Abstract
Imiquimod is an oral inducer of interferon (IFN) and several other proinflammatory cytokines and has been successfully used topically as an antiviral agent for the treatment of genital warts. We have investigated the molecular mechanisms by which imiquimod induces the expression of IFNs, IFN-stimulated genes (ISGs), and proinflammatory cytokines in vivo, using mice deficient in various components of the IFN signaling system. Mice deficient in the transcription factor interferon regulatory factor 1 (IRF-1) or in the serine/threonine protein kinase PKR responded normally to imiquimod, producing high levels of circulating IFN and induction of several ISGs. On the other hand, when mice deficient in STAT-1 were treated, a 32-fold reduction in the level of circulating IFN was observed, together with a lack of induction of 2-5 oligo adenylate synthetase (2-5 OAS) and IRF-1 genes. Interestingly, there was also a lack of induction of interleukin-6 (IL-6) gene expression, although tumor necrosis factor was induced and readily detected in serum. In mice deficient in the type I IFN receptor, imiquimod induced levels of IFN similar to those in control mice, but again, neither 2-5 OAS, IRF-1, nor IL-6 genes were induced in mutant mice. Our results suggest that STAT-1 plays a critical role in the mechanism of gene activation by imiquimod. Moreover, induction of IL-6 gene expression appears to be dependent on components of the IFN signaling cascade.
Interferons (IFNs) belong to a family of proteins involved in the regulation and function of the immune system and are important in both innate and specific immune responses against viruses, bacteria, and some parasites (43). Whereas type I IFNs are necessary for resistance to many viral infections, type II IFN, or IFN-γ, plays a key role in the innate immune response as well as in the development of some aspects of the cellular immune response (42). Animals deficient in type I IFN show an extremely high sensitivity to viral infection, with as much as a 1,000,000-fold reduction in the 50% lethal dose in the case of vesicular stomatitis virus (25). Mice that do not respond to IFN-γ have a defect in some macrophage functions (16–18), are more susceptible to some intracellular parasites (40, 41), have an impaired granulomatous reaction (30), and show some alterations in the balance between Th1- and Th2-type responses of T cells (44).
Drugs that modulate the expression of IFNs and other proinflammatory cytokines might be beneficial clinically by enhancing the immune response and could be used for the treatment of infectious diseases and cancer. Indeed, several reports describe the use of Mycobacterium bovis BCG as a means of boosting the immune response in order to treat neoplastic disorders and as a vaccine adjuvant (12).
Imiquimod is a low-molecular-weight compound that was originally described as a potent inducer of IFN when given orally to humans and mice (33). Applied topically, imiquimod is used for the treatment of genital warts caused by human papillomavirus, particularly in the female population (2, 3).
Little is known concerning the mechanism by which imiquimod can reverse the clinical manifestation of genital warts. Imiquimod is a very potent inducer of IFNs, and levels of circulating IFN activity as high as 2,000 U/ml have been detected in the sera of treated mice (14). Imiquimod can also induce the expression of a variety of other proinflammatory cytokines, such as interleukin-1 (IL-1), -6, and -12, tumor necrosis factor (TNF), etc., in vivo (23). It is not clear whether imiquimod’s antiviral action is due solely to the induction of IFNs and other cytokines known to have antiviral actions, such as TNF (28) and IL-12 (26, 34) or whether the induction of the IFN-stimulated genes (ISGs) necessary for inhibiting viral replication can be triggered directly by the drug. While it is likely that IFN is important for the beneficial effects of imiquimod in the case of genital warts, it is conceivable that IFN induction is only partially responsible for the actions ascribed to this drug. Moreover, such a broad induction of several mediators of the inflammatory response may also account for some of the undesirable effects observed in cancer patients who are treated orally with the drug.
Recently, a considerable amount of information concerning signaling within the IFN system has become available (36). Since high levels of both IFN-β and IFN-α mRNAs can be observed when cells are stimulated in the presence of protein synthesis inhibitors, it appears that activation of some latent transcription factors is necessary and sufficient for the induction of type I IFN gene expression (43). However, under some circumstances, induction of de novo protein synthesis of the transcription factor interferon regulatory factor 1 (IRF-1) might also be required for full induction of both IFN-β and IFN-α gene expression (11, 24, 27). Type I IFN signaling is mediated primarily via the activation of the trimeric ISGF-3 complex, assembled from inactive forms of STAT-1, STAT-2, and the DNA-binding protein p48 (39). Formation of ISGF-3 requires phosphorylation of STAT-1 and STAT-2 by JAK-1 and TYK-2 tyrosine kinases (35). A homodimer of phosphorylated STAT-1 appears to be the major signaling molecule in the IFN-γ pathway and utilizes the JAK-1 and JAK-2 tyrosine kinases (37). IRF-1 also contributes to IFN signaling, and it has been demonstrated that induction of some ISGs, especially by IFN-γ, requires IRF-1 (5, 20). In addition, IRF-1-deficient mouse embryonic fibroblasts are less sensitive to the antiviral action of both type I IFN and IFN-γ (20). Since IRF-1 is induced by a variety of other proinflammatory cytokines, such as TNF, IL-1 (11), IL-6 (15), and others, it has been proposed that it might play an important role in connecting the cytokine network (27).
