Abstract
Based on the acid-catalyzed reaction of functionalized aminoacetals with C-nucleophiles, a series of new diarylmethane derivatives with a taurine fragment were synthesized, the structure of which was established by NMR spectroscopy method.
Keywords: taurine, diarylmethanes, C-nucleophiles
Among a large number of synthetic and natural organic compounds, diarylmethane derivatives attract attention, which is due to their high biological activity and the use of a number of them as drugs, such as fendiline—an antianginal, hypotensive, antiarrhythmic, coronary dilating agent [1], antihistamine agent diphenhydramine [2] and methadone used as an analgesic, as well as in the treatment of drug dependence [3, 4]. Diarylmethane derivatives may be potential agents for the treatment of COVID-19 [5]. Diarylmethanes with two phenolic fragments have anti-inflammatory [6], antiviral [7, 8], antiproliferative [9], anti-HIV [10], anticancer [11], and antimicrobial [12] activity.
Taurine (2-aminoethanesulfonic acid), as a pharmacophore unit, is a part of taurocholic acid, which is involved into the fats emulsification [13–15], and the drug netobimine used in the treatment of helminthiases in animals [16, 17].
Combining two biologically active fragments in one molecule is a promising route for the synthesis of compounds with new pharmacological properties compared to the original structures. The synthesis of hybrid structures, including the taurine and diarylmethane fragments, seems to be relevant.
Previously, an original method was developed for the preparation of diarylmethane derivatives based on the acid-catalyzed reaction of 1-(3,3-diethoxybutyl)ureas with resorcinol and its derivatives [18]. Extending the boundaries of this method makes it possible to obtain previously unknown diarylmethane derivatives with a taurine fragment. The synthesis of starting acetals 3a–3e was carried out in several stages according to previously developed procedures [19]. The reaction of 2-chloroethanesulfonyl chloride 1 with amines in dichloromethane in the presence of triethylamine leads to vinylsulfonyl chlorides 2a–2d. Subsequent addition of 3,3-diethoxypropan-1-amine or 4,4-diethoxybutan-1-amine to the multiple bond (aza-Michael reaction) led to the formation of acetals 3a–3e (Scheme 1).
Scheme.
1.
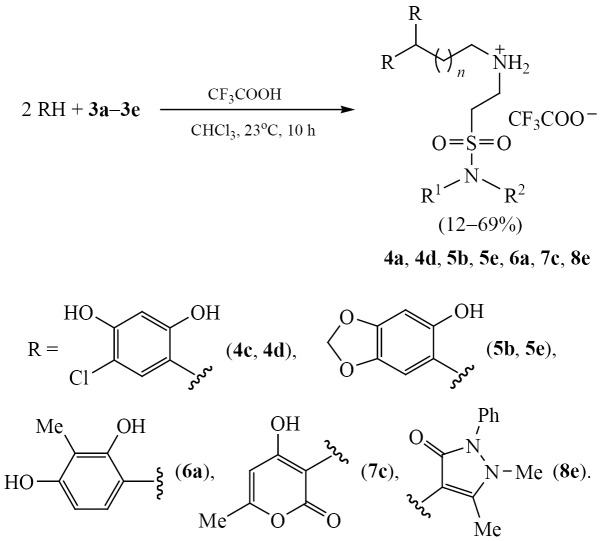
Phenols [4-chlororesorcinol, sesamol (1,3-benzodioxol-5-ol), 2-methylresorcinol] and heterocycles (antipyrine, 4-hydroxy-6-methyl-2H-pyran-2-one), which quite easily enter into electrophilic substitution reactions and show biological activity, were chosen as C-nucleophiles. The reactions of acetals 3c and 3d with 4-chlororersorcinol in chloroform in the presence of an excess of trifluoroacetic acid at room temperature led to the formation of diarylmethane derivatives 4c and 4d (Scheme 2). Compounds 5b, 5e, and 6a were obtained in a similar way by reacting acetals 3 with sesamol and 2-methylresorcinol, respectively. Compound 6a was isolated in only 12% yield, which is probably due to the formation of a large number of oligomers and polymers. Using 4-hydroxy-6-methyl-2H-pyran-2-one and antipyrine as nucleophiles, new representatives of dihetarylmethanes 7c and 8e were obtained.
