Abstract
Systematic review and meta-analysis are a gift to the modern researcher, delivering a crystallised understanding of the existing research data in any given space. This can include whether candidate drugs are likely to work or not and which are better than others, whether our models of disease have predictive value and how this might be improved and also how these all interact with disease pathophysiology.
Grappling with the literature needed for such analyses is becoming increasingly difficult as the number of publications grows. However, narrowing the focus of a review to reduce workload runs the risk of diminishing the generalisability of conclusions drawn from such increasingly specific analyses.
Moreover, at the same time as we gain greater insight into our topic, we also discover more about the flaws that undermine much scientific research. Systematic review and meta-analysis have also shown that the quality of much preclinical research is inadequate. Systematic review has helped reveal the extent of selection bias, performance bias, detection bias, attrition bias and low statistical power, raising questions about the validity of many preclinical research studies. This is perhaps the greatest virtue of systematic review and meta-analysis, the knowledge generated ultimately helps shed light on the limitations of existing research practice, and in doing so, helps bring reform and rigour to research across the sciences.
In this commentary, we explore the lessons that we have identified through the lens of preclinical systematic review and meta-analysis.
Keywords: systematic review, meta-analysis, preclinical
Introduction
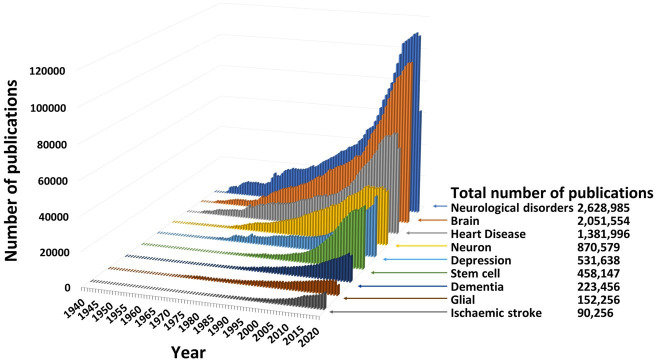
The rate of growth of knowledge claims and the literature in which these are communicated is now so great that we are faced with major data overload. For example, in the field of neurological disorders, more than 2.6 million papers were classified as relevant by PubMed ‘MeSH’ headings. Thousands more publications describe other similar fields of research (figure 1). No individual can read, let alone absorb and master all this detail, so we rely on summaries to gain an overview of a topic.
Figure 1.
Results of PubMed search performed on 3 March 2021 showing numbers of publications by year and totals for subject searches using ‘MeSH’ headings.
The traditional approach to summarising the literature has been the narrative review, where (at least conceptually) an expert, or group of experts, condenses the knowledge gained over one, or many lifetimes of work in a field. Journals value these reviews because they highlight areas of current development and attract many citations, raising the profile of the journal. Junior researchers like them because they provide a short-cut into a complex world they are just beginning to explore. Despite their undoubted value as important repositories of human wisdom, narrative reviews have limitations. In most instances, the literature described represents only a small proportion of the total evidence available, and it is rare for the reader to be able to discern the reasons for the selection of some papers and exclusion of others. It is also rare for narrative reviews to provide a quantitative summary of the underlying data to justify the conclusions which they reach. In both instances, we are forced to trust the judgement of the narrative review authors but are unable to judge the scientific basis of the claims made. An unacknowledged problem is that writing such reviews is a task often given to PhD students and junior post-doctoral researchers on starting a new project. While a great training tool, they cannot truly be considered expert reviews.
Systematic review and meta-analysis try to address these limitations. By clearly defining the scope of the review and providing replicable search criteria, their readers know explicitly what subject matter was assessed. By providing inclusion and exclusion criteria, systematic reviews also tell the reader what was considered important and what was not. Moreover, where performed, meta-analyses provide a formal environment for testing the statistical validity of claims made by the included literature. This includes assessment of whether or not publication bias might contribute to a falsely positive picture of a subject. By embracing the principles of Open Science,1 systematic reviews and meta-analyses have the additional advantage of permitting ongoing aggregation of data, an important consideration when faced with an ever-growing literature. However, this comes at a cost to the researcher. Because the process requires current searching and assessment of what is often a large body of literature, it is a demanding and time-consuming task. Consequently, systematic reviews usually have a very narrow focus and lack breadth. Ultimately, we need meta meta-research (meta-analysis of meta-analyses) to integrate these high-quality yet narrow snippets of information into research outputs with broader applicability. It should be remembered, however, that systematic review and meta-analysis contain a risk of error caused by the rigid application of procedure and that not all knowledge is a matter of data aggregation.
Importantly, asking pertinent questions, establishing effective criteria for inclusion and exclusion of publications and deciding what data to extract from a body of literature still requires expertise. It should also be noted that systematic reviews are not immune from human foibles. The principle of ‘garbage in, garbage out’ still applies. If this is recognised, systematic review and meta-analysis can provide a vehicle for assessing the consequences of poor science and may salvage important signals that might otherwise be missed if suboptimal science is discarded outright.
In this commentary, we discuss some of our key observations from the formative years of preclinical systematic review and meta-analysis research. We present these in the form of ‘lessons’ (box 1). These lessons are not about the scientific aims or conclusions drawn by individual papers but about the environment in which they are performed. We acknowledge that these might one day become the specific subjects of new systematic reviews and meta-analyses but to do so is beyond the scope of this commentary. Nevertheless, in the spirit of systematic review, where we provide examples to illustrate these lessons, we have selected data in an unbiased and transparent way.
Box 1. Summary of the lessons that have emerged from preclinical systematic review and meta-analysis.
Systematic review can compare interventions and assess preclinical models.
Literature overload limits research productivity.
Electronic databases have very different coverage.
Not all publications have value for meta-research.
The internal validity of many preclinical experiments is poor.
Publication bias is common.
Small preclinical studies present problems.
Promising reviews are rarely followed-up by their authors.
