Abstract
Background
Hepatitis B virus (HBV) infection is one of the global public problems. Among the known infection cases, HBV X protein (HBx) is one of the key inducements of viral replication and host infection. This study was aimed to uncover the role of protease activated receptor 2 (PAR2) on HBx-induced liver injury.
Methods
A PAR2-KO mouse model expressing HBx was constructed using hydrodynamics-based in vivo gene transfection method. In addition, pcDNA3.1-HBx was used to over-express HBx in LO2 cells. The effects of HBx overexpression on inflammation and mitochondria oxidative stress were evaluated.
Results
We found that PAR2 protein level was increased by HBx overexpression. The enforced HBx inhibited LO2 cells apoptosis. Meanwhile, HBx induced inflammation reactions through promoting the secretion of pro-inflammatory cytokines such as TNF-α, IL-6, and CXCL-2. Overexpressed HBx also resulted in mitochondria oxidative stress by upregulation of ROS level and downregulation of MMP and ATP. However, in FSLLRY-NH2 (PAR2 antagonist) treated LO2 cells or PAR2-KO mice, PAR2 blockade reversed the above adverse effects of HBx on liver cells or tissues.
Conclusion
Inhibition of PAR2 may suppress inflammation and mitochondria oxidative stress caused by HBx, pointing out the potential application values of PAR2 antagonist on the treatment of HBV infection in clinic.
Keywords: protease activated receptor 2, hepatitis B virus, hepatitis B virus X protein, inflammation, mitochondria oxidative stress
Introduction
Hepatitis B virus (HBV) is a hepatotropic DNA virus with a length of 3.2 kb, consisting of 4 open reading frames (S, C, P and X) and expressing 7 virus proteins.1,2 HBV infection is prevalent worldwide, and it remains one of the global public problems to threat people’s life.3 Over 350 million people carries HBV worldwide and one million patients may die each year due to HBV infection-related complications, such as cirrhosis and hepatocellular carcinoma (HCC).4,5 Currently, there are still no specific therapies for HBV infection except for antiviral drugs to repress the replication rate of virus particles.6 Therefore, exploring effective molecular targets may be helpful for the treatment of HBV infection-related diseases in clinic.
Among the known infection cases, HBV X protein (HBx) is one of the key inducements of viral replication and host infection.7 Numerous studies have reported that HBx can disrupt the progression of oxidative phosphorylation, suppress ATP production, and alter mitochondrial membranes permeability, eventually leading to liver cell damage.8,9 For example, Rahmani et al found that HBx is located in the mitochondrial of liver cells and HBx overexpression inhibits the mitochondrial membrane potential (MMP).8 The increased HBx elevates the level of reactive oxygen species (ROS) and the subsequent peroxidation, thereby promoting hyperplasia and metastasis of liver cells and inducing the development of HCC.9 In addition, the increased ROS level is generally strongly associated with inflammatory reactions in liver injuries,10 which is another crucial hallmark of HBV infection. Some chemokines and pro-inflammatory cytokines are confirmed to be deeply involved in the development of HBx-induced liver injury.11–17 The abnormal increased IL-6 and TNF-α are commonly observed in the tissues and serum of HCC patients, which can generally induce tumor growth and metastasis.11 The enhancement of IL-6 caused by HBx overexpression can induce cytotoxic T-cells proliferation, thus promoting liver inflammation and immune system dysfunction.12–14 Meanwhile, HBx induction has also been shown to trigger inflammation responses and fibrosis in liver tissues through upregulation of CXCL2 expression level.15–17 Therefore, targets that controlling HBx-induced inflammation, oxidative stress, and mitochondria injury may be surely urgent for HBV infection-related diseases therapies.
Proteinase activated receptor 2 (PAR2) is a member of seven-transmembrane G-protein coupled receptor family.18 Unlike the other three subtypes (PAR1, PAR3, and PAR4) that are sensitive to thrombin,19 PAR2 is generally activated by trypsin, tryptase and elastase.20 Previous studies have demonstrated that PAR2 may involve in the regulation of oxidative stress in hyperglycaemia and hypertension.21,22 Additionally, PAR2 activation has been also reported to mediate inflammation through modulation of IL-8, IL-6, and TNF-α in several types of human diseases, such as asthma,23 acute lung injury,24 allergic rhinitis,25 dementia,26 and myocarditis.27 More importantly, PAR2 is also shown to participate in HBV infection-related complications.28,29 High expression of PAR2 in HCC patients means the relatively low survival rate.28 PAR2 pepducin inhibitors administration can effectively repress liver fibrosis in a mouse model.29 However, studies on the interaction of PAR2 with HBx-induced liver injury are relatively rare.
In the current study, we determined the expression level of PAR2 in HBx overexpressed LO2 cells and C57BL/6-background PAR2-KO mice. Furthermore, the interaction of PAR2 inhibition and HBx overexpression on inflammation, oxidative stress, and mitochondria injury were also investigated in both in vitro and in vivo experiments. These findings may provide a clinical therapeutic method for HBV infection.
Materials and Methods
Cells and Treatment
American Type Culture Collection (Manassas, VA, USA) provided human normal hepatic cell line LO2. The obtained LO2 cells were then cultured in DMEM containing 10% FBS and 1% penicillin/streptomycin with 5% CO2 at 37°C. In accordance with Yip et al’s method,30 pcDNA3.1-HBx plasmid was constructed. Thereafter, the cultured LO2 cells were transfected with pcDNA3.1-HBx for 24 h at 37°C using Lipofectamine 3000 (Thermo Fisher Scientific, Waltham, MA, USA). Subsequently, the transfected LO2 cells were further treated with 200 or 400 μM FSLLRY-NH2 (FSL; PAR2 antagonist; CL Bio-Scientific Inc, Xi’an, China) for another 24 h. After FSL induction, LO2 cells were collected for the subsequent experiments.
HBx Overexpression Mouse Model
Wild-type (WT) C57BL/6 and PAR2-KO (C57BL/6 F2rl1(-/-)) mice purchased from Cyagen Biosciences (Guangzhou, China) were initially accustomed to laboratory environment for 7 days. In accordance with hydrodynamics-based in vivo gene transfection method,31,32 pcDNA3.1-HBx (250 µg) was injected into C57BL/6 WT and PAR2-KO mice (8 mice for each group) respectively through the tail vein within 4–8 sec. C57BL/6 WT mice (n = 8) injected with pcDNA3.1 empty vector were served as controls. Four days later, the mice were euthanized and eyeball blood and liver tissues were collected for the subsequent experiments. All animal experiments in this study were in strict accordance with the protocols stated in the Guide for the Care and Use of Laboratory Animals and approved by the ethical committee of Jinzhou Medical University.