Scheme.
2.
In conclusion, using the reactions of functionalized aminoacetals with C-nucleophiles, new di(het)arylmethane derivatives modified with a taurine fragment were synthesized. The proposed route for the synthesis of these compounds is simple and allows varying the substituents in both the taurine and diarylmethane moieties over a wide range.
EXPERIMENTAL
1Н and 13С NMR spectra were recorded on a Bruker MSL 400 spectrometer (400 and 150 MHz) relative to residual proton signals of the deuterated solvent (CDCl3, DMSO-d6). IR spectra were recorded on a Bruker Tensor 27 spectrometer from KBr pellets. Elemental analysis was performed on a Carlo Erba EA 1108 instrument. Melting points were determined in glass capillaries on a Stuart SMP 10 instrument.
General procedure for the synthesis of aminoacetals 3a–3e. To a mixture of 3.64 g (20 mmol) of 2-chloroethanesulfonyl chloride 1 and 6 mL of Et3N in 100 mL of methylene chloride was added 20 mmol of an amine under cooling (5–8°С). The reaction mixture was stirred at room temperature for 12 h, then washed with saturated aqueous sodium hydrogen carbonate solution (3×10 mL). The organic layer was separated and concentrated in vacuum. The resulting vinylsulfonamides 2а–2e were subjected without additional purification to the aza-Michael reaction with 20 mmol of aminoacetal (3,3-diethoxypropan-1-amine, 4,4-diethoxybutan-1-amine) in 30 mL of chloroform. The reaction mixture was refluxed for 25 h, after which the solvent was removed under reduced pressure. The reaction products were brown resinous substances.
2-[(3,3-Diethoxypropyl)amino]-N,N-diethylethane-1-sulfonamide (3a). Yield 5.77 g (93%). 1Н NMR spectrum (CDCl3), δ, ppm: 1.09‒1.14 m (12H, CH3), 1.64‒1.73 m (1H, CH2), 2.55‒2.63 m (1H, CH2), 2.83‒2.92 m (2H, CH2), 3.09‒3.23 m (8H, CH2), 3.37‒3.48 m (2H, CH2), 3.50‒3.69 m (2H, CH2), 4.55 t (1H, CH, 3JHH 5.3 Hz). 13С NMR spectrum (CDCl3), δС, ppm: 14.90, 15.71, 33.65, 41.84, 43.60, 44.81, 50.82, 61.13, 101.24. Found, %: C 50.46; H 9.90; N 8.88; S 10.37. C13H30N2O4S. Calculated, %: C 50.29; H 9.74; N 9.02; S 10.33.
3,3-Diethoxy-N-[2-( pyrrolidin-1-ylsulfonyl)ethyl]propan-1-amine (3b). Yield 5.36 g (87%). 1Н NMR spectrum (CDCl3), δ, ppm: 1.18 t (6H, СН3, 3JHH 7.1 Hz), 1.72‒1.82 m (2H, CH2), 1.88‒1.97 m (4H, CH2), 2.61‒2.74 m (2H, CH2), 2.93‒3.07 m (2H, CH2), 3.08‒3.19 m (2H, CH2), 3.23‒3.38 m (4H, CH2), 3.40‒3.54 m (2H, CH2), 3.57‒3.70 m (2H, CH2), 4.56 t (1H, CH, 3JHH 5.5 Hz). 13С NMR spectrum (CDCl3), δС, ppm: 15.28, 25.78, 33.82, 43.75, 45.26, 47.63, 49.17, 61.37, 101.73. Found, %: C, 50.41; H, 8.91; N, 9.24; S, 10.24. C13H28N2O4S. Calculated, %: C 50.62; H 9.15; N 9.08; S 10.40.