Lesson 1: systematic review can compare interventions and assess preclinical models
In most cases, systematic review and meta-analysis in the preclinical space are performed to ask the question ‘are we there yet?’. Is the preclinical data sufficient to support a move into the clinical sphere? A recent cross-sectional study (2015–2018) examining the epidemiology and reporting characteristics of preclinical systematic reviews found that 54% assessed pharmacological interventions and 46% assessed non-pharmacological (mainly cellular or surgical) interventions across 23 different disease domains.2 However, many studies do not just ask whether a single intervention works. Many also examine the relative efficacy within classes of interventions, how varying conditions employed during modelling modify the apparent efficacy of an intervention, and the validity of preclinical models themselves.
For example, a broad systematic review examining the efficacy of 1026 experimental treatments for stroke found that the best candidates were not always those taken to clinical trial. Additionally, as the breadth of experimental testing increased, effectiveness appeared to decrease.3 Another stroke study found that while systematic review supported treatment of ischaemic stroke with magnesium (anti-glutamatergic), melatonin (anti-oxidant) and minocyclin (anti-inflammatory), appropriately powered, randomised and blinded experiments across a range of ischaemic conditions were unable to detect any efficacy.4 This raises concerns about the true efficacy of many of the candidates that appear promising in systematic reviews. Conversely, in models of multiple sclerosis, systematic review has identified drugs effective across multiple outcome domains which have not been tested in humans suggesting clinical evaluation of these otherwise neglected drugs may be of value.5
Systematic review and meta-analysis have also been used to explore the experimental conditions most conducive to detection of benefit. For example, for stem cell therapies in renal disease, cardiac disease, stroke and spinal cord injury, therapeutic efficacy is inversely related to the size of the experimental animal, raising important questions about the doses that might be required for effective translation to humans.6 Others have used systematic review to support the face validity of models of, for example, cerebral palsy7 and xenograft models of colorectal cancer,8 or to justify calls for greater standardisation in preclinical experiments.9
Lesson 2: literature overload limits research productivity
The inclusivity of systematic reviewing presents a specific challenge, the person-power needed to find and sift publications for inclusion within the review. A utopian view would require us to perform broad reviews that examined the entire data set of a field of research. This would enable comparison of issues such as the strengths and weaknesses of our model systems, the relative merits of candidate drugs used within such models or the impact of interacting variables often present but not controlled for, such as anaesthesia in surgical models or staffing interactions during behavioural testing. Such broad studies are rare and probably unrealistic for most research teams until all publications are available in a searchable electronic format and automated text and data extraction tools mature. Asking a broad research question finds more publications than are easy to handle. As in science in general, asking a more specific question reduces the potential workload but at the probable expense of generalisability.
To illustrate this, we completed a simple and easily replicable search using PubMed’s ‘advanced’ search filter (figure 2). By searching for the neurological disorder ‘ischaemic stroke’ we can see that a large number of potentially relevant papers are found, much larger than any one reviewer could handle alone (78 730 total). Limiting the search to studies containing animals only and to studies listing particular treatments (hypothermia or aspirin), we reduce the number of studies identified with our search to a few hundred. This number is much more tractable for a systematic review project, but there is a risk that relevant papers are being missed in these narrower searches. That is, the increased specificity comes at the costs of some reduction in sensitivity.
Figure 2.

Results of simple searches performed in PubMed illustrating potential workload for systematic review and meta-analysis. Search completed on 19 January 2021 using the ‘advanced’ search option on the front page and the ‘species’ filter on the search results page.
We do not argue for less primary research but do argue that making such data more accessible for meta-research will improve future productivity and understanding. Importantly, consideration of logistics is critical when planning a systematic review. Automation tools are increasingly useful adjuncts to human effort, but the human effort is still substantial.
Lesson 3: electronic databases have very different coverage
Finding the publications would seem to be a simple task; after all, many electronic repositories/directories of the scientific literature are available for this purpose. However, no search engine provides access to a complete record of all published works. Search results with different tools vary with respect to subject matter and period of coverage, or how the indexing works (use of proprietary search and indexing algorithms and human curation).10 11 Changes to any of these databases or their indexing systems without public documentation also means that full reproducibility during future studies cannot be assured. Systematic reviewers must balance scientific purity against pragmatism.
Depending on your branch of science, you may have access to many literature databases. In our field, PubMed, EMBASE, Scopus (the latter two both owned by Elsevier) and Web of Science/BIOSIS are commonly used. While all cover biomedical and life science literature, their coverage differs substantially.11
To illustrate these issues of coverage and address additional questions below, we performed a simple search to find systematic reviews of preclinical therapeutic interventions to determine where these reviews found their primary data. On 3 March 2021, PubMed indexed 180 585 publications as systematic reviews. Of those with ‘Free full text’ available, PubMed’s inbuilt ‘other animal’ filter identified 5593 (3%) as potentially relevant to animal research. One thousand five hundred and seventy-two (0.9% of all PubMed returns, 28% of those filtered as ‘other animal’) self-identified as a systematic review, including the term in the article title. On inspection of these papers, we found 283 papers examining therapeutic interventions in preclinical disease models. We selected 20 of these at random for closer examination,12–31 their characteristics are summarised in table 1.
Table 1.
