Abstract
The Taeniidae tapeworms are a family of helminths that have a similar life cycle, with intermediate hosts developing characteristic cysts in visceral organs. We describe here a case in Pennsylvania, USA, of fatal Versteria infection in a muskrat (Ondatra zibethicus), which, to our knowledge, has not been reported to develop disease associated with infection. Postmortem examination revealed widespread tissue loss and replacement by solid-bodied cestode larvae with minimal adjacent inflammation in many visceral organs, most severe in the lungs, liver, and brain. Key morphologic features via histology included cephalic structures and short rostellar hooklets, which are characteristic for the genus. Genetic characterization confirmed the cestode as being an undescribed lineage of Versteria that has been implicated as the cause of severe morbidity and mortality in humans and nonhuman primates in North America. Considering the zoonotic significance of this pathogen, our report expands on the limited literature regarding disease caused by Versteria and emphasizes the need to identify the causative tapeworm more accurately, especially in rodent intermediate hosts given that previous reports do not have molecular confirmation of species.
Keywords: cestode, intermediate host, muskrats, Taeniidae, Versteria, wildlife health
Taeniid tapeworms represent some of the most medically important helminths in human and veterinary medicine.9,17 Historically, Taenia mustelae has been reported throughout North America and Europe, but genetic characterization of these parasites indicates that there are 2 distinct species, both of which are now included in the recently described genus Versteria.12,15 These species include the traditionally named Versteria mustelae in Europe, from which disease in intermediate hosts has not been described, and an undescribed Versteria sp. in North America that has resulted in fatal infections in various intermediate hosts.5,15 Given the unique implications within these species, further characterization of infection in these hosts is warranted.
Muskrats (Ondatra zibethicus) are medium-sized rodents (0.2–2 kg) that, despite their widespread distribution, have experienced population declines in parts of their range, particularly in the United States, possibly because of one or more factors, including habitat loss or degradation, predation or competition, or changes in hydrology.1,4 Muskrats are known intermediate hosts of multiple species of cestodes including Taenia spp. and Hydatigera taeniaeformis in the United States and Echinococcus spp. in Europe.7,16 Although there are some reports of muskrats infected with Versteria mustelae (reported as the old Taenia mustelae terminology), these reports predated the recent description of the Versteria genus, the split of V. mustelae in North America into 2 species, the potential for genetic analysis to confirm the parasite species, and the description of the associated lesions.12,15,18,20 The potential for zoonotic disease caused by one of the Versteria sp., and concern for muskrat disease threats in the United States, warrants additional study of the Versteria cestodes.2,13 We confirm here by molecular methods that muskrats can serve as intermediate hosts to the zoonotic Versteria sp., describe the phylogenetic relationship with other Versteria-infected individuals, and provide a histologic description of lesions in a muskrat.
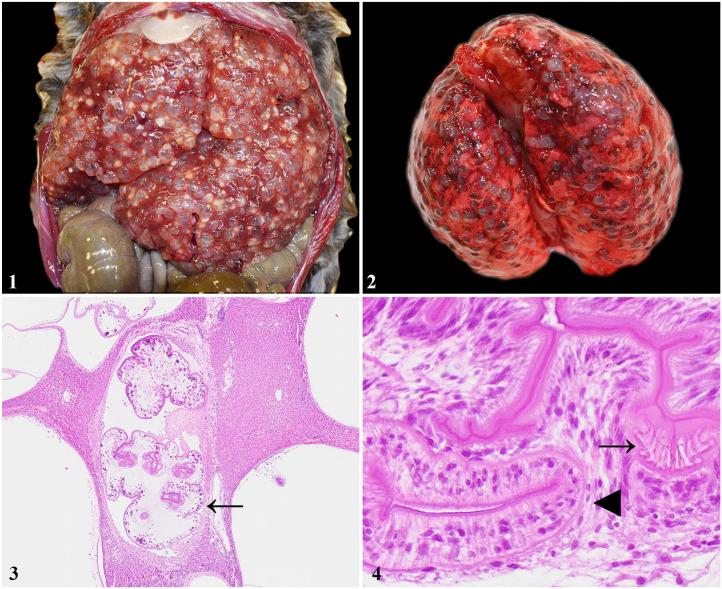
An adult, female muskrat was found deceased in Union County, Pennsylvania, USA, in June 2018. A postmortem examination was performed and showed the animal to be in fair body condition with scant adipose tissue but adequate musculature. The liver was diffusely effaced by dozens of 3–5-mm diameter, thin-walled cysts filled with clear, gelatinous fluid and containing <1-mm diameter white cestode larvae (Fig. 1). Similar cysts were present in smaller quantities in the lungs (Fig. 2), kidneys, spleen, ovaries, brain, and gastrointestinal tract.
