Abstract
Context
Premenopausal women with idiopathic osteoporosis (IOP) have abnormal skeletal microarchitecture and variable tissue-level bone formation rate (BFR).
Objectives
Compare 6 months (M) of teriparatide versus placebo on areal bone mineral density (aBMD) by dual-energy x-ray absorptiometry (DXA), bone turnover markers (BTMs) and BFR at 3M by quadruple-labeled transiliac biopsy. Characterize 12M and 24M effects of teriparatide on aBMD and whether BTMs and BFR predict response.
Design
6M phase 2 randomized controlled trial (RCT) followed by open extension.
Setting
Tertiary referral centers.
Patients
Premenopausal women with IOP.
Interventions
A total of 41 women were randomized to either teriparatide 20 mcg (n = 28) or placebo (n = 13). After 6M, those on placebo switched to teriparatide for 24M; those on teriparatide continued for 18M.
Main Outcome Measures
6M RCT: Between-group differences in lumbar spine (LS) aBMD (percent change from baseline), 3M BFR, and hypercalcemia. Open-label extension: Within-group change in LS aBMD over 12M and 24M. Secondary outcomes included aBMD change at other sites and relationship between BTMs, BFR, and changes in aBMD.
Findings
Over 6M, LS aBMD increased by 5.5% (95% CI: 3.83, 7.19) in teriparatide and 1.5% (95% CI: −0.73, 3.83) in placebo (P = 0.007). There were increases in 3M BTMs, and BFR (cancellous and endocortical BFR: between-groups P = 0.004). Over 24M, teriparatide increased LS aBMD by 13.2% (95% CI: 10.3, 16.2), total hip by 5.2% (95% CI: 3.7, 6.7) and femoral neck by 5.0% (95% CI: 3.2, 6.7; all P ≤ 0.001). Serum N-terminal propeptides of procollagen type 1 (P1NP) and 3M endocortical BFR were moderately associated with LS aBMD response. Teriparatide was well-tolerated.
Conclusions
Teriparatide increased BFR and formation markers and was associated with marked aBMD improvements in most premenopausal women (82%) with IOP.
Keywords: premenopausal osteoporosis, teriparatide, bone biopsy, bone turnover markers
Premenopausal idiopathic osteoporosis (IOP) is operationally defined as osteoporosis in young, otherwise healthy women with intact gonadal function and no secondary cause of bone loss or fragility (1). The clinical presentation of premenopausal IOP varies from an asymptomatic condition characterized by very low bone mineral density (BMD) and poor bone quality to a devastating condition with multiple low trauma vertebral and nonvertebral fractures (1, 2). We previously reported that premenopausal women with IOP have abnormal bone microarchitecture, with thinner cortices, fewer, thinner, more widely separated and heterogeneously distributed trabeculae (3-5), and a more rod-like trabecular structure with lower trabecular stiffness (3-5). Notably, the deficits in microstructure and stiffness were comparable in women with fractures and those with low BMD (3-5). Bone remodeling, assessed by serum bone formation markers (1) and bone formation rate (BFR) on tetracycline-labelled transiliac biopsies (3), showed normal or high bone turnover in some, while others had extremely low bone formation (3).
There is no approved therapy for premenopausal IOP. Teriparatide stimulates osteoblastic bone formation, increases BMD and reduces fracture incidence in postmenopausal osteoporosis (6) and in glucocorticoid-induced osteoporosis (GIOP) (7, 8). Teriparatide also increases BMD in men with IOP (9, 10). In a pilot study of 21 premenopausal women with IOP, teriparatide markedly increased serum bone formation markers and areal BMD (aBMD) of the spine and hip (11). At 18 months, micro-computed tomography (microCT) of transiliac biopsies (11) and high-resolution peripheral quantitative computed tomography (HR-pQCT) of the distal radius and tibia (12) revealed significant improvements in volumetric BMD, trabecular microarchitecture, and stiffness at the iliac crest, distal radius, and tibia. However, 20% of the participants had no increase in spine or hip aBMD. These women were characterized by lower baseline serum bone turnover markers (BTMs) and BFR on transiliac biopsies (11), suggesting that they might have primary osteoblast defects that attenuate response to teriparatide. As teriparatide is often selected because of its stimulatory effects on bone formation, it is important to determine whether it is effective in premenopausal women with IOP and low bone formation to assist physicians and patients in selecting therapy, should therapy be deemed necessary.
In this study, we aimed to establish the efficacy (aBMD and BTMs) and safety of 6 months of teriparatide versus placebo, to determine the effect of 3 months of teriparatide versus placebo on BFR assessed by quadruple tetracycline-labeled transiliac bone biopsy, to characterize the effect of 12 and 24 months of teriparatide on aBMD by dual-energy x-ray absorptiometry (DXA), and to determine the extent to which baseline and 3-month BFR and serum bone formation markers predict the BMD response to teriparatide.
Methods
Study design and participants
Premenopausal women with IOP were recruited by advertisement or self- or physician-referral. All participants underwent screening by telephone or in person. Those who met preliminary eligibility criteria and were interested in participating were invited for a screening visit to assess eligibility at 1 of 2 tertiary osteoporosis centers, Columbia University Medical Center, New York, New York and Creighton University, Omaha, Nebraska. After signing informed consent, they were evaluated on their usual calcium intake with serum and urine biochemistries, aBMD by DXA, and vertebral fracture assessment (VFA).
Eligible participants had to have regular menses, early follicular phase follicle stimulating hormone levels less than 20 mIU/mL, no historical or biochemical secondary cause of osteoporosis, and no clinical evidence of rare bone diseases (eg, osteogenesis imperfecta, Ehlers-Danlos). All had radiographically documented adult, low-trauma fractures and/or T-scores that were ≤−2.5 or Z-scores ≤−2.0 at the lumbar spine (LS), femoral neck (FN) or total hip (TH). Low trauma was defined as equivalent to a fall from a standing height or less; skull and digit fractures were excluded. At the request of the United States Food and Drug Administration (FDA), inclusion criteria varied by age. Participants aged 20 to 35 years had at least one major osteoporotic fracture and T-score or Z-score ≤−1.5. Those over age 35 required a history of fracture and/or T-score ≤−2.5 or Z-score ≤−2.0. A physician panel (E.S., A.C., R.R.R.) reviewed radiographic reports and assessed level of trauma associated with the fractures. Women were excluded if they had delivered a baby within the past 12 months or weaned a child within the previous 6 months. Women with secondary causes of osteoporosis (estrogen deficiency, eating disorders, osteomalacia, celiac or gastrointestinal disease), renal insufficiency, abnormal liver function tests, hyperparathyroidism, urinary calcium above 300 mg/g creatinine, serum 25-hydroxyvitamin D (25-OHD) levels below 20 ng/mL, specific drug exposures (glucocorticoids, anticonvulsants, anticoagulants, methotrexate, depot progesterone, gonadotrophin releasing hormone agonists) and current therapy for osteoporosis (raloxifene, bisphosphonates, calcitonin, teriparatide, denosumab) were excluded.
Randomization and masking
The study statistician created the randomization table using SAS PROC PLAN procedure (SAS version 9.2) in an approximately 3:1 ratio to teriparatide (n = 28) or placebo (n = 12), randomly permuted blocks of block size 2, 4 or 6, and stratified by whether they qualified by fractures or low BMD; only the Research Pharmacy had access to the randomization. Investigators, study personnel and participants were masked to treatment assignment until the 12-month visit when unblinding occurred to permit scheduling of future study procedures. Participants and study personnel remained masked to study outcomes until participants completed 24 months of teriparatide.
Change to trial design
After 18 participants enrolled, the Research Pharmacy informed the principal investigator (E.S.) that the study randomization table had been reversed. To maintain the number treated with teriparatide at 28 and blinding of subjects and study personnel to study assignment, an independent statistician generated an adaptive randomization table, increasing the sample size from 40 to 41 so that the remaining 23 patients would be randomized to teriparatide versus placebo in a 22:1 ratio. Ultimately, 28 women were randomized to teriparatide 20 mcg and 13 to placebo. The adaptive randomization scheme was approved by the FDA and both study Institutional Review Board (IRBs) (Columbia University and Creighton University).
Procedures
The Columbia University Medical Center IRB reviewed and approved the study (IRB AAAF2251); the Creighton University Medical Center approved the study after it was approved by Columbia. All participants provided their written informed consent. A Data and Safety Monitoring Committee was convened and met annually throughout the study.
Fasting morning serum was collected before the daily teriparatide dose at baseline, 3, 6, 12, 18, 24, and 30 months and stored at −80 oC for batch analyses performed in the Biomarkers Core of the Irving Institute for Clinical and Translational Research: serum N-terminal propeptides of procollagen type 1 (P1NP), osteocalcin (OCN), C-telopeptide (CTx), and insulin-like growth factor 1(IGF-1) (1, 3). Fasting serum calcium was assessed at each visit and 24-hour urinary calcium was assessed at 1 and 7 months (Quest Diagnostics).
BMD was measured by DXA (Discovery, Hologic Inc., Walton, MA) at baseline and 6-month intervals at the LS, TH, FN, and distal radius (DR) (1). Short-term coefficient of variation was 0.7% (LS) and 1.4% (FN) at Columbia and 1.5% (LS and FN) at Creighton. Phantoms were circulated between the study sites. T- and Z-scores were generated using the manufacturer’s database. Lack of response to teriparatide was defined as an absolute change in 12-month LS BMD below the manufacturer-provided least significant change (<0.026 g/cm2). Trabecular Bone Score (TBS), a textural analysis of LS BMD images, was assessed at L1-L4 using TBSiNsight software version 2.1.2.
Screening for moderate and severe vertebral fractures was performed by Vertebral Fracure Analysis (VFA) on lateral spine images (T4 to L5) using Hologic QDR Physician Viewer software. Participants were categorized as having vertebral fractures based on an International Society for Clinical Densitometry (ISCD)-certified densitometrist’s reading of the interpretable image using the Genant semiquantitative method, the currently recommended clinical technique. For the diagnosis of moderate (25%-40% reduction in vertebral height) or severe (>40% reduction in vertebral height) fractures, the sensitivity and specificity of VFA are comparable to spine radiographs (13).
Transiliac bone biopsy after tetracycline, which labels bone surfaces undergoing active formation, permits precise quantification of surface-based bone formation rate (BFR). With the quadruple-labeling protocol, one set of double demeclocycline labels (two 3-day courses separated by 12 days) is administered before treatment and a second set of double tetracycline labels is administered after 2.5 months of teriparatide/placebo. A single biopsy was performed in 38 participants after the second set of labels (14) to assess BFR before and during drug treatment, as described (14, 15). Biopsies were embedded, sectioned, and stained using established procedures (16, 17). Histomorphometry was performed with a digitizing image-analysis system (OsteoMeasure, Version 4.00C, OsteoMetrics, Inc, Atlanta, GA). All variables were calculated according to the American Society for Bone and Mineral Research recommendations (18, 19).
Data handling procedures
Data collected on case report forms were entered into Microsoft Excel (Microsoft Corporation, Redmond, WA) and imported to SAS (SAS Institute, Cary, NC) where categorical fields were coded to mutually exclusive and exhaustive values and continuous variables distributional characteristics assessed. Missing data were not imputed.