Characteristics of 20 randomly selected systematic reviews, including the disease or biological system of interest and the treatment/intervention being investigated. Total number of studies identified through systematic searches and number of studies included in each review’s final analysis were self-reported in each publication, percentage of studies included out of all studies identified was calculated by the authors of this paper
| Study (reference) | Disease/biological system | Treatment/intervention | # studies identified from searches | # studies included in final analysis | % studies included in final analysis |
| Albuquerque et al12 | Melanoma | Plant extracts | 1359 | 35 | 2.6 |
| Archambault et al13 | Neonatal hypoxic ischaemic encephalopathy (HIE) | Mesenchymal stem/stromal cells (MSCs) | 161 | 18 | 11.2 |
| Ashcraft et al14 | Cancer | Aerobic exercise | 466 | 53 | 11.4 |
| Auboire et al15 | Ischaemic stroke | Microbubbles (MBs) combined with ultrasound sonothrombolysis (STL) | 2506 | 16 | 0.6 |
| Cao et al16 | Gut microbiota | Anti-hyperglycaemic drugs | 4075 | 64 | 1.6 |
| Chen et al17 | Ischaemic stroke | Neural stem cells (NSCs) transplantation therapy | 2524 | 37 | 1.5 |
| Dong et al18 | Ischaemic stroke | Recombinant tissue plasminogen activator (rtPA) | 2128 | 47 | 2.2 |
| Gaubys et al19 | Regeneration of periodontal tissue complex | Stem cell therapy | 2099 | 10 | 0.5 |
| Janssen20 | Ischaemic stroke | Constraint-induced movement therapy (CIMT) | 3580 | 8 | 0.2 |
| Lambrecht21 | Anaemia | Animal husbandry and capture (AHC) | 9027 | 23 | 0.3 |
| Li et al22 | Hepatocellular carcinoma | Metformin | 573 | 13 | 2.3 |
| Liao et al23 | Injury to bone | Stem cell therapy | 202 | 20 | 9.9 |
| Ma et al24 | Ischaemic stroke | Xingnaojing injection (XNJI) | 392 | 23 | 5.9 |
| Senders et al25 | Glioma surgery | Agents for fluorescence-guided glioma surgery | 2619 | 105 | 4.0 |
| Silverblatt et al26 | Myocardial injury | Beta blockers, calcium channel blockers and antagonists of the renin–angiotensin system | 347 | 52 | 15.0 |
| Suen et al27 | Pulmonary arterial hypertension (PAH) | Regenerative cell therapies | 1368 | 45 | 3.3 |
| van der Bent et al28 | Heritable neurodegenerative and neuromuscular diseases | Antisense oligonucleotide (AON)-based therapies | 1330 | 95 | 7.1 |
| van der Spoel et al29 | Ischaemic heart disease | Stem cell therapy | 304 | 52 | 17.1 |
| Wei et al30 | Ischaemic stroke | Buyang Huanwu decoction (BHD) | 973 | 56 | 5.8 |
| Zhang et al31 | Vascular dementia | Acupuncture | 194 | 16 | 8.2 |
In these 20 studies, the authors had searched using the name of the intervention of interest within a broader pool of papers specific to a disease model in non-human animal species, giving little scope for ambiguity. The median number of databases searched by these reviews was 3 (range 1–7). The most commonly searched databases were PubMed, EMBASE and Web of Science. Examining the reason why publications were excluded from each review’s analysis shows that on average, 30% (range 0%–92.3%) of a review’s discarded papers were replicates found across multiple databases (median 214 replicates per review) (figure 3). The corollary of this is that many unique and potentially important papers would be missed if only a single database had been searched. The magnitude of this problem, and the extent to which adding a further database to be searched changes the conclusions of a meta-analysis, is unclear. Luijk32 found that for some reviews the global estimate of efficacy was lower when limited to studies identified through PubMed compared with all other databases; from PubMed and EMBASE compared with all other databases; and when inclusion was limited to English language publications. However, overall, there was no significant effect. Information scientists have been aware of such problems for decades. We should also remember—in the context of systematic reviews that aspire to assess all available data on a subject—that the English literature is not the only source of scientific data and it is critical that such language bias is avoided.33
Figure 3.

Screening data (extracted from PRISMA diagrams or text) from 20 randomly selected systematic reviews published between 2011 and 2020. Of the original 20 papers, three were excluded and replaced as the PRISMA diagrams contained mathematical errors. ‘Reasons for exclusion’ are expressed as a mean percentage of each review’s excluded publications. ‘Replicates’=studies found in multiple databases, only a single replicate is included in further screening and all other replicates are discarded, ‘irrelevant’=did not answer the research question asked by this review, ‘ineligible’=did not contain data useable by this review, ‘other’=papers excluded for reasons such as not having an available full text, being written in languages not included in the review or being a publication other than a primary research article. PRISMA, Preferred Reporting Items for Systematic Reviews and Meta-Analyses.
Lesson 4: not all publications have value for meta-research
Among several thousand publications found by electronic searching, examination of the search results and application of the systematic review inclusion and exclusion criteria also illustrates that the returns of electronic searches do not always provide a high yield of relevant data. We examined the total identified, excluded and included studies in our 20 randomly selected reviews (table 1) and noted the reasons nominated by review authors for their exclusions (figure 3). In the 20 reviews, each identified a large number of papers that might have been relevant (median 1345 publications identified per review, range 161–9027). However, on average, only 5.5% (range 0.2%–17.1%, median 36 publications per review) of these potentially relevant papers were included in each review’s final data set and their analyses. If we consider the other 94.5% of identified publications which were excluded per review (range 82.9%–99.8%, median 1279 excluded publications), we find that 61% of them (range 7.1%–99.9%, median 624 publications per review) were excluded as they were considered irrelevant when the abstracts were read by a screening team. Additionally, an average of 7% (range 0%–15.5%, median 15.5 publications) of each review’s excluded publications were excluded by the review authors because of omissions in data reporting. Usually this takes the form of reporting outcome measures that are not usable for meta-analysis or missing important parameters in the describing of methods. From the perspective of a systematic reviewer, this is frustrating. An inordinate amount of effort goes into evaluation of papers of little value. One could argue this is highly dependent on the sensitivity/specificity of the search terms used. However, it should be remembered that we selected the data for figure 3 because the authors of these systematic reviews were asking relatively unambiguous questions about very specific interventions. It is likely that studies asking more complex and ambiguous questions might have larger initial searches and exclude even more studies. This strongly suggests that better sensitivity and specificity require improvements to the indexing provided by the respective databases and the clarity of titles, abstracts and key words provided by originating authors.
