Abstract
Background
Tenecteplase has higher fibrin specificity with a longer half-life and the potential to achieve higher rates of recanalization than alteplase. A critical limitation of tenecteplase is no commercial use in Japan and no experience with its administration to Japanese patients.
Hypothesis
Tenecteplase is superior to alteplase in achieving recanalization on the initial angiogram when administered ≤4.5-hour of stroke onset in patients planned for mechanical thrombectomy (MT) in Japan where alteplase at the unique dose of 0.6mg/kg is officially used.
Methods
The Tenecteplase versus alteplase For LArge Vessel Occlusion Recanalization (T-FLAVOR) trial is an investigator-initiated, phase II, multicenter, prospective, randomized, open-label, masked-endpoint, superiority study. Eligibility criteria include acute ischemic stroke with pre-stroke modified Rankin Scale score ≤3 and large vessel occlusion (internal carotid artery, middle cerebral artery, or basilar artery) eligible for intravenous thrombolysis ≤4.5-hour and MT ≤6-hour of stroke onset. After completing the safety confirmation phase involving three patients who received non-masked tenecteplase (0.25 mg/kg), 220 patients will be randomized to two groups (1:1), intravenous alteplase (0.6mg/kg, n = 110) or tenecteplase (0.25mg/kg, n = 110), prior to MT.
Outcomes
In the safety confirmation phase, the primary outcome is symptomatic intracranial hemorrhage (sICH) ≤24-36-hour. In the randomized, comparative phase, the primary efficacy outcome is substantial angiographic reperfusion (mTICI grade 2b/2c/3) or absence of retrievable thrombus on the initial angiogram. The primary safety outcome is sICH ≤24-36-hour and 90-day mortality.
Discussion
T-FLAVOR may help determine if tenecteplase should be recommended as a routine clinical strategy before MT for Japanese stroke patients.
Trial registration
jRCTs051210055
Keywords: Ischemic stroke, thrombolysis, tissue plasminogen activator, tenecteplase, alteplase, mechanical thrombectomy, randomized trial
Introduction and rationale
Stroke thrombolysis with alteplase before mechanical thrombectomy (MT) often fails to recanalize large vessel occlusion. The rates of recanalization were reportedly 4% for the internal carotid artery (ICA), 32.3% for the M1-middle cerebral artery (MCA), and 30.8% for the M2-MCA after alteplase 0.9 mg/kg, 1 and 48.8% for the M1 and 62.5% for the M2 after alteplase 0.6 mg/kg. 2
Tenecteplase is a genetically modified form of alteplase, with higher fibrin specificity and a longer half-life, and is more resistant to plasminogen activator inhibitor than alteplase. Tenecteplase was reported to have higher recanalization rates, better early neurological improvement, and be at least as safe as alteplase.3–5 A higher pre-MT recanalization rate, reported in EXTEND-IA TNK, 5 would reduce the need for MT and improve patient outcomes. Even partial recanalization with IVT could allow faster successful recanalization with MT and reduce the risk of infarct growth and hemorrhagic transformation. The single bolus administration of tenecteplase offers substantial practical advantages over the 1-hour infusion of alteplase.
No drug company has or intends to obtain the license to supply tenecteplase in Japan. To gain support from the government by showing data on the effects of tenecteplase in a certain number of Japanese stroke patients, this investigator-initiated trial is planned.
Study purpose
To test the hypothesis that tenecteplase 0.25 mg/kg is superior to alteplase 0.6 mg/kg, the standard dose in Japan, in achieving recanalization on the initial angiogram when administered within 4.5 hours of stroke onset in patients planned for MT.
Methods and design
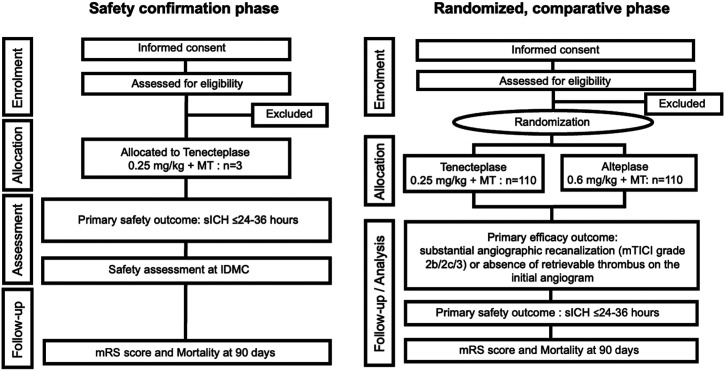
Tenecteplase versus alteplase For LArge Vessel Occlusion Recanalization (T-FLAVOR) trial is an investigator-initiated, phase II, multicenter, prospective, randomized, open-label, masked-endpoint, superiority study. Figure 1 shows the study flow chart. Trial methods are basically similar to those of EXTEND-IA TNK, 5 partly to facilitate integrated analysis of the trials.
