Abstract
Cancer cachexia is syndrome accompanying weight reduction, fat loss, muscle atrophy in patients with advanced cancer. Since tumor necrosis factor-α (TNF-α) played pivotal role in cancer cachexia, we hypothesized preemptive administration of TNF-α antibody might mitigate cancer cachexia. Detailed molecular mechanisms targeting muscle atrophy, cachexic inflammation, and catabolic catastrophe were explored whether TNF-α antibody can antagonize these cachexic mechanisms. Stimulated with preliminary finding human antibody, infliximab or adalimumab, significantly inhibited TNF-α as well as their signals relevant to cachexia in mice, preemptive administration of 1.5 mg/kg adalimumab was done in C-26-induced cancer cachexia. Adalimumab significantly mitigated cancer cachexia manifested with significantly lesser weight loss, leg muscle preservation, and higher survival compared to cachexia control (p<0.05). Significant ameliorating action of muscle atrophy were accompanied significant decreases of muscle-specific UPS like atrogin-1/MuRF-1, Pax-7, PCG-1α, and Mfn-2 after adalimumab (p<0.01) and significantly attenuated lipolysis with inhibition of ATGL HSL, and MMPs. Cachexic factors including IL-6 expression, serum IL-6, gp130, IL-6R, JAK2, and STAT3 were significantly inhibited with adalimumab (p<0.01). Genes implicated in cachexic inflammation like NF-κB, c-Jun/c-Fos, and MAPKs were significantly repressed, while mTOR/AKT was significantly increased adalimumab (p<0.05). Conclusively, preemptive administration of adalimumab can be tried in high risk to cancer cachexia.
Keywords: cancer cachexia, C-26 cell, adalimumab, muscle atrophy, preemptive administration
Introduction
Wasting of skeletal muscle and adipose tissue is a hallmark of cancer cachexia,(1) in which individual cancer cell affects host metabolism either by dysregulated metabolism or circulating factors from cancer cells. Mechanisms leading to muscle atrophy are very much complicated to abnormal immune system, nutrient impairment, sarcopenia, and organ dysfunction in addition to prominent anorexia. Ultimately, seriously reduced muscle mass is a serious symptom that can lead to death.(2–4) Besides of difficulty in treating advanced cancer causing cachexia, this complex, multi-factorial, and rather complicated nature of “cachexia” makes treatment very hard. Therefore, the treatment of cancer cachexia is a difficult disease that cannot be easily accessed due to the difficulty in recovering the underlying disease. However, if cancer cachexia can be alleviated, it can increase the quality of the patient, after which more amenable chemotherapy becomes possible, finally mitigation of cancer cachexia is important game changer for cancer patients.
As a scientific approach, many attempts have been made to inhibit cachexia either by targeting the inflammatory cytokines tumor necrosis factor-α (TNF-α) and IL-6, which are the molecular biologic mechanisms associated with the development of cancer cachexia(5–7) or adopting recent advancement of biotechnology, a number of promising agents, for instance, enobosarm as a selected androgen receptor modulator (SARM)(8) and anamorelin as a ghrelin agonist(9,10) are under Phase III studies now. Thinking of contributing pathogenesis, other agents such as infliximab as TNF-α antibody, tocilizumab as IL-6 receptor antibody(11) and bimagrumab as human antibody targeting Activin type II receptor(12) had been shown the possibility to reverse skeletal muscle. However, although some are still on clinical trial, others are already proven to be limited effect.
TNF-α played pivotal roles in causing cancer cachexia since TNF-α appears to promote muscle atrophy-associated genes such as muscle ring figure protein1 (MuRF-1) and Atrogin-1, resulting from the induction of E3 ligase genes that mediate the degradation of myofibrillar proteins by the ubiquitin proteasome pathway(13–17) and is involved in body weight homeostasis by increasing lipolysis and favoring muscle cell catabolism(18) Therefore, trial to block cancer cachexia-associated TNF-α can be a solution, for which rather diverse approaches to block TNF-α in cancer cachexia are on progress including nutrients, antioxidants,(19–21) phytochemicals,(22) and recent available biologics targeting TNF-α.
Supported with our preliminary study showing that among several kinds of TNF-α antibodies such as etanercept, infliximab, infliximab biosimilar, and adalimumab, preemptive administration of adalimumab exerted highest rescuing action against cachexia models in both exploration of cellular and animal models, in this study we explored their efficacy as well as anti-cachexic mechanisms of preemptive administration of adalimumab in cancer cachexia model using colon 26 adenocarcinoma cell (C-26). Though human antibody, preliminarily we found significant inhibiting action of adeither infliximab or alimumab human antibody in murine model.(23)
Materials and Methods
Cell culture
The cachexia-bearing C-26 is a murine colon carcinoma cell and was obtained from Cell Lines Service (no. 400156). The cells were cultured in RPMI-1640 medium supplemented with 10% heat inactivated fetal bovine serum (FBS, Sigma-Aldrich, St. Louis, MO) and 1% antibiotic/antimycotic solution (Sigma) at 37°C in a humidified atmosphere composed 5% CO2 incubator.
Mice model of cancer cachexia
Six-week-old male Balb/c mice were allocated into one of three experimental groups: a normal group (n = 10), a cachectic C-26 adenocarcinoma bearing group (n = 10), a cachectic C-26 adenocarcinoma bearing group with 1.5 mg/kg TNF-α antibody biologics, adalimumab (n = 10). Cachectic C-26 adenocarcinoma bearing groups were shaved on the dorsal side and injected in their right flank with either 1 × 107 C-26 cells. Animals were handled in an accredited animal facility in accordance with the AAALAC International Animal Care Policies of CHA Bio Complex (CHA University, Pangyo, Korea) after IRB approving (#2017-0501). Before the injection of C-26 cells into mice, cells were counted using a hemocytometer and suspended in 100 μl of sterilized phosphate-buffered saline(21,22) When injecting C-26 cells to mice, we used 1 cc insulin syringe (29 gauge, BD Ultra-FineTM needle). The recommended capacity of adalimumab to human is 80 mg in the first week. Converted to dose to be administered to mice, it was administered 1.5 mg/ml via an i.p. injection of a mixture of phosphate-buffered saline thrice in the first week of C-26 injection. All mice were monitored once every two to three days, including measuring body weight and food intake for three weeks. To check for the reduction of food intake due to anorexia, we limited feed only 100 g per each group every three days. The experiment was suspended before 4 weeks because of mortality related to cancer cachexia in this model.
Serum cytokine assays
To assess concentration of cytokine in serum, we collected the blood from mice, and separated the plasma after centrifuged at 3,000 rpm, 4°C for 30 min. IL-6 and TNF-α levels in serum were determined by a Mouse IL-6 Quantikine ELISA kit (M6000B) and a Mouse TNF-α Quantikine ELISA kit (MTA00B) all from R&D System (R&D Systems, Mineapolis, MN) according to the manufacturer’s instructions.
Reverse transcription PCR (RT-PCR)
Total RNA was isolated from the leg muscle of mice using TRIzol. RNA was transcribed into cDNA using cDNA synthesis kit (TOYOBO, Osaka, Japan). The primer sequences used for RT-PCR analysis are shown in Table 1 and glyceraldehyde 3-phosphate dehydrogenase (GAPDH) was used as an internal standard.
Table 1.