The inclusion of articles that are subsequently discovered to be fraudulent may also contribute to a systematic review if the review is performed prior to the fraudulent discovery. However, it is outside the scope of the reviewer to detect this given the lack of access to raw data, except for circumstances where individual subject data is used. The time of the systematic reviewer is of the least concern here, when set against the grant funding gone to waste, the exposure of animals to harms with little benefit in knowledge in return, and the efforts of research teams squandered by poor reporting.
Lesson 5: the internal validity of many preclinical experiments is poor
As stated by the late Doug Altman and colleagues ‘If the raw material is flawed then the conclusions of systematic reviews cannot be trusted’.34 The internal validity of a study is threatened by bias, ‘any process at any stage of inference tending to produce results that differ systematically from the true values’.35 In preclinical studies, as in clinical trials, we see selection bias, performance bias, detection bias and attrition bias. Moreover, most publications fail to include information that would allow a reader to know to what extent biases might have been prevented.4 36 37 Within the preclinical literature, a variety of tools have been used to assess overall quality and risk of bias. Within the 20 preclinical systematic reviews we randomly selected as illustrative of trends within the field, the majority used either the Collaborative Approach to Meta Analysis and Review of Animal Data from Experimental Studies (CAMARADES) quality score checklist38 or SYstematic Review Center for Laboratory animal Experimentation’s (SYRCLE) risk of bias tool.39 Two studies made no assessment of study quality or risk of bias. Worryingly, only one study assessed both, which raises the possibility that the other 17 studies were unaware that assessing a study’s quality and assessing the study’s risk of bias are not the same thing (table 2). Risk of bias tools assess a publication for the potential presence of specific biases (listed above). Quality scores assign yes/no answers to questions regarding disease model specific norms, randomisation, blinding, reporting ethics approvals or conflicts of interest to assess a paper’s overall adherence to good research practices. For each of the systematic reviews we assessed, included publications scored poorly against either of these types of tool. The highest median score using SYRCLE’s risk of bias tool was 5 out of 10, which is much lower than desirable. Reviews reporting a quality score were similar. No review using the CAMARADES quality rating scored a single included publication higher than 8 out of 10, with the median score being 4.
Table 2.
Median and range for quality/RoB scores were calculated for the studies included in each review
| Study (reference) | Assessed quality/RoB | Score for included publications, median (range) | Scoring system |
| Auboire et al15 | Yes—Quality | 4.5 (3–6) | CAMARADES |
| Chen et al17 | Yes—Quality | 5 (3–7) | CAMARADES |
| Dong et al18 | Yes—Quality | 4 (2–6) | CAMARADES |
| Janssen et al20 | Yes—Quality | 5 (2–6) | CAMARADES |
| Liao et al23 | Yes—Quality | 2.5 (1–4) | Jadad scale (modified) |
| Ma et al24 | Yes—Quality | 4 (3–6) | CAMARADES |
| van der Spoel et al29 | Yes—Quality | 1 (0–5) | Authors' custom scale |
| Wei et al30 | Yes—Quality | 3 (2–6) | CAMARADES |
| Zhang et al31 | Yes—Quality | 5.5 (4–8) | CAMARADES |
| Silverblatt et al26 | Yes—Both | 5 (3–8) | CAMARADES (modified) |
| 5 (1–10) | SYRCLE’s Risk of Bias Tool | ||
| Albuquerque et al12 | Yes—RoB | 62.9 (40–74.3) | ARRIVE |
| Archambault et al13 | Yes—RoB | 4 (4–8) | SYRCLE’s Risk of Bias Tool |
| Cao et al16 | Yes—RoB | 3 (2–4) | SYRCLE’s Risk of Bias Tool |
| Gaubys et al19 | Yes—RoB | 3 (1–4) | Cochrane’s Risk of Bias Tool |
| Lambrecht et al21 | Yes—RoB | 2 (1–4) | GRADE (modified) |
| Li et al22 | Yes—RoB | 1.5 (1–5) | SYRCLE’s Risk of Bias Tool |
| Suen et al27 | Yes—RoB | 1 (0–2) | SYRCLE’s Risk of Bias Tool |
| van der Bent et al28 | Yes—RoB | 2 (0–8) | SYRCLE’s Risk of Bias Tool |
| Ashcraft et al14 | No | – | – |
| Senders et al25 | No | – | – |
CAMARADES score is out of 10, Jadad scale is out of 5, SYRCLE’s Risk of Bias Tool is out of 10, ARRIVE score is a percentage, Cochrane’s Risk of Bias Tool is out of 7, GRADE score is out of 5, van der Spoel’s (2011) custom scale is out of 5.
ARRIVE, Animal Research: Reporting of In Vivo Experiments; CAMARADES, Collaborative Approach to Meta Analysis and Review of Animal Data from Experimental Studies; GRADE, Grading of Recommendations Assessment, Development, and Evaluation; RoB, Risk of bias; SYRCLE, SYstematic Review Center for Laboratory animal Experimentation.
While it can be argued that these poor assessments merely reflect a failure to report these issues and is of little consequence, the data suggest otherwise. Where systematic reviews have been able to stratify data by the presence or absence of randomisation and blinding (which should guard against bias), the detected effect sizes are often substantially reduced by the presence of these measures.38 40–46 Further, the increase in reporting which followed implementation of a change in editorial policy at nature was largely due to an increase in researchers reporting that they had not randomised, had not blinded or had not conducted a sample size calculation.47
Lesson 6: publication bias is common
Publication bias (also known as dissemination bias) is the phenomenon whereby studies with positive results are more likely to be published than research with negative or neutral results, causing an over-representation of positive findings in the literature. This distorts that literature, making interpretation and assignment of value to knowledge claims difficult. In 2011, a systematic review of over 4600 research papers from different disciplines found that the frequency of publishing positive findings rose by over 22% between 1990 and 2007, with the fields of clinical medicine, pharmacology and toxicology, and molecular biology the worst affected.48
In preclinical stroke research, estimates of the proportion of missing neutral or negative studies ranged from 5% to 36% for different candidate therapies and across the field this constituted an overstatement of efficacy (relative) of 31%.49 Excess significance (where there are more statistically significant results than should be expected) has been detected in most preclinical systematic reviews in neuroscience50 and publication bias probably exists in most of the preclinical literature.51 A 2019 Nature commentary co-signed by over 800 statisticians discussed that of these statistically significant papers dominating the literature, 51% were likely wrongly interpreted, and most of them likely interpreted their results with the false belief that a significant p value determines whether a result is ‘real’ or not.52 Because systematic reviews should find all the available data, a biased literature will lead to a biased synthesis of that evidence unless statistical methods are used to identify and where possible correct for this problem.50 53 However, despite systematic reviews being well positioned to take advantage of their comprehensive data collection to check for bias, Mueller and colleagues found that only 50% of preclinical systematic reviews chose to assess publication bias in their analysis.54 Of the 20 studies we analysed for this commentary, only 11 (55%) considered publication bias.