Aspartate Aminotransferase (AST) and Alanine Aminotransferase (ALT) Detection Assay
The collected blood samples (3 mL) were used to centrifuge (500 × g, 10 min) and the concentrations of AST and ALT were measured using the corresponding commercial kits (Sigma Aldrich, San Luis, MO, USA) under a dispensing peristaltic pump automatic biochemical analyzer (Roche, Basel, Switzerland).
Hematoxylin-Eosin (HE) Staining Assay
The collected liver tissues of mice were fixed in 4% paraformaldehyde for one day, followed by embedding in paraffin sectioned at 3 μm thickness. All the sections were stained with HE staining immediately and then were observed by a light microscopy.
Immunohistochemistry (IHC) Analysis
IHC staining was conducted using streptavidin-biotin-peroxidase complex method. Briefly, the mice liver samples were fixed, paraffin-embedded, dewaxed, rehydrated, and antigen retrieval. Then samples were stained with primary antibody rabbit anti-HBcAg (Abcam, Cambridge, UK; cat.no. ab115992; 1:200), followed by incubation with biotin-labelled goat anti-rabbit IgG (H+L) (Abcam; 1:100) for 30 min at 37°C. SABC reagent (Zhongshan Goldenbridge Biotechnology, Beijing, China) and DAB Substrate Kit (Wuhan Boster Biological Technology, Wuhan, China) were used to detect the immune complexes in the specimens according to the manufactures’ instructions. The positive signals were manifested as brown or tan staining. Pictures were taken under a light microscope (magnifications, × 400).
Western Blotting Analysis
The mice liver tissues or LO2 cells were lysed with RIPA buffer, and then determined the concentrations using a BCA Protein Assay Kit (Thermo Fisher Scientific), followed by separating the protein product using 10% SDS-PAGE and transferring into PVDF membrane. The membrane was incubated with the primary antibodies HBx (Abcam; cat.no. ab2741; 1:1000), PAR2 (Abcam; cat.no. ab180953; 1:1000), and β-actin (Abcam; cat.no. ab8227; 1:1000) at 4°C for overnight and then the secondary antibody for 1 h at room temperature. β-actin was used as the internal control. Immunoblotting was visualized using an ECL detection kit (Amersham Biosciences, Sweden).
5-Ethynyl-2’-Deoxyuridine (EdU) Assay
The proliferation of LO2 cells was measured by EdU assay. In brief, after fixation and permeabilization, the cells were incubated with EdU (50 µM) for 3 h at 37°C. Cell nuclei were stained with 4’,6-Diamidino-2-phenylindole dihydrochloride (Sigma Aldrich; cat.no. D8417; 1 µg/mL) for 10 min. The EdU-positive cells were determined using fluorescence microscopy.
Flow Cytometry Analysis
LO2 cells apoptosis was assessed by flow cytometric analysis. Briefly, the collected LO2 cells (1 × 105 cells/mL) were cultured in 96-well plates for 24 h, and then were stained with V-FITC and PI using an apoptosis detection kit (Thermo Fisher Scientific; cat. no. 88–8005-74) at 25°C for 20 min in the dark. The apoptotic cells were measured using a flow cytometer (BD Biosciences).
Meanwhile, flow cytometry analysis was also used for the ROS generation assay. Briefly, LO2 cells were stained with DCFH-DA (Sigma Aldrich; cat.no. D6883; 1µM) and incubated for 30 min in the dark at 37°C. After that, the stained cells were washed with PBS and the cell populations were analyzed using a flow cytometer.
Glutathione (GSH) Measurement Assay
The collected LO2 cells or the grinded liver tissue sections were centrifugated (500 × g, 10 min) and re-suspended in 5% ice-cold metaphosphoric acid (Sigma Aldrich) and then underwent ultrasonic processing. Thereafter, the cells were re-subjected to centrifugation at 14,000 × g for 5 min and the supernatant was mixed with o-phthalaldehyde-beta-mercaptoethanol and incubated for 15 min at room temperature. Fluorescent signals were recorded at 350 nm excitation and 420 nm emission.
MMP, H2O2, ATP, SOD, and MDA Determination Assay
LO2 cells or the grinded liver tissue sections were stained with tetramethyl rhodamine methyl ester (Sigma Aldrich; 200 µM) for 1 h at 37°C. Subsequently, the stained cells were washed with PBS and fluorescence was visualized using a fluorescence microscope. Fluorescent density was quantified using Image J to MMP level.
For the measurement of H2O2, LO2 cells or the grinded liver tissue sections were mixed with ferrous ammonium sulfate (500 mM), sorbitol (200 mM), xylenol orange (200 mM), and H2SO4 (50 mM) and incubated for 50 min. The level of complex Fe3+-xylenol orange was measured using a microplate reader at 560 nm.
According to manufacturer protocol, the corresponding commercial kits from Sigma Aldrich (San Luis, MO, USA) were used to determine the levels of ATP (cat.no. 119107), SOD (cat.no. 19160), and MDA (cat. no. MAK085) in LO2 cells or the grinded liver tissue sections.
Measurement for the Levels of TNF-α, IL-6, and CXCL-2
The mRNA expression of TNF-α, IL-6, and CXCL-2 was detected by qRT-PCR. In brief, total RNA was extracted from LO2 cells or the liver tissue sections using Trizol Reagent (Invitrogen). The cDNA was synthesized using RevertAid H Minus First Strand cDNA Synthesis Kit (Thermo Fisher Scientific, Waltham, MA, USA). Afterwards, cDNA was used to perform qRT-PCR analysis with DyNAmo Flash SYBR Green qPCR Kit (Thermo Fisher Scientific). The 2−ΔΔCt method was utilized to calculate the relative expression. GAPDH was used as the internal control. Meanwhile, the protein levels of these cytokines were measured by the corresponding commercial ELISA kits (EseBio, Shanghai, China).
