Abstract
Background: iron and calcium dysmetabolism, with hyperferritinemia, hypoferremia, hypocalcemia and anemia have been documented in the majority of COVID-19 patients at later/worse stages. Furthermore, complementary to ACE2, both sialic acid (SA) molecules and CD147 proved relevant host receptors for SARS-CoV-2 entry, which explains the viral attack to multiple types of cells, including erythrocytes, endothelium and neural tissue. Several authors advocated that cell ferroptosis may be the core and final cell degenerative mechanism.
Methods: a literature research was performed in several scientific search engines, such as PubMed Central, Cochrane Library, Chemical Abstract Service. More than 500 articles were retrieved until mid-December 2021, to highlight the available evidence about the investigated issues.
Results: based on COVID-19 literature data, we have highlighted a few pathophysiological mechanisms, associated with virus-based cation dysmetabolism, multi-organ attack, mitochondria degeneration and ferroptosis. Our suggested elucidated pathological sequence is: a) spike protein subunit S1 docking with sialylated membrane glycoproteins/receptors (ACE2, CD147), and S2 subunit fusion with the lipid layer; b) cell membrane morpho-functional changes due to the consequent electro-chemical variations and viroporin action, which induce an altered ion channel function and intracellular cation accumulation; c) additional intracellular iron concentration due to a deregulated hepcidin-ferroportin axis, with higher hepcidin levels. Viral invasion may also affect erythrocytes/erythroid precursors, endothelial cells and macrophages, through SA and CD147 receptors, with relative hemoglobin and iron/calcium dysmetabolism. AB0 blood group, hemochromatosis, or environmental elements may represent possible factors which affect individual susceptibility to COVID-19.
Conclusions: our literature analysis confirms the combined role of SA molecules, ACE2, CD147, viroporins and hepcidin in determining the cation dysmetabolism and final ferroptosis in the cells infected by SARS-CoV-2. The altered ion channels and electrochemical gradients of the cell membrane have a pivotal role in the virus entry and cell dysmetabolism, with subsequent multi-organ immune-inflammatory degeneration and erythrocyte/hemoglobin alterations.
Keywords: ferroptosis; cations; sialic acid; iron; ferritin; calcium; viroporins; voltage-gated calcium channels; cell membrane; CD147; ACE2; hepcidin; red blood cells; hemoglobin; mitochondria
1. Introduction
The present narrative review aims at providing a summary of the available evidence about a few peculiar aspects of COVID-19 pathophysiology, regarding: a) alterations of cation (iron and calcium mainly) metabolism, b) the role of sialic acid (SA) molecules, CD147, ACE2 and of the ion-channeling components, such as voltage-gated calcium channels (VGCC) and viroporins, in the morpho-functional chemical/electric alterations of the cell membrane caused by the viral attack, c) the contribution of hepcidin, red blood cell (RBC) and hemoglobin to the cation dysmetabolism, d) the consequent intracellular degeneration, ultimately resulting in both a mitochondrial degeneration and the ferroptosis process.
Our literature search addressed the published articles related to COVID-19, which reported data and elaborations concerning the pathophysiological elements described above.
We performed literature research retrieving and reviewing pertinent articles and documents from the following web-based scientific search engines: MEDLINE, PubMed Central, Cochrane Library, Google Scholar, ChemRxiv, MedRxiv, BioRxiv, Preprints, ResearchGate, Chemical Abstract Service, Genetic Home References and Human Metabolome Database. The words COVID-19 and/or SARS-CoV-2 were combined with the following headings and keywords: ferroptosis, iron, ferritin, transferrin, hepcidin, ferroportin, calcium, potassium, sodium, magnesium, hemoglobin, heme, hematology, erythrocyte, red blood cells (RBC), erythroblast, RDW, LDH, hemochromatosis, chelation, mitochondria, lactate, CD147, sialic acid (SA), VGCC, ion channels, viroporins.
We investigated the biomedical literature since January 2020 through mid-December 2021, and moreover we reviewed the earlier reference articles where basic concepts and data were available for the main topics covered in this review ( e.g., ferroptosis, iron, ferritin, SA, CD147, viroporins).
Nearly 500 articles were collected and reviewed, in order to extrapolate pertinent data and speculations concerning those clinical and instrumental (mainly laboratory biochemistry and computational biology) findings which pertain the investigated key-words.
2. Literature-based background
COVID-19 seems to be an extremely complex, variable systemic disease which has spread as pandemic worldwide, generating dramatic health and socio-economic problems. The responsible coronavirus, SARS-CoV-2, shares some of the pathomechanisms with the previous SARS-CoV-1 virus, but COVID-19 features peculiar clinical and instrumental findings.
A series of pathophysiology mechanisms have been described in these patients, mostly based on virus-induced immune-inflammatory pathways and on lung pathological consequences. In fact, a high body of evidence has been published about the multi-organ viral attack, which is not solely based on the original immune degeneration induced by the virus. Beyond the conventional pro-inflammatory pathways, other basic and possible pathomechanisms have been proposed in the early literature about COVID-19, 1 based on the preliminary scientific data.
Several authors have highlighted that iron dysmetabolism (and probable blood components denaturation), together with cell membrane morpho-functional deregulation, represent specific early biochemical phenomena in this infection. 1 – 6
Hyperferritinemia, low serum iron and low total iron binding capacity (TIBC) have been definitely linked to COVID-19 severity, and these elements have been considered relevant prognostic factors. 1 , 7 – 14 Similarly, a series of possible treatments aimed at controlling iron dysmetabolism have been proposed. 15 – 19 Together with these biochemical changes, a number of RBC alterations have been described, both in terms of hemoglobin decrease/denaturation, and in terms of RBC altered morphology/functionality. 1 , 20 – 23
Another well-documented cation dysmetabolism, especially in later stages is represented by lower serum calcium levels, which is equally a proven negative prognostic factor in COVID-19 patients 24 – 32 ; additionally, early autopsies also highlighted a higher representation of calcium deposits in many tissues. 33
Regulation of transmembrane pumps and calcium influx/efflux is generally of great importance in many viral infections. 34 Furthermore, past literature data has proven how excessive intracellular calcium does contribute to mitochondria dysfunction and to ferroptosis degeneration. 35
In view of the clinical relevance of excessive intracellular calcium, the use of calcium channel blockers has been repeatedly suggested in the patients affected by COVID-19. 36 – 41
In confirmation of the possible value of these drugs, a recently published retrospective cohort study among 4569 hospitalized hypertensive patients with COVID-19 showed a dramatic reduction of mortality in patients treated with calcium channel blockers (risk ratio [RR]: 0.32, 95% confidence interval [CI]: 0.13-0.76, P = 0.0058). 42
Decreased calcium levels in blood, in absence of a documented hypercalciuria, indicate a detrimental increased concentration of this cation inside the cells. The movement of this mineral across cellular membranes is highly regulated through the VGCC, which have an important role also in multiple pathological clinical conditions involving muscular, neural, cardiac and endothelial cells, just to name a few. 43 – 48
Interestingly, patients with later stages of COVID-19 often show altered serum concentration of other cations, such as sodium, potassium and magnesium. 49 – 52
Pathophysiology of this disease and, more specifically, cation dysmetabolism is linked to a series of viral interactions with human cell membrane receptors.
Beyond the ascertained role of ACE2, several authors have reported the determinant role of another peculiar receptor, namely CD147, in the multi-organ viral attack. 1 , 53 – 57
CD147, also termed Basigin or EMPRIM, is regarded as a relevant SARS-CoV-2 entry port with a specific role in a few tissues. 1 , 53 , 58 However, it is worth mentioning that a minority of literature publications denies a direct role of this receptor in the whole viral process. 59
In fact, CD147 is represented in several human tissues and for example it is widely localized on the erythrocyte membrane (nearly 2000-3000 receptors per cell are described, 60 erythroblasts, endothelial cells, brain etc. 1 ; as it is supposed that SARS-CoV-2 may affect also RBC, the putative role of CD147 is sustainable. 61
A few publications focused on CD147 in COVID-19; in fact, this diffusely distributed cell membrane receptor is a well-known mediator in several diseases, where it exhibits a pleiotropic function. 62 – 64
Cluster of Differentiation 147, or CD147, is a highly glycosylated transmembrane glycoprotein belonging to the immunoglobulin superfamily, compelling for the Ok blood group system. The immunoglobulin superfamily comprehends proteins with at least one Ig domain and is of fundamental importance in intercellular communication. 65
This transmembrane protein is dedicated to recognizing molecules inside the cell (cis-recognition), and extracellularly (trans-recognition). Various isoforms of human CD147 are produced by differential splicing and differences in transcription initiation sites. Isoform-1 has three Ig domains, located in the retina. Isoform-2, the most common, has two Ig domains. 66 Intriguingly, human platelets also express CD147, and a few authors observed a SARS-CoV-2 spike protein-dependent platelet activation and aggregation, which is in fact CD147-mediated. 55
CD147 was also found to contribute as a chaperone of plasma membrane transport of monocarboxylate transporters, hence a few authors postulated the possibility that CD147 might participate in ACE2-virus interaction, by a re-localization to the same compartment of the two receptors. 56
Furthermore, the well-known role of CD147 in inflammatory processes, together with its upregulation during hypoxic states, 1 , 67 may contribute to the deregulated inflammatory pathways of this disease in several organs. More specifically, it was found that the viral interaction with CD147 may lead to some documented detrimental viral effects on endothelium 68 – 70 and on RBC and their precursors. 1 , 21 , 61 , 71 , 72
In addition, it has been observed that CD147 expression levels correlate with SARS-CoV-2 infection extent, vascular damage and an increased expression of vascular endothelial growth factor and thrombosis. 73
Likewise, cardiovascular tissues and endothelial cells express putative genes for SARS-CoV-2 infection, including the ones regarding ACE2 and Basigin/CD147. However, ACE2 decreases with age in some tissues, whereas basigin/CD147 increases with age in endothelial cells, suggesting that the expression of this specific receptor in the vasculature might explain the heightened risk for severe vascular diseases with age. 69 , 74 Interestingly, in most COVID-19 autopsy studies a diffused micro- and macro-vascular thrombosis was shown. 75
Beyond the importance of ACE2 and CD147 receptors, several authors have pointed out how SA role should be re-assessed and emphasized in COVID-19. Early in May 2020, the determinant function of SA in SARS-CoV-2 transmembrane attack was highlighted. 76 More recently, several authors have similarly remarked a series of SA-based interaction pathways between the virus and the host cell. 57 , 77 – 83
Sialic acids are ubiquitous structural polysaccharides that are typically found attached to the N and O terminal positions of the cell membrane glycoproteins. Their structure is unique in the class of glycans: these alpha-keto acids have nine carbon atoms and this helps them to play a critical role in several intrinsic and extrinsic cell interactions. The SA family includes many derivatives of neuraminic acid and currently more than 50 structural variants for SA have been found in nature 84 ; this molecule has also been implicated in various pathophysiological processes such as oncogenesis and microbial pathogenesis. 85 In fact, several viruses attack host cells by binding with particular sialylated glycans, which become cell adhesion molecules, to mediate cell entry. 86
Of great importance, it has been recognized that SA may be expressed on the outer layer of cell membrane as molecules located on specific wall glycoproteins, but also on the surface of ACE-2 and CD147 receptors. 87 Sialo-glycoconjugates expressed on cell surfaces serve also as ligands or receptors for specific intrinsic or extrinsic SA lectins.
Host cell receptors undergo evolutionary alterations to avoid rapidly emerging pathogens while maintaining critical endogenous function. Similarly, most viruses have evolved to express enzymes that can cleave the interactions with these SA receptors, helping to release them from the infected host cells. These sialidases act as decoy receptors, which bind to virions, preventing their access to epithelial cells as well. 85
The presence or absence of an appropriate host SA receptor is a major determinant of the viral host tropism, including the specific host tissue and cell types that viruses can infect. Host receptors are therefore one of the keys to understanding susceptibility to a particular virus and to determining the body systems that are likely to be infected and the type of clinical outcome. 83 , 88 , 89
Blood AB0 group, as well as blood viscosity and erythrocyte shape, are strictly dependent upon the RBC wall negative electrical charge, which is induced mainly by the SA molecules; hence RBC morphology strictly relates to the configuration and density of these negatively charged molecules on their membrane. 90 – 95
With reference to the RBC changes induced by pathogens, it was also demonstrated that several viral glycoproteins may interact with SA on the erythrocytes of various species, resulting in agglutination. 85 , 96
The expression and distribution of these SA-based receptors differ according to their location within the body, cell type, and their functional role. Humans predominantly express SA α2,6-Gal receptors in the ciliated and non-ciliated epithelium of the respiratory tract, but also in blood, gut and neural system. 97 Additionally, age-dependent differences in the distribution of SA receptors have been reported in human subjects. 57
Beyond the cell membrane negative charge, also osmolarity and pH of the intra/extracellular space are significantly influenced by SA concentration and disposition. Hence, SA molecules are being considered of central importance in the SARS-CoV-2/cell docking and consequent infection. 76 , 83 , 89 , 98
Past research showed that electrical and pH changes due to SA deregulation or its interaction with pathogens, proved to interfere with cell membrane morpho-functional condition, for example resulting in spherical RBC shape and consequently in altered blood viscosity. 76 , 99 , 100
There is growing evidence that sialylated compounds present in the cellular glycocalyx may serve as an important factor in the mechanism of COVID-19 infection. A few studies have specifically focused on the glycosylation process of the two subunits of the spike protein, S1 (which facilitates the attachment to the host cell receptor through the receptor binding domain, RBD) and S2 (which mediates the fusion of viral to human cell membrane). 101 , 102
In a very recent paper, unexpected changes in the glycosylation of the S1 RBD have been shown, which can explain the crucial role of SA molecules in viral binding to ACE2, CD147 and to the glycoproteins of the host cell membrane. 103
Equally, in a computational biology paper it has been reported that the average estimated binding affinity is much higher for SA molecules (both free or on ACE2) docking with the furins and the cleaved spike protein subunit S1, than for any other receptor/compound on the cell wall. Interestingly, they found that binding of negatively charged SA molecules of host glycoproteins to the SARS-CoV-2 S protein will target the furins more thoroughly than the ACE2s, which may increase the efficiency of furin cleavage. 104
In general, coronaviruses bind SAs on the RBC and on the epithelium of upper respiratory tract through the hemagglutinin glycoproteins. The higher or lower concentration of these molecules on the various cell membranes, which may also depend upon a genetic constitution, is supposed to play a role in different diseases as well as in COVID-19. 6 , 105 , 106
Blood AB0 group determination is based also upon SA distribution on RBC wall glycoproteins and there is some contrasting evidence about the possibility that AB0 group may have a prognostic role in patients affected by COVID-19. 107
Interestingly, the blood group A has been found to have a more preferential SARS-CoV-2/RBD docking with the erythrocytes and with the cells of the respiratory epithelium. 108 Similarly, it has been proposed to link both the possible 0 group protection, and the A group higher susceptibility, to the different glycosylated part of the erythrocyte membrane (hence to the SA component). 109
However, some opposing data are reported in other studies where it is shown that SA molecules content does not depend on AB0 blood type. 110 Overall, several factors influence AB0 group determination, thus much more evidence is likely needed before drawing sound conclusions about this topic.
As a result of the described dysfunctional metabolic pathways in RBC, erythroblasts and hemoglobin, an alteration of iron metabolism and possibly also of the oxygen-transport function is expected. In fact, low levels of hemoglobin have been found in worse clinical stages, 7 , 111 – 113 while, conversely, higher hemoglobin levels at the time of the hospital admittance were associated to worse prognosis. 114 , 115
Furthermore, a number of publications have documented a clear increase in the figures of a few biomarkers which express dysfunctionality of hemoglobin and RBC, such as bilirubin, lactate dehydrogenase (LDH) and red cell distribution width (RDW). 1 , 115 – 118
Based on the evidence above, a multiple pathway-hypothesis has been published in the early 2021 to explain erythrocyte and hemoglobin denaturation in these cases. 119
Additionally, considering the possible autoimmune hemolysis (and piastrinopenia) consequent to the viral interaction with RBC receptors (namely CD147 and sialylated membrane glycoproteins), 120 – 123 the possibility of free circulating heme has been taken in consideration with reference to the hypothetical pathomechanisms of COVID-19. 1 , 124 – 130
More recently, an indirect demonstration of the SARS-CoV-2-induced hemolysis has been reported through liquid chromatography, with a documented increase of protoporphyrin IX in blood of COVID-19 patients. 131
Overall, scientific research has early focused on the possible role of hemoglobin morpho-functional alterations in this disease, as reported above, though a homogenous and direct evidence about hemoglobin dysregulation in COVID-19 has not been reached; similarly, literature reports contrasting papers about the related hypoxia and about the altered hemoglobin dissociation-curves. 4 , 132 – 139
At the same time, also the critical debate about hypoxemic hypoxia and about the high-altitude pulmonary edema (HAPE)-like condition in COVID-19 patients has attracted some scientific interest. 140 – 142 In fact, the dysfunctionality of RBC and hemoglobin has been proposed as primary cause of hypoxemic hypoxia, in presence of normal lung functionality during the early phase of COVID-19; the subsequent lung parenchyma deterioration in the late phase, with pneumolysis and interstitial pneumonia, would finally contribute to the ARDS phenomenon and to the deteriorated hypoxic state. 1 , 143 – 146
Iron and hemoglobin metabolism are strictly related; if the latter presents some denaturation, it is expected that there is some repercussion on iron biochemical pathways. However, this is a reminder here that iron physiology is strictly regulated by the axis hepcidin/ferroportin. Hepcidin is the master-regulator molecule in tissue and serum iron metabolism. 147 This hormone acts on the transmembrane ferroportin molecule, regulating the iron inflow/outflow, hence regulating serum iron and TIBC, together with ferritin concentration.
On one hand the finding of a hyperconcentration of hepcidin likely reflects the inflammatory state in these patients; on the other hand the encountered molecular similarity between the tail of the viral spike protein and the hepcidin hormone may contribute to the overall ferritin rise. 148
In presence of a hepcidin-mimicking action by the spike protein on the iron metabolism, it is argued that an over-blocking action on ferroportin may occur. The consequent iron accumulation in the tissues, with blood iron deprivation, may contribute to the findings of hyperferritinemia, hypoferremia and low TIBC in most COVID-19 patients, especially at later/worse stages. 8 , 149
Together with the occurrence of cell membrane morpho-functional alterations caused by external stimuli, viroporin activity represents another general viral pathomechanism which may contribute to the intracellular cation overload. By means of viroporin channeling action towards the cell membrane, cations (calcium mainly) may tend to enter the cytoplasm, while on the other hand viral replication and subsequent viruses’ extracellular release is facilitated. 150 – 152
In COVID-19 these viral proteins were shown to respectively oligomerize and accumulate in the endoplasmic reticulum and in the Golgi apparatus; subsequently they can interact with the VGCC, based on the level of cell transmembrane charge potential, which ultimately leads to an alteration of the same cell membrane electro-chemical activity. 153 – 156
A few channels may be subsequently opened by viroporins from the inside of cell membrane, thus favoring the passage of extracellular cations into the cell and subsequent virus expulsion which results in NLRP3 inflammasome activation and cell apoptosis. 153 – 155
A number of viroporins have been described in coronaviruses and more specifically in SARS-CoV-2 infection, being protein E (from the envelope), ORF-3a and ORF-8a the most relevant ones. 61 , 153 – 155 The ion-channeling action of these viroporins exacerbate calcium/iron intracellular accumulation, tending to reduce the transmembrane voltage furthermore. 154 , 157 These complex phenomena significantly condition both SARS-CoV-2 virulence and the microenvironment of host cell, thus contributing to the final cell ferroptosis.