Figures 1–4.
Systemic Versteria sp. infection in a muskrat (Ondatra zibethica). Figure 1. Dozens of tapeworm cysts in the liver. Figure 2. Tapeworm cysts throughout the lungs. Figure 3. In the liver, the cysts effaced the parenchyma and contained polycephalic tapeworm larvae covered by a thin capsule/bladder cell (arrow). H&E. Figure 4. In the liver, the rostellum of 1 scolex containing 2 rows of short hooks (arrow) and adjacent sucker (arrowhead) characteristic of Versteria sp. H&E.
Samples of brain, lung, liver, kidney, spleen, pancreas, heart, uterus, ovary, intestines, and colon were collected and placed into neutral-buffered formalin for histopathology and into 70% ethanol for molecular analysis. The formalin-fixed sections were processed routinely, stained with H&E, and evaluated by light microscopy. In the brain, lung, liver, kidney, spleen, and colonic serosa, there were one to dozens of 0.75–5-mm diameter cysts lined by a thin, fibrous capsule. Within these capsules were cross or sagittal sections of distinct, solid-bodied cestode larvae covered by a serrated tegument overlying tattered flocculent material and clusters of round-to-oval, calcareous corpuscles. The cestodes lacked a digestive tract and contained up to 4 invaginated, small scolices (polycephalic cysticerci; Fig. 3) and 1–4 suckers (Fig. 4). Short rostellar hooks (up to 11 µm long) were often, when visible, arranged in 2 rows (Fig. 4). Rather than distinct, compact larvae, some cysticerci bladders contained similarly described cestode tegument but with an empty center as a result of the absence of coelomic structures. The compressed adjacent tissue was often fibrotic and edematous, with rare, mixed inflammatory cells, primarily lymphocytes and histiocytes. Fibrous capsules with larvae rarely contained abundant, acute hemorrhage. The polycephalic larvae containing a small scolex and 2 rows of very short rostellar hooks were characteristic for Versteria spp. 15
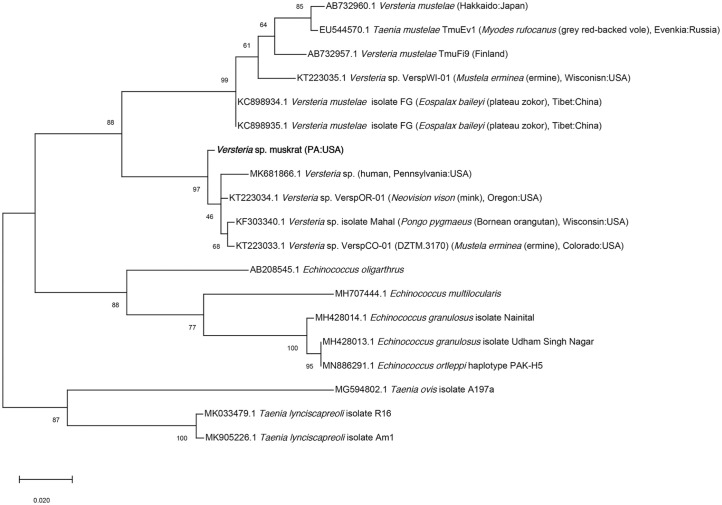
DNA was extracted from cysts (DNeasy blood and tissue kit; Qiagen). Partial cytochrome oxidase subunit 1 gene sequence was amplified as described previously. 11 The amplicon was extracted from the gel (QIAquick gel extraction kit; Qiagen) and submitted to Genewiz (South Plainfield, NJ, USA) for bidirectional sequencing. Geneious (v.11.5.1; Biomatters) was used to clean and generate a consensus sequence. Related sequences were obtained from GenBank, and a phylogenetic tree was constructed using maximum likelihood algorithms using the Tamura–Nei parameter model and pairwise deletion in MEGA X. 19 The muskrat Versteria sp. sequence (396 bp) was most similar (99%) to Versteria specimens from a mink (Neovison vison) in Oregon (KT223034) and ermine (Mustela erminea) from Colorado (KT223033). Phylogenetically, this parasite was in a clade with other Versteria specimens from North American hosts, including wild mink and ermine, a captive orangutan, and humans (Fig. 5).
