstrated non-additional benefit of EGFR antibodies in non-selected OSCC, and no biomarkers were reported to predict tumour response to EGFR antibodies (SCOPE1,30 31 RTOG0436,32 EORTC power trial),33 except the only phase II study reported a modest increase in response rate with cetuximab.34 Signs of efficacy of cetuximab in EGFR amplified positive lung SCC are encouraging35 and this drug has been approved in advanced HNSCC.36 37 Apart from targeting EGFR, cetuximab also stimulates antibody-dependent cellular cytotoxicity. The extent to which these antibodies blocking downstream signalling pathway relative to enzymatic tyrosine kinase inhibitors (TKIs) is not clear. For EGFR TKIs, four small clinical phase III trials reported the objective response rate in unselected patients with advanced OSCC of 2.8%–16.7%.38–41 Since our data revealed a broader dependency on the ERBB family in SCC, pan-ERBB inhibitors may have greater efficacy. One phase II study of afatinib (NCT02353936) demonstrated modest efficacy with manageable toxicity in platinum-resistant OSCC. Afatinib was also reported to improve PFS versus methotrexate (2.6 vs 1.7 months) in HNSCC and is FDA approved42 43 in HNSCC and lung SCC following progression on platinum-based chemotherapy. Biomarker studies showed that EGFR gene copy number aberrations (polysomy or amplification) and overexpression might potentially be used in predicting the efficacy in patients treated with EGFR TKIs.42 44 Our study also found a positive correlation between afatinib response in OSCC and EGFR/ERBB3 dependency as well as ERBB mRNA expression.
Clinical trials are currently testing combination treatment with EGFR and CDK4/6 inhibitor in HNSCC. Phase I/II clinical trials have demonstrated that ribociclib45 or palbociclib46 in combination with cetuximab is safe (NCT02429089) and resulted in a robust tumour response rate and prolongation of PFS and OS (NCT02101034).47 However, another randomised phase II trial failed to demonstrate a statistically significant benefit in PFS and OS of the palbociclib/cetuximab combination over cetuximab alone in unselected patients with human papillomavirus (HPV)-negative HNSCC (NCT02499120) (JCO 2019, 37:15_suppl 6013). These two phase II trials underscored the potential clinical value of biomarkers to select patients most likely to benefit (NCT02101034; NCT02499120). In addition, single-agent antibodies may not be sufficient to block ERBB2-mediated activation of cell cycle signalling. Pan-ERBB inhibitor lapatinib has recently been shown to synergise with CDK inhibition in HER2-associated malignancies.48 Until now, only one case was reported in an advanced lung SCC patient with EGFRL861Q and CDK4 amplification responding to afatinib combined with palbociclib treatment.49 Our in vivo experiments showed a significant interaction between afatinib and palbociclib treatment. Mechanistically, our gene expression analysis suggested that the degree of repression of the cell cycle/E2F pathway is greater with the combination than monotherapy. As such, the success of this combination may be best attributed to the cooperative effects on the same pathway leading to more substantial blockade. As such, this combination shows analogy to combinations such as those targeting BRAF and MEK in melanoma and colorectal cancer in order to enhance effective blockade of a single cellular pathway.50–52
One potential challenge with combination therapy is the tolerability of regimens, particular with similar classes of TKIs. For examples, the combination of dabrafenib with trametinib in melanoma had an increased incidence of adverse events compared with dabrafenib plus placebo.52 Similarly, a recent study investigating combined BRAF, EGFR and MEK inhibition in BRAFV600E-mutant colorectal cancer observed greater adverse events with triplet treatment group than doublet group, necessitating dose reductions and interruptions/delays.50 The most commonly reported adverse events (AEs) with ERBB TKIs are GI and dermatological in nature and include diarrhoea, skin rash, mucosal inflammation (eg, stomatitis and mucositis) and paronychia.53 In contrast, the most common AEs of palbociclib includes neutropenia, leucopenia, anaemia and fatigue.26 Evaluation of the tolerability of these combinations will need to be evaluated prospectively.