Statistical Analysis
The data normality test was performed using Kolmogorov–Smirnov method. If the data conformed to the normal distribution and homogeneity of variance, one-way ANOVA followed by Tukey’s multiple comparisons test was used for comparison among multiple groups. Data were presented as means ± SD. SPSS 22.0 software (Chicago, USA) was used for data processing. P-value less than 0.05 indicated a statistically significant difference.
Results
Overexpression of HBx Elevates PAR2 Protein Level in LO2 Cells
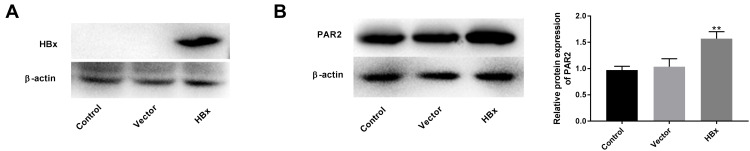
As shown in Figure 1A, the protein level of HBx was overexpressed in the HBx group compared to that of vector group, suggesting that HBx was transfected into LO2 cells successfully. Thereafter, we found that the enforced HBx further elevated PAR2 protein level (1.57 ± 0.11) in LO2 cells (Figure 1B, P < 0.01).
Figure 1.
Overexpression of HBx elevates PAR2 protein level in LO2 cells. (A) The protein level of HBx in LO2 cells transfected with pcDNA3.1-HBx was measured by Western blotting. (B) The protein level of PAR2 in LO2 cells transfected with pcDNA3.1-HBx was measured by Western blotting. **P < 0.01 vs the vector group. The experiments were performed in triplicate.
Inhibition of PAR2 Reverses the Influences of HBx Overexpression on the Proliferation and Apoptosis of LO2 Cells
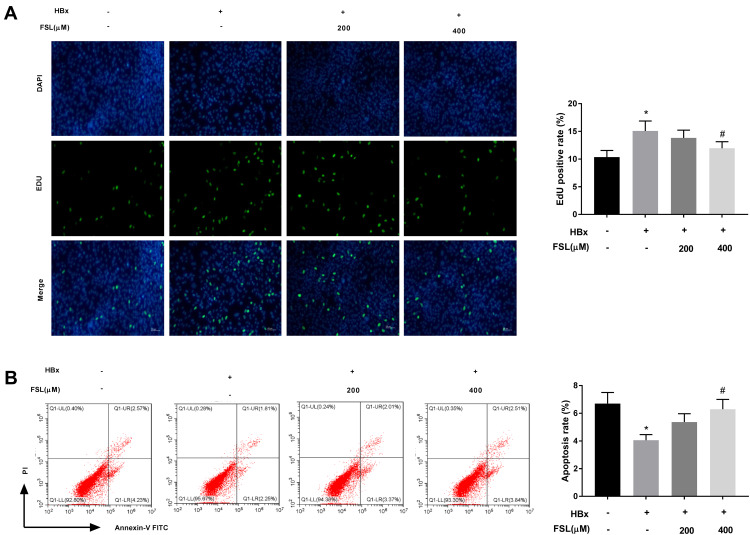
To further investigate the interaction between PAR2 and HBx on cellular processes such as proliferation and apoptosis, PAR2 antagonist FSL used to repress PAR2 was added to the HBx overexpressed LO2 cells. As illustrated in Figure 2A and B, we demonstrated that HBx overexpression significantly accelerated EdU positive rate (15.08 ± 1.76) but repressed apoptosis rate (4.03 ± 0.41) of LO2 cells (P < 0.05). Interestingly, addition of 400 μM FSL remarkably reversed the promoting effect of HBx overexpression on LO2 cell proliferation (11.96 ± 1.20), and the inhibiting effect on apoptosis (6.27 ± 0.67) (P < 0.05).
Figure 2.
Inhibition of PAR2 reverses the influences of HBx overexpression on the proliferation and apoptosis of LO2 cells. (A) The proliferation of LO2 cells under different treatments was measured by EdU assay. (B) The apoptosis of LO2 cells under different treatments was analysed using a flow cytometer. *P < 0.05 vs the HBx(-) + FSL(-) group. #P < 0.05 vs the HBx(+) + FSL(-) group. The experiments were performed in triplicate.
Suppression of PAR2 Attenuates HBx-Induced Mitochondria Oxidative Stress in LO2 Cells
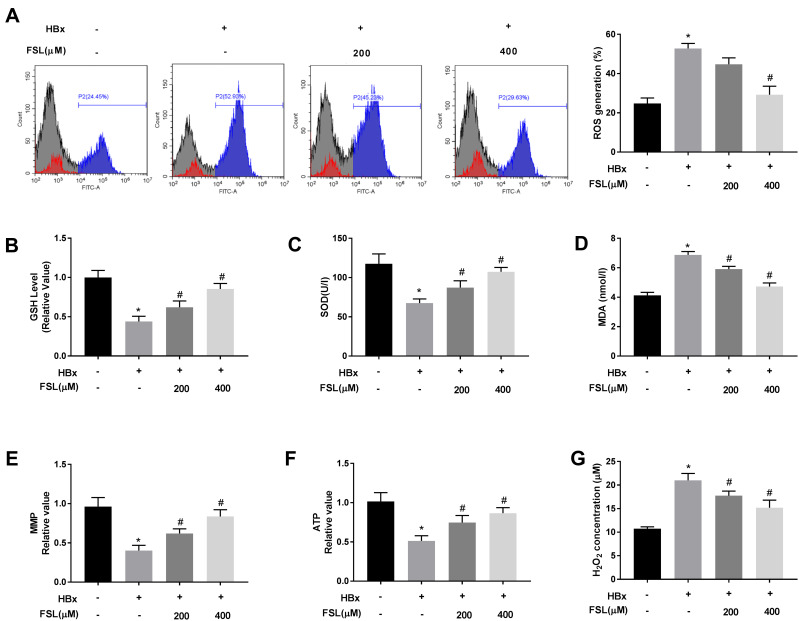
It is notable that HBx is generally closely associated with mitochondria oxidative stress.8,33–36 Therefore, the effects of PAR2 inhibition on HBx-induced oxidative stress were explored. As shown in Figure 3A-D, the levels of ROS (52.80 ± 2.08) and MDA (6.87 ± 0.19) were increased, and the concentrations of GSH (0.44 ± 0.05) and SOD (67.50 ± 4.36) were decreased by HBx overexpression (P < 0.05). As expected, FSL treatment especially 400 μM of FSL significantly alleviated the oxidative stress (ROS, 29.23 ± 3.56; GSH, 0.85 ± 0.06; SOD, 107.47 ± 4.49; MDA, 4.72 ± 0.20) caused by HBx (P < 0.05). Similarly, HBx overexpression reduced MMP (0.40 ± 0.05) and ATP (0.51 ± 0.05), but elevated H2O2 concentration (21.01 ± 1.18) in LO2 cells, while FSL treatment also reversed these situations caused by HBx overexpression (MMP, 0.84 ± 0.07; ATP, 0.87 ± 0.06; H2O2, 15.2 ± 1.30) (Figure 3E-G, P < 0.05), which indicated that PAR2 suppression protected LO2 cell mitochondria against HBx overexpression resulted injuries.
