Abstract
Background and objectives
The risks of postoperative risk of epilepsy after a craniotomy is widely believed to be raised. A study is warranted to quantify the risks for any neurosurgical indication. In this unselected register-based nationwide cohort study with virtually complete follow-up, the short-term and long-term cumulative risks of postoperative de novo epilepsy for all major neurosurgical indications were estimated.
Methods
The study was based on 8948 first-time craniotomy patients in Denmark 1 January 2005 to 31 December 2015 with follow-up until 31 December 2016. The patients were classified according to their underlying neurosurgical pathology. Patients with preoperative epilepsy were excluded. The postcraniotomy risks of de novo epilepsy were estimated using the Aalen-Johansen estimator in a multistate model.
Results
The overall cumulative 1-year risk of postcraniotomy de novo epilepsy was 13.9% (95% CI 13.2 to 14.6). For patients with intracranial tumour the cumulative 1-year risk was 15.4% (95% CI 14.4 to 16.5), for spontaneous intracranial haemorrhage 11.3% (95% CI 10.1 to 12.6), for traumatic intracranial haemorrhage 11.1% (95% CI 9.6 to 12.9), for cerebral abscess 27.6% (95% CI 22.8 to 33.5) and for congenital malformations 3.8% (95% CI 1.3 to 11.7). The 6-month, 1-year and 5-year risks for all major indications by specific subtypes are provided.
Conclusions
The cumulative risk of de novo epilepsy following craniotomy is high for patients with any indication for craniotomy, as compared with the background population. The results provide comprehensive data to support future recommendations regarding prophylactic antiepileptic treatment and driving restrictions.
Keywords: epilepsy, neurosurgery
Introduction
In 1996, the American neurosurgeon Vertosick wrote: ‘You ain’t never the same when the air hits your brain’,1 this being well exemplified by the known acute and long-term complication of postoperative de novo epilepsy after craniotomy. The risk of de novo epilepsy is of relevance to the question of prophylactic antiepileptic treatment and driving restrictions following craniotomy, as well as the general disease burden associated with having epilepsy. Guidelines and recommendations regarding these matters are highly variable between countries due to the hitherto scarce investigations of the risk of postoperative de novo epilepsy after a craniotomy. Like in many other countries, prophylactic antiepileptic medicine or driving restrictions in relation to craniotomy is not used in Denmark
The major indications for craniotomy, being intracranial tumour, spontaneous or traumatic haemorrhage, or intracerebral abscess, will in themselves constitute a significant risk of epilepsy (5%–35%).2–7 A craniotomy is a surgical procedure with removal of an intracranial mass lesion or closure of vascular anomalies through an opening of the skull. Due to the inevitable cortical trauma, gliosis, and changes in microcirculation following a craniotomy, it is reasonable to believe that the procedure may carry a risk of de novo epilepsy. At the same time preoperative epilepsy may be reduced by removal of a mass lesion. These coexisting effects are illustrated by a Swedish study of 113 meningioma patients with at least 7 years of follow-up, reporting that eight of the 21 patients with preoperative epilepsy were free of epilepsy after the surgery (38%), whereas 13 of the remaining 92 patients without preoperative epilepsy developed postoperative de novo epilepsy(14%).6 A much higher risk of 59% of de novo epilepsy within 27 months after craniotomy was reported among 141 patients with the highly malignant glioblastoma multiforme,5 which suggests high variability according to subtypes of indication.
Studies specifically investigating the risk of epilepsy following a craniotomy in patients without preoperative epilepsy are very scarce, nevertheless, it is highly relevant to investigate these risks from both a clinical and society perspective. The aim of this study was therefore to investigate the cumulative risk of postoperative de novo epilepsy in craniotomy patients in a nationwide, unselected cohort, encompassing all patients who underwent a craniotomy in Denmark during 2005–2015. Moreover, we estimated the cumulative risks for subgroups of craniotomy indications, where in most, the risk of postoperative de novo epilepsy is hitherto unknown.
Methods
Data sources
The Civil Registration System (CRS)8 contains demographic information on every individual living in Denmark since 1968. All individuals are given a unique social security number allowing for linkages between registers. The National Patient Register (NPR)9 covers all hospital admittances and discharge diagnoses since 1977; furthermore, since 1995 it also includes emergency room visits, outpatient contacts and surgeries, providing information on diseases according to the International Classification of Diseases (ICD) and surgeries according to Nordic Medico-Statistical Committee Classification of Surgical Procedures. Diagnoses given by general practitioners and private specialist clinics are not available from the NPR. The Danish Cancer Registry10 contains information on cancer diagnoses in Denmark since 1943. Both ICD codes and ICD for Oncology (ICD-O) codes are available, and therefore histological information is available for all surgically treated neurological tumours. The National Prescription Register11 has recorded information on all prescribed medicine in Denmark since 1995, according to the Anatomical Therapeutic Chemical Classification System (ATC), providing information on antiepileptic drugs.
Study population
The study population consisted of patients who underwent their first craniotomy in one of the neurosurgical departments in Denmark from 1 January 2005, until 31 December 2015. The patients were followed until 31 December 2016, which ensured at least 12 months of follow-up. Figure 1 illustrates how the cohort was defined.
Figure 1.