Cellular bioenergetics undergo major re-arrangements during viral infections; mitochondria represent the core-organelles for cell energy production and they typically undergo major changes in aging and in chronic degenerative diseases. 158 – 160
In general, the whole cell metabolism is strictly regulated by mitochondria activity and, equally, mitophagy is the driving process of cell apoptosis. 161 SARS-CoV-2 was shown to interfere significantly with mitochondrial activity, 1 , 160 , 162 – 164 which results in an increased blood level of a few specific biomarkers related to mitochondria oxidative stress, such as lactate, free radicals and LDH. 1 , 162 , 164 – 168 Past literature showed that the deregulated pathways of cation (iron and calcium mainly) metabolism invariably induce a relevant mitochondria degeneration, with altered mitophagy. 35 , 169 – 174
The complex degenerative sequences occurring in the infected cell, influenced by cation overwhelming concentration, may lead to several deranged biochemical pathways and ultimately to a specific type of apoptosis, named ferroptosis. The term ferroptosis refers to a peculiar form of cell apoptosis and it has been introduced in 2012. 175
This degenerative process takes place in case of excessive intracellular iron accumulation (under the form of ferritin and hemosiderin). Ferroptotic mechanisms are mostly based on lipoperoxidation and on mitochondria degeneration; they are considered of extreme relevance in a series of chronic degenerative diseases (such as neurodegeneration and cancer), 176 , 177 and more broadly in many biochemical pathways which are proper of cell senescence, 178 but also of viral infections. 179 , 180
An increasing number of articles have been showing the pivotal role of ferroptosis in the pathophysiology of COVID-19. In fact, based on the evidence above, the SARS-CoV-2-induced intracellular cation overconcentration may easily lead to a progressive deregulation of mitophagy, with a documented consequent ferroptosis-based cell degeneration in later stages and in worst scenarios. 1 , 2 , 15 , 18 , 181 , 182
Viruses were shown to generally alter iron metabolism, inducing somehow a form of iron deposition in the host cells; COVID-19 features a similar cell condition, which may be considered a sort of evolutionary and protective mechanism for viruses to survive and proliferate. 183 – 188
The high levels of intracellular calcium and iron in these patients, especially in later stages, significantly contribute to a number of dysfunctional metabolic pathways, both at mitochondrial level and at greater cell level. Cations notoriously undergo a strict homeodynamic interaction, influencing each other as to the intra/extra-cellular dynamics and concentration. Their accumulation in the affected cells is synergistically permitted by a series of mechanisms, which have been detailed in the scientific literature (see above) and will be highlighted furthermore in this paper.
In COVID-19 deceased patients different types of tissues are affected by iron deposition at autopsy examinations, regardless of virus localization. 189 – 192 More specifically, the post-mortem examinations documented iron-overloaded reticuloendothelial system, bone marrow, liver, lungs and generally iron-laded macrophages (hemophagocytosis). In fact, in association with the typical immune-mediated pathologic findings (e.g. leukocyte infiltrate etc.), hemophagocytosis represents another extremely common finding in bone marrow of critical or deceased COVID-19 patients, which may reinforce the hypothesis of a frequently occurring macrophage activation syndrome in this disease. 193 , 194
With reference to the pulmonary involvement in viral diseases, it was proven that iron and calcium altered homeostasis in lung cells may represent one of the factors to explain the onset of this interstitial infective-inflammatory phenomenon, which is characterized by alveolar macrophages laden with ferritin. 1 , 195 , 196
Obviously, intracellular cation accumulation in COVID-19 patients is also the result of the virus-based immune-inflammatory processes. Beside this explanation, we have highlighted that other SARS-CoV-2 pathomechanisms, based on cell membrane dysfunctionality and cations, may represent the core culprit of this disease.
In fact, virus interaction with cell membrane receptors, namely ACE 2, CD147 and SA molecules, proved to disrupt the whole cell membrane metabolic activity, through a profound deregulation of the transmembrane electric potential. The consequent intracellular cation accumulation and cell morphology/function alterations end up in the ferroptotic process, which likely represents the unifying terminal pathway at the root of the multi-organ attack operated by this virus.
3. Unifying pathophysiological hypotheses
Based on structural and functional studies, it has been highlighted that SARS-CoV-2 spike protein binds optimally three main host membrane receptors: ACE2, SA molecules and CD147 1 , 56 , 57 , 76 , 106 , 197 ; similarly, it has been ascertained that the viral spike protein features an aminoacidic polybasic structure, which allows its functional processing by the human hydrolases and furin enzymes, so to favor the final anchoring of the virus to the cell membrane.
More specifically, the human cell transmembrane protease serin 2 (TMPRSS2), codified by the TMPRSS2 gene, contributes to the division of the spike protein in subunit S1 and subunit S2. 198
Similarly, the viral interactome with human cell membrane includes specific furin activity addressed to S1 subunit which exposes the RBD part. 199 In fact, furin cleavage is necessary to allow the exposure of the fusion sequences of the spike protein with cell membranes, which preludes to virus entry in the cell. The viral RBD is unique in terms of affinity to the molecules of SA which are found on cell membrane glycoproteins and on ACE2 and CD147.
The molecular and computational analyses of RBD/human receptors interaction have shown that single ACE2 target would not suffice to explain the great diffusion and entry capability of this virus. Thus, the viral interaction also with SA and CD147 represents a fundamental additional route to explain the multi-tissue diffusibility of this virus.
The documented deranged cation (iron/calcium) metabolism is a consequence of the viral interaction with the cell membrane receptors and with the ion-channels, as we have previously described and will detail further.
Iron homeostasis can be profoundly altered by different types of infections and by the concomitant inflammation. Similarly, hepcidin production is increased by inflammatory cytokines, especially IL-6 and IL-1b, which are typically over-released in more advanced stages of COVID-19, during the so-called “cytokine storm”. 200
Hepcidin essentially downregulates ferroportin and therefore determines hypoferremia and the sequestration of iron at cellular (macrophage in primis) level, leading to the typical anemia of any inflammatory state.
A growing body of evidence highlights how serum levels of iron, TIBC, ferritin, C-reactive protein and hepcidin tend to correlate with the severity of inflammation and, specifically with the prognosis of COVID-19. 1 , 7 – 14 , 201 – 204
As a reminder, it is acknowledged that the increase of blood ferritin expresses a compensatory mechanism to neutralize free circulating heme and Fe3+; in fact, the latter contributes also to the formation of methemoglobin, which would decrease hemoglobin O2-carrying function, hence deteriorating hypoxia furthermore in these patients.
A series of deranged metabolic pathways may take place at the root of the altered cation intracellular overload and of the multi-organ ferroptosis: a) altered ion (calcium and iron mainly) channel function, via cell membrane morpho-functional electro-chemical alterations and via viral viroporin channeling activity; b) higher hepcidin level, as result of the viral pro-inflammatory action and possibly via a hepcidin-mimicking action of the viral spike protein; c) viral invasion through the binding of SA molecules, ACE2 and CD147 of multiple types of cells, including erythrocytes/erythroid precursors, endothelial cells and macrophages, with relative hemoglobin and iron deregulated metabolism.
Here below we review in detail the main interplaying factors which intervene in COVID-19 cation/ferroptosis-based pathophysiology.
a) Sialic acid
In SARS-CoV-2 interactome with host receptors, SA component of membrane glycoproteins and of receptors is considered of utmost importance due to its ubiquitous location. This molecule represents a fundamental mediator of cell metabolism derangement in several chronic degenerative diseases 205 , 206
The primary event in COVID-19 infection is the attachment of the virus particle to the surface of the host cell, which is mediated also by sialylated cell surface receptors on several types of cells, mainly in the respiratory tract in humans. Viruses interacting with humans preferably show a α2,6 binding to host cell receptors. 97
Recent experimental evidence has shown the ability of the SARS-CoV-2 Spike protein to bind SA molecules embedded in the membrane glycoproteins. In view of its negative charge, SA has a role in the determination of cell membrane electrical charge and in the consequent binding to the electrically positive charged spike protein S1. 207 , 208
Although the spike protein binding region has been identified in its N-terminal domain, this viral part is considerable as a dipole from an electrical point of view. In fact, due to the different aminoacidic composition, S1 segment (the so called RBD) has an overall positive charge, with 111 positive and 99 negative amino acids respectively; conversely S2 has a negative charge. 207
It is known that human cells have a negatively charged membrane, mostly due to SA molecules, to the ion pump activities and to the extra/intra-cellular pH. The average cell membrane resting potential is around -40/-70 mV. 209 Beyond this value expressed in mV, a more correct expression of this potential should be in (pico-)siemens (1/ohm), being this measure unit more reliably related to the conductance of the cell membrane. 210 , 211
Electrical properties of cells strictly relate to physiology and pathology, and literature data have clearly shown that microbes alter ion channel activity, cytoplasm activities and deformability. 211 Furthermore, scientific research has extensively documented the importance of SA in cell membrane interaction specifically with outer organisms or molecules. 212 , 213
From this point of view, erythrocytes were specifically investigated to detect their possible deformation when under pathogen attack or under specific biophysical/biochemical stressors; a different resistance and cell rigidity was found following to membrane and cytoplasm electrical changes. 95 , 214 , 215
Several publications have examined the virus-host cell interactome, elucidating the determinant role of SA for SARS-CoV-2 entry in the cell. 6 , 78 , 81 – 83 In fact, virus attack is mediated by the contact of the S1 subunit with the SA molecules; as a result of this docking, a dipole is formed, including SA negatively charged and S1 positively charged; this involves a variation of the local membrane potential. In view of the formation of numerous similar dipoles, the overall cell membrane potential undergoes remarkable electrochemical variations, lowering the total negative charge. This fundamental SA-based membrane change contributes to cation dysmetabolism, through a deregulation of ion channeling activity, as it was shown in numerous publications concerning electro-chemical physiology of human cell membrane 216 – 218
As to COVID-19 pathophysiology, it has been documented that the virus attack to cell membranes occurs starting through the formation of a fusion nucleus, derived from the spike protein and the host cell membrane; more specifically electrostatic bonds and hydrogen bridges favor this preliminary step.
The attacked human cells subsequently release the hydrolases (especially the TMPRSS2), which in turn cleave the spike protein creating the subunit S1 and S2, probably at the level of the aminoacidic interval 681-684, 219 or at the level of 861-865 segment. 220
On the other hand, human furins unveil the RBD in S1, which is then ready to dock with the cell membrane receptors. The subsequent transmembrane docking occurs between the positively charged [N(+) terminal] S1 RBD to the [C(-) terminal] receptors (ACE2, CD147, SA). 207 , 221 – 224
As the virus RBD interaction with the sialylated cell membrane glycoproteins/receptors (ACE2 and CD147) results in a lower electro-negative membrane potential, the consequent dysfunction of the membrane ionic pumps conditions the whole pathophysiology of COVID-19.
The past investigation of cell physiology clearly showed that SA concentration and its disposition in cluster (hence the resulting membrane potential) strictly influence VGCC function. 216 , 217 Once the electro-chemical variations mentioned above occur, the greater opening of the VGCC lead to iron/calcium hyperconcentration inside the cell. 218
With relevance to iron metabolism, it has been documented that any perturbation of the membrane potential and of ion channels lead also to an altered ferroportin function. 225 In COVID-19 this possible functional alteration of the membrane, and specifically of ferroportin, contributes furthermore to the intracellular ferritin accumulation, also in view of the deregulated axis with hepcidin.
Beside the cell membrane functional electro-chemical changes caused by the interaction between S1 and S2 subunits with host cell, also morphologic changes of membrane and cytoplasm may occur. For example, in septic conditions membrane SA concentration tends to decrease, which leads RBC to become spherical and to decrease their intercellular repulsion; these variations tend to increase blood viscosity and this finding constitutes one of the pro-coagulant elements which can be encountered in these patients. 226
Analogue morphologic changes of the whole cell may occur in many more tissues: this phenomenon depends upon the milieu pH, the membrane potential charge, the concentration and conformation of the receptors, which also influence the osmotic gradient. 98
b) CD147
Together with SA, also CD147 transmembrane receptor glycoprotein plays a role in COVID-19 pathogenicity. 1 , 53 , 54 , 62 , 227 – 230 The receptor activity of this sialylated transmembrane glycoprotein has been studied in a large number of diseases. 66 , 67 , 231 , 232
In case of SARS-CoV-2 attack, numerous effects have been documented in tissues and organs where ACE2 receptors are few if present at all (e.g. erythrocytes, leukocytes, platelets, endothelium, cardiomyocytes, neural cells, kidney). 61
Of interest, CD147 is the receptor malaria parasite uses to enter cells. As COVID-19 infection rate seems to be much lower in those countries where malaria is more endemic, 233 – 235 it is argued that some competition for the same receptor may likely contribute to explain the low infection rate in these African populations. Similarly, in these countries sickle cell anemia is widely diffused, which is considered a protective evolutionary mechanism. Also, this phenotype may be a possible additional protective factor from COVID-19 in the same populations of malaria areas. 235 – 237
A long series of publications have regarded the possible SARS-CoV-2 interaction with erythrocytes and the consequent hemoglobin dysfunction, with release of free circulating hemoglobin/heme. 21 , 22 , 126 , 130 , 238 , 239 More importantly, a few researchers highlighted clear erythrocyte membrane alterations in COVID-19 patients. 4 These and previous data seem to demonstrate furthermore the CD147/SA-based interaction of SARS-CoV-2 with RBC which, at least partially, explains the consequent hemoglobin/iron cycle alteration. A preliminary report has also evidenced a significantly higher CD147 surface expression (and an increased oxidative stress) in erythrocytes of COVID-19 patients in comparison to normal subjects. 240
Actually, higher RDW and lower hemoglobin figures have been highlighted, especially at later stages; moreover RDW is considered an important prognostic factor in this disease. 241 – 244 High values of RDW typically indicate an altered synthesis of RBC precursors, and a number of authors confirmed this deranged erythropoiesis also in COVID-19. 1 , 72 , 245 , 246
Further to the documented role of CD147 in the viral attack to the cell membrane, this receptor was found to interact with cyclophilin A, which is one of the human proteins used by SARS-CoV-2 for its intracellular replication. This interaction regulates cytokine secretion and chemotaxis of inflammatory cells, which facilitate the infection of the host cell. 62 , 247 , 248
c) Viroporins
As previously elucidated, viral encoded membrane pore forming protein (viroporins) may play a relevant role in the intracellular viral attack. Intra/extracellular pH and the transmembrane potential charge (both of them influenced by SA molecule activity and by the ion concentration) impact the cell membrane depolarization of these ion-channeling viroporins.
Viroporins are chronologically activated after the virus entry and do facilitate viral replication; they are known to exert a channeling action from the inner side of the host cellular membrane directed towards the extracellular space. 150 , 151 The modulation by viroporins on the opening/closure of multiple pores in the cell membrane tends to regulate cation transmembrane movement, but also endoplasmic reticulum cation release. 5 , 249
In fact, these small hydrophobic proteins are encoded by the virus and are oligomerized in the membrane of host cells, leading to the formation of hydrophilic pores. This activity disrupts several cellular functions, including membrane permeability, calcium homeostasis, membrane remodeling and glycoprotein trafficking. 150 , 151
One of the main functions of viroporins during viral replication is to participate in virion morphogenesis and release from host cells. Furthermore, some viroporins are involved in virus entry and genome replication.
The existence of viroporins was originally suggested following the observation that virus-infected cells become permeable to ions and small molecules. New members of this expanding family of viral proteins have been described, from both RNA and DNA viruses. These proteins are crucial for viral pathogenicity due to their involvement in different stages of the viral life cycle. Their main function is to participate in the assembly of viral particles and their release from the infected cells. Typically, deletion of a viroporin-encoding gene from a viral genome dramatically reduces viral progeny formation and viral pathogenicity, emphasizing the essential role of these proteins in the viral-human cell interaction. 150
SARS-CoV-2 viroporins, mainly type E, act to re-potentiate the channeling action and ultimately the entry of cations and new viruses in the cell. In fact, after the early depolarization of cell membrane due to S1 (especially) and S2 docking, virus entry and calcium/iron influx take place through ion channels (e.g., VGCC). Once the cation-enriched gradient grows up, the trans-channel ion movement from the extracellular space tends to decrease. Intracellular viroporin channeling action tend to immediately re-activate virus and cation entry against the gradient, thus ultimately favoring virus intra/extra-cellular diffusion and ultimately cell ferroptosis.
d) Calcium and iron dysmetabolism
A number of cations show a low blood level during COVID-19 course. 32 Interestingly, calcium represents one of the most investigated minerals in this disease and hypocalcemia has been repeatedly reported in these patients, 26 , 250 – 252 especially at later stages. 24 – 28 , 30 , 33
The low serum calcium level lays for a probable progressive intracellular deposition of this mineral, as no specific hypercalciuria has been detected. The prognostic role of hypocalcemia, 27 , 29 – 31 together with the significantly dysregulated calcium metabolism/signaling in COVID-19 patients, have been investigated in two recent reviews. 253 , 254
Physiologically, calcium intra/extra-cellular balance and homeodynamics are strictly depending on the so called VGCC. The latter represent highly regulated cell proton pump mechanisms. Excessive intracellular calcium has been related to several chronic degenerative diseases, mostly linked to peroxynitrite accumulation and oxidative stress. 173 , 255 , 256
In COVID-19, the documented hypocalcemia and the consequent intracellular hyperconcentration of calcium may originate a series of derangements, mostly through mitochondria deregulation, as already highlighted in other diseases. 257 – 259
Of interest, it was shown that a specific genetic susceptibility linked to VGCC may predispose to Kawasaki autoimmune disease, 45 which may occur among children in the current pandemic. 260 Neuronal cells may similarly suffer from a deregulated calcium and iron concentration, as these cations intervene in the conductivity process; overall, neuro-sensorial disturbs, including loss of smell and taste, have been extensively reported in the vast majority of COVID-19 cases. 261 – 264
Calcium is essential to most viruses as to their processes of entry, replication and diffusion. 34 For example in case of SARS-CoV-1 infection, a calcium-dependent transmembrane invasion was demonstrated 265 and it was proven that generally any alteration in host cells calcium homeostasis reflects upon viral pathogenicity and diffusion. 35 , 266
More in detail, it has been ascertained that viral replication is also based on a sort of hijacking of a few cation-based host cell processes, which mainly pertain mitochondria. This mitochondria deregulation results in a number of biochemical degenerative processes, such as NLRP3 inflammasome activation and a deregulated cell apoptosis (ferroptosis namely). 34 , 267
As previously reported, a similar mitochondria “kidnapping” and degeneration due to SARS-CoV-2 infection has been described and hyper-concentrated iron and calcium on one side mediate this detrimental effect, 2 , 164 , 268 on the other side stimulate ferroptosis. 35 , 44 , 269
In normal conditions body iron is mostly located in the prosthetic group of heme; hyperferritinemia reflects excessive iron availability, but also it could hypothetically derive from damaged tissues. 7 , 270 Thus, in order to assess iron metabolism properly, additional biomarkers (such as TIBC and other transferrin-related markers) should be investigated, so to have more surrogate findings which mirror iron deposition and free circulating iron. 271
In these conditions of hyperconcentration of this metal inside the cell, free cellular iron (Fe3+) can easily form free radicals, for example through Fenton and Haber-Weiss reactions, thus a series of detrimental biochemical pathways may be activated, including pro-coagulative cascades. 272 The same transferrin molecule has been recognized as an important pro-coagulant factor in COVID-19, 273 probably due to its interaction with several clotting factors. 274
Of interest, the “protective” mechanism of iron sequestration under the form of ferritin (especially in macrophages of lungs, liver etc.) can impair erythropoiesis and new hemoglobin production, which may complicate anemic hypoxia in patients with COVID-19. 271
Physiologically, in mitochondria iron represents a fundamental co-factor of several enzyme-based reactions; furthermore Fe2+ is transformed in its bioavailable form by a cluster iron-sulfur (Fe/S) along the heme synthesis pathways. 275 When iron is in excess, its altered pathways lead to a much higher production of free radicals; furthermore heme (thus hemoglobin) formation decreases, originating a sort of sideroblastic anemia in the case of COVID-19 patients. 1
Ferritin overloading cells leads to ferroptosis, which could be considered also an evolutive protection mechanism, aimed at reducing both free serum iron and its availability to viruses and other pathogens. Likely, ferroptotic mechanisms are upregulated in similar conditions of ferritinophagy, high amounts of free cell iron, deregulated hepcidin/ferroportin axis and especially exaggerated cation entry.
e) Cell membrane electrochemical changes and ion pumps
Following to the described morpho-dynamic viral interaction with cell membrane, and after the significant reduction of the transmembrane electronegative potential, the charge change relevantly impacts ionic pump regulation. The docking between S1 and S2 with membrane receptors (S1) and phospholipid layers (S2), as specified above, are mostly based on the binding with SA molecules and it leads to an altered ion channeling activity. VGCC modification usually takes place, permitting the excessive entry of calcium and, consequently, of Fe2+ ions. 61 , 216 – 218 Lastly, as previously documented, the subsequent intervention of the viroporins contributes to create new pores in the cell membrane, allowing further cations to enter, additionally facilitating virus diffusion.