Figure 1.
Study assessment flow chart. AEs, adverse events; ICH, intracranial hemorrhage; IDMC, independent data monitoring committee; mTICI, modified treatment in cerebral infarction; mRS, modified Rankin Scale; MT, mechanical thrombectomy; sICH, symptomatic intracranial hemorrhage; NIHSS, National Institutes of Health Stroke Scale
Patient population
Inclusion criteria
1. Acute ischemic stroke
2. Age ≥ 20 years
3. Eligible to start IVT within 4.5 hours of stroke onset
4. Arterial occlusion on CTA or MRA of the ICA, M1, M2, or basilar artery
5. Eligible to start arterial puncture for MT within 6 hours of stroke onset
6. Written, informed consent obtained from patient or proxies
Exclusion criteria
1. Pre-stroke modified Rankin Scale (mRS) score ≥ 4
2. >4.5 hours from stroke onset or last known well time
3. Contraindication to contrast agents
4. Any terminal illness with expected survival of less than 1 year
5. Breastfeeding or possibly pregnant
6. Participating or planning to participate in other clinical trials during the trial period
7. Considered ineligible for trial enrollment by the investigators
Intervention
Japan has no experience using tenecteplase. Therefore, the safety confirmation phase, when non-masked tenecteplase using 0.25 mg/kg is administered to three patients, precedes the main trial; the numbers followed the policy of the Advanced Medical Technology Development authentication system by the Ministry of Health, Labour and Welfare, Japan.
In the randomized, comparative phase, 220 patients will be randomized in a 1:1 ratio using a web-based randomization system to two groups, intravenous tenecteplase (0.25 mg/kg, maximum 25 mg, administered as a bolus over approximately 10 s) or intravenous alteplase (at the standard dose in Japan, 0.6 mg/kg, up to a maximum of 60 mg, 10% as bolus and the remainder infused over 1 hour), before MT. All patients will be transferred to the angio-suite after standard imaging for final vessel assessment. Written, informed consent will be obtained from all eligible patients or from a legal representative.
Clinical and radiological assessments
Neurological severity will be measured using the National Institutes of Health Stroke Scale (NIHSS) before randomization and 24–36 hours and 72 hours after stroke onset. The mRS will be assessed at 72 hours and at 90 days.
In addition to the initial MRI/CT prior to randomization, follow-up MRI will be performed after 18–30 hours to identify intracranial hemorrhage (ICH) and final infarct volume. Recanalization on the initial catheter angiogram will be assessed using the modified Thrombolysis In Cerebral Infarction (mTICI) scale with the primary outcome if there is recanalization of > 50% of the affected territory (mTICI 2b/2c/3) 6 or the absence of retrievable thrombus. Symptomatic ICH (sICH) is defined as parenchymal hematoma type 2 within 36 hours of treatment combined with a ≥4-point increase in the NIHSS score (representing substantial neurological deterioration). 7 Radiological outcome measures, including recanalization on the initial catheter angiography, will be centrally analyzed, masked to treatment allocation.
Primary and secondary outcomes
Efficacy and safety end-points are listed in Table 1 (simplified) and the supplemental material (complete).
Table 1.
Efficacy and safety assessment (simplified).
| Primary efficacy outcome |
| Substantial angiographic recanalization (mTICI 2b/2c/3) or absence of retrievable thrombus on the initial angiogram |
| Secondary efficacy outcomes |
| ≥8 points reduction in NIHSS score or reaching 0–1 at 72 hours after thrombolysis |
| Modified Rankin Scale (mRS) score at 90 days |
| Safety outcome |
| sICH within 24–36 hours |
| Major bleeding within 90 days |
| Mortality at 90 days |
| SAEs within 90 days |
In the safety confirmation phase, the above four safety outcomes plus severe adverse events (SAEs) and symptomatic intracranial hemorrhage (sICH) within 72 hours will be assessed.
mTICI, modified treatment in cerebral infarction; NIHSS, National Institutes of Health Stroke Scale
Data monitoring
Central data monitoring is conducted with a validated electronic data capture system. On-site or remote monitoring including source document verification will be conducted as necessary.