Statistical analysis
The study had 3 prespecified aims. The first was to establish the efficacy and safety of 6 months of teriparatide versus placebo in premenopausal women with IOP. The primary efficacy outcome was the between-group difference in mean change in LS aBMD (g/cm2) expressed as percent change from baseline to 6 months. Key secondary outcomes were the between-group differences in mean change in TH, FN, and DR aBMD (percent change from baseline) and absolute change in serum BTMs (P1NP, OCN, and CTx) from baseline to 3 and 6 months. Primary safety outcomes were between-group differences in the number in each group with episodes of pre-dose hypercalcemia or hypercalciuria. Secondary safety outcomes were differences in numbers of participants endorsing symptoms on the visit checklist.
The second aim of the study was to determine the effect of 3 months of teriparatide on bone remodeling at the tissue level. The primary outcome variables were the between-group difference in BFR at the cancellous, endocortical, and intracortical surfaces assessed on the 3-month quadruple tetracycline-labeled biopsy.
The third aim of the study was to determine the extent to which 12 and 24 months of teriparatide increases aBMD compared to baseline in premenopausal women with IOP. The primary outcome variable was within-group change in LS aBMD (percent change from baseline) to 12 and 24 months. Key secondary outcomes were within-group change in TH, FN, and DR aBMD (percent change from baseline) to 12 and 24 months, pattern of change in serum BTMs (P1NP, OCN, and CTx) between baseline and 24 months, and the extent to which these BTMs, baseline BFR (in all participants) and 3-month BFR (in participants randomized to teriparatide-first) were associated with change in aBMD at 12 and 24 months. There were no changes to outcomes after the trial began.
Analysis of covariance (ANCOVA) was used to examine serial changes in BMD, unadjusted and adjusted for baseline BMD, age and BMI. ANCOVA was also used to examine serial changes in BTMs and biochemistries. Baseline and 3-month biopsy parameters were compared with paired Student t tests. Because not all variables were normally distributed (Kolmogorov-Smirnov test), Spearman correlations were used to test associations between specific anthropometric, biochemical, and histomorphometric characteristics (baseline and 3 months) and response to teriparatide unless otherwise indicated. Student t tests were used to compare responders and nonresponders. All data are expressed as mean ± 95% confidence intervals (CI), unless otherwise indicated. P values < 0.05 were considered statistically significant. No adjustment for multiple comparisons was applied.
Sample size for the aBMD outcomes was estimated, whenever possible, from preliminary data (11). For the primary endpoint of between-group difference in LS aBMD at 6 months, 41 women randomized 3:1 to teriparatide, assuming a 10% dropout rate and an alpha level of 5%, provided >90% power to detect a difference of 3.7 ± 3.3% in the teriparatide group versus 0.8 ± 2.9% in the placebo group, based on change in aBMD in 18 untreated women with IOP followed for 4 years on calcium (1000-1500 mg/day) and vitamin D (800-1000 IU/day; unpublished data). For the secondary efficacy endpoints of between-group differences in TH, FN, and DR, we did not expect to have sufficient power to detect differences at 6 months. For the primary safety outcome, the between-group difference in the number of people in each assigned group reporting at least one hypercalcemia or hypercalciuria event, we did not have sufficient power to detect group differences in event rate because of the rarity of TPTD-related safety events observed in our pilot study and reported by others in the literature. Our small sample size provided minimal power to detect any but the most dramatic differences in safety profile between groups. For aBMD by DXA after 12 and 24 months of TPTD, we had >86% power to detect changes from baseline at all sites (11).
Role of the funding source
This study was supported by the FDA Orphan Products Clinical Trials Grants Program (R01 FD003902). The principal investigators, Dr. Cohen and Dr. Shane, designed and conducted the study. Eli Lilly, USA, supplied teriparatide and identical placebo but no other financial support, and had no role in study design, collection, analysis, and interpretation of data, writing of the report, or decision to submit for publication. Statisticians at Columbia University performed all analyses. This study was also supported by R03 AR064016 (Cohen), K24 AR052665 (Shane) and K23 AR054127 (Cohen), the Simon-Strauss Foundation and the Thomas L. Kempner Jr. and Katheryn C. Patterson Foundation. Dr. Cohen and Dr. Shane had full access to all data in the study and had final responsibility for the decision to submit for publication.
Results
From April 24, 2012 to June 15, 2016, 140 patients were evaluated for inclusion. Fifty-three declined participation, most commonly because they decided against pharmacologic therapy or time and travel commitments. In addition, 46 patients did not meet inclusion/exclusion criteria. Therefore, 41 patients were randomly assigned to teriparatide (n = 28) or placebo (n = 13; Fig. 1). All received calcium supplementation to achieve a total daily intake of 1200 mg/day and vitamin D 900 units daily throughout the study. Injections were self-administered in the morning or evening according to preference. Participants were required to use effective contraception during the study; 15 chose estrogen-based contraception.
Figure 1.
Participant disposition. * Women allocated to teriparatide-first completed 24 months of teripataride at the 24-month visit. ** Women allocated to placebo-first completed 24 months of teriparatide at the 30-month visit. Withdrawn by investigator: 1 patient was removed after the 12-month visit and 2 patients were removed after the 18-month visit because they met predefined bone loss stopping criteria of >5% at any site and 1 patient was removed after the 18-month visit because of worsening nephrolithiasis.
Among 32 participants enrolled based on fracture history, 25 reported multiple fractures in adulthood; 15 had vertebral, 9 had hip, 2 had pelvic, 9 had rib, 14 had upper extremity, and 16 had lower extremity fractures. Three participants (all in the teriparatide-first group) had prior exposure to bisphosphonates. Two took alendronate, 1 for 2 years, stopping 3 years before enrolling and 1 for 9 months, stopping 6 months before enrolling. One took risedronate for 6 months, 4 years before enrolling. One took teriparatide for 2 months after a hip fracture 12 months before enrolling.
All participants (100%) completed 6 months in their assigned group (Fig. 1). All placebo-first participants switched to teriparatide. Two participants withdrew, 1 before the 12-month and 1 before the 18-month visit. Four participants were withdrawn by the investigators, 3 because they met predefined stopping criteria of >5% bone loss and 1 for nephrolithiasis. In total, 40 women (97.5%) completed 12 months and 35 women (85.4%) completed 24 months of teriparatide.
Teriparatide versus placebo for 6 months
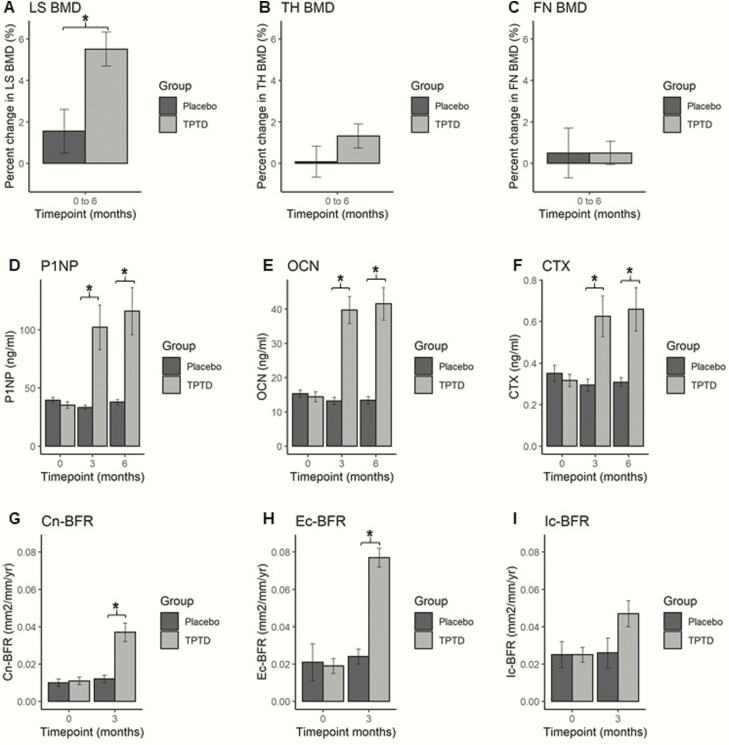
The teriparatide and placebo groups did not differ at baseline (Table 1), except that TH and FN aBMD were higher in the placebo-first group. The 6-month results are shown in Fig. 2 and Table 2. LS aBMD (Fig. 2A) increased by 5.5% (95% CI: 3.83, 7.19) in the teriparatide and did not change [+1.5% (95% CI: −0.73, 3.83)] in the placebo group (between-groups P = 0.007), with no difference at the TH (Fig. 2B), FN (Fig. 2C), or DR. The results were unchanged after adjusting for age, BMI, and baseline BMD. There was a 3-fold increase in serum P1NP (Fig. 2D) and serum OCN (Fig. 2E) in the teriparatide group at 3 and 6 months, with no change in the placebo group (between-groups P both < 0.001). In the teriparatide group, serum CTx approximately doubled (Fig. 2F) at 3 and 6 months, whereas it declined slightly in the placebo group (between-groups P = 0.024 and P = 0.028 respectively). Serum IGF-1 did not change in either group. The quadruple-labeled bone biopsy confirmed that serum BTMs reflected tissue level changes. In the teriparatide group, cancellous BFR (Fig. 2G) increased by 3.3-fold (P ≤ 0.001), while the placebo group did not change (between-groups P = 0.004). Similarly, endocortical BFR (Fig. 2H) increased 4-fold in the teriparatide group (P ≤ 0.001), with no change in the placebo group (between-groups P = 0.004). Intracortical BFR (Fig. 2I) increased by 1.9-fold in the teriparatide group (P ≤ 0.001), with no change in the placebo group (between-groups P = 0.14).
Table 1.