Primer sequences in this experiment
| Primer | Forward | Reverse |
|---|---|---|
| TNF-α | TTC TAT GGC CCA GAC CCT CA | CTC CAA AGT AGA CCT GCC CG |
| TNF-R1 | ATGAGAAATCCCAGGATGCAG | ACAGACGTTCACGATGCAGGTG |
| FasL | GGC CTA GAG GGC CGG ACC AA | ATG GGC CAC ACT CCT CGG CT |
| IL-6 | GGG ACT GAT GCT GGT GAC AA | TAA CGC ACT AGG TTT GCC GA |
| IL-6Rα | CCT GCT TCC GGA AGA ACC CC | TGA TAC CAC AAG GTT GGC AG |
| gp130 | TGT CAG CAC CAA GGA TTT | GTA GCT GAC CAT ACA TGA AGT G |
| Atrogin-1 | CTG AAT AGC ATC CAG ATC AGC AGG | TTG ATA AAG TCT TGA GGG GAA AGT G |
| MuRF-1 | AAA TGC TAT GGA GAA CCT GGA | GTC CTT GGA AGA TGC TTT GTA A |
| MMP-2 | GAG TAA GGG GAT CGC CGT GCA | AAG AGG TTG CAA CTC TCC TTG G |
| MMP-9 | AGC AGT CTC TAC GGC CGG CTT | TCC GCT TCG GGT CCG TAC ACG |
| GAPDH | AAT GTA TCC GTT GTG GAT CT | TCC ACC ACC CTG TTG CTG TA |
Western blotting
The leg muscle tissue was finely ground with liquid nitrogen and dissolved in Cell lysis buffer to make protein lysate. All primary antibodies were diluted 1:1,000 and all secondary antibodies were diluted 1:2,000. Primary antibodies for TNF-α (Cell Signaling, Danvers, MA), tumor necrosis factor-receptor 1 (TNF-R1; Santa Cruz Biotechnology, Santa Cruz, CA), tumor necrosis factor receptor type-1 associated death domain protein (TRADD; Cell Signaling), fas-associated protein with death domain (FADD; Santa Cruz Biotechnology), interleukin-6 (IL-6, Abcam, ab6672; Dawinbio, Hanam, Korea), interleukin-6 receptor α (IL-6Rα; Santa Cruz Biotechnology), phosphor-Janus kinase 2 (p-JAK2; Cell Signaling), phosphor-signal transducer and activator of transcription 3 (p-STAT3; Santa Cruz Biotechnology), atrogin-1 (a muscle-specific F-box protein; ECM Biosciences), muscle ring finger protein-1 (MuRF-1); Santa Cruz Biotechnology), ubiquitin (Cell Signaling), inhibitor of nuclear factor kappa-B kinase subunit beta (Ikκβ; Cell Signaling), phosphor-nuclear factor of kappa light polypeptide gene enhancer in B-cells inhibitor α (p-IκBα; Cell Signaling), phosphor-nuclear factor kappa-light-chain-enhancer of activated B cells p50 (p-NF-κB p50; Santa Cruz Biotechnology), phosphor-nuclear factor kappa-light-chain-enhancer of activated B cells p65 (p-NF-κB p65; Santa Cruz Biotechnology), c-Jun (Santa Cruz Biotechnology), c-Fos (Cell Signaling), phosphor-extracellular signal-regulated kinases (p-ERK; Santa Cruz Biotechnology), phosphor-c-jun N-terminal kinase (p-JNK; Santa Cruz Biotechnology), phosphor-p38 mitogen-activated protein kinases (p-p38 MAPKs; Cell Signaling, #9211), phosphatidylinositol-4-5-biphosphate 3-kinase (p-PI3K p85/p55; Cell Signaling), protein kinase B (p-Akt; Cell Signaling), phosphor-mechanistic target of rapamycin (p-mTOR; Cell Signaling), phosphor-5'AMP-activated protein kinase (p-AMPKα 1/2; Santa Cruz Biotechnology), peroxisome proliferator activated receptor gamma coactivator-1alpha (PCG-1α) (Santa Cruz Biotechnology), mitofusin-2 (Mnf-1; Santa Cruz Biotechnology), paired box protein-7 (Pax7; Santa Cruz Biotechnology), myogenic differentiation D (MyoD; Santa Cruz Biotechnology), adipose triglyceride lipase (ATGL; Cell Signaling), hormone-sensitive lipase (HSL; Cell Signaling), sterol response element binding protein-1 (Srebp1; Santa Cruz Biotechnology), cleaved caspase-8 (Cell Signaling), phosphatase and tensin homolog (PTEN; Cell Signaling), and α-tubulin (Santa Cruz Biotechnology) were incubated overnight at 4°C. α-Tubulin was used as a loading control. Images were visualized using SuperSignalTM West Pico PLUS Chemiluminescent Substrate (Thermo-Fisher Scientific, Waltham, MA) in Image Quant LAS 4000.
Immunohistochemical staining
Paraffin blocks were processed deparaffinized and rehydrated with graded alcohol. Tissue sections were heated in pressure jars filled antigen retrieval solution (0.01 M sodium citrate buffer, pH 6.0) in a microwave for 3 min. The slides were cooled to lukewarm and washed in PBS. The slides were blocked for 1 h and incubated overnight with the primary antibody. The slides were washed in PBS and applied to each section secondary HRP-conjugated anti-rabbit antibody. Finally, mount cover slips on slides using antifade reagent.
Terminal deoxynucleotidyl transferase-mediated dUTP nick-end labeling (TUNEL) staining
To visualize apoptosis, TUNEL staining was conducted on tissue slices using DeadEndTM Fluorometric TUNEL System (G3250; Promega, Madison, WI) according to the manufacturer’s instructions.
Zymography
Enzymatic activities of matrix metalloproteinases (MMP)-2 and MMP-9 were assayed by gelatin zymography. Samples were electrophoresed on gelatin-containing 10% SDS-polyacrylamide gels. The gel was washed twice with washing buffer (2.5% Triton X-100, 5 mM CaCl2, 1 μM ZnCl2, 50 mM Tris-HCl, pH 7.5). This was followed by treatment with incubation buffer (1% Triton X-100, 5 mM CaCl2, 1 μM ZnCl2, 50 mM Tris-HCl, pH 7.5) and incubate for overnight at 37°C. And then, the gel was stained with Coomassie brilliant blue R-250 staining solution (BIORAD), and destained using destaining solution (methanol, acetic acid, H2O). The MMP appearing on the gel signified 72 kDa (MMP-2) and 92 kDa (MMP-9).
Statistics
Results are expressed as the mean ± SD. Statistical analyses of the data were performed using Graphpad (GraphPad Software, San Diego, CA) and SPSS software (ver. 12.0, Chicago, IL). All other experiments were performed on triplicated independent occasions. The data were analyzed by one-way analysis of variance (ANOVA) tests, and the statistical significance between groups was determined by Tukey’s multiple comparison test. Significance were considered at p<0.05.