Figure 5.
Phylogenetic relationship with related cestodes of the Versteria sp. sequence obtained from the hepatic cysts in a muskrat.
The Taeniidae family, in the order Cyclophyllidea, is the largest order of tapeworms with thousands of species. 10 Historically, the family contained 2 genera, Taenia and Echinococcus, but now also contains Hydatigera and Versteria. 15 These genera are well-known in human and veterinary medicine given their potential to cause fatal disease or economic losses to livestock producers.9,14 Generally, the life cycle of taeniid tapeworms is similar, with carnivorous definitive hosts harboring adult cestodes in the intestines. These adults pass proglottids filled with eggs into the environment in the host’s feces, which are ingested by intermediate hosts. The tapeworm larvae develop as metacestodes in the intermediate host’s viscera. This stage is considered the most important from a health perspective because these cysticerci and coenura can be fatal in certain species. 17
Based on morphology, T. mustelae (Gmelin, 1790) was considered distinct from all other Taenia species and was, by some accounts, more closely related to Echinococcus than other Taenia species.9,15 Within the last decade, a new genus (Versteria) was proposed to formally include, among others, the previously recognized T. mustelae (and synonyms Fimbriotaenia mustelae and Taenia tenuicollis). 15 This parasite uses various members of the weasel family as definitive hosts and rodents as intermediate hosts. 12
Recent phylogenetic work on V. mustelae suggested that it represents at least 2 species. One species has retained the name and is found primarily in Eurasia, although based on molecular analyses, there is one confirmed report of the Eurasian V. mustelae in North America (an ermine from Wisconsin). 12 Interestingly, fatal disseminated cases of V. mustelae in intermediate hosts in Eurasia have not been reported. The other Versteria sp. in North America is currently undescribed, is zoonotic, and is only known to occur in North America. 12 Known definitive hosts for the zoonotic Versteria sp. include mink in Oregon and ermine in Colorado. 12 Although there are numerous reports of V. mustelae (=T. mustelae) from mustelids throughout North America, it is unknown which of the 2 Versteria spp. they represent. The taxonomic uncertainty of this group complicates our understanding of the natural history of the 2 Versteria spp. given that past reports of V. mustelae in both definitive and intermediate hosts in North America could be either species.
Our report confirms that muskrats are intermediate hosts for the undescribed zoonotic Versteria sp. This Versteria sp. has been implicated in severe morbidity and/or mortality of humans and nonhuman primates in North America. These reports include 3 people with pulmonary and hepatic cysts in New Brunswick, Canada and Pennsylvania, USA that were believed to be immunosuppressed, either through iatrogenic immunosuppressive treatment or other comorbidities.2,3,5,13 In 2 cases, there was a history of living in close proximity to a variety of wildlife species including fishers (Pekania pennanti) and other mustelids.2,13 An orangutan (Pongo pygmaeus) at a Wisconsin zoo died from Versteria cysts in the lungs, liver, and spleen without an obvious predisposing health condition. 8 Exposure in all these cases was presumably accidental ingestion of feces from infected definitive hosts.