In order to better understand the mechanism of gene activation triggered by imiquimod, we have investigated the induction of IFN and ISGs, as well as the induction of some cytokines, in mice deficient in several components of the IFN system, upon oral treatment with the drug. Our results suggest that imiquimod uses STAT-1 as a key signaling molecule. Moreover, we show, for the first time, that a component of the IFN signaling cascade, likely to be STAT-1, plays an important role in the induction of IL-6 by imiquimod.
MATERIALS AND METHODS
Mice.
All mouse lineages were on a pure 129/SV background, and their primary characterization has been described earlier (IRF-10/0 [29], PKR0/0 [45], STAT-10/0 [9], and type I IFN receptor0/0 [IFNR0/0] [25]). Animals were housed and maintained in animal facilities according to institutional regulations and used between the ages of 4 and 6 weeks.
Imiquimod and treatment of mice.
The compound imiquimod {S-26463/-3; 1-(2-methyl-propyl)-1H-imidazo[4,5-c]quinoline-4-amine} was provided by 3M Pharmaceuticals (St. Paul, Minn.). The drug was diluted in water to a concentration of 1 mg/ml, and mice were treated orally with 1 mg/kg of body weight or 20 μl of stock solution (1 mg/ml) (average weight of mice, 20 g). Animals were sacrificed at different times, and blood and organs were collected. Sera were stored at −20°C, and organs were stored at −70°C until use.
IFN titration.
To determine the levels of IFN in the serum, antiviral activity was measured by inhibition of cytopathic effect in immortalized mouse embryonic fibroblasts deficient in either the IFN-γ receptor (4) or the type I IFN receptor (24a). The former cell line allows for the detection of type I IFN activity only, whereas the latter allows for the detection of IFN-γ activity. Cells were seeded into 96-well plates and incubated for 24 h in the presence of twofold serial dilutions of serum and were then challenged with encephalomyocarditis virus. For the challenge, cells were infected with the highest dilution of the stock of virus that killed 100% of cells in 100% of wells in 36 h. The reciprocal of the highest dilution of serum showing protection of 50% of the cells compared to the controls was considered to be 1 U of IFN.
Cytokine detection in sera.
The levels of IL-6, TNF, and IL-12 in the sera of control or treated mice was determined by enzyme-linked immunosorbent assay (ELISA) according to the recommendations of the manufacturer (R&D Systems, Minneapolis, Minn.). The data presented are from one representative experiment with three independent titrations.
RNA extraction and Northern blot analysis.
For RNA extraction, frozen organs were homogenized in a solution containing guanidinium thiocyanate (7). Total RNA was fractionated on 1% denaturing agarose gels and transferred to a Hybond membrane (Amersham). Hybridization was carried out with [α-32P]dCTP-labeled probes prepared by the random primer procedure (RediPrime; Amersham) (a reference for each probe is provided in the corresponding figure legend). To normalize the amounts of RNA loaded, filters were stripped and rehybridized with an [α-32P]dCTP-labeled glyceraldehyde-3-phosphate dehydrogenase (GAPDH) cDNA probe (10).
RESULTS
In order to investigate the mechanism by which imiquimod can induce the expression of the IFN genes, we treated mice deficient in several components of the IFN system with imiquimod and compared the levels of circulating IFN with those in treated wild-type mice. The only difference beyond the limit of sensitivity of the assay was observed in STAT-1-deficient mice, which showed a reduction of more than 32-fold in the level of circulating IFN. This reduction was observed in three independent experiments, one of which is shown in Table 1. When the titration of the antiviral activity present in the sera of treated mice was performed in cell lines derived from mouse embryonic fibroblasts deficient in the type I IFN receptor, no detectable antiviral activity was observed, suggesting that, if any, imiquimod induces less than 20 U of IFN-γ/ml, at least under our experimental conditions (data not shown).
TABLE 1.
IFN levels in sera of imiquimod-treated micea
| IFN system component | IFN level (U/ml) in serum of:
|
FRb | |||||
|---|---|---|---|---|---|---|---|
| Mutant
|
Wild type
|
||||||
| Mouse 1 | Mouse 2 | Mean | Mouse 1 | Mouse 2 | Mean | ||
| IRF-1 | 640 | 320 | 480 | 320 | 320 | 320 | 0.66 |
| PKR | 320 | 320 | 320 | 640 | 640 | 640 | 2 |
| STAT-1 | <20 | <20 | <20 | 960 | 320 | 640 | >32 |
| Type I IFNR | 320 | 640 | 480 | 640 | 1,280 | 960 | 2 |
Imiquimod was administered orally (1 mg/kg), and mice were killed 2 or 8 h after treatment.