The presence of publication bias is important. New studies using biased foundational data run the risk of unnecessary repetition of futile science that has been performed but not reported. This wastes valuable resources, leads to unethical use of animals and ultimately puts humans at risk if, consequently, they are recruited into misguided clinical trials.55 56
Among the standard tools in assessment of publication bias are the funnel plot, and derivatives such as Egger’s regression. These detect asymmetry in the distribution of the effect size of a result compared with a measure of its precision. In the absence of bias, the distribution is ‘funnel’ shaped and symmetrical about its mean with small imprecise studies, most influenced by random variation, distributed broadly towards the base. When publication bias is present, smaller, less precise studies, reporting negative or neutral results, will be missing and the distribution becomes skewed.57
Lesson 7: small preclinical studies present problems
In 3145 preclinical stroke studies undertaken on 45 476 animals, the average cohort size was 7 animals per treatment group.58 In 2016, using similar data from the CAMARADES data set (mean group size=8), it was calculated that the statistical power of the preclinical stroke literature is only 45%, implying that half of the studies investigating a hypothesis which was correct would fail to find statistical evidence to support this. This is still, however, much larger than the 23% median power calculated across >700 neuroscience experiments.59 This problem is not unique to preclinical research. One review of data held within the Cochrane Reviews data set concluded that in 70% of 14 886 meta-analyses, all the publications included in the analyses were underpowered.60 Because small underpowered studies have a low chance of detecting a real effect, the statistical power of an experiment is a critical determinant of its value to users of the data. Meta-analysis can overcome this individual lack of power.
However, small sample size is also associated with more insidious risks. Publication bias is not the only possible explanation for funnel plot asymmetry. Small-study effects, where smaller studies show greater effects than larger studies are also detected this way. In both clinical and preclinical settings, this can occur because of heterogeneity between subjects in different sized studies.50 61 Such heterogeneity can occur for many reasons. For example, a small hospital-based study may not provide as good a representation of patients receiving a treatment as a large population-based study. Moreover, the hospital patients may have additional undeclared reasons for their selection into the study. In preclinical studies, it has been argued that heterogeneity between studies can be a good thing if it broadens the biological base of a series of experiments to improve generalisability.62 The caveat is that this benefit is only realised when research teams cooperate in data pooling or multicenter studies or when meta-research is conducted. If the heterogeneity is present, for example, because of attrition bias due to selective reporting of results in a subset of studies, it is clearly not beneficial. One study shows that attrition bias can inflate effect sizes by 25%–175% with the inflation greatest for the smallest studies.63 Moreover, because biases tend to co-occur in small studies, they have a disproportionate chance of being published and providing a falsely positive impression in preclinical meta-analyses.64 These issues leave the systematic reviewer with a number of important quandaries; when is the body of evidence big enough for systematic review or meta-analysis to add value to a field of research; do imperfect studies provide value despite their imperfections; should small studies be excluded because of an assumption of bias or is such an assumption an even bigger risk to finding the truth? At present these questions remain unanswered.
Lesson 8: promising reviews are rarely followed-up by their authors
Within the 20 preclinical therapeutic meta-analyses we randomly selected, examination of the citations of these papers within PubMed (11 May 2021) revealed that many had been extensively cited. For example; 91 citations29; 44 citations14; 27 citations25; 24 citations30. However, for most, the citations provided no evidence of direct experimental follow-up (preclinical or clinical) of the principal findings by the systematic review team (15/20). While lack of time for follow-up is one potential explanation for this, especially with the more recent reviews, a disturbing alternative is that many systematic reviewers are not embedded within the experimental science teams where the knowledge they generate would have most value. These reviews may have been undertaken simply for the sake of doing a study, instead of for their most valuable purpose, to inform biological knowledge and improve experimental studies.
Moreover, while most authors (65%) concluded that the intervention being studied was effective, few (20%) also concluded that the evidence available was robust (table 3). This demonstrates that systematic reviewers are aware that the quality of their data does not lend itself to robust conclusions. We use the term robust here in the sense defined in the Oxford English Dictionary—strong; able to survive being used a lot and not likely to break—in order to capture the gestalt of a range of quality/risk of bias scoring systems and author conclusions that point towards an interpretation that the authors believed their analysis to have sufficiently answered their question or not. This robustness was our conclusion from their reviews and is of necessity subjective. However, it is perturbing that these studies do not provoke further research, as this might lend more weight to these positive findings and possibly lead to effective therapeutics being carried through to clinical trials. Where a team conducting a systematic review is able to interact with teams conducting the primary research, the quality and impact of subsequent research is enhanced (see for example, McCann et al65).
Table 3.
