Abstract
The α(1,6)fucose residue attached to the N-glycoprotein core is suspected to play an essential role in the progression of several types of cancer. Lectins remain the first choice for probing glycan modifications, although they may lack specificity. Thus, efforts have been made to identify new lectins with a narrower core fucose (CF) detection profile. Here, we present a comparison of the classical Aleuria aurantia lectin (AAL), Lens culinaris agglutinin (LCA) and Aspergillus oryzae lectin (AOL) with the newer Pholiota squarrosa lectin (PhoSL), which has been described as being specific for core fucosylated N-glycans. To this end, we studied the binding profiles of the four lectins using mammalian glycan arrays from the Consortium of Functional Glycomics. To validate their glycan specificity, we probed AOL, LCA and PhoSL in western-blot assays using protein extracts from eight common colorectal cancer (CRC) lines and colorectal biopsies from a small cohort of patients with CRC. The results showed that (i) LCA and PhoSL were the most specific lectins for detecting the presence of CF in a concentration-dependent manner; (ii) PhoSL exhibited the highest N-glycan sequence restriction, with preferential binding to core fucosylated paucimannosidic-type N-glycans, (iii) the recognition ability of PhoSL was highly influenced by the presence of terminal N-acetyl-lactosamine; (iv) LCA bound to paucimannosidic, bi-antennary and tri-antennary core fucosylated N-glycans and (v) AOL and AAL exhibited broader specificity towards fucosylation. Together, our results support the choice of LCA as the most appropriate lectin for CF detection, as validated in protein extracts from CRC cell lines and tissue specimens from patients with CRC.
Keywords: core fucosylation, fucose lectins, glycan array, lactosamine lectins, specific recognition
Introduction
Glycosylation of cell-surface proteins is recognized as the most common and complex post-translational modification of proteins (Spiro 2002). Nearly 1–2% of the total human genome comprises glycogenes (Freeze 2006). Moreover, >50% of encoded proteins, including membrane-bound species, are known to be glycosylated (Apweiler et al. 1999; Zafar et al. 2011) with carbohydrate contents ranging from 1 to >85% by total weight. Glycan chains are attached to the protein backbone through eithe Asn (N) or Ser/Thr (S/T) residues. Specifically, N-glycans are attached by an N-glycosidic bond to the carboxylic group of Asn (N) residues in the sequon N-X-S/T, whereas O-glycans are attached to the side chains of Ser (S) or Thr (T) residues.
The importance of glycosylation in protein folding, localization and organelle trafficking is now widely understood (Xu and Ng 2015; Fisher, Spencer, et al. 2019a; Fisher, Thomas-Oates, et al. 2019b), and changes in the patterns of glycosylation are widely established to modulate cell fate, maturation and differentiation, signaling, growth and death (Saldova et al. 2008; Arcinas et al. 2009; Banerjee 2012; Gu et al. 2012; Christiansen et al. 2014), all of which are important hallmarks of carcinogenesis and cancer progression. However, despite substantial advances in understanding of th effects of glycosylation changes on cellular dynamics, the separation and identification of N-glycans remains challenging. Indeed, detection and monitoring of alterations in glycan expression remain elusive, owing to the similarities and complex structures of N-glycans and the arduous, low-throughput and time-consuming processes required for their separation and subsequent characterization.
Fucosylation is one of the most important oligosaccharide modifications involved in cell–cell and cell–matrix adhesion, as well as in the modulation of receptor–ligand binding and downstream responses. To date, 13 fucosyltransferases have been identified in the human genome. FucT-1 and FucT-2 are α(1,2)fucosyltransferases responsible for synthesis of the ABO blood group antigens (Larsen et al. 1990). FucT-3 to FucT-7 and FucT-9 belong to the α(1,3/4)fucosyltransferase family, which catalyzes the addition of fucose via α(1,3)linkages (FucT-4, FucT-5, FucT-6, FucT-7 and FucT-9) and α(1,3/4)linkages (FucT-3) (Javaud et al. 2003). FucT-8 is the only known mammalian α(1,6)fucosyltransferase catalyzing the transfer of fucose from GDP-L-fucose with α(1,6)linkages to the innermost Asn-linked GlcNAc residue of chitobiose disaccharide in N-glycans (Javaud et al. 2000), thus leading to core fucosylation. The related FucT-10 and FucT-11 correspond to an ancient evolutionary branch of α(1,3/4)fucosyltransferases that show special core fucosylation activity via addition of α(1,3)fucose to the innermost GlcNAc of bi-antennary N-oligosaccharides, although these enzymes do not compete with FucT-8 (Mollicone et al. 2009). Finally, POFUT1 and POFUT2 are endoplasmic fucosyltransferases implicated in the O-fucosylation of S/T residues on epidermal growth factor-like repeats and thrombospondin type 1 repeats, respectively (Loriol et al. 2006).
Alterations in core fucosylation have been described in various types of cancer. In this regard, increases in core fucosylated proteins have been detected in nonsmall cell lung cancer (Honma et al. 2015), hepatocarcinoma (Wang et al. 2015), breast cancer (Tu et al. 2017), melanoma (Agrawal et al. 2017) and glioblastoma (Shergalis et al. 2018). In recent years, the relationship between FucT-8 expression and microRNA-198 has also been investigated (Wang, Wang, et al. 2014a; Wang et al. 2019). More specifically, increased α(1,6)fucosylated α-fetoprotein (AFP-L3) is considered a cancer biomarker of hepatocarcinoma (Leerapun et al. 2007). Moreover, a decrease in core fucosylation contributes to malignancy in gastric cancer (Zhao et al. 2014) and is essential for Toll-like receptor signaling in macrophages (Nakayama et al. 2019). Along these lines, our group has reported increased activity and expression of FucT-8 during progression of colorectal cancer (CRC) (Muinelo-Romay et al. 2008), a finding consistent with the hypothesis that overexpression or increased activity of FucT-8 may play a relevant role in promoting epithelium–mesenchymal transition in some tumors (Mehta et al. 2016; Tu et al. 2017) including CRC (Muinelo-Romay et al. 2011a, 2011b). We have also demonstrated a significant increase in GDP-L-fucose transporter protein (FUCT1) expression in the tumor tissue of patients with CRC (Villar-Portela et al. 2013). This FUCT1 upregulation in the Golgi complex during malignant transformation may increase the availability of the donor substrate GDP-L-fucose, thus leading to overexpression of fucosylated structures.
Epitope-directed monoclonal antibodies are one of the most useful tools for rapid and inexpensive detection of glycoproteins. Owing to the clinical relevance of fucose modifications in the distal positions of glycoproteins, several commercial antibodies for detecting Lewis antigens and ABO blood groups have been developed. Hence, simple monofucosylated, bifucosylated or more complex structures, as well as their corresponding sialylated and/or sulfated counterparts, are routinely detected with monoclonal antibodies (Vanák et al. 1989; Torrado et al. 1992; Nyström et al. 2007; Sundin et al. 2007; Yu et al. 2012; Noble et al. 2013). In contrast, few commercial antibodies are available for the detection of core fucosylation, and the available antibodies specifically target core α(1,3)fucose residues, which is a common modification in plants but is not present in mammals (Baïet et al. 2011). Unfortunately, although α(1,6)core-fucosylated proteins have gained importance as tumor biomarkers, none of the efforts to obtain antibodies have been successful, and the murine monoclonal IgG against cell surface core fucosylated epitopes of Dictyostelium discoideum, which was developed by Crandall and Newell (1989) and subsequently successfully tested (Srikrishna et al. 1997), is no longer available.
Lectins are a second tool for the characterization of glycan epitopes. Lectins have been extensively used for the detection and enrichment of glycoproteins; however, they have several drawbacks. Lectin selectivity is not as high as those of monoclonal antibodies (Schreiber 2002). The lectin-binding affinity, measured as the dissociation constant, is usually at the micromolar level, whereas the typical antibody binding affinity is in the nanomolar–picomolar range (Cummings and Etzler 2009; Landry et al. 2012, 2015). The specificity of lectins is complex, because they can recognize cognate motifs not necessarily coincident with primary motifs in the binding pocket (McCarter et al. 2013); accordingly, the same lectin may show different affinity towards two glycoproteins bearing the same primary glycan epitope (Lee et al. 2010). Despite these intrinsic limitations, lectin-based approaches are particularly useful because of their affordability, ability to discriminate among sugar bonds and the possibility of fractionating complex glycan mixtures for further analysis.
Given that lectins can detect several epitopes, new lectin species capable of specifically recognizing core fucose (CF)-containing glycans should be a valuable tool in glycobiological research. Among the few commercially available lectins, those that can recognize the core fucosylated motif include Lens culinaris agglutinin (LCA), Pisum sativum agglutinin (PSA), Aleuria aurantia lectin (AAL) and Aspergillus oryzae lectin (AOL). Some other small lectins of fungal (Rhizopus stolonifera lectin, RSL) or marine origin (Hypnea japonica lectin, HJL; Bryothamnion triquetrum lectin, BTL) have been described as having excellent specificities for recognizing the presence of CF (Oda et al. 2003; Okuyama et al. 2009; do Nascimento et al. 2015, respectively). Additionally, a lectin of fungal origin, Pholiota squarrosa lectin (PhoSL), has also been described as specific for α(1,6)fucosylated N-glycans but no other types of fucosylated oligosaccharides (Kobayashi et al. 2012). The panel of lectins with known CF specificity (summarized in Table I) is expected to continue to grow.
Table I.
Lectins exhibit different affinities depending of the fucose linkage type
| Lectin | Name | Fucose detection | References |
|---|---|---|---|
| AAL | Aleuria aurantia lectin | Fucα(1,2), Fucα(1,3/4), Fucα(1,6) | Matsumura et al. (2009) |
| AOL | Aspergillus oryzae lectin | Fucα(1,2), Fucα(1,3/4), Fucα (1,6) | Matsumura et al. (2009) |
| AFL | Aspergillus fumigatus lectin | Fucα(1,2), Fucα(1,3/4) | Houser et al. (2013) |
| AAA | Angilla angilla agglutinin | Fucα(1,2), Fucα(1,4) | Baldus et al. (1996) |
| LTL | Lotus tetragonolobus lectin | Fucα(1,2), Fucα(1,3) | Yan et al. (1997) |
| UEA-I | Ulex europaeus agglutinin-I | Fucα(1,2) | Matsumoto and Osawa (1969) |
| LCA | Lens culinaris agglutinin | Fucα(1,6) | Tateno et al. (2009) |
| PSA | Pisum sativum agglutinin | Fucα(1,6) | Tateno et al. (2009) |
| BTL | Bryothamnion triquetrum lectin | Fucα(1,6) | Ainouz et al. (1995), do Nascimento et al. (2015) |
| PhoSL | Pholiota squarrosa lectin | Fucα(1,6) | Kobayashi et al. (2012) |
| RSL | Rhizopus stolonifer lectin | Fucα(1,6) | Oda et al. (2003) |
| HJL | Hypnea japonica lectin | Fucα(1,6) | Okuyama et al. (2009) |
Common assays for assessing their binding recognition pattern usually employ glycan array assay or FAC.
In the present report, we studied the specificity of PhoSL towards α(1,6)fucosylated N-glycans by testing 610 different N-glycan, O-glycan and glycolipid oligosaccharide structures printed onto mammalian glycan array v5.1 from the Consortium for Functional Glycomics (CFG, USA). Subsequently, we compared the recognition pattern of PhoSL with that of AAL, AOL and LCA. In brief, we demonstrated that PhoSL and LCA are the most specific lectins for detecting the presence of CF, and terminal lactosamine mediates the core-fucose recognition by PhoS. Furthermore, we examined the use of PhoSL in the blotting of protein extracts to gain insight into its biochemical utility.
Results
Bond specificity of PhoSL towards core fucose in glycan array assays
More than 600 oligosaccharides belonging to N-glycans, O-glycans and glycolipids with important characteristic structural motifs, such as ABO groups, are represented in the CFG array version 5.1 (Table SI). Three different concentrations of PhoSL (20, 50 and 200 μg/mL) were assayed in this glycan microarray. The CFG stored data for AAL (0.1, 1.0, 10 and 100 μg/mL), AOL (0.1, 1.0, 10 and 100 μg/mL) and LCA (1.0, 10 and 100 μg/mL), all obtained with version 5.0 of the CFG array (Table SII), were used to compare the recognition profiles of these four lectins. The spacer linkers providing the amine group for the covalent binding with the NHS-activated glass surface are shown in Table SIII. In addition, raw screening data for each lectin and concentration can be found in the public CFG database:
http://www.functionalglycomics.org/glycomics/publicdata/select-edScreens.jsp (PhoSL: array request 2928; investigator: Almudena Fernández-Briera).
http://www.functionalglycomics.org/glycomics/publicdata/select-edScreens.jsp (LCA, AOL and AAL: array request 2342).
The depiction of the normalized average fluorescence triggered by biotin-labeled PhoSL (Figure 1D) showed lectin preferentially recognizing core fucosylated structures with high selectivity (Figure 1D, fuchsia signals), although the binding to some noncore fucosylated oligosaccharides emitted a subtle basal signal requiring further consideration. In contrast, the biotin-labeled LCA recognized three types of glycan structures (Figure 1C): two corresponded to the presence of CF, alone or accompanied by another fucose in medial or distal position (fuchsia and gray signals, respectively), and another group of lower signal intensity corresponded to non fucosylated glycans (blue signals). The glycan-binding specificities of biotin-labeled AOL and AAL were highly similar (Figure 1A and B), showing virtually complete recognition of fucosylated oligosaccharides with only subtle differences.
Fig. 1.