The data supporting dependence on multiple ERBBs across the SCC lineage raised the potential question of lineage-specific transcriptional regulators that may promote ERBB activation. Previous studies reported that squamous lineage factors TP63 and SOX2 promote SCC progression in part by binding to the super-enhancers of EGFR.21 54 We identified a function relationship between KLF5, recently established to act as part of a core regulatory circuit that controls epigenetic and transcription patterns in OSCC,22 and ERBBs in OSCC and other SCCs. We found that KLF5 localises to several loci at EGFR, ERBB2 and ERBB3 with evince of H3K27ac signalling in several OSCC cancer models. Coupled to our experimental data with KLF5 targeting, these data suggest that KLF5 expression could help identify patients where pan-ERBB therapy could act as part of an effective combination
In summary, our study provides compelling evidence that combination treatment with ERBB and CDK4/6 in squamous cell cancer may benefit OSCC. KLF5 could be considered as a potential biomarker to predict the sensitivity to ERBBs inhibitors. We encourage clinical investigation of combination treatment in oesophageal and other SCCs.
Acknowledgments
The authors would like to thank members of the Bass laboratory for insightful discussions. The research was supported by the Twomey Family Fellowship in Oesophageal Cancer Research (JZ). AB was supported by the National Institutes of Health (NIH) Grants R01 CA196932 and R01 CA187119. AR, K-KW and AB were supported by the NIH Grant P01 CA098101. XZ was supported by the NIH Grant R00 CA215244.
Footnotes
JZ and ZZ contributed equally.
ZW and AB contributed equally.
Contributors: Conceptualisation: AB, JZ, ZW and ZZ. Methodology: JZ, ZW, ZZ, JM, AN, LG, YX and HL. Validation: JZ, ZW, AN, LG and KP. Formal analysis: ZW, ZZ, JM and YX. Investigation: ZW, JZ, ZZ and LG. Resources: ZW, JZ, ZZ, JM, XZ and YZ. Data curation: ZW, ZZ and XZ. Writing-original draft: JZ, ZW, ZZ and AB. Supervision: HN, AR, JAD, MM, K-KW and AB.
Funding: This study was funded by National Institutes of Health (Grant number: CA098101, CA187119, CA196932 and R00 CA215244).
Competing interests: AB receives research funding from Bayer, Merck and Novartis and is a consultant to Earli, and HelixNano and a cofounder of Signet Therapeutics. K-KW is a founder and equity holder of G1 Therapeutics, and he has consulting/sponsored research agreements with MedImmune, Takeda, TargImmune, BMS, AstraZeneca, Janssen, Pfizer, Novartis, Merck, Ono and Array.
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Data availability statement
Data are available in a public, open access repository. Genome-wide CRISPR dependency data (20Q2 Achiles_gene_effect file) and RNA interference (RNAi) combined dependency data (D2_combined_gene_dep_scores file) were downloaded from Broad Institute DepMap web portal (https://depmap.org/portal/download/). Cancer cell information, including mutation, gene expression, gene-level relative copy number data were retrieved from the Cancer Cell Line Encyclopedia (CCLE) project. These multi-omics data can be downloaded from Broad Institute DepMap web portal (https://depmap.org/portal/download/). Drug sensitivity Area under the ROC Curve (AUC) data were retrieved from Genomics of Drug Sensitivity in Cancer (GDSC- https://www.cancerrxgene.org/), Cancer Target Discovery and Development dataset (CTD2- https://ocg.cancer.gov/programs/ctd2/data-portal), and PRISM repurposing screen 20Q2 release (PRISM). Those datasets can be accessed from Broad Institute DepMap web portal (https://depmap.org/portal/download/).
Ethics statements
Patient consent for publication
Not required.
Ethics approval
Dana-Farber IACUC.