3,3-Diethoxy-N-[2-(morpholin-4-ylsulfonyl)ethyl]propan-1-amine (3c). Yield 6.16 g (95%). 1Н NMR spectrum (CDCl3), δ, ppm: 1.16 t (6H, СН3, 3JHH 7.1 Hz), 1.70‒1.72 m (2H, CH2), 2.61‒2.73 m (2H, CH2), 2.99‒3.15 m (4H, CH2), 3.18‒3.27 m (4H, CH2), 3.41‒3.53 m (2H, CH2), 3.55‒3.65 m (2H, CH2), 3.68‒3.75 m (4H, CH2), 4.55 t (1H, CH, 3JHH 5.5 Hz). 13С NMR spectrum (CDCl3), δС, ppm: 15.24, 33.63, 43.32, 45.13, 45.62, 48.59, 61.32, 66.41, 101.62. Found, %: C 48.24; H 8.81; N 8.69; S 10.04. C13H28N2O5S. Calculated, %: C 48.13; H 8.70; N 8.63; S 9.88.
2-[(3,3-Diethoxypropyl)amino]-N-hexylethanesulfonamide (3d). Yield 4.87 g (72%). 1Н NMR spectrum (CDCl3), δ, ppm: 0.84 t (3H, СН3, 3JHH 6.8 Hz), 1.16 t (6H, 3JHH 7.1 Hz), 1.22‒1.33 m (6H, CH2), 1.46‒ 1.56 m (2H, CH2), 1.71‒1.81 m (2H, CH2), 2.60‒2.73 m (2H, CH2), 2.99‒3.08 m (4H, CH2), 3.09‒3.16 m (2H, CH2), 3.41‒3.51 m (2H, CH2), 3.56‒3.65 m (2H, CH2), 4.53 t (1H, CH, 3JHH 5.4 Hz). 13С NMR spectrum (CDCl3), δС, ppm: 13.41, 14.81, 22.05, 25.92, 29.86, 30.97, 33.38, 42.55, 43.66, 44.69, 50.85, 60.53, 101.15. Found, %: C 53.34; H 10.01; N 8.15; S 9.61. C15H34N2O4S. Calculated, %: C 53.22; H 10.12; N 8.28; S 9.47.
2-[(4,4-Diethoxybutyl)amino]-N-hexylethanesulfonamide (3e). Yield 5.28 g (75%). 1Н NMR spectrum (CDCl3), δ, ppm: 0.80 t (3H, СН3, 3JHH 6.8 Hz), 1.10 t (6H, 3JHH 7.1 Hz), 1.16‒1.31 m (6H, CH2), 1.42‒1.60 m (6H, CH2), 2.50‒2.58 m (2H, CH2), 2.93‒3.05 m (4H, CH2), 3.05‒3.11 m (2H, CH2), 3.36‒3.47 m (2H, CH2), 3.50‒3.61 m (2H, CH2), 4.39 t (1H, CH, 3JHH 5.4 Hz). Found, %: C 54.76; H 10.51; N 8.08; S 9.22. C16H36N2O4S. Calculated, %: C 54.51; H 10.29; N 7.95; S 9.10.
General procedure for the synthesis of compounds 4c, 4d, 5b, 5e, 6a, 7c, 8e. To a mixture of 1.6 mmol of acetal 3a–3e in 10 mL of chloroform was added 3.2 mmol of phenol and 1 mL of trifluoroacetic acid. The reaction mixture was stirred for 72 h at room temperature, then the solvent was removed under reduced pressure. The residue was washed with 10 mL of diethyl ether. The resulting white powder was dried under reduced pressure.
3,3-Bis(2,4-dihydroxy-5-chlorophenyl)-N-[2-(morpholin-4-ylsulfonyl)ethyl]propane-1-aminium trifluoroacetate (4c). Yield 0.70 g (69%), mp 187–188°C. IR spectrum, ν, cm–1: 1504, 1680, 3056, 3130. 1Н NMR spectrum (DMSO-d6), δ, ppm: 2.09‒2.20 m (2H, CH2), 2.77‒2.83 m (4H, CH2), 2.84‒2.88 m (2H, CH2), 3.14‒3.19 m (2H, CH2), 3.21‒3.26 m (2H, CH2), 3.36‒3.46 m (4H, CH2), 4.27 t (1H, CH, 3JHH 7.6 Hz), 6.50 s (2НAr), 6.90 s (2НAr). Found, %: C 43.65; H 4.48; Cl 11.01; N 4.54; S 4.87. C23H27Cl2F3N2O9S. Calculated, %: C 43.47; H 4.28; Cl 11.16; N 4.41; S 5.05.