Conclusions drawn by authors of systematic reviews on the effectiveness of studied therapies and the robustness of the included data, n=20
| Total reviews | % of reviews | References | |
| Conclusion drawn on treatment | |||
| Effective | 13 | 65 | 12 13 16 17 19 22–24 26 27 29–31 |
| Unsure | 6 | 30 | 14 15 18 21 25 28 |
| Not effective | 1 | 5 | 20 |
| Conclusion drawn on robustness of work | |||
| Robust evidence, results could be refined further but is not essential | 4 | 20 | 17 19 29 31 |
| More evidence would be beneficial | 8 | 40 | 12 13 20 22–24 27 30 |
| Not enough evidence, more needed | 7 | 35 | 14–16 21 25 26 28 |
| Not reported | 1 | 5 | 18 |
Conclusions
Preclinical systematic review and meta-analysis are in its infancy and, like preclinical science itself, are busy absorbing lessons already learnt by other fields and earlier generations of scientists. The lessons presented above highlight considerations of the conduct of systematic reviews and where they can be improved to provide more informative information for the many scientific fields these reviews are important for. Data overload is common to all modern science and is a symptom of its successes. Dealing with that data overload requires a number of strategies. In the short term, we can ask ever more specific questions, but this focus occurs at the expense of the generalisability of our conclusions. We must build better ways of storing, indexing, retrieving and ensuring the availability of the data we generate. For meta-research in particular, it is important to be able to readily find and extract the data from its accompanying commentary. While systematic review and meta-analysis have played an important part in identifying the presence and importance of a variety of biases in the preclinical literature, until they are eliminated by better experimental conduct and reporting, we need to remain vigilant to their potential influence within our reviews. The recent advance in pre-registration of preclinical studies may help with improved experimental conduct and reporting. Moreover, because the foundations of our field include exposing the influence of poor scientific behaviours, we should adopt only the highest standards for our own work and expect that, as we learn more, these standards are likely to become more rigorous across the broader field of science. These increased standards would provide greater definiteness to the conclusions reached by systematic review and meta-analysis.
Footnotes
Contributors: DWH conceptualised and led the preparation of the manuscript. DWH and AAMR contributed equally to the data acquisition and analysis. DWH, AAMR, BAS, LML and MM contributed equally to drafting and editing of the manuscript the manuscript. All authors are accountable for the content of the manuscript and provided approval for publication.
Funding: The authors received funding from a Royal Hobart Hospital Research Foundation Grant.
Competing interests: None declared.
Provenance and peer review: Not commissioned; externally peer reviewed.
The data and associated code have been made available at https://doi.org/10.5281/zenodo.5010706.
Open material: The data and associated code have been made available at https://doi.org/10.5281/zenodo.5010706.
Data availability statement
Data are available in a public, open access repository.
Ethics statements
Patient consent for publication
Not required.
Ethics approval
This study does not involve human or animal participants.
References
- 1.Burgelman J-C, Pascu C, Szkuta K, et al. Open science, open data, and open scholarship: European policies to make science fit for the twenty-first century. Front Big Data 2019;2:43. 10.3389/fdata.2019.00043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hunniford VT, Montroy J, Fergusson DA, et al. Epidemiology and reporting characteristics of preclinical systematic reviews. PLoS Biol 2021;19:e3001177. 10.1371/journal.pbio.3001177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.O'Collins VE, Macleod MR, Donnan GA, et al. 1,026 experimental treatments in acute stroke. Ann Neurol 2006;59:467–77. 10.1002/ana.20741 [DOI] [PubMed] [Google Scholar]
- 4.O'Collins VE, Macleod MR, Cox SF, et al. Preclinical drug evaluation for combination therapy in acute stroke using systematic review, meta-analysis, and subsequent experimental testing. J Cereb Blood Flow Metab 2011;31:962–75. 10.1038/jcbfm.2010.184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hooijmans CR, Hlavica M, Schuler FAF, et al. Remyelination promoting therapies in multiple sclerosis animal models: a systematic review and meta-analysis. Sci Rep 2019;9:822. 10.1038/s41598-018-35734-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zwetsloot P-P, Antonic-Baker A, Gremmels H, et al. Combined meta-analysis of preclinical cell therapy studies shows overlapping effect modifiers for multiple diseases. BMJ Open Sci 2021;5:e100061. 10.1136/bmjos-2020-100061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Basilious A, Yager J, Fehlings MG. Neurological outcomes of animal models of uterine artery ligation and relevance to human intrauterine growth restriction: a systematic review. Dev Med Child Neurol 2015;57:420–30. 10.1111/dmcn.12599 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brown KM, Xue A, Mittal A, et al. Patient-derived xenograft models of colorectal cancer in pre-clinical research: a systematic review. Oncotarget 2016;7:66212–25. 10.18632/oncotarget.11184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bansal S, Keah NM, Neuwirth AL, et al. Large animal models of meniscus repair and regeneration: a systematic review of the state of the field. Tissue Eng Part C Methods 2017;23:661–72. 10.1089/ten.TEC.2017.0080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.M: G. Google Scholar to overshadow them all? comparing the sizes of 12 academic search engines and bibliographic databases. Scientometrics 2019;118:177–214. [Google Scholar]
- 11.Falagas ME, Pitsouni EI, Malietzis GA, et al. Comparison of PubMed, Scopus, web of science, and Google Scholar: strengths and weaknesses. Faseb J 2008;22:338–42. 10.1096/fj.07-9492LSF [DOI] [PubMed] [Google Scholar]