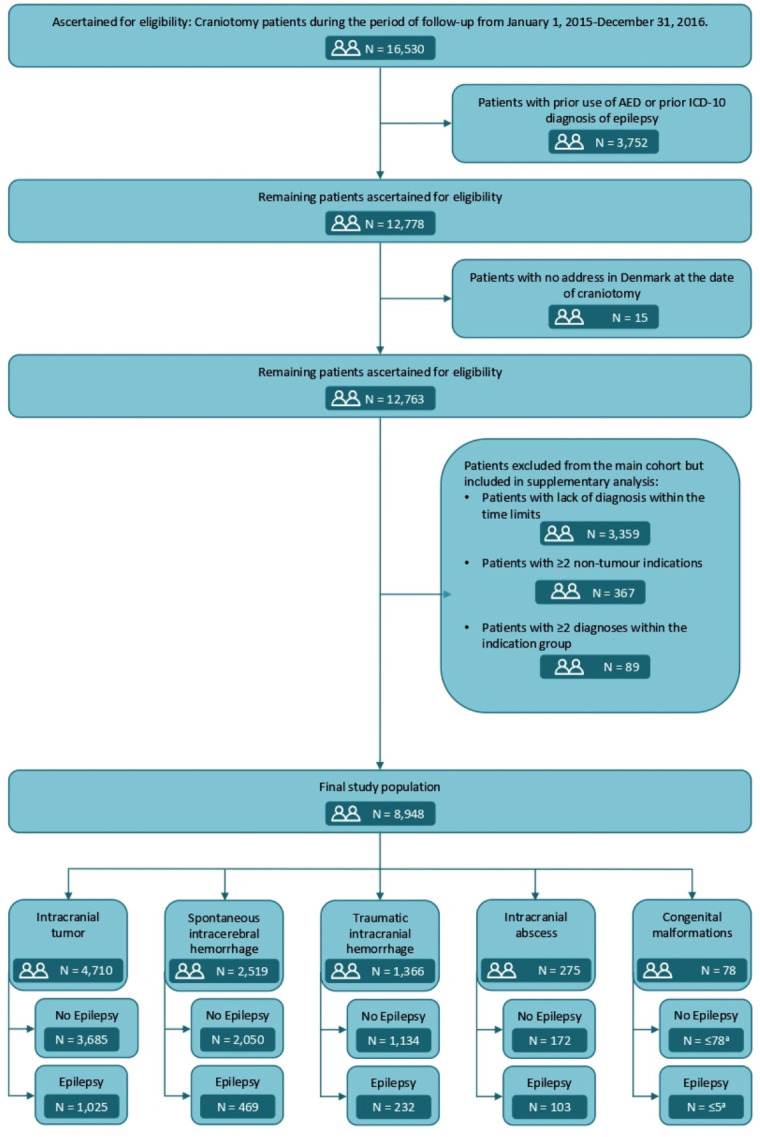
Flow diagram illustrating the selection steps of the study population. All numbers are at end of follow-up, 31 December 2016. aNot shown due to the General Data Protection Regulations. ICD, International Classification of Diseases.AED denotes antiepileptic drugs.
According to current guidelines, prophylactic antiepileptic drugs before craniotomy are not used in Denmark. The patients were identified from the procedure codes of craniotomies, which are shown in online supplemental table 1. The cohort members were allocated to one of the following groups, according to the indication for craniotomy: intracranial tumour, spontaneous intracranial haemorrhage, or traumatic intracranial haemorrhage, intracranial abscess, or congenital malformations, and further divided by subtype. The ICD-10 and ICD-0-codes are presented in online supplemental tables 2 and 3. The patients had to be diagnosed with one of the relevant diagnoses no more than 30 days prior to the craniotomy. For the intracranial tumour group, a time interval between diagnosis and craniotomy up to 90 days was allowed to accommodate for administrative delay of registration of the histological diagnosis. If a patient was diagnosed with both a brain tumour and another indication for craniotomy, such as for instance intracranial haemorrhage, it was assumed that the tumour was the primary indication, and therefore the patient was classified as such. Patients diagnosed with congenital malformations any time before craniotomy were included. In one of the additional analyses, we included an extra cohort of patients treated with endovascular closure of ruptured and unruptured aneurism instead of ligature of the aneurysm by a craniotomy defined by a diagnosis of ruptured or unruptured aneurysm in combination with the procedure code for endovascular occlusion of intracranial aneurysm (KAAL00).
jnnp-2021-326968supp001.pdf (361.7KB, pdf)
Exclusion criteria from the primary analyses
Patients who had been diagnosed with epilepsy or had used antiepileptic drugs (ATC: N03A) prior to the craniotomy (n=3752), or who did not have an address in Denmark at the time of craniotomy (n=15) were excluded completely from the study. Patients were excluded from the main cohort, but included in supplementary analyses of excluded patients, if (1) none of the above-mentioned indications for craniotomy could be assessed (n=3359) within the time limits, (2) patients with more than one indication (none of which was a tumour) for craniotomy, as it was impossible to determine the primary indication for craniotomy (n=367) and (3) patients with more than one diagnosis within the same group of pathologies, for instance, two different types of intracranial bleedings, or two different tumour subtypes (n=89). The selection steps of the study population are illustrated in figure 1 and details and definitions of exclusion criteria for the primary analyses are provided in online supplemental table 4.
Ascertainment of postoperative de novo epilepsy
For the primary analyses, we identified cohort members with postoperative de novo epilepsy using the following ICD-10 codes: G40 epilepsy, G41 status epilepticus, R252 seizures. In an additional analysis, cohort members with de novo epilepsy were identified by their use of antiepileptic drugs (N03A) instead of by diagnosis.
Statistical analyses
The cohort members were followed from the day of craniotomy, until the first of the following events: death, emigration, declared missing person in the CRS or end of follow-up 31 December 2016. During follow-up it was observed if, and when, patients were diagnosed with epilepsy. The 6-month, 1-year and 5-year postoperative probabilities of being (1) alive without epilepsy, (2) alive with epilepsy, (3) deceased without epilepsy or (4) deceased with epilepsy were estimated using the Aalen-Johansen estimator in a multistate model. The model is shown in online supplemental figure 1. The cumulative risk of postoperative epilepsy was estimated using a multistate model where states (2) and (4) were considered as one state. The cumulative risk reflects the risk of postoperative epilepsy taking into account the competing risks of mortality. That is, a low cumulative risk can reflect a low rate of postoperative epilepsy or a high rate of postoperative epilepsy in combination with a high mortality. Furthermore, to investigate the long-term effect of craniotomy, the cumulative risk of epilepsy at 5 years postoperative was estimated in the subpopulation of patients that were alive and not diagnosed with epilepsy at 1 year postoperative. Descriptions and rationales for the additional analyses are provided in online supplemental material. All analyses were conducted using SAS V.9.4 (SAS Institute) and R V.3.5.2.