Data and safety monitoring board
An independent data monitoring committee (IDMC) will oversee the conduct of the trial. In the safety confirmation phase, all safety end-points will be mandatorily analyzed for each patient. The principal investigator evaluates the safety outcome within 72 hours for each patient and reports to the IDMC. When the IDMC approves the safety of tenecteplase for all 3 patients, the steering committee will proceed to the randomized, comparative phase (Figure 1). In the randomized, comparative phase, an interim safety evaluation will be conducted by the IDMC at the time of enrollment of 100 patients. If there are concerns about participant safety, the IDMC will make a recommendation to the steering committee about continuing, stopping, or modifying the trial.
Sample size estimates
Pre-angiographic recanalization rates of 24% in the tenecteplase arm and 10% in the alteplase arm were estimated based on the reported rates (22% and 10%, respectively) in EXTEND-IA TNK. 5 An estimated sample size of 99 patients in each treatment arm had 80% power to detect a significant difference of angiographic recanalization (mTICI 2b/2c/3) on the initial angiogram at a two-sided significance threshold of p = 0.1. Accounting for 10% possible treatment failures, protocol violations, and dropouts, a total of 220 patients (110 per treatment arm) will be recruited.
Statistical analyses
The analysis of the primary efficacy outcome includes both full-analysis and per-protocol sets. Superiority of tenecteplase is established if the lower end of the above 95% confidence interval for the proportion of patients with angiographic recanalization is greater than zero. The analysis of safety outcomes includes the safety analysis set, and the proportion of patients with sICH and mortality will be compared between the tenecteplase and alteplase arms using binary logistic regression analysis. An NIHSS score reduction of ≥8 points or reaching 0–1 at 72 hours after thrombolysis will be compared between the two arms adjusted for age and baseline NIHSS score using binary logistic regression analysis. Secondary and tertiary outcome analyses will be carried out according to standard statistical principles for comparisons of parametric and nonparametric distributions, as appropriate.
Study organization and funding
T-FLAVOR is organized by central coordinating centers located at the National Cerebral and Cardiovascular Center (NCVC) and Kyorin University and conducted in 13 other hospitals in Japan (supplemental material). The Advanced Medical Technology Development authentication system approved the trial in September, 2021. T-FLAVOR receives funding support from the Japan Agency for Medical Research and Development (21lk0201109h0002) and the Intramural Research Fund for Cardiovascular Diseases of NCVC (21-4-2).
Discussion
T-FLAVOR is a trial of stroke IVT for the Japanese population; thus, selection of the optimal dose considering racial background is important. Tenecteplase at a dose of 0.25 mg/kg was reportedly superior to 0.1 mg/kg in efficacy 8 and 0.4 mg/kg in safety,9,10 and there appears to be a great deal of its off-label use for stroke in western countries. 11
East Asian populations were in the minority in most comparative trials between tenecteplase and alteplase 0.9 mg/kg for acute ischemic stroke, although alteplase 0.6 mg/kg reportedly did not show the noninferiority with respect to death and disability at 90 days compared to alteplase 0.9 mg/kg. 12 The efficacy or safety of 0.25 mg/kg tenecteplase, a recommended dose in guidelines,13,14 should be verified in every racial group. The results of T-FLAVOR could have a major impact on acute stroke treatment in East Asia.
If we can prove that 0.25 mg/kg tenecteplase is safer than 0.6 mg/kg alteplase, the safety of 0.25 mg/kg tenecteplase compared to both standard and low-dose alteplase will be reinforced. Currently, low-dose of alteplase is in official use in Japan for stroke thrombolysis. However, if the results of the T-FLAVOR trial are as expected, globally-standard dose of tenecteplase will be available for use in stroke treatment in Japan. We can disseminate common and sharable information on stroke treatment from Japan to many other countries in near future.
Summary and Conclusions
T-FLAVOR will show whether tenecteplase is superior to alteplase in achieving recanalization on the initial angiogram when administered within 4.5 hours of ischemic stroke onset in patients planned for MT in Japan.
Acknowledgements
We would like to thank all participating hospitals and local investigators for contributing to T-FLAVOR.