FR, fold reduction, calculated as the mean from the wild-type group divided by the mean from the mutant group.
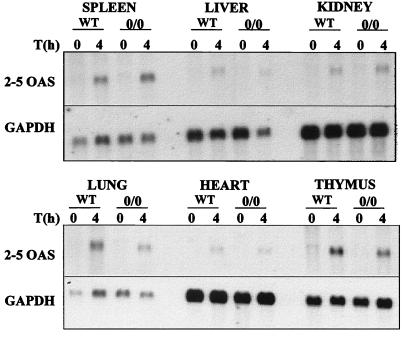
We next investigated the induction of some ISGs in imiquimod-treated mice. It has been speculated that IRF-1 might have some positive effect in regulating transcription of the 2-5 oligo adenylate synthetase (2-5 OAS) genes (27, 31). To investigate whether IRF-1 would be necessary for the induction of the 2-5 OAS genes by imiquimod, we determined the levels of 2-5 OAS in wild-type and IRF-1-deficient mice. Figure 1 shows the steady-state mRNA levels of 2-5 OAS in various organs of wild-type or IRF-10/0 mice treated for 4 h with imiquimod. Whereas very low constitutive expression of 2-5 OAS was observed in both wild-type and mutant mice, its expression was readily induced by imiquimod, with comparable levels of mRNA observed in both groups of mice.
FIG. 1.
Levels of 2-5 OAS mRNA in organs of imiquimod-treated mice. Wild-type or IRF-10/0 mice were treated orally with imiquimod (1 mg/kg) for 4 h, and total RNAs from the organs indicated were collected and fractionated on a 1% agarose-formaldehyde gel. RNA was transferred to a nylon membrane and hybridized with a 2-5 OAS cDNA probe (31) labeled with [α-32P]dCTP by random priming (RediPrime; Amersham). After autoradiography, filters were stripped and hybridized with a 32P-labeled GAPDH cDNA probe (10) to control for RNA loading.
We also determined the levels of mRNAs for the interferon-stimulated IRF-1, PKR, 1-8, and IRF-2 genes in the spleens, lungs, thymuses, livers, and hearts of wild-type, IRF-10/0, and PKR0/0 mice after treatment with imiquimod for 2 or 8 h. Whereas differences in the basal levels of mRNAs among organs were observed, induction of these genes in wild-type mice was comparable with that in both IRF-1 and PKR mutant mice (data not shown).
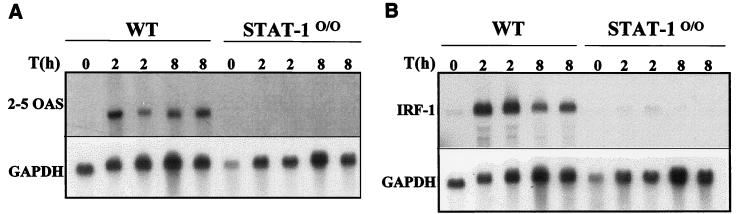
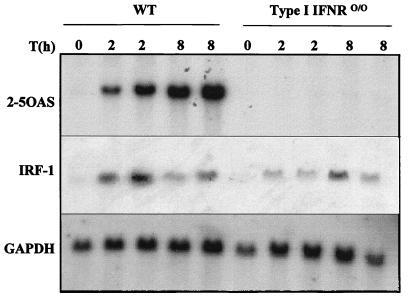
In contrast, induction of ISGs was severely impaired in STAT-10/0 mice. Induction of 2-5 OAS mRNA (Fig. 2A) or IRF-1 mRNA (Fig. 2B) was not detected in mutant mice, whereas increased levels of both mRNAs were observed in wild-type mice treated for 2 or 8 h with imiquimod. In mice deficient in the type I IFN receptor, there was also no induction of 2-5 OAS gene expression, and a modest augmentation of IRF-1 mRNA was detected, albeit not as high as in the wild-type counterparts (Fig. 3).
FIG. 2.
Levels of 2-5 OAS (A) and IRF-1 (B) mRNAs in the livers of imiquimod-treated mice. Wild-type or STAT-10/0 mice were treated orally with imiquimod (1 mg/kg) for 2 or 8 h, total RNAs were collected and fractionated on a 1% agarose-formaldehyde gel, and RNA was transferred to a nylon membrane. (A) The filter was hybridized with a 2-5 OAS cDNA probe (31). (B) The filter was hybridized with an IRF-1 cDNA probe (32) labeled with [α-32P]dCTP by random priming (RediPrime; Amersham). After autoradiography, filters were stripped and hybridized with a 32P-labeled GAPDH cDNA probe (10) to control for RNA loading.
FIG. 3.
Levels of 2-5 OAS and IRF-1 mRNAs in the spleens of imiquimod-treated mice. Wild-type or IFNR0/0 mice were treated orally with imiquimod (1 mg/kg) for 2 or 8 h. Total RNAs were collected from spleens and fractionated on a 1% agarose-formaldehyde gel, and RNAs were transferred to a nylon membrane. The filter was serially hybridized with [α-32P]dCTP-labeled cDNA probes for 2-5 OAS (31), IRF-1 (32), and GAPDH (10) (to control for equal RNA loading).