The fibrous tissue adjacent to Versteria sp. cysts in this muskrat contained only scant inflammation as part of the host immune response, which is similar to other reports of taeniid cysts in intermediate hosts. 17 Morbidity and mortality of intermediate hosts infected with cestodes are typically the result of overt organ failure caused by replacement or compression of the parenchyma by the cysts themselves, with severe clinical disease based largely on the specific tissues affected as well as the overall parasite burden. 14 Severe anaphylaxis can occur when cysts burst, and infection can lead to secondary bacterial or fungal infections. 14 Although rare, definitive hosts can also show signs of morbidity as reported in a European mink (Mustela lutreola) with a very high V. mustelae burden that was cachexic, presumably as a result of the parasites. 6
Understanding the taxonomic relationship and identification options for cestode cysts of different species in animals is critical considering the potential for fatal zoonotic disease. Since the establishment of the Versteria genus, there have been reports of fatal infection in intermediate hosts, including humans, captive animals, and now free-ranging wildlife in North America.2,8,13 Expanding our knowledge of taeniid cestodes is critical to understand potential predisposing factors to infection as well as the mechanisms of transmission and disease to guide prevention and treatment efforts. Considering the challenges of definitive identification using histology alone, consulting with experienced parasitologists by veterinarians, physicians, and pathologists is warranted as well as considering additional ancillary testing, such as molecular characterization, to confirm the identity of these parasites.
Acknowledgments
We thank the state wildlife agencies that support the Southeastern Cooperative Wildlife Disease Study, notably the Pennsylvania Game Commission. We also thank K. Garrett for assistance with molecular testing and the University of California–Davis School of Veterinary Medicine Histology and Digital Pathology Laboratories. Any use of trade, firm, or product names is for descriptive purposes only and does not imply endorsement by the U.S. Government.
Footnotes
Declaration of conflicting interests: The authors declared no potential conflict of interest with respect to the research, authorship, and/or publication of this article.
Funding: Funding was provided, in part, by the state wildlife agencies that support SCWDS via the Federal Aid to Wildlife Restoration Act (50. Stat. 19). The authors received no additional financial support for the research, authorship, and/or publication of this article.
ORCID iDs: Kevin D. Niedringhaus  https://orcid.org/0000-0002-9560-5621
https://orcid.org/0000-0002-9560-5621
W. David Walter  https://orcid.org/0000-0003-3068-1073
https://orcid.org/0000-0003-3068-1073
Contributor Information
Kevin D. Niedringhaus, Southeastern Cooperative Wildlife Disease Study, College of Veterinary Medicine, University of Georgia, Athens, GA, USA; Veterinary Medical Teaching Hospital, University of California–Davis, Davis, CA, USA.
Laken S. Ganoe, Pennsylvania Cooperative Fish and Wildlife Research Unit, Department of Ecosystem Science and Management, The Pennsylvania State University, University Park, PA, USA The University of Rhode Island, Kingston, RI, USA.
Matthew Lovallo, College of the Environmental and Life Sciences, Department of Natural Resources Science, Pennsylvania Game Commission, Bureau of Wildlife Management, Harrisburg, PA, USA.
W. David Walter, U.S. Geological Survey, Pennsylvania Cooperative Fish and Wildlife Research Unit, The Pennsylvania State University, University Park, PA, USA.
Michael J. Yabsley, Southeastern Cooperative Wildlife Disease Study, College of Veterinary Medicine, University of Georgia, Athens, GA, USA Warnell School of Forestry and Natural Resources, University of Georgia, Athens, GA, USA.