- 12.Albuquerque KRS, Pacheco NM, Del Rosario Loyo Casao T, et al. Applicability of plant extracts in preclinical studies of melanoma: a systematic review. Mediators Inflamm 2018;2018:6797924 10.1155/2018/6797924 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Archambault J, Moreira A, McDaniel D, et al. Therapeutic potential of mesenchymal stromal cells for hypoxic ischemic encephalopathy: a systematic review and meta-analysis of preclinical studies. PLoS One 2017;12:e0189895. 10.1371/journal.pone.0189895 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ashcraft KA, Peace RM, Betof AS, et al. Efficacy and mechanisms of aerobic exercise on cancer initiation, progression, and metastasis: a critical systematic review of in vivo preclinical data. Cancer Res 2016;76:4032–50. 10.1158/0008-5472.CAN-16-0887 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Auboire L, Sennoga CA, Hyvelin J-M, et al. Microbubbles combined with ultrasound therapy in ischemic stroke: a systematic review of in-vivo preclinical studies. PLoS One 2018;13:e0191788. 10.1371/journal.pone.0191788 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cao TTB, Wu K-C, Hsu J-L, et al. Effects of non-insulin anti-hyperglycemic agents on gut microbiota: a systematic review on human and animal studies. Front Endocrinol 2020;11:573891. 10.3389/fendo.2020.573891 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chen L, Zhang G, Gu Y, et al. Meta-analysis and systematic review of neural stem cells therapy for experimental ischemia stroke in preclinical studies. Sci Rep 2016;6:32291. 10.1038/srep32291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dong M-X, Hu Q-C, Shen P, et al. Recombinant tissue plasminogen activator induces neurological side effects independent on thrombolysis in mechanical animal models of focal cerebral infarction: a systematic review and meta-analysis. PLoS One 2016;11:e0158848. 10.1371/journal.pone.0158848 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gaubys A, Papeckys V, Pranskunas M. Use of autologous stem cells for the regeneration of periodontal defects in animal studies: a systematic review and meta-analysis. J Oral Maxillofac Res 2018;9:e3. 10.5037/jomr.2018.9203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Janssen H, Speare S, Spratt NJ, et al. Exploring the efficacy of constraint in animal models of stroke: meta-analysis and systematic review of the current evidence. Neurorehabil Neural Repair 2013;27:3–12. 10.1177/1545968312449696 [DOI] [PubMed] [Google Scholar]
- 21.Lambrecht NJ, Wilson ML, Jones AD. Assessing the impact of animal husbandry and capture on anemia among women and children in low- and middle-income countries: a systematic review. Adv Nutr 2019;10:331–44. 10.1093/advances/nmy080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Li J, Hernanda PY, Bramer WM, et al. Anti-tumor effects of metformin in animal models of hepatocellular carcinoma: a systematic review and meta-analysis. PLoS One 2015;10:e0127967. 10.1371/journal.pone.0127967 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Liao Y, Zhang X-L, Li L, et al. Stem cell therapy for bone repair: a systematic review and meta-analysis of preclinical studies with large animal models. Br J Clin Pharmacol 2014;78:718–26. 10.1111/bcp.12382 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ma R, Ma X, Wen J, et al. Preclinical evidence and mechanism of Xingnaojing injection for cerebral ischemia: a systematic review and meta-analysis of animal studies. Evid Based Complement Alternat Med 2018;2018:9624175. 10.1155/2018/9624175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Senders JT, Muskens IS, Schnoor R, et al. Agents for fluorescence-guided glioma surgery: a systematic review of preclinical and clinical results. Acta Neurochir 2017;159:151–67. 10.1007/s00701-016-3028-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Silverblatt JA, Ziff OJ, Dancy L, et al. Therapies to limit myocardial injury in animal models of myocarditis: a systematic review and meta-analysis. Basic Res Cardiol 2019;114:48. 10.1007/s00395-019-0754-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Suen CM, Stewart DJ, Montroy J, et al. Regenerative cell therapy for pulmonary arterial hypertension in animal models: a systematic review. Stem Cell Res Ther 2019;10:75. 10.1186/s13287-019-1172-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.van der Bent ML, Paulino da Silva Filho O, van Luijk J, et al. Assisted delivery of antisense therapeutics in animal models of heritable neurodegenerative and neuromuscular disorders: a systematic review and meta-analysis. Sci Rep 2018;8:4181. 10.1038/s41598-018-22316-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.van der Spoel TIG, Jansen of Lorkeers SJ, Agostoni P, et al. Human relevance of pre-clinical studies in stem cell therapy: systematic review and meta-analysis of large animal models of ischaemic heart disease. Cardiovasc Res 2011;91:649–58. 10.1093/cvr/cvr113 [DOI] [PubMed] [Google Scholar]
- 30.Wei R-L, Teng H-J, Yin B, et al. A systematic review and meta-analysis of buyang huanwu decoction in animal model of focal cerebral ischemia. Evid Based Complement Alternat Med 2013;2013:138484. 10.1155/2013/138484 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhang Z-Y, Liu Z, Deng H-H, et al. Effects of acupuncture on vascular dementia (Vd) animal models: a systematic review and meta-analysis. BMC Complement Altern Med 2018;18:302. 10.1186/s12906-018-2345-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.AKRv: L. The next steps towards responsible animal based-research. evaluation of strategies to improve scientific quality and responsible animal use in research. Radboud University, 2017. [Google Scholar]
- 33.Aalbers MB. Creative destruction through the Anglo-American hegemony: a non-Anglo-American view on publications, Referees and language. Area 2004;36:319–22. 10.1111/j.0004-0894.2004.00229.x [DOI] [Google Scholar]
- 34.Jüni P, Altman DG, Egger M. Systematic reviews in health care: assessing the quality of controlled clinical trials. BMJ 2001;323:42–6. 10.1136/bmj.323.7303.42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Murphy EA. The logic of medicine. Am J Med 1979;66:907–9. 10.1016/0002-9343(79)90443-1 [DOI] [PubMed] [Google Scholar]