Baseline Characteristics of all Participants, Those Randomized to Teriparatide-First and Those Randomized to Placebo-First, Analysed by Intention-to-Treat; Mean (95% CI) or N (%)
| All Patients (N = 41) | Teriparatide-First (N = 28) | Placebo-First (N = 13) | P Value | |
|---|---|---|---|---|
| Columbia | 32 | 23 | 9 | |
| Creighton | 9 | 5 | 4 | |
| Characteristics | ||||
| Age, years | 36.9 (34.5, 39.3) | 37.8 (34.8, 40.8) | 35.1 (30.7, 39.4) | 0.29 |
| Weight, kg | 58.4 (55.0, 61.8) | 56.5 (52.4, 60.3) | 62.7 (55.9, 69.5) | 0.08 |
| Height, cm | 162.2 (160.0, 164.5) | 162.1 (159.4, 164.7) | 162.5 (157.7, 167.4) | 0.86 |
| Body mass index (BMI), kg/m2 | 22.2 (20.9, 23.5) | 21.4 (20.2, 22.7) | 24.0 (20.7, 27.2) | 0.072 |
| Adult low trauma fractures, n (%) | 32 (80) | 21 (78) | 11 (85) | 0.6 |
| Number of adult low trauma fractures | 3.1 (2.1, 4.1) | 2.8 (1.6, 3.9) | 3.8 (1.6, 5.9) | 0.3 |
| Bone measurements by DXA | ||||
| Lumbar spine BMD, g/cm2 | 0.79 (0.76, 0..82) | 0.77 (0.74, 0.81) | 0.82 (0.76, 0.89) | 0.10 |
| Lumbar spine BMD Z-score | −2.19 (−0.49, −1.90) | −2.29 (−2.62, −1.95) | −1.99 (−2.61, −1.37) | 0.34 |
| Total hip BMD, g/cm2 | 0.76 (0.73, 0.79) | 0.74 (0.71, 0.78) | 0.81 (0.75, 0.88) | 0.03 |
| Total hip BMD Z-score | −1.31 (−1.55, −1.08) | −1.47 (−1.73, −1.20) | −0.96 (−1.48, −0.44) | 0.05 |
| Femoral neck BMD, g/cm2 | 0.64 (0.61, 0.68) | 0.62 (0.59, 0.66) | 0.69 (0.63, 0.76) | 0.045 |
| Femoral neck BMD Z-score | −1.59 (−1.86, −1.32) | −1.75 (−2.08, −1.41) | −1.23 (−1.69, −0.77) | 0.07 |
| Distal radius BMD, g/cm2 | 0.67 (0.66, 0.69) | 0.67 (0.64, 0.69) | 0.69 (0.66, 0.72) | 0.19 |
| Distal radius BMD Z-score | 0.04 (−0.26, 0.34) | −0.08 (−0.48, 0.33) | 0.30 (−0.13, 0.73) | 0.25 |
| Trabecular bone score (TBS; L1L4) | 1.28 (1.25, 1.32) | 1.28 (1.23, 1.32) | 1.30 (1.25, 1.34) | 0.61 |
| Biochemistries | ||||
| Serum N-terminal propeptide of procollagen type 1 (P1NP), ng/mL | 36.7 (32.6, 40.8) | 35.3 (29.6, 41.0) | 39.5 (34.4, 44.6) | 0.55 |
| Serum osteocalcin (OCN), ng/mL | 14.7 (12.6, 16.8) | 14.4 (11.4, 17.4) | 15.3 (12.9, 17.7) | 0.34 |
| Serum C-telopeptide (CTx), ng/mL | 0.327 (0.277, 0.378) | 0.317 (0.250, 0.384) | 0.350 (0.265, 0.434) | 0.70 |
| Serum 25-OHD, nmol/L | 98.1 (89.4, 106.8) | 95.6 (85.9, 105.3) | 103.3 (83.6, 123.1) | 0.42 |
| Serum parathyroid hormone, pmol/L | 2.06 (1.87, 2.26) | 1.94 (1.74, 2.14) | 2.32 (1.88, 2.76) | 0.06 |
| Serum calcium,* mmol/L | 2.32 (2.27, 2.35) | 2.30 (2.30, 2.32) | 2.32 (2.22, 2.40) | 0.91 |
| Urine calcium, mmol/molCr | 371.3 (311.4, 431.3) | 375.3 (292.4, 458.4) | 358.0 (254.2, 461.8) | 0.79 |
| Urine calcium, mg/gCr | 131.3 (110.1, 152.5) | 132.7 (103.4, 162.1) | 126.6 (89.9, 163.3) | 0.96 |
| Serum insulin-like growth factor 1 (IGF-1), ng/mL** | 124.2 (112.4, 136.0) | 124.4 (108.8, 140.0) | 123.7 (104.2, 143.3) | 0.09 |
| IGF-1 Z-score | −0.48 (−0.84, −0.12) | −0.28 (−0.68, 0.13) | −0.92 (−1.71, −0.14) | 0.09 |
| Bone biopsy measurements | ||||
| Number (N) | 38 | 27 | 11 | |
| Cancellous BFR, mm2/mm/yr | 0.011 (0.008, 0.014) | 0.011 (0.007, 0.015) | 0.010 (0.006, 0.015) | 0.82 |
| Endocortical BFR, mm2/mm/yr | 0.020 (0.013, 0.026) | 0.019 (0.011, 0.027) | 0.021 (0.010, 0.033) | 0.77 |
| Intracortical BFR, mm2/mm/yr | 0.025 (0.018, 0.033) | 0.025 (0.016, 0.034) | 0.025 (0.009, 0.041) | 0.99 |
| Cancellous MdPm (%) | 5.47 (4.00, 6.93) | 5.51 (3.56, 7.45) | 5.37 (3.19, 7.54) | 0.93 |
| Endocortical MdPm (%) | 9.83 (6.85, 12.8) | 9.77 (5.92, 13.6) | 9.95 (4.89, 15.0) | 0.96 |
| Intracortical MdPm (%) | 9.82 (6.87, 12.8) | 9.28 (6.02, 12.5) | 11.1 (3.84, 18.4) | 0.57 |
Abbreviations: 25-OHD, 25-hydroxyvitamin D; BFR, bone formation rate; BMD, bone mineral density; DXA, dual-energy x-ray absorptiometry; MdPm, mineralizing perimeter.
*Albumin-adjusted; **Immulite restandardized
Figure 2.
Six-month placebo-controlled trial of teriparatide versus placebo. A.) Lumbar spine (LS) BMD; B.) Total hip (TH) BMD; C.) Femoral neck (FN) BMD; D.) Serum P1NP; E.) Serum osteocalcin (OCN); F.) Serum C-telopeptide (CTX); G.) Cancellous bone formation rate (Cn-BFR); H.) Endocortical BFR (Ec-BFR); and I.) Intracortical BFR (Ic-BFR). Placebo: Black bars; Teriparatide: Gray bars. * P < 0.05 for between-groups comparison.
Table 2.
Six Months of Teriparatide Versus Placebo: Effects on aBMD, Trabecular Bone Score (TBS), Bone Turnover Markers (BTMs) and Bone Formation on Transiliac Biopsy in Premenopausal IOP, Mean (95% CI)
| Time | Teriparatide (N = 28) | Placebo (N = 13) | Difference | |
|---|---|---|---|---|
| Aim 1: Six months of teriparatide versus placebo: Effects on aBMD by DXA, Biochemistries, and TBS | ||||
| Primary Outcomes | ||||
| Lumbar Spine aBMD, g/cm2 | Baseline | 0.77 | 0.82 | −0.05 (−0.12, 0.01) |
| 6M | 0.81 | 0.84 | −0.02 (−0.09, 0.04) | |
| % Change | 5.51 | 1.55 | 3.96 (1.16, 6.76) | |
| Key Secondary Outcomes | ||||
| Total Hip aBMD, g/cm2 | Baseline | 0.74 | 0.81 | −0.07 (−0.13, −0.007) |
| 6M | 0.75 | 0.81 | −0.06 (−0.12, 0.002) | |
| % Change | 1.32 | 0.08 | 1.24 (−0.79, 3.28) | |
| Femoral Neck aBMD, g/cm2 | Baseline | 0.62 | 0.69 | −0.07 (−0.14, −0.002) |
| 6M | 0.63 | 0.70 | −0.06 (−0.13, 0.003) | |
| % Change | 0.50 | 0.50 | 0.00 (−2.34, 2.34) | |
| Distal Radius (1/3 site) aBMD, g/cm2 | Baseline | 0.67 | 0.69 | −0.02 (−0.06, 0.01) |
| 6M | 0.67 | 0.68 | −0.01 (−0.05, 0.03) | |
| % Change | 0.13 | −0.98 | 1.11 (−0.47, 2.69) | |
| P1NP, ng/mL | Baseline | 35.3 | 39.5 | −4.2 (−13.0, 4.6) |
| 3M | 102.1 | 33.3 | 68.8 (13.2, 124.4) | |
| 6M | 116.0 | 37.8 | 78.1 (18.5, 137.8) | |
| Osteocalcin (OCN), ng/mL | Baseline | 14.4 | 15.3 | −0.88 (−5.39, 3.63) |
| 3M | 39.7 | 13.2 | 26.5 (15.0, 38.0) | |
| 6M | 41.5 | 13.4 | 28.1 (14.0, 42.2) | |
| C-telopeptide (CTx), ng/mL | Baseline | 0.317 | 0.350 | −0.03 (−0.14, 0.08) |
| 3M | 0.625 | 0.294 | 0.33 (0.05, 0.62) | |
| 6M | 0.659 | 0.308 | 0.35 (0.04, 0.66) | |
| Other Outcomes | ||||
| Trabecular Bone Score (TBS; Exploratory) | Baseline | 1.28 | 1.30 | −0.02 (−0.09, 0.06) |
| 6M | 1.31 | 1.31 | 0.00 (−0.07, 0.07) | |
| IGF-1, ng/mL | Baseline | 124.4 | 123.7 | 0.66 (−24.8, 26.1) |
| 3M | 112.3 | 107.8 | 4.5 (−21.0, 30.0) | |
| 6M | 115.6 | 116.1 | −0.50 (−27.2, 26.2) | |
| Aim 2: Six months of teriparatide versus placebo: Tissue-based Bone Formation on Three-month Quadruple Biopsy | ||||
| Primary Outcomes | N = 27 | N = 11 | ||
| Cancellous BFR/BS, mm2/mm/yr | Baseline | 0.011 | 0.010 | 0.001 (−0.006, 0.007) |
| 3M | 0.037 | 0.012 | 0.025 (0.008, 0.04) | |
| Endocortical BFR/BS, mm2/mm/yr | Baseline | 0.019 | 0.021 | −0.002 (−0.017, 0.01) |
| 3M | 0.077 | 0.024 | 0.053 (0.02, 0.09) | |
| Intracortical BFR/BS, mm2/mm/yr | Baseline | 0.025 | 0.025 | −0.0001 (−0.017, 0.02) |
| 3M | 0.047 | 0.026 | 0.021 (−0.007, 0.05) | |
| Cancellous MdPm (%) | Baseline | 5.51 | 5.37 | 0.14 (−3.13, 3.41) |
| 3M | 16.7 | 6.06 | 10.7 (3.57, 17.8) | |
| Endocortical MdPm (%) | Baseline | 9.77 | 9.95 | −0.18 (−6.85, 6.49) |
| 3M | 33.8 | 10.2 | 23.6 (10.5, 36.7) | |
| Intracortical MdPm (%) | Baseline | 9.28 | 11.1 | −1.86 (−8.41, 4.70) |
| 3M | 15.6 | 9.03 | 6.57 (−1.82, 15.0) |
Abbreviations: aBMD, areal bone mineral density; BFR, bone formation rate; DXA, dual-energy x-ray absorptiometry; IGF-1, insulin-like growth factor 1; IOP, idiopathic osteoporosis; MdPm, mineralizing perimeter; P1NP, N-terminal propeptides of procollagen type 1.