Results
Preemptive administration of adalimumab significantly ameliorated cancer cachexia showing lesser muscle atrophy
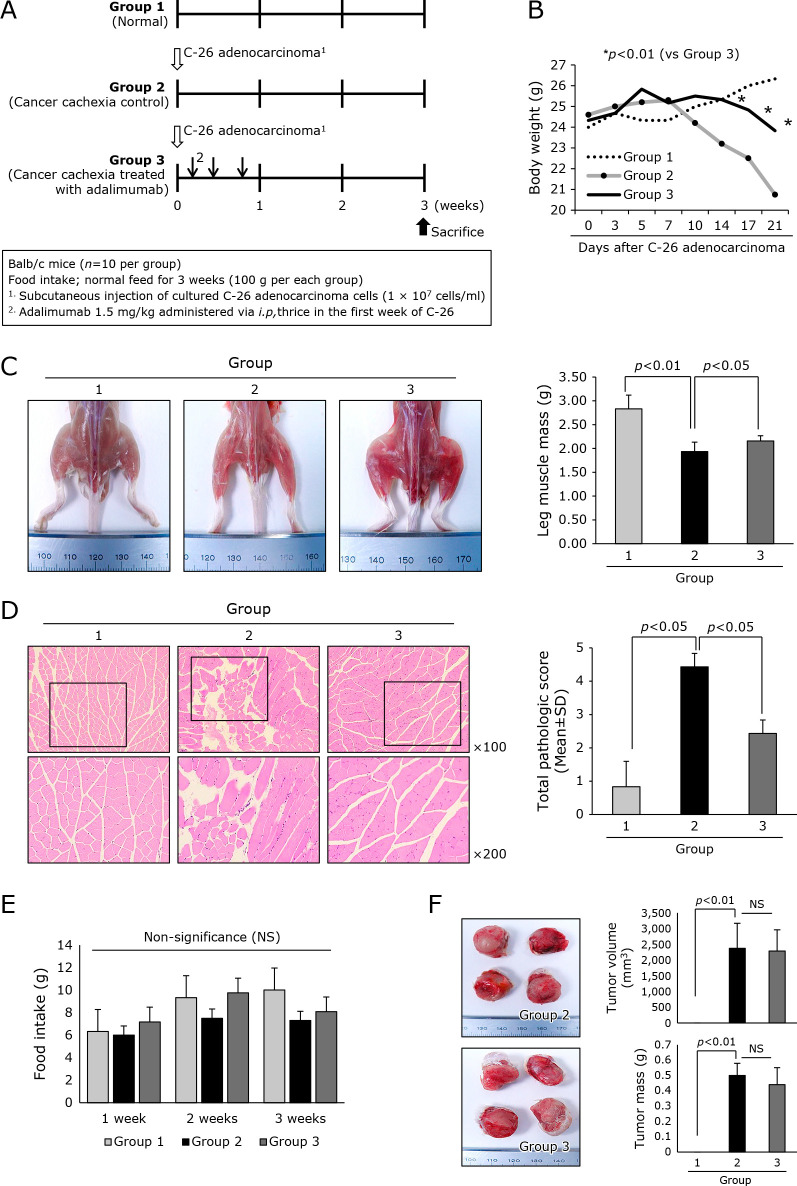
In order to document whether blocking of TNF-α with adalimumab can ameliorated C-26 adenocarcinoma-induced cancer cachexia, we set three groups, normal control, C-26 group, and C-26 group treated with thrice i.p. injection of adalimumab, 1.5 mg/kg (Fig. 1A). Through preliminary study, we found C-26 adenocarcinoma injection led to mortality due to severe cancer cachexia, accompanied with significant weight loss, about 25%, around 4–5 weeks of cell transplantation. As seen in Fig. 1B, control mice showed significant loss of body weight after 3 weeks (p<0.01). However, group treated with adalimumab (Group 3) preemptively showed statistically significant difference with Group 2 in the changes of body weight (p<0.05). In this rescuing action shown in Group, neither food intake nor mean size of transplanted tumors were differed between Group 2 and Group 3, signifying cancer cachexia was not the results of decreased food intake or excess tumor growth (Fig. 1E and F). On the other hand, gross morphology of mice thigh and gastronemus muscle was quite different, signifying ameliorating effect of cancer cachexia in Group 3 was presented less muscle atrophy (p<0.01, Fig. 1C). These findings were further documented with histology of leg muscle. As seen in Fig. 1E, muscle bundles in cancer cachexia were significantly reduced, whereas muscle bundles were significantly preserved in adalimumab treated group (p<0.05). Other points of muscle degeneration, inflammation, and shrinkage were significantly ameliorated in Group 3. Further evaluation of muscle degeneration was done with immunohistochemical staining of IgG. As shown in Supplemental Fig. 1*, IgG staining was significantly increased in Group 2, while no significant change was noted in Group 3. All of these results consistently suggested muscle atrophy relevant to cancer cachexia was ameliorated in group treated with preemptive adalimumab compared to control (p<0.01).
Fig. 1.
Efficacy of i.p. administered adalimumab, 1.5 mg/kg, on mitigating C26 adenocarcinoma-induced cancer cachexia. (A) Schematic protocol for experiment Balb/c mice were administered with 1 × 107 cells/ml on side of abdomen. Three times intraperitoneal injections of adalimumab in the first week of C-26 cells injection were given on the first week of cell administration. (B) Daily measurement of body weight according to group. Significant difference of whole body weight was noted after 2 weeks of C-26 cells injection (p<0.01). (C) Representational photo showing both thigh and leg muscle Using imaging analysis, individual muscle mass of leg was measured and averaged according to group. Statistically significant decreases in muscle mass were noted in Group 2 control (p<0.01). (D) Histopathology of leg gastrocnemius muscle. The pathological scores including muscle bundle size, presence of inflammation, and degree of muscle change were shown according to group, ×40 magnification. (E) Mean amounts of daily food intake were averaged according to group. No significant difference in amounts of pellet diet was noted between groups. (F) The mean size and volume of resected C-26 adenocarcinoma after 3 weeks. No significance in tumorigenesis was noted between groups.
Inhibition of muscle-related proteasome degradation with preemptive administration of adalimumab
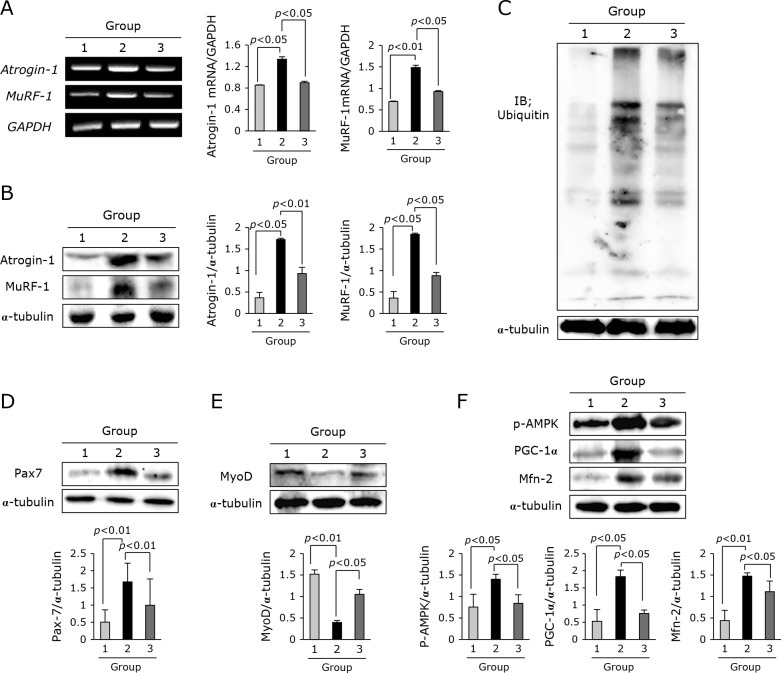
The significant differences in mice leg muscle between C-26 cells-induced cancer cachexia and adalimumab pretreated cancer cachexia noted in Fig. 1 led us to investigate muscle atrophy-related genetic changes between groups. As seen in Fig. 2A, first, we have checked atrogin-1 and MuRF-1, representational genes denoting muscle atrophy in cancer cachexia, and found either atrogin-1 or MuRF-1 mRNA was significantly increased in leg muscle of C-26 adenocarcinoma-induced cancer cachexia (p<0.05). However, Group treated with adalimumab showed significant decreases in atrogin-1 and MuRF-1 mRNA expression. These changes were further confirmed with Western blot as shown in Fig. 2B. Since these genes are related to proteasomic ubiquitin ligase, we repeated blotting for ubiquitin and inhibiting ubiquitin ligases was noted in Group 3 compared to Group 2 (Fig. 2C). Also, we have confirmed Pax7 gene signifying muscle degeneration was significantly increased in Group 2, but significantly decreased in Group 3 (p<0.01, Fig. 2D). On the other hand, MyoD was significantly decreased in Group 2, but it was significantly increased in Group 3 (p<0.05, Fig. 2E). All of these muscle degeneration and atrophy was further reflected with the changes of p-AMPK, PGC-1α, and mitofucin-2 (Mfn-2) genes implicated in either mitochondrial changes or energy expenditure. As seen in Fig. 2F, adalimumab significantly ameliorated the genetic changes occurred in cancer cachexia in this matter (p<0.01).