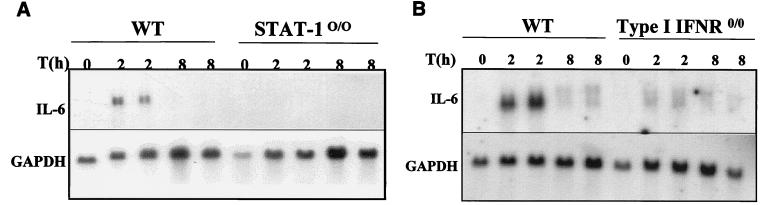
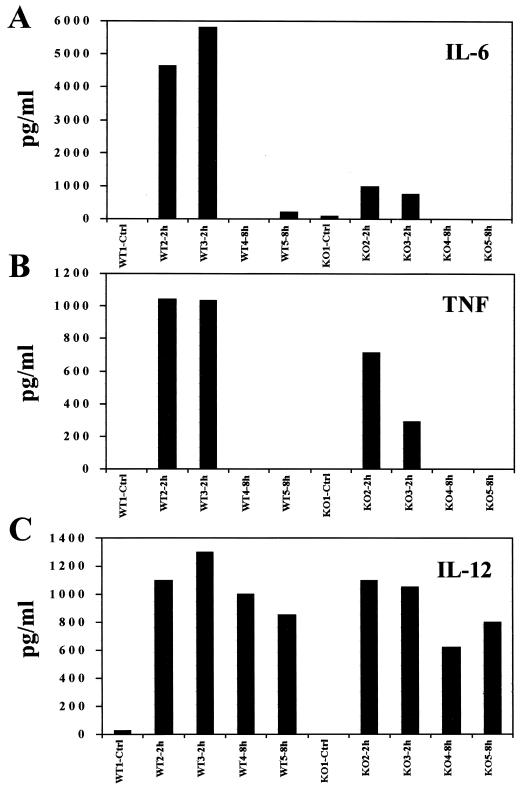
Induction of the IL-6 gene is thought to be achieved primarily by activation of the transcription factors NF-κB and NF-IL6 (8, 21), which act cooperatively to regulate gene expression. To date, there is no evidence suggesting that any component of the IFN signaling cascade would play a role in regulating the expression of the IL-6 gene. Since IL-6 is one of the proinflammatory cytokines that are up-regulated upon treatment with imiquimod (23), we investigated the levels of IL-6 mRNA as a possible positive control for a normal response to imiquimod in STAT-10/0 mice. However, imiquimod failed to increase the levels of IL-6 mRNA in this group of mutant mice, in contrast to a clear induction in wild-type mice treated for 2 h (Fig. 4A). A possible role of some component(s) of the IFN system in induction of the IL-6 gene expression was further substantiated by the observation that mice deficient in the type I IFN receptor also failed to exhibit increased levels of IL-6 mRNA in response to imiquimod (Fig. 4B). Moreover, there was a clear correlation between the level of IL-6 mRNA and the amount of circulating protein, as revealed by an ELISA using sera from wild-type or STAT-10/0 treated mice. Figure 5A shows the levels of circulating IL-6 in all individual mice. Again, there is a dramatic difference in the levels of IL-6 between wild-type and mutant mice treated with imiquimod for 2 h. Interestingly, TNF was elevated in treated mutant mice, although not to the same extent as in wild-type treated mice (Fig. 5B). Finally, IL-12 was induced equally in wild-type and mutant mice, suggesting that STAT-1 does not play a role in its induction by imiquimod (Fig. 5C).
FIG. 4.
Levels of IL-6 mRNA in the spleens of imiquimod-treated mice. Wild-type, STAT-10/0 (A), or IFNR0/0 (B) mice were treated orally with imiquimod (1 mg/kg) for 2 or 8 h, total RNAs were collected and fractionated on a 1% agarose-formaldehyde gel, and RNAs were transferred to a nylon membrane. Filters were hybridized with an IL-6 cDNA probe (6) labeled with [α-32P]dCTP by random priming (RediPrime; Amersham). After autoradiography, filters were stripped and hybridized with a 32P-labeled GAPDH cDNA probe (10) to control for RNA loading.
FIG. 5.
Levels of circulating IL-6 (A), TNF (B), and IL-12 (C) in the sera of imiquimod-treated mice. Wild-type (WT) or STAT-10/0 (KO) mice were treated orally with imiquimod (1 mg/kg) for 2 or 8 h, and cytokine levels in the serum of each animal were measured by ELISA (R&D Systems).
DISCUSSION
Induction of the IFN genes and other proinflammatory cytokines is a necessary step in the process of resolving an infection by a virus or another pathogen. We have investigated the mechanism by which imiquimod, a potent immune response modifier, can trigger the expression of the IFN genes, ISGs, and other proinflammatory cytokines. Using animals deficient in various components of the IFN signaling pathway, we evaluated the contributions of such molecules in mediating imiquimod’s activity in vivo.