Biophysical studies have recently highlighted that SARS-CoV-2 itself owns its electrical properties and generates an electro-magnetic field (EMF), which can interact with the polarity of the host cell membrane; this possible interplay urges a better understanding of the whole electrical and biochemical process occurring during the infection. 276
The detrimental chain of biochemical events caused by cation intracellular overload, such as Fenton reaction with Fe3+ formation, H2O transformation into hydroxyl free radical and NO transformation into the toxic ONOO (peroxynitrite) compound has been described in COVID-19 as well, especially concomitant to cytokine storm. 277 Moreover a few authors have advocated an involvement of hemoglobin denaturation, with free heme/iron release, at the root of the free radical production. 3
Lastly, the intracellular migration of iron and calcium ions is responsible for the activation of the inflammasome NLRP3, 278 which typically contributes to worst scenarios of this disease.
Altered ion exchange ultimately generates lipoperoxydation and oxidative stress (the root of ferroptosis), consuming glutathione and deeply damaging mitochondria. The consequent dysregulated mitophagy accelerates apoptosis of the infected cells. 176 , 177
A computational study has elucidated that host cell membrane interaction with the SARS-CoV-2 viroporin channeling action features a cation selectivity, being sodium, potassium and calcium the most facilitated ions in the transmembrane movement; moreover, the authors showed that transmembrane voltage influences the pore dimension and the transition rate (thus the intracellular accumulation) of cations. 156 Similar findings had been discovered for viroporins in general, thus confirming the importance of the electrochemical gradient of cell membrane in ion balance. 150 , 279 , 280
Also virus fusion, invasion and replication are greatly enhanced by the progressively more favorable intracellular cation-enriched microenvironment. 156
As mentioned above, the blood levels of other cations, such as potassium and sodium, have been shown to decrease in COVID-19 patients. Generally coronaviruses, and more specifically SARS-CoV-2, have been found to exert a channeling activity via viroporin E and ORF3a, 61 which leads to an intracellular K+ overload. 153 , 281 Of note, as calcium inflow is physiologically linked to potassium movement, a sort of vicious circle may arise, inducing an augmentation of the calcium concentration inside the cell.
As we have seen, iron and calcium influence mitochondrial function and several metabolic processes. Additionally, cellular iron stimulates calcium signaling and vice-versa, which impacts also ferroptosis. 44 , 269 , 282
Through VGCC, also iron (and other metals) may enter cells, as biophysical studies have documented. 283 – 289 Neurodegenerative diseases are paradigmatic examples of cell intoxication with iron and other metals, through VGCC dysfunction, 282 and calcium channels were shown to favor glutamate accumulation in neuronal diseases, giving rise to the so-termed “oxytosis”, which originally described ferroptosis. 290
The virus-based intracellular cation engulfment affects also the two-pore cation channels in the lysosomal membrane, thus reducing the endo-lysosomal “digestive” function against microbes. 291
In the light of the evidence above, scientific research is reserving a major attention to the relationship between iron and calcium on one side, and the virus-host cell membrane electro-chemical interactions on the other side. 104 , 154 , 271 , 276 , 292
f) Hepcidin and ferroportin
Hepcidin is the master-regulator peptide hormone in iron metabolism. Basically, hepcidin is to iron what insulin is to glucose and an alteration of its interaction with ferroportin has been linked to a series of chronic degenerative diseases. 147 From the first reports on hepcidin discover, 293 , 294 the understanding of iron homeostasis has become more and more precise, revealing a large number of metabolic activity. Iron circulates inside-outside the cell in different manners: introduction by extracellular transferrin capitation and internalization, or by divalent metal channel DMT1. 295
Intracellular iron exists in two forms, Fe2+ and Fe3+; as Fe3+ is the toxic form more available to generate free radicals, normally cell embeds this ion in the ferritin molecule inside specific vesicles. A minority of Fe3+ is deposited under the form of hemosiderin. This equilibrium between the two iron forms is unbalanced in presence of excessive hepcidin activity. Also, hyper-formation and storage of ferritin, based on the hyperconcentration of hepcidin molecules, requires a relevant consumption of ATP, with consequently increases mitochondrial dysmetabolism.
Once in the plasmatic and cytoplasmic compartment, iron is a labile ion that could interact with numerous molecules, stimulating, and inhibiting different processes. A homeostatic mechanism exists with regards to intra/extra-cellular iron concentration. Hepcidin has the role of blocking and internalize the transmembrane ferroportin, preventing iron efflux. If this excessive intracellular uptake is combined with a reduction of efflux, an overcharge of intracellular labile iron with ferritin hyper-concentration and formation of toxic free radicals may occur. 296
With concern to the immune-inflammatory derangement which takes place in COVID-19, a direct link has been highlighted between viral ACE2 receptor internalization and hepcidin-IL6 activation through the NF-kB system. 203 , 278
This mechanism suggests the occurrence of a vicious cycle, where transferrin uptake and hepcidin overexpression cause a persistent activation of NF-kB as a consequence of the intracellular iron augmentation. Literature data put in evidence that also CD147 receptors stimulate NF-kB system, 297 , 298 suggesting that both ACE2 and CD147 act on the same system and consequently generate the same cascade of events within the inflammasome NLRP3. More in general, inflammasomes include a class of intracellular proteins involved in inflammatory reaction in most chronic and acute inflammation processes, such as obesity, diabetes, stroke, cancer, 299 hypoxia and thrombosis. 300
Similarly, it was proven 301 , 302 that the NLRP3 inflammasome is directly activated and related to the NF-kB system and, more interestingly, to the intracellular labile iron, suggesting a complex interaction with ACE2 and CD147 receptors in COVID-19 infection.
Beyond the strict relationship between (chronic) inflammation and hepcidin/ferroportin axis activity, with an anemia pattern and an increased level of circulating hepcidin, 303 , 304 COVID-19 has been associated with a marked increase of serum hepcidin level in worse patients. 201 – 203
As previously described, early publications about COVID-19 1 , 148 reported about a similarity between the aminoacidic sequence of SARS-CoV-2 spike cytoplasmic tail and the hepcidin molecule. Hence the virus could perhaps block ferroportin-based iron extracellular transport and an accumulation of iron inside the cell could occur.
Note that hypoferremia and hyperferritinemia along the course of the disease should deactivate hepcidin activity and reduce its blood levels. As this is not the case in the studies cited above, the viral pathomimicry could at least partially justify the iron deregulated axis in these patients.
g) Hemoglobin, heme
Nearly 75% of human iron is contained in hemoglobin; in view of the iron deranged metabolism in COVID-19, a number of articles have investigated a possible hemoglobin denaturation in these patients 1 , 3 , 22 , 61 , 124 , 126 – 128 Though no sound evidence of a direct hemoglobin viral attack has been documented so far, free circulating heme has been described in a few papers 130 , 238 , 239 ; moreover, some recruitment of hemoglobin and its metabolites (hemin and protoporphyrin IX) by a few SARS-CoV-2 proteins was documented in a computational biology study. 239
An analogue scientific debate is underway about the alteration of the dissociation curves of hemoglobin in these patients, regardless of whether hypoxia may not be regarded as solely generated by pneumolysis/lung disease. 143 – 145 , 305
h) Potential environmental, genetic and microbiome susceptibility factors
Beside AB0 group and sickle cell conformation, several other factors may predispose to SARS-CoV-2 pathogenicity.
Specific chromosome variants or sequences have been linked to worse prognosis, but also genetically-determined hemochromatosis is being considered of some importance. In fact, liver biopsies in seriously ill patients, or during autopsies, have shown a remarkable intracellular iron accumulation, which clearly reminds hemochromatosis histopathologic features. 191 Similarly, a ferroptotic pattern has been encountered in a fatal case of myocarditis 190 and a typical pattern hemophagocytosis with iron-laden bone marrow cells was documented in a large series of autopsies. 194
Hereditary hemochromatosis is characterized by an accumulation of ferritin in several tissues, which may be clinically asymptomatic or less pronounced in heterozygosis type, more aggressive in homozygosis.
The incidence of heterozygote hemochromatosis has been reported as high as 5.4-13.5% in the general U.S.A. population 306 and, more specifically, a review 307 found the following prevalence percentages in the general population: 5% as compound heterozygotes (C282Y/H63D mutation), 1.5% homozygous for the H63D mutation, 3.6% were C282Y heterozygotes, and 5.2% were H63D heterozygotes. With reference to world areas, the frequency of the C282Y heterozygosity ranges from 9.2% in Europeans to much lower figures in Asia, Africa and Middle East. Conversely, the H63D carrier frequency was documented as 22% in European populations.
In case of SARS-CoV-2 contagion, the subject with hemochromatosis may undergo a more complex course; being this disease latent in the vast majority of the individuals, it could be of interest to investigate its presence in the patients showing an atypical (e.g. young, or apparently healthy subjects) complicated infection. 1 , 308 , 309
Environmental factors have equally been called into question as possible disruptors in the course of this infection. In the past years a number of articles have evidenced in animals and humans the possible role of EMF on the dysfunctional pathways of VGCC, on peroxynitrite formation, oxidative stress and on other biochemical pathways at the root of cation dysmetabolism. 310 – 320
Additionally, in the last decades scientific literature has documented the influence of EMF on viral activity, erythrocyte/viroporins metabolism, mitochondria and immunity. 321 – 325
The possible interaction of 5G technology with human health is being debated as well, raising contrasting speculations and evidence, mostly due to the limited knowledge about this relatively new EMF typology. 326 , 327 More recently, a few specific papers focused on the possible role of peculiar types of EMF in SARS-CoV-2 pathogenicity, 328 , 329 and a recent publication has presented some data about the detrimental interaction of radiofrequencies specifically with VGCC and with cation metabolism, erythrocytes, hemoglobin, coagulation, immune system and oxidative stress. 330
In view of the role of both the cell membrane electro-chemical changes and the cations in COVID-19, some more research could be performed to assess any possible inter-relationship of polluting environmental factors with VGCC, cellular membrane and cytoplasm ionic changes.
Microbiome is another potential element of interest in this infection. Microbiome regulates a large number of bodily biochemical pathways and it is considered of utmost importance in several chronic degenerative diseases and, more generally, in cell senescence. 331 , 332
A few authors have highlighted an analogue specific role of microbiome in COVID-19, some of them focusing on the pathogenicity of lipopolysaccharides (LPSs); LPSs are known byproducts of gram-negative bacteria which derive from an altered microbiome and gut permeability, inducing endotoxemia. 333 , 334 A strict relationship between LPSs and ferroptosis has been already established in past literature 335 – 338 ; similarly, it was documented that LPS interaction with spike proteins may be mediated by SA receptor activity, 339 which seems to reinforce the role of SA molecule in the LPS-mediated ferroptotic processes in these specific patients.
In fact, a high binding affinity between LPSs and SA has been repeatedly demonstrated, 339 – 342 hence the complex interaction among LPS, SA and SARS-CoV-2 spike protein, with the resulting pro-ferroptosis pathways, might deserve more attention in the future scientific research.
Summarizing the evidence and the hypotheses
Overall, the hyperinflammatory reaction which characterizes the worst scenarios of COVID-19 may be regarded as an expression of the immune-inflammatory derangement, which in turn could depend upon the cascade of the biochemical pathways that characterize intracellular cation accumulation and cell ferroptotic mechanisms. The very final detrimental degenerative cell events are then represented by excessive free radical formation (peroxynitrite above all), 277 glutathione depletion, membrane lipoperoxidation, mitochondria degeneration, partial metabolic shift to anaerobic glycolysis, marked activation of the inflammasome NRLP3 and NF-kB with subsequent inflammatory cytokine cascade.
At the very beginning of this sequence of pathomechanisms, it is possible to recognize the relevant role of the electrochemical changes induced by the spike proteins on the cell membrane, via glycosylated receptors and ion channeling alterations.
4. Discussion
The finding, now confirmed by many publications, of low levels of hemoglobin, serum iron and calcium, together with the very high levels of ferritinemia in critical or deceased patients, highlights the major derangement of cation metabolism in these patients. Similarly, higher figures of LDH, lactate and RDW express both the involvement of mitochondria, and some form of degeneration of RBC and of their precursors.
The intervention of the negatively charged SA, both on the receptors ACE2 and CD147 and on the sialylated membrane glycoproteins, proves extremely relevant in the mediation of the docking process with the spike proteins.
This docking interaction induces a decrease of the negative potential of the cell membrane and, as consequence, trans-membrane cation channels may alter their permeability so that, on a multi-tissue level, different types of cells undergo morpho-functional changes. Of course, the latter depend upon several other factors, including pH and osmolarity of the intra-extracellular space. 98
Hepcidin, as a result of both the hyperinflammatory state and of a possible mimicking action by the viral spike protein, remarkably contributes to ferritin and hemosiderin accumulation in the tissues, by reducing ferroportin activity.
Lastly, viral viroporins and other viral pathomechanisms contribute to the accumulation of the cations inside the cell, thus contributing to viral replication/extra-cellular release and cell degeneration.
The resulting calcium and iron hyper-influx impairs homeodynamics of a series of cellular (mitochondrial primarily) pathways, and also favors a dramatic free radical increase. Excessive ferroptosis represents the ending outcome of these degenerative changes in many tissues (e.g., lung, endothelium, heart).
Erythrocyte degenerative electrochemical changes of the membrane, with morpho-functional alterations (such as spherocytosis, altered erythropoiesis, hemoglobin dysfunctionality) may deserve some specific attention. In fact, iron metabolism strictly depends upon RBC and hemoglobin and several authors have documented some degree of (autoimmune-like) hemolysis, with an increased RBC destruction which takes place in the reticuloendothelial system and in specific organs (spleen, liver etc.). Lastly, some literature data point out the free heme circulation in patients affected by COVID-19.
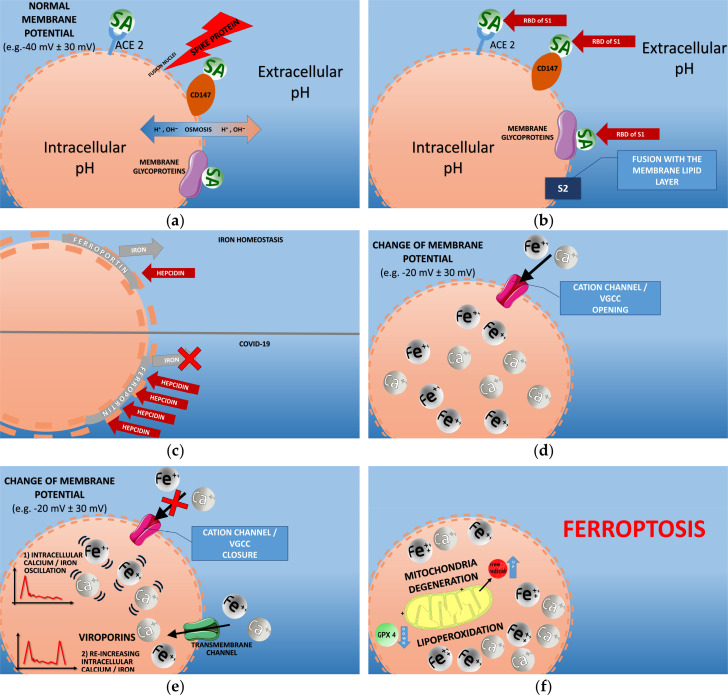
To summarize the probable events at the root of cation imbalance and ultimately of ferroptosis, we have reported the main pathological steps in Table 1 and in Figure 1.
Table 1.
Main pathological steps of the SARS-CoV-2 attack to cell membrane and of consequent cell degeneration.
|
Figure 1. a) Docking approach of the spike protein with the host cell membrane, which presents three main receptors: ACE2, CD147 and Sialic Acid (SA); SA is expressed on the membrane glycoproteins and on the outer site of the other two receptors. b) The two subunits of the spike protein (S1 and S2), derived from priming and cleavage by hydrolases (TMPRSS2) and furins, contact host cell membrane: S1 receptor binding domain (RBD) attach ACE2 and CD147 through SA and directly SA on the membrane glycoproteins; S2 enters the lipid layer of the cell membrane. c) Upper part: normal hepcidin-ferroportin axis; lower part: COVID-19 situation, with hyper-concentrated plasma hepcidin molecules that bind the extracellular portion of the transmembrane ferroportin, thus blocking iron extracellular transport and favoring intracellular ferritin accumulation. d) Decrease of the membrane potential (less negatively charged), which is altered by the formation of several dipoles between SA negative and S1 positive; consequent opening of the cation channels, especially of the voltage gated calcium channels (VGCC), with intracellular entry of cations, primarily calcium and iron. e) Cell homeostasis, after cation entry, rapidly brings closure of VGCC and cation channels (1 intracellular oscillation with one peak of cations); simultaneous viroporin action of membrane channeling which brings opening of new channels and re-entry of cations (2 intracellular oscillation with a second peak of cations). f) Ferroptosis: excessive cation concentration, increase of free radicals, depletion of glutathione peroxidase 4 (GPX4), lipoperoxidation of membranes and organelles, mitochondria degeneration.
The pathophysiology sequence of the virus-host cell interaction which has been proposed above, is based on the basic knowledge of the spike protein attack to cell membrane 207 , 221 – 224 and focusing on SA, CD147, ACE2, hepcidin, viroporins and on the electrochemical changes happening on the membrane, with subsequent cation imbalance and ferroptosis.
Cell ferroptosis is the final step of the cascade described above. This specific cell death process is caused by a pronounced lipoperoxidative transformation of several cell components, with glutathione peroxidase 4 (GPX4) depletion; finally a deep dysregulation of mitophagy with mitochondrial degeneration occur, inducing an accelerated and deregulated apoptosis. 343
Cell membrane altered polarization and the overload of cations can also affect blood coagulation, endothelial and neural functionality; more generally the electro-chemical events occurring at the cell wall significantly impact the metabolic pathways of a number of organs.
Most of the biochemistry alterations reported above are also linked to the relevant immune-inflammatory process which takes place in these patients. In fact, SARS-CoV-2 infection leads to the typical inflammasome activation, with NF-kB activation and interleukins/TNF-alfa increase; these pathways involve extremely higher hepcidin production with intracellular iron sequestration, which favors viral replication. However, in comparison to other inflammatory/infective processes, ferritin peaks higher figures and in a shorter time in the course of this disease. 1 , 203 , 344 , 345
Hence, ferritin is regarded both as a typical consequence and marker of the SARS-CoV-2-induced inflammatory cascade, and as a potential pathogenic mediator of the viral infection.
Furthermore, ferritin was found to have a different biochemical composition (higher protein component) when derived from inflammatory diseases, whereas in these cases it is expected a higher ferric component and a different composition in its two H and L subunits. 346 In fact, for a long time now, scientific research has shown how viruses tend to take control of iron metabolism and of VGCC, so to establish a preferential microenvironment for their growth and multiplication. 183 – 186
With reference to the possible therapeutic proposals targeting the basic pathophysiology steps elucidated above, literature highlights a number of options to address this cation dysregulation, based on chelation for example. 15 , 185 , 347 Equally, a large series of drugs and natural compounds have been tested, or advocated, to target the sialylated receptors, hepcidin/ferroportin axis and the cation channels deregulation.