Fig. 2.
Muscle related gene changes according to group. (A) RT-PCR for atrogin-1 and MuRF-1 mRNA. (B) Western blot for atrogin-1 and MuRF-1. (C) Western blot for proteasome ubiquitin ligases. (D) Western blot for Pax7, muscle transcription factor. (E) Western blot for MyoD for muscle regeneration. (F) Western blot for p-AMPK, PCG-1α, and Mfn-2.
Adalimumab significantly blocked cancer cachexia-related TNF-α and its signaling
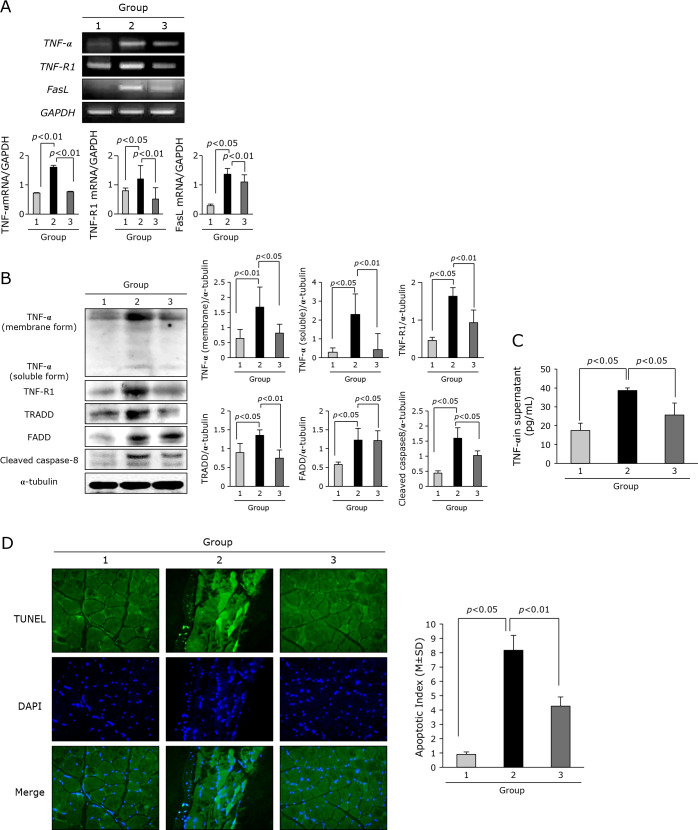
According to group, the expression of TNF-α and its receptor and signaling was measured. As seen in Fig. 3A and B, the expressions of TNF-α, TNF-receptor 1, and FasL mRNA were all significantly increased in cancer cachexia group, but these expressions were significantly decreased in group treated with adalimumab (p<0.05). Also, on Western blot for TNF-α, membrane bound and soluble form), TNF-R1 as well as signaling molecules, TRADD, FADD, cleaved caspase-8, respectively, increased expressions noted in Group 2 were all significantly decreased in Group 3 (p<0.05, Fig. 3B). When we measured TNF-α levels according to group, TNF-α in sera from each group was significantly increased in Group 2, but significantly decreased in Group 3 (p<0.05, Fig. 3C). Since all of these data suggested TNF-α might contributed to cancer cachexia, whereas adalimumab significantly blocked cachexic effect of TNF-α, we further hypothesized apoptosis in muscle might be differ between groups. As seen in Fig. 3D, apoptotic index in mice leg muscle was significantly increased in Group 2, but significantly decreased in Group 3 (p<0.01).
Fig. 3.
Assay for the changes of TNF-α and its receptor including signal molecules and TUNEL for apoptosis. (A) RT-PCR for TNF-α, TNF-R1, and FasL mRNA. (B) Western blot for TNF-α, membranous form and soluble form, TNF-R1, TRADD, FADD, and cleaved capsapse-8. (C) ELISA for sera levels of TNF-α according to group. (D) TUNEL to assess apoptosis Apoptotic index was shown according to group, ×40 magnification.
Adalimumab significantly attenuated cancer cachexia-related lipolysis and protease
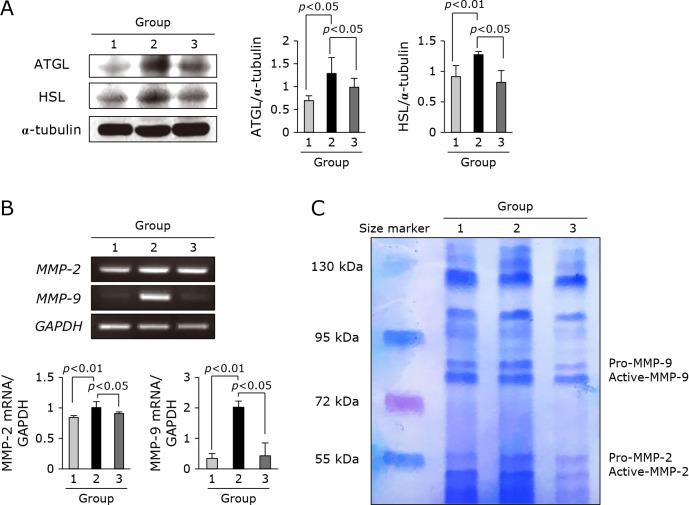
As much as muscle atrophy, lipolysis is a prominent phenomenon noted in cancer cachexia. As seen in Fig. 4A, ATGL and HSL expressions were significantly increased in adipose tissues obtained from Group 2, but significantly attenuated in group treated with adalimumab (p<0.05). These findings were further reflected with changes in fatty acid synthase (FAS) and Srebp-1 (Fig. 4B). Combined with these muscle degradation and lipolysis, systemic activation of MPs further aggravated cachexia. In this matter, when we checked MMP-2 and MMP-9 mRNA, these MMPs were significantly decreased with adalimumab administration (p<0.05, Fig. 4C). The activity of MMPs was measured with zymography. As seen in Fig. 4D, preemptive adalimumab significantly attenuated cachexia-induced MMPs, especially MMP-9.
Fig. 4.
Changes of gene involved in lipolysis and protease. (A) Western blot for ATGL and HSL. (B) RT-PCR for MMP-2 and MMP-9 mRNA. (C) Zymography for MMP activities.