Results
Study population
A total of 8948 craniotomy patients were eligible for inclusion during follow-up. Of these, 4710 (53%) were diagnosed with a tumour, 2519 (28%) with a spontaneous haemorrhage, 1366 (15%) with a traumatic intracranial haemorrhage, 275 (3%) with an intracranial abscess and 78 (1%) with a congenital malformation. The median follow-up time for the cohort members was 2.8 years (range 1 day to 12 years). The average age at craniotomy was 55.6 years and 4528 (51%) were women. For the tumour group the average age of craniotomy was 56.9 years and 2477 (53%) were women, for the spontaneous haemorrhage patients the corresponding numbers were 55.3 years and 1458 (58%) women, for the traumatic intracranial haemorrhage patients it was 54.5 years and 426 (31%) women, for the group of patients with intracranial abscess it was 48.8 years and 115 (42%) women, and for the group of patients with congenital malformations it was 28.0 years and 52 (67%) women. At the end of follow-up 1833 patients had been diagnosed with epilepsy: 1025 in the tumour group, 469 among the spontaneous haemorrhages, 232 among traumatic intracranial haemorrhages, 103 among intracranial abscess and <5 for congenital malformations (figure 1). The distribution of outcome diagnoses was: DG40 1490 patients, DG41 50 patients and R252 293 patients.
Overall risk of postcraniotomy de novo epilepsy
Figure 2 shows the estimated probabilities of being alive without epilepsy, alive with epilepsy, deceased without epilepsy or deceased with epilepsy in all craniotomy patients from time of surgery until 10 years postoperative. In online supplemental table 5 these probabilities are presented at 6 months, 1 year and 5 years after surgery by indication for craniotomy. The overall 6-month postoperative risk of being alive with epilepsy was 8.4% (95% CI 7.9 to 9.0) and deceased with epilepsy 1.3% (95% CI 1.1 to 1.5), resulting in a cumulative risk of de novo epilepsy for all craniotomy patients of 9.7% (95% CI 9.1 to 10.3). The overall 1-year postoperative risk of being alive with epilepsy was 10.7% (95% CI 10.1 to 11.4) and deceased with epilepsy 3.2% (95% CI 2.8 to 3.6), resulting in a cumulative risk of de novo epilepsy for all craniotomy patients of 13.9% (95% CI 13.2 to 14.6). The 5-year postoperative risk of being alive with epilepsy was 10.6% (95% CI 9.9 to 11.3) and deceased with epilepsy 9.8% (95% CI 9.1 to 10.4) resulting in a cumulative risk of 20.4% (95% CI 19.5 to 21.3).
Figure 2.
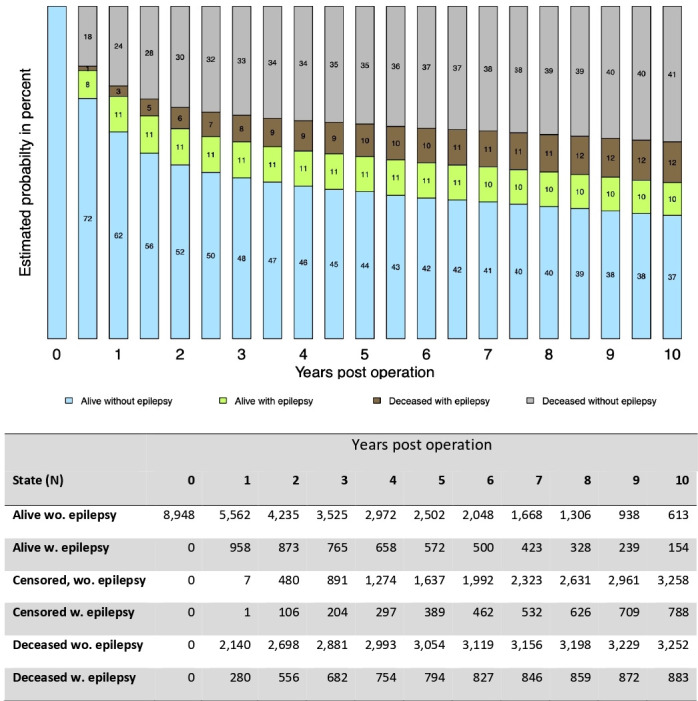
Estimated probabilities for being alive without epilepsy, alive with epilepsy, deceased with epilepsy, deceased without epilepsy, by year after the craniotomy in all neurosurgical patients in Denmark, 2005–2016. Number of patients by each year and state is shown below the figure.
Table 1 presents the 6-month, 1-year and 5-year cumulative risks of postoperative de novo epilepsy for all major indications for craniotomy in Denmark, during the period 2005–2016. The corresponding 5-year risks among patients being alive and without epilepsy at 1 year postoperative by type of indication are presented in table 2.
Table 1.