References
- 1. Bray F, Ferlay J, Soerjomataram I, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2018;68:394–424. 10.3322/caac.21492 [DOI] [PubMed] [Google Scholar]
- 2. Kelly RJ. Emerging multimodality approaches to treat localized esophageal cancer. J Natl Compr Canc Netw 2019;17:1009–14. 10.6004/jnccn.2019.7337 [DOI] [PubMed] [Google Scholar]
- 3. Kato K, Cho BC, Takahashi M, et al. Nivolumab versus chemotherapy in patients with advanced oesophageal squamous cell carcinoma refractory or intolerant to previous chemotherapy (ATTRACTION-3): a multicentre, randomised, open-label, phase 3 trial. Lancet Oncol 2019;20:1506–17. 10.1016/S1470-2045(19)30626-6 [DOI] [PubMed] [Google Scholar]
- 4. Shah MA, Kojima T, Hochhauser D, et al. Efficacy and safety of pembrolizumab for heavily pretreated patients with advanced, metastatic adenocarcinoma or squamous cell carcinoma of the esophagus: the phase 2 KEYNOTE-180 study. JAMA Oncol 2019;5:546–50. 10.1001/jamaoncol.2018.5441 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Cancer Genome Atlas Research Network, Analysis Working Group: Asan University, BC Cancer Agency, et al. Integrated genomic characterization of oesophageal carcinoma. Nature 2017;541:169–75. 10.1038/nature20805 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Gao Y-B, Chen Z-L, Li J-G, et al. Genetic landscape of esophageal squamous cell carcinoma. Nat Genet 2014;46:1097–102. 10.1038/ng.3076 [DOI] [PubMed] [Google Scholar]
- 7. Sawada G, Niida A, Uchi R, et al. Genomic Landscape of Esophageal Squamous Cell Carcinoma in a Japanese Population. Gastroenterology 2016;150:1171–82. 10.1053/j.gastro.2016.01.035 [DOI] [PubMed] [Google Scholar]
- 8. Dotto GP, Rustgi AK. Squamous cell cancers: a unified perspective on biology and genetics. Cancer Cell 2016;29:622–37. 10.1016/j.ccell.2016.04.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Campbell JD, Yau C, Bowlby R, et al. Genomic, pathway network, and immunologic features distinguishing squamous carcinomas. Cell Rep 2018;23:194–212. 10.1016/j.celrep.2018.03.063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Klein ME, Kovatcheva M, Davis LE, et al. Cdk4/6 inhibitors: the mechanism of action may not be as simple as once thought. Cancer Cell 2018;34:9–20. 10.1016/j.ccell.2018.03.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Karasic TB, O'Hara MH, Teitelbaum UR, et al. Phase II trial of Palbociclib in patients with advanced esophageal or gastric cancer. Oncologist 2020;25:e1864–8. 10.1634/theoncologist.2020-0681 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Zhou J, Wu Z, Wong G, et al. CDK4/6 or MAPK blockade enhances efficacy of EGFR inhibition in oesophageal squamous cell carcinoma. Nat Commun 2017;8:13897. 10.1038/ncomms13897 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Malorni L, Curigliano G, Minisini AM, et al. Palbociclib as single agent or in combination with the endocrine therapy received before disease progression for estrogen receptor-positive, HER2-negative metastatic breast cancer: TREnd trial. Ann Oncol 2018;29:1748–54. 10.1093/annonc/mdy214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Rossi L, Biagioni C, McCartney A, et al. Clinical outcomes after palbociclib with or without endocrine therapy in postmenopausal women with hormone receptor positive and HER2-negative metastatic breast cancer enrolled in the TREnd trial. Breast Cancer Res 2019;21:71. 10.1186/s13058-019-1149-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Mao P, Cohen O, Kowalski KJ, et al. Acquired FGFR and FGF Alterations Confer Resistance to Estrogen Receptor (ER) Targeted Therapy in ER+ Metastatic Breast Cancer. Clin Cancer Res 2020;26:5974–89. 10.1158/1078-0432.CCR-19-3958 [DOI] [PubMed] [Google Scholar]