Figure 3.
Suppression of PAR2 attenuates HBx-induced mitochondria oxidative stress in LO2 cells. (A) ROS generation assay was performed using flow cytometry analysis. (B) The level of GSH in LO2 cells under different treatments. (C) The level of SOD in LO2 cells under different treatments was measured by a commercial kit. (D) The level of MDA in LO2 cells under different treatments was measured by a commercial kit. (E) MMP value in LO2 cells under different treatments. (F) ATP value in LO2 cells under different treatments. (G) H2O2 concentration in LO2 cells under different treatments. *P < 0.05 vs the HBx(-) + FSL(-) group. #P < 0.05 vs the HBx(+) + FSL(-) group. The experiments were performed in triplicate.
Decreased PAR2 Represses Inflammation Responses in HBx Overexpressed LO2 Cells
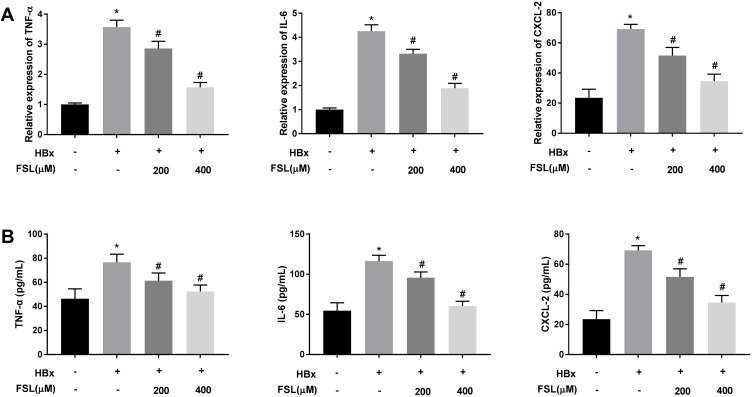
Changes of mitochondrial permeability generally lead to cell apoptosis and enhance the replication of HBV in return, eventually accelerating the development of liver inflammation.36–38 As presented in Figure 4A and B, the mRNA expression and protein levels of TNF-α, IL-6, and CXCL-2 in HBx overexpressed LO2 cells were significantly elevated (P < 0.05). Unsurprisingly, both 200 μM and 400 μM FSL reversed the promoting effects of HBx overexpression on inflammation reactions in LO2 cells (P < 0.05).
Figure 4.
Decreased PAR2 represses inflammation responses in HBx overexpressed LO2 cells. (A) The mRNA expression of TNF-α, IL-6, and CXCL-2 in LO2 cells under different treatments was detected by qRT-PCR. (B) The protein level of TNF-α, IL-6, and CXCL-2 in LO2 cells under different treatments was measured by ELISA. *P < 0.05 vs the HBx(-) + FSL(-) group. #P < 0.05 vs the HBx(+) + FSL(-) group. The experiments were performed in triplicate.
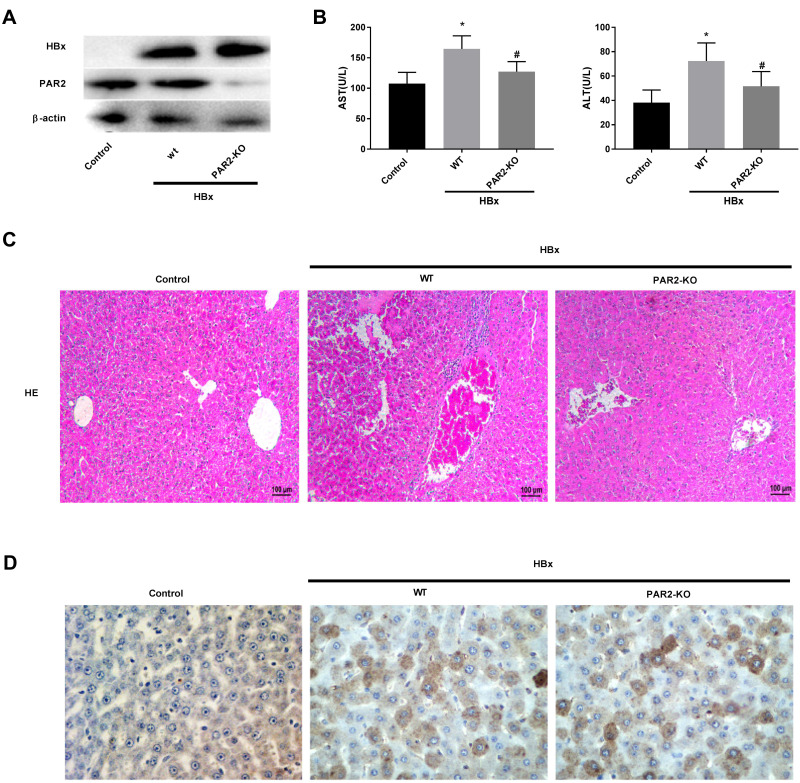
PAR2 Knockdown Alleviates HBx-Induced Liver Injuries in a Mouse Model
As shown in Figure 5A, we found that transfection of pcDNA3.1-HBx remarkably increased the protein levels of HBx and PAR2 in C57BL/6 WT mice, however, PAR2 protein level in PAR2-KO mice was elevated slightly. These results indicated that HBx gene was successfully transfected into the liver cells of experimental mice. Meanwhile, our experimental data also showed increased concentrations of AST and ALT in HBx overexpressed C57BL/6 WT mice (Figure 5B, P < 0.05). As expected, the levels of these two aminotransferases in PAR2-KO mice were relatively lower than those of C57BL/6 WT mice (P < 0.05). HE staining assay was used to determine the histopathological changes of liver tissues after transfection of pcDNA3.1-HBx. As illustrated in Figure 5C, the liver tissues of C57BL/6 WT mice transfected with pcDNA3.1-HBx showed severe inflammatory cell infiltrations and edema. PAR2 knockdown attenuated the liver injury of mice caused by HBx overexpression. HBcAg is one of the major antigens of HBV, which is usually considered a signal of HBV replication, especially in the hepatocytes in vivo. The expression level of HBcAg in the liver tissue can directly reflect the quantity of HBV particles.39 Therefore, HBcAg expression was detected with immunohistochemistry. As shown in Figure 5D, the expression of HBcAg in the control group was negative, while it was positively expressed in liver cell cytoplasm of C57BL/6 WT or PAR2-KO mice transfected with pcDNA3.1-HBx.