More robust data are needed before drawing definitive conclusions about the role of intracellular cation accumulation in the onset and perpetuation of the inflammatory and immune-mediated processes of this infection. However, through this narrative review we aimed at addressing the electro-chemical pathomechanisms which are at the root of the viral attack, and we highlighted as well the host cell membrane morpho-functional changes which relate to cation imbalance.
Furthermore, newer insights were provided about the role of SA, the CD147 receptor, about the hepcidin-ferroportin axis deregulation and the erythrocyte/hemoglobin altered metabolism.
Some contrasting literature data were reported on the specific subjects’ different susceptibility to the viral attack, based on determined genetic mutations, blood groups/diseases, specific receptors expression. 71 , 191 , 309 , 348 – 355 Beyond a specific possible TMPRSS2-related predisposition on a genetic or epigenetic (e.g. diabetic and obese patients) basis, 356 the majority of these speculations refer both to the variability of the membrane expression of certain receptors (namely SA and CD147), and to the consequent cation dysmetabolism.
For example, past research has linked blood group to susceptibility to viral infections for a number of reasons, mostly connected to membrane receptors. 60
Basically, AB0 blood group glycans were found to modulate SA recognition on erythrocytes and it was reported that there are approximately 2 million AB0 glycan antigen sites on each RBC and 50 million SA molecules per erythrocyte. 94
Similarly to CD147 expression on RBC wall, also different SA molecules represent the terminal part of glycoproteins which are expressed in variable formulas and clusters on the surface of several kinds of cell, thus on the RBC membranes as well. Interestingly, SA has possible substituent groups, especially in position 5 of the carbon atom, which are of extremely variable nature. 357 This peculiarity remarkably modifies both the charge (which remains always negative) and the stereochemistry of the SA molecule from the primary to the quaternary conformational structure; additionally, further substitutions may occur in the overall glycoproteic molecule and, of importance, different concentrations of SA molecules have been highlighted in the various kinds of cells and individuals. 105
In a speculative review the possible protective and detrimental role of 0 and A blood group respectively, was linked to a lower (0 group) or higher (non-0 group) attack from innate immunity. This reaction would take place based on the different distribution of the erythrocyte membrane glycoproteins. 109
AB0 antigens are known to modulate cellular interactions with outer molecules, microbes and parasites (such as plasmodium falciparum), not as ligands, but by stabilizing other glycans such as SA on the cell surface in clusters, thus influencing their accessibility for outer glycan-binding proteins. 94
As previously elucidated, these biochemical differences among individuals in terms of SA and CD147, together with the related RBC evolutionary changes, could partly explain the reduced pathogenicity of COVID-19 in those countries where malaria and sickle cell disease are more endemic.
Furthermore, an overlap of the gene loci for the AB0 system and the loci for the iron metabolism has been elicited. 348 This genetic connection between cations and blood type may represent another element of discussion within the findings concerning individual genetic susceptibility.
Anyway, other authors reported no specific difference in terms of SA content in the context of the different AB0 blood types, whereas they anyway found that patients with sickle cell anemia had significantly lower SA values in comparison to the erythrocytes from healthy subjects. 110
In view of the wide distribution of SA molecules and of CD147 receptors on RBC, platelets and endothelium, and because of the well-known detrimental role of cation imbalance on the coagulative cascade, 358 , 359 these factors should be regarded of importance also to explain the artero-venous thromboembolic complications which may occur in COVID-19, with the formation of cation-based fibrinolysis-resistant intravascular parafibrin.
Basically, beside the traditional vision of micro-macro-thromboses based on coagulation abnormalities deriving from a number of immune-inflammatory derangements, a panoply of pro-thrombotic factors, partly based on RBC/hemoglobin dysfunction, may intervene.
Other typical clinical findings of these patients, such as hypoxemic hypoxia, pneumonia, most immune-inflammatory processes and cytokine storm/ARDS, could be equally regarded as late expressions of a multi-organ (blood, endothelium, liver and neural system included) disease, where RBC altered morphology, function and clearance, together with ferroptosis in other cell types, seem to play a major role.
In the latest weeks the newly emerged Omicron variant of SARS-CoV-2 has been extensively studied. A much higher diffusibility and a lower pathogenicity of this viral strain over the previous ones has been highlighted so far. 360
From the biophysical point of view, it has just been recorded that this variant shows a series of mutations which increase the overall positive electric charge of S protein, more specifically of S2 subunit. 361 , 362
Actually, the present review has shown how the electrical interaction between the spike protein (especially RBD of S1) and cell membrane is pivotal to determine especially pathogenicity of this viral infection. This feature derives from the formation of the new dipoles created by RBD-SA molecules, which brings the membrane electrochemical changes mentioned above, and in turn generates ion channels dysfunction.
As Omicron variant expresses a higher positive charge especially at the level of S2, it may be expected an easier cell penetration through the lipofilic fusion with cell membrane, whereas a lower ion-channeling dysfunction (putatively a lower pathogenicity) could occur.
Based on the reported evidence here, it is expected biomedical research may deepen the meaning of the interplaying pathophysiology factors elucidated in this review: cell membrane potential and ion channels, sialylated receptors, cations, RBC, hemoglobin, hepcidin. The resulting deranged ferroptosis may represent the main and ultimate cell degenerative process which characterizes the multi-organ SARS-CoV-2 attack and the final lung involvement.
Data availability
There are no underlying data associated with this article.
Acknowledgements
We sincerely thank Roberto Colucci for his valuable help in the preparation of the figure and Giovanni d’Errico for the text revision.
Funding Statement
The author(s) declared that no grants were involved in supporting this work.
[version 1; peer review: 1 approved
References
- 1. Cavezzi A, Troiani E, Corrao S: COVID-19: hemoglobin, iron, and hypoxia beyond inflammation. A narrative review. Clin Pract. 2020 May 19;10(2):24–30. 10.4081/cp.2020.1271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Edeas M, Saleh J, Peyssonnaux C: Iron: Innocent bystander or vicious culprit in COVID-19 pathogenesis?. Int. J. Infect. Dis. 2020 Aug;97:303–305. 10.1016/j.ijid.2020.05.110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Wenzhong L, Hualan L: COVID-19: captures iron and generates reactive oxygen species to damage the human immune system. Autoimmunity. 2021 May 19;54(4):213–224. 10.1080/08916934.2021.1913581 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Thomas T, Stefanoni D, Dzieciatkowska M, et al. : Evidence of Structural Protein Damage and Membrane Lipid Remodeling in Red Blood Cells from COVID-19 Patients. J. Proteome Res. 2020 Nov 6;19(11):4455–4469. 10.1021/acs.jproteome.0c00606 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Saurabh K, Solovchuk M, Sheu TW-H: Investigating ion transport inside the pentameric ion channel encoded in COVID-19 E protein. Phys. Rev. E. 2020 Nov;102(5–1):052408. 10.1103/PhysRevE.102.052408 [DOI] [PubMed] [Google Scholar]
- 6. Nguyen L, McCord KA, Bui DT, et al. : Sialic acid-containing glycolipids mediate binding and viral entry of SARS-CoV-2. Nat. Chem. Biol. 2021 Nov 9. [DOI] [PubMed] [Google Scholar]
- 7. Taneri PE, Gómez-Ochoa SA, Llanaj E, et al. : Anemia and iron metabolism in COVID-19: a systematic review and meta-analysis. Eur. J. Epidemiol. 2020 Aug;35(8):763–773. 10.1007/s10654-020-00678-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Henry BM, Oliveira MHS, Benoit S, et al. : Hematologic, biochemical and immune biomarker abnormalities associated with severe illness and mortality in coronavirus disease 2019 (COVID-19): a meta-analysis. Clin. Chem. Lab. Med. 2020 Jun 25;58(7):1021–1028. 10.1515/cclm-2020-0369 [DOI] [PubMed] [Google Scholar]
- 9. Deng F, Zhang L, Lyu L, et al. : Increased levels of ferritin on admission predicts intensive care unit mortality in patients with COVID-19. Med. Clin. (Barc.). 2021 Apr 9;156(7):324–331. 10.1016/j.medcli.2020.11.030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Raman N, Kv P, Ashta KK, et al. : Ferritin and Hemoglobin as Predictors of Fatal Outcome in COVID-19: Two Sides of the Same Coin. J. Assoc. Physicians India. 2021 Aug;69(8):11–12. [PubMed] [Google Scholar]
- 11. Cheng L, Li H, Li L, et al. : Ferritin in the coronavirus disease 2019 (COVID-19): A systematic review and meta-analysis. J. Clin. Lab. Anal. 2020 Oct;34(10):e23618. 10.1002/jcla.23618 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Zhao K, Huang J, Dai D, et al. : Serum Iron Level as a Potential Predictor of Coronavirus Disease 2019 Severity and Mortality: A Retrospective Study. Open Forum Infect. Dis. 2020 Jul 1;7(7):ofaa250. 10.1093/ofid/ofaa250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Bellmann-Weiler R, Lanser L, Barket R, et al. : Prevalence and Predictive Value of Anemia and Dysregulated Iron Homeostasis in Patients with COVID-19 Infection. J. Clin. Med. 2020 Jul 29;9(8):E2429. 10.3390/jcm9082429 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Lv Y, Chen L, Liang X, et al. : Association between iron status and the risk of adverse outcomes in COVID-19. Clin. Nutr. 2021 May;40(5):3462–3469. 10.1016/j.clnu.2020.11.033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Mandolesi S, Cavezzi A, d’Alessandro A, et al. : Could iron-chelation with EDTA and bloodletting/transfusion be a therapeutic option in COVID-19 worst scenario? Proposal for a protocol. JTAVR. 2020 [cited 2021 Dec 13];5(1). 10.24019/jtavr.99 Reference Source [DOI] [Google Scholar]
- 16. Abobaker A: Can iron chelation as an adjunct treatment of COVID-19 improve the clinical outcome?. Eur. J. Clin. Pharmacol. 2020 Nov;76(11):1619–1620. 10.1007/s00228-020-02942-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Vlahakos VD, Marathias KP, Arkadopoulos N, et al. : Hyperferritinemia in patients with COVID-19: An opportunity for iron chelation?. Artif. Organs. 2021 Feb;45(2):163–167. 10.1111/aor.13812 [DOI] [PubMed] [Google Scholar]
- 18. Habib HM, Ibrahim S, Zaim A, et al. : The role of iron in the pathogenesis of COVID-19 and possible treatment with lactoferrin and other iron chelators. Biomed. Pharmacother. 2021 Apr;136:111228. 10.1016/j.biopha.2021.111228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Chakurkar V, Rajapurkar M, Lele S, et al. : Increased serum catalytic iron may mediate tissue injury and death in patients with COVID-19. Sci. Rep. 2021 Oct 4;11(1):19618. 10.1038/s41598-021-99142-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Lippi G, Mattiuzzi C: Hemoglobin value may be decreased in patients with severe coronavirus disease 2019. Hematol. Transfus. Cell Ther. 2020 Apr;42(2):116–117. 10.1016/j.htct.2020.03.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Berzuini A, Bianco C, Migliorini AC, et al. : Red blood cell morphology in patients with COVID-19-related anaemia. Blood Transfus. 2021 Jan 19 [cited 2021 Dec 14];19:34–36. 10.2450/2020.0242-20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Kubánková M, Hohberger B, Hoffmanns J, et al. : Physical phenotype of blood cells is altered in COVID-19. Biophys. J. 2021 Jul 20;120(14):2838–2847. 10.1016/j.bpj.2021.05.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Rawal Institute of Health Sciences Islamabad. Chaudhry ZR, Rasheed S, et al. : Corona virus lowers hemoglobin more in severe infection than mild COVID-19 infection. TPMJ. 2021 Aug 10;28(08):1211–1214. 10.29309/TPMJ/2021.28.08.6523 [DOI] [Google Scholar]
- 24. Sun J-K, Zhang W-H, Zou L, et al. : Serum calcium as a biomarker of clinical severity and prognosis in patients with coronavirus disease 2019. Aging. 2020 Jun 25;12(12):11287–11295. 10.18632/aging.103526 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Wu Y, Hou B, Liu J, et al. : Risk Factors Associated With Long-Term Hospitalization in Patients With COVID-19: A Single-Centered, Retrospective Study. Front. Med. 2020 Jun 9;7:315. 10.3389/fmed.2020.00315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Pal R, Ram S, Zohmangaihi D, et al. : High Prevalence of Hypocalcemia in Non-severe COVID-19 Patients: A Retrospective Case-Control Study. Front. Med. 2021 Jan 7;7:590805. 10.3389/fmed.2020.590805 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Cappellini F, Brivio R, Casati M, et al. : Low levels of total and ionized calcium in blood of COVID-19 patients. Clinical Chemistry and Laboratory Medicine (CCLM). 2020 Aug 27;58(9):e171–e173. 10.1515/cclm-2020-0611 [DOI] [PubMed] [Google Scholar]
- 28. Crespi B, Alcock J: Conflicts over calcium and the treatment of COVID-19. Evolution, Medicine, and Public Health. 2021 Feb 26;9(1):149–156. 10.1093/emph/eoaa046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Bennouar S, Cherif AB, Kessira A, et al. : Vitamin D Deficiency and Low Serum Calcium as Predictors of Poor Prognosis in Patients with Severe COVID-19. J. Am. Coll. Nutr. 2021 Feb;40(2):104–110. 10.1080/07315724.2020.1856013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Zhou X, Chen D, Wang L, et al. : Low serum calcium: a new, important indicator of COVID-19 patients from mild/moderate to severe/critical. Biosci. Rep. 2020 Nov 30;40:BSR20202690. 10.1042/BSR20202690 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Liu J, Han P, Wu J, et al. : Prevalence and predictive value of hypocalcemia in severe COVID-19 patients. J. Infect. Public Health. 2020 Sep;13(9):1224–1228. 10.1016/j.jiph.2020.05.029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Lippi G, South AM, Henry BM: Electrolyte imbalances in patients with severe coronavirus disease 2019 (COVID-19). Ann. Clin. Biochem. 2020 May;57(3):262–265. 10.1177/0004563220922255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. COVID-19 Autopsy : Electronic address: anapat.hrc@salud.madrid.org. The first COVID-19 autopsy in Spain performed during the early stages of the pandemic. Rev. Esp. Patol. 2020 Sep;53(3):182–187. 10.1016/j.patol.2020.05.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Chen X, Cao R, Zhong W: Host Calcium Channels and Pumps in Viral Infections. Cells. 2019 Dec 30;9(1):E94. 10.3390/cells9010094 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Maher P, Leyen K, Dey PN, et al. : The role of Ca2+ in cell death caused by oxidative glutamate toxicity and ferroptosis. Cell Calcium. 2018 Mar;70:47–55. 10.1016/j.ceca.2017.05.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Balasubramanyam M: COVID-19: Is it time to revisit the research on calcium channel drug targets?. EMJ Diabet. 2020 Jun 8 [cited 2021 Mar 21]. 10.33590/emjdiabet/200608 Reference Source [DOI] [Google Scholar]
- 37. Zhang L-K, Sun Y, Zeng H, et al. : Calcium channel blocker amlodipine besylate therapy is associated with reduced case fatality rate of COVID-19 patients with hypertension. Cell Discov. 2020 Dec 22;6(1):96. 10.1038/s41421-020-00235-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Straus MR, Bidon M, Tang T, et al. : FDA approved calcium channel blockers inhibit SARS-CoV-2 infectivity in epithelial lung cells. Microbiology. 2020 Jul [cited 2021 Mar 21]. 10.1101/2020.07.21.214577 [DOI] [Google Scholar]
- 39. Solaimanzadeh I: Nifedipine and Amlodipine Are Associated With Improved Mortality and Decreased Risk for Intubation and Mechanical Ventilation in Elderly Patients Hospitalized for COVID-19. Cureus. 2020 May 12 [cited 2021 Mar 21];12:e8069. 10.7759/cureus.8069 Reference Source [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Straus MR, Bidon MK, Tang T, et al. : Inhibitors of L-Type Calcium Channels Show Therapeutic Potential for Treating SARS-CoV-2 Infections by Preventing Virus Entry and Spread. ACS Infect. Dis. 2021 Oct 8;7(10):2807–2815. 10.1021/acsinfecdis.1c00023 [DOI] [PubMed] [Google Scholar]
- 41. Alsagaff MY, Mulia EPB, Maghfirah I, et al. : Association of calcium channel blocker use with clinical outcome of COVID-19: A meta-analysis. Diabetes Metab. Syndr. 2021 Oct;15(5):102210. 10.1016/j.dsx.2021.102210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Peng C, Wang H, Guo Y-F, et al. : Calcium channel blockers improve prognosis of patients with coronavirus disease 2019 and hypertension. Chin. Med. J. 2021 Jun 16;134(13):1602–1609. 10.1097/CM9.0000000000001479 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Mattson MP: Calcium and neurodegeneration. Aging Cell. 2007 Jun;6(3):337–350. 10.1111/j.1474-9726.2007.00275.x [DOI] [PubMed] [Google Scholar]
- 44. Cataldi M: The Changing Landscape of Voltage-Gated Calcium Channels in Neurovascular Disorders and in Neurodegenerative Diseases. CN. 2013 Apr 1;11(3):276–297. 10.2174/1570159X11311030004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Shimizu C, Eleftherohorinou H, Wright VJ, et al. : Genetic Variation in the SLC8A1 Calcium Signaling Pathway Is Associated With Susceptibility to Kawasaki Disease and Coronary Artery Abnormalities. Circ. Cardiovasc. Genet. 2016 Dec;9(6):559–568. 10.1161/CIRCGENETICS.116.001533 [DOI] [PubMed] [Google Scholar]
- 46. Andrade A, Brennecke A, Mallat S, et al. : Genetic Associations between Voltage-Gated Calcium Channels and Psychiatric Disorders. IJMS. 2019 Jul 19;20(14):3537. 10.3390/ijms20143537 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Zamponi GW, Striessnig J, Koschak A, et al. : The Physiology, Pathology, and Pharmacology of Voltage-Gated Calcium Channels and Their Future Therapeutic Potential. Pharmacol. Rev. 2015 Oct;67(4):821–870. 10.1124/pr.114.009654 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Zamponi GW: Targeting voltage-gated calcium channels in neurological and psychiatric diseases. Nat. Rev. Drug Discov. 2016 Jan;15(1):19–34. 10.1038/nrd.2015.5 [DOI] [PubMed] [Google Scholar]
- 49. Hu W, Lv X, Li C, et al. : Disorders of sodium balance and its clinical implications in COVID-19 patients: a multicenter retrospective study. Intern. Emerg. Med. 2021 Jun;16(4):853–862. 10.1007/s11739-020-02515-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Berni A, Malandrino D, Corona G, et al. : Serum sodium alterations in SARS CoV-2 (COVID-19) infection: impact on patient outcome. Eur. J. Endocrinol. 2021 May 28;185(1):137–144. 10.1530/EJE-20-1447 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Stevens JS, Moses AA, Nickolas TL, et al. : Increased Mortality Associated with Hypermagnesemia in Severe COVID-19 Illness. Kidney360. 2021 Jul 29;2(7):1087–1094. 10.34067/KID.0002592021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Causton HC: SARS-CoV2 Infection and the Importance of Potassium Balance. Front Med (Lausanne). 2021;8:744697. 10.3389/fmed.2021.744697 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Wang K, Chen W, Zhang Z, et al. : CD147-spike protein is a novel route for SARS-CoV-2 infection to host cells. Signal Transduct. Target. Ther. 2020 Dec 4;5(1):283. 10.1038/s41392-020-00426-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Ulrich H, Pillat MM: CD147 as a Target for COVID-19 Treatment: Suggested Effects of Azithromycin and Stem Cell Engagement. Stem Cell Rev. Rep. 2020 Apr 20;16:434–440. 10.1007/s12015-020-09976-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Maugeri N, De Lorenzo R, Clementi N, et al. : Unconventional CD147-dependent platelet activation elicited by SARS-CoV-2 in COVID-19. J. Thromb. Haemost. 2021 Oct 28;20:434–448. 10.1111/jth.15575 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Fenizia C, Galbiati S, Vanetti C, et al. : SARS-CoV-2 Entry: At the Crossroads of CD147 and ACE2. Cells. 2021 Jun 8;10(6):1434. 10.3390/cells10061434 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Evans JP, Liu S-L: Role of host factors in SARS-CoV-2 entry. J. Biol. Chem. 2021 Jul;297(1):100847. 10.1016/j.jbc.2021.100847 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Helal MA, Shouman S, Abdelwaly A, et al. : Molecular basis of the potential interaction of SARS-CoV-2 spike protein to CD147 in COVID-19 associated-lymphopenia. J. Biomol. Struct. Dyn. 2020 Sep 16;1–11. 10.1080/07391102.2020.1822208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Ragotte RJ, Pulido D, Donnellan FR, et al. : Human Basigin (CD147) Does Not Directly Interact with SARS-CoV-2 Spike Glycoprotein. Frieman MB, editor. mSphere. 2021 Aug 25 [cited 2021 Dec 12];6(4):e0064721. 10.1128/mSphere.00647-21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Cooling L: Blood Groups in Infection and Host Susceptibility. Clin. Microbiol. Rev. 2015 Jul 1;28(3):801–870. 10.1128/CMR.00109-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Loh D: The potential of melatonin in the prevention and attenuation of oxidative hemolysis and myocardial injury from cd147 SARS-CoV-2 spike protein receptor binding. Melatonin Res. 2020 Jun 15;3(3):380–416. 10.32794/mr11250069 [DOI] [Google Scholar]
- 62. Radzikowska U, Ding M, Tan G, et al. : Distribution of ACE2, CD147, CD26, and other SARS-CoV-2 associated molecules in tissues and immune cells in health and in asthma, COPD, obesity, hypertension, and COVID-19 risk factors. Allergy. 2020 Nov;75(11):2829–2845. 10.1111/all.14429 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Behl T, Kaur I, Aleya L, et al. : CD147-spike protein interaction in COVID-19: Get the ball rolling with a novel receptor and therapeutic target. Sci. Total Environ. 2021 Dec 1;808:152072. 10.1016/j.scitotenv.2021.152072 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Lecca P, Carpentieri B, Sylos Labini P, et al. : Analysis of SARS-CoV-2 protein interactome map. Workshop on Integrative Data Analysis in Systems Biology (IDASB 2021) presented at: IEEE BIBM 2021. 2021 12/12.