- 16. McCartney A, Migliaccio I, Bonechi M, et al. Mechanisms of resistance to CDK4/6 inhibitors: potential implications and biomarkers for clinical practice. Front Oncol 2019;9:666. 10.3389/fonc.2019.00666 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Tsherniak A, Vazquez F, Montgomery PG, et al. Defining a cancer dependency MAP. Cell 2017;170:564–76. 10.1016/j.cell.2017.06.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. McFarland JM, Ho ZV, Kugener G, et al. Improved estimation of cancer dependencies from large-scale RNAi screens using model-based normalization and data integration. Nat Commun 2018;9:4610. 10.1038/s41467-018-06916-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Wilson TR, Lee DY, Berry L, et al. Neuregulin-1-mediated autocrine signaling underlies sensitivity to HER2 kinase inhibitors in a subset of human cancers. Cancer Cell 2011;20:158–72. 10.1016/j.ccr.2011.07.011 [DOI] [PubMed] [Google Scholar]
- 20. Yadav B, Wennerberg K, Aittokallio T, et al. Searching for drug synergy in complex dose-response landscapes using an interaction potency model. Comput Struct Biotechnol J 2015;13:504–13. 10.1016/j.csbj.2015.09.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Jiang Y, Jiang Y-Y, Xie J-J, et al. Co-activation of super-enhancer-driven CCAT1 by TP63 and SOX2 promotes squamous cancer progression. Nat Commun 2018;9:3619. 10.1038/s41467-018-06081-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Jiang Y-Y, Jiang Y, Li C-Q, et al. TP63, Sox2, and KLF5 establish a core regulatory circuitry that controls epigenetic and transcription patterns in esophageal squamous cell carcinoma cell lines. Gastroenterology 2020;159:1311–27. 10.1053/j.gastro.2020.06.050 [DOI] [PubMed] [Google Scholar]
- 23. Edelman MJ, Redman MW, Albain KS, et al. SWOG S1400C (NCT02154490)-A Phase II Study of Palbociclib for Previously Treated Cell Cycle Gene Alteration-Positive Patients with Stage IV Squamous Cell Lung Cancer (Lung-MAP Substudy). J Thorac Oncol 2019;14:1853–9. 10.1016/j.jtho.2019.06.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Gougis P, Moreau Bachelard C, Kamal M, et al. Clinical development of molecular targeted therapy in head and neck squamous cell carcinoma. JNCI Cancer Spectr 2019;3:pkz055. 10.1093/jncics/pkz055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Finn RS, Crown JP, Lang I, et al. The cyclin-dependent kinase 4/6 inhibitor palbociclib in combination with letrozole versus letrozole alone as first-line treatment of oestrogen receptor-positive, HER2-negative, advanced breast cancer (PALOMA-1/TRIO-18): a randomised phase 2 study. Lancet Oncol 2015;16:25–35. 10.1016/S1470-2045(14)71159-3 [DOI] [PubMed] [Google Scholar]
- 26. Finn RS, Martin M, Rugo HS, et al. Palbociclib and letrozole in advanced breast cancer. N Engl J Med 2016;375:1925–36. 10.1056/NEJMoa1607303 [DOI] [PubMed] [Google Scholar]
- 27. Su D, Zhang D, Jin J, et al. Identification of predictors of drug sensitivity using patient-derived models of esophageal squamous cell carcinoma. Nat Commun 2019;10:5076. 10.1038/s41467-019-12846-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Cristofanilli M, Turner NC, Bondarenko I, et al. Fulvestrant plus palbociclib versus fulvestrant plus placebo for treatment of hormone-receptor-positive, HER2-negative metastatic breast cancer that progressed on previous endocrine therapy (PALOMA-3): final analysis of the multicentre, double-blind, phase 3 randomised controlled trial. Lancet Oncol 2016;17:425–39. 10.1016/S1470-2045(15)00613-0 [DOI] [PubMed] [Google Scholar]
- 29. VanArsdale T, Boshoff C, Arndt KT, et al. Molecular pathways: targeting the cyclin D-CDK4/6 axis for cancer treatment. Clin Cancer Res 2015;21:2905–10. 10.1158/1078-0432.CCR-14-0816 [DOI] [PubMed] [Google Scholar]
- 30. Crosby T, Hurt CN, Falk S, et al. Chemoradiotherapy with or without cetuximab in patients with oesophageal cancer (SCOPE1): a multicentre, phase 2/3 randomised trial. Lancet Oncol 2013;14:627–37. 10.1016/S1470-2045(13)70136-0 [DOI] [PubMed] [Google Scholar]