Footnotes
Funding: T-FLAVOR trial receives funding support from the Japan Agency for Medical Research and Development (21lk0201109h0002) and the Intramural Research Fund for Cardiovascular Diseases of the National Cerebral and Cardiovascular Center (21-4-2).
Ethical approval: Ethical approval for this study was obtained from Kyoto University Certified Review Board (ref. CRB5180002).
Informed consent: Written informed consent is obtained for all subjects before the study
Guarantor: KTo
Contributorship: HK, KI, and KTa wrote the first version of the study protocol. All authors contributed to further protocol development. MI, MF, and HY contributed data collection/management and study coordination. KO planned statistical analysis. MK, NS and TN were involved in coordination of the study. MS contributed imaging analysis techniques advise/design. NH controlled study drug.TH and KTo contributed study conception, funding, study design, supervision, ethics and regulatory approvals, and protocol development. All authors reviewed and edited the manuscript and approved the final version of the manuscript.
ORCID iDs
Hiroyuki Kawano https://orcid.org/0000-0001-8497-3659
Nobuyuki Sakai https://orcid.org/0000-0002-3289-1210
References
- 1.Bhatia R, Hill MD, Shobha N, et al. Low rates of acute recanalization with intravenous recombinant tissue plasminogen activator in ischemic stroke. Stroke 2010; 41: 2254–2258. [DOI] [PubMed] [Google Scholar]
- 2.Hirano T, Sasaki M, Mori E, et al. Residual vessel length on magnetic resonance angiography identifies poor responders to alteplase in acute middle cerebral artery occlusion patients. Stroke 2010; 41: 2828–2833. [DOI] [PubMed] [Google Scholar]
- 3.Coutts SB, Berge E, Campbell BC, et al. Tenecteplase for the treatment of acute ischemic stroke: a review of completed and ongoing randomized controlled trials. International Journal of Stroke 2018; 13: 885–892. [DOI] [PubMed] [Google Scholar]
- 4.Vishnu VY, Padma Srivastava MV. Innovations in acute stroke reperfusion strategies. Annals of Indian Academy of Neurology 2019; 22: 6–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Campbell BCV, Mitchell PJ, Churilov L, et al. Tenecteplase versus alteplase before thrombectomy for ischemic stroke. New England Journal of Medicine 2018; 378: 1573–1582. [DOI] [PubMed] [Google Scholar]
- 6.Goyal M, Fargen KM, Turk AS, et al. 2C or not 2C: defining an improved revascularization grading scale and the need for standardization of angiography outcomes in stroke trials. Journal of NeuroInterventional Surgery 2014; 6: 83–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wahlgren N, Ahmed N, Dávalos A, et al. Thrombolysis with alteplase for acute ischaemic stroke in the safe implementation of thrombolysis in stroke-monitoring study (SITS-MOST): an observational study. The Lancet 2007; 369: 275–282. [DOI] [PubMed] [Google Scholar]
- 8.Persons M, Spratt N, Bivard A, et al. A randomized trial of tenecteplase versus alteplase for acute ischemic stroke. N Engl J Med 2012; 366: 1099–1107. [DOI] [PubMed] [Google Scholar]
- 9.Haley EC, Jr, Thompson JLP, Grotta JC, et al. Phase IIB/III trial of tenecteplase in acute ischemic stroke. Stroke 2010; 41: 707–711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Campbell BCV, Mitchell PJ, Churilov L, et al. Effect of intravenous tenecteplase dose on cerebral reperfusion before thrombectomy in patients with large vessel occlusion ischemic stroke. JAMA 2020; 323: 1257–1265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhong CS, Beharry J, Salazar D, et al. Routine use of tenecteplase for thrombolysis in acute ischemic stroke. Stroke 2021; 52: 1087–1090. [DOI] [PubMed] [Google Scholar]
- 12.Anderson CS, Robinson T, Lindley RI, et al. Low-dose versus standard-dose intravenous alteplase in acute ischemic stroke. New England Journal of Medicine 2016; 374: 2313–2323. [DOI] [PubMed] [Google Scholar]
- 13.Powers WJ, Rabinstein AA, Ackerson T, et al. Guidelines for the early management of patients with acute ischemic stroke: 2019 update to the 2018 guidelines for the early management of acute ischemic stroke: a guideline for healthcare professionals from the American heart association/American stroke association. Stroke 2019; 50: e344–e418. [DOI] [PubMed] [Google Scholar]
- 14.https://informme.org.au/en/Guidelines/Clinical-Guidelines-for-Stroke-Management