The 6-month, 1-year and 5-year cumulative risks (CRs) of postoperative de novo epilepsy by indication for craniotomy in Denmark, 2005–2016
| Cranial neurosurgical disease |
Patients (n) | Average age at craniotomy | Patients with epilepsy | CR* 6 months, % (95% CI) | CR* 1 year, % (95% CI) |
CR* 5 years, % (95% CI) |
| All patients | 8948 | 55.6 | 1833 | 9.7 (9.1 to 10.3) | 13.9 (13.2 to 14.6) | 20.4 (19.5 to 21.3) |
| All intracranial tumours | 4710‡ | 56.9 | 1025 | 11.0 (10.1 to 11.9) | 15.4 (14.4 to 16.5) | 21.8 (20.6 to 23.0) |
| Astrocytoma | 245 | 34.4 | 73 | 10.2 (7.0 to 14.8) | 16.3 (12.3 to 21.7) | 29.4 (24.0 to 36.0) |
| Cranial nerves | 316 | 50.6 | 7 | 0.9 (0.3 to 2.9) | 1.3 (0.5 to 3.4) | 1.7 (0.7 to 4.0) |
| Embryonal | 104 | 14.7 | 21 | 7.7 (4.0 to 15) | 9.6 (5.3 to 17.3) | 18.1 (11.9 to 27.6) |
| Glioblastoma | 1593 | 62.4 | 481 | 16.5 (14.7 to 18.4) | 23.6 (21.6 to 25.7) | 30.8 (28.6 to 33.2) |
| Meningioma | 1245 | 58.9 | 239 | 9.4 (7.9 to 11.2) | 12.1 (10.4 to 14.0) | 19.0 (16.9 to 21.4) |
| Mesenchymal | 105 | 49.7 | 10 | 6.7 (3.3 to 13.6) | 7.6 (3.9 to 14.8) | 8.7 (4.6 to 16.2) |
| Metastasis | 746 | 64.1 | 91 | 7.1 (5.5 to 9.2) | 9.9 (8.0 to 12.3) | 12.0 (9.9 to 14.6) |
| Oligodendroglioma | 193 | 53.6 | 81 | 16.6 (12.1 to 22.8) | 25.4 (19.9 to 32.3) | 43.6 (36.9 to 51.6) |
| All spontaneous intracranial haemorrhages | 2519 | 55.3 | 469 | 6.9 (5.9 to 7.9) | 11.3 (10.1 to 12.6) | 18.3 (16.8 to 19.9) |
| Aneurysm, ruptured | 740 | 54.8 | 115 | 5.3 (3.9 to 7.2) | 9.7 (7.8 to 12.1) | 15.4 (12.9 to 18.3) |
| Aneurysm, non-ruptured | 434 | 55.2 | 36 | 3.7 (2.3 to 6.0) | 5.3 (3.6 to 7.9) | 8.3 (6.0 to 11.4) |
| AVM or Moya-Moya disease | 165 | 41.3 | 43 | 12.7 (8.5 to 19.0) | 13.9 (9.5 to 20.4) | 24.8 (18.9 to 32.7) |
| Intracerebral haemorrhage | 1180 | 57.7 | 275 | 8.2 (6.8 to 10.0) | 14.1 (12.2 to 16.2) | 22.9 (20.6 to 25.5) |
| All traumatic intracranial haemorrhages | 1366 | 54.5 | 232 | 8.4 (7.1 to 10.0) | 11.1 (9.6 to 12.9) | 17.0 (15.1 to 19.2) |
| Cerebral contusion | 214 | 49.8 | 53 | 8.0 (5.0 to 12.5) | 13.6 (9.7 to 19.1) | 25.1 (19.8 to 31.9) |
| Epidural haematoma | 277 | 31.8 | 34 | 6.1 (3.9 to 9.7) | 7.6 (5.0 to 11.5) | 11.9 (8.6 to 16.4) |
| Subdural haematoma | 875 | 62.9 | 145 | 9.3 (7.5 to 11.4) | 11.7 (9.7 to 14.0) | 16.7 (14.3 to 19.4) |
| Intracranial abscess | 275 | 48.8 | 103 | 21.5 (17.1 to 26.9) | 27.6 (22.8 to 33.5) | 35.8 (30.4 to 42.1) |
| Congenital malformations | 78 | 28.0 | NA† | 3.8 (1.3 to 11.7) | 3.8 (1.3 to 11.7) | 5.6 (2.1 to 14.7) |
*Cumulative risks represent the risks of postoperative de novo epilepsy among patients alive with epilepsy and deceased patients with epileps.
†Not shown due to small numbers.
‡No analyses done for the six small cancer groups (oligoastrocytoma ependymal, pineal region, germ cell, sellar region and miscellaneous).
AVM, arteriovenous malformation of cerebral vessels.
Table 2.
The 5-year cumulative risk of postoperative de novo epilepsy by indication for craniotomy among the subgroup of patients being alive without epilepsy at 1 year after craniotomy
| Intracranial surgical disease | Total patients | Patients with epilepsy | CR* at 5 years, % (95% CI) |
| All patients | 5562 | 594 | 10.5 (9.7 to 11.4) |
| All tumours | 2749‡ | 301 | 11.0 (9.8 to 12.3) |
| Astrocytoma | 175 | 33 | 18.3 (13.0 to 25.6) |
| Cranial nerves | 306 | <5† | 0.4 (0.1 to 3.0) |
| Embryonal | 80 | 11 | 11.0 (5.7 to 21.3) |
| Glioblastoma | 592 | 107 | 19.6 (16.5 to 23.4) |
| Meningioma | 1022 | 89 | 8.5 (6.8 to 10.6) |
| Mesenchymal | 93 | <5† | 1.2 (0.2 to 8.5) |
| Metastasis | 241 | 17 | 6.5 (4.0 to 10.5) |
| Oligodendroglioma | 101 | 32 | 34.8 (26.0 to 46.6) |
| All spontaneous haemorrhages | 1727 | 185 | 10.2 (8.8 to 11.9) |
| Cerebral aneurysm, ruptured | 532 | 43 | 7.8 (5.8 to 10.6) |
| Cerebral aneurysm, non-ruptured | 399 | 13 | 3.3 (1.9 to 5.7) |
| AVM or Moya-Moya disease | 136 | 20 | 13.2 (8.3 to 20.9) |
| Intracerebral haemorrhage | 660 | 109 | 15.8 (13.0 to 19.1) |
| All traumatic haemorrhages | 828 | 80 | 9.7 (7.8 to 12.1) |
| Cerebral contusion | 117 | 24 | 20.9 (14.4 to 30.4) |
| Epidural haemorrhage | 230 | 13 | 5.1 (2.9 to 9.1) |
| Traumatic subdural haemorrhage | 481 | 43 | 9.1 (6.7 to 12.4) |
| Abscess | 183 | 27 | 12.2 (8.0 to 18.7) |
| Congenital malformations | 75 | <5† | 1.8 (0.3 to 12.8) |
*Cumulative risks represent the risks of postoperative de novo epilepsy among patients alive with epilepsy and deceased patients with epilepsy.
†Not shown due to small numbers.
‡No analyses done for the six small cancer groups (oligoastrocytoma, ependymal, pineal region, germ cell, sellar region and miscellaneous).
AVM, arteriovenous malformation of cerebral vessels; ICH, intracerebral haemorrhage.