Extension study: teriparatide for 24 months
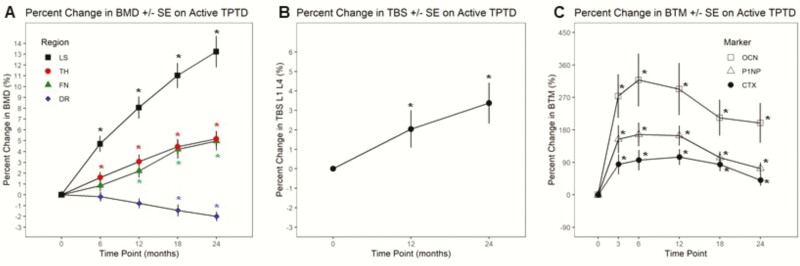
The within-group effects of 12 and 24 months of teriparatide in all patients compared with baseline are shown in Fig. 3 and Table 3. LS aBMD (Fig. 3A) increased by 8.1% (95% CI: 6.0, 10.1) at 12 months and 13.2% (95% CI: 10.3, 16.2) at 24 months (both P < 0.001). TH aBMD increased by 3.1% (95% CI: 1.8, 4.4) at 12 months and 5.2% (95% CI: 3.7, 6.7) at 24 months (both P < 0.001). FN aBMD increased by 2.2% (95% CI: 0.96, 3.4) at 12 months and 5.0% (95% CI: 3.2, 6.7) at 24 months (both P ≤ 0.001). DR aBMD decreased by 0.8 (95% CI: 0.13, 1.7) at 12 months (P = 0.08) and 2.0% (95% CI: 1.1, 2.9) at 24 months (P < 0.001). The results did not differ according to use of estrogen-based contraception.
Figure 3.
Effect of 24 months of teriparatide expressed as percent change from baseline (± SE) on A.) BMD of the lumbar spine (LS), total hip (TH), femoral neck (FN), and distal radius (DR); B.) Trabecular bone score (TBS); and C.) Serum bone turnover markers: osteocalcin (OCN), P1NP, C-telopeptide (CTx). * P < 0.05 vs baseline.
Table 3.
Within-Group Effects of Teriparatide Over 24 Months Expressed as Percent Change From Baseline, Mean ± SD (95% CI); N = 41
| 3 Months | 6 Months | 12 Months | 18 Months | 24 Months | |
|---|---|---|---|---|---|
| aBMD by DXA and Trabecular Bone Score (% change from baseline) | |||||
| Lumbar Spine | n/a | 4.7 ± 4.7 *** (3.2, 6.2) | 8.1 ± 6.4 *** (6.0, 10.1) | 11.0 ± 7.1 *** (8.7, 13.4) | 13.2 ± 8.5 *** (10.3, 16.2) |
| Total Hip | n/a | 1.6 ± 3.1 ** (0.6, 2.6) | 3.1 ± 4.0 *** (1.8, 4.4) | 4.5 ± 4.2 *** (3.0, 5.9) | 5.2 ± 4.3 *** (3.7, 6.7) |
| Femoral Neck | n/a | 0.9 ± 3.2 (−0.2, 1.9) | 2.2 ± 3.7 *** (1.0, 3.4) | 4.2 ± 5.1 *** (2.5, 5.9) | 5.0 ± 5.0 *** (3.2, 6.7) |
| Distal Radius | n/a | −0.2 ± 2.7 (−1.1, 0.7) | −0.8 ± 2.7 (−1.7, 0.1) | −1.4 ± 3.2 ** (−2.6, −0.3) | −2.0 ± 2.6 *** (−2.9, −1.1) |
| Trabecular Bone Score | n/a | n/a | 2.0 ± 5.2 * (0.1, 4.0) | n/a | 3.4 ± 5.6 ** (1.3, 5.5) |
| Bone Turnover Markers (% change from baseline) | |||||
| P1NP | 154 ± 230 *** (76, 232) | 167 ± 202 *** (102, 233) | 164 ± 170 *** (108, 220) | 103 ± 101 *** (69, 137) | 73 ± 99 *** (40, 106) |
| Osteocalcin | 274 ± 360 *** (152, 396) | 318 ± 454 *** (171, 466) | 293 ± 442 *** (148, 439) | 214 ± 301 *** (113, 314) | 199 ± 329 *** (88, 311) |
| C-telopeptide | 85 ± 166 ** (28, 141) | 96 ± 176 *** (39, 153) | 104 ± 134 *** (60, 149) | 84 ± 114 *** (46, 122) | 41 ± 95 * (9, 73) |
Abbreviations: aBMD, areal bone mineral density; DXA, dual-energy x-ray absorptiometry; P1NP, N-terminal propeptides of procollagen type 1.
* P ≤ 0.05 ** P ≤ 0.01; *** P ≤ 0.001
TBS provides information on spine microarchitecture (20), with higher TBS indicating better and low TBS indicating worse microarchitecture (21, 22). At baseline, mean TBS was 1.29 (95% CI:1.25, 1.32), consistent with partial degradation of trabecular microarchitecture (1.230-1.309) (23). TBS (Fig. 3B) increased by 2.0% (95% CI: 0.10, 4.0) at 12 months (P = 0.049) and 3.4% (95% CI: 1.3, 5.5) at 24 months (P = 0.003) into the normal range (>1.310) at 1.32 (95% CI: 1.29, 1.35) and 1.34 (95% CI: 1.30, 1.38), respectively (23).
Serum P1NP and OCN (Fig. 3C) increased by 3 months, remained elevated during the first year and declined during the second year (all P < 0.001). Serum CTx followed a similar pattern with less marked increases (P = 0.04 to ≤0.001). All 3 remained above baseline at 24 months, P1NP by 73% (95% CI: 40, 106), OCN by 199% (95% CI: 88, 310) and CTx by 41% (95% CI: 9, 72).
The extent to which serum BTMs predicted BMD response to teriparatide at 12 and 24 months is shown in Table 4. Baseline BTMs were not associated with teriparatide response. Serum P1NP at 3 months and change in serum P1NP (baseline to 3 months) were directly associated with increases in LS aBMD at both 12 and 24 months and in TH aBMD at 24 months. Serum OCN at 3 months and absolute change in serum OCN between baseline and 3 months were directly associated with increases in LS aBMD at both 12 and 24 months. In addition, absolute change in serum OCN was associated with teriparatide response at 24 months at the TH and FN. These correlations were statistically significant (P values, 0.027-0.002) though moderate, with r values ranging from 0.403 to 0.518 (Pearson R2: 0.025-0.353). Serum CTx did not associate with teriparatide response.
Table 4.
BMD at 12 and 24 Months; Correlations Between Baseline, 3-Month, and Absolute Change Between Baseline and 3-Month Measures of Bone Turnover Markers and Bone Formation Rate (BFR)*
| 12M and 24M Absolute Change in aBMD by DXA on Active Teriparatide | |||||||
|---|---|---|---|---|---|---|---|
| 12M LS BMD | 12M TH BMD | 12M FN BMD | 24M LS BMD | 24M TH BMD | 24M FN BMD | ||
| Baseline Serum P1NP | r | 0.118 | 0.056 | −0.249 | 0.158 | 0.197 | 0.083 |
| p | 0.480 | 0.743 | 0.143 | 0.372 | 0.271 | 0.653 | |
| 3M Serum P1NP | r | 0.472 | 0.070 | −0.038 | 0.479 | 0.419 | 0.266 |
| p | 0.005 | 0.698 | 0.836 | 0.007 | 0.024 | 0.171 | |
| 3M Absolute Change in Serum P1NP | r | 0.408 | 0.054 | −0.053 | 0.508 | 0.489 | 0.305 |
| p | 0.018 | 0.770 | 0.775 | 0.005 | 0.008 | 0.121 | |
| Baseline Serum OCN | r | 0.281 | 0.199 | −0.087 | 0.219 | 0.071 | −0.058 |
| p | 0.088 | 0.237 | 0.614 | 0.213 | 0.695 | 0.751 | |
| 3M Serum OCN | r | 0.518 | 0.156 | 0.025 | 0.403 | 0.276 | 0.184 |
| p | 0.002 | 0.386 | 0.894 | 0.027 | 0.147 | 0.347 | |
| 3M Absolute Change in Serum OCN |
r | 0.421 | 0.150 | 0.048 | 0.445 | 0.467 | 0.460 |
| p | 0.015 | 0.413 | 0.797 | 0.016 | 0.012 | 0.016 | |
| Baseline Serum CTx | r | 0.256 | −0.053 | −0.087 | 0.230 | 0.181 | 0.234 |
| p | 0.121 | 0.754 | 0.613 | 0.190 | 0.312 | 0.197 | |
| 3M Serum CTx | r | 0.296 | −0.125 | 0.044 | 0.314 | 0.225 | 0.179 |
| p | 0.089 | 0.488 | 0.809 | 0.091 | 0.241 | 0.362 | |
| 3M Absolute Change in Serum CTx |
r | 0.024 | −0.157 | 0.108 | 0.156 | 0.144 | 0.136 |
| p | 0.893 | 0.391 | 0.564 | 0.419 | 0.464 | 0.500 | |
| Baseline BFR Cancellous | r | 0.267 | 0.252 | 0.061 | 0.244 | 0.389 | 0.236 |
| p | 0.121 | 0.151 | 0.734 | 0.179 | 0.031 | 0.210 | |
| 3M BFR Cancellous (active only) | r | 0.355 | 0.318 | 0.141 | 0.244 | 0.448 | 0.438 |
| p | 0.089 | 0.130 | 0.521 | 0.273 | 0.037 | 0.047 | |
| 3M Absolute Change in BFR Cancellous (active only) | r | 0.243 | 0.327 | 0.200 | 0.151 | 0.383 | 0.459 |
| p | 0.264 | 0.128 | 0.371 | 0.514 | 0.086 | 0.042 | |
| Baseline BFR Endocortical | r | 0.338 | 0.192 | 0.120 | 0.365 | 0.476 | 0.490 |
| p | 0.054 | 0.292 | 0.519 | 0.047 | 0.009 | 0.008 | |
| 3M BFR Endocortical (active only) | r | 0.408 | 0.524 | 0.106 | 0.319 | 0.510 | 0.408 |
| p | 0.048 | 0.008 | 0.631 | 0.148 | 0.015 | 0.066 | |
| 3M Absolute Change in BFR Endocortical (active) | r | 0.502 | 0.538 | 0.306 | 0.394 | 0.439 | 0.421 |
| p | 0.017 | 0.01 | 0.177 | 0.086 | 0.053 | 0.073 | |
| Baseline BFR Intracortical | r | 0.100 | 0.101 | 0.467 | 0.053 | 0.372 | 0.528 |
| p | 0.572 | 0.577 | 0.007 | 0.776 | 0.043 | 0.003 | |
| 3M BFR Intracortical (active only) | r | 0.052 | −0.001 | 0.121 | 0.150 | 0.256 | 0.292 |
| p | 0.809 | 0.997 | 0.582 | 0.506 | 0.251 | 0.198 | |
| 3M Absolute Change in BFR Intracortical (active) | r | 0.151 | 0.144 | −0.001 | 0.269 | 0.277 | 0.156 |
| p | 0.503 | 0.523 | 0.995 | 0.251 | 0.238 | 0.523 | |
| Compliance (injections given / injections expected) | r | 0.160 | −0.014 | −0.271 | 0.038 | 0.071 | −0.226 |
| P | 0.436 | 0.944 | 0.190 | 0.854 | 0.729 | 0.277 |
Abbreviations: aBMD, areal bone mineral density; BFR, bone formation rate; BMD, bone mineral density; CTx, C-telopeptide; DXA, dual-energy x-ray absorptiometry; FN, femoral neck; LS, lumbar spine; M, month; OCN, osteocalcin; P1NP, N-terminal propeptides of procollagen type 1.