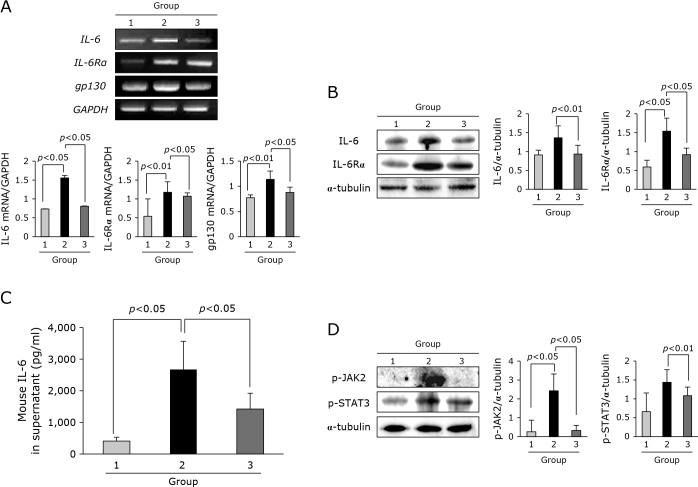
Block of IL-6 driven cancer cachexia by adalimumab
As much as surges of TNF-α observed in cancer cachexia, IL-6 and its signaling mechanism had been implicated in its pathogenesis. As shown in Fig. 5A, the expressions of IL-6, IL-6R, and gp130 mRNA were significantly increased in Group 2, but these expressions were significantly decreased in Group 3. On Western blot for these IL-6 and its receptor, same finding was noted (Fig. 5B). ELISA levels of IL-6 were also significantly increased in sera of Group 2, but statistically significantly decreased in Group 3 (p<0.05, Fig. 5C). Also, when explored about IL-6 signaling, the expressions of phosphorylated JAK2 and STAT3 were significantly increased in Group 2, but significantly decreased in Group 3 (p<0.05, Fig. 5D).
Fig. 5.
IL-6 and its signal according to group. (A) RT-PCR for IL-6, IL-6Rα, and gp130 mRNA. (B) Western blot for IL-6 and IL-6Rα. (C) ELISA for sera levels of IL-6 according to group. (D) Western blot for p-JAK2 and p-STAT3.
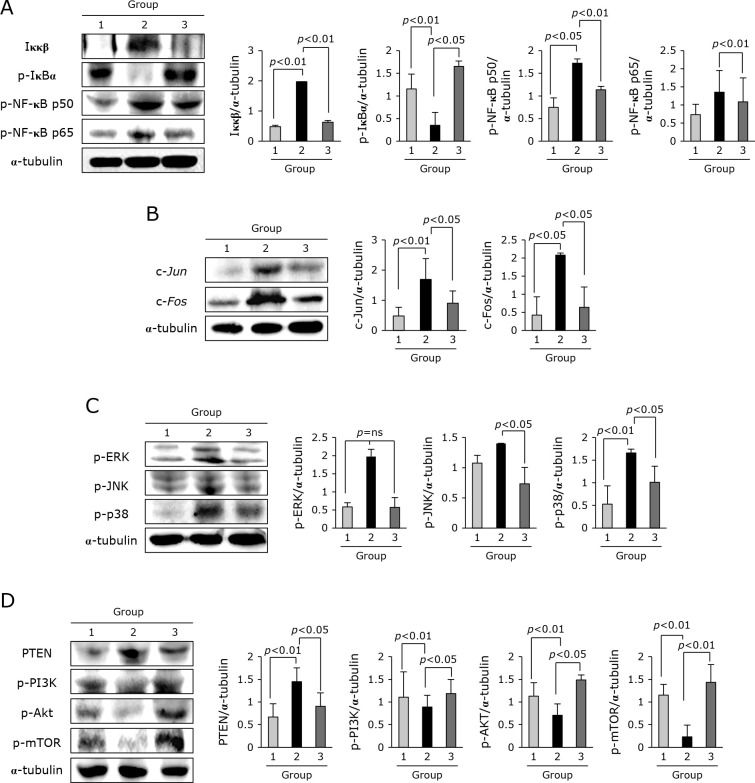
Signaling molecules principally implicated in cancer cachexia progression including cahcexic inflammation and catabolism
All gross as well as underlying molecular genes implicated in cancer cachexia were consistently ameliorated with TNF-α antibody. Therefore, since we have the curiosity whether adalimumab also influenced on the changes of signaling molecules. We proceed to compare NF-κB, AP-1, MAPK, and mTOR signaling molecules and found that significant inhibition of NF-κB transcription factors was noted in Group 3 (p<0.01, Fig. 6A), significant decreases in either c-Jun or c-Fos (p<0.05, Fig. 6B), and inhibition of MAPKs such as ERK1/2, JNK, and p38 (p<0.05, Fig. 6C). On the other hand, repressed PI3K, AKT, and mTOR essential in regeneration against cachexia was all significantly restored in Group 3 (p<0.05, Fig. 6D).
Fig. 6.
Expression levels of transcription factor NF-kB, acute phase response gene, MAPKs, and genes involved in cell growth. (A) Western blot for Ikκβ, p-IκBα, p-NF-κB p50, and p-NF-κB P65. (B) Western blot for c-Jun and c-Fos. (C) Western blot for p-ERK1/2, p-JNK, and p-p38. (D) Western blot for PTEN, p-PI3K, p-AKT, and p-mTOR.
Discussion
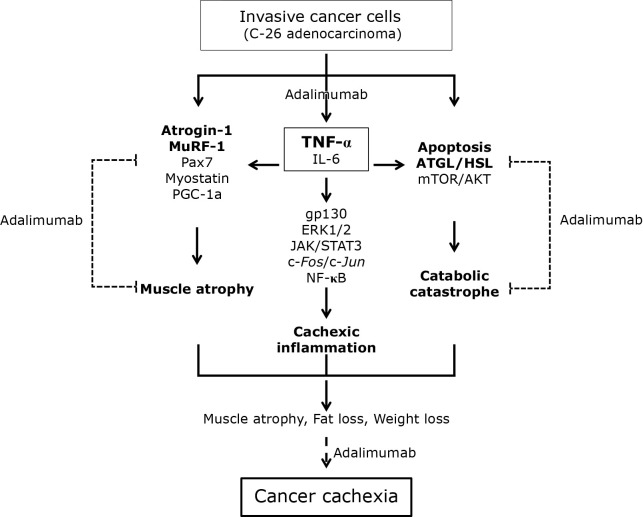
In this study, we have documented the possibility of adalimumab, biologics consisted of humanized TNF-α antibody actively prescribed for the treatment of inflammatory bowel disease, rheumatic arthritis, and psoriasis, as ameliorating agent for cancer cachexia. Though reported earlier that similar biologics, infliximab or etanercept, failed in clinical trial in advanced patients suffering from lung cancer, we dare to conclude that adalimumab can be tried for rescuing from troublesome cancer cachexia in preemptive consideration rather than post-event treatment in patients with high risk and patients with invasive cancer. As summarized in Fig. 7, all the molecular changes implicated in the pathogenesis of cancer cachexia, consisting of muscle atrophy, cachexic inflammation, and catabolic catastrophe, are all significantly ameliorated with earlier three times preemptive administration of adalimumab. Though the consensus when to administer, how much dosing, and how long to administer is still not determined, from our current study, earlier preemptive intervention of adalimumab should be chosen to attenuate the molecular, pathogenic, and contributing events of cancer cachexia. Though no overt biomarker to predict cancer cachexia is available yet, we inferred earlier intervention to mitigate possible mechanisms of cancer cachexia in patients carrying higher risk of cancer cachexia due to invasive nature should be considered. Though required further clinical trial, we dare to stress that preemptive administration of TNF-α antibody can rescue from cancer cachexia in patients suffering from advanced stage of cancer vulnerable to cancer cachexia.
Fig. 7.
Schematic presentation summarizing anti-cachexic effect of adalimumab in C26 adenocarcinoma-induced cancer cachexia. Pre-emptive administration of adalimumab exerted significant protection from muscle atrophy, inflammation, and catabolic disaster.