- 65. Williams AF, Barclay AN: The immunoglobulin superfamily--domains for cell surface recognition. Annu. Rev. Immunol. 1988;6:381–405. 10.1146/annurev.iy.06.040188.002121 [DOI] [PubMed] [Google Scholar]
- 66. Muramatsu T: Basigin (CD147), a multifunctional transmembrane glycoprotein with various binding partners. J. Biochem. 2016 May;159(5):481–490. 10.1093/jb/mvv127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Zhu X, Song Z, Zhang S, et al. : CD147: a novel modulator of inflammatory and immune disorders. Curr. Med. Chem. 2014;21(19):2138–2145. 10.2174/0929867321666131227163352 [DOI] [PubMed] [Google Scholar]
- 68. Varga Z, Flammer AJ, Steiger P, et al. : Endothelial cell infection and endotheliitis in COVID-19. Lancet. 2020 May 2;395(10234):1417–1418. 10.1016/S0140-6736(20)30937-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Ahmetaj-Shala B, Vaja R, Atanur SS, et al. : Cardiorenal Tissues Express SARS-CoV-2 Entry Genes and Basigin (BSG/CD147) Increases With Age in Endothelial Cells. JACC Basic Transl. Sci. 2020 Nov;5(11):1111–1123. 10.1016/j.jacbts.2020.09.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Dalan R, Boehm BO: The implications of COVID-19 infection on the endothelium: A metabolic vascular perspective. Diabetes Metab. Res. Rev. 2021 Mar;37(3):e3402. 10.1002/dmrr.3402 [DOI] [PubMed] [Google Scholar]
- 71. Iesa M a M, Osman MEM, Hassan MA, et al. : SARS-CoV-2 and Plasmodium falciparum common immunodominant regions may explain low COVID-19 incidence in the malaria-endemic belt. New Microbes New Infect. 2020 Nov;38:100817. 10.1016/j.nmni.2020.100817 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Shahbaz S, Xu L, Osman M, et al. : Erythroid precursors and progenitors suppress adaptive immunity and get invaded by SARS-CoV-2. Stem Cell Reports. 2021 May 11;16(5):1165–1181. 10.1016/j.stemcr.2021.04.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Bortolotti D, Simioni C, Neri LM, et al. : Relevance of VEGF and CD147 in different SARS-CoV-2 positive digestive tracts characterized by thrombotic damage. FASEB J. 2021 Dec;35(12):e21969. 10.1096/fj.202100821RRR [DOI] [PubMed] [Google Scholar]
- 74. Corrao S, Pinelli K, Vacca M, et al. : Type 2 Diabetes Mellitus and COVID-19: A Narrative Review. Front. Endocrinol (Lausanne). 2021;12:609470. 10.3389/fendo.2021.609470 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. D’Errico S, Zanon M, Montanaro M, et al. : More than Pneumonia: Distinctive Features of SARS-Cov-2 Infection. From Autopsy Findings to Clinical Implications: A Systematic Review. Microorganisms. 2020 Oct 23;8(11):E1642. 10.3390/microorganisms8111642 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Menicagli R, Limodio M: COVID-19 Solution. Int. J. Prev. Med. 2020;11:73. 10.4103/ijpvm.IJPVM_227_20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Robson B: Bioinformatics studies on a function of the SARS-CoV-2 spike glycoprotein as the binding of host sialic acid glycans. Comput. Biol. Med. 2020 Jul;122:103849. 10.1016/j.compbiomed.2020.103849 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Sun X-L: The role of cell surface sialic acids for SARS-CoV-2 infection. Glycobiology. 2021 Nov 18;31(10):1245–1253. 10.1093/glycob/cwab032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Kim C-H: SARS-CoV-2 Evolutionary Adaptation toward Host Entry and Recognition of Receptor O-Acetyl Sialylation in Virus-Host Interaction. Int. J. Mol. Sci. 2020 Jun 26;21(12):E4549. 10.3390/ijms21124549 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Pruimboom L: SARS-CoV 2; Possible alternative virus receptors and pathophysiological determinants. Med. Hypotheses. 2021 Jan;146:110368. 10.1016/j.mehy.2020.110368 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Wielgat P, Rogowski K, Godlewska K, et al. : Coronaviruses: Is Sialic Acid a Gate to the Eye of Cytokine Storm? From the Entry to the Effects. Cells. 2020 Aug 25;9(9):E1963. 10.3390/cells9091963 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Morniroli D, Giannì ML, Consales A, et al. : Human Sialome and Coronavirus Disease-2019 (COVID-19) Pandemic: An Understated Correlation?. Front. Immunol. 2020;11:1480. 10.3389/fimmu.2020.01480 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Uraki R, Kawaoka Y: Host glycolipids in SARS-CoV-2 entry. Nat Chem Biol. 2021 Nov 9 [cited 2021 Dec 14]. Reference Source [DOI] [PubMed] [Google Scholar]
- 84. Varki A, Schauer R: Sialic Acids. Varki A, Cummings RD, Esko JD, et al., editors. Essentials of Glycobiology. 2nd ed. Cold Spring Harbor (NY): Cold Spring Harbor Laboratory Press;2009 [cited 2021 Dec 28]. Reference Source [Google Scholar]
- 85. Kuchipudi SV, Nelli RK, Gontu A, et al. : Sialic Acid Receptors: The Key to Solving the Enigma of Zoonotic Virus Spillover. Viruses. 2021 Feb 8;13(2):262. 10.3390/v13020262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Li Y, Liu D, Wang Y, et al. : The Importance of Glycans of Viral and Host Proteins in Enveloped Virus Infection. Front. Immunol. 2021 Apr 29;12:638573. 10.3389/fimmu.2021.638573 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Jackson CB, Farzan M, Chen B, et al. : Mechanisms of SARS-CoV-2 entry into cells. Nat. Rev. Mol. Cell Biol. 2022 Jan;23(1):3–20. 10.1038/s41580-021-00418-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Silva-Filho JC, Melo CGF, Oliveira JL: The influence of ABO blood groups on COVID-19 susceptibility and severity: A molecular hypothesis based on carbohydrate-carbohydrate interactions. Med. Hypotheses. 2020 Nov;144:110155. 10.1016/j.mehy.2020.110155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Dhar C, Sasmal A, Diaz S, et al. : Are sialic acids involved in COVID-19 pathogenesis?. Glycobiology. 2021 Sep 20;31(9):1068–1071. 10.1093/glycob/cwab063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Eylar EH, Madoff MA, Brody OV, et al. : The contribution of sialic acid to the surface charge of the erythrocyte. J. Biol. Chem. 1962 Jun;237:1992–2000. 10.1016/S0021-9258(19)73972-6 [DOI] [PubMed] [Google Scholar]
- 91. Udoh AE: Distribution of sialic acid between sialoglycoproteins and other membrane components of different erythrocyte phenotypes. Acta Physiol. Hung. 1991;78(3):265–273. [PubMed] [Google Scholar]
- 92. Izumida Y, Seiyama A, Maeda N: Erythrocyte aggregation: bridging by macromolecules and electrostatic repulsion by sialic acid. Biochim. Biophys. Acta. 1991 Aug 26;1067(2):221–226. 10.1016/0005-2736(91)90047-C [DOI] [PubMed] [Google Scholar]
- 93. Vayá A, Martínez Triguero M, Ricart A, et al. : Erythrocyte aggregability and AB0 blood groups. Clin. Hemorheol. Microcirc. 2009;41(1):67–72. 10.3233/CH-2009-1163 [DOI] [PubMed] [Google Scholar]
- 94. Cohen M, Hurtado-Ziola N, Varki A: ABO blood group glycans modulate sialic acid recognition on erythrocytes. Blood. 2009 Oct 22;114(17):3668–3676. 10.1182/blood-2009-06-227041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Piagnerelli M, Boudjeltia KZ, Brohee D, et al. : Alterations of red blood cell shape and sialic acid membrane content in septic patients. Crit. Care Med. 2003 Aug;31(8):2156–2162. 10.1097/01.CCM.0000079608.00875.14 [DOI] [PubMed] [Google Scholar]
- 96. Graaf M, Fouchier RAM: Role of receptor binding specificity in influenza A virus transmission and pathogenesis. EMBO J. 2014 Apr 16;33(8):823–841. 10.1002/embj.201387442 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Matrosovich M, Herrler G, Klenk HD: Sialic Acid Receptors of Viruses. SialoGlyco Chemistry and Biology II. 2013 Jul 20;367:1–28. 10.1007/128_2013_466 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Cicconetti F, Sestili P, Madiai V, et al. : Extracellular pH, osmolarity, temperature and humidity could discourage SARS-CoV-2 cell docking and propagation via intercellular signaling pathways. PeerJ. 2021;9:e12227. 10.7717/peerj.12227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Alonso DM: Characterization of cationic conductances of human erythrocytes and their involvement in health and disease [phdthesis]. Sorbonne Université;2019 [cited 2021 Dec 28]. Reference Source [Google Scholar]
- 100. Glaser R, Fujii T, Müller P, et al. : Erythrocyte shape dynamics: influence of electrolyte conditions and membrane potential. Biomed. Biochim. Acta. 1987;46(2–3):S327–S333. [PubMed] [Google Scholar]
- 101. Huang Y, Yang C, Xu X-F, et al. : Structural and functional properties of SARS-CoV-2 spike protein: potential antivirus drug development for COVID-19. Acta Pharmacol. Sin. 2020 Sep;41(9):1141–1149. 10.1038/s41401-020-0485-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Zhao X, Chen H, Wang H: Glycans of SARS-CoV-2 Spike Protein in Virus Infection and Antibody Production. Front. Mol. Biosci. 2021;8:629873. 10.3389/fmolb.2021.629873 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Sepahvandi A, Ghaffari M, Bahmanpour AH, et al. : COVID-19: insights into virus–receptor interactions. Mol. Biomed. 2021 Apr 10;2:10. 10.1186/s43556-021-00033-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Wang C-W, Lee OK, Fischer WB, et al. : Screening coronavirus and human proteins for sialic acid binding sites using a docking approach. AIMSBPOA. 2021;8(3):248–263. 10.3934/biophy.2021019 [DOI] [Google Scholar]
- 105. Ghosh S: Sialic acid and biology of life: An introduction. Sialic Acids and Sialoglycoconjugates in the Biology of Life, Health and Disease. 2020;1–61. 10.1016/B978-0-12-816126-5.00001-9 [DOI] [Google Scholar]
- 106. Chu H, Hu B, Huang X, et al. : Host and viral determinants for efficient SARS-CoV-2 infection of the human lung. Nat. Commun. 2021 Jan 8;12(1):134. 10.1038/s41467-020-20457-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Severe Covid-19 GWAS Group. Ellinghaus D, Degenhardt F, et al. : Genomewide Association Study of Severe Covid-19 with Respiratory Failure. N. Engl. J. Med. 2020 Oct 15;383(16):1522–1534. 10.1056/NEJMoa2020283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Wu S-C, Arthur CM, Wang J, et al. : The SARS-CoV-2 receptor-binding domain preferentially recognizes blood group A. Blood Adv. 2021 Mar 9;5(5):1305–1309. 10.1182/bloodadvances.2020003259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Arend P: Why blood group A individuals are at risk whereas blood group O individuals are protected from SARS-CoV-2 (COVID-19) infection: A hypothesis regarding how the virus invades the human body via ABO(H) blood group-determining carbohydrates. Immunobiology. 2021 May;226(3):152027. 10.1016/j.imbio.2020.152027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Aminoff D, Anderson J, Dabich L, et al. : Sialic acid content of erythrocytes in normal individuals and patients with certain hematologic disorders. Am. J. Hematol. 1980;9(4):381–389. 10.1002/ajh.2830090405 [DOI] [PubMed] [Google Scholar]
- 111. Karimi Shahri M, Niazkar HR, Rad F: COVID-19 and hematology findings based on the current evidences: A puzzle with many missing pieces. Int. J. Lab. Hematol. 2021 Apr;43(2):160–168. 10.1111/ijlh.13412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Tao Z, Xu J, Chen W, et al. : Anemia is associated with severe illness in COVID-19: A retrospective cohort study. J. Med. Virol. 2021 Mar;93(3):1478–1488. 10.1002/jmv.26444 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Kuno T, Miyamoto Y, Iwagami M, et al. : The association of hemoglobin drop with in-hospital outcomes in COVID-19 patients. QJM. 2021 Oct 1;114:789–794. 10.1093/qjmed/hcab251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Ballaz SJ, Pulgar-Sánchez M, Chamorro K, et al. : Common laboratory tests as indicators of COVID-19 severity on admission at high altitude: a single-center retrospective study in Quito (ECUADOR). Clin. Chem. Lab. Med. 2021 Jul 27;59(8):e326–e329. 10.1515/cclm-2021-0156 [DOI] [PubMed] [Google Scholar]
- 115. Adamidi ES, Mitsis K, Nikita KS: Artificial intelligence in clinical care amidst COVID-19 pandemic: A systematic review. Comput. Struct. Biotechnol. J. 2021;19:2833–2850. 10.1016/j.csbj.2021.05.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Jandaghian S, Vaezi A, Manteghinejad A, et al. : Red Blood Cell Distribution Width (RDW) as a Predictor of In-Hospital Mortality in COVID-19 Patients; a Cross Sectional Study. Arch. Acad. Emerg. Med. 2021;9(1):e67. 10.22037/aaem.v9i1.1325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Cihakova D, Streiff MB, Menez SP, et al. : High-value laboratory testing for hospitalized COVID-19 patients: a review. Futur. Virol. 2021 Sep;16:691–705. 10.2217/fvl-2020-0316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Saberiyan M, Safi A, Kamel A, et al. : An Overview on the Common Laboratory Parameter Alterations and their Related Molecular Pathways in Screening for COVID-19 Patients. Clin. Lab. 2020 Oct 1;66(10) 10.7754/Clin.Lab.2020.200705 [DOI] [PubMed] [Google Scholar]
- 119. Goud PT, Bai D, Abu-Soud HM: A Multiple-Hit Hypothesis Involving Reactive Oxygen Species and Myeloperoxidase Explains Clinical Deterioration and Fatality in COVID-19. Int. J. Biol. Sci. 2021;17(1):62–72. 10.7150/ijbs.51811 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Sahu KK, Borogovac A, Cerny J: COVID-19 related immune hemolysis and thrombocytopenia. J. Med. Virol. 2021 Feb;93(2):1164–1170. 10.1002/jmv.26402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Lazarian G, Quinquenel A, Bellal M, et al. : Autoimmune haemolytic anaemia associated with COVID-19 infection. Br. J. Haematol. 2020 Jul;190(1):29–31. 10.1111/bjh.16794 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Abrahams L: Covid-19: acquired acute porphyria?. Preprints. 2021 Jul [cited 2021 Dec 28]. Reference Source
- 123. Algassim AA, Elghazaly AA, Alnahdi AS, et al. : Prognostic significance of hemoglobin level and autoimmune hemolytic anemia in SARS-CoV-2 infection. Ann. Hematol. 2021 Jan;100(1):37–43. 10.1007/s00277-020-04256-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Majeed A, Shajar MA: Is hemoglobin the missing link in the pathogenesis of COVID-19?. APIC. 2020 May 7;24(1):9–12. 10.35975/apic.v24i1.1216 [DOI] [Google Scholar]
- 125. Lancman G, Marcellino BK, Thibaud S, et al. : Coombs-negative hemolytic anemia and elevated plasma hemoglobin levels in COVID-19. Ann. Hematol. 2021 Mar;100(3):833–835. 10.1007/s00277-020-04202-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Su W-L, Lin C-P, Hang H-C, et al. : Desaturation and heme elevation during COVID-19 infection: A potential prognostic factor of heme oxygenase-1. J. Microbiol. Immunol. Infect. 2021 Feb;54(1):113–116. 10.1016/j.jmii.2020.10.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Comentale G, Manzo R, Pilato E: Sars-Cov-2 interference in HEME production: is it the time for an early predictive biomarker?. J. Mol. Med. 2020 Aug;98(8):1053–1054. 10.1007/s00109-020-01945-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Hopp M-T, Domingo-Fernández D, Gadiya Y, et al. : Linking COVID-19 and Heme-Driven Pathophysiologies: A Combined Computational-Experimental Approach. Biomolecules. 2021 Apr 27;11(5):644. 10.3390/biom11050644 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. San Juan I, Bruzzone C, Bizkarguenaga M, et al. : Abnormal concentration of porphyrins in serum from COVID-19 patients. Br. J. Haematol. 2020 Sep;190(5):e265–e267. 10.1111/bjh.17060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. Rapozzi V, Juarranz A, Habib A, et al. : Is haem the real target of COVID-19?. Photodiagn. Photodyn. Ther. 2021 Sep;35:102381. 10.1016/j.pdpdt.2021.102381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. Courrol LC, Oliveira Silva FR, Masilamani V: SARS-CoV-2, hemoglobin and protoporphyrin IX: Interactions and perspectives. Photodiagn. Photodyn. Ther. 2021 Jun;34:102324. 10.1016/j.pdpdt.2021.102324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132. DeMartino AW, Rose JJ, Amdahl MB, et al. : No evidence of hemoglobin damage by SARS-CoV-2 infection. Haematologica. 2020 Sep 10;105:2769–2773. 10.3324/haematol.2020.264267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133. Dhont S, Derom E, Van Braeckel E, et al. : The pathophysiology of “happy” hypoxemia in COVID-19. Respir. Res. 2020 Jul 28;21(1):198. 10.1186/s12931-020-01462-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134. Böning D, Kuebler WM, Bloch W: The oxygen dissociation curve of blood in COVID-19. Am. J. Physiol. Lung Cell. Mol. Physiol. 2021 Aug 1;321(2):L349–L357. 10.1152/ajplung.00079.2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135. Gille T, Sesé L, Aubourg E, et al. : The Affinity of Hemoglobin for Oxygen Is Not Altered During COVID-19. Front. Physiol. 2021;12:578708. 10.3389/fphys.2021.578708 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136. Vogel DJ, Formenti F, Retter AJ, et al. : A left shift in the oxyhaemoglobin dissociation curve in patients with severe coronavirus disease 2019 (COVID-19). Br. J. Haematol. 2020 Nov;191(3):390–393. 10.1111/bjh.17128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137. Daniel Y, Hunt BJ, Retter A, et al. : Haemoglobin oxygen affinity in patients with severe COVID-19 infection. Br. J. Haematol. 2020 Aug;190(3):e126–e127. 10.1111/bjh.16888 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138. Shakoori TA, Hafeez MM, Malik A: COULD COVID-19 BE A HEMOGLOBINOPATHY?. Acta Clin. Croat. 2020 Dec;59(4):740–744. 10.20471/acc.2020.59.04.21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139. Woyke S, Rauch S, Ströhle M, et al. : Modulation of Hb-O2 affinity to improve hypoxemia in COVID-19 patients. Clin. Nutr. 2021 Jan;40(1):38–39. 