*Significance denoted by bold font
We next examined baseline BFR at cancellous, endocortical, and intracortical surfaces as a predictor of BMD response in all participants, and 3-month and absolute change in BFR from baseline in the 27 participants randomized to teriparatide-first who were biopsied on teriparatide (Table 4). Endocortical BFR was the most consistent predictor of aBMD response. Baseline endocortical BFR tended to correlate with 12-month change in LS aBMD and correlated with 24-month change in aBMD at the LS, TH, and FN. Endocortical BFR at 3 months was directly associated with teriparatide response at 12 months at the LS and TH but not FN, and at 24 months at the TH. In addition, the 3-month change in endocortical BFR was associated with 12-month response at the LS and TH, but not FN, and not at 24 months. Similar to BTMs, relationships were statistically significant (P values, 0.048-0.003) but modest, with r values ranging between 0.365 to 0.538 (Pearson R2: 0.141 to 0.446).
Consistent with our pilot study (11), response to teriparatide varied. Of 40 women who completed 12 months of teriparatide, 8 (20%) did not demonstrate a 12-month increase in LS BMD above the least significant change (0.026 g/cm2). To examine characteristics of nonresponse (Table 5), we excluded 1 patient who did not take study medication (compliance 37% at 12 months), lowering nonresponse to 18%. LS aBMD in nonresponders fell, on average, by 0.007 g/cm2 (95% CI: −0.046, 0.031) and increased by 0.080 g/cm2 (95% CI: 0.068, 0.091) in responders. Nonresponders also had no increase at the FN and a trend toward a smaller TH increase. BMI was higher in nonresponders. More nonresponders were randomized to placebo-first, although the difference was not statistically significant. Compliance with study drug injections was excellent and did not differ. BTMs did not differ between nonresponders and responders at baseline or 3 months, nor did the absolute change in BTMs (baseline to 3 months). Baseline BFR was 58% lower in nonresponders at the cancellous surface (P = 0.14), 68% lower at the endocortical surface (P = 0.10) and 44% lower at the intracortical surface (P = 0.24), but none of the differences was statistically significant. Only 3 nonresponders were biopsied on teriparatide, thus providing insufficient data to evaluate whether responders and nonresponders differed by BFR after 3 months of teriparatide or by change in BFR between baseline and 3 months.
Table 5.
Characteristics of Responders and Nonresponders to Teriparatide, Mean (95% CI) or N (%)
| Timepoint | Responders (n = 32) | Nonresponders (n = 7) | P | |
|---|---|---|---|---|
| Characteristics | ||||
| Age, years | Baseline | 37.2 (34.5, 39.9) | 35.9 (27.9, 43.8) | 0.67 |
| Weight, kg | Baseline | 57.2 (53.7, 60.8) | 65.2 (53.1, 77.3) | 0.08 |
| Height, cm | Baseline | 163.1 (160.5, 165.8) | 158.6 (154.2, 163.0) | 0.14 |
| Body mass index, kg/m2 | Baseline | 21.5 (20.4, 22.6) | 26.1 (20.4, 31.8) | 0.006 |
| Adult low trauma fractures, n | Baseline | 3.2 (2.0, 4.3) | 2.9 (0.2, 5.5) | 0.83 |
| Active n (%) | 25 (76) | 3 (43) | 0.17 | |
| Placebo n (%) | 8 (24) | 4 (57) | ||
| Compliance | 12M | 94.9 | 94.7 | 0.95 |
| Areal BMD by DXA, g/cm 2 | ||||
| Lumbar spine | Baseline | 0.779 (0.745, 0.814) | 0.838 (0.781, 0.893) | 0.14 |
| 12M | 0.861 (0.823, 0.899) | 0.830 (0.749, 0.911) | 0.48 | |
| 12M Absolute Change | 0.08 (0.068, 0.091) | −0.007 (−0.046, 0.031) | <0.0001 | |
| Total Hip | Baseline | 0.749 (0.721, 0.776) | 0.825 (0.685, 0.965) | 0.055 |
| 12M | 0.771 (0.742, 0.801) | 0.833 (0.691, 0.975) | 0.13 | |
| 12M Absolute Change | 0.027 (0.016, 0.037) | 0.008 (−0.016, 0.033) | 0.13 | |
| Femoral Neck | Baseline | 0.634 (0.601, 0.667) | 0.692 (0.571, 0.814) | 0.16 |
| 12M | 0.652 (0.614, 0.689) | 0.680 (0.563, 0.798) | 0.53 | |
| 12M Absolute Change | 0.020 (0.012, 0.029) | −0.012 (−0.022, −0.003) | 0.0009 | |
| Distal Radius (1/3 site) | Baseline | 0.671 (0.652, 0.689) | 0.657 (0.587, 0.727) | 0.58 |
| 12M | 0.665 (0.644, 0.685) | 0.653 (0.590, 0.715) | 0.61 | |
| 12M Absolute Change | −0.004 (−0.012, 0.003) | −0.009 (−0.026, 0.008) | 0.56 | |
| TBS (L1-L4) | Baseline | 1.28 (1.24, 1.32) | 1.33 (1.25, 1.40) | 0.30 |
| 12M | 1.32 (1.28, 1.36) | 1.32, (1.23, 1.41) | 0.98 | |
| 12M Absolute Change | 2.66 (0.49, 4.83) | −0.57 (−6.75, 5.61) | 0.19 | |
| Serum Biochemistry | ||||
| 25-OHD, nmol/L | Baseline | 101.3 (91.8, 110.8) | 93.3 (72.0, 114.5) | 0.48 |
| Parathyroid hormone, pmol/L | Baseline | 2.02 (1.80, 2.23) | 2.17 (1.58, 2.76) | 0.53 |
| Calcium,* mmol/L | Baseline | 2.32 (2.27, 2.35) | 2.32 (2.30, 2.37) | 0.59 |
| IGF-1 | Baseline | 123.5 (109.8, 137.2) | 139.1 (88.6, 189.7) | 0.37 |
| IGF-1 Z-score | Baseline | −0.51 (−0.86, −0.16) | −0.34 (−2.11, 1.42) | 0.73 |
| C-telopeptide, ng/mL | Baseline | 0.317 (0.26, 0.37) | 0.29 (0.17, 0.42) | 0.69 |
| 3M | 0.576 (0.40, 0.75) | 0.470 (−0.05, 0.99) | 0.63 | |
| 3M Absolute Change | 0.22 (0.07, 0.38) | 0.17 (−0.20, 0.53) | 0.76 | |
| P1NP, ng/mL | Baseline | 36.2 (31.2, 41.2) | 36.7 (31.2, 42.1) | 0.93 |
| 3M | 96.9 (62.5, 131.2) | 75.3 (7.2, 143.4) | 0.62 | |
| 3M Absolute Change | 57.5 (23.2, 91.8) | 40.2 (−21.4, 101.8) | 0.68 | |
| Osteocalcin, ng/mL | Baseline | 14.4 (11.8, 16.9) | 14.0 (10.9, 17.1) | 0.89 |
| 3M | 39.1 (31.6, 46.5) | 31.3 (3.3, 59.3) | 0.43 | |
| 3M Absolute Change | 23.9 (17.7, 30.1) | 18.4 (−6.5, 43.4) | 0.51 | |
| Bone biopsy measurements | ||||
| Cancellous BFR, mm2/mm/yr | Baseline | 0.012 (0.008, 0.015) | 0.005 (0.0002, 0.011) | 0.14 |
| Endocortical BFR, mm2/mm/yr | Baseline | 0.022 (0.015, 0.030) | 0.007 0.003, 0.016) | 0.10 |
| Intracortical BFR, mm2/mm/yr | Baseline | 0.027 (0.019, 0.036) | 0.015 (−0.004, 0.033) | 0.24 |
| Cancellous MdPm (%) | Baseline | 5.84 (4.15, 7.53) | 2.87 (0.73, 5.00) | 0.17 |
| Endocortical MdPm (%) | Baseline | 10.7 (7.24, 14.1) | 4.13 (−0.67, 8.93) | 0.14 |
| Intracortical MdPm (%) | Baseline | 10.6 (7.26, 14.0) | 5.61 (−1.71, 12.9) | 0.25 |
Bold indicates P values < 0.05.
Abbreviations: 25-OHD, 25-hydroxyvitamin D; BFR, bone formation rate; BMD, bone mineral density; DXA, dual-energy x-ray absorptiometry; IGF-1, insulin-like growth factor 1; MdPm, mineralizing perimeter; P1NP, N-terminal propeptides of procollagen type 1; TBS, trabecular bone score.
Fractures, adverse events, and compliance
Moderate or severe vertebral fractures were detected by baseline VFA in 12 of the 40 women. In addition, 3 women had vertebral fractures on spine radiographs that were not visible on VFA. There were no incident vertebral fractures during the first 6 months. One participant in the teriparatide group sustained a low trauma nonvertebral metatarsal fracture (Table 6). During the 24-month teriparatide period, 1 participant with a history of multiple vertebral fractures and chronic back pain had a magnetic resonance imaging (MRI) scan performed by her primary physician that revealed a new inferior end-plate fracture of L2 not visualized on the per protocol VFA obtained at the end of the study. One participant had a low trauma rib fracture and 2 had traumatic fractures (metatarsal, skull).
Table 6.