Induction of the type I IFN genes can be observed in vivo during viral infection or by treatment with the synthetic double-stranded RNA poly(I) · poly(C). These two inducers apparently use different mechanisms, which are independent of IRF-1 (29, 45), for IFN gene activation (29). We observed that induction of type I IFN genes by imiquimod, like that by viruses and poly(I) · poly(C), is independent of the transcription factor IRF-1 and the double-stranded RNA-dependent protein kinase PKR.
Viral induction of type I IFN genes requires some signaling component(s) that is activated via the type I IFN receptor, as demonstrated by the fact that IFN induction by viruses is reduced in mice deficient in the type I IFN receptor (46). It has been speculated that small amounts of IFN induced by a virus function as a positive feedback mechanism, allowing full expression of the IFN genes. The observation that IFN facilitates its own induction was described earlier as priming (1). In agreement with these observations and with data observed by Megyeri and coworkers (23) showing that IFN induction by imiquimod and viruses requires tyrosine kinase activity, we found that induction of type I IFN by imiquimod was abrogated in mice deficient in STAT-1 (Table 1). Since both STAT-1 itself and ISGF-3, a STAT-1-containing complex, have very low affinities for the type I IFN promoters (19), it is likely that STAT-1 is involved in the induction of the type I IFN genes by activating some intermediate factor, as, for example, another IRF family member.
We have also observed that induction of type I IFN genes by imiquimod was similarly efficient in wild-type mice and mice deficient in the type I IFN receptor (Table 1). This observation suggests that full activation of the type I IFN promoter can be achieved even in the absence of a type I IFN response. Based on these observations, we can conclude that, for full induction of type I IFN genes, small amounts of type I IFN, which would facilitate its own synthesis, can be replaced by some other factor(s) whose expression is STAT-1 dependent and is induced by imiquimod.
Induction of several ISGs (IRF-1, PKR, 2-5 OAS, and 1-8 genes) was not affected by lack of IRF-1 or PKR (data not shown). It has been demonstrated that induction of the IRF-1 gene is driven by an IFN-responsive element, designated IR, with which a STAT-1-containing complex interacts and also by an NF-κB binding element (13, 38). Since NF-κB is a natural substrate for PKR (45), we investigated whether induction of IRF-1 by imiquimod requires PKR as an activator of NF-κB, which, in turn, would mediate induction of IRF-1. The fact that no differences in steady-state IRF-1 mRNA levels in the spleen after imiquimod treatment were observed between wild-type and PKR-deficient mice suggests that, at least for the induction of IRF-1, imiquimod does not use PKR as a possible activator of NF-κB.
The observation that IRF-1 mRNA was not detectable in STAT-10/0 (Fig. 2B) mice but was elevated in imiquimod-treated type I IFN receptor-deficient mice (Fig. 3) could be explained either by direct activation of STAT-1 by imiquimod or, alternatively, by induction of IFN-γ, which in turn would activate STAT-1. Although we failed to detect IFN-γ in the sera of treated mice (<20 U/ml), it is possible that IFN-γ activity below our detection limit is present in the organs of treated mice. In fact, under some circumstances, IFN-γ activity can be demonstrated in mice treated with imiquimod (40a). Finally, the fact that imiquimod failed to increase the levels of 2-5 OAS mRNA in mice deficient in the type I IFN receptor (Fig. 3) suggests that assembly of ISGF-3 is dependent on type I IFN induction.
To control for the induction of genes that are known to be regulated by factors other than those involved in the IFN system, we investigated the levels of IL-6, TNF, and IL-12 in mice deficient in STAT-1. To our surprise, there was no induction of IL-6 in STAT-1-deficient mice, in contrast to a sharp increase in IL-6 mRNA levels in wild-type mice, after treatment with imiquimod for 2 h (Fig. 4A). This result was confirmed by determination of the levels of circulating cytokines in both mutant and wild-type mice. Furthermore, the lack of IL-6 induction by imiquimod in type I IFN receptor-deficient mice also corroborates the idea that the induced expression of the IL-6 gene in imiquimod-treated mice requires a component(s) of the IFN signaling cascade.
It has been demonstrated by several groups that regulation of IL-6 gene expression is mediated primarily by NF-κB and NF-IL6 (8). In addition, TNF was one of the first inducers of IL-6 to be identified (22). Since we observed an increase in TNF levels upon treatment of STAT-1-deficient mice with imiquimod, and since there is no evidence for the activation of STAT-dependent pathways by TNF, one would predict that induction of IL-6 should not be affected by the lack of STAT-1.
Since we detected a lack of induction of IL-6 in STAT-1-deficient mice, we decided to measure the levels of IL-6 mRNA in type I IFN receptor-deficient mice treated with imiquimod. Again, there were only traces of induction of IL-6 mRNA in the spleens of mutant mice, but in wild-type animals, imiquimod treatment led to an increase in IL-6 mRNA levels (Fig. 4B).