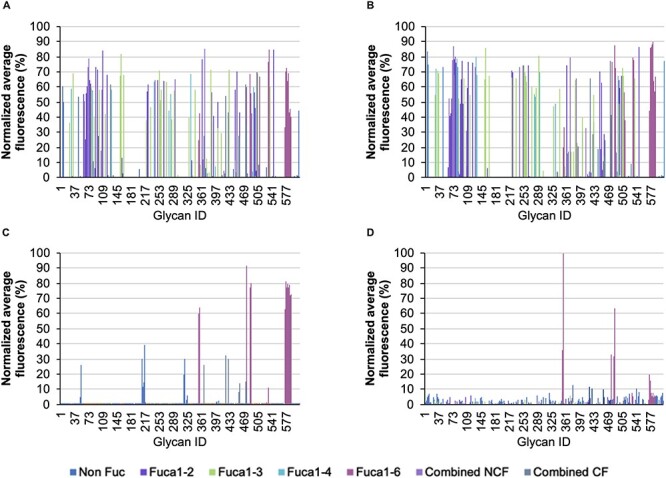
Relative average binding intensities of (A) AOL, (B) AAL, (C) LCA and (D) Pholiota PhoSL to glycan structures contained in the mammalian glycan arrays from the CFG v5.0 for AOL, ALL and LCA and CFG v.5.1 for PhoSL. Glycan signals without fucosylation are colored in blue, those containing α(1,2)Fuc are colored in purple, those containing α(1,3)Fuc are colored in green, those containing α(1,4)Fuc are colored in cyan, those containing α(1,6)Fuc are colored in fuchsia, those containing at least two different fucoses, but not core fucose are colored in lavender and those containing at least two different fucoses, one of it in the core position, are colored in metallic gray.
To elucidate the detailed binding profiles of the four lectins analyzed, we created a list of ten glycan motifs that are fucosylated in humans. Their structural descriptions and array sequences are shown in Table II. We then recorded the presence or absence of each motif in the glycan structures of the array that scored positive results (i.e., reaching a relative average intensity equal to or above the limit of detection (LOD) defined for each lectin). The results are summarized in Table III, according to the fucose linkage present in each motif. PhoSL showed the highest specificity for core fucosylated N-glycans, as 59.1% of the total oligosaccharides with positive events in the glycan array were single core fucosylated. For LCA, this percentage was 48.1%, whereas AAL and AOL showed the broadest nonspecificity towards CF (18.4% and 16.9% core fucosylated oligosaccharides recognized, respectively). Of note, although AAL and AOL detected the most glycans (n = 158 and 172 structures, respectively; Figure 1), only a small fraction of them were single core fucosylated. Additionally, we recorded glycans with two or more fucose residues, a result indicative of the presence of CF along with Lewis or ABO motifs (although this group also included the noncore fucosylated Leb and Ley motifs; combined, CF; Table III). In that case, AAL, AOL and LCA recognized the same number of structures (n = 9), whereas PhoSL showed lower binding to these glycans (four recognized structures), thus suggesting a possible influence of the terminal sequence in the recognition of CF, as discussed in the next section.
Table II.
Secondary structure of mammalian fucosylated epitopes
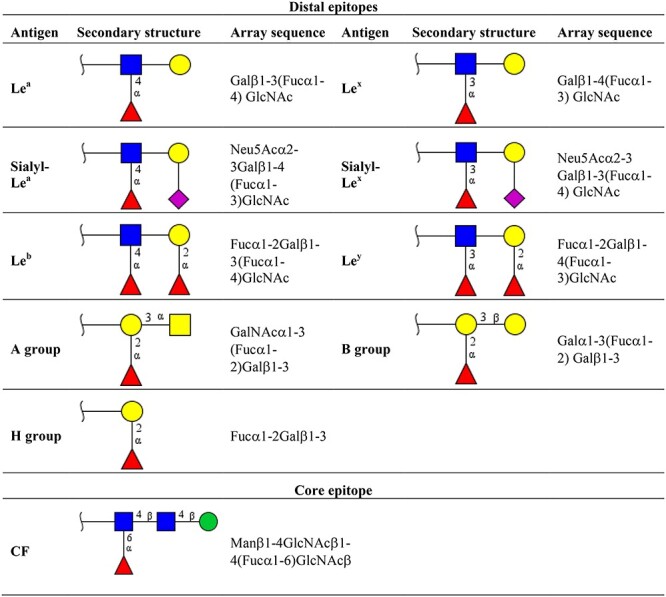
|
Distal epitopes can be present either in the terminal glycan chain or in the middle of the polylactosamine extension. The synthesis of Lewis (Le) antigens is catalyzed by FucT-1, FucT-2, FucT-3, FucT-4, Fuct-5, FucT-6, FucT-7 and FucT-9. Biosynthesis of ABO groups is catalyzed by FucT-1 and FucT-2. Addition of CF epitope with the specific linkage α(1,6) is catalyzed by the unique FucT-8 enzyme. Structures are illustrated according to the SNFG notation:  Gal (Galactose);
Gal (Galactose);  Man (Mannose);
Man (Mannose);  Fuc (Fucose);
Fuc (Fucose);  GlcNAc (N-Acetylglucosamine);
GlcNAc (N-Acetylglucosamine);  GalNAc (N-Acetylgalactosamine);
GalNAc (N-Acetylgalactosamine);  Neu5Ac (N-Acetylneuraminic acid).
Neu5Ac (N-Acetylneuraminic acid).
Table III.
Summary of the binding specificity of the four lectins assayed
| n | Non fucosylated | α(1,2)Fuc | α(1,3)Fuc | α(1,4)Fuc | α(1,6)Fuc | Combined | ||
|---|---|---|---|---|---|---|---|---|
| NCF | CF | |||||||
| AOL | 172 | 10 (5.8%) | 51 (29.7%) | 38 (22.1%) | 12 (7.0%) | 29 (16.9%) | 23 (13.4%) | 9 (5.2%) |
| AAL | 158 | 7 (4.4%) | 44 (27.8%) | 39 (24.7%) | 11 (7.0%) | 29 (18.4%) | 19 (12.0%) | 9 (5.7%) |
| LCA | 52 | 18 (34.6%) | — | — | — | 25 (48.1%) | — | 9 (17.3%) |
| PhoSL | 22 | 5 (22.7%) | ¯ | ¯ | ¯ | 13 (59.1%) | ¯ | 4 (18.2%) |
Only structures with a relative average intensity above the LOD (n) were considered for further analysis. Fucosylated oligosaccharides are displayed according to the linkage type. When two or more fucose residues were present, oligosaccharides are designated as either combined NCF, if CF is absent, or combined CF, if the glycan structure has a CF. Absolute number of recognized structures and relative percentage are given for comparison.
Secondary binding of PhoSL is mediated by terminal N-acetyl-lactosamine (Galβ1–4GlcNAc)
The results were quite different with regards to non fucosylated structures, for which the binding preferences of lectins for secondary epitopes played a role in the detection of glycans. All five non fucosylated structures above the LOD that were detected by PhoSL (Table III) incorporated the terminal N-acetyl-lactosamine sequence (Galβ1–4GlcNAc). These five glycans, identified with the numerals #379, #351, #319, #541 and #547 in array version 5.1, as listed in Table SI, had a relative average intensity of 13%, 8%, 8%, 11% and 8%, respectively (Figure 2). The most strongly targeted glycan by PhoSL (100%) was the core fucosylated bi-antennary N-acetyl-lactosamine chitobiose trimannosyl core oligosaccharide (#353, Figure 2). Its relative average fluorescence binding intensity was 87% higher than that of the noncore fucosylated bi-antennary N-acetyl-lactosamine chitobiose trimannosyl oligosaccharide equivalent (#379), thus indicating the importance of CF in PhoSL glycan recognition. Similarly, the core fucosylated target (#353) had a binding intensity 52% stronger than that of the non N-acetyl-lactosamine trimannosyl chitobiose core oligosaccharide (#484) (Figure 2). Therefore, the secondary influence exerted by the N-acetyl-lactosamine epitope in PhoSL recognition cannot be ignored.
Fig. 2.
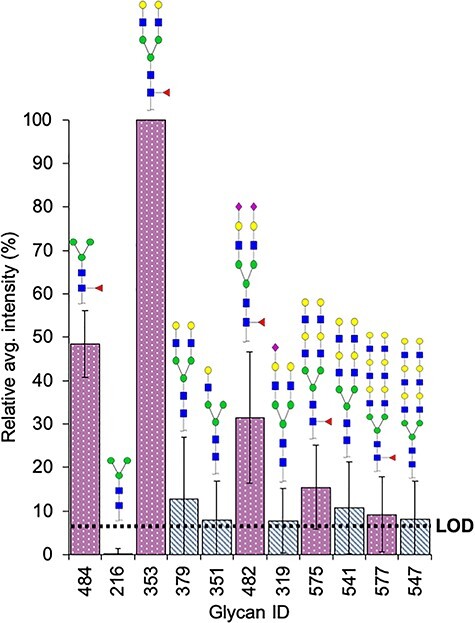
Relative average binding intensity of PhoSL to selected glycans of the glycan array v5.1 from the CFG. Bars of noncore fucosylated glycans with terminal Galβ(1-4)GlcNAc are colored in alternating white and metallic gray slanted lines, bars of the derived core fucosylated glycans are colored in fuchsia background with white dots. Dotted line means LOD = 8. The structures are illustrated accordingly the Symbol Nomenclature for Glycans (SNFG) notation:  Gal (Galactose);
Gal (Galactose);  Man (Mannose);
Man (Mannose);  Fuc (Fucose);
Fuc (Fucose);  GlcNAc (N-Acetylglucosamine);
GlcNAc (N-Acetylglucosamine);  Neu5Ac (N-Acetylneuraminic acid).
Neu5Ac (N-Acetylneuraminic acid).
Furthermore, removal of the CF from the trimannosyl chitobiose core oligosaccharide (#216), completely canceled out the fluorescence signal (Figure 2). Similarly, the addition of the Galβ1–4GlcNAc epitope to the noncore fucosylated trimannosyl chitobiose oligosaccharide (#216) increased the binding intensities in the resulting structures #351 (mono-antennary terminal N-acetyl-lactosamine) and #379 (bi-antennary terminal N-acetyl-lactosamine) (Figure 2). Indeed, whereas #216 was greatly below the LOD, the presence of one N-acetyl-lactosamine residue (#351) increased binding slightly above the LOD, and the incorporation of a new lactosamine unit (#379, with two lactosamine branches) further increased lectin affinity (Figure 2). In contrast, the addition of subsequent lactosamine epitopes significantly decreased the binding capacity of PhoSL, as observed with the polylactosamine bi-antennary N-glycans #541 and #547, two noncore fucosylated glycans recognized by PhoSL (Figure 2). Nevertheless, the signal intensities of the latter polylactosamine glycans increased when CF was also present (#575 and #577, respectively; Figure 2). Likewise, tri-antennary glycans were not recognized by PhoSL, even those with short chains including CF and terminal N-acetyl-lactosamine residues. Together, these results suggest a secondary recognition role of a terminal monolactosamine residue in the binding capacity of PhoSL.
With respect to LCA, 12 glycans were described as high mannose, of which seven attached to the array without the chitobiose core (*) were detected (#51, #208*, #209*, #210*, #211, #212, #213, #214*, #215*, #216, #316* and #317*; Table SII). Six other non fucosylated structures positive for LCA were complex N-glycans (#52, #53, #321, #325, #399 and #404). This finding is consistent with the classical description of LCA being able to bind high-mannose N-glycans (Allen et al. 1976), although the presence of CF is also known to greatly increase the binding affinity (Kornfeld et al. 1981). Similarly, AAL and AOL showed a weak ability to recognize the terminal α(2,3)-N-acetyl-neuraminic acid (Neu5) (structures #46, #442, #527 and #46, #442, #479 and #527, respectively; Table SII), as previously reported (Bergström et al. 2012). AAL and AOL also bound the fucose residues directly printed on the array (#6, #7; Table SII) as well as the specific O-fucosylation sequence GlcNAcβ1–3Fucα (#610, Table SII), as previously reported (Moloney et al. 2000).
The binding intensity of PhoSL and LCA in the glycan array assay depends on the type of fucose bond
Because the fluorescence signal intensity correlates with lectin binding affinity, we represented all the positive structures with equal or greater LOD score in a box plot (Figure 3). AOL (Figure 3A) and AAL (Figure 3B) did not show any statistically significant differences in fluorescence signal intensity according to the fucose position (P = 0.067 and P = 0.453, respectively, by Mann–Whitney U test), although AOL showed a slight preference for CF over other fucosylation types. In sharp contrast, for LCA (Figure 3C) and PhoSL (Figure 3D), the presence of a single CF in the oligosaccharides resulted in significantly greater binding capacity than that detected with non fucosylated oligosaccharides (P = 0.000 and P = 0.023, respectively). Among the structures recognized by LCA and PhosL, we identified two core fucosylated glycans that were outliers, although with opposing behaviors: glycan 38 (#531 in the glycan array 5.0, Table SII) was scarcely detected by LCA, whereas glycan 1 (#353 in the glycan array 5.1, Table SI) emitted the strongest signal for PhoSL. Structure #531 [GlcNAcβ1–2 Manα1–6(GlcNAcβ1–4)(GlcNAcβ1–2 Manα1–3)Manβ1–4GlcNAcβ1–4(Fucα1–6)GlcNAc] has a bisecting GlcNAc, a modification that increases the steric hindrance of LCA near the binding site (Rouwendal et al. 2007). However, #353 is the simplest oligosaccharide with a CF and a terminal monolactosamine in each of the two branches (Figure 2), thus demonstrating the synergistic effects of these two epitopes in PhoSL recognition.
Fig. 3.