- 31. Crosby T, Hurt CN, Falk S, et al. Long-term results and recurrence patterns from SCOPE-1: a phase II/III randomised trial of definitive chemoradiotherapy +/- cetuximab in oesophageal cancer. Br J Cancer 2017;116:709–16. 10.1038/bjc.2017.21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Suntharalingam M, Winter K, Ilson D, et al. Effect of the addition of cetuximab to paclitaxel, cisplatin, and radiation therapy for patients with esophageal cancer: the NRG oncology RTOG 0436 phase 3 randomized clinical trial. JAMA Oncol 2017;3:1520–8. 10.1001/jamaoncol.2017.1598 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Moehler M, Maderer A, Thuss-Patience PC, et al. Cisplatin and 5-fluorouracil with or without epidermal growth factor receptor inhibition panitumumab for patients with non-resectable, advanced or metastatic oesophageal squamous cell cancer: a prospective, open-label, randomised phase III AIO/EORTC trial (power). Ann Oncol 2020;31:228–35. 10.1016/j.annonc.2019.10.018 [DOI] [PubMed] [Google Scholar]
- 34. Lorenzen S, Schuster T, Porschen R, et al. Cetuximab plus cisplatin-5-fluorouracil versus cisplatin-5-fluorouracil alone in first-line metastatic squamous cell carcinoma of the esophagus: a randomized phase II study of the Arbeitsgemeinschaft Internistische Onkologie. Ann Oncol 2009;20:1667–73. 10.1093/annonc/mdp069 [DOI] [PubMed] [Google Scholar]
- 35. Herbst RS, Redman MW, Kim ES, et al. Cetuximab plus carboplatin and paclitaxel with or without bevacizumab versus carboplatin and paclitaxel with or without bevacizumab in advanced NSCLC (SWOG S0819): a randomised, phase 3 study. Lancet Oncol 2018;19:101–14. 10.1016/S1470-2045(17)30694-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Bonner JA, Harari PM, Giralt J, et al. Radiotherapy plus cetuximab for squamous-cell carcinoma of the head and neck. N Engl J Med 2006;354:567–78. 10.1056/NEJMoa053422 [DOI] [PubMed] [Google Scholar]
- 37. Bonner JA, Harari PM, Giralt J, et al. Radiotherapy plus cetuximab for locoregionally advanced head and neck cancer: 5-year survival data from a phase 3 randomised trial, and relation between cetuximab-induced rash and survival. Lancet Oncol 2010;11:21–8. 10.1016/S1470-2045(09)70311-0 [DOI] [PubMed] [Google Scholar]
- 38. Adelstein DJ, Rodriguez CP, Rybicki LA, et al. A phase II trial of gefitinib for recurrent or metastatic cancer of the esophagus or gastroesophageal junction. Invest New Drugs 2012;30:1684–9. 10.1007/s10637-011-9736-z [DOI] [PubMed] [Google Scholar]
- 39. Ilson DH, Kelsen D, Shah M, et al. A phase 2 trial of erlotinib in patients with previously treated squamous cell and adenocarcinoma of the esophagus. Cancer 2011;117:1409–14. 10.1002/cncr.25602 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Huang J, Fan Q, Lu P, et al. Icotinib in Patients with Pretreated Advanced Esophageal Squamous Cell Carcinoma with EGFR Overexpression or EGFR Gene Amplification: A Single-Arm, Multicenter Phase 2 Study. J Thorac Oncol 2016;11:910–7. 10.1016/j.jtho.2016.02.020 [DOI] [PubMed] [Google Scholar]
- 41. Janmaat ML, Gallegos-Ruiz MI, Rodriguez JA, et al. Predictive factors for outcome in a phase II study of gefitinib in second-line treatment of advanced esophageal cancer patients. J Clin Oncol 2006;24:1612–9. 10.1200/JCO.2005.03.4900 [DOI] [PubMed] [Google Scholar]
- 42. Cohen EEW, Licitra LF, Burtness B, et al. Biomarkers predict enhanced clinical outcomes with afatinib versus methotrexate in patients with second-line recurrent and/or metastatic head and neck cancer. Ann Oncol 2017;28:2526–32. 10.1093/annonc/mdx344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Machiels J-PH, Haddad RI, Fayette J, et al. Afatinib versus methotrexate as second-line treatment in patients with recurrent or metastatic squamous-cell carcinoma of the head and neck progressing on or after platinum-based therapy (LUX-Head & Neck 1): an open-label, randomised phase 3 trial. Lancet Oncol 2015;16:583–94. 10.1016/S1470-2045(15)70124-5 [DOI] [PubMed] [Google Scholar]