Based on these results, we suggest that, in addition to NF-κB and NF-IL6, some alternative signaling pathway might contribute to the regulated expression of the IL-6 gene and that, at least under the conditions we tested, such a pathway is indispensable for the transcription activation of the IL-6 gene. Since elevated levels of TNF could be detected in STAT-1-deficient mice yet no induction of IL-6 could be demonstrated, we could also speculate that, in this system, STAT-1 might be important for full responsiveness to TNF.
ACKNOWLEDGMENTS
We thank Eduardo Pires for performing the IRF-1 Northern blot for type I IFN receptor-deficient mice, Lionel Bethancourt for assistance with figures, and all members of the laboratory for helpful discussions. We also thank Charles Weissmann for providing mutant mice (PKR0/0 and IFNR0/0) and Andrew Simpson, Adam Goodman, and Kenneth Gollob for critical reading of the manuscript.
This work was supported by 3M Pharmaceuticals, PADCT/CNPq, FAPEMIG, WHO, and FAPESP.
REFERENCES
- 1.Abreu S L, Bancroft F C, Stewart W E. Interferon priming. Effects on interferon messenger RNA. J Biol Chem. 1979;254:4114–4118. [PubMed] [Google Scholar]
- 2.Baker G E, Tyring S K. Therapeutic approaches to papillomavirus infections. Dermatol Clin. 1997;15:331–340. doi: 10.1016/s0733-8635(05)70441-1. [DOI] [PubMed] [Google Scholar]
- 3.Beutner K R, Ferenczy A. Therapeutic approaches to genital warts. Am J Med. 1997;102:28–37. doi: 10.1016/s0002-9343(97)00181-2. [DOI] [PubMed] [Google Scholar]
- 4.Bohni R, Hemmi S, Aguet M. Signaling steps involving the cytoplasmic domain of the interferon-gamma receptor alpha-subunit are not species-specific. J Biol Chem. 1994;269:14541–14545. [PubMed] [Google Scholar]
- 5.Briken V, Ruffner H, Schultz U, Schwarz A, Reis L F L, Strehlow I, Decker T, Staeheli P. Interferon regulatory factor 1 is required for mouse Gbp gene activation by gamma interferon. Mol Cell Biol. 1995;15:975–982. doi: 10.1128/mcb.15.2.975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chiu C P, Moulds C, Coffman R L, Rennick D, Lee F. Multiple biological activities are expressed by a mouse interleukin 6 cDNA clone isolated from bone marrow stromal cells. Proc Natl Acad Sci USA. 1988;85:7099–7103. doi: 10.1073/pnas.85.19.7099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chomczynski P, Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987;162:156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- 8.Dendorfer U, Oettgen P, Libermann T A. Multiple regulatory elements in the interleukin-6 gene mediate induction by prostaglandins, cyclic AMP, and lipopolysaccharide. Mol Cell Biol. 1994;14:4443–4454. doi: 10.1128/mcb.14.7.4443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Durbin J E, Hackenmiller R, Simon M C, Levy D E. Targeted disruption of the mouse Stat1 gene results in compromised innate immunity to viral disease. Cell. 1996;84:443–450. doi: 10.1016/s0092-8674(00)81289-1. [DOI] [PubMed] [Google Scholar]
- 10.Fort P, Marty L, Piechaczyk M, el Sabrouty S, Dani C, Jeanteur P, Blanchard J M. Various rat adult tissues express only one major mRNA species from the glyceraldehyde-3-phosphate-dehydrogenase multigenic family. Nucleic Acids Res. 1985;13:1431–1442. doi: 10.1093/nar/13.5.1431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fujita T, Reis L F L, Watanabe N, Kimura Y, Taniguchi T, Vilcek J. Induction of the transcription factor IRF-1 and interferon-beta mRNAs by cytokines and activators of second-messenger pathways. Proc Natl Acad Sci USA. 1989;86:9936–9940. doi: 10.1073/pnas.86.24.9936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Grange J M, Stanford J L, Rook G A. Tuberculosis and cancer: parallels in host responses and therapeutic approaches? Lancet. 1995;345:1350–1352. doi: 10.1016/s0140-6736(95)92542-2. [DOI] [PubMed] [Google Scholar]