Figure 5.
PAR2 knockdown alleviates HBx-induced liver injuries in a mouse model (n = 8 in each group). (A) The protein levels of HBx and PAR2 in PAR2-KO mice after injection of pcDNA3.1-HBx. (B) The levels of AST and ALT in PAR2-KO mice after injection of pcDNA3.1-HBx were measured by the corresponding commercial kits. *P < 0.05 vs the control mice group. #P < 0.05 vs the WT mice + pcDNA3.1-HBx group. (C) The HE staining and (D) immunohistochemistry assay of PAR2-KO mice after injection of pcDNA3.1-HBx.
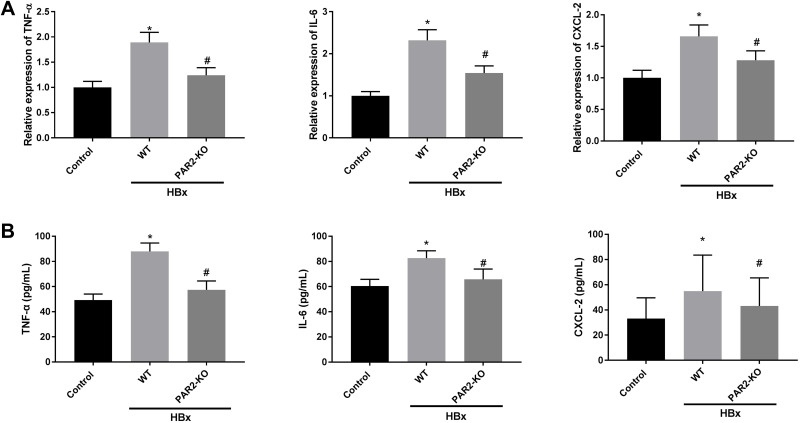
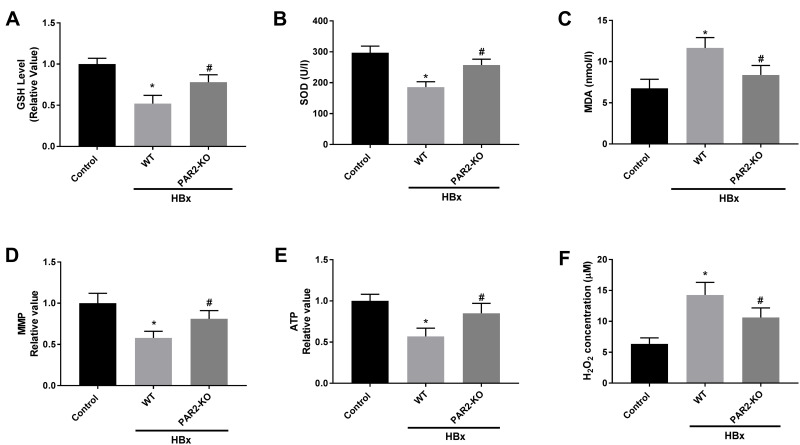
Inflammation and Mitochondria Oxidative Stress Caused by HBx Overexpression are Improved in PAR2-KO Mice
We further explored the interaction between PAR2 and HBx on inflammation, oxidative stress, and mitochondria injury in a PAR2-KO mouse model. As shown in Figures 6A, B and Figures 7A–F, HBx overexpression significantly enhanced inflammation responses, elevated oxidative stress levels, and accelerated the mitochondria injuries in C57BL/6 WT mice (P < 0.05). However, in PAR2-KO mice, the inflammation, oxidative stress, and mitochondria injury caused by pcDNA3.1-HBx transfection were all improved (P < 0.05).
Figure 6.
Inflammation caused by HBx is improved in PAR2-KO mice (n = 8 in each group). (A) The mRNA expression of TNF-α, IL-6, and CXCL-2 in liver tissues of PAR2-KO mice was detected by qRT-PCR. (B) The protein level of TNF-α, IL-6, and CXCL-2 in liver tissues of PAR2-KO mice was measured by ELISA. *P < 0.05 vs the control mice group. #P < 0.05 vs the WT mice + pcDNA3.1-HBx group.
Figure 7.
Mitochondria oxidative stress caused by HBx is alleviated in PAR2-KO mice (n = 8 in each group). (A) The level of GSH in liver tissues of PAR2-KO mice. (B) The level of SOD in liver tissues of PAR2-KO mice was measured by a commercial kit. (C) The level of MDA in liver tissues of PAR2-KO mice was measured by a commercial kit. (D) MMP value in liver tissues of PAR2-KO mice. (E) ATP value in liver tissues of PAR2-KO mice. (F) H2O2 concentration in liver tissues of PAR2-KO mice. *P < 0.05 vs the control mice group. #P < 0.05 vs the WT mice + pcDNA3.1-HBx group.
Discussion
HBV infection remains a global public problem in the current world due to the high mortality caused by the two HBV infection-related complication including liver cirrhosis and HCC.3–5 HBx overexpression is a considerable driver of HBV infection.40 It is noticed that HBx induction is generally accompanied by the occurrence and development of inflammation, oxidative stress, and mitochondria injury.8,9,11 In this study, we explored the interaction of PAR2 inhibition and HBx overexpression, and the role of PAR2 inhibition on inflammation and mitochondrial oxidative stress. Our study indicated that PAR2 was increased by exposure to HBx and inhibition of PAR2 attenuated HBx overexpression-induced inflammation and mitochondria injury, which may provide evidence of the potential of PAR2 as a treatment target against HBV infection.