10.1016/j.clnu.2020.04.036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140. Solaimanzadeh I: Acetazolamide, Nifedipine and Phosphodiesterase Inhibitors: Rationale for Their Utilization as Adjunctive Countermeasures in the Treatment of Coronavirus Disease 2019 (COVID-19). Cureus. 12(3):e7343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141. Geier MR, Geier DA: Respiratory conditions in coronavirus disease 2019 (COVID-19): Important considerations regarding novel treatment strategies to reduce mortality. Med. Hypotheses. 2020 Apr 22;140:109760–109760. 10.1016/j.mehy.2020.109760 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142. Luks AM, Swenson ER: COVID-19 Lung Injury and High-Altitude Pulmonary Edema. A False Equation with Dangerous Implications. Ann. Am. Thorac. Soc. 2020 Aug;17(8):918–921. 10.1513/AnnalsATS.202004-327CME [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143. Gattinoni L, Chiumello D, Rossi S: COVID-19 pneumonia: ARDS or not?. Crit. Care. 2020 Apr 16;24(1):154–154. 10.1186/s13054-020-02880-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144. Herrmann J, Mori V, Bates JHT, et al. : Modeling lung perfusion abnormalities to explain early COVID-19 hypoxemia. Nat. Commun. 2020 Dec;11(1):4883. 10.1038/s41467-020-18672-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145. Kyle-Sidell C: COVID-19 Lung Injury and “Typical” Acute Respiratory Distress Syndrome: The Danger of Presumed Equivalency. Ann. Am. Thorac. Soc. 2020 Sep;17(9):1171–1172. 10.1513/AnnalsATS.202005-405LE [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146. Rubano JA, Maloney LM, Simon J, et al. : An Evolving Clinical Need: Discordant Oxygenation Measurements of Intubated COVID-19 Patients. Ann. Biomed. Eng. 2021 Mar;49(3):959–963. 10.1007/s10439-020-02722-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147. Ganz T, Nemeth E: Hepcidin and iron homeostasis. Biochim. Biophys. Acta. 2012 Sep;1823(9):1434–1443. 10.1016/j.bbamcr.2012.01.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148. Ehsani S: COVID-19 and iron dysregulation: distant sequence similarity between hepcidin and the novel coronavirus spike glycoprotein. Biol. Direct. 2020 Oct 16;15(1):19. 10.1186/s13062-020-00275-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149. Hippchen T, Altamura S, Muckenthaler MU, et al. : Hypoferremia is Associated With Increased Hospitalization and Oxygen Demand in COVID-19 Patients. Hemasphere. 2020 Dec;4(6):e492. 10.1097/HS9.0000000000000492 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150. Nieva JL, Madan V, Carrasco L: Viroporins: structure and biological functions. Nat. Rev. Microbiol. 2012 Jul 2;10(8):563–574. 10.1038/nrmicro2820 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151. Nieto-Torres JL, Verdiá-Báguena C, Castaño-Rodriguez C, et al. : Relevance of Viroporin Ion Channel Activity on Viral Replication and Pathogenesis. Viruses. 2015 Jul 3;7(7):3552–3573. 10.3390/v7072786 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152. Schoeman D, Fielding BC: Coronavirus envelope protein: current knowledge. Virol. J. 2019 May 27;16(1):69. 10.1186/s12985-019-1182-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153. Ren Y, Shu T, Wu D, et al. : The ORF3a protein of SARS-CoV-2 induces apoptosis in cells. Cell. Mol. Immunol. 2020 Aug;17(8):881–883. 10.1038/s41423-020-0485-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154. Cao Y, Yang R, Lee I, et al. : Characterization of the SARS-CoV-2 E Protein: Sequence, Structure, Viroporin, and Inhibitors. Protein Sci. 2021 Jun;30(6):1114–1130. 10.1002/pro.4075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155. Yu H-G, Sizemore G, Smoot K, et al. : Detecting SARS-CoV-2 Orf3a and E ion channel activity in COVID-19 blood samples. J. Clin. Transl. Sci. 2021;5(1):e196. 10.1017/cts.2021.856 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156. Cao Y, Yang R, Wang W, et al. : Computational Study of the Ion and Water Permeation and Transport Mechanisms of the SARS-CoV-2 Pentameric E Protein Channel. Front. Mol. Biosci. 2020 Sep 23;7:565797. 10.3389/fmolb.2020.565797 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157. Kern DM, Sorum B, Mali SS, et al. : Cryo-EM structure of SARS-CoV-2 ORF3a in lipid nanodiscs. Nat. Struct. Mol. Biol. 2021 Jul;28(7):573–582. 10.1038/s41594-021-00619-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158. Know L: Mitochondria and the future of medicine: the key to understanding disease, chronic illness, aging, and life itself. White River Junction, Vermont: Chelsea Green Publishing;2018;257. [Google Scholar]
- 159. Cavezzi A: Medicine and Phlebolymphology: Time to Change?. J. Clin. Med. 2020 Dec 18;9(12):E4091. 10.3390/jcm9124091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160. Shenoy S: Coronavirus (Covid-19) sepsis: revisiting mitochondrial dysfunction in pathogenesis, aging, inflammation, and mortality. Inflamm. Res. 2020 Nov;69(11):1077–1085. 10.1007/s00011-020-01389-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161. Ma K, Chen G, Li W, et al. : Mitophagy, Mitochondrial Homeostasis, and Cell Fate. Front. Cell Dev. Biol. 2020;8:467. 10.3389/fcell.2020.00467 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162. Saleh J, Peyssonnaux C, Singh KK, et al. : Mitochondria and microbiota dysfunction in COVID-19 pathogenesis. Mitochondrion. 2020 Sep;54:1–7. 10.1016/j.mito.2020.06.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163. Singh KK, Chaubey G, Chen JY, et al. : Decoding SARS-CoV-2 hijacking of host mitochondria in COVID-19 pathogenesis. Am. J. Physiol. Cell Physiol. 2020 Aug 1;319(2):C258–C267. 10.1152/ajpcell.00224.2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164. Soria-Castro E, Soto ME, Guarner-Lans V, et al. : The kidnapping of mitochondrial function associated with the SARS-CoV-2 infection. Histol. Histopathol. 2021 Jun 16;18354. [DOI] [PubMed] [Google Scholar]
- 165. Burtscher J, Cappellano G, Omori A, et al. : Mitochondria: In the Cross Fire of SARS-CoV-2 and Immunity. iScience. 2020 Oct 23;23(10):101631. 10.1016/j.isci.2020.101631 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166. Henry BM, Aggarwal G, Wong J, et al. : Lactate dehydrogenase levels predict coronavirus disease 2019 (COVID-19) severity and mortality: A pooled analysis. Am. J. Emerg. Med. 2020 Sep;38(9):1722–1726. 10.1016/j.ajem.2020.05.073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167. Iepsen UW, Plovsing RR, Tjelle K, et al. : The role of lactate in sepsis and COVID-19: Perspective from contracting skeletal muscle metabolism. Exp. Physiol. 2021 May 31. 10.1113/EP089474 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168. Wu J: Tackle the free radicals damage in COVID-19. Nitric Oxide. 2020 Sep 1;102:39–41. 10.1016/j.niox.2020.06.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169. Bernardi P: Mitochondrial transport of cations: channels, exchangers, and permeability transition. Physiol. Rev. 1999 Oct;79(4):1127–1155. 10.1152/physrev.1999.79.4.1127 [DOI] [PubMed] [Google Scholar]
- 170. Paul BT, Manz DH, Torti FM, et al. : Mitochondria and Iron: current questions. Expert. Rev. Hematol. 2017 Jan;10(1):65–79. 10.1080/17474086.2016.1268047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171. Battaglia AM, Chirillo R, Aversa I, et al. : Ferroptosis and Cancer: Mitochondria Meet the “Iron Maiden” Cell Death. Cells. 2020 Jun 20;9(6):E1505. 10.3390/cells9061505 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172. Galaris D, Barbouti A, Pantopoulos K: Iron homeostasis and oxidative stress: An intimate relationship. Biochim. Biophys. Acta, Mol. Cell Res. 2019 Dec;1866(12):118535. 10.1016/j.bbamcr.2019.118535 [DOI] [PubMed] [Google Scholar]
- 173. Bertero E, Maack C: Calcium Signaling and Reactive Oxygen Species in Mitochondria. Circ. Res. 2018 May 11;122(10):1460–1478. 10.1161/CIRCRESAHA.118.310082 [DOI] [PubMed] [Google Scholar]
- 174. Orrenius S, Gogvadze V, Zhivotovsky B: Calcium and mitochondria in the regulation of cell death. Biochem. Biophys. Res. Commun. 2015 Apr 24;460(1):72–81. 10.1016/j.bbrc.2015.01.137 [DOI] [PubMed] [Google Scholar]
- 175. Dixon SJ: Ferroptosis: bug or feature?. Immunol. Rev. 2017 May;277(1):150–157. 10.1111/imr.12533 [DOI] [PubMed] [Google Scholar]
- 176. Cao JY, Dixon SJ: Mechanisms of ferroptosis. Cell. Mol. Life Sci. 2016 Jun;73(11–12):2195–2209. 10.1007/s00018-016-2194-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177. Li J, Cao F, Yin H-L, et al. : Ferroptosis: past, present and future. Cell Death Dis. 2020 Feb 3;11(2):88. 10.1038/s41419-020-2298-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178. Toyokuni S, Yanatori I, Kong Y, et al. : Ferroptosis at the crossroads of infection, aging and cancer. Cancer Sci. 2020 Aug;111(8):2665–2671. 10.1111/cas.14496 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179. Hino K, Nishina S, Sasaki K, et al. : Mitochondrial damage and iron metabolic dysregulation in hepatitis C virus infection. Free Radic. Biol. Med. 2019 Mar;133:193–199. 10.1016/j.freeradbiomed.2018.09.044 [DOI] [PubMed] [Google Scholar]
- 180. Sekine S, Ito K, Watanabe H, et al. : Mitochondrial iron accumulation exacerbates hepatic toxicity caused by hepatitis C virus core protein. Toxicol. Appl. Pharmacol. 2015 Feb 1;282(3):237–243. 10.1016/j.taap.2014.12.004 [DOI] [PubMed] [Google Scholar]
- 181. Yang M, Lai CL: SARS-CoV-2 infection: can ferroptosis be a potential treatment target for multiple organ involvement?. Cell Death Dis. 2020 Nov 25;6(1):130–136. 10.1038/s41420-020-00369-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182. Fratta Pasini AM, Stranieri C, Girelli D, et al. : Is Ferroptosis a Key Component of the Process Leading to Multiorgan Damage in COVID-19?. Antioxidants (Basel). 2021 Oct 25;10(11):1677. 10.3390/antiox10111677 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183. Yilmaz N, Eren E: Covid-19’s passion for iron and fear of oxygen: Perhaps covid- 19 craves the atmospheric environment in ancient times. 2020.
- 184. Menshawey R, Menshawey E, Alserr AHK, et al. : Low iron mitigates viral survival: insights from evolution, genetics, and pandemics—a review of current hypothesis. Egypt. J. Med. Hum. Genet. 2020 Dec;21(1):75. 10.1186/s43042-020-00114-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185. Liu W, Zhang S, Nekhai S, et al. : Depriving Iron Supply to the Virus Represents a Promising Adjuvant Therapeutic Against Viral Survival. Curr. Clin. Microbiol. Rep. 2020 Apr 20;7:13–19. 10.1007/s40588-020-00140-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186. Schmidt S: The role of iron in viral infections. Frontiers in bioscience (Landmark edition). 2020 Jan 1;25:893–911. 10.2741/4839 [DOI] [PubMed] [Google Scholar]
- 187. Drakesmith H, Prentice A: Viral infection and iron metabolism. Nat. Rev. Microbiol. 2008 Jul;6(7):541–552. 10.1038/nrmicro1930 [DOI] [PubMed] [Google Scholar]
- 188. Amaral EP, Namasivayam S: Emerging Role for Ferroptosis in Infectious Diseases. Adv. Exp. Med. Biol. 2021;1301:59–79. 10.1007/978-3-030-62026-4_5 [DOI] [PubMed] [Google Scholar]
- 189. Dorward DA, Russell CD, Um IH, et al. : Tissue-Specific Immunopathology in Fatal COVID-19. Am. J. Respir. Crit. Care Med. 2021 Jan 15;203(2):192–201. 10.1164/rccm.202008-3265OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190. Jacobs W, Lammens M, Kerckhofs A, et al. : Fatal lymphocytic cardiac damage in coronavirus disease 2019 (COVID-19): autopsy reveals a ferroptosis signature. ESC Heart Fail. 2020 Sep 22;7:3772–3781. 10.1002/ehf2.12958 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191. Del Nonno F, Nardacci R, Colombo D, et al. : Hepatic Failure in COVID-19: Is Iron Overload the Dangerous Trigger?. Cells. 2021 May 4;10(5):1103. 10.3390/cells10051103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192. Colafrancesco S, Alessandri C, Conti F, et al. : COVID-19 gone bad: A new character in the spectrum of the hyperferritinemic syndrome?. Autoimmun. Rev. 2020 May 5;19:102573. 10.1016/j.autrev.2020.102573 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193. Harris CK, Hung YP, Nielsen GP, et al. : Bone Marrow and Peripheral Blood Findings in Patients Infected by SARS-CoV-2. Am. J. Clin. Pathol. 2021 Jan 27;155:627–637. 10.1093/ajcp/aqaa274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194. Swoboda J, Wittschieber D, Sanft J, et al. : Bone marrow haemophagocytosis indicates severe infection with severe acute respiratory syndrome coronavirus 2. Histopathology. 2021 Apr;78(5):727–737. 10.1111/his.14281 [DOI] [PubMed] [Google Scholar]
- 195. Neves J, Haider T, Gassmann M, et al. : Iron Homeostasis in the Lungs-A Balance between Health and Disease. Pharmaceuticals (Basel). 2019 Jan 1;12(1) 10.3390/ph12010005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196. Ray A, Jaiswal A, Dutta J, et al. : A looming role of mitochondrial calcium in dictating the lung epithelial integrity and pathophysiology of lung diseases. Mitochondrion. 2020 Nov;55:111–121. 10.1016/j.mito.2020.09.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197. Zamorano Cuervo N, Grandvaux N: ACE2: Evidence of role as entry receptor for SARS-CoV-2 and implications in comorbidities. elife. 2020 Nov 9;9:e61390. 10.7554/eLife.61390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198. Takeda M: Proteolytic activation of SARS-CoV-2 spike protein. Microbiol. Immunol. 2021 Sep 25;66:15–23. 10.1111/1348-0421.12945 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199. Seyran M, Takayama K, Uversky VN, et al. : The structural basis of accelerated host cell entry by SARS-CoV-2†. FEBS J. 2021 Sep;288(17):5010–5020. 10.1111/febs.15651 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200. Kim JS, Lee JY, Yang JW, et al. : Immunopathogenesis and treatment of cytokine storm in COVID-19. Theranostics. 2021;11(1):316–329. 10.7150/thno.49713 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201. Zhou C, Chen Y, Ji Y, et al. : Increased Serum Levels of Hepcidin and Ferritin Are Associated with Severity of COVID-19. Med. Sci. Monit. 2020 Sep 26;26:e926178. 10.12659/MSM.926178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202. Nai A, Lorè NI, Pagani A, et al. : Hepcidin levels predict Covid-19 severity and mortality in a cohort of hospitalized Italian patients. Am. J. Hematol. 202 Jan 1;96(1):E32–E35. 10.1002/ajh.26027 [DOI] [PubMed] [Google Scholar]
- 203. Banchini F, Vallisa D, Maniscalco P, et al. : Iron overload and Hepcidin overexpression could play a key role in COVID infection, and may explain vulnerability in elderly, diabetics, and obese patients. Acta Biomed. 2020 Sep 7;91(3):e2020013. 10.23750/abm.v91i3.9826 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204. Bastin A, Shiri H, Zanganeh S, et al. : Iron Chelator or Iron Supplement Consumption in COVID-19? The Role of Iron with Severity Infection. Biol. Trace Elem. Res. 2021 Nov 25 [cited 2021 Dec 28]. 10.1007/s12011-021-03048-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205. Visser EA, Moons SJ, Timmermans SBPE, et al. : Sialic acid O-acetylation: From biosynthesis to roles in health and disease. J. Biol. Chem. 2021 Aug;297(2):100906. 10.1016/j.jbc.2021.100906 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206. Schauer R, Kamerling JP: Exploration of the Sialic Acid World. Adv. Carbohydr. Chem. Biochem. 2018;75:1–213. 10.1016/bs.accb.2018.09.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207. Pawłowski PH, Institute of Biochemistry and Biophysics, Polish Academy of Sciences, Warszawa, Poland : Charged amino acids may promote coronavirus SARS-CoV-2 fusion with the host cell. AIMS Biophysics. 2021;8(1):111–121. 10.3934/biophy.2021008 [DOI] [Google Scholar]
- 208. Corrêa Giron C, Laaksonen A, Barroso da Silva FL: On the interactions of the receptor-binding domain of SARS-CoV-1 and SARS-CoV-2 spike proteins with monoclonal antibodies and the receptor ACE2. Virus Res. 2020 Aug;285:198021. 10.1016/j.virusres.2020.198021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209. Tamagawa H: Mathematical expression of membrane potential based on Ling’s adsorption theory is approximately the same as the Goldman–Hodgkin–Katz equation. J. Biol. Phys. 2019 Mar;45(1):13–30. 10.1007/s10867-018-9512-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210. Hammond C: Cellular and molecular neurophysiology. Fourth edition. Amsterdam, Boston: Elsevier/AP, Academic Press is an imprint of Elsevier;2015;433. [Google Scholar]
- 211. Mansor M, Ahmad M: Single Cell Electrical Characterization Techniques. IJMS. 2015 Jun 4;16(12):12686–12712. 10.3390/ijms160612686 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212. Cheng B, Xie R, Dong L, et al. : Metabolic Remodeling of Cell-Surface Sialic Acids: Principles, Applications, and Recent Advances. Chembiochem. 2016 Jan 1;17(1):11–27. 10.1002/cbic.201500344 [DOI] [PubMed] [Google Scholar]
- 213. Schnaar RL: The Biology of Gangliosides. Adv. Carbohydr. Chem. Biochem. 2019;76:113–148. 10.1016/bs.accb.2018.09.002 [DOI] [PubMed] [Google Scholar]
- 214. Katsumoto Y, Tatsumi K, Doi T, et al. : Electrical classification of single red blood cell deformability in high-shear microchannel flows. Int. J. Heat Fluid Flow. 2010 Dec;31(6):985–995. 10.1016/j.ijheatfluidflow.2010.02.019 [DOI] [Google Scholar]
- 215. Aoki T: A Comprehensive Review of Our Current Understanding of Red Blood Cell (RBC) Glycoproteins. Membranes (Basel). 2017 Sep 29;7(4):E56. 10.3390/membranes7040056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216. Lazniewska J, Weiss N: Glycosylation of voltage-gated calcium channels in health and disease. Biochim. Biophys. Acta Biomembr. 2017 May;1859(5):662–668. 10.1016/j.bbamem.2017.01.018 [DOI] [PubMed] [Google Scholar]