Intracranial tumours
The 6-month, 1-year and 5-year postoperative cumulative risks of de novo epilepsy were 11.0% (95% CI 10.1 to 11.9), 15.4% (95% CI 14.4 to 16.5) and 21.8% (95% CI 20.6 to 23.0), respectively (table 1). Meningiomas and glioblastomas dominated the group with respectively 1245 and 1593 patients. The specific 1-year postoperative cumulative risk of de novo epilepsy was 16.3% (95% CI 12.3 to 21.7) for astrocytoma, 1.3% (95% CI 0.5 to 3.4) for tumours of the cranial nerves, 9.6% (95% CI 5.3 to 17.3) for embryonal tumours, 23.6% (95% CI 21.6 to 25.7) for glioblastoma, 12.1% (95% CI 10.4 to 14.0) for meningioma, 7.6% (95% CI 3.9 to 14.8) for tumours of mesenchymal origin, 9.9% (95% CI 8.0 to 12.3) for metastases and 25.4% (95% CI 19.9 to 32.3) for oligodendroglioma. It is noteworthy, that for all tumours, except tumours of the cranial nerves and of mesenchymal origin, a substantial increase is seen from 6 months to 1 year and even from 1 year to 5 years after the craniotomy. The 1 year to 5 years increase is reflected in the high 5-year risks that were seen among the subgroup of patients being alive and without epilepsy at 1 year postoperative (table 2).
Spontaneous intracranial haemorrhage
The 6-month postoperative cumulative risk of de novo epilepsy among all 2519 patients with spontaneous intracranial haemorrhages was 6.9% (95% CI 5.9 to 7.9), 11.3% (95% CI 10.1 to 12.6) at 1 year postoperative and 18.3% (95% CI 16.8 to 19.9) at 5 years postoperative (table 1). Among the 1180 patients with intracerebral haemorrhage, the 1-year postoperative cumulative risk of de novo epilepsy was 14.1% (95% CI 12.2 to 16.2). Among the 740 patients with ruptured cerebral aneurysm, the 1-year risk was 9.7% (95% CI 7.8 to 12.1), and 5.3% (95% CI 3.6 to 7.9) for the 434 patients with a non-ruptured cerebral aneurysm. For patients with arteriovenous malformations (165) the 1-year risk was 13.9% (95% CI 9.5 to 20.4). High 5-year risks were also seen among the subgroup of patients being alive and without epilepsy at 1 year postoperative (table 2).
Traumatic intracranial haemorrhage
As shown in table 1, the 6-month postoperative cumulative risk of de novo epilepsy among all 1366 patients with traumatic intracranial haemorrhages was 8.4% (95% CI 7.1 to 10.0), 11.1% (95% CI 9.6 to 12.9) at 1 year postoperative and 17.0% (95% CI 15.1 to 19.2) at 5 years postoperative. For the 277 patients with epidural haemorrhage the 1-year postoperative cumulative risk of de novo epilepsy was 7.6% (95% CI 5.0 to 11.5). Among the 875 patients with traumatic subdural haemorrhage the corresponding risks was 11.7% (95% CI 9.7 to 14.0), and for the 214 patients with cerebral contusion the corresponding risks was 13.6% (95% CI 9.7 to 19.1). High 5-year risks were also seen among the subgroup of patients being alive and without epilepsy at 1 year postoperative (table 2).
Other indications for craniotomy
The 6-month postoperative cumulative risk of de novo epilepsy among the 275 patients with intracranial abscess was as high as 21.5% (95% CI 17.1 to 26.9) with 27.6% (95% CI 22.8 to 33.5) at 1 year postoperative and 35.8% (95% CI 30.4 to 42.1) at 5 years postoperative (table 1). Among the 78 patients with congenital malformations (eg, Chiari syndrome or Dandy-Walker malformation), the corresponding risk was 3.8% (95% CI 1.3 to 11.7) at both 6 months and 1 year postoperative, and 5.6% (95% CI 2.1 to 14.7) at 5 years postoperative (table 1).
Additional analyses
Craniotomy-related risk versus risk related to the indication for the craniotomy
In order to investigate how much of the risk of epilepsy could be attributed to the craniotomy itself, we investigated the risk of epilepsy among patients who, instead of surgical ligature by a craniotomy, underwent endovascular treatment for a cerebral aneurysm. Results are presented in table 3. Patients who underwent endovascular treatment had lower 5-year risks (ruptured: 6.7%, 95% CI 5.2 to 8.6, non-ruptured: 5.6%; 95% CI 4.0 to 8.0) compared with patients treated by surgical ligature (ruptured: 15.4%, 95% CI 12.9 to 18.3; non-ruptured 8.3%, 95% CI 6.0 to 11.4). Thus, the difference in these risks may reflect the risk of the craniotomy itself.
Table 3.
The 6-month, 1-year and 5-year cumulative risks (CRs) of de novo epilepsy after endovascular treatment of an intracranial aneurysm
| Endovascular treatment | Total no. of patients | Patients with epilepsy | CR* 6 months, % (95% CI) |
CR* 1 year, % (95% CI) |
CR* 5 years, % (95% CI) |
| Aneurysm, ruptured | 885 | 62 | 2.9 (2.0 to 4.3) | 4.1 (3.0 to 5.6) | 6.7 (5.2 to 8.6) |
| Aneurysm, non-ruptured | 599 | 35 | 3.7 (2.4 to 5.5) | 3.8 (2.6 to 5.7) | 5.6 (4.0 to 8.0) |
*Cumulative risks are the summation of patients alive with epilepsy and deceased with epilepsy.
Infratentorial versus supratentorial craniotomies
It is widely believed that infratentorial craniotomies and brain pathologies rarely lead to epilepsy, but this dogma lacks scientific ground. In order to enlighten this question, the cumulative risks of epilepsy after supratentorial craniotomies were compared with the risks after infratentorial craniotomies. The results are presented in table 4. Although the results, as expected, did confirm significantly higher risks among patients who underwent supratentorial craniotomies (1-year risk 13.5%, 95% CI12.6 to 14.6 and 5-year risk 19.6%, 95% CI18.4 to 20.8), infratentorial craniotomies still carried considerable risks of postoperative epilepsy: 1-year risk: 3.7%, 95% CI 2.1 to 6.2 and 5-year risk: 7.8%, 95% CI5.4 to 11.3.
Table 4.
The 6-month, 1-year and 5-year cumulative risks (CRs) of postoperative de novo epilepsy in neurosurgical patients according to the craniotomy being supratentorial or infratentorial
| Total no. of patients* | Patients with epilepsy | CR 6 months, % (95% CI) |
CR 1 year, % (95% CI) |
CR 5 years, % (95% CI) |
|
| Supratentorial | 4677 | 917 | 9.5 (8.7 to 10.4) | 13.5 (12.6 to 14.6) | 19.6 (18.4 to 20.8) |
| Infratentorial | 355 | 30 | 3.4 (1.9 to 5.9) | 3.7 (2.1 to 6.2) | 7.8 (5.4 to 11.3) |
*Patients in whom it was impossible to distinguish between infratentorial or supratentorial craniotomies from either the procedure or diagnostic code were excluded from this analysis.