Since muscle wasting occurs systemically in older people as either a physiological response to malnutrition or one of manifestations in many diseases such as chronic obstructive pulmonary disorder, diabetes mellitus, chronic renal failure, heart failure, sepsis, burns as well as advanced cancer,(26) the loss of muscle mass and strength developed first, then led to vicious circle of further losses of muscle function, aggravated to reduced quality of life and mortality, in which excess protein breakdown and reduced protein synthesis prevails. Therefore, therapeutic approach targets either to compensate inhibiting protein breakdown or to increase protein synthesis should be provided. Though either nutrient enrichment or optimal exercise might be a basic approach to prevent or slow muscle atrophy, the reality is not so easy because of cachexia developed after advanced underlying chronic disease and wasting condition not responsive to general treatment. This is why preemptive intervention should be considered. Recent advancement in either enhanced understanding of cellular mechanisms regulating protein balance in muscle/fat, specifically cytokines, or the feasible application of several promising therapeutic agents to combat muscle wasting might raise the possibility to prevent or mitigate cachexia(27,28) Nobody guess that exercise might be the only validated treatment, reduces various types of atrophy, but it can’t be a practical option for bed-ridden, frail, sarcopenic patients suffering advanced cancer. Therefore, there is unmet medical need to develop drugs or intervention to increase muscle regeneration or inhibit atrophy. In this matter, research on exploring the molecular mechanisms underlying muscle atrophy during cancer cachexia has expanded in the last years to discover several potential therapeutic targets.(29) In detail, agents increasing the levels of PGC-1α, transcription factor jun-B (JUNB) or SIRT1 to slow muscle wasting in various catabolic status,(30) myostatin to antagonize “myostatin - activin A - GDF-11” signaling as an autocrine factor limiting muscle size,(31) IGF-1 analogues, ghrelin mimetic,(32,33) β2-adrenoreceptor agonists,(34) and androgen/selective androgen receptor modulators(35) are under active development. With substantial progress, some such as myostatin and activin A antagonists are under high expectation, but future use of these agents is still under expectation with warranty of ultimate safety.
In the current study, under the hope to develop “safe and effective multi-mechanistic anti-cachexia drug”, using RNAseq to discover critical path to cancer cachexia in C-26 adenocarcinoma model, we reached to document the application of biologics targeting “TNF-α” and “IL-6”. Also supported with our investigation that infliximab or adalimumab could improve wasting symptoms in pediatric Crohn’s disease and strong data showing wasting condition reflecting the severity of in inflammatory bowel disease much better than current inflammation biomarkers such as CRP, ESR, and fecal calprotectin led us to investigate TNF-α antibody for ameliorating action against cachexia. Before our investigation, though Jatoi et al.(36) tried placebo-controlled double blinded trial of etanercept for 63 patients with incurable malignancy having weight loss of >2.27 kg over 2 months and/or daily intake of <20 calories/kg, etanercept did not palliate cancer anorexia/weight loss syndrome. Also, 3 years later, additional study placebo controlled double-blind trial of infliximab for cancer associated weight loss in non-small cell lung cancer patients was done by same investigator, Jatoi et al.,(35) but studies were suspended due to no inhibition of muscle wasting accompanied with increased fatigue and inferior global quality of life, deeming our expectation. In spite of these negative trials, we inferred they just explored the therapeutic efficacy of TNF-α antibody in very late stage and evaluated cross-sectional effects after TNF-α antibody, that is, too late stage and too narrow parameter. Before our study, we have tried all three TNF-α antibodies, etanercept, infliximab and remisima as infliximab biosimilar, and adalimumab, highest inhibition of muscle atrophy as well as general condition was noted in group administered with adalimumab preemptively. As noted in our study, preemptive administration of adalimumab has achieved either quantitative (muscle mass and weight preservation) or qualitative (low mortality with high quality of life) advantages over C-26 adenocarcinoma-induced severe wasting condition. In another model showing wasting condition, APCMin/+ mice developing intestinal polyposis due to APC gene mutation and LLC cell-induced cachexia model, similar outcome was obtained, preemptive administration of adalimumab significantly rescued wasting condition in accordant with mitigated polyposis and ameliorated muscle atrophy (data not shown). Hence, the common features drawn from our trial with adalimumab were that they did not change either food intake or tumor growth, but significantly amelioration of cachexia. Conclusively, although previous two studies by Jatoi et al.,(34,35) concluding TNF-α antibodies were not effective against cancer cachexia, the limitations of their clinical studies were that they only focused on appetite, weight gain, and tumor staging under intervention during obscure stage of cancer, simply mentioned as advanced malignancy was included.
In the current study, adalimumab administration played concerted roles in ameliorating cancer cachexia through limiting cachexia-associated inflammation, inhibiting muscle atrophy, and mitigating other catabolic catastrophe as summarized in Fig. 7. Interestingly either food intake or tumor growth was not influenced at all relevant to cachexia improvement. IL-6 and interferon-γ in addition to TNF-α led to increased ubiquitin dependent proteolysis and resulted in increased degradation of skeletal muscle. Since the ubiquitin proteasome system (UPS), an ATP-dependent proteolytic system mediating the degradation of target proteins by the conjugation of ubiquitin molecules(15) is especially the main regulatory mechanism of protein degradation in skeletal muscle, in detail, two muscle specific E3 ubiquitin ligase, MuRF-1 and muscle atrophy F-box, MAFbx/atrogin-1, have been shown to be implicated in the regulation of skeletal muscle atrophy, up-regulated during cancer cachexia and regarded as molecular target for treating cancer cachexia.(38,39) As presented in Fig. 2A–C, atrogin-1 and MuRF-1 are significantly elevated in leg muscles during cancer cachexia progression, lesser in group treated with preemptive adalimumab. In addition to these UPS, increased expressions of Pax7, transcription factor for muscle regeneration, contributed to compensate atrophy,(40,41) PGC-1α,(42,43) and Mfn-2(44) noted in cancer cachexia were significantly decreased with adalimumab administration, validating anti-cachexic effect of adalimumab (Fig. 2D and F).
In addition to muscle changes, near loss of intestinal or subcutaneous or genital fat is additional prominent finding noted in cancer cachexia mostly due to increased lipases and lipid droplet-associated protein expression, showing adipose tissue lipolysis and deranged energy metabolism during cancer cachexia.(45–47) In our study, over-expressions of ATGL and HSL noted in cancer cachexia significantly decreased with preemptive adalimumab. These findings were further featured with the changes of fat related transcription factor, Srebp-1. Usually, the pathological features of adipose tissue existing in cancer cachexia are shrunken, heterozygous sized, and fibrotic adipocytes,(48) so called ultrastructure of 'slimmed' adipocytes. However, in our study, the expressions of FAS and Srebp-1c were increased in cancer cachexia, whereas significantly decreased with adalimumab, suggesting effective compensatory mechanisms to lipolysis were intervened to cover cancer cachexia with adalimumab. However, further detailed measurements including content of C/EBP-α, acetyl CoA carboxylase, stearoyl CoA desaturase-1, and glycerol-3-phosphate acyl transferase should be followed for full explanation regarding anti-lipolysis of adalimumab.(49) Furthermore, significant inhibition of MMP-9 with adalimumab seems to be also very contributing action against either cancer cachexia, since MMP expressions were prominently changed during either cancer cachexia or cardiac muscle atrophy.(50)
All of these beneficial contributions of adalimumab should be imposed with pertinent modulation of signal transduction regarding metabolism advantages. First, encouraging mTOR and AKT signaling to overcome sarcopenia with adalimumab, silver linings on the horizon by Ebner and Haehling,(51) is recommended since long-term use of mTOR inhibitors led to a marked loss of muscle mass.(52) As summarized by several investigators(53–55) and ours in the current study, molecular substrates and mechanisms underlying the dysregulation of skeletal muscle synthesis and degradation included proinflammatory cytokines such as TNF-α, IL-1, IL-6 and related signaling transduction systems such as NF-κB, MAPKs, acute phase reactants, and myostatin- activin- SMAD pathways(54) Though not studied in the current investigation, metastatic cancer to bone increased TGF-β and related mycostatin contributed muscle atrophy via FOXO activation. However, though still under clinical trial, myostatin inhibition did not show anticipating outcome.(55)
Conclusively, even though mentioned above that clinical trials of similar TNF-α antibodies, etanercept or infliximab, did not achieve any improvement of cancer cachexia, we believe future trial with adalimumab covering the underlying pathogenic events of cancer cachexia with significant improvement of each catabolic catastrophe, cachexic inflammation, and muscle atrophy should be planned to ameliorate cancer cachexia through well-designed RCT. Quite Similar to our previous publication,(56) important lesson from our preclinical trial under the concept of preemption that rather than patients with far advanced stage, preemptive administration of adalimumab might be considered under the pertinent biomarkers or clinical predictive findings suggestive of being cancer cachexia since correcting cachexic mechanisms rather than removing cachexic conditions seem to be practical.