Justin D. Brown, Department of Veterinary and Biomedical Sciences, The Pennsylvania State University, University Park, PA, USA
References
- 1. Ahlers AA, Heske EJ. Empirical evidence for declines in muskrat populations across the United States. J Wildl Manage 2017;81:1408–1416. [Google Scholar]
- 2. Barkati S, et al. First human case of metacestode infection caused by Versteria sp. in a kidney transplant recipient. Clin Infect Dis 2019;68:680–683. [DOI] [PubMed] [Google Scholar]
- 3. Connor DH, et al. Disseminated parasitosis in an immunosuppressed patient. Possibly a mutated sparganum. Arch Pathol Lab Med 1976;100:65–68. [PubMed] [Google Scholar]
- 4. Danell K. Introductions of aquatic rodents: lessons of the muskrat Ondatra zibethicus invasion. Wildl Biol 1996;2:213–220. [Google Scholar]
- 5. Deplazes P, et al. Wildlife-transmitted Taenia and Versteria cysticercosis and coenurosis in humans and other primates. Int J Parasitol Parasites Wildl 2019;9:342–358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Fournier-Chambrillon C, et al. Severe parasitism by Versteria mustelae (Gmelin, 1790) in the critically endangered European mink Mustela lutreola (Linnaeus, 1761) in Spain. Parasitol Res 2018;117:3347–3350. [DOI] [PubMed] [Google Scholar]
- 7. Ganoe LS, et al. A review of pathogens, diseases, and contaminants of muskrats (Ondatra zibethicus) in North America. Front Vet Sci 2020;7:233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Goldberg TL, et al. Fatal metacestode infection in a Bornean orangutan caused by unknown Versteria species. Emerg Infect Dis 2014;20:109–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Hoberg EP. Taenia tapeworms: their biology, evolution and socioeconomic significance. Microbes Infect 2002;4:859–866. [DOI] [PubMed] [Google Scholar]
- 10. Hoberg EP. Phylogeny of Taenia: species definitions and origins of human parasites. Parasitol Int 2006;55(Suppl):S23–S30. [DOI] [PubMed] [Google Scholar]
- 11. Lee S-U, et al. Molecular phylogeny of parasitic Platyhelminthes based on sequences of partial 28S rDNA D1 and mitochondrial cytochrome c oxidase subunit I. Korean J Parasitol 2007;45:181–189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Lee LM, et al. Definitive hosts of Versteria tapeworms (Cestoda: Taeniidae) causing fatal infection in North America. Emerg Infect Dis 2016;22:707–710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Lehman B, et al. Disseminated metacestode Versteria species infection in woman, Pennsylvania, USA. Emerg Infect Dis 2019;25:1429–1431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Moro P, Schantz PM. Echinococcosis: a review. Int J Infect Dis 2009;13:125–133. [DOI] [PubMed] [Google Scholar]
- 15. Nakao M, et al. Molecular phylogeny of the genus Taenia (Cestoda: Taeniidae): proposals for the resurrection of Hydatigera Lamarck,1816 and the creation of a new genus Versteria. Int J Parasitol 2013;43:427–437. [DOI] [PubMed] [Google Scholar]
- 16. Oksanen A, et al. The geographical distribution and prevalence of Echinococcus multilocularis in animals in the European Union and adjacent countries: a systematic review and meta-analysis. Parasit Vectors 2016;9:519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Roberts LS, et al. Tapeworms. In: Johnson C, ed. Foundations of Parasitology. 9th ed. McGraw-Hill, 2013:325–348. [Google Scholar]
- 18. Senger CM, Bates JW. The occurrence of Hymenolepis evaginata and H. ondatrae in Utah muskrats. Proc Helminthol Soc Wash 1957;24:141–142. [Google Scholar]
- 19. Stecher G, et al. Molecular evolutionary genetics analysis (MEGA) for macOS. Mol Biol Evol 2020;37:1237–1239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Todd KS, Jr, et al. The muskrat, Ondatra zibethicus, as a host of Taenia mustelae in Illinois. J Parasitol 1978;64:523. [Google Scholar]