Increasing researches have shown that HBx may lead to mitochondrial oxidative stress and the liver injuries through suppression of ATP production, MMP and promotion of ROS level.8,9 HBx accelerates the loss of Mcl-1 protein through caspase-3 cascade and plays its pro-apoptotic effect upon exposure to oxidative stress.41 Meanwhile, PAR2 overexpression is confirmed to enhance oxidative stress in hyperglycaemia and hypertension.21,22 PAR2 also induces oxidative stress and inflammation in skin photoaging through AKT/NF-κB/FoxO6 signaling pathway.42 In the current study, we found that antagonism of PAR2 not only reversed the promoting effects of HBx induction on the levels of ROS, MDA, and H2O2, but also partly eliminated the inhibiting effects of HBx overexpression on MMP and the levels of GSH, SOD, and ATP. These results uncovered the potential role of PAR2 inhibition on the prevention of oxidative stress injury caused by HBx in liver cells.
Excessive ROS production can affect cell proliferation, differentiation, and gene mutations, thus promoting the occurrence of HCC.43 HBx is considered to modulate apoptosis in hepatocytes by modulating protein interactions and the cell cycle.33 HBx also has been found to inhibit renal tubular cells proliferation, promote apoptosis, and activate the PI3K/Akt pathway.44 In this study, we found HBx overexpression promoted the proliferation but inhibited the apoptosis of LO2 cells. Taken together, we believed that HBx infection can induce mitochondrial dysfunction through promoting hyperoxide productions, and the balance of proliferation and apoptosis of normal hepatic cells is disrupted, thereby leading to normal hepatic cells for malignant transformation and triggering the occurrence of HCC. Blocking of PAR2 could effectively attenuate the malignant effects of HBx infection on normal hepatic cells.
Additionally, excessively increased ROS level is reported to stimulate inflammation reactions through promoting the secretion of pro-inflammatory-related cytokines such as IL-6, TNF-α, and CXCL2.12–17 Importantly, the increased level of IL-6 disrupts the normal cell cycle of liver cell to retard the rate of liver regeneration,12 and subsequent cytotoxic T-cells aggregated to liver tissues further aggravates liver damage and inflammation responses.12–14 PAR2 is reported to be closely associated with inflammation in liver fibrosis, and overexpressed PAR2 can elevate the concentrations of IL-8, IL-6, and TNF-α.29 PAR2 also plays important roles in inflammatory and allergic responses, and development in innate and adaptive immunity.45 In bone marrow-derived macrophages, PAR2 activation promotes M1 macrophage polarization and inflammation through the FOXO1-dependent pathway.46 In the current study, we found that decreased PAR2 significantly eliminated the promoting effects of HBx overexpression on the expression levels of IL-6, TNF-α, and CXCL2. It is commonly recognized that the concentrations of AST and ALT in liver tissues reflect the degree of liver injury.47,48 Our findings demonstrated that both the levels of AST and ALT in PAR2-KO mice tissues were significantly declined compared to those of WT mice. All above results uncovered that inhibition of PAR2 may attenuate the liver injury caused by HBx.
In addition, HBx has been reported to interact with many signaling pathways in liver tissues, such as AKT/FOXO1,49 JAK1/STATs,50 and ERK/NF-κB.48 Meanwhile, PAR2 is shown to be closely associated with some downstream pathways in various human diseases, such as PAR2-PI3K/Akt/mTOR in kidney tubular epithelial inflammation,51 PAR2-MEK-ERK1/2-FOS/MYC/STAT3-COX2 signaling in ovarian cancer,52 and PAR2-ERK/c-jun/AP-1 pathway in colon cancer.53 Notably, some of these pathways are confirmed to combine with PAR2 in liver-related diseases, such as PAR2-NF-κB in liver cirrhosis54 and PAR2-ERK/AP-1 in hepatoma.55 We speculated that PAR2 may also interact with HBx via these signaling pathways in liver tissues.
There are also some limitations in this study. First, HBx-caused liver injury is a very complex process, and the detailed action mechanism of PAR2 blocking on HBx-induced liver cells, such as cell proliferation, apoptosis, oxidative stress and inflammation is still needed to be explore. Second, the method for HBx-induced mouse model should be further optimized.
Conclusion
Taken together, this study indicates that PAR2 blockade may inhibit inflammation, and mitochondria oxidative stress caused by HBx. Our findings may provide novel insights into the mechanisms of HBV infection.
Acknowledgment
Bin Li is now affiliated with the Department of Pathogenic Biology, School of Basic Medicine, Jinzhou Medical University, Jinzhou City, Liaoning Province, 121001, People’s Republic of China.
Funding Statement
No funding was received.
Data Sharing Statement
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
Ethics Approval and Informed Consent
All animal experiments in this study were in strict accordance with the protocols stated in the Guide for the Care and Use of Laboratory Animals and approved by the ethical committee of Jinzhou Medical University.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest for this work.