- 217. Ednie AR, Bennett ES: Modulation of voltage-gated ion channels by sialylation. Compr. Physiol. 2012 Apr;2(2):1269–1301. 10.1002/cphy.c110044 [DOI] [PubMed] [Google Scholar]
- 218. Catterall WA: Structure and regulation of voltage-gated Ca2+ channels. Annu. Rev. Cell Dev. Biol. 2000;16:521–555. 10.1146/annurev.cellbio.16.1.521 [DOI] [PubMed] [Google Scholar]
- 219. Lemmin T, Kalbermatter D, Harder D, et al. : Structures and dynamics of the novel S1/S2 protease cleavage site loop of the SARS-CoV-2 spike glycoprotein. J. Struct. Biol. X. 2020;4:100038. 10.1016/j.yjsbx.2020.100038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220. Winstone H, Lista MJ, Reid AC, et al. : The Polybasic Cleavage Site in SARS-CoV-2 Spike Modulates Viral Sensitivity to Type I Interferon and IFITM2. J. Virol. 2021 Apr 12;95(9):e02422–e02420. 10.1128/JVI.02422-20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221. Xia S, Lan Q, Su S, et al. : The role of furin cleavage site in SARS-CoV-2 spike protein-mediated membrane fusion in the presence or absence of trypsin. Sig. Transduct. Target Ther. 2020 Dec;5(1):92. 10.1038/s41392-020-0184-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222. Shang J, Wan Y, Liu C, et al. : Structure of mouse coronavirus spike protein complexed with receptor reveals mechanism for viral entry. Poon L, editor. PLoS Pathog. 2020 Mar 9;16(3):e1008392. 10.1371/journal.ppat.1008392 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223. Chambers JP, Yu J, Valdes JJ, et al. : SARS-CoV-2, Early Entry Events. Franciosa G, editor. J. Pathog. 2020 Nov 24;2020:1–11. 10.1155/2020/9238696 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224. Benton DJ, Wrobel AG, Xu P, et al. : Receptor binding and priming of the spike protein of SARS-CoV-2 for membrane fusion. Nature. 2020 Dec 10;588(7837):327–330. 10.1038/s41586-020-2772-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225. Li S, Yang Y, Li W: Human ferroportin mediates proton-coupled active transport of iron. Blood Adv. 2020 Oct 13;4(19):4758–4768. 10.1182/bloodadvances.2020001864 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226. Nader E, Nougier C, Boisson C, et al. : Increased blood viscosity and red blood cell aggregation in patients with COVID-19. Am. J. Hematol. 2021 Dec 23. 10.1002/ajh.26440 [DOI] [PubMed] [Google Scholar]
- 227. Faghihi H: CD147 as an alternative binding site for the spike protein on the surface of SARS-CoV-2. Eur. Rev. Med. Pharmacol. Sci. 2020 Dec;24(23):11992–11994. 10.26355/eurrev_202012_23985 [DOI] [PubMed] [Google Scholar]
- 228. Su H, Wan C, Wang Z-D, et al. : Expression of CD147 and Cyclophilin A in Kidneys of Patients with COVID-19. CJASN. 2021 Apr 7;16(4):618–619. 10.2215/CJN.09440620 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229. Gubernatorova EO, Gorshkova EA, Polinova AI, et al. : IL-6: Relevance for immunopathology of SARS-CoV-2. Cytokine Growth Factor Rev. 2020 Jun;53:13–24. 10.1016/j.cytogfr.2020.05.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230. Joshi S, Joshi M, Degani MS: Tackling SARS-CoV-2: proposed targets and repurposed drugs. Future Med. Chem. 2020 Sep;12(17):1579–1601. 10.4155/fmc-2020-0147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231. Pennings GJ, Kritharides L: CD147 in cardiovascular disease and thrombosis. Semin. Thromb. Hemost. 2014 Oct;40(7):747–755. 10.1055/s-0034-1390001 [DOI] [PubMed] [Google Scholar]
- 232. Xin X, Zeng X, Gu H, et al. : CD147/EMMPRIN overexpression and prognosis in cancer: A systematic review and meta-analysis. Sci. Rep. 2016 Sep 9;6:32804. 10.1038/srep32804 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233. Heuschen A-K, Lu G, Razum O, et al. : Public health-relevant consequences of the COVID-19 pandemic on malaria in sub-Saharan Africa: a scoping review. Malar. J. 2021 Dec;20(1):339. 10.1186/s12936-021-03872-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234. Achan J, Serwanga A, Wanzira H, et al. : Current malaria infection, previous malaria exposure, and clinical profiles and outcomes of COVID-19 in a setting of high malaria transmission: an exploratory cohort study in Uganda. The Lancet Microbe. 2021 Oct; S2666524721002408. 10.2139/ssrn.3844848 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235. Krishan K, Kanchan T: Novel Coronavirus (SARS-CoV-2) resistance in African populations: A cause worth exploring: Novel coronavirus resistance in African population. Acta Bio Medica Atenei Parmensis. 2020 Sep 7;91(3):e2020023. 10.23750/abm.v91i3.9872 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236. Hussain FA, Njoku FU, Saraf SL, et al. : COVID-19 infection in patients with sickle cell disease. Br. J. Haematol. 2020 Jun;189(5):851–852. 10.1111/bjh.16734 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237. Balanchivadze N, Kudirka AA, Askar S, et al. : Impact of COVID-19 Infection on 24 Patients with Sickle Cell Disease. One Center Urban Experience, Detroit, MI, USA. Hemoglobin. 2020 Jul;44(4):284–289. 10.1080/03630269.2020.1797775 [DOI] [PubMed] [Google Scholar]
- 238. Singh RD, Barry MA, Croatt AJ, et al. : The spike protein of SARS-CoV-2 virus induces heme oxygenase-1: Pathophysiologic implications. Biochim. Biophys. Acta Mol. basis Dis. 2021 Dec 14;1868:166322. 10.1016/j.bbadis.2021.166322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239. Lechuga GC, Souza-Silva F, Sacramento CQ, et al. : SARS-CoV-2 Proteins Bind to Hemoglobin and Its Metabolites. Int. J. Mol. Sci. 2021 Aug 21;22(16):9035. 10.3390/ijms22169035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240. Murphy P, Mullen E, Bergin S, et al. : Red Blood Cells from COVID-19 Patients Show Evidence of Increased Oxidative Stress and Increased Lactate Influx. Blood. 2021 Nov 5;138(Supplement 1):928–928. 10.1182/blood-2021-146305 [DOI] [Google Scholar]
- 241. Wang C, Zhang H, Cao X, et al. : Red cell distribution width (RDW): a prognostic indicator of severe COVID-19. Ann. Transl. Med. 2020 Oct;8(19):1230. 10.21037/atm-20-6090 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242. Foy BH, Carlson JCT, Reinertsen E, et al. : Association of Red Blood Cell Distribution Width With Mortality Risk in Hospitalized Adults With SARS-CoV-2 Infection. JAMA Netw. Open. 2020 Sep 1;3(9):e2022058. 10.1001/jamanetworkopen.2020.22058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243. Lorente L, Martín MM, Argueso M, et al. : Association between red blood cell distribution width and mortality of COVID-19 patients. Anaesth Crit Care. Pain Med. 2021 Feb;40(1):100777. 10.1016/j.accpm.2020.10.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244. Atik D, Kaya HB: EVALUATION OF THE RELATIONSHIP OF MPV, RDW AND PVI PARAMETERS WITH DISEASE SEVERITY IN COVID-19 PATIENTS. Acta Clin. Croat. 2021 Mar;60(1):103–114. 10.20471/acc.2021.60.01.15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245. Huerga Encabo H, Grey W, Garcia-Albornoz M, et al. : Human Erythroid Progenitors Are Directly Infected by SARS-CoV-2: Implications for Emerging Erythropoiesis in Severe COVID-19 Patients. Stem Cell Reports. 2021 Mar;16(3):428–436. 10.1016/j.stemcr.2021.02.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246. Bernardes JP, Mishra N, Tran F, et al. : Longitudinal Multi-omics Analyses Identify Responses of Megakaryocytes, Erythroid Cells, and Plasmablasts as Hallmarks of Severe COVID-19. Immunity. 2020 Dec 15;53(6):1296–1314.e9. 10.1016/j.immuni.2020.11.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247. Liu C, Brunn A, Zhu D: Cyclophilin A and CD147: novel therapeutic targets for the treatment of COVID-19. Med. Drug Discov. 2020 Sep;7:100056. 10.1016/j.medidd.2020.100056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248. Gil C, Ginex T, Maestro I, et al. : COVID-19: Drug Targets and Potential Treatments. J. Med. Chem. 2020 Nov 12;63(21):12359–12386. 10.1021/acs.jmedchem.0c00606 [DOI] [PubMed] [Google Scholar]
- 249. Hyser JM, Estes MK: Pathophysiological Consequences of Calcium-Conducting Viroporins. Annu Rev Virol. 2015 Nov;2(1):473–496. 10.1146/annurev-virology-100114-054846 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250. Yang C, Ma X, Wu J, et al. : Low serum calcium and phosphorus and their clinical performance in detecting COVID-19 patients. J. Med. Virol. 2021 Mar;93(3):1639–1651. 10.1002/jmv.26515 [DOI] [PubMed] [Google Scholar]
- 251. Singh VP, Khatua B, El-Kurdi B, et al. : Mechanistic basis and therapeutic relevance of hypocalcemia during severe COVID-19 infection. Endocrine. 2020 Dec;70(3):461–462. 10.1007/s12020-020-02530-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252. Ouyang S-M, Zhu H-Q, Xie Y-N, et al. : Temporal changes in laboratory markers of survivors and non-survivors of adult inpatients with COVID-19. BMC Infect. Dis. 2020 Dec;20(1):952. 10.1186/s12879-020-05678-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253. Saurav S, Tanwar J, Ahuja K, et al. : Dysregulation of host cell calcium signaling during viral infections: Emerging paradigm with high clinical relevance. Mol. Asp. Med. 2021 Oct;81:101004. 10.1016/j.mam.2021.101004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254. Danta CC: SARS-CoV-2, Hypoxia, and Calcium Signaling: The Consequences and Therapeutic Options. ACS Pharmacol. Transl. Sci. 2021 Feb 12;4(1):400–402. 10.1021/acsptsci.0c00219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255. Virág L, Szabó E, Gergely P, et al. : Peroxynitrite-induced cytotoxicity: mechanism and opportunities for intervention. Toxicol. Lett. 2003 Apr 11;140-141:113–124. 10.1016/S0378-4274(02)00508-8 [DOI] [PubMed] [Google Scholar]
- 256. Chakraborti T, Das S, Mondal M, et al. : Oxidant, mitochondria and calcium: an overview. Cell. Signal. 1999 Feb;11(2):77–85. 10.1016/S0898-6568(98)00025-4 [DOI] [PubMed] [Google Scholar]
- 257. Calvo-Rodriguez M, Bacskai BJ: Mitochondria and Calcium in Alzheimer’s Disease: From Cell Signaling to Neuronal Cell Death. Trends Neurosci. 2021 Feb;44(2):136–151. 10.1016/j.tins.2020.10.004 [DOI] [PubMed] [Google Scholar]
- 258. Rossi A, Pizzo P, Filadi R: Calcium, mitochondria and cell metabolism: A functional triangle in bioenergetics. Biochim. Biophys. Acta, Mol. Cell Res. 2019 Jul;1866(7):1068–1078. 10.1016/j.bbamcr.2018.10.016 [DOI] [PubMed] [Google Scholar]
- 259. Madreiter-Sokolowski CT, Thomas C, Ristow M: Interrelation between ROS and Ca2+ in aging and age-related diseases. Redox Biol. 2020 Sep;36:101678. 10.1016/j.redox.2020.101678 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260. Kabeerdoss J, Pilania RK, Karkhele R, et al. : Severe COVID-19, multisystem inflammatory syndrome in children, and Kawasaki disease: immunological mechanisms, clinical manifestations and management. Rheumatol. Int. 2021 Jan;41(1):19–32. 10.1007/s00296-020-04749-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261. Filippo L, Doga M, Frara S, et al. : Hypocalcemia in COVID-19: Prevalence, clinical significance and therapeutic implications. Rev. Endocr. Metab. Disord. 2021 Apr 13. 10.1007/s11154-021-09655-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 262. Mullol J, Alobid I, Mariño-Sánchez F, et al. : The Loss of Smell and Taste in the COVID-19 Outbreak: a Tale of Many Countries. Curr. Allergy Asthma Rep. 2020 Aug 3;20(10):61. 10.1007/s11882-020-00961-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263. Melley LE, Bress E, Polan E: Hypogeusia as the initial presenting symptom of COVID-19. BMJ Case Rep. 2020 May;13(5):e236080. 10.1136/bcr-2020-236080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264. Zhang Q, Shan KS, Abdollahi S, et al. : Anosmia and Ageusia as the Only Indicators of Coronavirus Disease 2019 (COVID-19). Cureus. 2020 May 1 [cited 2021 Dec 29];12:e7918. 10.7759/cureus.7918 Reference Source [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265. Lai AL, Millet JK, Daniel S, et al. : The SARS-CoV Fusion Peptide Forms an Extended Bipartite Fusion Platform that Perturbs Membrane Order in a Calcium-Dependent Manner. J. Mol. Biol. 2017 Dec 8;429(24):3875–3892. 10.1016/j.jmb.2017.10.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 266. Horng T: Calcium signaling and mitochondrial destabilization in the triggering of the NLRP3 inflammasome. Trends Immunol. 2014 Jun;35(6):253–261. 10.1016/j.it.2014.02.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 267. Chakravarty K, Antontsev VG, Khotimchenko M, et al. : Accelerated Repurposing and Drug Development of Pulmonary Hypertension Therapies for COVID-19 Treatment Using an AI-Integrated Biosimulation Platform. Molecules. 2021 Mar 29;26(7):1912. 10.3390/molecules26071912 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 268. Mehrzadi S, Karimi MY, Fatemi A, et al. : SARS-CoV-2 and other coronaviruses negatively influence mitochondrial quality control: beneficial effects of melatonin. Pharmacol. Ther. 2021 Aug;224:107825. 10.1016/j.pharmthera.2021.107825 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 269. Pompella A, Maellaro E, Benedetti A, et al. : Glutathione depletion, lipid peroxidation, and the antecedents of ferroptosis: What about cellular calcium ?. Free Radic. Biol. Med. 2019 Nov 1;143:221–222. 10.1016/j.freeradbiomed.2019.08.004 [DOI] [PubMed] [Google Scholar]
- 270. Altschul DJ, Esenwa C, Haranhalli N, et al. : Predictors of mortality for patients with COVID-19 and large vessel occlusion. Interv. Neuroradiol. 2020 Oct;26(5):623–628. 10.1177/1591019920954603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 271. Yilmaz N, Eren E, Öz C, et al. : COVID-19 and Iron Metabolism: Traditional Review. Turkiye Klinikleri. J. Med. Sci. 2021;41(2):176–188. [Google Scholar]
- 272. Giannis D, Ziogas IA, Gianni P: Coagulation disorders in coronavirus infected patients: COVID-19, SARS-CoV-1, MERS-CoV and lessons from the past. J. Clin. Virol. 2020 Jun;127:104362. 10.1016/j.jcv.2020.104362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 273. McLaughlin K-M, Bechtel M, Bojkova D, et al. : COVID-19-Related Coagulopathy-Is Transferrin a Missing Link?. Diagnostics (Basel). 2020 Jul 30;10(8):E539. 10.3390/diagnostics10080539 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 274. Tang X, Zhang Z, Fang M, et al. : Transferrin plays a central role in coagulation balance by interacting with clotting factors. Cell Res. 2020 Feb;30(2):119–132. 10.1038/s41422-019-0260-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 275. Gao J, Zhou Q, Wu D, et al. : Mitochondrial iron metabolism and its role in diseases. Clin. Chim. Acta. 2021 Feb;513:6–12. 10.1016/j.cca.2020.12.005 [DOI] [PubMed] [Google Scholar]
- 276. Al Sarkhi K: The Link between Electrical Properties of COVID-19 and Electromagnetic Radiation. Biotechnology to Combat COVID-19 [Working Title]. IntechOpen;2021 [cited 2021 Dec 29]. 10.5772/intechopen.96815 Reference Source [DOI] [Google Scholar]
- 277. Lysenkov SP, Muzhenya DV, Tuguz AR, et al. : Participation of nitrogen oxide and its metabolites in the genesis of hyperimmune inflammation in COVID-19. Chin. J. Physiol. 2021 Aug;64(4):167–176. 10.4103/cjp.cjp_38_21 [DOI] [PubMed] [Google Scholar]
- 278. Banchini F: COVID-19 and NF-kB: The Hepcidin paradox and the Iron Storm - reply. Acta Biomed. 2020;91(4):e2020137. 10.23750/abm.v91i4.10904 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 279. Bratko D, Daub CD, Leung K, et al. : Effect of Field Direction on Electrowetting in a Nanopore. J. Am. Chem. Soc. 2007 Mar 1;129(9):2504–2510. 10.1021/ja0659370 [DOI] [PubMed] [Google Scholar]
- 280. Catterall WA: Ion Channel Voltage Sensors: Structure, Function, and Pathophysiology. Neuron. 2010 Sep;67(6):915–928. 10.1016/j.neuron.2010.08.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 281. McClenaghan C, Hanson A, Lee S-J, et al. : Coronavirus Proteins as Ion Channels: Current and Potential Research. Front. Immunol. 2020 Oct 9;11:573339. 10.3389/fimmu.2020.573339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 282. Núñez MT, Hidalgo C: Noxious Iron–Calcium Connections in Neurodegeneration. Front. Neurosci. 2019 Feb 12;13:48. 10.3389/fnins.2019.00048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 283. Gaasch JA, Geldenhuys WJ, Lockman PR, et al. : Voltage-gated calcium channels provide an alternate route for iron uptake in neuronal cell cultures. Neurochem. Res. 2007 Oct;32(10):1686–1693. 10.1007/s11064-007-9313-1 [DOI] [PubMed] [Google Scholar]
- 284. Chattipakorn N: Calcium channels and iron uptake into the heart. WJC. 2011;3(7):215–218. 10.4330/wjc.v3.i7.215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 285. Lewerenz J, Ates G, Methner A, et al. : Oxytosis/Ferroptosis-(Re-) Emerging Roles for Oxidative Stress-Dependent Non-apoptotic Cell Death in Diseases of the Central Nervous System. Front. Neurosci. 2018;12:214. 10.3389/fnins.2018.00214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 286. Bostanci MÖ, Bagirici F: Blocking of L-type calcium channels protects hippocampal and nigral neurons against iron neurotoxicity The role of L-type calcium channels in iron-induced neurotoxicity. Int. J. Neurosci. 2013 Dec;123(12):876–882. 10.3109/00207454.2013.813510 [DOI] [PubMed] [Google Scholar]
- 287. Oudit GY, Trivieri MG, Khaper N, et al. : Role of L-type Ca2+ channels in iron transport and iron-overload cardiomyopathy. J. Mol. Med. 2006 May;84(5):349–364. 10.1007/s00109-005-0029-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 288. Oudit GY, Sun H, Trivieri MG, et al. : L-type Ca2+ channels provide a major pathway for iron entry into cardiomyocytes in iron-overload cardiomyopathy. Nat. Med. 2003 Sep;9(9):1187–1194. 10.1038/nm920 [DOI] [PubMed] [Google Scholar]