3,3-Bis(2,4-hydroxy-5-chlorophenyl)-N-[2-(N-hexylsulfamoyl)ethyl]propane-1-aminium trifluoroacetate (4d). Yield 0.30 g (29%), mp 70‒72°C. IR spectrum, ν, cm–1: 1503, 1685, 3035, 3275, 3157. 1Н NMR spectrum (DMSO-d6), δ, ppm: 0.82‒0.92 m (3H, CH2), 1.17‒1.35 m (6H, CH2), 1.38‒1.54 m (2H, CH2), 2.05‒2.22 m (2H, CH2), 2.88‒2.99 m (4H, CH2), 3.14‒3.26 m (4H, CH2), 4.26‒4.31 m (1H, CH), 6.48 s (2HAr), 6.91 s (2HAr). 13С NMR spectrum (DMSO-d6), δС, ppm: 14.33, 22.46, 26.18, 29.94, 30.01, 30.14, 31.26, 34.37, 42.85, 47.54, 48.58, 104.26, 109.68, 117.05 q (1JСF 295.9 Hz) 122.15, 128.88, 152.12, 154.78, 158.84 к (2JСF 33.3 Hz). Found, %: C 48.39; H 5.60; Cl 10.79; N 4.16; S 5.12. C26H35Cl2F3N2O7S. Calculated, %: C 48.23; H 5.45; Cl 10.95; N 4.33; S 4.95.
3,3-Bis(6-hydroxybenzo[d][1,3]dioxol-5-yl)-N-[2-(pyrolidin-1-ylsulfonyl)ethyl]propane-1-aminium trifluoroacetate (5b). Yield 0.66 g (68%), mp 106‒107°C. IR spectrum, ν, cm–1: 1504, 1682, 3076, 3136. 1Н NMR spectrum (DMSO-d6), δ, ppm: 0.86‒0.93 m (4H, CH2), 2.32‒2.38 m (2H, CH2), 2.65‒2.71 m (2H, CH2), 2.79‒2.89 m (2H, CH2), 3.18‒3.24 m (6H, CH2), 4.44 t (1H, CH, 3JHH 6.6 Hz), 5.85 d (4H, CH2, 3JHH 6.4 Hz), 6.43 s (2HAr), 6.66 s (2HAr). Found, %: C 49.77; H 4.99; N 4.76; S 5.41. C25H29F3N2O10S. Calculated, %: C 49.50; H 4.82; N 4.62; S 5.29.
N-[2-(N-Hexylsulfamoyl)ethyl]-4,4-bis(6-hydroxybenzo[d][1,3]dioxol-5-yl)butane-1-aminium trifluoroacetate (5e). Yield 0.64 g (59%), mp 97‒99°C. IR spectrum, ν, cm–1: 1503, 1680, 3076, 3132. 1Н NMR spectrum (DMSO-d6), δ, ppm: 1.17 t (3H, CH3, 3JHH 6.8 Hz), 1.17‒1.32 m (8H, CH2), 1.59‒1.68 m (2H, CH2), 2.23‒2.41 m (2H, CH2), 2.82‒2.97 m (2H, CH2), 3.02‒3.12 m (2H, CH2), 3.14‒3.22 m (2H, CH2), 3.98‒4.12 m (2H, CH2), 4.23 t (1H, CH, 3JHH 6.7 Hz), 4.34‒4.52 m (2H, CH2), 5.84 д (4H, CH2, 3JHH 6.5 Hz), 6.38 s (2HAr), 6.68 с (2HAr). Found, %: C 51.89; H 5.56; N 4.40; S 5.12. C28H37F3N2O10S. Calculated, %: C 51.69; H 5.73; N 4.31; S 4.93.