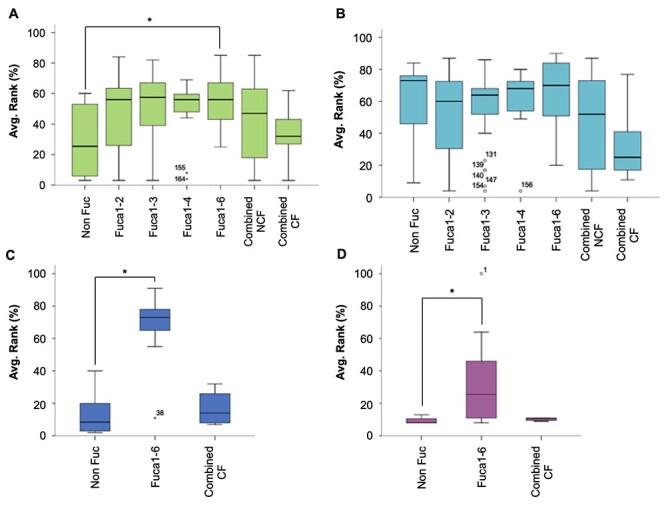
Box plot distribution of the relative average binding intensities depending on the fucose linkage in the fucosylated structures at the CFG glycan array v5.0 for (A) AOL (green), (B) AAL (cyan) and (C) LCA (blue) and v5.1 for (D) PhoSL (fuchsia). When two or more fucose residues were present, the structure was categorized either as combined NCF (noncore fucose) if there is no core fucose or combined CF (core fucose) if one of the fucose residues was linked in α(1,6) position. (*) means a statistically significant difference accordingly the U Mann–Whitney test (P < 0.05).
We also studied the binding intensity of the four lectins to structures with two or more fucose residues (combined CF group, Table III), but we detected no significant differences between combined CF and combined non-CF (NCF) groups (Figure 3). Indeed, the presence of CF with another fucose residue in the same oligosaccharide chain abrogated the lectin binding. Thus, the LCA and PhoSL lectin affinity essentially decreased to the baseline level for non fucose structures (Figure 3C and D, respectively) or to the affinity exhibited by combined NCF oligosaccharides in the case of AOL (Figure 3A) and AAL (Figure 3B).
PhoSL binding and fluorescence intensity according to core fucosylation
To gain insight into which core fucosylated oligosaccharide structures included in the glycan arrays were mainly recognized by the lectins assayed, we selected 33 core fucosylated structures and their corresponding noncore fucosylated counterparts, then paired and ordered them from 1 to 33 according to the typical epitopes commonly reported as important biomarker glycans (numeration and schematic structures can be seen in Table SIV): core structures (1–8), sialylated N-glycans (9–10), Lewis and ABO terminal groups (11–18), as well as bi-antennary (19–26) and tri-antennary (27–33) complex N-glycans. Plotting of the relative average binding intensities showed that AOL and AAL (Figure 4A and B, respectively) mainly detected core fucosylated bi-antennary complex N-glycans (2B) and, to a lesser extent, the tri-antennary group (3B). Moreover, terminal Lewis and ABO determinants showed the highest disparities in their relative average intensities. In addition, the group of core fucosylated structures with terminal sialylation (♦, Figure 4A and B; #9 and #10 oligosaccharides, Table SIV) were also recognized, and their average affinity was 68% and 48%, respectively, for AOL and 87% and 76%, respectively, for AAL. These values are in line with those obtained for several other structural motifs (Figure 4A and B).
Fig. 4.
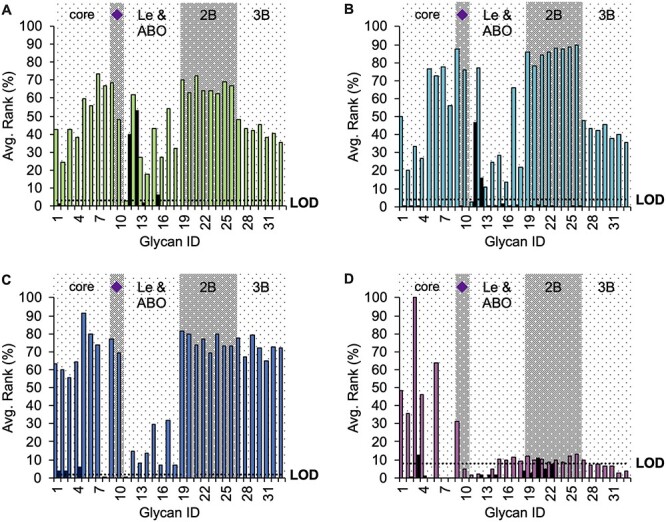
Relative average binding intensities comparing the core fucosylated structures (colored bars) with the corresponding noncore fucosylated glycan (black bar) for (A) AOL (green), (B) AAL (cyan), (C) LCA (blue) and (D) PhoSL (fuchsia). All the structures (numbered in the horizontal axis) were also categorized according to common considerations found in the specialized literature: core means short paucimannosidic oligosaccharides; purple diamond refers to distal sialylated glycans; Le and ABO are glycan structures having the Lewis or blood group epitopes in at least one of their antennas; 2B and 3B are di- and tri-antennary glycans, respectively. Complete structures can be seen in Table SIV. Dotted line refers to the LOD calculated for each lectin.
However, LCA was able to detect the fucosylated core of the bi- and tri-antennary complex N-glycans with high relative average intensities (65–82%) (Figure 4C). This result contrasts with previous evidence of an absence of LCA binding to the tri-antennary N-glycans (Montreuil et al. 1983; Aoyagi et al. 1993). With regard to the group of the terminal Lewis and ABO epitopes, structures #15 and #17, corresponding to the β(1–4)isomer of B and H blood epitopes, respectively, showed the highest binding capacity, although these values were much lower than those for the other glycan groups. Likewise, sialylated glycans showed strong signals as well as the simplest core structures, among which the highest signal was for structure #5, which is characterized by three terminal GlcNAc residues whose elongation gives rise to tri-antennary complex N-glycans.
PhoSL exhibited maximal affinity for the simplest core fucosylated N-oligosaccharides: the bi-antennary Galβ(1–4)GlcNAc trimannosylchitobiose (structure #3, Figure 4D, Table SIV; #353, Table SI; binding = 100%, Figure 2), followed by the core fucosylated trilactosamine trimannosylchitobiose (structure #6, Figure 4D, Table SIV; #485, Table SI;), from which the lactosamine tri-antennary complex N-glycans arise, that showed a signal of 64% (structure #6, Figure 4D; #485, Table SI). Next was the core fucosylated trimannosylchitobiose (structure #1, Figure 4D; #484, Table SI; binding = 48%, Figure 2) and the core fucosylated Galβ(1–3)GlcNAc trimannosylchitobiose (structure #4, Figure 4D; #354, Table SI; binding = 46%). The core fucosylated terminal bi-α(2,6)sialylated oligosaccharide also provided a high intensity signal (structure #9, Figure 4D; #482, Table SI; binding = 31%), whereas the signal for its isomeric bi-α(2,3)sialylated counterpart was almost undetectable (structure #10, Figure 4D; #483, Table SI; binding = 5%). The signal affinity for core fucosylated N-glycans bearing Lewis and ABO epitopes (structures #11–#18, Figure 4D; #419, #472, #454, #457, #427, #455, #420 and #426, Table SI) was below that for the LOD, with the exception of core fucosylated structures with B or H epitopes (structures #15–#18, Figure 4D; #427, #455, #420 and #426, Table SI). Furthermore, the eight core fucosylated polylactosamine bi-antennary complex N-glycans (structures #19–#26, Figure 4D; #574–#581 in Table SI), were scarcely detected. Finally, core fucosylated polylactosamine tri-antennary polylactosamine (structures #27–#33, Figure 4D; #582–#588 in Table SI) complex N-glycans were not recognized at all, with the exception of the shortest #27 structure.
Overall, these results indicated that PhoSL preferentially binds simple core fucosylated N-glycans, lectin discriminates anomeric configurations, and the presence of CF influences lectin affinity for complex N-glycans.
PhoSL binds fucose-null HCT116 colorectal cancer cells, on the basis of lectin blotting
To complement the lectin profiling provided by the CFG array, we used AAL, LCA and PhoSL to perform lectin blots of protein extracts from eight CRC cell lines (HT29, Caco-2, SW480, SW620, DLD-1, HCT116, COLO205 adherent growth fraction and COLO205 suspension growth fraction). This set of CRC lines covers a broad range of CRC histopathological stages.
The DLD-1 and COLO205 cell lines displayed the most intense ligand detection signals with AAL, whereas HCT116 showed no binding (Figure 5A). The profiles of LCA blots were similar to those of AAL, with negative staining for HCT116 and the highest staining for DLD-1 and COLO205 lines (Figure 5B). Nevertheless, PhoSL showed a different binding pattern. Despite a maximum binding intensity signal on both adherent and nonadherent COLO205 cells, PhoSL (1) had clearly lower binding preference towards DLD-1 protein preparations, and (2) its lectin blots were positive for the HCT116 line (Figure 5C). Because HCT116 is considered defective in fucosylation, owing to the deletion of exons 5–7 in the GMD gene (Nakayama et al. 2013), results with AAL/LCA (Figure 5A and B), but not those with PhoSL (Figure 5C), were consistent with the absence of fucose residues.
Fig. 5.
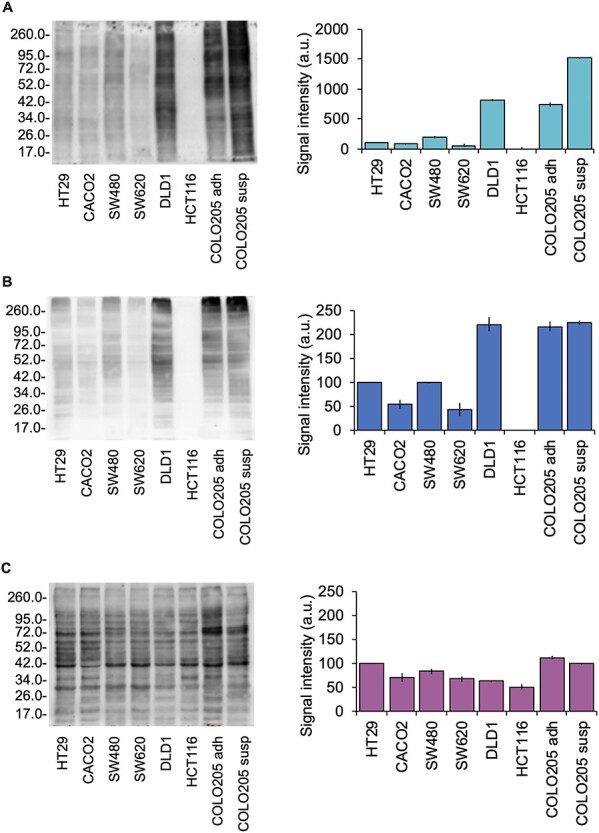
Lectin blot from a selection of 8 CRC cell lines at different tumor stages and fucosylation expression. COLO205 was harvested in two fractions (adherent subclones, adh, and suspension-growing subclones, susp) from the same cell culture. Representative lectin blots using (A) AAL (cyan), (B) LCA (blue) and (C) PhoSL (fuchsia) were performed from the same protein extracts and at the same protein content. Plot bars next to each figure represent the mean relative chemiluminiscence intensity using Coomassie R-250 dyer as protein loading control ± standard deviation from three experiments.
Next, to explain the positive results of PhoSL in the HCT116 line, we analyzed the presence or absence of fucosylated N-glycans in HCT116 extracts with matrix-assisted laser desorption/ionization mass spectrometry (MALDI MS). In the two technical replicates examined (Figure 6), no fucosylated structures were detected among the HCT116 glycoproteins. However, MS data indicated the presence of terminal monolactosamine structures (m/z = 1501.54, 1663.58, 1704.62, 1866.66, 1892.61, 2028.71, 2231.79, 2347.72, 2393.73, 2393.85, 266.83, 2712.86 and 2758.86; Figure 6). Structures corresponding to the m/z peaks at 1501.53 and 1663.58 were present on the glycan array (#351 and #379, respectively; Table SI) and were detected by PhoSL (Figure 2).
Fig. 6.
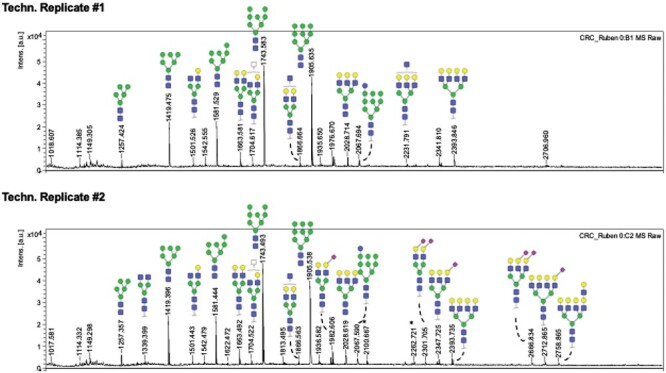
MALDI MS pictures of two technical replicates with N-glycans released from a HCT116 CRC cell line protein extract. None of the identified signals subjected to MS/MS fragmentation was compatible with the presence of a fucose residue, neither distal, medial nor core fucose. HCT116 is considered as a negative control for fucosylation. Selected structures are illustrated accordingly the SNFG notation:  Gal (Galactose);
Gal (Galactose);  Man (Mannose);
Man (Mannose);  Fuc (Fucose);
Fuc (Fucose);  GlcNAc (N-Acetylglucosamine); □ HexNAc (N-Acetylhexosamine);
GlcNAc (N-Acetylglucosamine); □ HexNAc (N-Acetylhexosamine);  Neu5Ac (N-Acetylneuraminic acid).