Acknowledgments
This work was supported by Korea Institute of Planning and Evaluation for Technology in Food, Agriculture, Forestry and Fisheries (IPET) through High Value-added Food Technology Development Program, funded by Ministry of Agriculture, Food and Rural Affairs (MAFRA) (116015-03-1-CG00) and granting from California Walnut Commission.
Abbreviations
- ATF6
ER stress transcription factor activating transcription factor 6
- ATGL
adipose triglyceride lipase
- C-26
colon adenocarcinoma 26
- FASN
fatty acid synthase
- FOXO
members of the class O of forkhead box transcription factors
- HSL
hormone sensitive lipase
- IL-6/IL-6R
interleukin-6/interleukin-6 receptor
- JAK2
Janus kinase 2
- MAPK
mitogen activated protein kinase
- MMPs
matrix metalloproteinases
- Mfn-2
mitofusin-2
- MMPs
matrix metalloproteinases
- mTOR
mammalian target of rapamycin
- MuRF-1
muscle ring figure protein1
- NF-kB
nuclear factor-kappa B
- Pax-7
paired protein-7
- PCG-1α
peroxisome proliferator activator receptor gamma coactivator protein-1 alpha
- SREBP
sterol response element binding protein
- TNF-α
tumor necrosis factor-alpha
- TGF-β
transforming growth factor-beta
- UPS
ubiquitin proteasome system
Conflict of Interest
No potential conflicts of interest were disclosed.
Supplementary Material
References
- 1.Shyh-Chang N. Metabolic changes during cancer cachexia pathogenesis. Adv Exp Med Biol 2019; 1026: 233–249. [DOI] [PubMed] [Google Scholar]
- 2.Porporato PE. Understanding cachexia is a cancer metabolism syndrome. Oncogenesis 2016; 5: e200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Anandavadivelan P, Lagergren P. Cachexia in patients with oesophageal cancer. Nat Rev Clin Oncol 2016; 13: 185–198. [DOI] [PubMed] [Google Scholar]
- 4.Morley JE, von Haehling S, Anker SD. Are we closer to having drugs to treat muscle wasting disease? J Cachexia Sarcopenia Muscle 2014; 5: 83–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhou W, Jiang ZW, Tian J, Jiang J, Li N, Li NS. Role of NF-kappaB and cytokine in experimental cancer cachexia. World J Gastroenterol 2003; 9: 1567–1570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ponnusamy S, Sullivan RD, Thiyagarajan T, Tillmann H, Getzenberg RH, Narayanan R. Tissue selective androgen receptor modulators (SARMs) increase pelvic floor muscle mass in ovariectomized mice. J Cell Biochem 2017; 118: 640–646. [DOI] [PubMed] [Google Scholar]
- 7.Sidaway P. CAR T cell therapy efficacious against B-ALL across age groups. Nat Rev Clin Oncol 2018; 15: 199. [DOI] [PubMed] [Google Scholar]
- 8.Currow DC, Abernethy AP. Anamorelin hydrochloride in the treatment of cacner anorexia-cachexia syndrome. Future Oncol 2014; 10: 789–802. [DOI] [PubMed] [Google Scholar]
- 9.Ando K, Takahashi T, Koto M, et al. Tocilizumab, a proposed therapy for the cachexia of Interleukin6-expressing lung cancer. PLoS One 2014; 9: e102436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rooks DS, Laurent T, Praestgaard J, Rasmussen S, Bartlett M, Tankó LB. Effect of bimagrumab on thigh muscle volume and composition in men with casting-induced atrophy. J Cchexia Sarcopenia Muscle 2017; 8: 727–734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Li YP, Chen Y, John J, et al. TNF-alpha acts via p38 MAPK to stimulate expression of the ubiquitin ligase atrogin1/MAFbx in skeletal muscle. FASEB J 2005; 19: 362–370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Frost RA, Nystrom GJ, Jefferson LS, Lang CH. Hormone, cytokine, and nutritional regulation of sepsis-induced increases in atrogin-1 and MuRF1 in skeletal muscle. Am J Physiol Endocrinol Metab 2007; 292: E501–E512. [DOI] [PubMed] [Google Scholar]
- 13.Moylan JS, Smith JD, Chambers MA, McLoughlin TJ, Reid MB. TNF induction of atrogin-1/MAFbx mRNA depends on Foxo4 expression but not AKT-Foxo1/3 signaling. Am J Physiol Cell Physiol 2008; 295: C986–C993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sishi BJ, Engelbrecht AM. Tumor necrosis factor alpha (TNF-α) inactivates the PI3-kinase/PKB pathway and induces atrophy and apoptosis in L6 myotubes. Cytokine 2011; 54: 173–184. [DOI] [PubMed] [Google Scholar]
- 15.Yuan L, Han J, Meng Q, et al. Muscle-specific E3 ubiquitin ligases are involved in muscle atrophy of cancer cachexia: an in vitro and in vivo study. Oncol Rep 2015; 33: 2261–2268. [DOI] [PubMed] [Google Scholar]
- 16.Li YP, Lecker SH, Chen Y, Waddel ID, Goldberg AL, Reid MB. TNF-alpha increases ubiquitin-conjugating activity in skeletal muscle by up-regulating UbcH2/E220k. FASEB J 2003; 17: 1048–1057. [DOI] [PubMed] [Google Scholar]
- 17.Assi M, Derbré F, Lefeuvre-Orfila L, Rébillard A. Antioxidant supplementation accelerates cachexia development by promoting tumor growth in C26 tumor-bearing mice. Free Rad Biol Med 2016; 91: 204–214. [DOI] [PubMed] [Google Scholar]
- 18.Dun YL, Zhou XL, Guan HS, et al. Low molecular weight guluronate prevents TNF-α-induced oxidative damage and mitochondrial dysfunction in C2C12 skeletal muscle cells. Food Funct 2015; 6: 3056–3064. [DOI] [PubMed] [Google Scholar]
- 19.Wang H, Li TL, Hsia S, Su IL, Chan YL, Wu CJ. Skeletal muscle atrophy is attenuated in tumor-bearing mice under chemotherapy by treatment with fish oil and selenium. Oncotarget 2015; 6: 7758–7773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Taksande BG, Gawande DY, Chopde CT, Umekar MJ, Kotagale NR. Agmatine ameliorates adjuvant induced arthritis and inflammatory cachexia in rats. Biomed Pharmacother 2017; 86: 271–278. [DOI] [PubMed] [Google Scholar]
- 21.Acharyya S, Ladner KJ, Nelsen LL, et al. Cancer cachexia is regulated by selective targeting of skeletal muscle gene products. J Clin Invest 2004: 114: 370–378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Murphy KT, Chee A, Trieu J, Naim T, Lynch GS. Importance of functional and metabolic impairments in the characterization of the C-26 murinemodelof cancer cachexia. Dis Model Mech 2012; 5: 533–545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kim YJ, Hong KS, Chung JW, Kim JH, Hahm KB. Prevention of colitis-associated carcinogenesis with infliximab. Cancer Prev Res (Phila) 2010; 10: 1314–1333. [DOI] [PubMed] [Google Scholar]
- 24.Cohen S, Nathan JA, Goldberg AL. Muscle wasting in disease: molecular mechanisms and promising therapies. Nat Rev Drug Discov 2015; 104: 58–74. [DOI] [PubMed] [Google Scholar]
- 25.Jackman RW, Kandarian SC. The molecular basis of skeletal muscle atrophy. Am J Physiol Cell Physiol 2004; 287: C834–C843. [DOI] [PubMed] [Google Scholar]
- 26.Mitch WE, Goldberg AL. Mechanisms of muscle wasting. The role of the ubiquitin-proteasome pathway. N Engl J Med 1996; 335: 1897–1905. [DOI] [PubMed] [Google Scholar]