†Cumulative risks are the summation of patients alive with epilepsy and deceased with epilepsy.
Risk of epilepsy measured by the use of anti-epileptic drugs
In order to investigate if our results would change significantly by changing the outcome measure from a diagnosis of epilepsy to the use of antiepileptic drugs, the cumulative risks of postoperative treatment with antiepileptic drugs were estimated as well. The overall 6-month postoperative risk was 13.2% (95% CI 12.5 to 13.9), the 1-year risk was 19.0% (95% CI 18.2 to 19.9) and the 5-year risk was 27.9% (95% CI 27.0 to 28.9). The cumulative risks for all of the indications for craniotomies are presented in online supplemental table 6. Generally, the cumulative risks measured by treatment with antiepileptic drugs were higher than the risks measured by diagnosis, embryonal tumours being the only exception.
Age and epilepsy risk
As shown in table 1, there is a great variation between ages in the different groups of neurosurgical diseases. In order to investigate if the observed differences in risk was driven by an underlying age-associated risk of epilepsy, we performed age-stratified analyses of the 6-month, 1-year and 5-year postoperative cumulative risk of de novo epilepsy, which are all presented in online supplemental table 7. The 1-year risks were: age group 0–9 years 9.8% (95% CI 6.8 to 14.2), 10–19 years 10.0% (95% CI 7.1 to 14.0), 20–44 years 13.9% (95% CI 12.2 to 15.8), 45–59 years 16.0% (95% CI 14.7 to 17.5), 60–69 years 14.1% (95% CI 12.8 to 15.6) and for 70+ years 11.7% (95% CI 10.3 to 13.2). Hence, the stratified analyses did not show any major differences between the age groups.
Finally, the cumulative risks of postoperative de novo epilepsy were estimated among the patients not meeting inclusion criteria for the primary analyses are presented in online supplemental tables 8–10.
Discussion
Principal findings
In this unselected, nationwide cohort study encompassing 8948 patients, we found high cumulative risks of de novo epilepsy following craniotomy, as compared with the previously reported risk among the background population. The overall 1-year cumulative risk of de novo epilepsy after craniotomy was 13.9% (95% CI 13.2 to 14.6) and after 5 years 20.4% (95% CI 19.5 to 21.3). The cumulative 6-month, 1-year and 5-year risks for each of the neurosurgical diseases leading to craniotomy exhibited considerable variation owing to the indications for craniotomy. Notably, both short-term and long-term risks were surprisingly high for most of the indications for craniotomy.
Comparison with other studies
Intracranial tumours
There are few comparable studies, mainly concerning meningioma and glioblastoma. In line with our findings, a Swedish single-centre study,6 including 113 meningioma patients reported a 2-year cumulative risk of postoperative epilepsy of 14% (95% CI not provided), whereas we found a 1-year risk of 12.1% (95% CI 10.4 to 14.0). Meningiomas are a very common type of intracranial tumour with an overall prevalence of 97.5/100.000.12 As the vast majority of meningiomas are benign and slow-growing tumours, most of them are treated conservatively, but this approach is highly variable and debated. The high risk of postcraniotomy epilepsy needs to be considered when balancing risks and benefits regarding surgical treatment in the individual meningioma patient, particularly if asymptomatic.
A prospective single-centre study in China reported a 1-year cumulative incidence of epilepsy of approximately 50% after craniotomy based on 184 glioblastoma patients.5 In comparison, we found a 1-year postoperative cumulative risk of de novo epilepsy of 23.6% (95% CI 21.6 to 25.7).
The very high risks seen in both studies may be explained by the fact that the glioblastoma is a diffusely infiltrating tumour. The Chinese study seems to report on a study population different to ours as gross total resection was obtained in only 41% of the patients, whereas surgical treatment in Denmark is mainly offered if a resection grade of at least 80% is considered possible.
Spontaneous intracranial haemorrhage
Our finding of a 1-year cumulative postoperative risk of de novo epilepsy of 9.7% (95% CI 7.8 to 12.1), after surgical treatment of a ruptured aneurysm, is in line with the finding of a Finish cohort study, that reported a 1-year cumulative incidence of 8% among 876 patients with similar indication for craniotomy (95% CI not provided).3 The authors of the International Subarachnoid Aneurysm Trial reported overall risks of epilepsy among the 2143 patients with ruptured aneurysm to be 10.9% during the follow-up time of up to 14 years.2 Among the 1073 patients who underwent endovascular treatment, the overall risk was 8.3%, whereas 13.6% of the 1070 surgically treated patients suffered a seizure (p=0.014). Despite the differences in study design and statistical methods, these findings seem comparable to our findings.
We found a rather high 1-year postoperative risk of de novo epilepsy of 14.1% (95% CI 12.2 to 16.2) among patients with intracerebral haemorrhage. Only two cohort studies could be found in the literature investigating the risk of epilepsy among patients with intracerebral haemorrhage, one being a multicentre study including 265 patients13 and the other including 615 patients.4 The first study reported a 1-year actual risk of 20%, and the other a cumulative incidence of 6.9% (95% CI 5.0 to 8.9). However, the study populations were not entirely comparable to the cohort of this study as they consisted of both surgically and conservatively treated patients. No comparable studies could be found on the postcraniotomy risk of epilepsy in patients with arteriovenous malformations.
Traumatic intracranial haemorrhage
We were not able to identify any studies in the literature that evaluated the postcraniotomy risk of epilepsy in patients with traumatic intracranial haemorrhages, but a recent review reported a pooled incidence of post-cranioplasty seizures of 5.1% (95% CI 2.6% to 8.2%).14 The cranioplasty is surgical re-insertion of the cranial flap, which was removed (craniectomy) in relation to the most severe of head injuries in order to manage elevated intracranial pressure. Thus, re-insertion of the cranial flap does not involve surgery beneath the dura mater. This is mostly comparable to craniotomy for an epidural haematoma, for which we found the 6-month postoperative risk to be 6.1% (95% CI 3.9 to 9.7). A large population-based study on post-traumatic seizures in Olmsted County, Minnesota, reported a standardised incidence ratio of 17.0 (95% CI 12.3 to 23.6) after severe head injuries, which is very much in line with our finding of an overall cumulative 5-year risk of 17.0 (95% CI 15.1 to 19.2).7 Yet, it was not reported how many of the patients underwent a craniotomy.