Fractures and Adverse Events
| 6 Months of Teriparatide Versus Placebo (Number/Percent) | 24 Months of Teriparatide (Number/Percent) | |||
|---|---|---|---|---|
| Type of Event | Teriparatide- First | Placebo-First | Difference | |
| # of patients | 28 | 13 | 41 | |
| Anticipated Adverse Events | ||||
| Hypercalcemia, Hypercalciuria, and Genitourinary | ||||
| One serum calcium level >10.5 mg/dL | 1 (3.6) | 0 | 0.04 (−0.19, 0.18) | 1 (2.4) |
| ≥2 serum calcium levels>10.5 mg/dL | 0 | 0 | 0 (−0.23, 0.12) | 0 |
| Hypercalciuria | 1 (3.6) | 0 | 0.04 (−0.19, 0.18) | 2 (4.9) |
| Kidney stones | 0 | 0 | 0 (−0.23, 0.12) | 3 (7.3) |
| Hematuria | 0 | 0 | 0 (−0.23, 0.12) | 1 (2.4) |
| Injection Site Reactions | ||||
| Redness | 20 (71.4) | 1 (7.7) | 0.64 (0.32, 0.78) | 25 (61.0) |
| Swelling | 1 (3.6) | 0 | 0.04 (−0.19, 0.18) | 3 (7.3) |
| Itching | 7 (25.0) | 1 (7.7) | 0.17 (−0.11, 0.37) | 10 (24.4) |
| Bruising | 18 (64.3) | 8 (61.5) | 0.03 (−0.25, 0.33) | 37 (90.2) |
| Incident Fractures | ||||
| Vertebral (Clinical*) | 0 | 0 | 0 (−0.23, 0.12) | 1 (7.7) |
| Vertebral (VFA**) | 0 | 0 | 0 (−0.23, 0.12) | 0 |
| Nonvertebral | 1 (3.6) | 0 | 0 (−0.23, 0.12) | 4 (9.8) |
| Nonvertebral (Fragility) | 1 (3.6) | 0 | 0 (−0.23, 0.12) | 2 (4.9) |
| Study Emergent Adverse Events | ||||
| Serious Adverse Events | ||||
| Any | 0 | 1 (7.7) | 0.08 (−0.06, 0.33) | 0 |
| Possibly related to treatment | 0 | 0 | 0 (−0.23, 0.12) | 0 |
| Gastrointestinal | ||||
| Nausea | 6 (21.4) | 3 (23.1) | 0.02 (−0.22, 0.31) | 12 (29.3) |
| Vomiting | 1 (3.6) | 1 (7.7) | 0.04 (−0.11, 0.30) | 6 (14.6) |
| Constipation | 8 (28.6) | 4 (30.8) | 0.02 (−0.24, 0.32) | 17 (41.5) |
| Loss of appetite | 3 (10.7) | 5 (38.5) | 0.28 (0.01, 0.55) | 7 (17.1) |
| Diarrhea | 1 (3.6) | 2 (15.4) | 0.006 (−0.16, 0.27) | 5 (12.2) |
| Stomach upset | 4 (14.3) | 1 (7.7) | 0.07 (−0.20, 0.25) | 10 (24.4) |
| Musculoskeletal | ||||
| Muscle weakness | 7 (25.0) | 3 (23.1) | 0.02 (−0.28, 0.26) | 11 (26.8) |
| Leg cramps | 6 (21.4) | 4 (30.8) | 0.09 (−0.16, 0.38) | 9 (22.0) |
| Pain | 19 (67.9) | 8 (61.5) | 0.06 (−0.21, 0.36) | 34 (82.9) |
| Myalgia | 4 (14.3) | 1 (7.7) | 0.07 (−0.20, 0.25) | 8 (19.5) |
| Nervous system | ||||
| Dizziness | 9 (32.1) | 1 (7.7) | 0.24 (−0.05, 0.44) | 12 (29.3) |
| Headache | 13 (46.4) | 5 (38.5) | 0.08 (−0.23, 0.35) | 29 (70.7) |
| Lightheadedness | 13 (46.4) | 3 (23.1) | 0.23 (−0.09, 0.47) | 20 (48.8) |
| Miscellaneous | ||||
| Fatigue | 11 (39.3) | 5 (38.5) | 0.008 (−0.30, 0.29) | 23 (56.1) |
| Fever | 1 (3.6) | 3 (23.1) | 0.20 (−0.01, 0.47) | 2 (4.9) |
| Dyspnea | 2 (7.1) | 0 (0) | 0.07 (−0.16, 0.23) | 4 (9.8) |
| Fast heartbeat | 8 (28.6) | 1 (7.7) | 0.21 (−0.08, 0.40) | 14 (34.1) |
| Low energy | 12 (42.9) | 3 (23.1) | 0.20 (−0.12, 0.43) | 22 (53.7) |
| Sore throat | 4 (14.3) | 2 (15.4) | 0.01 (−0.19, 0.29) | 10 (24.4) |
| Anxiety | 8 (28.6) | 3 (23.1) | 0.05 (−0.25, 0.29) | 19 (46.3) |
Adverse events were assessed at each visit by interview (Table 6). Teriparatide was well-tolerated. During the first 6 months, 1 participant on placebo was admitted for an illness unrelated to osteoporosis or study drug. One participant on teriparatide had an episode of hypercalcemia (calcium >10.2 mg/dL) and another had an episode of hypercalciuria (>300 mg/g creatinine) that resolved after per protocol reduction of calcium supplements. Injection site reactions and vomiting were reported by more in the teriparatide than the placebo arm (both P < 0.001). During the 18 to 24 months of teriparatide that followed, there were no serious adverse events. One additional participant had an episode of hypercalciuria that resolved after lowering calcium supplements. Three had nephrolithiasis; 1 woman with a history of kidney stones developed 2 new stones and was withdrawn after 18 months of teriparatide. Two patients without stones at baseline had asymptomatic incident stones (3.0 mm and 2.2 mm) documented on 24-month computed tomography (CT) scans. More than 50% reported redness and bruising at the injection site, musculoskeletal pain, headache, fatigue, and low energy.
During the 6-month RCT, compliance was greater than 90% and did not differ between groups. In the 24-month open-label trial, compliance averaged 95% at 6 months, 93% at 12 months, and 89% at 24 months.
Discussion
Teriparatide was associated with marked improvements in spine and hip aBMD in the majority of premenopausal women with IOP. Compared to placebo, quadruple tetracycline-labeled transiliac bone biopsies acquired after 3 months of teriparatide revealed significant increases in BFR at cancellous and endocortical surfaces, reflected biochemically by significant increases in formation markers. At 6 months, there was a significant increase in LS aBMD compared to placebo, with no significant differences at the hip and forearm. Treatment for 24 months was associated with large increases in LS aBMD, significant increases in FN and TH aBMD, and a small but significant decrease in DR aBMD. Spine trabecular microarchitecture, assessed by TBS, also improved significantly. Serum P1NP, OCN, and CTx increased significantly by 3 months, remained elevated during the first year and, although they declined during the second year, remained significantly above baseline at 24 months. Despite being adherent to teriparatide, 7 women (18%) did not respond.
The effects of teriparatide on aBMD in premenopausal women with IOP are consistent with its effects in more common forms of osteoporosis. Teriparatide increases aBMD and reduces incident vertebral and nonvertebral fractures in postmenopausal women (24) and has also been shown to increase aBMD in hypogonadal men (25) and men with IOP (9, 25). Teriparatide also increases aBMD and reduces fractures in glucocorticoid-induced osteoporosis (8, 26, 27). TBS predicts fracture risk (23) and correlates with bone strength parameters in premenopausal women and men with IOP (28). The effects of teriparatide on TBS in this study are consistent with other populations. The 2.0% and 3.4% increases we documented after 12 and 24 months of teriparatide were comparable to the 2.7% to 4.3% increases observed with teriparatide (29-31) or abaloparatide (32) in postmenopausal women.
There are few studies of teriparatide in premenopausal women. Parathyroid hormone(1-34) maintains aBMD in those receiving gonadotrophin-releasing hormone agonists (33) and increases aBMD to a greater extent than alendronate in those on glucocorticoids (26). Teriparatide increases bone formation and density in adult women with anorexia nervosa (34) and in adults with osteogenesis imperfecta (35). A retrospective cohort study of 32 women with pregnancy- and lactation-associated osteoporosis reported a significantly greater increase in LS aBMD in women who took teriparatide than those who declined therapy (36).
The results of this study are consistent with our pilot study of 21 premenopausal women with IOP in many respects (11). The 13.2% mean increase in LS aBMD was slightly higher than the 10.8% increase previously seen. The 5.2% and 5.0% increases at the TH and FN were comparable, though slightly lower than the 6.2% and 7.6% previously observed. Formerly, DR aBMD decreased transiently, while this study found a linear 2% decrease by 24 months. The pattern of change and degree of rise in serum P1NP and CTx were similar but in the current study, BTMs did not return to baseline by 24 months.
The 18% nonresponse to teriparatide was similar, but the characteristics of nonresponders differed between our studies. In this study, nonresponders had significantly higher BMI, whereas in the previous study, BMI was slightly higher in nonresponders but not statistically significant (24.5 ± 4.5 vs 21.5 ± 2.8 kg/m2; P = 0.1). In postmenopausal women, higher weight and BMI have been associated with nonresponse to teriparatide (37). In our previous study, baseline cancellous BFR was 80% lower in nonresponders, a statistically significant difference. In addition, baseline OCN and CTx levels were lower, P1NP increase was blunted and delayed and serum IGF-1 levels were higher, leading us to conclude that the nonresponders might be resistant to teriparatide’s osteoanabolic actions at the osteoblast level. In the current study, although baseline BFR was 58% lower at the cancellous and 68% lower at the endocortical surface, the differences were not statistically significant, serum IGF1 and BTMs did not differ at baseline or 3 months. Nonresponse to teriparatide was therefore not clearly related to low bone formation at baseline. All women enrolled were normally menstruating/eugonadal. Thus, it is possible that the absence of the high bone remodeling state associated with menopause led to a higher nonresponse rate than has been seen in postmenopausal teriparatide-treated populations. Of the 7 women who were compliant but did not respond to teriparatide, only 3 were randomized to teriparatide-first and biopsied on teriparatide, so there are insufficient data on whether they differed by BFR at 3 months or by change between baseline and 3 months. Compliance did not differ between responders and nonresponders in either study. The reasons for the differences between studies are unclear. However, it is reassuring that women with low bone formation in our study did respond to an osteoanabolic drug like teriparatide.
In postmenopausal women and men, BMD effects of teriparatide dissipate after discontinuation unless followed by antiresorptive therapy and therefore subsequent consolidation therapy is required after osteoanabolic drugs and denosumab. In postmenopausal women, estrogen prevents bone loss after discontinuing teriparatide (38, 39). Endogenous estrogen may prevent bone loss in premenopausal women who resume menstruating after discontinuing gonadotropin-releasing hormone agonists and parathyroid hormone(1-34) (40). It is therefore possible that premenopausal women with IOP and normal menses might have sufficient endogenous estrogen to prevent post-teriparatide bone loss. However, in 15 premenopausal women evaluated 2 years after completing teriparatide, we observed significant losses (4.8%) in LS aBMD, although declines at the TH and FN were small and not significant (−1.1% and −1.4%, respectively) (41). Those with significant bone loss were older (46 versus 38 years), had had larger increases in aBMD on teriparatide and higher cancellous BFR on transiliac biopsies performed before and after 18 months of therapy. Unfortunately, at the most relevant time for clinical decision making, namely completion of teriparatide, serum BTMs did not differ between those who lost or maintained bone mass. Thus, we conclude that a treatment plan to initiate teriparatide in a young woman with severe osteoporosis should include management after teriparatide is completed, particularly in older premenopausal women who may have declining endogenous estrogen production. Possible interventions to prevent bone loss could include estrogen (if estrogen-deficient), bisphosphonates (if pregnancy is not being considered), or denosumab, although the same need for consolidation therapy applies after stopping denosumab (42-47). In this regard, after completing teriparatide, the majority of our study participants enrolled in a study of denosumab, which will be completed in the next year.