N-[2-(N,N-Diethylsulfamoyl)ethyl]3,3-bis(2,4-dihydroxy-3-methylphenyl)propane-1-aminium trifluoroacetate (6a). Yield 0.11 g (12%), mp 114‒116°C. IR spectrum, ν, cm–1: 1503, 1682, 3056, 3274, 3148. 1Н NMR spectrum (DMSO-d6), δ, ppm: 1.10 t (6H, CH3, 3JHH 7.0 Hz), 1.98 s (3H, CH3), 1.99‒2.02 m (2H, CH2), 2.05‒2.23 m (2H, CH2), 2.77‒2.92 m (2H, CH2), 3.16–3.30 m (2H, CH2), 3.36–3.45 m (4H, CH2), 4.46 t (1H, CH, 3JHH 7.6 Hz), 6.32 d (2HAr, 3JHH 8.4 Hz), 6.73 d (2HAr, 3JHH 8.4 Hz). Found, %: C 51.60; H 5.89; N 4.89; S, 5.67. C25H35F3N2O8S. Calculated, %: C 51.72; H 6.08; N 4.82; S 5.52.
3,3-Bis(4-hydroxy-6-methyl-2-oxo-2H-pyran-3-yl)-N-[2-(morpholin-4-ylsulfonyl)ethyl]propane-1-aminium trifluoroacetate (7c). Yield 0.46 g (48%), mp 167‒169°C. IR spectrum, ν, cm–1: 1504, 1676, 3059, 3089. 1Н NMR spectrum (DMSO-d6), δ, ppm: 1.59‒1.73 m (2H, CH2), 2.10 s (6H, CH3), 2.23‒2.36 m (2H, CH2), 2.48‒2.70 m (2H, CH2), 2.79‒2.87 m (4H, CH2), 2.94‒3.03 m (2H, CH2), 3.19‒3.26 m (4H, CH2), 4.29 t (1H, CH, 3JHH 8.1 Hz), 5.88 s (2H, СН). Found, %: C 46.33; H 4.98; N 4.79; S 5.18. C23H29F3N2O11S. Calculated, %: C 46.15; H 4.88; N 4.68; S 5.36.
4,4-Bis(1,5-dimethyl-3-oxo-2-phenyl-2,3-dihydro-1H-pyrazol-4-yl)-N-[2-(N-hexylsulfamoyl)ethyl]butane-1-aminium trifluoroacetate (8e). Yield 0.48 g (40%), mp 93‒94°C. IR spectrum, ν, cm–1: 1501, 1685, 3035, 3117. 1Н NMR spectrum (CDCl3), δ, ppm: 0.90 t (3H, CH3, 3JHH 6.9 Hz), 1.22‒1.39 m (6H, CH2), 1.46‒1.61 m (2H, CH2), 1.74‒1.92 m (2H, CH2), 2.13‒2.26 m (2H, CH2), 2.45 s (6H, CH3), 3.00‒3.17 m (4H, CH2), 3.32 s (6H, CH3), 3.43‒3.58 m (4H, CH2), 3.79 t (1H, CH, 3JHH 8.3 Hz), 7.35‒7.43 m (4HAr), 7.44‒7.60 m (6HAr). 13С NMR spectrum (CDCl3), δС, ppm: 10.93, 13.96, 22.50, 24.30, 26.19, 28.42, 29.10, 29.15, 29.70, 30.19, 31.32, 34.28, 42.79, 43.14, 47.72, 48.21, 107.32, 115.23 q (1JСF 294.7 Hz), 126.78, 129.56, 129.76, 132.23, 149.80, 161.42 q (2JСF 35.0 Hz),161.94. Found, %: C 57.63; H 6.65; N 11.04; S 4.45. C36H49F3N6O6S. Calculated, %: C 57.58; H 6.58; N 11.19; S 4.27.
ACKNOWLEDGMENTS
The work was performed using the equipment of the Spectral Analytical Center of the Federal Research Center “Kazan Scientific Center of the Russian Academy of Sciences.”
CONFLICT OF INTEREST
No conflict of interest was declared by the authors.