Long-term risk of postcraniotomy epilepsy
We found that patients in almost all of the main and subgroups of diseases also had high long-term risk of postoperative de novo epilepsy. For comparison, a Danish cohort study from 2007 estimated the overall 5-year prevalence proportion of epilepsy in the background population to be 0.6% with a slight variation according to age between 0.4% and 0.8%.15 Only tumours of the cranial nerves are within this range (0.4%) and mesenchymal tumours and congenital malformations are close, 1.2% and 1.8%, respectively. We also found the 5-year risk of the background population to be negligible compared with the long-term risk among neurosurgical patients.
As illustrated in table 3, the postcraniotomy risk of de novo epilepsy is composed by a risk attributable to the craniotomy itself and by the risk related to the indication for the craniotomy. The long-term risk owes more to the indication for the craniotomy than to the operation itself. Obviously, the high long-term risks are expected for patients with continuously growing tumours, particularly the ones of infiltrating nature. We speculate, that the high long-term risks also seen for spontaneous and traumatic intracranial haemorrhages may be explained by a long-standing state of neuroinflammation and gliosis, which previously has been connected with epilepsy.16 17 This line of thought is supported by the finding that epidural haematoma displays a much lower 5-year risk (11.9%, 95% CI 8.6 to 16.4) than cerebral contusions (25.1%, 95% CI 19.8 to 31.9). Furthermore, a higher 5-year postoperative risk is seen in patients with ruptured compared with unruptured aneurisms (15.4%, 95% CI 12.9 to 18.3% and 8.3%, 95% CI 6.0 to 11.4, respectively), supposedly due to the long-standing neuroinflammatory effects of subarachnoid haemorrhage.18 Finally, the high 5-year risk of 35.8% (95% CI 30.4 to 42.1) for intracranial abscess, a highly inflammatory condition, also supports this notion.
Infratentorial versus supratentorial craniotomies
We observed that patients with infratentorial craniotomies had a considerable risk of developing postoperative de novo epilepsy (table 4). Although much lower than the risk following supratentorial craniotomies, this is an interesting finding, as origin of epileptic seizures outside the cerebral cortex is controversial. Part of the explanation may be that posterior fossa pathologies often produce secondary supratentorial effects, such as hydrocephalus. Furthermore, craniotomies coded as infratentorial sometimes include combined approaches, such as trans tentorial approaches, with exposure of the temporal lobe cortex. No other studies could be found reporting postoperative risks of epilepsy after infratentorial craniotomy.
Strengths and limitations
Several design characteristics strengthens the validity of our results. The access to unselected nationwide information on the entire population in Denmark ensured that all patients from all neurosurgical centres in Denmark were included, which counteract selection-centre and single-centre bias and ensures sufficient sample size for subgroup analyses and estimation of long-term risks. Furthermore, we were able to exclude patients with epilepsy or antiepileptic drug use prior to the craniotomy. The mandatory reporting to the registers for all private and public hospitals ensured virtually complete follow-up.
As in all register-based studies, misclassification of diagnoses constitutes a risk. A Danish validation study reported a positive predictive value for epilepsy in the NPR of 81%, whereas the negative predictive value, sensitivity, and specificity for this algorithm is unknown, given the study design.19 We observed that patients who did not meet our inclusion criteria of either diagnosis or time limits had lower risk of postoperative de novo epilepsy (online supplemental tables 8–10). Thus, we most likely reduced misclassification by using restrictive inclusion criteria based on concurrent registration of both the disease and the craniotomy. However, the excluded patients still had a much higher risk than the background population. Therefore, the general message of high short-term and long-term risks of postcraniotomy epilepsy also apply for the excluded group.
The finding of higher risks of postoperative de novo epilepsy when using antiepileptic drugs as outcome measure may suggest that the risk is somewhat underestimated when using diagnoses as outcome measure. A possible reason for this is the lack of information on epilepsy diagnoses given by general practitioners and specialist practice in the NPR.
On the other hand, the higher risks may also reflect the fact that antiepileptic drugs are used for other indications than epilepsy. Therefore, patients with a hospital diagnosis of bipolar affective disorder, trigeminal neuralgia, neuropathies, generalised anxiety disorder or migraine were excluded if diagnosed before the date of craniotomy and censored if diagnosed during follow-up, as described in the supplementary material. As the NPR only include hospital diagnoses, not diagnoses from specialty practices, the patients treated for these conditions in neurology or psychiatry specialty practices were included in the analysis, which may be another possible explanation of the higher number.
Either way, the use of both diagnoses and antiepileptic drugs as outcome measures supports the notion of high risks of postcraniotomy de novo epilepsy, as compared with the general population. The findings in this study were furthermore in line with the reported high risks in the few previous studies in the literature, which report even higher risks based on clinical data from patient files. If anything, the effect of the potential misclassification draws towards an underestimated risk.
Implications for clinicians, policymakers and future research
In conclusion, the cumulative risks of postoperative de novo epilepsy are high for patients with any of the indications for craniotomy. The disease-specific short-term and long-term risks of postoperative de novo epilepsy adds significantly to the current scarce literature. Our results call for a discussion of the clinical recommendations for prophylactic antiepileptic treatment in relation to the craniotomy. Furthermore, the high short-term and long-term risks warrants revision of the divergent guidelines on driving restrictions following neurosurgical procedures, for which data of sufficient strength has been lacking so far. Future research should be directed at understanding the signalling pathways and regulation of neuroinflammation leading to epilepsy in order to identify modifiable factors.20
Footnotes
Contributors: Planning of the study: TNM, KF and MM. Conduct of the study:JVH and JW conducted the statistical analyses, all authors were engaged in analysis and interpretation of the results. Reporting of the work: the manuscript was written by LG and TNM, all authors assisted in critical revision and all authors approved the final version of the manuscript.