- 27.Aversa Z, Costelli P, Muscaritoli M. Cancer-induced muscle wasting: latest findings in prevention and treatment. Ther Adv Med Oncol 2017; 9: 369–382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sandri M, Lin J, Handschin C, et al. PGC-1 alpha protects skeletal muscle from atrophy by suppressing FoxO3 action and atrophy-specific gene transcription. Proc Natl Acad Sci U S A 2006; 103: 16260–16265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.McPherron AC, Lawler AM, Lee SJ. Regulation of skeletal muscle mass in mice by a new TGF-beta superfamily member. Nature 1997; 387: 83–90. [DOI] [PubMed] [Google Scholar]
- 30.Dunshea FR, Chung CS, Owens PC, Ballard JF, Walton PE. Insulin-like growth factor-1 and analogues increase growth in artificially-reared neonatal pigs. Br J Nutr 2002; 87: 587–593. [DOI] [PubMed] [Google Scholar]
- 31.Porporato PE, Filigheddu N, Reano S, et al. Acylated and unacylated ghrelin impair skeletal muscle atrophy in mice. J Clin Invest 2013; 123: 611–622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lynch GS, Ryall JG. Role of beta-adrenoceptor signaling in skeletal muscle: implications for muscle wasting and disease. Physiol Rev 2008; 88: 729–767. [DOI] [PubMed] [Google Scholar]
- 33.Zilbermint MF, Dobs AS. Nonsteroidal selective androgen receptor modulator Ostarine in cancer cachexia. Future Oncol 2009; 9: 1211–1220. [DOI] [PubMed] [Google Scholar]
- 34.Jatoi, A, Dakhil SR, Nguyen PL, et al. A placebo-controlled double blind trial of etanercept for the cancer anorexia/weight loss syndrome: results from N00C1 from the North Central Cancer Treatment Group. Cancer 2007; 110: 1396–1403. [DOI] [PubMed] [Google Scholar]
- 35.Jatoi A, Ritter HL, Dueck A, et al. A placebo-controlled, double-blind trial of infliximab for cancer-associated weight loss in elderly and/or poor performance non-small cell lung cancer patients (N01C9). Lung Cancer 2010; 68: 234–239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Murton AJ, Constantin D, Greenhaff PL. The involvement of the ubiquitin proteasome system in human skeletal muscle remodelling and atrophy. Biochim Biophys Acta 2008; 1782: 730–743. [DOI] [PubMed] [Google Scholar]
- 37.Rom O, Reznick AZ. The role of E3 ubiquitin-ligases MuRF-1 and MAFbx in loss of skeletal muscle mass. Free Rad Biol Med 2016; 98: 218–230. [DOI] [PubMed] [Google Scholar]
- 38.Brzeszczyńska J, Johns N, Schilb A, et al. Loss of oxidative defense and potential blockade of satellite cell maturation in the skeletal muscle of patients with cancer but not in the healthy elderly. Aging (Albany NY) 2016; 8: 1690–1702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.He WA, Berardi E, Cardillo VM, et al. NF-κB-mediated Pax7 dysregulation in the muscle microenvironment promotes cancer cachexia. J Clin Invest 2013; 123: 4821–4835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lu JJ, Wang Q, Xie LH, Zhang Q, Sun SH. Tumor necrosis factor-like weak induce of apoptosis regulates quadriceps muscle atrophy and fiber-type alteration in a rat model of chronic obstructive pulmonary disease. Tob Induc Dis 2017; 15: 43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tanaka K, Tanaka M, Takegaki J, Fujino H. Preventive effects of electrical stimulation on inflammation-induced muscle mitochondrial dysfunction. Acta Histochem 2016; 118: 464–470. [DOI] [PubMed] [Google Scholar]
- 42.Xi QL, Zhang B, Jiang Y, et al. Mitofusin-2 prevents skeletal muscle wasting in cancer cachexia. Oncol Lett 2016; 12: 4013–4020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Silvério R, Lira FS, Oyama LM, et al. Lipases and lipid droplet-associated protein expression in subcutaneous white adipose tissue of cachectic patients with cancer. Lipids Health Dis 2017; 16: 159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kliewer KL, Ke JY, Tian M, Cole RM, Andriage RR, Beruly MA. Adipose tissue lipolysis and energy etabolism in early cancer cachexia in mice. Cancer Biol Ther 2015; 16: 886–897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tsoli M, Swarbrick MM, Robertson GR. Lipolytic and thermogenic depletion of adipose tissue in cancer cachexia. Semin Cell Develop Biol 2016; 54: 68–81. [DOI] [PubMed] [Google Scholar]
- 46.Bing C, Russell S, Becket E, et al. Adipose atrophy in cancer cachexia: morphologic and molecular analysis of adipose tissue in tumor-bearing mice. Br J Cancer 2006; 95: 1028–1037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Inadera H, Nagai S, Dong HY, Matsushima K. Molecular analysis of lipid-depleting factor in a colon-26-inoculated cancer cachexia model. Int J Cancer 2002; 101: 37–45. [DOI] [PubMed] [Google Scholar]
- 48.Devine RD, Bicer S, Reiser PJ, Velten M, Wold LE. Metalloproteinase expression is altered in cardiac and skeletal muscle in cancer cachexia. Am J Physiol Heart Circ Physiol 2015; 309: H685–H691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ebner N, von Haehling S. Silver linings on the horizon: highlights from the 10th Cachexia Conference. J Cachexia Sarcopenia Muscle 2018; 9: 176–182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Gyawali B, Shimokata T, Honda K, et al. Muscle wasting associated with the long-term use of mTOR inhibitors. Mol Clin Oncol 2016; 5: 641–646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Miyamoto Y, Hanna DL, Zhang W, Baba H, Lenz HJ. Molecular pathways: cachexia signaling—a targeted approach to cancer treatment. Clinical Cancer Res 2016; 22: 3999–4004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bohnert KR, Gallot YS, Sato S, Xiang G, Hindi SM, Kumar A. Inhibition of ER stress and unfolding protein esponse pathways causes skeletal muscle wasting during cancer cachexia. FASEB J 2016; 30: 3053–3068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Chen MC, Hsu WL, Hwang PA, Chen YL, Chou TC. Combined administration of fucoidan ameliorates tumor and chemotheraphy-induced skeletal muscle atrophy in bladder cancer-bearing mice. Oncotarget 2016; 7: 51608–51618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Mathew SJ. InACTIVatINg cancer cachexia. Dis Model Mech 2011; 4: 283–285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Guttridge DC. A TGF-β pathway associated with cancer cachexia. Nat Med 2015; 21: 1248–1249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.An JM, Kang EA, Han YM, et al. Dietary intake of probiotic kimchi ameliorated IL-6-driven cancer cachexia. J Clin Biochem Nutr 2019; 65: 109–117. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.