- 13.Haque S J, Williams B R. Identification and characterization of an interferon (IFN)-stimulated response element-IFN-stimulated gene factor 3-independent signaling pathway for IFN-alpha. J Biol Chem. 1994;269:19523–19529. [PubMed] [Google Scholar]
- 14.Harrison C J, Miller R L, Bernstein D I. Posttherapy suppression of genital herpes simplex virus (HSV) recurrences and enhancement of HSV-specific T-cell memory by imiquimod in guinea pigs. Antimicrob Agents Chemother. 1994;38:2059–2064. doi: 10.1128/aac.38.9.2059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Harroch S, Revel M, Chebath J. Induction by interleukin-6 of interferon regulatory factor 1 (IRF-1) gene expression through the palindromic interferon response element pIRE and cell type-dependent control of IRF-1 binding to DNA. EMBO J. 1994;13:1942–1949. doi: 10.1002/j.1460-2075.1994.tb06463.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Huang S, Hendriks W, Althage A, Hemmi S, Bluethmann H, Kamijo R, Vilcek J, Zinkernagel R M, Aguet M. Immune response in mice that lack the interferon-gamma receptor [see comments] Science. 1993;259:1742–1745. doi: 10.1126/science.8456301. [DOI] [PubMed] [Google Scholar]
- 17.Kamijo R, Harada H, Matsuyama T, Bosland M, Gerecitano J, Shapiro D, Le J, Koh S I, Kimura T, Green S J. Requirement for transcription factor IRF-1 in NO synthase induction in macrophages. Science. 1994;263:1612–1615. doi: 10.1126/science.7510419. [DOI] [PubMed] [Google Scholar]
- 18.Kamijo R, Shapiro D, Le J, Huang S, Aguet M, Vilcek J. Generation of nitric oxide and induction of major histocompatibility complex class II antigen in macrophages from mice lacking the interferon gamma receptor. Proc Natl Acad Sci USA. 1993;90:6626–6630. doi: 10.1073/pnas.90.14.6626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kessler D S, Levy D E, Darnell J E J. Two interferon-induced nuclear factors bind a single promoter element in interferon-stimulated genes. Proc Natl Acad Sci USA. 1988;85:8521–8525. doi: 10.1073/pnas.85.22.8521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kimura T, Nakayama K, Penninger J, Kitagawa M, Harada H, Matsuyama T, Tanaka N, Kamijo R, Vilcek J, Mak T W. Involvement of the IRF-1 transcription factor in antiviral responses to interferons. Science. 1994;264:1921–1924. doi: 10.1126/science.8009222. [DOI] [PubMed] [Google Scholar]
- 21.Kishimoto T, Akira S, Taga T. IL-6 receptor and mechanism of signal transduction. Int J Immunopharmacol. 1992;14:431–438. doi: 10.1016/0192-0561(92)90173-i. [DOI] [PubMed] [Google Scholar]
- 22.Kohase M, Henriksen-Destefano D, May L T, Vilcek J, Sehgal P B. Induction of beta 2-interferon by tumor necrosis factor: a homeostatic mechanism in the control of cell proliferation. Cell. 1986;45:659–666. doi: 10.1016/0092-8674(86)90780-4. [DOI] [PubMed] [Google Scholar]
- 23.Megyeri K, Au W C, Rosztoczy I, Raj N B, Miller R L, Tomai M A, Pitha P M. Stimulation of interferon and cytokine gene expression by imiquimod and stimulation by Sendai virus utilize similar signal transduction pathways. Mol Cell Biol. 1995;15:2207–2218. doi: 10.1128/mcb.15.4.2207. . (Erratum, 15:2905.) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Miyamoto M, Fujita T, Kimura Y, Maruyama M, Harada H, Sudo Y, Miyata T, Taniguchi T. Regulated expression of a gene encoding a nuclear factor, IRF-1, that specifically binds to IFN-beta gene regulatory elements. Cell. 1988;54:903–913. doi: 10.1016/s0092-8674(88)91307-4. [DOI] [PubMed] [Google Scholar]
- 24a.Mueller, U., L. F. L. Reis, and M. Aguet. Unpublished data.