- 44. Petty RD, Dahle-Smith A, Stevenson DAJ, et al. Gefitinib and EGFR gene copy number aberrations in esophageal cancer. J Clin Oncol 2017;35:2279–87. 10.1200/JCO.2016.70.3934 [DOI] [PubMed] [Google Scholar]
- 45. Seront E, Schmitz S, Papier M, et al. Phase 1 study evaluating the association of the cyclin-dependent kinase 4/6 inhibitor Ribociclib and cetuximab in recurrent/metastatic p16-negative squamous cell carcinoma of the head and neck. Front Oncol 2019;9:155. 10.3389/fonc.2019.00155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Michel L, Ley J, Wildes TM, et al. Phase I trial of palbociclib, a selective cyclin dependent kinase 4/6 inhibitor, in combination with cetuximab in patients with recurrent/metastatic head and neck squamous cell carcinoma. Oral Oncol 2016;58:41–8. 10.1016/j.oraloncology.2016.05.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Adkins D, Ley J, Neupane P, et al. Palbociclib and cetuximab in platinum-resistant and in cetuximab-resistant human papillomavirus-unrelated head and neck cancer: a multicentre, multigroup, phase 2 trial. Lancet Oncol 2019;20:1295–305. 10.1016/S1470-2045(19)30405-X [DOI] [PubMed] [Google Scholar]
- 48. Goel S, Wang Q, Watt AC, et al. Overcoming therapeutic resistance in HER2-positive breast cancers with CDK4/6 inhibitors. Cancer Cell 2016;29:255–69. 10.1016/j.ccell.2016.02.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Jiang H, Luo N, Zhang X, et al. EGFR L861Q and CDK4 amplification responding to afatinib combined with palbociclib treatment in a patient with advanced lung squamous cell carcinoma. Lung Cancer 2020;145:216–8. 10.1016/j.lungcan.2020.04.001 [DOI] [PubMed] [Google Scholar]
- 50. Corcoran RB, André T, Atreya CE, et al. Combined BRAF, EGFR, and MEK Inhibition in Patients with BRAFV600E-Mutant Colorectal Cancer. Cancer Discov 2018;8:428–43. 10.1158/2159-8290.CD-17-1226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Long GV, Hauschild A, Santinami M, et al. Adjuvant dabrafenib plus trametinib in stage III BRAF-mutated melanoma. N Engl J Med 2017;377:1813–23. 10.1056/NEJMoa1708539 [DOI] [PubMed] [Google Scholar]
- 52. Long GV, Stroyakovskiy D, Gogas H, et al. Combined BRAF and MEK inhibition versus BRAF inhibition alone in melanoma. N Engl J Med 2014;371:1877–88. 10.1056/NEJMoa1406037 [DOI] [PubMed] [Google Scholar]
- 53. Melosky B, Hirsh V. Management of common toxicities in metastatic NSCLC related to anti-lung cancer therapies with EGFR-TKIs. Front Oncol 2014;4:238. 10.3389/fonc.2014.00238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Holcakova J, Nekulova M, Orzol P, et al. Δnp63 activates EGFR signaling to induce loss of adhesion in triple-negative basal-like breast cancer cells. Breast Cancer Res Treat 2017;163:475–84. 10.1007/s10549-017-4216-6 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
gutjnl-2020-323276supp001.pdf (62.4KB, pdf)
gutjnl-2020-323276supp002.pdf (15.8MB, pdf)
Data Availability Statement
Data are available in a public, open access repository. Genome-wide CRISPR dependency data (20Q2 Achiles_gene_effect file) and RNA interference (RNAi) combined dependency data (D2_combined_gene_dep_scores file) were downloaded from Broad Institute DepMap web portal (https://depmap.org/portal/download/). Cancer cell information, including mutation, gene expression, gene-level relative copy number data were retrieved from the Cancer Cell Line Encyclopedia (CCLE) project. These multi-omics data can be downloaded from Broad Institute DepMap web portal (https://depmap.org/portal/download/). Drug sensitivity Area under the ROC Curve (AUC) data were retrieved from Genomics of Drug Sensitivity in Cancer (GDSC- https://www.cancerrxgene.org/), Cancer Target Discovery and Development dataset (CTD2- https://ocg.cancer.gov/programs/ctd2/data-portal), and PRISM repurposing screen 20Q2 release (PRISM). Those datasets can be accessed from Broad Institute DepMap web portal (https://depmap.org/portal/download/).