In managing premenopausal osteoporosis, it is important to evaluate for secondary causes of bone loss and treat specifically. Conservative therapy focusing on optimizing calcium and caloric intake, vitamin D status, and exercise, and avoiding skeletal toxins, such as tobacco and excess alcohol, should be the first step. Pharmacologic therapy should be reserved for women with major fractures, severe bone loss or progressive bone loss, and/or fractures despite conservative therapy. Although this was true for all our participants, our research has shown that premenopausal women with IOP have comparable and severe deficits in bone microarchitecture and strength, whether they have had fractures or low BMD by DXA (3-5). Several issues should be considered when contemplating drug therapy. First, evidence that drug therapy reduces fracture incidence in premenopausal women with either idiopathic (primary) or secondary osteoporosis is lacking. Moreover, fracture data are unlikely to be available in the near future because premenopausal osteoporosis is so uncommon and adequately powered studies would need to be very large, and therefore extremely expensive. Therefore, at the present time, treatment decisions must be based on demonstrated efficacy in other populations, which may or may not be generalizable to premenopausal women. Second, a substantial proportion of premenopausal women with IOP have extremely low bone formation (1, 11, 48). Thus, sequential or combined approaches with an osteoanabolic drug to stimulate bone formation and improve microarchitecture, followed by antiresorptive therapy to consolidate and maintain aBMD may be more desirable than starting with antiresorptives that would further depress bone remodeling. Recent randomized trials and comparative studies between anabolic and antiresorptive drugs have led to increasing attention to sequential or combined approaches for postmenopausal osteoporosis, particularly for women with recent clinical or radiographic vertebral fractures, multiple fractures, and those starting with very low BMD (49-52). Two sequential anabolic/antiresorptive regimens, abaloparatide and alendronate, and romosozumab and denosumab, reduce incident fractures in postmenopausal osteoporosis (53, 54). Third, a substantial number of premenopausal women did not respond to teriparatide with an increase in BMD in either of our studies for reasons that are unclear and difficult to predict before starting therapy. However, it is not clear that measurement of P1NP or OCN after 3 months of teriparatide would be helpful to identify women who are not responding optimally before the 1-year point is reached.
Although injection site reactions were common, teriparatide was generally well-tolerated. The few isolated episodes of mild hypercalcemia and hypercalciuria resolved after calcium supplements were reduced. However, 3 women developed new renal stones. In 2, asymptomatic stones were detected on per protocol CT imaging at 24 months; these stones were not visible on baseline CT imaging. The third had a previous history of nephrolithiasis, developed symptoms and withdrew after 18 months of teriparatide. In contrast, 4 women with a prior history of nephrolithiasis remained stone-free. There were no increases in serum uric acid. Although we excluded women with urinary calcium excretion above 300 mg/gCr, it is possible that levels below that cutpoint might have contributed to the higher than expected number with nephrolithiasis. The higher physiologic calcium absorption in estrogen-replete premenopausal women may also predispose them to hypercalciuria while taking teriparatide (55). If teriparatide is considered for a premenopausal woman with a history of nephrolithiasis, care should be taken to avoid hypercalciuria.
This study has several strengths. The combination of the 6-month comparison of teriparatide and placebo and the 24-month extension on teriparatide for all participants provide critical efficacy data in an understudied population of young women with IOP. Inclusion of an untreated control group for the initial 6 months and the quadruple tetracycline-labeled transiliac bone biopsy also established that those randomized to teriparatide experienced highly significant increases in tissue-based BFR and serum formation markers. Mechanistically, serum P1NP and osteocalcin at 3 months, and the absolute change in serum P1NP and OCN from baseline to 3 months, predicted response to teriparatide. Endocortical BFR at 3 months and the absolute change in endocortical BFR between baseline and 3 months were the best biopsy predictors.
This study also has limitations. All our patients were severely affected with either multiple fractures or had extremely low BMD or both, and in our pilot study, teriparatide increased aBMD. Therefore, a study that randomized women to active drug or placebo for 24 months would have been unethical and few women would have agreed to enroll. Because IOP in premenopausal women is very uncommon, we could not evaluate the effect of teriparatide on fracture incidence, which is critical to acceptance of any therapeutic intervention for osteoporosis. Although compliance was excellent, it is possible that nonresponders were less compliant than responders. The error in the randomization table and subsequent adaptive randomization could have impacted blinding of study personnel.
In summary, this study confirms that teriparatide is associated with marked improvements in spine and hip bone density in the majority of premenopausal women with IOP. Quadruple tetracycline-labeled transiliac biopsies demonstrated that teriparatide caused large increases in tissue-level bone formation at cancellous and endocortical surfaces that were associated with significant increases in aBMD at 12 and 24 months. Spine trabecular microarchitecture by TBS also improved. The 18% rate of nonresponse to teriparatide was comparable to the 19.5% rate previously observed, although not clearly associated with low pretreatment bone formation. Under ideal circumstances, larger studies that determine whether teriparatide or newly available biosimilars reduce fracture incidence in premenopausal IOP would be a next step. In the meantime, however, these data are encouraging and support the use of teriparatide in premenopausal women with severe osteoporosis.
Acknowledgments
We thank the members of the Data and Safety Monitoring Committee: Dr. Mishaela Rubin (CUIMC, Division of Endocrinology), Dr. Judith Korner (CUIMC, Division of Endocrinology), Emilia Bagiella, PhD (Director, The Center for Biostatistics at the Icahn School of Medicine at Mount Sinai), and the study’s medical monitor, Dr. Shonni Silverberg (CUIMC, Division of Endocrinology)
Financial Support: The study was supported by the United States Food and Drug Administration (FDA) Orphan Products Clinical Trials Grants Program (R01 FD003902). Eli Lilly, USA, supplied teriparatide and identical placebo.
Clinical Trial Information: Trial Registration ClinicalTrials.gov No. NCT01440803.
Glossary
Abbreviations
- 25-OHD
25-hydroxyvitamin D
- aBMD
areal bone mineral density
- ANCOVA
analysis of covariance
- BFR
bone formation rate
- BMD
bone mineral density
- BTM
bone turnover marker
- CI
confidence interval
- CT
computed tomography
- CTx
C-telopeptide
- DR
distal radius
- DXA
dual-energy x-ray absorptiometry
- FDA
United States Food and Drug Administration
- FN
femoral neck
- IGF-1
insulin-like growth factor 1
- IOP
idiopathic osteoporosis
- IRB
Institutional Review Board
- LS
lumbar spine
- M
month
- MRI
magnetic resonance imaging
- OCN
osteocalcin
- P1NP
N-terminal propeptides of procollagen type 1
- RCT
randomized controlled trial
- TBS
trabecular bone score
- TH
total hip
- VFA
vertebral fracture assessment
Contributor Information
Adi Cohen, Department of Medicine, Columbia University College of Physicians & Surgeons, New York, New York.
Stephanie Shiau, Department of Epidemiology, Columbia University, Mailman School of Public Health, New York, New York.
Nandini Nair, Department of Medicine, Columbia University College of Physicians & Surgeons, New York, New York.
Robert R Recker, Department of Medicine, Creighton University Medical Center, Omaha, Nebraska.
Joan M Lappe, Department of Medicine, Creighton University Medical Center, Omaha, Nebraska.
David W Dempster, Department of Pathology and Cell Biology, Columbia University College of Physicians & Surgeons, New York, New York; Regional Bone Center, Helen Hayes Hospital, West Haverstraw, New York.
Thomas L Nickolas, Department of Medicine, Columbia University College of Physicians & Surgeons, New York, New York.
Hua Zhou, Regional Bone Center, Helen Hayes Hospital, West Haverstraw, New York.
Sanchita Agarwal, Department of Medicine, Columbia University College of Physicians & Surgeons, New York, New York.
Mafo Kamanda-Kosseh, Department of Medicine, Columbia University College of Physicians & Surgeons, New York, New York.
Mariana Bucovsky, Department of Medicine, Columbia University College of Physicians & Surgeons, New York, New York.
John M Williams, Department of Medicine, Columbia University College of Physicians & Surgeons, New York, New York.
Donald J McMahon, Department of Medicine, Columbia University College of Physicians & Surgeons, New York, New York.
Julie Stubby, Department of Medicine, Creighton University Medical Center, Omaha, Nebraska.
Elizabeth Shane, Department of Medicine, Columbia University College of Physicians & Surgeons, New York, New York.
Additional Information
Disclosure Summary: A.C., E.S., R.R.R. and J.M.L. receive research support from Amgen and Eli Lilly. D.W.D. receives research support and consulting fees from Amgen, Eli Lilly and Radius Health.
Data Availability
The datasets generated during and/or analyzed during the current study are not publicly available but are available from the corresponding author on reasonable request.