Neu5Ac (N-Acetylneuraminic acid).
PhoSL, AAL and LCA positively correlate with FucT-8 expression, on the basis of lectin blotting of CRC patient samples
Because PhoSL has been reported to have a strong preference for CF (Kobayashi et al. 2012), the monitoring of FucT-8 may be useful in biological studies. Indeed, if a positive correlation between FucT-8 expression and core fucosylation were demonstrated, PhoSL might serve as a clinical tool in pathological screening. Therefore, we blotted membrane protein extracts from biopsies of four patients with CRC (Figure 7A) and assayed for FucT-8 expression (Figure 7B) and staining with PhoSL (Figure 7C) LCA (Figure 7D) and AAL (Figure 7E). Both healthy and tumoral specimens from patients with CRC showed a positive correlation between lectin binding and FucT-8 expression according to the Pearson coefficient (0.60 for PhoSL, 0.63 for LCA and 0.61 for AAL). Specifically, all three correlations were statistically significant (P < 0.05 for PhoSL and AAL, P < 0.01 for LCA). Consequently, these preliminary results on CRC samples suggest the potential utility of PhoSL to trace the FucT-8 status in clinical studies.
Fig. 7.
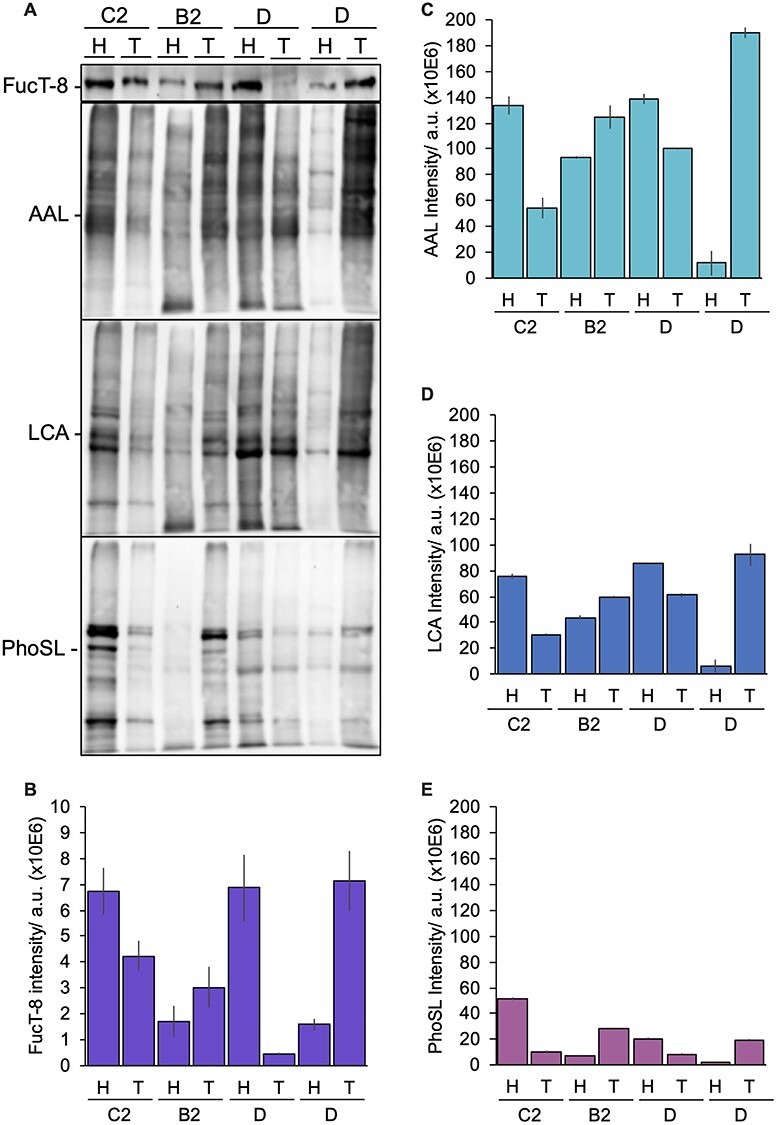
Expression of FucT-8 enzyme and lectin blot from CRC patients. (A) Four biopsies from different patients at different Dukes tumor stages (B2, C2 and D) were separated by SDS-PAGE, quantifying (B) FucT-8 protein expression (violet) and fucose content with (C) AAL (cyan), (D) LCA (blue) and (E) PhoSL (fuchsia) lectins. Plot bars represent the mean relative chemiluminiscence intensity (using the Coomassie R-250 dyer as protein loading control) ± standard deviation from two experiments.
Discussion
Core fucosylation of glycoproteins is a common post-translational modification in human tissues that is altered under several pathological conditions, particularly cancer development (Miyoshi et al. 2012). To date, neither lectin nor immunological alternatives for unambiguous detection of the α(1,6)fucosyl linkage are available. Therefore, there is a need for either novel lectins with strict binding specificity and/or monoclonal antibodies against CF.
The profiling of lectin specificity is usually performed by frontal affinity chromatography (FAC) using lectin-immobilized beds through which a mixture of free glycans is forced (Tateno et al. 2007; Kasai 2014). However, one of the main disadvantages of this approach is that the nonreducing ends of glycans are easily available for lectin interaction without the steric hindrance exerted by the peptide backbone. Hence, when the epitope of interest is the innermost fucose, the branched chains can introduce strong steric restrictions that hinder lectin approximation. However, in a free glycans, this effect would be absent at the reducing end, and thus lectin adhesion towards the core fucosylated epitope of the oligosaccharide would be facilitated. Serna and collaborators, by using fucosyltransferases, first described this phenomenon in the chemical synthesis of N-glycans (Serna et al. 2011). Likewise, in other novel CF-specific lectins, such as BPL, this steric hindrance of branching has been invoked to explain the lack of binding to any bisected N-glycans (do Nascimento et al. 2015). In a more recent publication, the glycan moiety has been found to decisively affect the elution profile of a mixture of peptides, in comparison with the use of equivalent glycopeptides in wheat germ agglutinin lectin chromatography coupled to mass spectrometry (Zhu et al. 2016). However, these authors investigated the possible influence of nonspecific interactions between the lectin and the peptide moiety and found no effects. Despite this evidence, nonspecific interactions in lectin recognition may become dominant, particularly with undigested glycoproteins (Martín del Valle and Galán 2002; Lee et al. 2010). Interestingly, although only GlcNAc on the α(1,3)mannose arm of N-glycans was considered essential for FucT-8-dependent core fucosylation, recent reports using glycopeptides instead of free N-glycans have demonstrated that FucT-8 also interacts favorably with the peptide moiety (Yang et al. 2017).
In agreement with this hypothesis, we emphasize the need for a more detailed characterization of the stereochemical hindrances that lectins encounter in glycan recognition as well as for systematic profiling of their specificity based on glycoproteins instead of free N-glycans. In this sense, it is advisable to revisit the importance of adapting FAC for N-glycans by mimicking their natural state instead of having a free-derivatized reducing end. To this end, glycan arrays have been demonstrated to be more advantageous than FAC in lectin/core-fucose binding characterization, because lectin binding to the CF motif is hindered in a manner that resembles the natural exposition of the motif. Additionally, the use of buffers supplemented with detergents and washing steps weakens the nonspecific adsorption onto the array, thus eliminating or at least decreasing the risk of artifacts (Haab and Yue 2011). Certainly, the influence of the protein backbone on lectin–glycan recognition remains to be investigated. In this regard, the glycan arrays developed by the CFG allowed us to detect the broad ranges of different structures recognized by the fucose-oriented lectins AOL, AAL, LCA and PhoSL. With more than 600 glycan sequences, the CFG glycan array generated useful information on isomeric and anomeric configurations of fucose links and other common glycan moieties that are preferentially recognized by the set of lectins tested. To our knowledge, this is the most comprehensive approach undertaken to compare lectin–glycan interactions with CF.
AOL has been reported to bind α(1,6)fucosylated glycans more strongly than other lectins (Matsumura et al. 2007; Matsumura et al. 2009). This binding specificity led to its initial use in the development of immunofluorescence probes for CF evaluation (Mun et al. 2012). However, although AOL has been described by Matsumura and collaborators as more specific for CF than AAL, our results showed that AOL and AAL displayed an overall similar pattern of binding to fucosylated structures on the microarray (Figures 1A and B, 3A and B, 4A and B). Therefore, our results contrast with previous evidence in that AOL did not clearly demonstrate a stronger preference for CF, as seen in the relative binding intensity for the different fucose linkage types (Figure 3A). Furthermore, in a recent analysis, the glycan microarray of an antipodocalyxin mouse IgG2a antibody was positive with AOL, AAL, LCA and PhoSL, but only AOL remained positive for the defucosylated counterpart mIgG2a-f antipodocalyxin (Itai et al. 2018).
AAL is also commonly used for detecting the presence of protein fucosylation (Wang, Chen, et al. 2014b). Herein, among other fucosylated oligosaccharides, it was found to bind heterogeneous core fucosylated glycan structures (Figure 4B), producing fluorescence signals in both the glycan array (Figure 3B) and lectin blots (Figures 5A and 7A). Despite these advantages, AAL is also capable of binding with similar strength and preference to fucose residues regardless of the type of linkage (Figures 1B and 3B). This finding prompts concerns regarding the mutual glycosyltransferase regulation reported in numerous studies (Saldova et al. 2011; Groux-Degroote et al. 2014; Kurimoto et al. 2014; Huang et al. 2020); therefore, it is uncertain whether changes in lectin reactivity can be assigned to the modulation of FucT-8 expression or whether the detected alterations are a consequence of the regulation of one or more fucosyltransferases, as could be inferred from the observation of concomitant elevation of CF and terminal Lewis antigens in several cancers (Zhou et al. 2017; Jia et al. 2018; Li et al. 2020; Park et al. 2020).
Core fucosylation is believed to strengthen LCA binding, on the basis of evidence provided by FAC and glycan arrays (Kinoshita et al. 1991; Tateno et al. 2009). Furthermore, the structural analysis of lectin–ligand binding also supported enhancement through CF of LCA recognition, because CF interacts with a hydrophobic region outside the lectin recognition pocket and properly orients the epitope for binding (Sokolowski et al. 1997). Therefore, although the main determinant for LCA binding is X-1,2-Manα-Man, CF plays a pivotal role in lectin recognition. Perhaps the absence of core fucosylated glycans on previous glycan microarray versions prevented finding the influence of CF on LCA recognition (Maupin et al. 2012). Nevertheless, our results with CFG microarray version 5.0 allowed us to determine that core fucosylation, but no other fucose positions, enhances the binding preference of LCA (Figures 1C and 3C). Thus, given our observations, LCA appears to specifically detect CF, because it was not optimal for detecting the presence of this moiety in other positions or linkage types (Figure 3C), and it did not detect glycans with two or more fucose residues (Figure 4C, Le and ABO group). Furthermore, it was less affected by the presence of secondary binding epitopes that may be altered during carcinogenesis and cancer progression, as described for lactosamination in general and polylactosamination in particular (Chakraborty and Pawelek 2003; Chen et al. 2014; Jiang et al. 2019). In summary, LCA should remain as the first option in screening for CF epitopes, because is readily available, and it demonstrated a better recognition specificity towards not only simple N-glycans, but also bi- and tri-antennary N-glycans (Figure 4C). However, the preference of LCA for high-mannose N-glycans described in the literature (Zhao et al. 2008; Holst et al. 2016) and our results (Figure 4C) may be problematic, not because these N-glycoproteins are dominant in intracellular protein extracts and participate in the endoplasmic reticulum-Golgi-membrane N-glycoprotein maturation pathway, but because they are the result of incomplete N-glycoprotein processing (Zhao et al. 2008) as well as aberrations in the glycosylation machinery during cancer development. Thus, their presence among cell surface and extracellular glycoproteins may be elevated (Chik et al. 2014; Kaprio et al. 2015).
Unlike the other three lectins, PhoSL does not have a reliable natural source for extraction and purification; therefore, we used chemical synthesis according to Kobayashi and coworkers, who have also demonstrated that both the natural lectin and its chemically synthesized analog behave almost identically in terms of glycan recognition (Kobayashi et al. 2012). The main binding motif of PhoSL has recently been identified by NMR and X-ray diffraction using the disaccharide Fucα(1–6)GlcNAc and the trisaccharide Fucα(1–6)[GlcNAcβ(1–4)]GlcNAc (Yamasaki et al. 2019). The CF and the outermost GlcNAc residues have been found to interact through extensive hydrophobic and hydrogen-bonding contacts in the sugar-binding pocket. Moreover, a previous study has described the relevance of the fucosylated chitobiose core in lectin–sugar binding and the interaction of the galactose located in the 1,6-branch of the N-glycan with PhoSL, involving hydrogen-bonding with Asp33 in the protein backbone (Cabanettes et al. 2018). To our knowledge, this is the first report suggesting that both CF and Galβ1–4GlcNAc may play roles in PhoSL recognition ability. Indeed, our results add new support to this hypothesis, because they indicated that Galβ1–4GlcNAc is recognized by PhoSL as a secondary epitope capable of affecting the binding strength of the recognized N-glycans, regardless of whether they are core fucosylated (Figure 2).