References
- 1.Seeger C, Mason WS. Molecular biology of hepatitis B virus infection. Virology. 2015;479-480:672–686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Seeger C, Mason WS. Hepatitis B virus biology. Microbiol Mol Biol Rev. 2000;64(1):51–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yang S, Yu C, Chen P, et al. Protective immune barrier against hepatitis B is needed in individuals born before infant HBV vaccination program in China. Sci Rep. 2015;5:18334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ganem D, Prince AM. Hepatitis B virus infection–natural history and clinical consequences. N Engl J Med. 2004;350(11):1118–1129. [DOI] [PubMed] [Google Scholar]
- 5.Slagle BL, Andrisani OM, Bouchard MJ, Lee CG, Ou JH, Siddiqui A. Technical standards for hepatitis B virus X protein (HBx) research. Hepatology. 2015;61(4):1416–1424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Terrault NA, Bzowej NH, Chang KM, Hwang JP, Jonas MM, Murad MH. AASLD guidelines for treatment of chronic hepatitis B. Hepatology. 2016;63(1):261–283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Martin-Vilchez S, Moreno-Otero R, Sanz-Cameno P. Effects of hepatitis B virus X protein on chronic hepatitis B pathophysiology. Med Clin (Barc). 2013;140(11):508–513. [DOI] [PubMed] [Google Scholar]
- 8.Rahmani Z, Huh KW, Lasher R, Siddiqui A. Hepatitis B virus X protein colocalizes to mitochondria with a human voltage-dependent anion channel, HVDAC3, and alters its transmembrane potential. J Virol. 2000;74(6):2840–2846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lee YI, Hwang JM, Im JH, et al. Human hepatitis B virus-X protein alters mitochondrial function and physiology in human liver cells. J Biol Chem. 2004;279(15):15460–15471. [DOI] [PubMed] [Google Scholar]
- 10.Jaeschke H. Reactive oxygen and mechanisms of inflammatory liver injury: present concepts. J Gastroenterol Hepatol. 2011;26(Suppl 1):173–179. [DOI] [PubMed] [Google Scholar]
- 11.Wong VW, Yu J, Cheng AS, et al. High serum interleukin-6 level predicts future hepatocellular carcinoma development in patients with chronic hepatitis B. Int J Cancer. 2009;124(12):2766–2770. [DOI] [PubMed] [Google Scholar]
- 12.Quetier I, Brezillon N, Duriez M, et al. Hepatitis B virus HBx protein impairs liver regeneration through enhanced expression of IL-6 in transgenic mice. J Hepatol. 2013;59(2):285–291. [DOI] [PubMed] [Google Scholar]
- 13.Gruden G, Carucci P, Lolli V, et al. Serum heat shock protein 27 levels in patients with hepatocellular carcinoma. Cell Stress Chaperones. 2013;18(2):235–241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lan T, Chang L, Wu L, Yuan YF. IL-6 Plays a Crucial Role in HBV Infection. J Clin Transl Hepatol. 2015;3(4):271–276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Xie L, Huang Y. Antagonism of RIP1 using necrostatin-1 (Nec-1) ameliorated damage and inflammation of HBV X protein (HBx) in human normal hepatocytes. Artif Cells Nanomed Biotechnol. 2019;47(1):1194–1199. [DOI] [PubMed] [Google Scholar]
- 16.Fan Y, Wang L, Dou X. Serum Monocyte Chemoattractant Protein-1 Predicts Liver Inflammation of Patients with Chronic Hepatitis B. Clin Lab. 2018;64(5):841–846. [DOI] [PubMed] [Google Scholar]
- 17.Luo MX, Wong SH, Chan MT, et al. Autophagy Mediates HBx-Induced Nuclear Factor-kappaB Activation and Release of IL-6, IL-8, and CXCL2 in Hepatocytes. J Cell Physiol. 2015;230(10):2382–2389. [DOI] [PubMed] [Google Scholar]
- 18.Frungieri MB, Albrecht M, Raemsch R, Mayerhofer A. The action of the mast cell product tryptase on cyclooxygenase-2 (COX2) and subsequent fibroblast proliferation involves activation of the extracellular signal-regulated kinase isoforms 1 and 2 (erk1/2). Cell Signal. 2005;17(4):525–533. [DOI] [PubMed] [Google Scholar]
- 19.Lee YJ, Kim SJ, Kwon KW, Lee WM, Im WJ, Sohn UD. Inhibitory effect of FSLLRY-NH2 on inflammatory responses induced by hydrogen peroxide in HepG2 cells. Arch Pharm Res. 2017;40(7):854–863. [DOI] [PubMed] [Google Scholar]
- 20.Antoniak S, Pawlinski R, Mackman N. Protease-activated receptors and myocardial infarction. IUBMB Life. 2011;63(6):383–389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.El-Daly M, Pulakazhi Venu VK, Saifeddine M, et al. Hyperglycaemic impairment of PAR2-mediated vasodilation: prevention by inhibition of aortic endothelial sodium-glucose-co-Transporter-2 and minimizing oxidative stress. Vascul Pharmacol. 2018;109:56–71. [DOI] [PubMed] [Google Scholar]
- 22.Aman M, Hirano M, Kanaide H, Hirano K. Upregulation of proteinase-activated receptor-2 and increased response to trypsin in endothelial cells after exposure to oxidative stress in rat aortas. J Vasc Res. 2010;47(6):494–506. [DOI] [PubMed] [Google Scholar]
- 23.Walker JK, DeFea KA. Role for beta-arrestin in mediating paradoxical beta2AR and PAR2 signaling in asthma. Curr Opin Pharmacol. 2014;16:142–147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rayees S, Rochford I, Joshi JC, Joshi B, Banerjee S, Mehta D. Macrophage TLR4 and PAR2 Signaling: role in Regulating Vascular Inflammatory Injury and Repair. Front Immunol. 2020;11:2091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lee HM, Kim HY, Kang HJ, et al. Up-regulation of protease-activated receptor 2 in allergic rhinitis. Ann Otol Rhinol Laryngol. 2007;116(7):554–558. [DOI] [PubMed] [Google Scholar]
- 26.Noorbakhsh F, Vergnolle N, McArthur JC, et al. Proteinase-activated receptor-2 induction by neuroinflammation prevents neuronal death during HIV infection. J Immunol. 2005;174(11):7320–7329. [DOI] [PubMed] [Google Scholar]