Literature Cited
- 1. Cohen A, Recker RR, Lappe J, et al. Premenopausal women with idiopathic low-trauma fractures and/or low bone mineral density. Osteoporos Int. 2012;23(1):171-182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Cohen A, Fleischer J, Freeby MJ, McMahon DJ, Irani D, Shane E. Clinical characteristics and medication use among premenopausal women with osteoporosis and low BMD: the experience of an osteoporosis referral center. J Womens Health. 2009;18(1):79-84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Cohen A, Dempster DW, Recker RR, et al. Abnormal bone microarchitecture and evidence of osteoblast dysfunction in premenopausal women with idiopathic osteoporosis. J Clin Endocrinol Metab. 2011;96(10):3095-3105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Cohen A, Lang TF, McMahon DJ, et al. Central QCT reveals lower volumetric BMD and stiffness in premenopausal women with idiopathic osteoporosis, regardless of fracture history. J Clin Endocrinol Metab. 2012;97(11):4244-4252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Cohen A, Liu XS, Stein EM, et al. Bone microarchitecture and stiffness in premenopausal women with idiopathic osteoporosis. J Clin Endocrinol Metab. 2009;94(11):4351-4360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Kraenzlin ME, Meier C. Parathyroid hormone analogues in the treatment of osteoporosis. Nat Rev Endocrinol. 2011;7(11):647-656. [DOI] [PubMed] [Google Scholar]
- 7. Grossman JM, Gordon R, Ranganath VK, et al. American College of Rheumatology 2010 recommendations for the prevention and treatment of glucocorticoid-induced osteoporosis. Arthritis Care Res. 2010;62(11):1515-1526. [DOI] [PubMed] [Google Scholar]
- 8. Saag KG, Shane E, Boonen S, et al. Teriparatide or alendronate in glucocorticoid-induced osteoporosis. N Engl J Med. 2007;357(20):2028-2039. [DOI] [PubMed] [Google Scholar]
- 9. Kurland ES, Cosman F, McMahon DJ, Rosen CJ, Lindsay R, Bilezikian JP. Parathyroid hormone as a therapy for idiopathic osteoporosis in men: effects on bone mineral density and bone markers. J Clin Endocrinol Metab. 2000;85(9):3069-3076. [DOI] [PubMed] [Google Scholar]
- 10. Orwoll ES, Scheele WH, Paul S, et al. The effect of teriparatide [human parathyroid hormone (1-34)] therapy on bone density in men with osteoporosis. J Bone Miner Res. 2003;18(1):9-17. [DOI] [PubMed] [Google Scholar]
- 11. Cohen A, Stein EM, Recker RR, et al. Teriparatide for idiopathic osteoporosis in premenopausal women: a pilot study. J Clin Endocrinol Metab. 2013;98(5):1971-1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Nishiyama K, Wang J, Young P, et al. Teriparatide is associated with improved microarchitecture and estimated bone strength in premenopausal women with idiopathic osteoporosis: an HR-pQCT study. Presented at: American Society for Bone and Mineral Research Annual Meeting; October 4-7, 2013; Baltimore, MD. [Google Scholar]
- 13. Lewiecki EM, Laster AJ. Clinical review: Clinical applications of vertebral fracture assessment by dual-energy x-ray absorptiometry. J Clin Endocrinol Metab. 2006;91(11):4215-4222. [DOI] [PubMed] [Google Scholar]
- 14. Lindsay R, Cosman F, Zhou H, et al. A novel tetracycline labeling schedule for longitudinal evaluation of the short-term effects of anabolic therapy with a single iliac crest bone biopsy: Early actions of teriparatide. J Bone Miner Res. 2006;21(3):366-373. [DOI] [PubMed] [Google Scholar]
- 15. Rubin MR, Dempster DW, SlineyJ, Jr., et al. PTH(1–84) administration reverses abnormal bone-remodeling dynamics and structure in hypoparathyroidism. J Bone Miner Res. 2011;26:2727-2736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Dempster DW. The contribution of trabecular architecture to cancellous bone quality. J Bone Miner Res. 2000;15(1):20-23. [DOI] [PubMed] [Google Scholar]
- 17. Dempster DW, Shane E. Bone quantification and dynamics of bone turnover. In: Becker KL, ed. Principles and Practice of Endocrinology and Metabolism. Philadelphia, PA: J.B. Lippincott Co; 2002:475-479. [Google Scholar]
- 18. Parfitt AM, Drezner MK, Glorieux FH, et al. Bone histomorphometry: standardization of nomenclature, symbols, and units. Report of the ASBMR Histomorphometry Nomenclature Committee. J Bone Miner Res. 1987;2(6):595-610. [DOI] [PubMed] [Google Scholar]
- 19. Dempster DW, Compston JE, Drezner MK, et al. Standardized nomenclature, symbols, and units for bone histomorphometry: A 2012 update of the report of the ASBMR Histomorphometry Nomenclature Committee. J Bone Miner Res. 2013;28(1):2-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Hans D, Barthe N, Boutroy S, Pothuaud L, Winzenrieth R, Krieg MA. Correlations between trabecular bone score, measured using anteroposterior dual-energy X-ray absorptiometry acquisition, and 3-dimensional parameters of bone microarchitecture: an experimental study on human cadaver vertebrae. J Clin Densitom. 2011;14(3):302-312. [DOI] [PubMed] [Google Scholar]
- 21. Silva BC, Leslie WD, Resch H, et al. Trabecular bone score: a noninvasive analytical method based upon the DXA image. J Bone Miner Res. 2014;29(3):518-530. [DOI] [PubMed] [Google Scholar]
- 22. Krohn K, Schwartz EN, Chung YS, Lewiecki EM. Dual-energy X-ray absorptiometry monitoring with trabecular bone score: 2019 ISCD official position. J Clin Densitom. 2019;22(4):501-505. [DOI] [PubMed] [Google Scholar]
- 23. McCloskey EV, Odén A, Harvey NC, et al. A meta-analysis of trabecular bone score in fracture risk prediction and its relationship to FRAX. J Bone Miner Res. 2016;31(5):940-948. [DOI] [PubMed] [Google Scholar]
- 24. Neer RM, Arnaud CD, Zanchetta JR, et al. Effect of parathyroid hormone (1-34) on fractures and bone mineral density in postmenopausal women with osteoporosis. N Engl J Med. 2001;344(19):1434-1441. [DOI] [PubMed] [Google Scholar]
- 25. Orwoll E, Teglbjærg CS, Langdahl BL, et al. A randomized, placebo-controlled study of the effects of denosumab for the treatment of men with low bone mineral density. J Clin Endocrinol Metab. 2012;97(9):3161-3169. [DOI] [PubMed] [Google Scholar]
- 26. Langdahl BL, Marin F, Shane E, et al. Teriparatide versus alendronate for treating glucocorticoid-induced osteoporosis: an analysis by gender and menopausal status. Osteoporos Int. 2009;20(12):2095-2104. [DOI] [PubMed] [Google Scholar]
- 27. Langdahl BL, Silverman S, Fujiwara S, et al. Real-world effectiveness of teriparatide on fracture reduction in patients with osteoporosis and comorbidities or risk factors for fractures: Integrated analysis of 4 prospective observational studies. Bone. 2018;116:58-66. [DOI] [PubMed] [Google Scholar]
- 28. Muschitz C, Kocijan R, Haschka J, et al. TBS reflects trabecular microarchitecture in premenopausal women and men with idiopathic osteoporosis and low-traumatic fractures. Bone. 2015;79:259-266. [DOI] [PubMed] [Google Scholar]
- 29. Saag KG, Agnusdei D, Hans D, et al. Trabecular bone score in patients with chronic glucocorticoid therapy-induced osteoporosis treated with alendronate or teriparatide. Arthritis Rheumatol. 2016;68(9):2122-2128. [DOI] [PubMed] [Google Scholar]
- 30. Senn C, Günther B, Popp AW, Perrelet R, Hans D, Lippuner K. Comparative effects of teriparatide and ibandronate on spine bone mineral density (BMD) and microarchitecture (TBS) in postmenopausal women with osteoporosis: a 2-year open-label study. Osteoporos Int. 2014;25(7):1945-1951. [DOI] [PubMed] [Google Scholar]
- 31. Tsai JN, Jiang LA, Lee H, Hans D, Leder BZ. Effects of teriparatide, denosumab, or both on spine trabecular microarchitecture in DATA-switch: a randomized controlled trial. J Clin Densitom. 2017;20(4):507-512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Bilezikian JP, Hattersley G, Fitzpatrick LA, et al. Abaloparatide-SC improves trabecular microarchitecture as assessed by trabecular bone score (TBS): A 24-week randomized clinical trial. Osteoporos Int. 2018;29(2):323-328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Finkelstein JS, Klibanski A, Arnold AL, Toth TL, Hornstein MD, Neer RM. Prevention of estrogen deficiency-related bone loss with human parathyroid hormone-(1-34): A randomized controlled trial. JAMA. 1998;280(12):1067-1073. [DOI] [PubMed] [Google Scholar]
- 34. Fazeli PK, Wang IS, Miller KK, et al. Teriparatide increases bone formation and bone mineral density in adult women with anorexia nervosa. J Clin Endocrinol Metab. 2014;99(4):1322-1329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Orwoll ES, Shapiro J, Veith S, et al. Evaluation of teriparatide treatment in adults with osteogenesis imperfecta. J Clin Invest. 2014;124(2):491-498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Hong N, Kim JE, Lee SJ, Kim SH, Rhee Y. Changes in bone mineral density and bone turnover markers during treatment with teriparatide in pregnancy- and lactation-associated osteoporosis. Clin Endocrinol. 2018;88(5):652-658. [DOI] [PubMed] [Google Scholar]
- 37. Heaney RP, Watson P. Variability in the measured response of bone to teriparatide. Osteoporos Int. 2011;22(6):1703-1708. [DOI] [PubMed] [Google Scholar]
- 38. Cosman F, Nieves J, Woelfert L, et al. Parathyroid hormone added to established hormone therapy: Effects on vertebral fracture and maintenance of bone mass after parathyroid hormone withdrawal. J Bone Miner Res. 2001;16(5):925-931. [DOI] [PubMed] [Google Scholar]
- 39. Lane NE, Sanchez S, Modin GW, Genant HK, Pierini E, Arnaud CD. Bone mass continues to increase at the hip after parathyroid hormone treatment is discontinued in glucocorticoid-induced osteoporosis: results of a randomized controlled clinical trial. J Bone Miner Res. 2000;15(5):944-951. [DOI] [PubMed] [Google Scholar]
- 40. Finkelstein JS, Arnold AL. Increases in bone mineral density after discontinuation of daily human parathyroid hormone and gonadotropin-releasing hormone analog administration in women with endometriosis. J Clin Endocrinol Metab. 1999;84(4):1214-1219. [DOI] [PubMed] [Google Scholar]
- 41. Cohen A, Kamanda-Kosseh M, Recker RR, et al. Bone density after teriparatide discontinuation in premenopausal idiopathic osteoporosis. J Clin Endocrinol Metab. 2015;100(11):4208-4214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Leder B. An essential warning: editorial on vertebral fractures after discontinuation of denosumab: a post hoc analysis of the randomized placebo-controlled FREEDOM trial, its extension. J Bone Mine Res. 2018;33(2):188-189. [DOI] [PubMed] [Google Scholar]
- 43. Leder BZ, Tsai JN, Jiang LA, Lee H. Importance of prompt antiresorptive therapy in postmenopausal women discontinuing teriparatide or denosumab: The Denosumab and Teriparatide Follow-up study (DATA-Follow-up). Bone. 2017;98:54-58. [DOI] [PubMed] [Google Scholar]
- 44. Leder BZ, Tsai JN, Uihlein AV, et al. Denosumab and teriparatide transitions in postmenopausal osteoporosis (the DATA-Switch study): Extension of a randomised controlled trial. Lancet. 2015;386(9999):1147-1155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. McClung MR, Wagman RB, Miller PD, Wang A, Lewiecki EM. Observations following discontinuation of long-term denosumab therapy. Osteoporos Int. 2017;28(5):1723-1732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Popp AW, Zysset PK, Lippuner K. Rebound-associated vertebral fractures after discontinuation of denosumab-from clinic and biomechanics. Osteoporos Int. 2016;27(5):1917-1921. [DOI] [PubMed] [Google Scholar]
- 47. Reid IR, Horne AM, Mihov B, Gamble GD. Bone loss after denosumab: Only partial protection with zoledronate. Calcif Tissue Int. 2017;101(4):371-374. [DOI] [PubMed] [Google Scholar]
- 48. Donovan MA, Dempster D, Zhou H, McMahon DJ, Fleischer J, Shane E. Low bone formation in premenopausal women with idiopathic osteoporosis. J Clin Endocrinol Metab. 2005;90(6):3331-3336. [DOI] [PubMed] [Google Scholar]
- 49. Cappola AR, Shoback DM. Osteoporosis therapy in postmenopausal women with high risk of fracture. JAMA. 2016;316(7):715-716. [DOI] [PubMed] [Google Scholar]
- 50. Cheng C, Wentworth K, Shoback DM. New frontiers in osteoporosis therapy. Annu Rev Med. 2020;71:277-288. [DOI] [PubMed] [Google Scholar]
- 51. Cosman F. The evolving role of anabolic therapy in the treatment of osteoporosis. Curr Opin Rheumatol. 2019;31(4):376-380. [DOI] [PubMed] [Google Scholar]
- 52. Leder BZ. Optimizing sequential and combined anabolic and antiresorptive osteoporosis therapy. JBMR Plus. 2018;2(2):62-68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Bone HG, Cosman F, Miller PD, et al. ACTIVExtend: 24 months of alendronate after 18 months of abaloparatide or placebo for postmenopausal osteoporosis. J Clin Endocrinol Metab. 2018;103(8):2949-2957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Cosman F, Crittenden DB, Ferrari S, et al. FRAME study: the foundation effect of building bone with 1 year of romosozumab leads to continued lower fracture risk after transition to denosumab. J Bone Miner Res. 2018;33(7):1219-1226. [DOI] [PubMed] [Google Scholar]
- 55. Heaney RP, Recker RR, Stegman MR, Moy AJ. Calcium absorption in women: relationships to calcium intake, estrogen status, and age. J Bone Miner Res. 1989;4(4):469-475. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets generated during and/or analyzed during the current study are not publicly available but are available from the corresponding author on reasonable request.