Despite the higher preference of PhoSL towards CF, we cannot ignore the importance of the effects of terminal lactosamine on binding capacity, although this phenomenon was not detected by Kobayashi and coworkers when they reported the identification, purification and behavior of this lectin (Kobayashi et al. 2012). In this regard, they did not find a single terminal galactosylated N-glycan recognized by PhoSL. The revision of the panel of N-glycans assayed by those authors in their seminal work allowed us to identify that their structure #405 was coincident with our #353, both of which comprise a core fucosylated paucimannosidic core with two Galβ1–4GlcNAc branches (Figure 2). Likewise, their equivalent defucosylated counterparts (#301 versus #379) were also present in both studies. These opposite results may be explained by determining whether mobile phase mass transfer effects should be considered under the experimental conditions of FAC (Schiel and Hage 2009; Iftekhar et al. 2019). Additionally, our glycan array results came from an average of three different lectin concentrations (from 20 to 200 μg/mL, in contrast to the concentrations used by Kobayashi et al. 2012 of 1–2.1 mg/mL), thus avoiding the possible effects of lectin concentration in the recognition signal, because greater lectin concentrations led to greater numbers and heterogeneity of the recognized structures.
However, the blotting of protein extracts from CRC cells by using LCA, AAL and PhoSL (Figure 5) indicated that PhoSL had the lowest binding and provided a positive signal for the human HCT116 CRC cell line. Of note, HCT116 is considered defective in fucosylation, either core, medial or terminal, as demonstrated in previous reports and our results by mass spectrometry (Figure 6). This phenomenon could not be explained if the relevance of terminal lactosamine in the PhoSL recognition capacity were neglected. In this regard, the absence of PhoSL binding to the afucosylated α-fetoprotein has been suggested as evidence of the selectivity of this lectin towards core fucosylation (Kobayashi et al. 2012). Early publications demonstrated that the major glycoform of α-fetoprotein from cord serum and benign liver diseases is a bi-antennary bi-α(2,3)sialylated N-glycan (Taketa 1990; Taketa et al. 1990). In contrast, patients diagnosed with hepatocellular carcinoma showed an altered pattern of α-fetoprotein glycoforms (Breborowicz et al. 1981), usually marked by elevated core fucosylation, thus leading to the discrimination of the AFP-L3 fraction band by using LCA (Taketa et al. 1990); this band has been proposed as a prognostic biomarker in hepatocellular carcinoma (Wong et al. 2015). Nevertheless, in-depth analysis of the α-fetoprotein glycoforms led to the identification of microheterogeneity; thus, monosialylated variants, and even partially and fully lactosaminated glycoforms, have been identified within fractions obtained after a lectin purification of α-fetoprotein (Johnson et al. 1999; Johnson et al. 2000; Ichikawa et al. 2006). In fact, a recent report has demonstrated higher variability than previously expected in α-fetoprotein glycoforms, depending on the natural source (Egashira et al. 2019). In that work, the authors found that the hepatocellular carcinoma cell line Huh7 produces mostly six bi-antennary, core fucosylated, bi-sialylated α-fetoprotein glycoforms, whereas the hepatocellular carcinoma cell line HepG2 produces a more complex mixture with the presence of bisecting core fucosylated N-glycans, monosialylated chains and bi-antennary truncated glycoforms. Additionally, the hepatocellular carcinoma cell line FUT8−/− HepG2 produces afucosylated alactosaminated α-fetoprotein with minor bisecting glycoforms. Moreover, analysis of the flow-through fraction from a LCA column chromatography of human cord serum α-fetoprotein has enabled the identification of truncated bi-antennary either monolactosaminated or monosialylated N-glycans. Interestingly, another study has identified a different population of fetoprotein glycoforms from that in the Huh7 cell line, with bi-antennary core fucosylated, lactosaminated N-glycans; tri-antennary core fucosylated, lactosaminated N-glycans; or bi-antennary bisecting core fucosylated, lactosaminated N-glycans (Nakagawa et al. 2008). Considering these reports together, we cannot predict a homogeneous composition across α-fetoprotein sources, despite their being from the same cell line, or within the AFP-L3 fraction bands. As suggested by our positive results of the analysis of the HCT116 proteome with PhoSL, determining the glycan microheterogeneity of a glycoprotein as α-fetoprotein is a step that cannot be underestimated, even if similar procedures and samples are used; furthermore a negative signal with PhoSL indicates an absence of core fucosylation but may also imply the absence of terminal lactosamination.
The analysis by lectin blotting of membrane protein extracts from biopsies of four patients with CRC (Figure 7) showed the correlation between PhoSL staining and FucT-8 expression, in agreement with previously published results of an immunochemistry study in nonsmall cell lung cancer, in which this lectin exhibited a binding affinity closely dependent on FucT-8 and GDP-mannose 4,6-dehydratase (GMD) protein expression (Honma et al. 2015). The prognosis of nonsmall cell lung cancer is strongly affected by FucT-8 enzyme expression (Chen et al. 2013), as is also the case with CRC (Muinelo-Romay et al. 2008, 2011a). Hence, the identification of glycoproteins affected by abnormal core fucosylation has gained translational interest in recent years. Thus, the availability of a lectin that easily enables comparison among tumoral stages would be of great clinical relevance.
The results of such a small sample size (four specimens) are insufficient to support use of PhoSL as a reference for screening for patient prognosis and outcome. However, our results suggest the potential utility of specific lectins in biomedicine. In this regard, PhoSL had the same range of specificity as LCA, a lectin with a well-demonstrated preference for α(1,6)fucose.
In conclusion, the present study provides deeper characterization of the N-glycan binding profiles of AAL, AOL, LCA and PhoSL. AAL and AOL were nondiscriminatory towards fucosylated motifs. In contrast, LCA had high affinity to CF and showed slight binding to high-mannose oligosaccharides without interfering with the detection of fucose. PhoSL, described as one of the most specific lectins for CF, mainly recognized structural variants of the α(1,6)fucosylated trimannosylchitobiose core. However, although the CF enhanced binding, the terminal N-acetyl-lactosamine residue was also found to have an important role in lectin specificity. The modulation of PhoSL binding specificity by N-acetyl-lactosamine is a mechanistic feature that cannot be ignored, because complex glycomic samples contain motifs that usually bear both lactosamine and CF residues. Consequently, the usefulness of PhoSL is more restricted than was initially expected.
Material and methods
Synthesis of biotin-PhoSL
PhoSL was chemically synthesized by Peptide 2.0 (Chantilly, VA, USA) through solid phase peptide synthesis, to 95% purity. The 40-mer sequence was NH2-APVPVTKLVCDGDTYKCTAYLDFGDGRWVAQWDTNVFHTG-OH. The biotin carboxylic functional group was activated by treatment with HBTU/HOBt and then was added to the resin for functionalizing the N-terminal side through direct biotin coupling. Before delivery, the crude synthesized peptide was purified by reverse-phase HPLC, and its sequence was determined by MALDI-MS. PhoSL was supplied as a white powder, which was suspended in phosphate buffered saline, divided into aliquots and stored at −20°C until use.
Cell culture
The CRC cell lines HT-29, Caco2, SW480, SW620, DLD1 and HCT116 were provided by the Liquid Biopsy Analysis Unit (Oncomet, Health Research Institute of Santiago de Compostela, Spain), research group of Muinelo-Romay, whereas COLO205 was kindly donated by Rodríguez-Berrocal, FJ (Department of Biochemistry, Genetics and Immunology, University of Vigo, Spain). All cell lines were from the American Type Culture Collection. Cell cultures were maintained in Dulbecco’s modified Eagle’s medium-high glucose (Sigma-Aldrich, Saint Louis, MO, USA) supplemented with 10% fetal bovine serum (Life Technologies, Grand Island, NY, USA) and penicillin–streptomycin 10,000 U/mL (Life Technologies, Grand Island, NY, USA). Cells were harvested in P100 Petri dishes (BD Falcon, Franklin Lakes, NJ, USA) at 37°C in a humidified incubator supplied with 5% CO2.
Cell passaging was performed when the cell population reached 80% confluence to the naked eye. Culture medium was removed by aspiration, and attached cells were washed twice with Dulbecco’s phosphate-buffered saline (0.2 g/L KCl, 0.2 g/L KH2PO4, 8.0 g/L NaCl and 1.15 g/L Na2HPO4) (Dulbecco and Vogt 1954). Cell detachment was then achieved with 0.25% trypsin/EDTA (Lonza, Basel, Switzerland). For the COLO205 cell line, cells in suspension cultures (COLO205 susp) were harvested from the culture medium by centrifugation for 5 min at 1500 × g and mixed with the adherent growing fraction (COLO205 adh).
Protein extraction from cell cultures
Cell lysates were obtained by direct addition of homemade RIPA buffer (0.1% SDS, 150 mM NaCl, 50 mM Tris–HCl, pH 8.5, 0.5% sodium deoxycholate, 1% Nonidet P-40, 2 mM Na3 VO4 and 4 mM NaF), supplemented immediately before use with complete protease inhibitor cocktail tablets (Roche Life Sciences, Penzberg, Bayern, Germany). Cell lysis was manually facilitated with a scraper, and the suspension was kept on ice for 30 min, with gentle vortexing at intervals. Then, cell debris and nonsolubilized material were removed by centrifugation at 10,000 × g for 10 min and stored at −20°C. All protein extracts were validated with BCA assays (Sigma-Aldrich, Saint Louis, MO, USA), with BSA (Sigma-Aldrich, Saint Louis, MO, USA) as the reference.
Preparation of colorectal tissue extracts
A total of 0.2–0.4 g of colorectal tissues from surgical resections of patients with CRC were homogenized in six volumes of 0.01 M Tris–HCl and 250 mM sucrose buffer (pH 7.4) in a Potter-Elvehjem homogenizer (Pobel, Madrid, Spain). The homogenate was centrifuged for 10 min at 500 × g at 4°C (Kontron T-124, rotor A8.24, Augsburg, Germany), and the supernatant was subsequently centrifuged at 33,000 × g for 60 min at 4°C. The pellet was resuspended in 1.5 mL of 0.01 M Tris–HCl buffer (pH 7.4) and centrifuged at 145,000 × g for 45 min at 4°C (Kontron T-1075, rotor TFT 80.4, Augsburg, Bayern, Germany). The final pellet containing the total cell membrane fraction was resuspended in 300 μL of 0.01 M Tris–HCl buffer (pH 7.4) and stored at −20°C. The protein content was determined as described for protein extracted from cell culture.
SDS-PAGE, western blotting and lectin blotting
A total of 20 μg of protein per sample was heated with 4× Laemmli loading buffer (Laemmli 1970) for 5 min at 100°C. SDS-PAGE with 10% polyacrylamide was used in all experiments. Protein bands in the 60–20 kDa range were adequately resolved with standard molecular weight markers (BlueStar PLUS Prestained Protein Marker, NIPPON Genetics Europe GmbH, Düren, Nordrhein-Westfalen, Germany). When necessary, protein bands were stained with Coomassie brilliant blue (0.1% Coomassie blue R250, 5% glacial acetic acid, 30% methanol). For western blotting, gels were blotted onto polyvinylidenedifluoride (PVDF) membranes. After blotting, the membranes were blocked for 1 h with 5% skimmed milk in Tris-buffered saline (TBS, 20 mM Tris and 150 mM NaCl, pH 7.4). A 1/500 dilution of mouse anti-FUT8 IgG (Proteintech, Chicago, IL, USA) in 0.05% Tween 20 in TBS (TBST) was incubated with the membranes at room temperature for 1 h. Development was performed by incubation with Clarity Western ECL detection reagent (Bio-Rad, Hercules, CA, USA) according to the manufacturer’s protocol. For lectin blotting, gels were blotted onto PVDF membranes. After blotting, the membranes were blocked for 1 h with 3% BSA in TBS. Then, 1/350 of biotin-PhoSL, 1/2000 of biotin-LCA and 1/2000 of biotin-AAL in TBST were sequentially assayed with a mild stripping step (15 g/L glycine and 1 g/L SDS, 0.01% Tween 20, pH 2.2) between each lectin blot. Three washing steps with TBST were then performed. Membranes were incubated with horseradish peroxidase-conjugated avidin (EliteVECTASTAIN ABC kit; Vector Laboratories, Peterborough, UK) for either 1 h (PhoSL, LCA) or 15 min (AAL). Development was performed as described above. The intensity of bands from either gels or membranes was quantified with Fiji software (Schindelin et al. 2012).
Glycan arrays
PhoSL glycan array screening was performed with the standard procedure used by the protein–glycan interaction core (H) of the CFG, as previously described (Blixt et al. 2004, Heimburg-Molinaro et al. 2011, 2012). The conditions for the glycan array and data acquisition based on the MIRAGE guidelines are summarized in Table SV (Liu et al. 2017). Briefly, three concentrations of PhoSL (20–50 and 200 μg/mL) were analyzed with a PA_v5.1 mammalian chip containing 610 different glycan structures. Biotin-PhoSL was diluted to an appropriate concentration in standard binding buffer (TSM, 1% BSA and 0.05% Tween 20). A volume of 70 μL was applied onto the array and incubated for 1 h at room temperature in a humidified chamber protected from light. The slide was rinsed four times in TSM washing buffer and four times in TSM buffer without Tween. Then, 70 μL of fluorescently labeled streptavidin in standard TSM binding buffer was added to the slide and incubated in the dark for 1 h at room temperature in a humidified chamber. Later, new washes were performed as described above and were followed by four washes in distilled water. After slides had been dried and scanned, the images were opened in Imagene software, and a grid was used to align the spots on the slide according to the biotin control spots. The amount of binding per spot was quantified and represented in random fluorescence units (RFU).
The relative binding intensities were calculated from the RFU values through a modified version of a previously described method (Millen et al. 2010). Briefly, experimental data were normalized to the percentages of the highest RFU value for each analysis. Subsequently the percentages for each glycan at different lectin concentrations were averaged to obtain an average value of the relative binding. A lectin–glycan event was considered positive only if its medium value of relative intensity was greater than the mean background relative average intensity value plus three standard deviations (formulation derived from the LOD definition from IUPAC 1997), where the mean background relative average intensity was the mean of the relative average intensity values less than 10% of the maximum relative average intensity (Figure S1). Finally, analysis of the glycan recognition pattern for each lectin was performed on the GlycoPattern platform (Agravat et al. 2014), which provided motifs, glycan searching, lectin similarity and glycan-binding-protein cross analysis.