- 27.Weithauser A, Bobbert P, Antoniak S, et al. Protease-activated receptor-2 regulates the innate immune response to viral infection in a coxsackievirus B3-induced myocarditis. J Am Coll Cardiol. 2013;62(19):1737–1745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chen P, Yang N, Xu L, Zhao F, Zhang M. Increased expression of protease-activated receptors 2 indicates poor prognosis in HBV related hepatocellular carcinoma. Infect Agent Cancer. 2019;14:39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shearer AM, Rana R, Austin K, et al. Targeting Liver Fibrosis with a Cell-penetrating Protease-activated Receptor-2 (PAR2) Pepducin. J Biol Chem. 2016;291(44):23188–23198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yip WK, Cheng AS, Zhu R, et al. Carboxyl-terminal truncated HBx regulates a distinct microRNA transcription program in hepatocellular carcinoma development. PLoS One. 2011;6(8):e22888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Liu F, Song Y, Liu D. Hydrodynamics-based transfection in animals by systemic administration of plasmid DNA. Gene Ther. 1999;6(7):1258–1266. [DOI] [PubMed] [Google Scholar]
- 32.Yang PL, Althage A, Chung J, Chisari FV. Hydrodynamic injection of viral DNA: a mouse model of acute hepatitis B virus infection. Proc Natl Acad Sci U S A. 2002;99(21):13825–13830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hu L, Chen L, Yang G, et al. HBx sensitizes cells to oxidative stress-induced apoptosis by accelerating the loss of Mcl-1 protein via caspase-3 cascade. Mol Cancer. 2011;10:43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Xie WH, Ding J, Xie XX, et al. Hepatitis B virus X protein promotes liver cell pyroptosis under oxidative stress through NLRP3 inflammasome activation. Inflamm Res. 2020;69(7):683–696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Casciano JC, Bouchard MJ. Hepatitis B virus X protein modulates cytosolic Ca(2+) signaling in primary human hepatocytes. Virus Res. 2018;246:23–27. [DOI] [PubMed] [Google Scholar]
- 36.Shirakata Y, Koike K. Hepatitis B virus X protein induces cell death by causing loss of mitochondrial membrane potential. J Biol Chem. 2003;278(24):22071–22078. [DOI] [PubMed] [Google Scholar]
- 37.Tan C, Guo H, Zheng M, Chen Y, Huang W. Involvement of mitochondrial permeability transition in hepatitis B virus replication. Virus Res. 2009;145(2):307–311. [DOI] [PubMed] [Google Scholar]
- 38.Gearhart TL, Bouchard MJ. Replication of the hepatitis B virus requires a calcium-dependent HBx-induced G1 phase arrest of hepatocytes. Virology. 2010;407(1):14–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bonino F, Ponzetto A, Veglio V, Bosio G, Zaffino MC, Rizzetto M. The immunological diagnosis of HBsAg liver disease by combined screening for the e system in the serum and the HB core and surface antigens in liver biopsy samples. Ric Clin Lab. 1977;7(4):365–372. [DOI] [PubMed] [Google Scholar]
- 40.Chen Y, Tian Z. HBV-Induced Immune Imbalance in the Development of HCC. Front Immunol. 2019;10:2048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hu L, Chen L, Yang G, et al. HBx sensitizes cells to oxidative stress-induced apoptosis by accelerating the loss of Mcl-1 protein via caspase-3 cascade. Mol Cancer. 2011;10(43):1476–4598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bang E, Kim DH, Chung HY. Protease-activated receptor 2 induces ROS-mediated inflammation through Akt-mediated NF-κB and FoxO6 modulation during skin photoaging. Redox Biol. 2021;44(102022):26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ling LR, Zheng DH, Zhang ZY, et al. Effect of HBx on inflammation and mitochondrial oxidative stress in mouse hepatocytes. Oncol Lett. 2020;19(4):2861–2869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.He P, Lei J, Miao JN, Wu D, Wang C. Cordyceps sinensis attenuates HBx-induced cell apoptosis in HK-2 cells through suppressing the PI3K/Akt pathway. Int J Mol Med. 2020;45(4):1261–1269. [DOI] [PubMed] [Google Scholar]
- 45.Shpacovitch V, Feld M, Hollenberg MD, Luger TA, Steinhoff M. Role of protease-activated receptors in inflammatory responses, innate and adaptive immunity. J Leukoc Biol. 2008;83(6):1309–1322. [DOI] [PubMed] [Google Scholar]
- 46.Chen L, Gao B, Zhang Y, et al. PAR2 promotes M1 macrophage polarization and inflammation via FOXO1 pathway. J Cell Biochem. 2019;120(6):9799–9809. [DOI] [PubMed] [Google Scholar]
- 47.Liang DY, Sha S, Yi Q, et al. Hepatitis B X protein upregulates decoy receptor 3 expression via the PI3K/NF-kappaB pathway. Cell Signal. 2019;62:109346. [DOI] [PubMed] [Google Scholar]
- 48.Xia L, Tian D, Huang W, et al. Upregulation of IL-23 expression in patients with chronic hepatitis B is mediated by the HBx/ERK/NF-kappaB pathway. J Immunol. 2012;188(2):753–764. [DOI] [PubMed] [Google Scholar]
- 49.Chiu AP, Tschida BR, Sham TT, et al. HBx-K130M/V131I Promotes Liver Cancer in Transgenic Mice via AKT/FOXO1 Signaling Pathway and Arachidonic Acid Metabolism. Mol Cancer Res. 2019;17(7):1582–1593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Xu QG, Yuan SX, Tao QF, et al. A novel HBx genotype serves as a preoperative predictor and fails to activate the JAK1/STATs pathway in hepatocellular carcinoma. J Hepatol. 2019;70(5):904–917. [DOI] [PubMed] [Google Scholar]
- 51.Du C, Zhang T, Xiao X, Shi Y, Duan H, Ren Y. Protease-activated receptor-2 promotes kidney tubular epithelial inflammation by inhibiting autophagy via the PI3K/Akt/mTOR signalling pathway. Biochem J. 2017;474(16):2733–2747. [DOI] [PubMed] [Google Scholar]
- 52.Jiang Y, Lim J, Wu KC, Xu W, Suen JY, Fairlie DP. PAR2 induces ovarian cancer cell motility by merging three signalling pathways to transactivate EGFR. Br J Pharmacol. 2021;178(4):913–932. [DOI] [PubMed] [Google Scholar]
- 53.Hu L, Xia L, Zhou H, et al. TF/FVIIa/PAR2 promotes cell proliferation and migration via PKCalpha and ERK-dependent c-Jun/AP-1 pathway in colon cancer cell line SW620. Tumour Biol. 2013;34(5):2573–2581. [DOI] [PubMed] [Google Scholar]
- 54.Mohamed DI, Khairy E, Khedr SA, Habib EK, Elayat WM, El-Kharashi OA. N-acetylcysteine (NAC) alleviates the peripheral neuropathy associated with liver cirrhosis via modulation of neural MEG3/PAR2/ NF-B axis. Neurochem Int. 2020;132:104602. [DOI] [PubMed] [Google Scholar]
- 55.Xie L, Zheng Y, Li X, et al. Enhanced proliferation of human hepatoma cells by PAR-2 agonists via the ERK/AP-1 pathway. Oncol Rep. 2012;28(5):1665–1672. [DOI] [PubMed] [Google Scholar]