- 36.Macleod MR, Lawson McLean A, Kyriakopoulou A, et al. Risk of bias in reports of in vivo research: a focus for improvement. PLoS Biol 2015;13:e1002273. 10.1371/journal.pbio.1002273 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kilkenny C, Parsons N, Kadyszewski E, et al. Survey of the quality of experimental design, statistical analysis and reporting of research using animals. PLoS One 2009;4:e7824. 10.1371/journal.pone.0007824 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Macleod MR, O'Collins T, Howells DW, et al. Pooling of animal experimental data reveals influence of study design and publication bias. Stroke 2004;35:1203–8. 10.1161/01.STR.0000125719.25853.20 [DOI] [PubMed] [Google Scholar]
- 39.Hooijmans CR, Rovers MM, de Vries RB, et al. Langendam MW: SYRCLE’s risk of bias tool for animal studies. BMC Med Res Methodol 2014;14:43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Macleod MR, van der Worp HB, Sena ES, et al. Evidence for the efficacy of NXY-059 in experimental focal cerebral ischaemia is confounded by study quality. Stroke 2008;39:2824–9. 10.1161/STROKEAHA.108.515957 [DOI] [PubMed] [Google Scholar]
- 41.Bebarta V, Luyten D, Heard K. Emergency medicine animal research: does use of randomization and blinding affect the results? Acad Emerg Med 2003;10:684–7. 10.1111/j.1553-2712.2003.tb00056.x [DOI] [PubMed] [Google Scholar]
- 42.Vesterinen HM, Sena ES, ffrench-Constant C, et al. Improving the translational hit of experimental treatments in multiple sclerosis. Mult Scler 2010;16:1044–55. 10.1177/1352458510379612 [DOI] [PubMed] [Google Scholar]
- 43.Hirst JA, Howick J, Aronson JK, et al. The need for randomization in animal trials: an overview of systematic reviews. PLoS One 2014;9:e98856. 10.1371/journal.pone.0098856 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sena E, van der Worp HB, Howells D, et al. How can we improve the pre-clinical development of drugs for stroke? Trends Neurosci 2007;30:433–9. 10.1016/j.tins.2007.06.009 [DOI] [PubMed] [Google Scholar]
- 45.Percie du Sert N, Hurst V, Ahluwalia A, et al. The ARRIVE guidelines 2.0: updated guidelines for reporting animal research. PLoS Biol 2020;18:e3000410. 10.1371/journal.pbio.3000410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Percie du Sert N, Ahluwalia A, Alam S, et al. Reporting animal research: explanation and elaboration for the ARRIVE guidelines 2.0. PLoS Biol 2020;18:e3000411. 10.1371/journal.pbio.3000411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.group TNC. Did a change in Nature journals’ editorial policy for life sciences research improve reporting? BMJ Open Science 2019;3:e000035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Fanelli D. Negative results are disappearing from most disciplines and countries. Scientometrics 2012;90:891–904. 10.1007/s11192-011-0494-7 [DOI] [Google Scholar]
- 49.Sena ES, van der Worp HB, Bath PMW, et al. Publication bias in reports of animal stroke studies leads to major overstatement of efficacy. PLoS Biol 2010;8:e1000344. 10.1371/journal.pbio.1000344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Tsilidis KK, Panagiotou OA, Sena ES, et al. Evaluation of excess significance bias in animal studies of neurological diseases. PLoS Biol 2013;11:e1001609. 10.1371/journal.pbio.1001609 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Korevaar DA, Hooft L, ter Riet G. Systematic reviews and meta-analyses of preclinical studies: publication bias in laboratory animal experiments. Lab Anim 2011;45:225–30. 10.1258/la.2011.010121 [DOI] [PubMed] [Google Scholar]
- 52.Amrhein V, Greenland S, McShane B. Scientists rise up against statistical significance. Nature 2019;567:305–7. 10.1038/d41586-019-00857-9 [DOI] [PubMed] [Google Scholar]
- 53.Lin L, Chu H. Quantifying publication bias in meta-analysis. Biometrics 2018;74:785–94. 10.1111/biom.12817 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Mueller KF, Briel M, Strech D, et al. Dissemination bias in systematic reviews of animal research: a systematic review. PLoS One 2014;9:e116016. 10.1371/journal.pone.0116016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Chalmers I, Bracken MB, Djulbegovic B, et al. How to increase value and reduce waste when research priorities are set. Lancet 2014;383:156–65. 10.1016/S0140-6736(13)62229-1 [DOI] [PubMed] [Google Scholar]
- 56.Joober R, Schmitz N, Annable L, et al. Publication bias: what are the challenges and can they be overcome? J Psychiatry Neurosci 2012;37:149–52. 10.1503/jpn.120065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Peters JL, Sutton AJ, Jones DR, et al. Comparison of two methods to detect publication bias in meta-analysis. JAMA 2006;295:676–80. 10.1001/jama.295.6.676 [DOI] [PubMed] [Google Scholar]
- 58.O’Collins VE, Donnan GA, Macleod MR, et al. Animal Models of Ischemic Stroke Versus Clinical Stroke: Comparison of Infarct Size, Cause, Location, Study Design, and Efficacy of Experimental Therapies. In: Animal Models for the Study of Human Disease. Academic Press 2017:481–523. [Google Scholar]
- 59.Dirnagl U: Thomas Willis Lecture: is translational stroke research broken. and if So, How Can We Fix It? Stroke 2016;47:2148–53. [DOI] [PubMed] [Google Scholar]
- 60.Turner RM, Bird SM, Higgins JPT. The impact of study size on meta-analyses: examination of underpowered studies in Cochrane reviews. PLoS One 2013;8:e59202. 10.1371/journal.pone.0059202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.IntHout J, Ioannidis JPA, Borm GF, et al. Small studies are more heterogeneous than large ones: a meta-meta-analysis. J Clin Epidemiol 2015;68:860–9. 10.1016/j.jclinepi.2015.03.017 [DOI] [PubMed] [Google Scholar]
- 62.Voelkl B, Vogt L, Sena ES, et al. Reproducibility of preclinical animal research improves with heterogeneity of study samples. PLoS Biol 2018;16:e2003693. 10.1371/journal.pbio.2003693 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Holman C, Piper SK, Grittner U, et al. Where have all the rodents gone? the effects of attrition in experimental research on cancer and stroke. PLoS Biol 2016;14:e1002331. 10.1371/journal.pbio.1002331 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Button KS, Ioannidis JPA, Mokrysz C, et al. Power failure: why small sample size undermines the reliability of neuroscience. Nat Rev Neurosci 2013;14:365–76. 10.1038/nrn3475 [DOI] [PubMed] [Google Scholar]
- 65.McCann SK, Cramond F, Macleod MR, et al. Systematic review and meta-analysis of the efficacy of interleukin-1 receptor antagonist in animal models of stroke: an update. Transl Stroke Res 2016;7:395–406. 10.1007/s12975-016-0489-z [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data are available in a public, open access repository.