TNM is responsible for the overall content as guarantor. The guarantor accepts full responsibility for the finished work and/or the conduct of the study, had access to the data, and controlled the decision to publish.
Disclaimer: The funders had no role in the study and the researchers were completely independent from the funders. All authors had full access to all of the data and can take responsibility for the integrity of the data and the accuracy of the data analyses.
Competing interests: None declared.
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Data availability statement
Data may be obtained from a third party and are not publicly available. According to the Danish data protection law, it is not allowed to share data from the Danish registers.
Ethics statements
Patient consent for publication
Not applicable.
Ethics approval
The study was purely based on the Danish National Registers to which the health carehealthcare system report data. Therefore, the study did not involve any contact to patients. All data were encrypted as standard, and results were only presented as aggregated data ensuring data protection of the individual, in accordance with the GDPR regulations. Danish law does not require patient ethical approval of register-based studies, but the study was approved by the Danish Data Protection Agency, no. 2015-57-0102.
References
- 1. Vertosick FT. When the air hits your brain: tales of neurosurgery: WW. Norton & Company, 1996. [Google Scholar]
- 2. Hart Y, Sneade M, Birks J, et al. Epilepsy after subarachnoid hemorrhage: the frequency of seizures after clip occlusion or coil embolization of a ruptured cerebral aneurysm: results from the International subarachnoid aneurysm trial. J Neurosurg 2011;115:1159–68. 10.3171/2011.6.JNS101836 [DOI] [PubMed] [Google Scholar]
- 3. Huttunen J, Kurki MI, von Und Zu Fraunberg M, et al. Epilepsy after aneurysmal subarachnoid hemorrhage: a population-based, long-term follow-up study. Neurology 2015;84:2229–37. 10.1212/WNL.0000000000001643 [DOI] [PubMed] [Google Scholar]
- 4. Lahti A-M, Saloheimo P, Huhtakangas J, et al. Poststroke epilepsy in long-term survivors of primary intracerebral hemorrhage. Neurology 2017;88:2169–75. 10.1212/WNL.0000000000004009 [DOI] [PubMed] [Google Scholar]
- 5. Liang S, Zhang J, Zhang S, et al. Epilepsy in adults with supratentorial glioblastoma: incidence and influence factors and prophylaxis in 184 patients. PLoS One 2016;11:e0158206. 10.1371/journal.pone.0158206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Xue H, Sveinsson O, Bartek J, et al. Long-term control and predictors of seizures in intracranial meningioma surgery: a population-based study. Acta Neurochir 2018;160:589–96. 10.1007/s00701-017-3434-3 [DOI] [PubMed] [Google Scholar]
- 7. Annegers JF, Hauser WA, Coan SP, et al. A population-based study of seizures after traumatic brain injuries. N Engl J Med 1998;338:20–4. 10.1056/NEJM199801013380104 [DOI] [PubMed] [Google Scholar]
- 8. Schmidt M, Pedersen L, Sørensen HT. The Danish civil registration system as a tool in epidemiology. Eur J Epidemiol 2014;29:541–9. 10.1007/s10654-014-9930-3 [DOI] [PubMed] [Google Scholar]
- 9. Lynge E, Sandegaard JL, Rebolj M. The Danish national patient register. Scand J Public Health 2011;39:30–3. 10.1177/1403494811401482 [DOI] [PubMed] [Google Scholar]
- 10. Gjerstorff ML. The Danish cancer registry. Scand J Public Health 2011;39:42–5. 10.1177/1403494810393562 [DOI] [PubMed] [Google Scholar]
- 11. Kildemoes HW, Sørensen HT, Hallas J. The Danish national prescription registry. Scand J Public Health 2011;39:38–41. 10.1177/1403494810394717 [DOI] [PubMed] [Google Scholar]
- 12. Wiemels J, Wrensch M, Claus EB. Epidemiology and etiology of meningioma. J Neurooncol 2010;99:307–14. 10.1007/s11060-010-0386-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Bladin CF, Alexandrov AV, Bellavance A, et al. Seizures after stroke: a prospective multicenter study. Arch Neurol 2000;57:1617–22. 10.1001/archneur.57.11.1617 [DOI] [PubMed] [Google Scholar]
- 14. Spencer R, Manivannan S, Sharouf F, et al. Risk factors for the development of seizures after cranioplasty in patients that sustained traumatic brain injury: a systematic review. Seizure 2019;69:11–16. 10.1016/j.seizure.2019.03.014 [DOI] [PubMed] [Google Scholar]
- 15. Christensen J, Vestergaard M, Pedersen MG, et al. Incidence and prevalence of epilepsy in Denmark. Epilepsy Res 2007;76:60–5. 10.1016/j.eplepsyres.2007.06.012 [DOI] [PubMed] [Google Scholar]
- 16. Sharma R, Leung WL, Zamani A, et al. Neuroinflammation in post-traumatic epilepsy: pathophysiology and tractable therapeutic targets. Brain Sci 2019;9. 10.3390/brainsci9110318. [Epub ahead of print: 09 Nov 2019]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Sanz P, Garcia-Gimeno MA. Reactive glia inflammatory signaling pathways and epilepsy. Int J Mol Sci 2020;21. 10.3390/ijms21114096. [Epub ahead of print: 08 Jun 2020]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Lucke-Wold BP, Logsdon AF, Manoranjan B, et al. Aneurysmal subarachnoid hemorrhage and neuroinflammation: a comprehensive review. Int J Mol Sci 2016;17:497. 10.3390/ijms17040497 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Christensen J, Vestergaard M, Olsen J, et al. Validation of epilepsy diagnoses in the Danish national Hospital register. Epilepsy Res 2007;75:162–70. 10.1016/j.eplepsyres.2007.05.009 [DOI] [PubMed] [Google Scholar]
- 20. Vezzani A, Balosso S, Ravizza T. Neuroinflammatory pathways as treatment targets and biomarkers in epilepsy. Nat Rev Neurol 2019;15:459–72. 10.1038/s41582-019-0217-x [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
jnnp-2021-326968supp001.pdf (361.7KB, pdf)
Data Availability Statement
Data may be obtained from a third party and are not publicly available. According to the Danish data protection law, it is not allowed to share data from the Danish registers.