Release of N-glycans from glycoproteins
Cell pellets from HCT116 (∼2 × 106 cells) were suspended in 100 μL milli-Q (mQ) water, then sonicated in a water bath for 30 min. Glycans were released with a modification of a PVDF-membrane based protocol (Burnina et al. 2013). Briefly, 2,5 × 105 cells/well in denaturation buffer (5.8 M GuHCl and 5 mM DTT) were loaded in duplicate onto preconditioned HTS 96-well plates with hydrophobic Immobilon-P PVDF membranes, then incubated for 30 min in a humidified box kept in an oven at 60°C. The plate was shaken for 5 min on a horizontal shaker before centrifugation (1 min, 500 × g). The wells were washed twice with 200 μL mQ water with 2 min incubation steps on a horizontal shaker and once with 200 μL 100 mM sodium bicarbonate. For N-glycan release, 50 μL 100 mM sodium bicarbonate and 1 mU PNGase F (Roche Life Sciences, Penzberg, Bayern, Germany) were added per well. The plate was incubated overnight at 37°C. Glycans were recovered into 96-well collection plates by centrifugation.
Derivatization of N-glycans and hydrophilic interaction liquid chromatography solid phase extraction glycan enrichment
Released N-glycans were derivatized with an adaptation of an ethyl esterification protocol that allows for differentiation between the α(2–3) and α(2–6) sialic acid linkages (Reiding et al. 2014). Briefly, 20 μL of released N-glycans was added to 100 μL of ethyl esterification reagent (0.25 M EDC and 0.25 M HOBt, 1:1 v/v) and incubated for 1 h at 37°C. Subsequently, 100 μL acetonitrile (ACN) was added, and the mixture was incubated in the freezer at −20°C for 15 min. Glycan purification was performed with hydrophilic interaction liquid chromatography cotton-solid phase extraction, as previously described (Selman et al. 2011). Pipette tips (20 μL) were packed with cotton threads, equilibrated (3 × 20 μL mQ water) and conditioned (3 × 20 μL 85% ACN). Samples were loaded by pipetting up and down, and the solid phase was then washed (3 × 20 μL 85% ACN/1% TFA and 3 × 20 μL 85% ACN). Elution was performed with 10 μL mQ water by pipetting up and down.
MALDI-TOF MS analysis
For mass spectrometric analysis, 5 μL of ethyl-esterified N-glycans was spotted onto a MALDI plate (Bruker Daltonics, Billerica, MA, USA) with 1 μL of 1 mg/mL superDHB in ACN/mQ water (1/1, v/v) containing 1 mM NaOH. Samples were allowed to dry at room temperature before insertion into the spectrometer. Matrix-assisted laser desorption/ionization-time-of-flight mass spectrometry (MALDI-TOF MS) spectra were acquired with an UltrafleXtreme™ mass spectrometer in positive-ion reflector mode, controlled by FlexControl 3.4 software build 119 (Bruker Daltonics, Billerica, MA, USA). The instrument was calibrated with a Bruker peptide calibration kit. Spectra were obtained over a mass window of m/z 1000–5000 with ion suppression below m/z 900 for a total of 10,000 shots (1000 Hz laser frequency, 200 shots per raster spot during complete random walk). Tandem mass spectrometry (MALDI-TOF-MS/MS) was performed for structural elucidation via fragmentation in gas-off TOF/TOF mode.
Data processing of MALDI-TOF-MS spectra
Two spectra from the HCT116 cell line were generated with an in-house developed script in Python 2.7.3 (Python Software Foundation; http://docs.python.org/py3k/reference/index.html). The spectra were internally recalibrated by using glycan peaks of known composition. Peaks with signal-to-noise > 2 were picked, and their structures were analyzed in GlycoWorkbench 2.1 (http://www.eurocarbdb.org/) with the Glyco-Peakfinder tool for generation of a glycan compositions list. Selected glycan compositions were confirmed by MS/MS. The final peak list overview is shown in Figure 6.
Statistical analysis
Microsoft Excel 2013 and IBM SPSS Statistics v26 were used for statistical analysis. Nonparametric Mann–Whitney U test was used for detecting differences among the fucose recognition groups in terms of relative average fluorescence intensity. P < 0.05 was considered statistically significant. The Pearson correlation coefficient was used for testing the linear correlation between the FucT-8 expression and the normalized lectin intensity. Either P < 0.05 or P < 0.01 was considered statistically significant.
Supplementary Material
Acknowledgements
RBL acknowledges doctoral grant AP-FPU12/03662 provided by the Ministerio de Educación y Ciencia, Spain. A.F.B. and E.G.M. are grateful for financial support provided under the projects “Contrato-Programa de Consolidación de Unidades de Investigación Competitivas CN 2011/024” and “Contrato-Programa de Consolidación de Grupos de Referencia Competitiva GRC 2014/019” from Xunta de Galicia, Spain. All authors acknowledge the resources provided by The CFG funded by NIGMS—GM62116, GM098791 and a EUREKA grant R01GM085447 to DFS. All authors are also grateful to Stephanie Holst-Bernal and Manfred Wuhrer (Center for Proteomics and Metabolomics, Leids Universitair Medisch Centrum, Leiden, Zuid-Holland, The Netherlands), for their collaborative efforts in the optimization, realization and interpretation of the mass spectrometry data of N-glycans, and to Richard Cummings and Jamie Heimburg-Molinaro (National Center for Functional Glycomics, Beth Israel Deaconess Medical Center, Harvard Medical School, Boston, Massachusetts, USA), for their revision of the lectin array information provided in the supplementary glycan microarray information template based on MIRAGE guidelines.
Contributor Information
López-Cortés Rubén, Doctoral Program in Methods and Applications in Life Sciences, Faculty of Biology, Universidade de Vigo, Campus Lagoas-Marcosende, Vigo, Pontevedra, Galicia ES36310, Spain.
Muinelo-Romay Laura, Liquid Biopsy Analysis Unit, Translational Medical Oncology (Oncomet), Health Research Institute of Santiago de Compostela (IDIS), CIBERONC, Travesía da Choupana, Santiago de Compostela, A Coruña, Galicia ES15706, Spain.
Fernández-Briera Almudena, Molecular Biomarkers, Biomedical Research Centre (CINBIO), Universidade de Vigo, Campus Lagoas-Marcosende, Vigo, Pontevedra, Galicia ES36310, Spain.
Gil Martín Emilio, Nutrition and Food Science Group, Department of Biochemistry, Genetics and Immunology, Faculty of Biology, Universidade de Vigo. Campus Lagoas-Marcosende, Vigo, Pontevedra, Galicia ES36310, Spain.
Conflict of interest statement
None declared.
References
- Agravat SB, Saltz JH, Cummings RD, Smith DF. 2014. GlycoPattern: a web platform for glycan array mining. Bioinformatics. 30:3417–3418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agrawal P, Fontanals-Cirera B, Sokolova E, Jacob S, Vaiana CA, Argibay D, Davalos V, McDermott M, Nayak S, Darvishian F et al. 2017. A systems biology approach identifies FUT8 as a driver of melanoma metastasis. Cancer Cell. 31:804–819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ainouz IL, Sampaio AH, Freitas ALP, Benevides NMB, Mapurunga S. 1995. Comparative study on hemagglutinins from the red algae Bryothamnion seaforthii and Bryothamnion triquetrum. R Bras Fisiol Veg. 7:15–19. [Google Scholar]
- Allen AK, Desai NN, Neuberger A. 1976. Purification of the glycoprotein lectin from the broad bean (Vicia faba) and a comparison of its properties with lectins of similar specificity. Biochem J. 155:127–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aoyagi Y, Suzuki Y, Igarashi K, Saitoh A, Oguro M, Yokota T, Mori S, Suda T, Isemura M, Asakura H. 1993. Carbohydrate structures of human alpha-fetoprotein of patients with hepatocellular carcinoma: presence of fucosylated and non-fucosylated triantennary glycans. Br J Cancer. 67:486–492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Apweiler R, Hermjakob H, Sharon N. 1999. On the frequency of protein glycosylation, as deduced from analysis of the SWISS-PROT database. Biochim Biophys Acta. 1473:4–8. [DOI] [PubMed] [Google Scholar]
- Arcinas A, Yen TY, Kebebew E, Macher BA. 2009. Cell surface and secreted protein profiles of human thyroid cancer cell lines reveal distinct glycoprotein patterns. J Proteome Res. 8:3958–3968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baïet B, Burel C, Saint-Jean B, Louvet R, Menu-Bouaouiche L, Kiefer-Meyer MC, Mathieu-Rivet E, Lefebvre T, Castel H, Carlier A et al. 2011. N-glycans of Phaeodactylum tricornutum diatom and functional characterization of its N-acetylglucosaminyltransferase I enzyme. J Biol Chem. 286:6152–6164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baldus SE, Thiele J, Park YO, Hanisch FG, Bara J, Fischer R. 1996. Characterization of the binding specificity of Anguilla anguilla agglutinin (AAA) in comparison to Ulex europaeus agglutinin I (UEA-I). Glycoconj J. 13:585–590. [DOI] [PubMed] [Google Scholar]
- Banerjee DK. 2012. N-glycans in cell survival and death: cross-talk between glycosyltransferases. Biochim Biophys Acta. 1820:1338–1346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergström M, Aström E, Påhlsson P, Ohlson S. 2012. Elucidating the selectivity of recombinant forms of Aleuria aurantia lectin using weak affinity chromatography. J Chromatogr B Analyt Technol Biomed Life Sci. 885:66–72. [DOI] [PubMed] [Google Scholar]
- Blixt O, Head S, Mondala T, Scanlan C, Huflejt ME, Alvarez R, Bryan MC, Fazio F, Calarese D, Stevens J et al. 2004. Printed covalent glycan array for ligand profiling of diverse glycan binding proteins. Proc Natl Acad Sci USA. 101:17033–17038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breborowicz J, Mackiewicz A, Breborowicz D. 1981. Microheterogeneity of α-fetoprotein in patient serum as demonstrated by lectin affinity electrophoresis. Scand J Immunol. 14:15–20. [DOI] [PubMed] [Google Scholar]
- Burnina I, Hoy E, Lynaugh H, Li H, Gong B. 2013. A cost-effective plate-based sample preparation for antibody N-glycan analysis. J Chromatogr A. 1307:201–206. [DOI] [PubMed] [Google Scholar]
- Cabanettes A, Perkarms L, Spies C, Unverzagt VA. 2018. Recognition of complex core-fucosylated N-glycans by a mini lectin. Angew Chem. 130:10335–10338. [DOI] [PubMed] [Google Scholar]
- Chakraborty AK, Pawelek JM. 2003. GnT-V, macrophage and cancer metastasis: a common link. Clin Exp Metastasis. 20:365–373. [DOI] [PubMed] [Google Scholar]
- Chen CH, Wang SH, Liu CH, Wu YL, Wang WJ, Huang J, Hung JS, Lai IR, Liang JT, Huang MC. 2014. β-1,4-galactosyltransferase III suppresses β1 integrin-mediated invasive phenotypes and negatively correlates with metastasis in colorectal cancer. Carcinogenesis. 35:1258–1266. [DOI] [PubMed] [Google Scholar]
- Chen CY, Jan YH, Juan YH, Yang CJ, Huang MS, Yu CJ, Yang PC, Hsiao M, Hsu TL, Wong CH. 2013. Fucosyltransferase 8 as a functional regulator of non-small cell lung cancer. Proc Natl Acad Sci USA. 110:630–635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chik JH, Zhou J, Moh ES, Christopherson R, Clarke SJ, Molloy MP, Packer NH. 2014. Comprehensive glycomics comparison between colon cancer cell cultures and tumours: implications for biomarker studies. J Proteomics. 108:146–162. [DOI] [PubMed] [Google Scholar]
- Christiansen MN, Chik J, Lee L, Anugraham M, Abrahams JL, Packer NH. 2014. Cell surface protein glycosylation in cancer. Proteomics. 14:525–546. [DOI] [PubMed] [Google Scholar]
- Crandall IE, Newell PC. 1989. Changes in cell surface glycoproteins during Dictyostelium development analysed using monoclonal antibodies. Development. 107:87–94. [DOI] [PubMed] [Google Scholar]
- Cummings RD, Etzler ME. 2009. Antibodies and lectins in glycan analysis. In: Varki A, Cummings RD, Esko JD, editors. Essentials of glycobiology. 2nd ed. Cold Spring Harbor (NY): Cold Spring Harbor Laboratory Press. Chapter 45. [PubMed] [Google Scholar]
- do Nascimento AS, Serna S, Beloqui A, Arda A, Sampaio AH, Walcher J, Ott D, Unverzagt C, Reichardt NC, Jimenez-Barbero J et al. 2015. Algal lectin binding to core (α1-6) fucosylated N-glycans: structural basis for specificity and production of recombinant protein. Glycobiology. 25:607–616. [DOI] [PubMed] [Google Scholar]