- 289. Angelova PR, Choi ML, Berezhnov AV, et al. : Alpha synuclein aggregation drives ferroptosis: an interplay of iron, calcium and lipid peroxidation. Cell Death Differ. 2020 Oct;27(10):2781–2796. 10.1038/s41418-020-0542-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 290. Murphy TH, Malouf AT, Sastre A, et al. : Calcium-dependent glutamate cytotoxicity in a neuronal cell line. Brain Res. 1988 Mar 22;444(2):325–332. 10.1016/0006-8993(88)90941-9 [DOI] [PubMed] [Google Scholar]
- 291. Grimm C, Tang R: Could an endo-lysosomal ion channel be the Achilles heel of SARS-CoV2?. Cell Calcium. 2020 Jun;88:102212. 10.1016/j.ceca.2020.102212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 292. Taheri M, Bahrami A, Habibi P, et al. : A Review on the Serum Electrolytes and Trace Elements Role in the Pathophysiology of COVID-19. Biol. Trace Elem. Res. 2021 Jul;199(7):2475–2481. 10.1007/s12011-020-02377-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 293. Krause A, Neitz S, Mägert HJ, et al. : LEAP-1, a novel highly disulfide-bonded human peptide, exhibits antimicrobial activity. FEBS Lett. 2000 Sep 1;480(2–3):147–150. 10.1016/S0014-5793(00)01920-7 [DOI] [PubMed] [Google Scholar]
- 294. Park CH, Valore EV, Waring AJ, et al. : Hepcidin, a urinary antimicrobial peptide synthesized in the liver. J. Biol. Chem. 2001 Mar 16;276(11):7806–7810. 10.1074/jbc.M008922200 [DOI] [PubMed] [Google Scholar]
- 295. Katsarou A, Pantopoulos K: Hepcidin Therapeutics. Pharmaceuticals (Basel). 2018 Nov 21;11(4):E127. 10.3390/ph11040127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 296. Dixon SJ, Lemberg KM, Lamprecht MR, et al. : Ferroptosis: an iron-dependent form of nonapoptotic cell death. Cell. 2012 May 25;149(5):1060–1072. 10.1016/j.cell.2012.03.042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 297. Landras A, Reger de Moura C, Jouenne F, et al. : CD147 Is a Promising Target of Tumor Progression and a Prognostic Biomarker. Cancers (Basel). 2019 Nov 16;11(11):1803. 10.3390/cancers11111803 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 298. Yu B, Zhang Y, Wu K, et al. : CD147 promotes progression of head and neck squamous cell carcinoma via NF-kappa B signaling. J. Cell. Mol. Med. 2019 Feb;23(2):954–966. 10.1111/jcmm.13996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 299. Bai B, Yang Y, Wang Q, et al. : NLRP3 inflammasome in endothelial dysfunction. Cell Death Dis. 2020 Sep 18;11(9):776. 10.1038/s41419-020-02985-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 300. Gupta N, Sahu A, Prabhakar A, et al. : Activation of NLRP3 inflammasome complex potentiates venous thrombosis in response to hypoxia. Proc. Natl. Acad. Sci. U. S. A. 2017 May 2;114(18):4763–4768. 10.1073/pnas.1620458114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 301. Boaru SG, Borkham-Kamphorst E, Van de Leur E, et al. : NLRP3 inflammasome expression is driven by NF-κB in cultured hepatocytes. Biochem. Biophys. Res. Commun. 2015 Mar 13;458(3):700–706. 10.1016/j.bbrc.2015.02.029 [DOI] [PubMed] [Google Scholar]
- 302. Nakamura K, Kawakami T, Yamamoto N, et al. : Activation of the NLRP3 inflammasome by cellular labile iron. Exp. Hematol. 2016 Feb;44(2):116–124. 10.1016/j.exphem.2015.11.002 [DOI] [PubMed] [Google Scholar]
- 303. Ginzburg YZ: Hepcidin-ferroportin axis in health and disease. Vitam. Horm. 2019;110:17–45. 10.1016/bs.vh.2019.01.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 304. Wang C-Y, Babitt JL: Hepcidin regulation in the anemia of inflammation. Curr. Opin. Hematol. 2016 May;23(3):189–197. 10.1097/MOH.0000000000000236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 305. Zubieta-Calleja G, Zubieta-DeUrioste N: Pneumolysis and “Silent Hypoxemia” in COVID-19. Indian J. Clin. Biochem. 2020 Nov 9;1–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 306. Merryweather-Clarke AT, Pointon JJ, Shearman JD, et al. : Global prevalence of putative haemochromatosis mutations. J. Med. Genet. 1997 Apr;34(4):275–278. 10.1136/jmg.34.4.275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 307. Hanson EH: HFE Gene and Hereditary Hemochromatosis: A HuGE Review. Am. J. Epidemiol. 2001 Aug 1;154(3):193–206. 10.1093/aje/154.3.193 [DOI] [PubMed] [Google Scholar]
- 308. Sfera A, Osorio C, Maguire G, et al. : COVID-19, ferrosenescence and neurodegeneration, a mini-review. Prog. Neuro-Psychopharmacol. Biol. Psychiatry. 2021 Jul 13;109:110230. 10.1016/j.pnpbp.2020.110230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 309. Riley MJ, Hicks SR, Irvine S, et al. : Hereditary haemochromatosis, haemophagocytic lymphohistiocytosis and COVID-19. Clin. Infect. Pract. 2020 Oct;7-8:100052. 10.1016/j.clinpr.2020.100052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 310. Pall ML: Electromagnetic fields act via activation of voltage-gated calcium channels to produce beneficial or adverse effects. J. Cell. Mol. Med. 2013 Aug;17(8):958–965. 10.1111/jcmm.12088 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 311. Santini SJ, Cordone V, Falone S, et al. : Role of Mitochondria in the Oxidative Stress Induced by Electromagnetic Fields: Focus on Reproductive Systems. Oxidative Med. Cell. Longev. 2018;2018:5076271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 312. Pall ML: Wi-Fi is an important threat to human health. Environ. Res. 2018 Jul;164:405–416. 10.1016/j.envres.2018.01.035 [DOI] [PubMed] [Google Scholar]
- 313. Prucha J, Krusek J, Dittert I, et al. : Acute exposure to high-induction electromagnetic field affects activity of model peripheral sensory neurons. J. Cell. Mol. Med. 2018 Feb;22(2):1355–1362. 10.1111/jcmm.13423 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 314. Doyon PR, Johansson O: Electromagnetic fields may act via calcineurin inhibition to suppress immunity, thereby increasing risk for opportunistic infection: Conceivable mechanisms of action. Med. Hypotheses. 2017 Sep;106:71–87. 10.1016/j.mehy.2017.06.028 [DOI] [PubMed] [Google Scholar]
- 315. García-Sancho J, Montero M, Alvarez J, et al. : Effects of extremely-low-frequency electromagnetic fields on ion transport in several mammalian cells. Bioelectromagnetics. 1994;15(6):579–588. 10.1002/bem.2250150611 [DOI] [PubMed] [Google Scholar]
- 316. Lai H: Exposure to Static and Extremely-Low Frequency Electromagnetic Fields and Cellular Free Radicals. Electromagn. Biol. Med. 2019;38(4):231–248. 10.1080/15368378.2019.1656645 [DOI] [PubMed] [Google Scholar]
- 317. Shao T: EMF-cancer link: the ferritin hypothesis. Med. Hypotheses. 1993 Jul;41(1):28–30. 10.1016/0306-9877(93)90028-O [DOI] [PubMed] [Google Scholar]
- 318. El-Maleky NF, Ebrahim RH: Effects of exposure to electromagnetic field from mobile phone on serum hepcidin and iron status in male albino rats. Electromagn. Biol. Med. 2019;38(1):66–73. 10.1080/15368378.2018.1531423 [DOI] [PubMed] [Google Scholar]
- 319. Çetkin M, Demirel C, Kızılkan N, et al. : Evaluation of the mobile phone electromagnetic radiation on serum iron parameters in rats. Afr. Health Sci. 2017 Mar;17(1):186–190. 10.4314/ahs.v17i1.23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 320. Choe M, Choe W, Cha S, et al. : Changes of cationic transport in AtCAX5 transformant yeast by electromagnetic field environments. J. Biol. Phys. 2018 Sep;44(3):433–448. 10.1007/s10867-018-9500-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 321. Nguyen THP, Pham VTH, Baulin V, et al. : The effect of a high frequency electromagnetic field in the microwave range on red blood cells. Sci. Rep. 2017 Sep 7;7(1):10798. 10.1038/s41598-017-11288-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 322. Bordiushkov IN, Goroshinskaia IA, Frantsiiants EM, et al. : Structural-functional changes in lymphocyte ans erythrocyte membranes after exposure to alternating magnetic field. Vopr. Med. Khim. 2000 Feb;46(1):72–80. [PubMed] [Google Scholar]
- 323. Baieth HEA: Physical parameters of blood as a non - newtonian fluid. Int. J. Biomed. Sci. 2008 Dec;4(4):323–329. [PMC free article] [PubMed] [Google Scholar]
- 324. Havas M: Radiation from wireless technology affects the blood, the heart, and the autonomic nervous system. Rev. Environ. Health. 2013;28(2–3):75–84. 10.1515/reveh-2013-0004 [DOI] [PubMed] [Google Scholar]
- 325. Kivrak E, Yurt K, Kaplan A, et al. : Effects of electromagnetic fields exposure on the antioxidant defense system. J Microsc Ultrastruct. 2017;5(4):167–176. 10.1016/j.jmau.2017.07.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 326. Kostoff RN, Heroux P, Aschner M, et al. : Adverse health effects of 5G mobile networking technology under real-life conditions. Toxicol. Lett. 2020 May 1;323:35–40. 10.1016/j.toxlet.2020.01.020 [DOI] [PubMed] [Google Scholar]
- 327. Bushberg JT, Chou CK, Foster KR, et al. : IEEE Committee on Man and Radiation-COMAR Technical Information Statement: Health and Safety Issues Concerning Exposure of the General Public to Electromagnetic Energy from 5G Wireless Communications Networks. Health Phys. 2020 Aug;119(2):236–246. 10.1097/HP.0000000000001301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 328. Mordachev VI: Correlation between the potential electromagnetic pollution level and the danger of COVID-19. 4G/5G/6G can be safe for people. Doklady Belorusskogo gosudarstvennogo universiteta informatiki i radioèlektroniki. 2020 Jun 25;18(4):96–112. 10.35596/1729-7648-2020-18-4-96-112 [DOI] [Google Scholar]
- 329. tsiang angela. Havas M: COVID-19 Attributed Cases and Deaths are Statistically Higher in States and Counties with 5th Generation Millimeter Wave Wireless Telecommunications in the United States. MRAJ. 2021 [cited 2021 Dec 29];9(4). 10.18103/mra.v9i4.2371 Reference Source [DOI] [Google Scholar]
- 330. Rubik B, Brown RR: Evidence for a connection between coronavirus disease-19 and exposure to radiofrequency radiation from wireless communications including 5G. J. Clin. Transl. Res. 2021 Oct 26;7(5):666–681. [PMC free article] [PubMed] [Google Scholar]
- 331. Lynch SV, Pedersen O: The Human Intestinal Microbiome in Health and Disease. N. Engl. J. Med. 2016 Dec 15;375(24):2369–2379. 10.1056/NEJMra1600266 [DOI] [PubMed] [Google Scholar]
- 332. López-Otín C, Kroemer G: Hallmarks of Health. Cell. 2021 Jan 7;184(1):33–63. 10.1016/j.cell.2020.11.034 [DOI] [PubMed] [Google Scholar]
- 333. Petruk G, Puthia M, Petrlova J, et al. : SARS-CoV-2 spike protein binds to bacterial lipopolysaccharide and boosts proinflammatory activity. J. Mol. Cell Biol. 2020 Oct 12;12(12):916–932. 10.1093/jmcb/mjaa067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 334. Aquino-Martinez R, Hernández-Vigueras S: Severe COVID-19 Lung Infection in Older People and Periodontitis. J. Clin. Med. 2021 Jan 14;10(2):E279. 10.3390/jcm10020279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 335. Li N, Wang W, Zhou H, et al. : Ferritinophagy-mediated ferroptosis is involved in sepsis-induced cardiac injury. Free Radic. Biol. Med. 2020 Nov 20;160:303–318. 10.1016/j.freeradbiomed.2020.08.009 [DOI] [PubMed] [Google Scholar]
- 336. Kell DB, Pretorius E: No effects without causes: the Iron Dysregulation and Dormant Microbes hypothesis for chronic, inflammatory diseases. Biol. Rev. Camb. Philos. Soc. 2018 Aug;93(3):1518–1557. 10.1111/brv.12407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 337. Liu P, Feng Y, Li H, et al. : Ferrostatin-1 alleviates lipopolysaccharide-induced acute lung injury via inhibiting ferroptosis. Cell. Mol. Biol. Lett. 2020 Dec;25(1):10. 10.1186/s11658-020-00205-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 338. Fang J, Kong B, Shuai W, et al. : Ferroportin-mediated ferroptosis involved in new-onset atrial fibrillation with LPS-induced endotoxemia. Eur. J. Pharmacol. 2021 Dec 15;913:174622. 10.1016/j.ejphar.2021.174622 [DOI] [PubMed] [Google Scholar]
- 339. Yang C-C, Yao C-A, Yang J-C, et al. : Sialic Acid Rescues Repurified Lipopolysaccharide-Induced Acute Renal Failure via Inhibiting TLR4/PKC/gp91-Mediated Endoplasmic Reticulum Stress, Apoptosis, Autophagy, and Pyroptosis Signaling. Toxicol. Sci. 2014 Sep 1;141(1):155–165. 10.1093/toxsci/kfu121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 340. Hsu S-P, Chen C-C, Chien C-T: Pretreatment of Sialic Acid Efficiently Prevents Lipopolysaccharide-Induced Acute Renal Failure and Suppresses TLR4/gp91-Mediated Apoptotic Signaling. Kidney Blood Press. Res. 2016;41(3):267–277. 10.1159/000443430 [DOI] [PubMed] [Google Scholar]
- 341. Ho C-H, Hsu S-P, Yang C-C, et al. : Sialic acid reduces acute endotoxemia-induced liver dysfunction in the rat. Shock. 2009 Aug;32(2):228–235. 10.1097/SHK.0b013e318197118e [DOI] [PubMed] [Google Scholar]
- 342. Feng C, Stamatos NM, Dragan AI, et al. : Sialyl residues modulate LPS-mediated signaling through the Toll-like receptor 4 complex. PLoS One. 2012;7(4):e32359. 10.1371/journal.pone.0032359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 343. Hirschhorn T, Stockwell BR: The development of the concept of ferroptosis. Free Radic. Biol. Med. 2019 Mar;133:130–143. 10.1016/j.freeradbiomed.2018.09.043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 344. Banchini F, Cattaneo GM, Capelli P: Serum ferritin levels in inflammation: a retrospective comparative analysis between COVID-19 and emergency surgical non-COVID-19 patients. World J. Emerg. Surg. 2021 Mar 8;16(1):9. 10.1186/s13017-021-00354-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 345. Lin Z, Long F, Yang Y, et al. : Serum ferritin as an independent risk factor for severity in COVID-19 patients. J. Infect. 2020 Oct;81(4):647–679. 10.1016/j.jinf.2020.06.053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 346. Gómez-Pastora J, Weigand M, Kim J, et al. : Hyperferritinemia in critically ill COVID-19 patients – Is ferritin the product of inflammation or a pathogenic mediator?. Clin. Chim. Acta. 2020 Oct;509:249–251. 10.1016/j.cca.2020.06.033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 347. Abbas A, Mostafa A, Yousof E, et al. : Use of Iron Chelators to Reduce the Severity of COVID-19. Thromb. Haemost Res. 2020 May 24;4:1042. [Google Scholar]
- 348. Lawaczeck R: COVID-19 and Body Iron: A Survey on Phenomenological and Genetic Correlations. ACS Chem. Neurosci. 2020 Dec 16;11(24):3996–4000. 10.1021/acschemneuro.0c00572 [DOI] [PubMed] [Google Scholar]
- 349. Liu N, Zhang T, Ma L, et al. : The impact of ABO blood group on COVID-19 infection risk and mortality: A systematic review and meta-analysis. Blood Rev. 2021 Jul;48:100785. 10.1016/j.blre.2020.100785 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 350. Zhang Y, Garner R, Salehi S, et al. : Association between ABO blood types and coronavirus disease 2019 (COVID-19), genetic associations, and underlying molecular mechanisms: a literature review of 23 studies. Ann. Hematol. 2021 May;100(5):1123–1132. 10.1007/s00277-021-04489-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 351. Shibeeb S, Khan A: ABO blood group association and COVID-19. COVID-19 susceptibility and severity: A review. Hematol. Transfus. Cell Ther. 2021 Sep 14. 10.1016/j.htct.2021.07.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 352. Ray JG, Schull MJ, Vermeulen MJ, et al. : Association Between ABO and Rh Blood Groups and SARS-CoV-2 Infection or Severe COVID-19 Illness: A Population-Based Cohort Study. Ann. Intern. Med. 2021 Mar;174(3):308–315. 10.7326/M20-4511 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 353. Coluk Y, Hizli O, Gunaydın S, et al. : Association of Blood Subgroups With PCR Test Positivity and Lung Involvement in Patients With COVID-19. Cureus. 2021 Mar 29;13(3):e14172. 10.7759/cureus.14172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 354. Ishaq U, Malik A, Malik J, et al. : Association of ABO blood group with COVID-19 severity, acute phase reactants and mortality. PLoS One. 2021;16(12):e0261432. 10.1371/journal.pone.0261432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 355. Gursoy V, Avci S: Effect of ABO blood groups on length of hospital stay according to age in Covid-19 patients. Hematol. Transfus. Cell Ther. 2021 Nov 29. 10.1016/j.htct.2021.08.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 356. Singh H, Choudhari R, Nema V, et al. : ACE2 and TMPRSS2 polymorphisms in various diseases with special reference to its impact on COVID-19 disease. Microb. Pathog. 2021 Jan;150:104621. 10.1016/j.micpath.2020.104621 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 357. Lee W-L, Wang P-H: Aberrant sialylation in ovarian cancers. J. Chin. Med. Assoc. 2020 Apr;83(4):337–344. 10.1097/JCMA.0000000000000252 [DOI] [PubMed] [Google Scholar]
- 358. Lipinski B, Pretorius E: Iron-induced fibrin in cardiovascular disease. Curr. Neurovasc. Res. 2013 Aug;10(3):269–274. 10.2174/15672026113109990016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 359. Yilmaz N, Eren E: COVID-19: iron ferroptosis parafibrin. 2020 [cited 2021 Jan 1]. 10.13140/RG.2.2.16046.33607 [DOI]
- 360. Meo SA, Meo AS, Al-Jassir FF, et al. : Omicron SARS-CoV-2 new variant: global prevalence and biological and clinical characteristics. Eur. Rev. Med. Pharmacol. Sci. 2021 Dec;25(24):8012–8018. 10.26355/eurrev_202112_27652 [DOI] [PubMed] [Google Scholar]
- 361. Pawłowski P: SARS-CoV-2 variant Omicron (B.1.1.529) is in a rising trend of mutations increasing the positive electric charge in crucial regions of the spike protein S. Acta Biochim. Pol. 2021 Dec 14. 10.18388/abp.2020_6072 [DOI] [PubMed] [Google Scholar]
- 362. Pawłowski PH: Additional Positive Electric Residues in the Crucial Spike Glycoprotein S Regions of the New SARS-CoV-2 Variants. Infect Drug Resist. 2021;14:5099–5105. 10.2147/IDR.S342068 [DOI] [PMC free article] [PubMed] [Google Scholar]