- 25.Mueller U, Steinhoff U, Reis L F, Hemmi S, Pavlovic J, Zinkernagel R M, Aguet M. Functional role of type I and type II interferons in antiviral defense. Science. 1994;264:1918–1921. doi: 10.1126/science.8009221. [DOI] [PubMed] [Google Scholar]
- 26.Ozmen L, Aguet M, Trinchieri G, Garotta G. The in vivo antiviral activity of interleukin-12 is mediated by gamma interferon. J Virol. 1995;69:8147–8150. doi: 10.1128/jvi.69.12.8147-8150.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Reis L F L, Harada H, Wolchok J D, Taniguchi T, Vilcek J. Critical role of a common transcription factor, IRF-1, in the regulation of IFN-beta and IFN-inducible genes. EMBO J. 1992;11:185–193. doi: 10.1002/j.1460-2075.1992.tb05041.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Reis L F L, Lee T H, Ho L T, Vilcek J. Tumor necrosis factor acts synergistically with autocrine interferon-beta and increases interferon-beta mRNA levels in human fibroblasts. J Biol Chem. 1989;264:16351–16354. [PubMed] [Google Scholar]
- 29.Reis L F L, Ruffner H, Stark G, Aguet M, Weissmann C. Mice devoid of interferon regulatory factor 1 (IRF-1) show normal expression of type I interferon genes. EMBO J. 1994;13:4798–4806. doi: 10.1002/j.1460-2075.1994.tb06805.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rezende S A, Oliveira V R, Silva A M, Alves J B, Gocs A M, Reis L F L. Mice lacking the gamma interferon receptor have an impaired granulomatous reaction to Schistosoma mansoni infection. Infect Immun. 1997;65:3457–3461. doi: 10.1128/iai.65.8.3457-3461.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rutherford M N, Kumar A, Nissim A, Chebath J, Williams B R. The murine 2-5A synthetase locus: three distinct transcripts from two linked genes. Nucleic Acids Res. 1991;19:1917–1924. doi: 10.1093/nar/19.8.1917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sailer A, Nagata K, Naf D, Aebi M, Weissmann C. Interferon regulatory factor-1 (IRF-1) activates the synthetic IRF-1-responsive sequence (GAAAGT)4 in Saccharomyces cerevisiae. Gene Expr. 1992;2:329–337. [PMC free article] [PubMed] [Google Scholar]
- 33.Savage P, Horton V, Moore J, Owens M, Witt P, Gore M E. A phase I clinical trial of imiquimod, an oral interferon inducer, administered daily. Br J Cancer. 1996;74:1482–1486. doi: 10.1038/bjc.1996.569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Schijns V E, Haagmans B L, Horzinek M C. IL-12 stimulates an antiviral type 1 cytokine response but lacks adjuvant activity in IFN-gamma-receptor-deficient mice. J Immunol. 1995;155:2525–2532. [PubMed] [Google Scholar]
- 35.Schindler C, Shuai K, Prezioso V R, Darnell J E J. Interferon-dependent tyrosine phosphorylation of a latent cytoplasmic transcription factor [see comments] Science. 1992;257:809–813. doi: 10.1126/science.1496401. [DOI] [PubMed] [Google Scholar]
- 36.Sen G C, Lengyel P. The interferon system. A bird’s eye view of its biochemistry. J Biol Chem. 1992;267:5017–5020. [PubMed] [Google Scholar]
- 37.Shuai K, Stark G R, Kerr I M, Darnell J E J. A single phosphotyrosine residue of Stat91 required for gene activation by interferon-gamma [see comments] Science. 1993;261:1744–1746. doi: 10.1126/science.7690989. [DOI] [PubMed] [Google Scholar]
- 38.Sims S H, Cha Y, Romine M F, Gao P Q, Gottlieb K, Deisseroth A B. A novel interferon-inducible domain: structural and functional analysis of the human interferon regulatory factor 1 gene promoter. Mol Cell Biol. 1993;13:690–702. doi: 10.1128/mcb.13.1.690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Stark G R, Kerr I M. Interferon-dependent signaling pathways; DNA elements, transcription factors, mutations, and effects of viral proteins. J Interferon Res. 1992;12:147–151. doi: 10.1089/jir.1992.12.147. [DOI] [PubMed] [Google Scholar]
- 40.Swihart K, Fruth U, Messmer N, Hug K, Behin R, Huang S, Del Giudice G, Aguet M, Louis J A. Mice from a genetically resistant background lacking the interferon gamma receptor are susceptible to infection with Leishmania major but mount a polarized T helper cell 1-type CD4+ T cell response. J Exp Med. 1995;181:961–971. doi: 10.1084/jem.181.3.961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40a.Tomai, M. Unpublished data.
- 41.Tsuji M, Miyahira Y, Nussenzweig R S, Aguet M, Reichel M, Zavala F. Development of antimalaria immunity in mice lacking IFN-gamma receptor. J Immunol. 1995;154:5338–5344. [PubMed] [Google Scholar]
- 42.Vilcek J, Aguet M, Reis L F L. Knockouts of interferons. Interferon receptors and interferon signaling components. In: Durum S K, Muegge K, editors. Contemporary immunology: cytokine knockouts. Totowa, N.J: Humana Press Inc.; 1998. pp. 207–225. [Google Scholar]
- 43.Vilcek J, Sen G. IFNs and other cytokines. In: Fields B N, Knipe D M, Howley P M, editors. Virology. Philadelphia, Pa: Lippincott-Raven; 1996. pp. 375–399. [Google Scholar]
- 44.Wang Z E, Reiner S L, Zheng S, Dalton D K, Locksley R M. CD4+ effector cells default to the Th2 pathway in interferon gamma-deficient mice infected with Leishmania major. J Exp Med. 1994;179:1367–1371. doi: 10.1084/jem.179.4.1367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yang Y L, Reis L F L, Pavlovic J, Aguzzi A, Schafer R, Kumar A, Williams B R, Aguet M, Weissmann C. Deficient signaling in mice devoid of double-stranded RNA-dependent protein kinase. EMBO J. 1995;14:6095–6106. doi: 10.1002/j.1460-2075.1995.tb00300.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yang Y L, Weissmann C. Induction of type 1 interferon by virus or double-stranded RNA is defective in mice devoid of type 1 IFN receptor. J Interferon Cytokine Res. 1997;17(Suppl. 2):S52. . (Abstract.) [Google Scholar]