- Dulbecco R, Vogt M. 1954. Plaque formation and isolation of pure lines with poliomyelitis viruses. J Exp Med. 99:167–182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egashira Y, Suganuma M, Kataoka Y, Higa Y, Ide N, Morishita K, Kamada Y, Gu J, Fukagawa K, Miyoshi E. 2019. Establishment and characterization of a fucosylated α-fetoprotein-specific monoclonal antibody: a potential application for clinical research. Sci Rep. 9:12359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher P, Spencer H, Thomas-Oates J, Wood AJ, Ungar D. 2019a. Modeling glycan processing reveals Golgi-enzyme homeostasis upon trafficking defects and cellular differentiation. Cell Rep. 27:1231–1243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher P, Thomas-Oates J, Wood AJ, Ungar D. 2019b. The N-glycosylation processing potential of the mammalian Golgi apparatus. Front Cell Dev Biol. 7:157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freeze HH. 2006. Genetic defects in the human glycome. Nat Rev Genet. 7:537–551. [DOI] [PubMed] [Google Scholar]
- Groux-Degroote S, Wavelet C, Krzewinski-Recchi MA, Portier L, Mortuaire M, Mihalache A, Trinchera M, Delannoy P, Malagolini N, Chiricolo M et al. 2014. B4GALNT2 gene expression controls the biosynthesis of Sda and sialyl Lewis X antigens in healthy and cancer human gastrointestinal tract. Int J Biochem Cell Biol. 53:442–449. [DOI] [PubMed] [Google Scholar]
- Gu J, Isaji T, Xu Q, Kariya Y, Gu W, Fukuda T, Du Y. 2012. Potential roles of N-glycosylation in cell adhesión. Glycoconj J. 29:599–607. [DOI] [PubMed] [Google Scholar]
- Haab BB, Yue T. 2011. High-throughput studies of protein glycoforms using antibody-lectin sandwich arrays. Methods Mol Biol. 785:223–236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heimburg-Molinaro J, Song X, Smith DF, Cummings RD. 2011. Preparation and analysis of glycan microarrays. Curr Protoc Protein Sci. Chapter 12: Unit12.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heimburg-Molinaro J, Tappert M, Song X, Lasanajak Y, Air G, Smith DF, Cummings RD. 2012. Probing virus-glycan interactions using glycan microarrays. Mets Mol Biol. 808:251–267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holst S, Deuss AJ, van Pelt GW, van Vliet SJ, Garcia-Vallejo JJ, Koeleman CA, Deelder AM, Mesker WE, Tollenaar RA, Rombouts Y et al. 2016. N-glycosylation profiling of colorectal cancer cell lines reveals association of fucosylation with differentiation and caudal type homebox 1 (CDX1)/villin mRNA expression. Mol Cell Proteomics. 15:124–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honma R, Kinoshita I, Miyoshi E, Tomaru U, Matsuno Y, Shimizu Y, Takeuchi S, Kobayashi Y, Kaga K, Taniguchi N et al. 2015. Expression of fucosyltransferase 8 is associated with an unfavorable clinical outcome in non-small cell lung cancers. Oncology. 88:298–308. [DOI] [PubMed] [Google Scholar]
- Houser J, Komarek J, Kostlanova N, Cioci G, Varrot A, Kerr SC, Lahmann M, Balloy V, Fahy JV, Chignard M. 2013. A soluble fucose-specific lectin from Aspergillus fumigatus conidia—structure, specificity and possible role in fungal pathogenicity. PLoS One. 8:e83077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang G, Li Z, Li Y, Liu G, Sun S, Gu J, Kameyama A, Li W, Dong W. 2020. Loss of core fucosylation in both ST6GAL1 and its substrate enhances glycoprotein sialylation in mice. Biochem J. 477:1179–1201. [DOI] [PubMed] [Google Scholar]
- Ichikawa E, Kuriyama S, Yuji J, Masaki T, Uchida N, Nishioka M, Taketa K. 2006. Further resolution of alpha-fetoprotein glycoforms by two-dimensional isoelectric focusing and lectin affinity electrophoresis. Electrophoresis. 27:3480–3487. [DOI] [PubMed] [Google Scholar]
- Iftekhar S, Ovbude ST, Hage DS. 2019. Kinetic analysis by affinity chromatography. Front Chem. 7:673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itai S, Ohishi T, Kaneko MK, Yamada S, Abe S, Nakamura T, Yanaka M, Chang YW, Ohba SI, Nishioka Y et al. 2018. Anti-podocalyxin antibody exerts antitumor effects via antibody-dependent cellular cytotoxicity in mouse xenograft models of oral squamous cell carcinoma. Oncotarget. 9:22480–22497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- IUPAC . 1997. In: McNaught AD, Wilkinson A, editors. Compendium of Chemical Terminology. 2nd ed. The "Gold Book". Oxford (UK): Blackwell Scientific Publications. [Google Scholar]
- Javaud C, Dupuy F, Maftah A, Julien R, Petit JM. 2003. The fucosyltransferase gene family: an amazing summary of the underlying mechanisms of gene evolution. Genetica. 118:157–170. [PubMed] [Google Scholar]
- Javaud C, Dupuy F, Maftah A, Michalski JC, Oriol R, Petit JM, Julien R. 2000. Ancestral exonic organization of FUT8, the gene encoding the alpha6-fucosyltransferase, reveals successive peptide domains which suggest a particular three-dimensional core structure for the alpha6-fucosyltransferase family. Mol Biol Evol. 17:1661–1672. [DOI] [PubMed] [Google Scholar]
- Jia L, Zhang J, Ma T, Guo Y, Yu Y, Cui J. 2018. The function of fucosylation in progression of lung cancer. Front Oncol. 8:565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang XN, Dang YF, Gong FL, Guo XL. 2019. Role and regulation mechanism of Gal-3 in non-small cell lung cancer and its potential clinical therapeutic significance. Chem Biol Interact. 309:108724. [DOI] [PubMed] [Google Scholar]
- Johnson PJ, Poon TC, Hjelm NM, Ho CS, Ho SK, Welby C, Stevenson D, Patel T, Parekh R, Townsend RR. 1999. Glycan composition of serum alpha-fetoprotein in patients with hepatocellular carcinoma and non-seminomatous germ cell tumour. Br J Cancer. 81:1188–1195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson PJ, Poon TC, Hjelm NM, Ho CS, Blake C, Ho SK. 2000. Structures of disease-specific serum alpha-fetoprotein isoforms. Br J Cancer. 83:1330–1337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaprio T, Satomaa T, Heiskanen A, Hokke CH, Deelder AM, Mustonen H, Hagström J, Carpen O, Saarinen J, Haglund C. 2015. N-glycomic profiling as a tool to separate rectal adenomas from carcinomas. Mol Cell Proteomics. 14:277–288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasai K. 2014. Frontal affinity chromatography: a unique research tool for biospecific interaction that promotes glycobiology. Proc Jpn Acad Ser B Phys Biol Sci. 90:215–234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinoshita N, Ohno M, Nishiura T, Fujii S, Nishikawa A, Kawakami Y, Uozumi N, Taniguchi N. 1991. Glycosylation at the fab portion of myeloma immunoglobulin G and increased fucosylated biantennary sugar chains: structural analysis by high-performance liquid chromatography and antibody-lectin enzyme immunoassay using Lens culinaris agglutinin. Cancer Res. 51:5888–5892. [PubMed] [Google Scholar]
- Kobayashi Y, Tateno H, Dohra H, Moriwaki K, Miyoshi E, Hirabayashi J, Kawagishi H. 2012. A novel core fucose-specific lectin from the mushroom Pholiota squarrosa. J Biol Chem. 287:33973–33982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kornfeld K, Reitman ML, Kornfeld R. 1981. The carbohydrate-binding specificity of pea and lentil lectins. Fucose is an important determinant. J Biol Chem. 256:6633–6640. [PubMed] [Google Scholar]
- Kurimoto A, Kitazume S, Kizuka Y, Nakajima K, Oka R, Fujinawa R, Korekane H, Yamaguchi Y, Wada Y, Taniguchi N. 2014. The absence of core fucose up-regulates GnT-III and Wnt target genes: a possible mechanism for an adaptive response in terms of glycan function. J Biol Chem. 289:11704–11714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli UK. 1970. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 227:680–685. [DOI] [PubMed] [Google Scholar]
- Landry JP, Fei Y, Zhu X. 2012. Simultaneous measurement of 10,000 protein-ligand affinity constants using microarray-based kinetic constant assays. Assay Drug Dev Technol. 10:250–259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landry JP, Ke Y, Yu G-L, Zhu XD. 2015. Measuring affinity constants of 1,450 monoclonal antibodies to peptide targets with a microarray-based label-free assay platform. J Immunol Methods. 417:86–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larsen RD, Ernst LK, Nair RP, Lowe JB. 1990. Molecular cloning, sequence, and expression of a human GDP-L-fucose:Beta-Dgalactoside 2-alpha-L-fucosyltransferase cDNA that can form the H blood group antigen. Proc Natl Acad Sci USA. 87:6674–6678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee A, Nakano M, Hincapie M, Kolarich D, Baker MS, Hancock WS, Packer NH. 2010. The lectin riddle: glycoproteins fractionated from complex mixtures have similar glycomic profiles. OMICS. 14:487–499. [DOI] [PubMed] [Google Scholar]
- Leerapun A, Suravarapu SV, Bida JP, Clark RJ, Sanders EL, Mettler TA, Stadheim LM, Aderca I, Moser CD, Nagorney DM et al. 2007. The utility of Lens culinaris agglutinin-reactive alpha-fetoprotein in the diagnosis of hepatocellular carcinoma: evaluation in a United States referral population. Clin Gastroenterol Hepatol. 5:394–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li F, Zhao S, Cui Y, Guo T, Qiang J, Xie Q, Yu W, Guo W, Deng W, Gu C et al. 2020. α1,6-Fucosyltransferase (FUT8) regulates the cancer-promoting capacity of cancer-associated fibroblasts (CAFs) by modifying EGFR core fucosylation (CF) in non-small cell lung cancer (NSCLC). Am J Cancer Res. 10:816–837. [PMC free article] [PubMed] [Google Scholar]
- Liu Y, McBride R, Stoll M, Palma AS, Silva L, Agravat S, Aoki-Kinoshita KF, Campbell MP, Costello CE, Dell A et al. 2017. The minimum information required for a glycomics experiment (MIRAGE) project: improving the standards for reporting glycan microarray-based data. Glycobiology. 27:280–284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loriol C, Dupuy F, Rampal R, Dlugosz MA, Haltiwanger RS, Maftah A, Germot A. 2006. Molecular evolution of protein O-fucosyltransferase genes and splice variants. Glycobiology. 16:736–747. [DOI] [PubMed] [Google Scholar]
- Martín del Valle EM, Galán MA. 2002. Effect of the spacer arm in affinity chromatography: Determination of adsorption characteristics and flow rate effect. Ind Eng Chem Res. 41:2296–2304. [Google Scholar]
- Matsumoto I, Osawa T. 1969. Purification and characterization of an anti-H(O) phytohemagglutinin of Ulex europeus. Biochim Biophys Acta. 194:180–189. [DOI] [PubMed] [Google Scholar]
- Matsumura K, Higashida K, Hata Y, Kominami J, Nakamura-Tsuruta S, Hirabayashi J. 2009. Comparative analysis of oligosaccharide specificities of fucose-specific lectins from Aspergillus oryzae and Aleuria aurantia using frontal affinity chromatography. Anal Biochem. 386:217–221. [DOI] [PubMed] [Google Scholar]
- Matsumura K, Higashida K, Ishida H, Hata Y, Yamamoto K, Shigeta M, Mizuno-Horikawa Y, Wang X, Miyoshi E, Gu J et al. 2007. Carbohydrate binding specificity of a fucose-specific lectin from Aspergillus oryzae: a novel probe for core fucose. J Biol Chem. 282:15700–15708. [DOI] [PubMed] [Google Scholar]
- Maupin KA, Liden D, Haab BB. 2012. The fine specificity of mannose-binding and galactose-binding lectins revealed using outlier motif analysis of glycan array data. Glycobiology. 22:160–169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarter C, Kletter D, Tang H, Partyka K, Ma Y, Singh S, Yadav J, Bern M, Haab BB. 2013. Prediction of glycan motifs using quantitative analysis of multi-lectin binding: motifs on MUC1 produced by cultured pancreatic cancer cells. Proteomics Clin Appl. 7:632–641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehta A, Comunale MA, Rawat S, Casciano JC, Lamontagne J, Herrera H, Ramanathan A, Betesh L, Wang M, Norton P et al. 2016. Intrinsic hepatocyte dedifferentiation is accompanied by upregulation of mesenchymal markers, protein sialylation and core alpha 1,6 linked fucosylation. Sci Rep. 6:27965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Millen SH, Lewallen DM, Herr AB, Iyer SS, Weiss AA. 2010. Identification and characterization of the carbohydrate ligands recognized by pertussis toxin through glycan microarray and surface plasmon resonance. Biochemistry. 49:5954–5967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyoshi E, Moriwaki K, Terao N, Tan CC, Terao M, Nakagawa T, Matsumoto H, Shinzaki S, Kamada Y. 2012. Fucosylation is a promising target for cancer diagnosis and therapy. Biomolecules. 2:34–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mollicone R, Moore SE, Bovin N, Garcia-Rosasco M, Candelier JJ, Martinez-Duncker I, Oriol R. 2009. Activity, splice variants, conserved peptide motifs, and phylogeny of two new alpha1,3-fucosyltransferase families (FUT10 and FUT11). J Biol Chem. 284:4723–4738. [DOI] [PubMed] [Google Scholar]