REFERENCES
- 1.Schäfer N., Belz G.G., Stauch M., Schneider B. Deutsch Medizinische Wochenschrift. 2008;105:1253. doi: 10.1055/s-2008-1070851. [DOI] [PubMed] [Google Scholar]
- 2.Albert K.S., Hallmark M.R., Sakmar E., Weidler D.J., Wagner J.G. J. Pharmacokinet. Biopharm. 1975;3:159. doi: 10.1007/BF01067905. [DOI] [PubMed] [Google Scholar]
- 3.Ferrari A., Coccia C., Bertolini A., Sternieri E. Pharmacol. Res. 2004;50:551. doi: 10.1016/j.phrs.2004.05.002. [DOI] [PubMed] [Google Scholar]
- 4.Lugo R.A., Satterfield K.L., Kern S.E. J. Pain Palliat. Care Pharmacother. 2005;19:13. doi: 10.1080/J354v19n04_05. [DOI] [PubMed] [Google Scholar]
- 5.Hu X., Chen C.Z., Xu M., Hu Z., Guo H., Itkin Z., Shinn P., Ivin P., Leek M., Liang T.J., Shen M., Zheng W., Hall M.D. ACS Med. Chem. Lett. 2021;12:1267. doi: 10.1021/acsmedchemlett.1c00263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cardinal S., Paquet-Côté P.-A., Azelmat J., Bouchard C., Grenier D., Voyer N. Bioorg. Med. Chem. 2017;25:2043. doi: 10.1016/j.bmc.2017.01.050. [DOI] [PubMed] [Google Scholar]
- 7.Sumoto K., Mibu N., Yokomizo K., Uyeda M. Chem. Pharm. Bull. (Tokyo) 2002;50:298. doi: 10.1248/cpb.50.298. [DOI] [PubMed] [Google Scholar]
- 8.Mibu N., Yokomizo K., Miyata T., Sumoto K.J. Heterocycl. Chem. 2010;47:1434. doi: 10.1002/jhet.457. [DOI] [Google Scholar]
- 9.Pericherla K., Shirazi A.N., Kameshwara Rao V., Tiwari R.K., DaSilva N., McCaffrey K.T., Beni Y.A., González-Sarrías A., Seeram N.P., Parang K., Kumar A. Bioorg. Med. Chem. Lett. 2013;23:5329. doi: 10.1016/j.bmcl.2013.07.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chauthe S.K., Bharate S.B., Sabde S., Mitra D., Bhutani K.K., Singh I.P. Bioorg. Med. Chem. 2010;18:2029. doi: 10.1016/j.bmc.2010.01.023. [DOI] [PubMed] [Google Scholar]
- 11.Chauthe S.K., Bharate S.B., Periyasamy G., Khanna A., Bhutani K.K., Mishra P.D., Singh I.P. Bioorg. Med. Chem. Lett. 2012;22:2251. doi: 10.1016/j.bmcl.2012.01.089. [DOI] [PubMed] [Google Scholar]
- 12.Bouthenet E., Oh K.-B., Park S., Nagi N.K., Lee H.-S., Matthews S.E. Bioorg. Med. Chem. Lett. 2011;21:7142. doi: 10.1016/j.bmcl.2011.09.072. [DOI] [PubMed] [Google Scholar]
- 13.Schuller-Levis G.B., Park E. FEMS Microbiol. Lett. 2003;226:195. doi: 10.1016/S0378-1097(03)00611-6. [DOI] [PubMed] [Google Scholar]
- 14.Brosnan J.T., Brosnan M.E. J. Nutr. 2006;136:1636. doi: 10.1093/jn/136.6.1636S. [DOI] [Google Scholar]
- 15.Ridlon J.M., Wolf P.G., Gaskins H.R. Gut Microbes. 2016;7:201. doi: 10.1080/19490976.2016.1150414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Martínez-Valladares M., Martín-Ramos E., EstebanBallesteros M., Balaña-Fouce R., Rojo-Vázquez F.A. Parasite. 2021;28:71. doi: 10.1051/parasite/2021068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gokbulut C., McKellar Q.A. Vet. Parasitol. 2018;261:27. doi: 10.1016/j.vetpar.2018.08.002. [DOI] [PubMed] [Google Scholar]
- 18.Smolobochkin, A.V., Gazizov, A.S., Burilov, A.R., and Pudovik, M.A., J. Chem., 2019, p. 1. 10.1155/2019/3424319
- 19.Smolobochkin, A.V., Muravyeva, E.A., Vagapova, L.I., Knyazeva, I.R., Voronina, J.K., Burilov, A.R., Pudovik, M.A., Gildebrant, A.V., Sazykin, I.S., Sazykina, M.A., and Gazizov, A.S., Chem. Biodivers., 2019, vol. 16, p. e1800490. 10.1002/cbdv.201800490 [DOI] [PubMed]