- Moloney DJ, Shair LH, Lu FM, Xia J, Locke R, Matta KL, Haltiwanger RS. 2000. Mammalian Notch1 is modified with two unusual forms of O-linked glycosylation found on epidermal growth factor-like modules. J Biol Chem. 275:9604–9611. [DOI] [PubMed] [Google Scholar]
- Montreuil J, Debray P, Debeire P, Delannoy P. 1983. Lectins as oligosaccharide receptors. In: Popper H, Reutter W, Kottgen E, Gudat F, editors. Structural Carbohydrates in the Liver. Boston, The Hauge, Dordrecht, Lancaster: MTP Press Limited. p. 239–258. [Google Scholar]
- Muinelo-Romay L, Vázquez-Martín C, Villar-Portela S, Cuevas E, Gil-Martín E, Fernández-Briera A. 2008. Expression and enzyme activity of alpha(1,6)fucosyltransferase in human colorectal cancer. In J Cancer. 123:641–646. [DOI] [PubMed] [Google Scholar]
- Muinelo-Romay L, Villar-Portela S, Cuevas E, Gil-Martín E, Fernández-Briera A. 2011a. α(1,6)fucosyltransferase expression is an independent prognostic factor for disease-free survival in colorectal carcinoma. Hum Pathol. 42:1740–1750. [DOI] [PubMed] [Google Scholar]
- Muinelo-Romay L, Villar-Portela S, Cuevas E, Gil-Martín E, Fernández-Briera A. 2011b. Identification of α(1,6)fucosylated proteins differentially expressed in human colorectal cancer. BMC Cancer. 11:508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mun JY, Lee KJ, Kim YJ, Kwon O, Kim SJ, Lee SG, Park WS, Heo WD, Oh DB. 2012. Development of fluorescent probes for the detection of fucosylated N-glycans using an Aspergillus oryzae lectin. Appl Microbiol Biotechnol. 93:251–260. [DOI] [PubMed] [Google Scholar]
- Nakagawa T, Miyoshi E, Yakushijin T, Hiramatsu N, Igura T, Hayashi N, Taniguchi N, Kondo A. 2008. Glycomic analysis of α-fetoprotein L3 in hepatoma cell lines and hepatocellular carcinoma patients. J Proteome Res. 7:2222–2233. [DOI] [PubMed] [Google Scholar]
- Nakayama K, Moriwaki K, Imai T, Shinzaki S, Kamada Y, Murata K, Miyoshi E. 2013. Mutation of GDP-mannose-4,6-dehydratase in colorectal cancer metastasis. PLoS One. 8:e70298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakayama K, Wakamatsu K, Fujii H, Shinzaki S, Takamatsu S, Kitazume S, Kamada Y, Takehara T, Taniguchi N, Miyoshi E. 2019. Core fucose is essential glycosylation for CD14-dependent toll-like receptor 4 and toll-like receptor 2 signalling in macrophages. J Biochem. 165:227–237. [DOI] [PubMed] [Google Scholar]
- Noble P, Spendlove I, Harding S, Parsons T, Durrant LG. 2013. Therapeutic targeting of Lewis(y) and Lewis(b) with a novel monoclonal antibody 692/29. PLoS One. 8:e54892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nyström K, Grahn A, Lindh M, Brytting M, Mandel U, Larson G, Olofsson S. 2007. Virus-induced transcriptional activation of host FUT genes associated with neo-expression of ley in cytomegalovirus-infected and sialyl-Lex in varicella-zoster virus-infected diploid human cells. Glycobiology. 17:355–366. [DOI] [PubMed] [Google Scholar]
- Oda Y, Senaha T, Matsuno Y, Nakajima K, Naka R, Kinoshita M, Honda E, Furuta I, Kakehi K. 2003. A new fungal lectin recognizing alpha(1-6)-linked fucose in the N-glycan. J Biol Chem. 278:32439–32447. [DOI] [PubMed] [Google Scholar]
- Okuyama S, Nakamura-Tsuruta S, Tateno H, Hirabayashi J, Matsubara K, Hori K. 2009. Strict binding specificity of small-sized lectins from the red alga Hypnea japonica for core (alpha1-6) fucosylated N-glycans. Biosci Biotechnol Biochem. 73:912–920. [DOI] [PubMed] [Google Scholar]
- Park S, Lim JM, Chun JN, Lee S, Kim TM, Kim DW, Kim SY, Bae DJ, Bae SM, So I et al. 2020. Altered expression of fucosylation pathway genes is associated with poor prognosis and tumor metastasis in non-small cell lung cancer. Int J Oncol. 56:559–567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reiding KR, Blank D, Kuijper DM, Deelder AM, Wuhrer M. 2014. High-throughput profiling of protein N-glycosylation by MALDI-TOF-MS employing linkage-specific sialic acid esterification. Anal Chem. 86:5784–5793. [DOI] [PubMed] [Google Scholar]
- Rouwendal GJ, Wuhrer M, Florack DE, Koeleman CA, Deelder AM, Bakker H, Stoopen GM, van Die I, Helsper JP, Hokke CH et al. 2007. Efficient introduction of a bisecting GlcNAc residue in tobacco N-glycans by expression of the gene encoding human N-acetylglucosaminyltransferase III. Glycobiology. 17:334–444. [DOI] [PubMed] [Google Scholar]
- Saldova R, Wormald MR, Dwek RA, Rudd PM. 2008. Glycosylation changes on serum glycoproteins in ovarian cancer may contribute to disease pathogenesis. Dis Markers. 25:219–232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saldova R, Fan Y, Fitzpatrick JM, Watson RW, Rudd PM. 2011. Core fucosylation and alpha2-3 sialylation in serum N-glycome is significantly increased in prostate cancer comparing to benign prostate hyperplasia. Glycobiology. 21:195–205. [DOI] [PubMed] [Google Scholar]
- Schiel JE, Hage DS. 2009. Kinetic studies of biological interactions by affinity chromatography. J Sep Sci. 32:1507–1522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schindelin J, Arganda-Carreras I, Frise E. 2012. Fiji: an open-source platform for biological-image analysis. Nat Methods. 9:676–682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schreiber G. 2002. Kinetic studies of protein-protein interactions. Curr Opin Struct Biol. 12:41–47. [DOI] [PubMed] [Google Scholar]
- Selman MH, Hemayatkar M, Deelder AM, Wuhrer M. 2011. Cotton HILIC SPE microtips for microscale purification and enrichment of glycans and glycopeptides. Anal Chem. 83:2492–2499. [DOI] [PubMed] [Google Scholar]
- Serna S, Yan S, Martin-Lomas M, Wilson IB, Reichardt NC. 2011. Fucosyltransferases as synthetic tools: glycan array-based substrate selection and core fucosylation of synthetic N-glycans. J Am Chem Soc. 133:16495–16502. [DOI] [PubMed] [Google Scholar]
- Shergalis A, Bankhead A 3rd, Luesakul U, Muangsin N, Neamati N. 2018. Current challenges and opportunities in treating glioblastoma. Pharmacol Rev. 70:412–445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sokolowski T, Peters T, Pérez S, Imberty A. 1997. Conformational analysis of biantennary glycans and molecular modeling of their complexes with lentil lectin. J Mol Graph Model. 15:37–42 54. [DOI] [PubMed] [Google Scholar]
- Spiro RG. 2002. Protein glycosylation: nature, distribution, enzymatic formation, and disease implications of glycopeptide bonds. Glycobiology. 12:43–56. [DOI] [PubMed] [Google Scholar]
- Srikrishna G, Varki NM, Newell PC, Varki A, Freeze HH. 1997. An IgG monoclonal antibody against Dictyostelium discoideum glycoproteins specifically recognizes Fucα1,6GlcNAcβ in the core of N-linked glycans—localized expression of core-fucosylated glycoconjugates in human tissues. J BiolChem. 272:25743–25752. [DOI] [PubMed] [Google Scholar]
- Sundin M, Ringdén O, Sundberg B, Nava S, Götherström C, Le Blanc K. 2007. No alloantibodies against mesenchymal stromal cells, but presence of anti-fetal calf serum antibodies, after transplantation in allogeneic hematopoietic stem cell recipients. Haematologica. 92:1208–1215. [DOI] [PubMed] [Google Scholar]
- Taketa K. 1990. Alpha-fetoprotein: reevaluation in hepatology. Hepatology. 12:1420–1432. [DOI] [PubMed] [Google Scholar]
- Taketa K, Sekiya C, Namiki M, Akamatsu K, Ohta Y, Endo Y, Kosaka K. 1990. Lectin-reactive profiles of alpha-fetoprotein characterizing hepatocellular carcinoma and related conditions. Gastroenterology. 99:508–518. [DOI] [PubMed] [Google Scholar]
- Tateno H, Nakamura-Tsuruta S, Hirabayashi J. 2007. Frontal affinity chromatography: sugar-protein interactions. Nat Protoc. 2:2529–2537. [DOI] [PubMed] [Google Scholar]
- Tateno H, Nakamura-Tsuruta S, Hirabayashi J. 2009. Comparative analysis of core-fucose-binding lectins from Lens culinaris and Pisum sativum using frontal affinity chromatography. Glycobiology. 19:527–536. [DOI] [PubMed] [Google Scholar]
- Torrado J, Correa P, Ruiz B, Bernardi P, Zavala D, Bara J. 1992. Lewis antigen alterations in gastric cancer precursors. Gastroenterology. 102:424–430. [DOI] [PubMed] [Google Scholar]
- Tu CF, Wu MY, Lin YC, Kannagi R, Yang RB. 2017. FUT8 promotes breast cancer cell invasiveness by remodeling TGF-β receptor core fucosylation. Breast Cancer Res. 19:111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanák J, Drímalová D, Smyslová O, Nĕmec M, Viklický V, Wisniewski K. 1989. Detection of blood group a antigen expression in human colon cancer using monoclonal antibodies with different specificities. Neoplasma. 36:479–488. [PubMed] [Google Scholar]
- Villar-Portela S, Muinelo-Romay L, Cuevas E, Gil-Martín E, Fernández-Briera A. 2013. FX enzyme and GDP-L-Fuc transporter expression in colorectal cancer. Histopathology. 63:174–186. [DOI] [PubMed] [Google Scholar]
- Wang M, Wang J, Kong X, Chen H, Wang Y, Qin M, Lin Y, Chen H, Xu J, Hong J et al. 2014a. MiR-198 represses tumor growth and metastasis in colorectal cancer by targeting fucosyl transferase 8. Sci Rep. 4:6145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang S, Zhang X, Yang C, Xu S. 2019. MicroRNA-198-5p inhibits the migration and invasion of non-small lung cancer cells by targeting fucosyltransferase 8. Clin Exp Pharmacol Physiol. 46:955–967. [DOI] [PubMed] [Google Scholar]
- Wang X, Chen J, Li QK, Peskoe SB, Zhang B, Choi C, Platz EA, Zhang H. 2014b. Overexpression of α (1,6) fucosyltransferase associated with aggressive prostate cancer. Glycobiology. 24:935–944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Fukuda T, Isaji T, Lu J, Im S, Hang Q, Gu W, Hou S, Ohtsubo K, Gu J. 2015. Loss of α1,6-fucosyltransferase inhibits chemical-induced hepatocellular carcinoma and tumorigenesis by down-regulating several cell signaling pathways. FASEB J. 29:3217–3227. [DOI] [PubMed] [Google Scholar]
- Wong RJ, Ahmed A, Gish RG. 2015. Elevated alpha-fetoprotein: differential diagnosis—hepatocellular carcinoma and other disorders. Clin Liver Dis. 19:309–323. [DOI] [PubMed] [Google Scholar]
- Xu C, Ng DT. 2015. Glycosylation-directed quality control of protein folding. Nat Rev Mol Cell Biol. 16:742–752. [DOI] [PubMed] [Google Scholar]
- Yamasaki K, Kubota T, Yamasaki T, Nagashima I, Shimizu H, Terada RI, Nishigami H, Kang J, Tateno M, Tateno H. 2019. Structural basis for specific recognition of core fucosylation in N-glycans by Pholiota squarrosa lectin (PhoSL). Glycobiology. 29:576–587. [DOI] [PubMed] [Google Scholar]
- Yan L, Wilkins PP, Alvarez-Manilla G, Do SI, Smith DF, Cummings RD. 1997. Immobilized Lotus tetragonolobus agglutinin binds oligosaccharides containing the Le(x) determinant. Glycoconj J. 14:45–55. [DOI] [PubMed] [Google Scholar]
- Yang Q, Zhang R, Cai H, Wang LX. 2017. Revisiting the substrate specificity of mammalian α1,6-fucosyltransferase reveals that it catalyzes core fucosylation of N-glycans lacking α1,3-arm GlcNAc. J Biol Chem. 292:14796–14803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu Y, Mishra S, Song X, Lasanajak Y, Bradley KC, Tappert MM, Air GM, Steinhauer DA, Halder S, Cotmore S et al. 2012. Functional glycomic analysis of human milk glycans reveals the presence of virus receptors and embryonic stem cell biomarkers. J Biol Chem. 287:44784–44799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zafar S, Nasir A, Bokhari H. 2011. Computational analysis reveals abundance of potential glycoproteins in archaea, bacteria and Eukarya. Bioinformation. 6:352–355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao YP, Xu XY, Fang M, Wang H, You Q, Yi CH, Ji J, Gu X, Zhou PT, Cheng C et al. 2014. Decreased core-fucosylation contributes to malignancy in gastric cancer. PLoS One. 9:e94536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao YY, Takahashi M, Gu JG, Miyoshi E, Matsumoto A, Kitazume S, Taniguchi N. 2008. Functional roles of N-glycans in cell signaling and cell adhesion in cancer. Cancer Sci. 99:1304–1310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou W, Ma H, Deng G, Tang L, Lu J, Chen X. 2017. Clinical significance and biological function of fucosyltransferase 2 in lung adenocarcinoma. Oncotarget. 8:97246–97259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu F, Clemmer DE, Trinidad JC. 2016. Characterization of lectin binding affinities via direct LC–MS profiling: implications for glycopeptide enrichment and separation strategies. Analyst. 142:6574. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


